Summary
Myxococcus xanthus is a social bacterium that preys on prokaryotic and eukaryotic microorganisms. Co-culture of M. xanthus with reference laboratory strains and field isolates of the legume symbiont Sinorhizobium meliloti revealed two different predatory patterns that resemble frontal and wolfpack attacks. Use of mutants impaired in the two types of M. xanthus surface motility (A or adventurous and S or social motility) and a csgA mutant, which is unable to form macroscopic travelling waves known as ripples, has demonstrated that both motility systems but not rippling are required for efficient predation. To avoid frontal attack and reduce killing rates, rhizobial cells require a functional expR gene. ExpR regulates expression of genes involved in a variety of functions. The use of S. meliloti mutants impaired in several of these functions revealed that the exopolysaccharide galactoglucan (EPS II) is the major determinant of the M. xanthus predatory pattern. The data also suggest that this biopolymer confers an ecological advantage to rhizobial survival in soil, which may have broad environmental implications.
Introduction
Planet sustainability depends on a high number of factors and, among others, on diverse inter kingdom interactions in the soil environment. One such interaction, the root-nodule endosymbiosis established between certain nitrogen-fixing α- and β-proteobacteria (collectively referred to as rhizobia) with leguminous plants, contributes the largest annual input of combined nitrogen into terrestrial ecosystems (Newton, 2000). Successful symbiotic associations depend on the capacity of rhizobia to survive in soil and establish competitive populations in the plant rhizosphere (Toro, 1996; Triplett and Sadowsky, 1992). The composition, structure and dynamics of rhizobial communities are shaped by diverse abiotic (e.g. drought, salt or acidic stresses) and biotic (e.g. compatibility with the legume host and competition with other soil organisms) variables (Zahran, 1999; Rangin et al., 2008; Lindström et al., 2010). However, the mechanisms used by rhizobia to sense, transduce and respond to most of these environmental cues are poorly understood.
Sinorhizobium meliloti, the symbiotic partner of agronomic forage legumes of the genera Medicago, Melillotus and Trigonella, is a genetically tractable model species for investigating rhizobial biology (Jones et al., 2007). Many of the known adaptive responses of S. meliloti to soil and plant stimuli are governed by the density-dependent ExpR/Sin quorum sensing system. Two transcriptional regulators (i.e. ExpR and SinR) and a synthase (SinI) of several N-acyl-L-homoserine lactone autoinducers (AHLs) activate the system (Marketon et al., 2002; Pellock et al., 2002; Gao et al., 2005). Upon activation by AHLs, the LuxR homolog ExpR regulates expression of a large array of genes involved in free-living and symbiotic functions such as transport of metal ions and small molecules, motility, chemotaxis, nitrogen fixation, and exopolysaccharide (EPS) biosynthesis (Marketon et al., 2003; Hoang et al., 2004; Hoang et al., 2008; Gurich and González, 2009).
S. meliloti produces two types of EPS; succinoglycan (EPS I) and galactoglucan (EPS II). ExpR is required for EPS II biosynthesis and fine-tunes EPS I levels. The low molecular weight fractions of these polymers have an interchangeable symbiotic function, being required in picomolar concentrations for successful colonization of Medicago nodules (González et al., 1996a,b). These results suggest that EPSs act as a signaling molecule during symbiosis, perhaps by modulating plant defense responses triggered by the invading bacteria. As an abundant cell-surface layer, EPSs are also expected to confer much broader ecological advantages to rhizobia such as facilitating bacterial attachment to surfaces, improving nutrient acquisition, and providing protection from abiotic stresses (e.g. oxidative stress) (Lehman and Long, 2013).
Parasitism and predation are expected to regulate microbial diversity in natural populations (Chesson, 2000). However, their impact on rhizobial communities has remained largely unexplored. Some rhizobiophages as well as antimicrobial peptides produced by Rhizobium etli alter the ecological balance of competing rhizobial strains in soil (Barnet, 1980; Hashem and Angle, 1988; Robleto et al., 1998). R. vitis and S. fredii strains are sensitive to predation by the ubiquitous, soil-dwelling, δ-proteobacterium Myxococcus xanthus (Morgan et al., 2010; Mendes-Soares and Velicer, 2013). Myxobacteria constitute a phylogenetic order of microorganisms characterized by predatory behavior on a wide range of microbial species (Dawid, 2000; Bull et al., 2002; Pham et al., 2005; Berleman et al., 2006; Morgan et al., 2010; Pérez et al., 2011), which lyse prey cells by a mixture of bioactive secondary metabolites and exoenzymes (Goldman et al., 2006; Berleman and Kirby, 2009; Xiao et al., 2011; Volz et al., 2012). Although the predatory activity of myxobacteria is usually referred to as a wolfpack attack, current evidence indicates that M. xanthus cells do not surround the prey colonies. Instead, they penetrate the colony and lyse cells directly. Macroscopic travelling waves known as ripples are often observed and thought to maximize predatory efficiency (Berleman et al., 2006; Berleman et al., 2008; Berleman and Kirby, 2009; Zhang et al., 2012). In contrast, results obtained about the role of the two M. xanthus motility systems are contradictory, suggesting that characteristics of the prey define specific motility requirements (Pham et al., 2005; Berleman et al., 2006).
In this work we studied the interaction of M. xanthus with a series of S. meliloti field isolates, reference laboratory strains, and genetically engineered derivatives. The results show that M. xanthus exhibits two different predatory patterns that are determined by the rhizobial EPS galactoglucan. Our results also show that EPS II reduces killing rates, thus revealing a novel role for these biopolymers in the ecology of endosymbiotic bacteria.
Results
M. xanthus exhibits two predatory patterns on S. meliloti
The predatory activity of M. xanthus wild-type (WT) strain DK1622 on S. meliloti was examined on an agar surface by spotting suspensions of each microorganism next to each other (Fig. 1A and Fig. S1A). M. xanthus cells moved towards all S. meliloti strains, but two different interaction patterns were observed. One of them is a frontal attack during which M. xanthus entered the prey colony at the closest point and lysed the S. meliloti colonies directly (Fig. 1A and Fig. S1A). Interestingly, susceptible rhizobial prey included current reference laboratory strains (Table 1) that formed dry, non-mucoid colonies (i.e. GR4b, Rm1021, Rm2011 and RMO17b). In contrast, colonies of uncharacterized S. meliloti field isolates (i.e. a series of AK strains) that had not been routinely subcultured, produced highly mucoid colonies which resisted M. xanthus attack (Fig. 1A and Fig. S1A). No zones of clearing were evident within the AK colonies 72 h after plating. Rather, myxobacterial cells surrounded the edges of these colonies in a manner resembling wolfpack hunting.
