SUMMARY
The selectins and their ligands are required for leukocyte extravasation during inflammation. Several glycoproteins have been suggested to bind to E-selectin in vitro but the complete identification of its physiological ligands has remained elusive. Here, we show using gene- and RNA-targeted loss-of-function that E-selectin ligand-1 (ESL-1), PSGL-1 and CD44 encompass all endothelial selectin ligand activity on neutrophils. PSGL-1 plays a major role in the initial leukocyte capture, while ESL-1 is critical to convert initial tethers into steady slow rolling. CD44 controls rolling velocity and mediates E-selectin-dependent redistribution of PSGL-1 and L-selectin to a major pole on slowly rolling leukocytes through p38 signaling. These results suggest distinct and dynamic contributions of these three glycoproteins in selectin-mediated neutrophil adhesion and signaling.
The selectins, a family of three C-type lectins, mediate interactions among leukocytes, platelets, and the endothelium (Ley, 2003; McEver, 2002; Vestweber and Blanks, 1999). P-selectin, stored in platelets and endothelial cells, is rapidly expressed on the cell surface upon stimulation, and mediates most leukocyte rolling in the first hour of an inflammatory challenge. L-selectin is constitutively expressed on the tip of leukocyte microvilli and plays critical roles in the homing of lymphocytes to lymph nodes. L-selectin may also participate in leukocyte recruitment in systemic venules through secondary tethers between circulating and adherent leukocytes (Sperandio et al., 2003). E-selectin expression is restricted to the endothelium, induced by inflammatory cytokines like TNF-α and IL-1, and mediates the slow leukocyte rolling in inflamed venules. E-selectin plays a major role in leukocyte recruitment to inflammatory sites through its close collaboration with P-selectin (Bullard et al., 1996; Frenette et al., 1996; Labow et al., 1994).
All three selectins bind to α(2,3)sialylated and α(1,3)fucosylated carbohydrate structures similar to the sialyl Lewis x (sLex ) determinant. While P-and L-selectins additionally require sulfation of the polypeptide or carbohydrate ligand, sLex appears to be sufficient for Ca2+-dependent binding to E-selectin. Several studies have indicated that the engagement of a selectin on its ligand can trigger signaling inside the leukocyte that may involve tyrosine phosphorylation and p38 mitogen-activated protein kinase (MAPK) activation, resulting in activation of the β2-integrins, and clustering of adhesion molecules on the cell surface (Evangelista et al., 1999; Green et al., 2004; Lo et al., 1991; Simon et al., 2000; Smith et al., 2004). Genetic deletion studies have revealed that P-selectin glycoprotein-1 (PSGL-1) was the major ligand for P-selectin (Xia et al., 2002; Yang et al., 1999).
The binding requirements of E-selectin to highly conserved, poorly immunogenic carbohydrates, have hampered efforts to generate inhibitory monoclonal antibodies, and the identification of physiological E-selectin ligands (ESLs) (Vestweber and Blanks, 1999). Since most cells can roll on E-selectin when transfected with a vector encoding an α(1,3)-fucosyltransferase gene that introduces sLex to the cell surface, it has been implied that E-selectin may interact indiscriminately with many glycoproteins and glycolipids.
Several glycostructures on various hematopoietic cells have indeed been proposed to bind to E-selectin using in vitro affinity purification techniques, including PSGL-1 (Moore et al., 1994), ESL-1 (Steegmaier et al., 1995), CD44 (Dimitroff et al., 2001), Lselectin (on human but not mouse) (Picker et al., 1991; Zollner et al., 1997), CD43 (Fuhlbrigge et al., 2006; Matsumoto et al., 2005), β2 integrins (Kotovuori et al., 1993), glycolipids (Alon et al., 1995) and other yet unidentified glycoproteins (Jones et al., 1997; Montoya et al., 1999). Of these, PSGL-1 has clearly been shown to contribute to E-selectin-mediated leukocyte rolling in vivo (Hirata et al., 2000; Xia et al., 2002). However, leukocyte rolling velocities, a trademark function of ESLs (Kunkel and Ley, 1996), were not altered in Selplg−/− mice (Xia et al., 2002). In addition, the fact that the overall phenotype of Selplg−/− mice resembles that of Selp−/− animals suggested that PSGL-1 largely functions as a P-selectin ligand, and implied the presence of other major ESLs. Our recent studies have revealed that CD44 was a physiological ESL mediating the slow leukocyte rolling on E-selectin in vivo and that it collaborated with PSGL-1 in neutrophil extravasation to inflamed sites (Katayama et al., 2005). However, the alterations in leukocyte behavior in mice lacking both CD44 and PSGL-1 were not as severe as in mice deficient in both endothelial selectins, a finding supporting the existence of at least three physiological ESLs or the compensation in gene knockout mice by other glycoprotein or glycolipid structures.
