Abstract
Aims—To determine whether neutrophil elastase and cathepsin G are expressed, at transcriptional or translational levels, in the bone marrow from a patient with Chediak-Higashi syndrome.
Methods—Blood neutrophils were isolated from three patients with Chediak-Higashi disease and bone marrow was collected from one. Cell lysates were analysed for neutrophil elastase and cathepsin G activity by enzyme linked immunosorbent assay and western immunoblotting. Northern blotting was used to detect messenger RNA (mRNA) for cathepsin G, elastase and β-actin in bone marrow extracts, and immunohistochemistry was used to localise the enzymes in marrow myeloid cells.
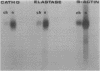
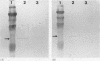
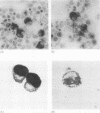
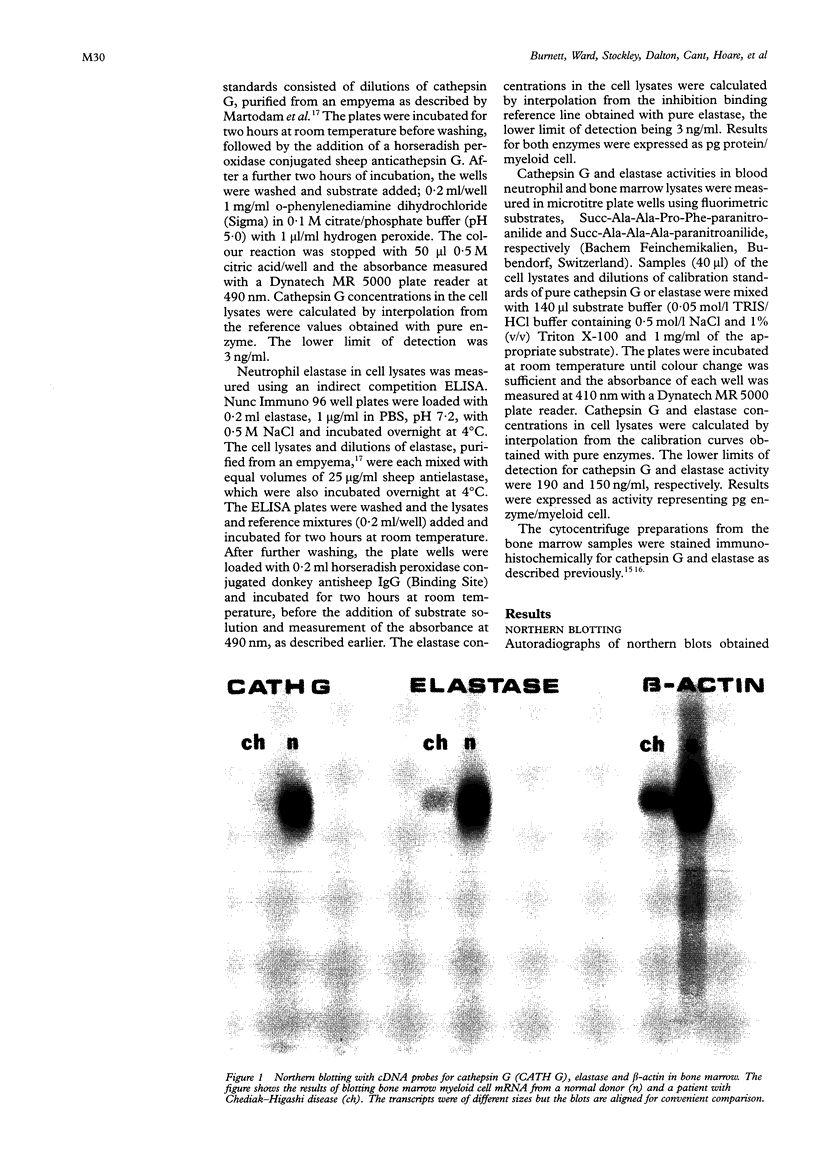
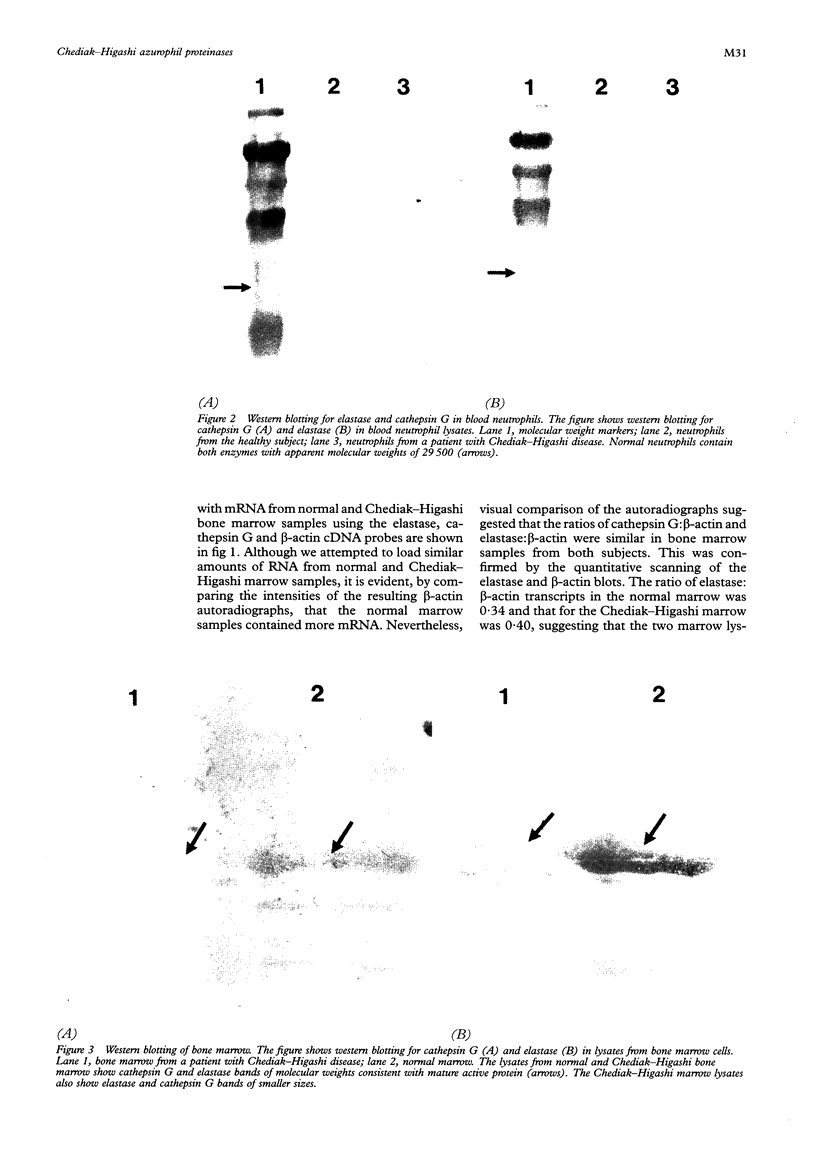
Results—Elastase and cathepsin G were not detected in blood neutrophils from the patients with Chediak-Higashi disease, but were present in bone marrow cells, although immunohistochemistry showed they were not within cytoplasmic granules. The concentrations of elastase and cathepsin G in Chediak-Higashi bone marrow were about 25 and 15%, respectively, of those in normal marrow. Quantitative scanning of northern blots showed that elastase and cathepsin G mRNA, corrected for β-actin mRNA, were expressed equally in normal marrow.
Conclusions—Transcription of elastase and cathepsin G mRNA in promyelocytes of patients with Chediak-Higashi disease is normal, but the protein products are deficient in these cells and absent in mature neutrophils. This suggests that the translated proteins are not packaged into azurophil granules but are degaded or secreted from the cells.