Fig. 1.
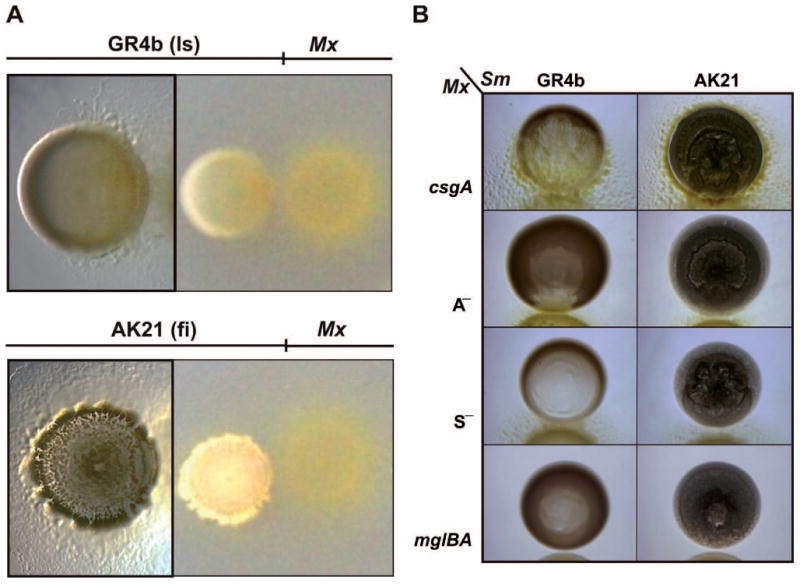
Predatory patterns of M. xanthus on S. meliloti. The non-mucoid reference laboratory strain (ls) GR4b and the mucoid S. meliloti field isolate (fi) AK21 were exposed to the M. xanthus (Mx) WT strain DK1622 (A) or mutants (B) impaired in rippling (csgA), A motility (A—), S motility (S—), and A— S— (mglBA) on CTT agar plates. Microscopic images of the S. meliloti-M. xanthus cocultures were taken 72 h after bacterial spotting. Images on the right of panel a were taken with a digital camera.
Table 1. Bacterial strains used in this study.
| Strains | Relevant characteristics | Source/ reference |
|---|---|---|
| M. Xanthus | ||
| DK1622 | Wild-type | (Kaiser, 1979) |
| DZ2 | Wild-type | (Campos and Zusman, 1975) |
| LS2442 | ΔcsgA | (Curtis et al., 2007) |
| MXH1777 | ΔaglU, A—, no adventurous motility | (White and Hartzell, 2000) |
| DK10410 | ΔpilA, S—, no social motility | (Wu and Kaiser, 1996) |
| DK6204 | ΔmglBA, A— S— | (Hartzell and Kaiser, 1991) |
| S. meliloti | ||
| AK21 | Field isolate (Aral Sea region, Russia) | RIAM1 |
| AK27 | Field isolate (Aral Sea region, Russia) | RIAM |
| AK70 | Field isolate (Aral Sea region, Russia) | RIAM |
| AK83 | Field isolate (Aral Sea region, Russia) | RIAM |
| GR4 | Field isolate (Granada, Spain) | (Casadesús and Olivares, 1979) |
| RMO17 | Field isolate (León, Spain) | (Villadas et al., 1995) |
| GR4b | GR4 spontaneous non mucoid variant | (Casadesús and Olivares, 1979) |
| RMO17b | RMO17 spontaneous non mucoid variant | (Villadas et al., 1995) |
| Rm1021 | SU47 derivative (reference strain); Smr(2) | (Meade and Singer, 1977) |
| Rm2011 | SU47 derivative; Smr | (Case et al., 1979) |
| 1021R | Rm1021 expR+; Smr | (Nogales et al., 2012) |
| 2011R | Rm2011 expR+; Smr | (Nogales et al., 2012) |
| Rm8530 | Rm1021 expR+; Smr | (Pellock et al., 2002) |
| Rm11601 | Rm8530 flaA flaB; Smr Hygr | (Gurich and González, 2009) |
| 11601W | Rm11601 wgeB::mini-Tn5; Smr Nmr, Hygr | (Nogales et al., 2012) |
| 8530W | Rm8530 wgeB::mini-Tn5; Smr Nmr | (Nogales et al., 2012) |
| Rm9020 | Rm8530 exoY::Tn5-132; Smr Otcr | (Glazebrook and Walker, 1989) |
Culture collection of All-Russia Institute of Agricultural Microbiology (St. Petersburg, Russia).
Smr, Hygr, Nmr, and Otcr indicate streptomycin, hygromycin, neomycin, and oxitetracycline resitance, respectively.
The distinctly different predatory patterns against S. meliloti GR4b (non-mucoid) and AK21 (mucoid) colonies were examined in more detail by time-lapse videomicroscopy (Movies S1-S3). The cell density at the interface of the M. xanthus and GR4b colonies increased after 6-12 h of incubation. M. xanthus was able to penetrate the rhizobial colony head on and massively lyse prey cells, as evidenced by progressive clarification of the interaction zone (Movie S1). Rippling behavior consisting of macroscopic travelling waves was rarely observed during this interaction (Movies S1, S4, and S5. Movies S4-13 are available at http://www.ugr.es/∼jdorado/movies.htm).
In marked contrast, S. meliloti AK21 cells responded to the approaching myxobacteria by producing EPS and retreating backwards. Upon contact, the high cell density at the interface lingered for over 24 h without evident rhizobial lysis (Movies S2 and S3). Instead, progressive migration of myxobacteria toward the distal edge of the AK21 colony was observed where the myxobacteria organized traveling waves and initiated prey lysis.
To elucidate whether these two interaction patterns were general properties of M. xanthus, mucoid and non-mucoid rhizobia were assayed against another WT strain, DZ2 (Table 1). As shown in Fig. S1B and Movies S6-S9, this strain exhibits predatory patterns similar to those reported for DK1622.