Among the aforementioned candidate ligands, ESL-1 was isolated by affinity purification techniques from myeloid cells (Steegmaier et al., 1995). ESL-1 protein sequence was found to be identical to a membrane sialoglycoprotein of the medial cisternae of the rat Golgi complex (MG-160 (Gonatas et al., 1989)) and the avian cysteine-rich FGF receptor (CFR (Burrus et al., 1992)). These glycoproteins are now known to be encoded by a single gene locus named Glg1 (Golgi complex-localized glycoprotein-1). Indeed, most ESL-1 is found in the Golgi apparatus while a relatively small fraction is expressed on the cell surface where it has been immunolocalized to microvilli (Steegmaier et al., 1997). In myeloid cells, ESL-1 is thought to be endowed with E-selectin binding activity notably through α(1,3) fucosylation (Huang et al., 2000). In other cell systems, the protein may be secreted as part of the TGF-β complex where it is bound through disulphide linkage to the latency-associated peptide of the TGF-β1 precursor (Olofsson et al., 1997). It may also associate intracellularly with several FGF family members (Kohl et al., 2000). These studies suggest important functions of ESL-1 outside the immune system.
Here, we have used short hairpin RNA (shRNA) interference specific to Glg1, delivered by lentiviral transduction, to knockdown ESL-1 specifically in hematopoietic cells deficient or sufficient in PSGL-1 and / or CD44. Strikingly, our results reveal that ESL-1 is the most versatile and powerful ESL, and that, together with PSGL-1 and CD44, they comprise all detectable ESL activity on neutrophils. Moreover, we show here that these three glycoproteins exhibit dynamic, specialized functions in selectindependent leukocyte rolling, adhesion, and signaling.
RESULTS
SPECIFIC KNOCKDOWN OF ESL-1 IN THE HEMATOPOIETIC SYSTEM
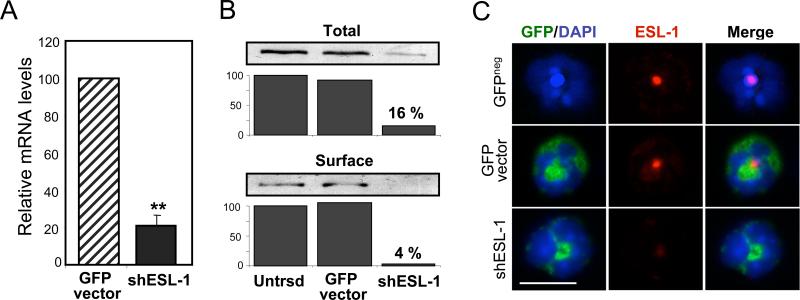
To knockdown ESL-1 expression specifically in the hematopoietic system, we generated independently two vectors encoding shRNA sequences specific to its intraluminal domain. The lentiviral vector separately expresses the green fluorescent protein (GFP), allowing efficient detection of transduced cells. Following preliminary transduction studies of myeloid (32D) and non-myeloid (bEnd.3) cells, we chose the most robust vector for all studies presented herein (Supplementary Fig.1). Transduction of 32D cells with this shESL-1 lentiviral vector profoundly reduced (by ~70%) ESL-1 mRNA levels as detected by quantitative PCR (Fig. 1A) and ESL-1 protein levels (by ~85%) in lysates of transduced cells sorted on the basis of GFP expression (Fig. 1B, upper panel). Since most ESL-1 is expressed in the Golgi apparatus, we biotinylated surface proteins to assess specifically the effect of shESL-1 on ESL-1 expressed on the cell membrane. We found that ESL-1 was virtually undetectable on the surface of shESL-1-transduced 32D cells (Fig.1B, lower panel). Importantly, transduction with a control vector did not alter ESL-1 protein levels relative to untransduced cells (Fig. 1B).
