Abstract
Aim—To investigate cytochrome P4501A1 (CYP1A1) polymorphism and susceptibility to emphysema and lung cancer.
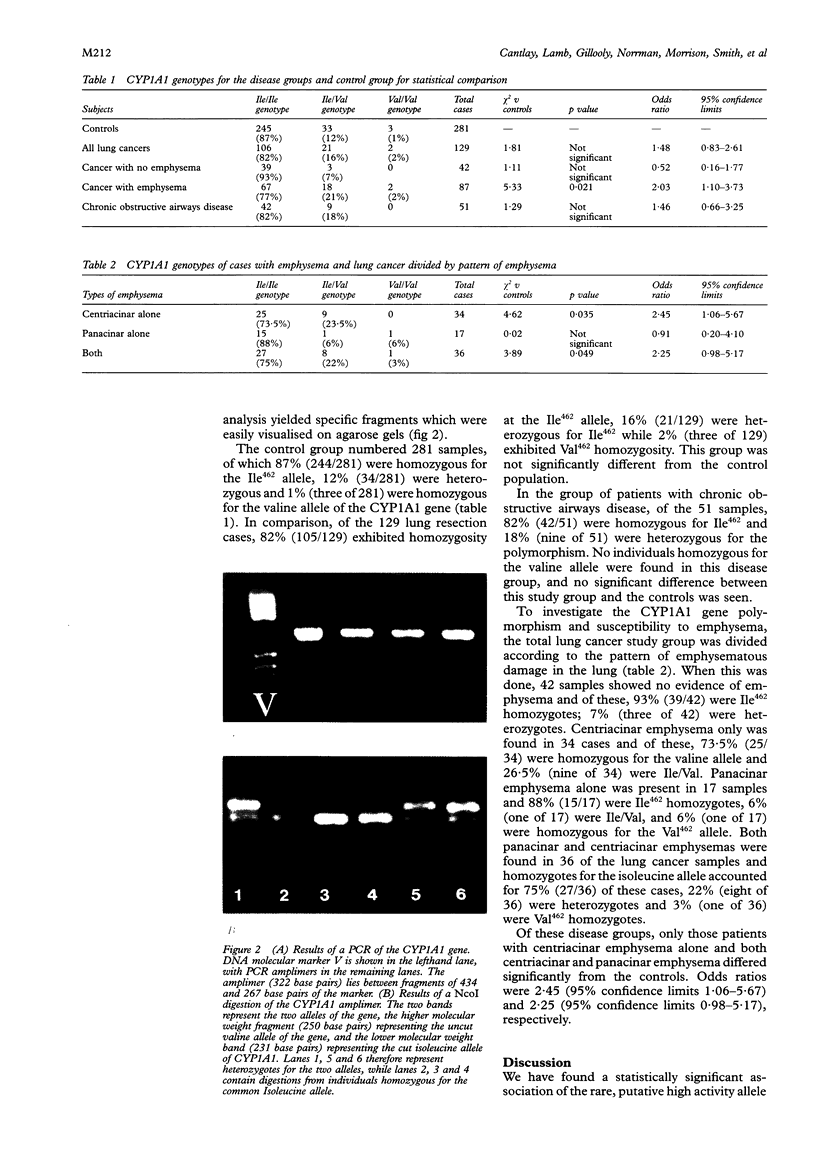
Methods—A novel polymerase chain reaction (PCR) for genotyping the CYP1A1 polymorphism, corresponding to putative low or high enzyme activity, was developed to genotype lung cancer resection samples which had been assessed macroscopically for the presence of centriacinar and panacinar emphysema. Samples were collected and genotyped from a group of patients with chronic obstructive airways disease. A control group of anonymous blood donations was genotyped to determine the basal levels of the polymorphism in the Scottish population.
Results—The high activity allele of the CYP1A1 gene is associated with susceptibility to centriacinar emphysema and lung cancer but not panacinar emphysema. CYP1A1 polymorphism is not linked to lung cancer in the absence of emphysema, nor to chronic obstructive airways disease which is the clinical manifestation of emphysema, particularly of the panacinar type.
Conclusions—Susceptibility to emphysema and lung cancer is associated with polymorphism of the P4501A1 gene. A trend towards damage of centriacinar pattern has been detected, which supports the theory that centriacinar emphysema results from local, direct damage to the respiratory bronchioles from exposure to cigarette smoke.