Rippling and motility were examined to deepen our understanding of the two M. xanthus predatory patterns. As rippling depends on the accumulation of small amounts of the C-signal (Li et al., 1992), a ΔcsgA mutant was assayed for its ability to prey on rhizobial strains. As shown in Fig. 1B and Movies S10-S13, this mutant exhibits predatory patterns similar to the parental strain against mucoid and non-mucoid rhizobia except for the absence of ripples. Furthermore, we investigated the role of motility on predation. M. xanthus glides on solid surfaces by using two different mechanisms, called adventurous (A) and social (S) motility (Hodgkin and Kaiser, 1979). Although these two mechanisms are genetically independent, both are controlled by the mglBA operon (Hartzell and Kaiser, 1991). Mutants impaired in either A or S motility as well as both systems are available (Table 1). When assayed for predator efficiency, it was observed that all had lost partial or complete ability to prey on rhizobia (Fig. 1B). This is especially evident in the case of the A— S— cells, because they cannot migrate toward the rhizobial colonies.
A functional rhizobial expR gene alleviates predation by M. Xanthus
The mucoid phenotype of S. meliloti colonies is primarily due to ExpR-dependent biosynthesis of EPS (Pellock et al., 2002; Marketon et al., 2003). PCR-amplified ExpR ORFs from the highly mucoid S. meliloti field isolates revealed no amino acid substitutions (Fig. S2). On the other hand, the expR gene of non-mucoid strains Rm1021 and Rm2011, both derived from S. meliloti isolate SU47, is disrupted by the 1 320 bp insertion sequence ISRm1 (Fig. 2). The well-characterized Rm8530/1021R and 2011R strains obtained by restoration of the WT expR gene through allelic exchange in Rm1021 and Rm2011, respectively, exhibited a highly mucoid phenotype (Fig. 2).
Fig. 2.
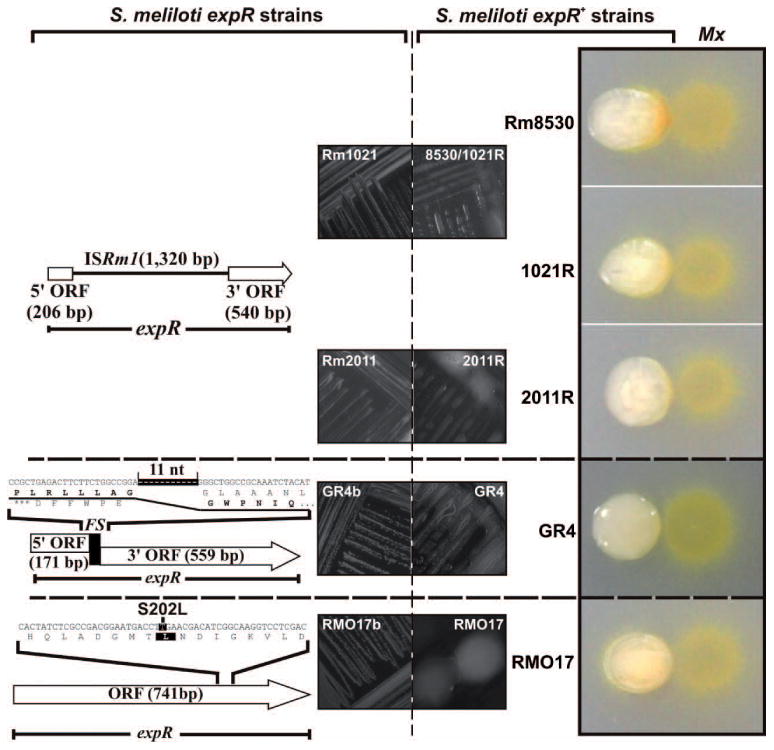
The wolfpack-like attack of M. xanthus DK1622 on S. meliloti requires a functional rhizobial expR gene. Diagrams of the spontaneous mutations of expR in the S. meliloti laboratory strains Rm1021 and Rm2011 (insertion of ISRm1), GR4b (frameshift deletion) and RMO17b (S202L), and their associated non-mucoid phenotypes on plates are shown in the left panels. M. xanthus preys on these strains by a frontal attack as shown in Fig. 1. The expR+ engineered derivatives (Rm8530, 1021R, 2011R) or natural variants (GR4 and RMO17) of these strains exhibit a mucoid phenotype and a wolfpack-like pattern in co-culture with M. xanthus (Mx) as shown in the images to the right.
Sequence analyses revealed different mutations in the S. meliloti GR4b and RMO17b expR alleles. The GR4b sequence has a frameshift mutation resulting from an internal 11-bp deletion whereas RMO17 has a single nucleotide substitution resulting in replacement of the ultraconserved Ser202 with a Leu residue (Fig. 2). Parental, mucoid isolates of the GR4b and RMO17b strains (GR4 and RMO17) (Fig. 2) were recovered from the freezer and DNA sequencing demonstrated that both strains harbor a WT expR gene (Fig. S2). The expR+ S. meliloti Rm8530, 1021R, 2011R, GR4 and RMO17 strains were then examined with the predation assay. Without exception, all exhibited the wolfpack-like predation pattern associated with mucoid, EPS-producing strains (Fig. 2).
We next monitored killing of isogenic Rm1021 and 1021R strains, which are non-mucoid and mucoid, respectively (Table 1), by WT M. xanthus strain DK1622. In this assay, predator cells were spotted on top of the prey colonies, which were previously grown on membrane filters placed on nutrient rich CTT agar. Survival of S. meliloti cells was determined with plate counts by taking advantage of prey streptomycin-resistance. Without the predator, populations of both S. meliloti strains increased to over 109 cells (Fig. 3, black lines). In the presence of WT M. xanthus, the Rm1021 population decreased to ∼2 × 104 cells within 24 h (Fig. 3A, red line), but did not decline further with longer incubation times. In contrast, ∼2 × 108 1021R cells survived at 24 h, and declined to 107 cells at 96 h (Fig. 3B, red line). These findings corroborate the photographs and videos showing that mucoid strains are more resistant to lysis. Furthermore, they demonstrate that a functional expR gene protects rhizobia from predation.
Fig. 3.