Figure 1. ESL-1 knockdown in 32D cells and circulating leukocytes.
32D cells transduced with the control or shESL vectors were sorted on the basis of GFP expression. (A) Q-PCR of RNA extracts from GFP+ cells. Data were normalized to the levels of GAPDH. (B) Western blot analyses of 32D cell lysates either untransduced (Untrsd) or transduced with the control or shESL-1 vectors. Percentages reflect the proportion ESL-1 protein detected in the shESL-1 groups relative to untransduced cells. Surface ESL-1 expression was virtually undetectable in the shESL–transduced group. Shown is one representative experiment of three. (C) Immunofluorescence staining of cytospin preparations of blood leukocytes from mice transplanted with BM cells untransduced or transduced with GFP control or shESL-1 vectors. Representative neutrophils expressing GFP (green) are depicted. Nuclei are stained with DAPI (blue). ESL-1 staining (red), detectable only in the Golgi apparatus of wild-type neutrophils, is markedly reduced in shESL-1-transduced neutrophils. Scale bar, 10 μm.
To assess the efficiency of shESL-1 knockdown in primary hematopoietic cells, we generated chimeric mice by transplantation of hematopoietic stem and progenitor cells. In these studies, we purified bone marrow-derived lineage-negative (Lin-) cells from wild-type, Selplg−/−, Cd44−/− or Selplg−/− / Cd44−/− (herein referred to as DKO) mice. Lin- cells were then transduced with the shESL-1 or GFP control vectors, and transplanted into lethally irradiated wild-type recipient mice. After a recovery of at least three weeks, the average frequencies of mature GFP+ blood leukocytes descendant from transduced progenitor cells ranged between ~10 to 25% of all circulating leukocytes in blood (Supplementary Table 1). ESL-1 downregulation did not have any major effect on the survival or growth of hematopoietic cells since the engraftment levels and percentages of circulating transduced leukocytes were comparable to those of the control group (Supplementary Table 1). Immunostaining of shESL-1-transduced blood leukocytes from transplanted animals revealed a marked reduction in ESL-1 expression in the perinuclear region corresponding to the Golgi apparatus (Fig. 1C). However, shESL-1 did not reduce the expression of other known selectin ligands (PSGL-1 and CD44; Supplementary Fig.2). These results validate this knockdown strategy to study the function of surface ESL-1 in mature leukocytes.
ESL-1 IS A MAJOR PHYSIOLOGICAL LIGAND FOR E-SELECTIN THAT COLLABORATES WITH PSGL-1
To assess the contribution of ESL-1 in leukocyte binding to E-selectin, we first analyzed the ability of transduced neutrophils from transplanted mice to bind to soluble E-selectin in a fluid-phase assay. In agreement with previous reports (Katayama et al., 2005; Xia et al., 2002), Selplg−/− neutrophils displayed a marked reduction in soluble Eselectin- IgM chimera binding which was further reduced by 50% in DKO neutrophils (Fig. 2B, inset). Absence of either ESL-1 or CD44 reduced binding to E-selectin by ~30%, and E-selectin binding was modestly reduced further by the combined deficiency of CD44 and ESL-1 (Fig. 2B). Binding of soluble P-selectin, in contrast, was not affected by the absence of ESL-1 (Supplementary Fig. 3). Strikingly, the ability of soluble E-selectin to bind to shESL-1-transduced neutrophils deficient in PSGL-1 was completely abrogated (Fig. 2A-B), suggesting a remarkable cooperation between these two glycoproteins for high affinity binding.
Figure 2. Contribution of ESL-1, PSGL-1 and CD44 in binding of soluble E-selectin.