Keywords: Chediak-Higashi syndrome
Keywords: elastase
Keywords: cathepsin G
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P. Solubilization in formamide protects RNA from degradation. Nucleic Acids Res. 1992 Jul 25;20(14):3791–3792. doi: 10.1093/nar/20.14.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J., Jenkins R., Burnett D. Immunohistochemical demonstration of leucocyte elastase in human tissues. J Clin Pathol. 1984 Oct;37(10):1114–1118. doi: 10.1136/jcp.37.10.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J., Jenkins R., Burnett D. Immunohistochemical localization of cathepsin G in human tissues. Am J Surg Pathol. 1985 May;9(5):338–343. doi: 10.1097/00000478-198505000-00003. [DOI] [PubMed] [Google Scholar]
- Farley D., Salvesen G., Travis J. Molecular cloning of human neutrophil elastase. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):3–7. [PubMed] [Google Scholar]
- Fouret P., du Bois R. M., Bernaudin J. F., Takahashi H., Ferrans V. J., Crystal R. G. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J Exp Med. 1989 Mar 1;169(3):833–845. doi: 10.1084/jem.169.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. S., Parmley R. T., Rice W. G., Kinkade J. M., Jr Heterogeneity of peroxidase-positive granules in normal human an Chédiak-Higashi neutrophils. J Histochem Cytochem. 1993 Jun;41(6):837–849. doi: 10.1177/41.6.8315276. [DOI] [PubMed] [Google Scholar]
- Heusel J. W., Scarpati E. M., Jenkins N. A., Gilbert D. J., Copeland N. G., Shapiro S. D., Ley T. J. Molecular cloning, chromosomal location, and tissue-specific expression of the murine cathepsin G gene. Blood. 1993 Mar 15;81(6):1614–1623. [PubMed] [Google Scholar]
- Hohn P. A., Popescu N. C., Hanson R. D., Salvesen G., Ley T. J. Genomic organization and chromosomal localization of the human cathepsin G gene. J Biol Chem. 1989 Aug 15;264(23):13412–13419. [PubMed] [Google Scholar]
- Jepsen L. V., Skottun T. A rapid one-step method for the isolation of human granulocytes from whole blood. Scand J Clin Lab Invest. 1982 May;42(3):235–238. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martodam R. R., Baugh R. J., Twumasi D. Y., Liener I. E. A rapid procedure for the large scale purification of elastase and cathepsin G from human sputum. Prep Biochem. 1979;9(1):15–31. doi: 10.1080/00327487908061669. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G., Enghild J. J. An unusual specificity in the activation of neutrophil serine proteinase zymogens. Biochemistry. 1990 Jun 5;29(22):5304–5308. doi: 10.1021/bi00474a013. [DOI] [PubMed] [Google Scholar]
- Takeuchi K. H., McGarry M. P., Swank R. T. Elastase and cathepsin G activities are present in immature bone marrow neutrophils and absent in late marrow and circulating neutrophils of beige (Chediak-Higashi) mice. J Exp Med. 1987 Nov 1;166(5):1362–1376. doi: 10.1084/jem.166.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K. H., Swank R. T. Inhibitors of elastase and cathepsin G in Chédiak-Higashi (beige) neutrophils. J Biol Chem. 1989 May 5;264(13):7431–7436. [PubMed] [Google Scholar]
- Takeuchi K., Wood H., Swank R. T. Lysosomal elastase and cathepsin G in beige mice. Neutrophils of beige (Chediak-Higashi) mice selectively lack lysosomal elastase and cathepsin G. J Exp Med. 1986 Mar 1;163(3):665–677. doi: 10.1084/jem.163.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Medcalf R. L., Fink T. M., Mattmann C., Lichter P., Jenne D. E. Three human elastase-like genes coordinately expressed in the myelomonocyte lineage are organized as a single genetic locus on 19pter. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8215–8219. doi: 10.1073/pnas.89.17.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]