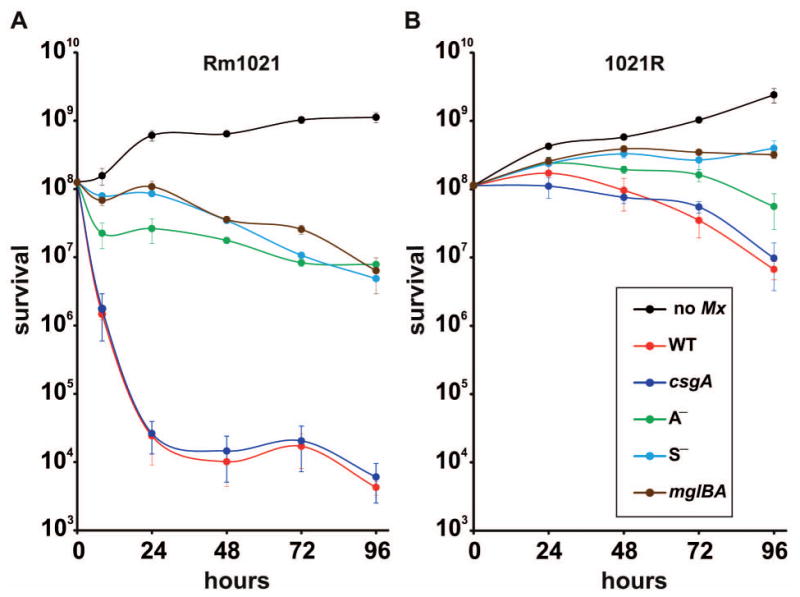
Survival of S. meliloti Rm1021 (A) or its expR+ derivative 1021R (B) upon coculture with WT M. xanthus strain DK1622. M. xanthus WT or different mutant strains (Table 1) were spotted on top of rhizobial colonies previously grown on membrane filters. As controls (no Mx, black lines), similar experiments were conducted in the absence of myxobacteria. At the times shown, S. meliloti cells on the filters were measured as colony-forming units (CFU). Results are means of counts from three different filters at each time-point and three independent dilution sets. Error bars indicate standard deviations.
Adventurous and social motility, but not rippling, are required for predation
The mutants impaired in rippling and motility were also assayed to quantify their predatory efficiencies on both 1021R and Rm1021 strains. The rhizobial killing curves by the csgA mutant were nearly identical to those of WT strain DK1622 (compare dark blue and red lines, Fig. 3). This result was expected against non-mucoid rhizobia because DK1622 exhibits no rippling during frontal attack. But it was surprising to see that rippling did not increase predator efficiency against mucoid strains where rippling is observed.
All motility mutants exhibit a fraction of the lytic activity compared with the WT strain. As shown in Fig. 3A (green line), a difference of three orders of magnitude was observed between the WT strain and the A— mutant against non-mucoid rhizobia. Although the difference between these two strains was much lower with the mucoid rhizobia, significant differences were still observed (Fig. 3B, green line). These differences were even more dramatic in the case of the S— and the A— S— mutants (Fig. 3, light blue and brown lines, respectively). Altogether these data indicate that optimal predation requires both motility systems.
Rhizobial galactoglucan confers resistance to predation and determines the predatory pattern
The S. meliloti ExpR regulon is required for widely diverse functions. We examined M. xanthus predation on well-characterized derivatives of expR+ strain Rm8530 lacking important genes in the ExpR regulon (Table 1) in order to identify the ExpR-dependent functions determining predatory pattern. Strain Rm11601 (expR+ flaA flaB) is impaired in swarming motility because it lacks flagella. Strain 8530W (expR+ wgeB) is blocked in galactoglucan synthesis due to a transposon insertion in a glycosyl transferase gene. Strain 11601W (expR+ flaA flaB wgeB) lacks both flagella and EPS II. Strain Rm9020 (expR+ exoY) lacks a glycosyl transferase involved in succinoglycan synthesis. Only mutations in the wgeB gene (also referred to as expE2) rendered strains susceptible to frontal attack in a manner similar to the non-mucoid laboratory strains (Fig. 4, compare center panel with others). We therefore conclude that EPS II, rather than functional flagella or EPS I, is the ExpR-dependent factor that confers resistance to predation and determines the M. xanthus predatory pattern.
Fig. 4.
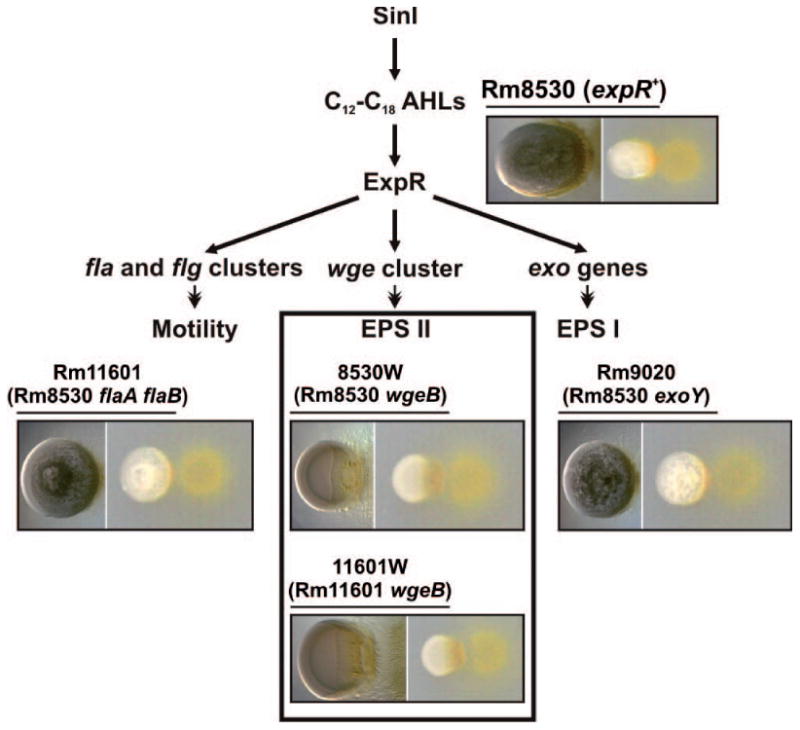
The rhizobial galactoglucan determines the predatory pattern of M. xanthus on S. meliloti. Simplified outline of the rhizobial functions influenced by the ExpR/Sin quorum sensing system. S. meliloti Rm8530 (expR+) and derivative strains harboring mutations impairing biosynthesis of EPS II/EPS I or motility (specified in brackets under the name of each strain) were subjected to predation tests on CTT agar. Images of the S. meliloti-M. xanthus cocultures taken 72 h after spotting are shown below the name of each rhizobial strain. Microscopic images of the S. meliloti colonies are shown to the left in each case. Only strains devoid of EPS II (inset) suffered a prolonged frontal attack.
Crude EPS was extracted from strains 8530 (EPS II-proficient) and 8530W (EPS II-deficient). DK1622 cells rapidly spread over the area were the extracts were spotted, but rippled only in the presence of EPS II (Fig. 5, left). In contrast, M. xanthus cells did not invade the surface containing only buffer (Fig. 5, right) and were markedly less aggressive in the spot lacking EPS II.
Fig. 5.