Flow cytometric analyses of E-selectin/IgM chimera binding to blood neutrophils obtained from mice transplanted with Lin- cells from wild-type, Cd44−/−, Selplg−/− or DKO donors that were transduced with GFP control or shESL-1 vectors. (A) Representative dot plots showing GFP expression (upper quadrants, green) and E-selectin binding in the control (top panels) or shESL (middle panels) groups. Control binding in the presence of EDTA in untransduced neutrophils was used to set the quadrants. Histograms (bottom panels) show overlays of E-selectin binding to neutrophils from the GFP control (light green) and shESL-1 (dark green) groups, as well as control binding in the presence of EDTA (grey) for each donor group. E-selectin binding was reduced to the levels of EDTA controls in the absence of both PSGL-1 and ESL-1. (B) Quantitative summary of E-selectin binding intensities in all eight groups. Inset shows expanded Eselectin binding for the Selplg−/− and DKO groups. n=6-18 mice per group. * p=0.08, ** p<0.0001 compared to the respective GFP vector controls; #, p<0,0001 compared to WT GFP vector; ‡, p<0.0001 compared to WT or CD44 GFP vector groups. §, p<0.0001 compared to WT or CD44 shESL-1 groups.
To assess the contribution of ESL-1 in leukocyte rolling in vivo, we examined cremasteric venules of TNF-α-stimulated mice transplanted with transduced Lin- cells using intravital microscopy. We tracked the behavior of transduced (GFP+) leukocytes using high-speed widefield fluorescence microscopy (Supplementary Fig. 4 and Video 1). The rolling frequency of transduced leukocytes was reduced by ~75% in the absence of PSGL-1 but was not affected by the absence of ESL-1 alone (Fig. 3A). ESL-1 knockdown in Cd44−/− leukocytes resulted in a marked increase in rolling frequencies, which, interestingly, is reminiscent of Sele−/− mice (Kunkel and Ley, 1996). In contrast, rolling was dramatically reduced (by ~93%) when both PSGL-1 and ESL-1 were absent from the leukocyte surface (Fig. 3A). Rolling frequencies tended to be further reduced when CD44 was also absent, although this did not reach statistical significance in a multigroup analysis of variance (Fig. 3A). These results indicate that PSGL-1 and ESL-1 are the major selectin ligands mediating the initial leukocyte tethering and rolling in vivo.
Figure 3. Intravital microscopy analyses of cremasteric venules from TNF-α-stimulated mice.
The behavior of GFP+ leukocytes, transduced with either the GFP control or shESL-1 vector, was tracked in real time using high-speed digital videomicroscopy between 150 to 210 min after TNF-α administration. (A) Rolling flux fractions. Inset shows expanded rolling fractions for the Selplg−/− and DKO groups. Data derived from 60 chimeric mice and 448 venules. n=6-11 mice per group; n=48-60 venules per group. * p ≤ 0.001, ** p<0.0001 compared to their respective GFP vector controls; #, p<0.0001 compared to wild type (WT) GFP vector; ‡, p<0.0001 compared to WT and Cd44−/− transduced with GFP vector; § p<0.0001 compared to WT or CD44 transduced with shESL-1. (B) Mean rolling velocity analyses. Data are derived from 1,759 GFP+ leukocytes. * p ≤ 0.002, ** p < 0.0001 compared to their respective GFP vector controls; # p<0.0001 compared to Cd44−/− and DKO transduced with GFP vector; ‡ p<0.0001; §, p<0.0001 compared to all other shESL-1 groups. (C) Cumulative histograms of rolling velocities. Shown are the velocity histograms for the groups transduced with GFP control (left panel) or shESL-1 vectors (right panel). Shown for comparison in the right panel is the histogram from wild-type leukocytes transduced with GFP control vector (discontinuous pink line).