Keywords: CYP1A1 polymorphism, emphysema, lung cancer, cigarette smoke
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttila S., Vainio H., Hietanen E., Camus A. M., Malaveille C., Brun G., Husgafvel-Pursiainen K., Heikkilä L., Karjalainen A., Bartsch H. Immunohistochemical detection of pulmonary cytochrome P450IA and metabolic activities associated with P450IA1 and P450IA2 isozymes in lung cancer patients. Environ Health Perspect. 1992 Nov;98:179–182. doi: 10.1289/ehp.9298179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlay A. M., Smith C. A., Wallace W. A., Yap P. L., Lamb D., Harrison D. J. Heterogeneous expression and polymorphic genotype of glutathione S-transferases in human lung. Thorax. 1994 Oct;49(10):1010–1014. doi: 10.1136/thx.49.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. L. Mechanisms of cell injury by activated oxygen species. Environ Health Perspect. 1994 Dec;102 (Suppl 10):17–24. doi: 10.1289/ehp.94102s1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly M., Lamb D. Airspace size in lungs of lifelong non-smokers: effect of age and sex. Thorax. 1993 Jan;48(1):39–43. doi: 10.1136/thx.48.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M. Molecular genetics of the N-acetyltransferases. Pharmacogenetics. 1993 Feb;3(1):45–50. doi: 10.1097/00008571-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Greaves I. A., Colebatch H. J. Observations on the pathogenesis of chronic airflow obstruction in smokers: implications for the detection of "early" lung disease. Thorax. 1986 Feb;41(2):81–87. doi: 10.1136/thx.41.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Watanabe J., Nakachi K., Kawajiri K. Genetic linkage of lung cancer-associated MspI polymorphisms with amino acid replacement in the heme binding region of the human cytochrome P450IA1 gene. J Biochem. 1991 Sep;110(3):407–411. doi: 10.1093/oxfordjournals.jbchem.a123594. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hirvonen A., Husgafvel-Pursiainen K., Karjalainen A., Anttila S., Vainio H. Point-mutational MspI and Ile-Val polymorphisms closely linked in the CYP1A1 gene: lack of association with susceptibility to lung cancer in a Finnish study population. Cancer Epidemiol Biomarkers Prev. 1992 Sep-Oct;1(6):485–489. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Biochemical links between cigarette smoking and pulmonary emphysema. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):285–293. doi: 10.1152/jappl.1983.55.2.285. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Gotoh O., Tagashira Y., Sogawa K., Fujii-Kuriyama Y. Titration of mRNAs for cytochrome P-450c and P-450d under drug-inductive conditions in rat livers by their specific probes of cloned DNAs. J Biol Chem. 1984 Aug 25;259(16):10145–10149. [PubMed] [Google Scholar]
- Kawajiri K., Nakachi K., Imai K., Watanabe J., Hayashi S. The CYP1A1 gene and cancer susceptibility. Crit Rev Oncol Hematol. 1993 Feb;14(1):77–87. doi: 10.1016/1040-8428(93)90007-q. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Nakachi K., Imai K., Yoshii A., Shinoda N., Watanabe J. Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P450IA1 gene. FEBS Lett. 1990 Apr 9;263(1):131–133. doi: 10.1016/0014-5793(90)80721-t. [DOI] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. Tissue-specific expression of the mouse dioxin-inducible P(1)450 and P(3)450 genes: differential transcriptional activation and mRNA stability in liver and extrahepatic tissues. Mol Cell Biol. 1986 May;6(5):1471–1477. doi: 10.1128/mcb.6.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. New colon cancer gene discovered. Science. 1993 May 7;260(5109):751–752. doi: 10.1126/science.8484115. [DOI] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. A. Pharmacogenetics: the slow, the rapid, and the ultrarapid. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):1983–1984. doi: 10.1073/pnas.91.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakachi K., Imai K., Hayashi S., Watanabe J., Kawajiri K. Genetic susceptibility to squamous cell carcinoma of the lung in relation to cigarette smoking dose. Cancer Res. 1991 Oct 1;51(19):5177–5180. [PubMed] [Google Scholar]
- Omiecinski C. J., Redlich C. A., Costa P. Induction and developmental expression of cytochrome P450IA1 messenger RNA in rat and human tissues: detection by the polymerase chain reaction. Cancer Res. 1990 Jul 15;50(14):4315–4321. [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Service R. F. Stalking the start of colon cancer. Science. 1994 Mar 18;263(5153):1559–1560. doi: 10.1126/science.8128240. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Smith G., Wolf C. R. Genetic polymorphisms in xenobiotic metabolism. Eur J Cancer. 1994;30A(13):1921–1935. doi: 10.1016/0959-8049(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Totti N., 3rd, McCusker K. T., Campbell E. J., Griffin G. L., Senior R. M. Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science. 1984 Jan 13;223(4632):169–171. doi: 10.1126/science.6318317. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Gordon L. I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990 Aug 15;76(4):655–663. [PubMed] [Google Scholar]