EPS II induces rippling. Crude EPS extracts obtained from S. meliloti 8530 (which synthesizes EPS II) and 8530W (impaired in EPS II production), or TM buffer were spotted next to M. xanthus DK1622 on CTT agar plates. Pictures were taken under a dissecting microscope after 72 h of incubation. The red box indicates an area of rippling. The arrows in the enlargement of the red box point to wave crests.
Discussion
Studies on the interaction between two soil bacteria, M. xanthus and S. meliloti, carried out under laboratory conditions have revealed that M. xanthus exhibits two different predatory patterns on S. meliloti in response to differences in the prey. In one of them, M. xanthus cells surround the prey and begin forming ripples at collision points (Fig. 1 and Movies S2 and S3). In the other pattern, myxobacterial cells move directly into the prey colony, lysing cells as they go (Fig. 1 and Movie S1). Rippling is not observed or is very faint in contrast to previous results with other types of prey (Berleman et al., 2006; Berleman et al., 2008; Berleman and Kirby, 2009; Morgan et al., 2010). The differences with rippling may be due to nutrient concentrations. While other studies employed starvation media, which induce rippling independently of predation, we used a rich medium that normally suppresses rippling.
Rippling has been reported to be a predatory behavior against E. coli and other prokaryotic and eukaryotic cells during consumption of macromolecular substrates (Berleman et al., 2006). In one approach, media with low and high nutrient concentrations were used to induce or inhibit rippling, respectively. Although predator efficiency was greater in media where M. xanthus ripples, the difference could have been caused by other factors such as differing levels of extracellular hydrolytic enzymes between the two media (Berleman et al., 2006). In a second approach, the authors showed that a mutant impaired in S motility neither rippled nor was an effective predator. This mutant has dramatically less motility so the difference could have been due to loss of motility rather than loss of rippling. To obtain more definitive data, we performed all our assays under the same nutrient conditions with a ΔcsgA mutant, which has lost the ability to ripple while retaining A and S motility. Although, it has been postulated that rippling increases the rate of spreading over prey cells and allows the predator to remain on the prey longer (Zhang et al., 2012), the data presented here with the ΔcsgA mutant indicate that rippling does not help overcome the physical and chemical barrier conferred by galactoglucan nor does it improve prey lysis (Fig. 1B and 3). The induction of traveling waves by EPS extracts containing EPS II (Fig. 5) suggests that rippling might be a general response to certain macromolecules, as it has been reported for other polymers such as peptidoglycan, proteins, or chromosomal DNA (Berleman et al., 2006; Shimkets and Kaiser, 1982), which may be useful against some prey species but useless against others.
The role of each type of motility on predation was examined with mutants. S motility is important for both prey attack and prey lysis (Fig. 1B and 3), which is in agreement with the data obtained by Berleman et al. (2006). We also observed reduced prey lysis by A— mutants. Although it is expected that motility is necessary to attack the prey, it is not clear why A— S— cells or cells impaired in only one of the two motility systems have lost much of their capacity to lyse prey. We speculate that each motility system improves contact with the prey and allows distribution of hydrolytic enzymes. Surrounding the prey colony during a wolfpack-like attack seems to be the response of fully motile predator cells against highly resistant prey cells, even after the induction of ripples by galactoglucan.
We also focused on the prey defense mechanisms. Rhizobial cells producing EPS II are much more resistant to predation than those impaired in EPS II synthesis (Fig. 1-3). Mucoid cells are still able to grow in the presence of M. xanthus 24 h after the addition of the predator. In contrast, the number of non-mucoid rhizobial cells decreases about four orders of magnitude during the same period of time. However, it should be noted that S. meliloti must use other unidentified mechanisms to escape predation, because ∼104 non-mucoid cells persist for the next two days (Fig. 3). As EPS II production depends on ExpR, protection against predation can be added to the list of ExpR and EPS II functions. EPS II is commonly lost by rhizobia during subculture in the laboratory because of spontaneous mutations in the expR gene. Our data add new evidences supporting that EPSII provides an ecological advantage for successful survival of rhizobia in soil.
In summary, our findings show that M. xanthus approaches the nitrogen-fixing symbiont S. meliloti using two different predatory patterns that vary depending on the production of the EPS galactoglucan. Furthermore, galactoglucan protects rhizobia from lytic attack despite inducing rippling. Although fully motile cells are more efficient predators than mutants impaired in either of the two motility systems, identification of all the M. xanthus determinants mediating confrontation as well as the S. meliloti compensatory defense mechanisms are new challenges that will clarify the role of the predator in the dynamics of microbial soil populations. This observation may extend to other rhizobacteria. Since our experiments have been conducted on agar plates and, to our knowledge, there are no studies about the interaction between these two groups of bacteria in natural environments, the environmental significance of our findings remains to be elucidated.
Experimental Procedures
Bacterial strains, media and growth conditions
The bacterial strains used in this work, their source and relevant characteristics are listed in Table 1. CTT solid (1.5% Bacto-Agar) and liquid media were used to grow M. xanthus (Hodgkin and Kaiser, 1977) and for the predation experiments. Tryptone yeast (TY) solid (Beringer, 1974) or defined minimal media (Robertsen et al., 1981) were used for maintenance and growth of S. meliloti strains. When required, antibiotics were added to the media at a final concentration of 200 μg ml-1 streptomycin, 0.75 μg ml-1 oxytetracycline, 100 μg ml-1 hygromycin, or 120 μg ml-1 neomycin.
EPS isolation
Crude EPS extracts were obtained using a previously described protocol with some modifications (May and Chakrabarty, 1994). S. meliloti 8530 (WT for EPS II production) and 8530W (impaired in EPS II synthesis) were grown on minimal medium agar for 3 days at 30°C. Bacteria were recovered from each plate (5 plates per strain) by resuspension in 2 ml 0.9% NaCl, and centrifugation at room temperature for 5 min at 12 000 × g. The supernatant was carefully collected and EPS was precipitated at 4°C overnight with three volumes of ice-cold 95% ethanol. The precipitated material was centrifuged at 4°C for 15 min at 13 700 × g and the ethanol was carefully removed. The pellet was washed three times with ethanol, each time centrifuged for 15 min at 13 700 × g. Finally, the pellets were dried under vacuum, UV sterilized, and resuspended in 2 ml TM buffer (10 mM Tris-HCl [pH 7.6], 8 mM MgSO4) for biological assays.