DIFFERENTIAL CONTRIBUTIONS OF PSGL-1, ESL-1 AND CD44 IN LEUKOCYTE ROLLING DYNAMICS IN INFLAMED VENULES
E-selectin, but not P-selectin, controls slow leukocyte rolling on inflamed venules which may in turn serve to promote an efficient transition to firm adhesion and to keep extravasation localized to the inflamed area (Jung et al., 1998; Kunkel and Ley, 1996; Ley et al., 1998). While Selplg−/− leukocytes had rolling velocities similar to those of wild-type type animals (Fig. 3B-C), leukocytes deficient in CD44 or ESL-1 exhibited a significant increase in rolling velocities (Fig. 3B-C). In addition, the rolling velocities of leukocytes deficient in ESL-1 and either CD44 or PSGL-1 were further increased compared to those with any single deficiency, suggesting that ESL-1 cooperates with CD44 and PSGL-1 in controlling rolling velocities. Leukocyte velocities were further increased when all three glycoproteins were absent (18 ± 4 μm/s), to a level comparable to those observed in Sele−/− mice reconstituted with wild-type Lin- cells transduced with the control vector, (18 ± 1 μm/s, n=32 venules from 4 mice). These results suggest that these three glycoproteins comprise most, if not all, ESLs on leukocytes required to control the slow rolling mediated by E-selectin.
In addition to the changes in rolling velocities, shESL-1-transduced leukocytes exhibited a characteristic “skipping” behavior. Velocity tracings revealed cycles of short steady rolling periods followed by release and re-tethering further downstream, resulting in sharp and transient increases in velocity (Fig. 4A and Supplementary Videos 2 & 3). Consequently, leukocytes moving in this fashion failed to engage into steady rolling. Rolling kinetics of ESL-1-deficient cells sharply contrasted with the steady and slow motion of wild-type or Selplg−/− leukocytes, and the steady but faster rolling of Cd44−/− leukocytes (Fig. 4A). Quantitation of this rolling behavior revealed that approximately half of shESL-1-transduced leukocytes displayed such rolling kinetics, compared to only ~15% of wild-type or Cd44−/− leukocytes (Fig. 4B). It is important to note that the elevated rolling velocities found in the shESL-1 groups (Fig. 3B) were independent of these “skipping” kinetics since these velocity profiles are obtained from leukocytes interacting with the endothelium. These observations indicate distinct functions of these glycoproteins during leukocyte tethering and rolling in inflamed venules. While PSGL-1 is critical for the initial leukocyte capture and CD44 specializes in controlling leukocyte rolling velocities, ESL-1 cooperates with PSGL-1 and CD44 in both activities, and appears to play a major role in stabilizing leukocytes into steady rolling.
Figure 4. ESL-1 is required for steady rolling of leukocytes.
(A) “Skipping” rolling behavior of ESL-1-deficient leukocytes. Tracings show near instantaneous velocity tracking of wild-type or Cd44−/− leukocytes transduced with GFP control or shESL-1 vectors. Individual rolling velocities were determined every 0.2 s from digital recordings. Each line represents the fluctuation in velocity of 5 individual rolling cells per group over 5 s. Spikes in the shESL-1-transduced groups (right histograms) reflect the behavior of cells that transiently detach from the endothelium, and re-tether shortly after. (B) Percentages of GFP+ leukocytes displaying a “skipping” behavior from the groups represented in (A). Data were obtained from 780 leukocytes. n= 28-30 venules from 4 mice per group. *** p<0.0001.