Predation experiments
All M. xanthus and S. meliloti strains were grown in CTT broth (with appropriate antibiotic, when necessary) with vigorous shaking (300 rpm) at 30°C to an optical density at 600 nm (OD600) of 1. Cells were centrifuged, washed in CTT, and concentrated in fresh CTT to a final OD600 of 15 for M. xanthus and 5 for S. meliloti strains. Drops of 10 μl of the S. meliloti suspensions were deposited on the surface of CTT agar plates and allowed to dry. Next, drops of 10 μl of the different M. xanthus suspensions were spotted close to the S. meliloti spot, leaving a separation of no more than 1 mm between spots. Plates were incubated at 30°C and images were taken at different time-points (usually 24, 48, 72 and 96 h after plating) with a digital camera and an Olympus SZX7 dissecting microscope. The videomicroscopy experiments were performed as described in Pérez et al. (2011). Additionally, 10 μl drops (equivalent to an OD600 of 10 for rhizobial cultures) of crude S. meliloti EPS extracts were assayed on plates against M. xanthus as described above.
For cell counting and viability determinations, M. xanthus DK1622 and the reference strains S. meliloti Rm1021 (non-mucoid) and 1021R (mucoid) were grown as indicated above. Rhizobial cultures were diluted to a final OD600 of 0.2. Drops of 10 μl were deposited onto membrane filters (Isopore, Millipore) placed on the surface of CTT agar plates, and incubated at 30°C for 24 hours. After that time, 10 μl of concentrated cultures (OD600 of 15) from the different M. xanthus strains were deposited on top of the rhizobial colonies. At different time-points, filters were deposited in an eppendorf tube, trapped with the tube stoppers, and thoroughly washed with 1 ml of TM buffer. The filters were then centrifuged for 3 min at 12 000 rpm. Supernatants and filters were discarded and the pellets resuspended in 500 μl TM buffer. S. meliloti viable cells were counted by using the dilution method. TY agar supplemented with streptomycin was used for counting because it allows growth of S. meliloti while inhibiting M. xanthus growth.
DNA methods
The expR gene of the different S. meliloti strains used in this work was amplified with the proofreading DNA polymerase Phusion® (New England Biolabs. Ipswich MA, USA) using bacterial lysates as template and primers ExpR1 (5′-ATCCGATACCATGGGAGG-3′) and ExpR2 (5′-GGGCTGGCCGGATTC-3′) in standard PCR reactions as specified by the enzyme supplier. PCR products were cloned into the pGEM-T Easy vector (Promega, Fitchburg WI, USA) for Sanger sequencing.
Supplementary Material
Fig. S1. Predatory patterns of M. xanthus on S. meliloti. Different S. meliloti field isolates and reference laboratory strains were exposed to M. xanthus DK1622 (Mx DK1622) (A) and M. xanthus DZ2 (Mx DZ2) (B) on CTT agar plates. Images were taken with a digital camera 72 h after spotting on plates. Microscopic images of the same S. meliloti macrocolonies (boxed) are shown to the left of each panel.
Fig. S2. Sequence analysis of the S. meliloti expR gene. (A) Amplification of the expR alleles in different S. meliloti strains. The oligonucleotide primers used in the PCR reactions (ExpR1/ExpR2) and the expected size of the PCR products are indicated above the map of the S. meliloti expR genomic region. The ExpR ORF is drawn to scale. The ethidium bromide stained agarose gels of the PCR products are shown below with the names of the S. meliloti strains analyzed on top. Amplification of the Rm1021 expR failed because it is disrupted by the insertion sequence ISRm1 (1,320 bp). Sizes of the co-migrating DNA markers are indicated. (B) Multiple amino acid sequence alignment of the ExpR proteins. Host strains are indicated to the left. ExpR sequences were inferred by sequencing of the corresponding PCR products except those of the AK83 and Rm1021 strains which were obtained from the GeneBank database (accession numbers NC_015590 and NC_003047, respectively). Mutations resulting in a non-functional protein are shown in red. The ultraconserved S202 residue altered in the RMO17b ExpR is indicated by a double arrowhead.
Movie S1. Interaction between M. xanthus DK1622 (left) and S. meliloti GR4b (right). Time 0-48 h after spotting.
Movie S10. Interaction between M. xanthus ΔcsgA (right) and S. meliloti GR4b (left). Time 0-24 h after spotting.
Movie S11. Interaction between M. xanthus ΔcsgA (light area) and S. meliloti GR4b (dark area). Time 24-72 h after spotting.
Movie S12. Interaction between M. xanthus ΔcsgA (right) and S. meliloti AK21 (left). Time 0-24 h after spotting.
Movie S2. Interaction between M. xanthus DK1622 (left) and S. meliloti AK21 (right). Time 0-24 h after spotting.
Movie S3. Interaction between M. xanthus DK1622 (upper, light area) and S. meliloti AK21 (dark area). Time 24-72 h after spotting.
Movie S4. Interaction between M. xanthus DK1622 (right) and S. meliloti GR4b (left). Time 0-48 h after spotting.
Movie S5. Interaction between M. xanthus DK1622 (light area) and S. meliloti GR4b (dark area). Time 48-72 h after spotting.
Movie S6. Interaction between M. xanthus DZ2 (right) and S. meliloti GR4b (left). Time 0-48 h after spotting.
Movie S7. Interaction between M. xanthus DZ2 (light area) and S. meliloti GR4b (dark area). Time 48-72 h after spotting.
Movie S8. Interaction between M. xanthus DZ2 (right) and S. meliloti AK21 (left). Time 0-24 h after spotting.
Movie S9. Interaction between M. xanthus DZ2 (light area) and S. meliloti AK21 (dark area). Time 24-72 h after spotting.
Movie S13. Interaction between M. xanthus ΔcsgA (light area) and S. meliloti AK21 (dark area). Time 24-72 h after spotting.
Acknowledgments
This work has been supported by the ERDF-cofinanced grants BFU2012-33248 to JMD and CSD2009-00006 (Program Consolider-Ingenio 2010) to UGR and EEZ from the Spanish MINECO, and by the National Institute of General Medical Science of the National Institutes of Health (R01GM095826) and the National Science Foundation (MCB0742976) to LJS. We thank the high school students of the PIIISA-2012 program, and in particular G. Tarifa Álvarez, for some preliminary work, M. J. Soto for kindly providing the S. meliloti expR derivatives, the core facilities of EEZ-CSIC for sequencing, and O. Igoshin for help compressing the movies.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest
References
- Barnet YM. The effect of rhizobiophages on populations of Rhizobium trifolii in the root zone of clover plants. Can J Microbiol. 1980;26:572–576. doi: 10.1139/m80-101. [DOI] [PubMed] [Google Scholar]
- Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Berleman JE, Chuntley T, Cheung P, Kirby JR. Rippling is a predatory behavior in Myxococcus xanthus. J Bacteriol. 2006;188:5888–5895. doi: 10.1128/JB.00559-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleman JE, Kirby JR. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol Rev. 2009;33:942–957. doi: 10.1111/j.1574-6976.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleman JE, Scott J, Chuntley T, Kirby JR. Predataxis behavior in Myxococcus xanthus. Proc Natl Acad Sci USA. 2008;105:17127–17132. doi: 10.1073/pnas.0804387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull CT, Shetty KG, Subbarao KV. Interactions between myxobacteria, plant pathogenic fungi, and biocontrol agents. Plant Dis. 2002;86:889–896. doi: 10.1094/PDIS.2002.86.8.889. [DOI] [PubMed] [Google Scholar]
- Campos JM, Zusman D. Regulation of development in Myxococcus xanthus: effect of 3′:5′-cyclic AMP, ADP and nutrition. Proc Natl Acad Sci USA. 1975;72:518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesús J, Olivares J. Rough and fine linkage mapping of the Rhizobium meliloti chromosome. Mol Gen Genet. 1979;174:203–209. doi: 10.1007/BF00268356. [DOI] [PubMed] [Google Scholar]
- Casse F, Boucher C, Julliot JS, Michel M, Dénarié J. Identification and characterization of large plasmids in Rhizobium meliloti using agarose-gel electrophoresis. J Gen Microbiol. 1979;113:229–242. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:342–366. [Google Scholar]
- Curtis PD, Taylor RG, Welch RD, Shimkets LJ. Spatial organization of Myxococcus xanthus during fruiting body formation. J Bacteriol. 2007;189:9126–9130. doi: 10.1128/JB.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol Rev. 2000;24:403–427. doi: 10.1111/j.1574-6976.2000.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Gao M, Chen H, Eberhard A, Gronquist MR, Robinson JB, Rolfe BG, et al. sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. J Bacteriol. 2005;187:7931–7944. doi: 10.1128/JB.187.23.7931-7944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Walker GC. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 1989;204:398–418. doi: 10.1016/0076-6879(91)04021-f. [DOI] [PubMed] [Google Scholar]
- Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, et al. Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci USA. 2006;103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JE, Gregory MY, Walker GC. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene. 1996a;179:141–146. doi: 10.1016/s0378-1119(96)00322-8. [DOI] [PubMed] [Google Scholar]
- González JE, Reuhs BL, Walker GC. Low molecular weight EPSII of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996b;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurich N, González JE. Role of quorum sensing in Sinorhizobium meliloti-alfalfa symbiosis. J Bacteriol. 2009;191:4372–4382. doi: 10.1128/JB.00376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell P, Kaiser D. Upstream gene of the mgl operon controls the level of MglA protein in Myxococcus xanthus. J Bacteriol. 1991;173:7625–7635. doi: 10.1128/jb.173.23.7625-7635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem FM, Angle JS. Rhizobiophage effects on Bradyrhizobium japonicum nodulation and soybean growth. Soil Biol Biochem. 1988;20:69–73. [Google Scholar]
- Hoang HH, Becker A, González JE. The LuxR homolog ExpR, in combination with the Sin quorum sensing system, plays a central role in Sinorhizobium meliloti gene expression. J Bacteriol. 2004;186:5460–5472. doi: 10.1128/JB.186.16.5460-5472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang HH, Gurich N, González JE. Regulation of motility by the ExpR/Sin quorum sensing system in Sinorhizobium meliloti. J Bacteriol. 2008;190:861–871. doi: 10.1128/JB.01310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci USA. 1977;74:2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AP, Long SR. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J Bacteriol. 2013;195:5362–5369. doi: 10.1128/JB.00681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lee BU, Shimkets LJ. csgA expression entrains Myxococcus xanthus development. Genes Dev. 1992;6:401–410. doi: 10.1101/gad.6.3.401. [DOI] [PubMed] [Google Scholar]
- Lindström K, Murwira M, Willems A, Altier N. The biodiversity of beneficial microbe-host mutualism: the case of rhizobia. Res Microbiol. 2010;161:453–463. doi: 10.1016/j.resmic.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Marketon MM, Glenn SA, Eberhard A, González JE. Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J Bacteriol. 2003;185:325–231. doi: 10.1128/JB.185.1.325-331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marketon MM, Gronquist MR, Eberhard A, González JE. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J Bacteriol. 2002;184:5686–5695. doi: 10.1128/JB.184.20.5686-5695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May TB, Chakrabarty AM. Isolation and assay of Pseudomonas aeruginosa alginate. Methods Enzymol. 1994;235:295–304. doi: 10.1016/0076-6879(94)35148-1. [DOI] [PubMed] [Google Scholar]
- Meade HM, Signer ER. Genetic mapping of Rhizobium meliloti. Proc Natl Acad Sci USA. 1977;74:2076–2078. doi: 10.1073/pnas.74.5.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Soares H, Velicer GJ. Decomposing predation: testing for parameters that correlate with predatory performance by a social bacterium. Microb Ecol. 2013;65:415–423. doi: 10.1007/s00248-012-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AD, MacLean RC, Hiilesland KL, Velicer GJ. Comparative analysis of Myxococcus predation on soil bacteria. Appl Environ Microbiol. 2010;76:6920–6927. doi: 10.1128/AEM.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton WE. Nitrogen fixation in perspective. In: Pedrosa FO, Hungría M, Yates MG, Newton WE, editors. Nitrogen Fixation: from Molecules to Crop Productivity. Dordrecht, The Netherlands: Kluwer; 2000. pp. 3–8. [Google Scholar]
- Nogales J, Bernabéu-Roda L, Cuéllar V, Soto MJ. ExpR is not required for swarming but promotes sliding in Sinorhizobium meliloti. J Bacteriol. 2012;194:2027–2035. doi: 10.1128/JB.06524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock BJ, Teplitski M, Boinay RP, Bauer WD, Walker GC. A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J Bacteriol. 2002;184:5067–5076. doi: 10.1128/JB.184.18.5067-5076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez J, Muñoz-Dorado J, Braña AF, Shimkets LJ, Sevillano L, Santamaría RI. Myxococcus xanthus induces actinorhodin overproduction and aerial mycelium formation by Streptomyces coelicolor growth and development. Microb Biotechnol. 2011;4:175–183. doi: 10.1111/j.1751-7915.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VD, Shebelut CW, Diodati ME, Bull CT, Singer M. Mutations affecting predation ability of the soil bacterium Myxococcus xanthus. Microbiology. 2005;151:1865–1874. doi: 10.1099/mic.0.27824-0. [DOI] [PubMed] [Google Scholar]
- Rangin C, Brunel B, Cleyet-Marel JC, Perrineau MM, Béna G. Effects of Medicago truncatula genetic diversity, rhizobial competition, and strain effectiveness on the diversity of a natural Sinorhizobium species community. Appl Environ Microbiol. 2008;74:5653–5661. doi: 10.1128/AEM.01107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen BK, Aiman P, Darvill AG, McNeil M, Alberstein P. The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii. Plant Physiol. 1981;67:389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robleto EA, Kmiecik K, Oplinger ES, Nienhuis J, Triplett EW. Trifolitoxin production increases nodulation competitiveness of Rhizobium etli CE3 under agricultural conditions. Appl Environ Microbiol. 1998;64:2630–2633. doi: 10.1128/aem.64.7.2630-2633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets LJ, Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982;152:451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro N. Nodulation competitiveness in the Rhizobium-legume symbiosis. World J The authors declare no conflict of interest. 1996;12:157–162. doi: 10.1007/BF00364680. [DOI] [PubMed] [Google Scholar]
- Triplett EW, Sadowsky MJ. Genetics of competition for nodulation of legumes. Annu Rev Microbiol. 1992;46:399–428. doi: 10.1146/annurev.mi.46.100192.002151. [DOI] [PubMed] [Google Scholar]
- Villadas PJ, Velázquez E, Martínez-Molina E, Toro N. Identification of nodule-dominant Rhizobium meliloti strains carrying pRmeGR4b-type plasmid within indigenous soil populations by PCR using primers derived from specific DNA sequences. FEMS Microbiol Ecol. 1995;17:161–168. [Google Scholar]
- Volz C, Kegler C, Müller R. Enhancer binding proteins act as hetero-oligomers and link secondary metabolite production to myxococcal development, motility, and predation. Chem Biol. 2012;21:1447–1454. doi: 10.1016/j.chembiol.2012.09.010. [DOI] [PubMed] [Google Scholar]
- White DJ, Hartzell PL. AglU, a protein required for gliding motility and spore maturation of Myxococcus xanthus, is related to WD-repeat proteins. Mol Microbiol. 2000;36:662–678. doi: 10.1046/j.1365-2958.2000.01887.x. [DOI] [PubMed] [Google Scholar]
- Wu SS, Kaiser D. Makerless deletion of pil genes in Myxococcus xanthus generated by counterselection with the Bacillus subtilis sacB gene. J Bacteriol. 1996;178:5817–5821. doi: 10.1128/jb.178.19.5817-5821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Wei X, Ebright R, Wall D. Antibiotic production by myxobacteria plays a role in predation. J Bacteriol. 2011;193:4626–4633. doi: 10.1128/JB.05052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev. 1999;63:968–989. doi: 10.1128/mmbr.63.4.968-989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vaksman Z, Litwin DB, Shi P, Kaplan HB, Igoshin OA. The mechanistic basis of Myxococcus xanthus rippling behavior and its physiological role during predation. PLoS Comput Biol. 2012;8:e1002715. doi: 10.1371/journal.pcbi.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Predatory patterns of M. xanthus on S. meliloti. Different S. meliloti field isolates and reference laboratory strains were exposed to M. xanthus DK1622 (Mx DK1622) (A) and M. xanthus DZ2 (Mx DZ2) (B) on CTT agar plates. Images were taken with a digital camera 72 h after spotting on plates. Microscopic images of the same S. meliloti macrocolonies (boxed) are shown to the left of each panel.
Fig. S2. Sequence analysis of the S. meliloti expR gene. (A) Amplification of the expR alleles in different S. meliloti strains. The oligonucleotide primers used in the PCR reactions (ExpR1/ExpR2) and the expected size of the PCR products are indicated above the map of the S. meliloti expR genomic region. The ExpR ORF is drawn to scale. The ethidium bromide stained agarose gels of the PCR products are shown below with the names of the S. meliloti strains analyzed on top. Amplification of the Rm1021 expR failed because it is disrupted by the insertion sequence ISRm1 (1,320 bp). Sizes of the co-migrating DNA markers are indicated. (B) Multiple amino acid sequence alignment of the ExpR proteins. Host strains are indicated to the left. ExpR sequences were inferred by sequencing of the corresponding PCR products except those of the AK83 and Rm1021 strains which were obtained from the GeneBank database (accession numbers NC_015590 and NC_003047, respectively). Mutations resulting in a non-functional protein are shown in red. The ultraconserved S202 residue altered in the RMO17b ExpR is indicated by a double arrowhead.
Movie S1. Interaction between M. xanthus DK1622 (left) and S. meliloti GR4b (right). Time 0-48 h after spotting.
Movie S10. Interaction between M. xanthus ΔcsgA (right) and S. meliloti GR4b (left). Time 0-24 h after spotting.
Movie S11. Interaction between M. xanthus ΔcsgA (light area) and S. meliloti GR4b (dark area). Time 24-72 h after spotting.
Movie S12. Interaction between M. xanthus ΔcsgA (right) and S. meliloti AK21 (left). Time 0-24 h after spotting.
Movie S2. Interaction between M. xanthus DK1622 (left) and S. meliloti AK21 (right). Time 0-24 h after spotting.
Movie S3. Interaction between M. xanthus DK1622 (upper, light area) and S. meliloti AK21 (dark area). Time 24-72 h after spotting.
Movie S4. Interaction between M. xanthus DK1622 (right) and S. meliloti GR4b (left). Time 0-48 h after spotting.
Movie S5. Interaction between M. xanthus DK1622 (light area) and S. meliloti GR4b (dark area). Time 48-72 h after spotting.
Movie S6. Interaction between M. xanthus DZ2 (right) and S. meliloti GR4b (left). Time 0-48 h after spotting.
Movie S7. Interaction between M. xanthus DZ2 (light area) and S. meliloti GR4b (dark area). Time 48-72 h after spotting.
Movie S8. Interaction between M. xanthus DZ2 (right) and S. meliloti AK21 (left). Time 0-24 h after spotting.
Movie S9. Interaction between M. xanthus DZ2 (light area) and S. meliloti AK21 (dark area). Time 24-72 h after spotting.
Movie S13. Interaction between M. xanthus ΔcsgA (light area) and S. meliloti AK21 (dark area). Time 24-72 h after spotting.