IMPAIRED ADHESION AND RECRUITMENT OF NEUTROPHILS TO INFLAMMATORY SITES IN THE ABSENCE OF PSGL-1, ESL-1 AND CD44
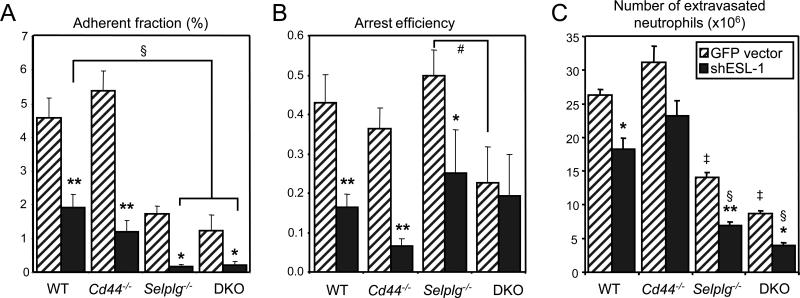
Slow rolling leukocytes integrate signals from chemokines and E-selectin presented by the endothelium, resulting in their firm arrest on inflamed venules (Chesnutt et al., 2006; Laudanna et al., 2002; Ley et al., 1998; Smith et al., 2004). We therefore monitored the contributions of PSGL-1, ESL-1 and CD44 in the arrest of leukocytes in TNF-α-treated cremasteric venules by intravital microscopy. Absence of PSGL-1 or ESL-1 markedly reduced of the number of arrested leukocytes, and the absence of both PSGL-1 and ESL-1 further reduced leukocyte arrest to almost undetectable levels (Fig. 5A). However, analyses of the arrest efficiencies (i.e. the fraction of rolling leukocytes that undergo firm arrest) revealed a dramatic influence of ESL-1 alone (reduction by ~63%) or with CD44 (reduction by ~86%), but no effect of PSGL-1 (Fig. 5B). Arrest fractions and efficiencies in Sele−/− mice (2.8 ± 0.8% and 0.20 ± 0.06%, respectively, in n=30 venules from 4 mice) were comparable to those found in the absence of ESL-1 alone (p>0.05 for both parameters). These results underscore a major role for ESL-1 and CD44 in the transition from rolling to firm arrest, and support the notion that PSGL-1, while critical to initiate rolling, does not significantly contribute to the arrest of leukocytes that are already rolling.
Figure 5. Contributions of PSGL-1, ESL-1 and CD44 in the adhesion and extravasation of leukocytes.
(A) The adherent fraction reflects the proportion of firmly adherent GFP+ leukocytes out of all circulating GFP+ leukocytes counted over 1 min. Data were derived from 448 venules in 60 chimeric mice. n=6-11 mice, n=48-60 venules per group. (B) Arrest efficiencies reflect the proportion of rolling GFP+ that undergo firm arrest. Data were derived from 354 venules from 60 mice. n=6-11 mice, n= 13-69 venules per group. (C) Neutrophil extravasation into inflammatory sites was determined in thioglycollate induced peritonitis. Extravasated PMNs were estimated 8h after i.p. injection of thioglycollate in mice transplanted with bone marrow cells transduced with control or shESL-1 vector. The proportions of transduced neutrophils (GFP+ Gr-1hi) in the circulation and in the peritoneum was determined by FACS analysis, and used to calculate the extravasation efficiencies (see Supplementary Fig.5 and Table 2). Data from 54 mice, n=5-9 mice per group. * p<0.05, ** p<0.001 compared to the respective GFP vector controls; # p=0.0076; ‡ p<0.0001 compared to wild-type or Cd44−/− transduced with GFP vector. § p<0.0001 compared to wild-type or Cd44−/− transduced with shESL-1.
To assess whether PSGL-1, ESL-1 and CD44 are the physiological selectin ligands mediating neutrophil extravasation to inflammatory sites, mice were injected with thioglycollate, a chemical that induces a severe peritoneal inflammation. The number of neutrophils in the peritoneal exudate was evaluated 8h after injection and the percentage of GFP+ control and shESL-1 neutrophils (defined as Gr-1hi) relative to those of untransduced (GFPneg) cells, were determined in the blood and peritoneal exudate by FACS analysis. Since the number of transduced leukocytes is variable among transplanted animals, we determined the ratio of GFP+ Gr-1hi neutrophils in the exudate versus blood to estimate the efficiency of extravasation (see Supplementary Fig. 5 and Table 2 for details). PSGL-1-deficient or shESL-1-transduced neutrophils showed a significant but moderate reduction in their ability to extravasate. However, the number of peritoneal DKO neutrophils was strongly reduced (by ~65%) compared to wild-type levels (Fig. 5C). Remarkably, the absence of all three glycoproteins dramatically impaired neutrophil recruitment (> 85%) to the peritoneum, to an extent similar to that found in animals deficient in both endothelial selectins (Bullard et al., 1996; Frenette et al., 1996). These results indicate that PSGL-1, ESL-1 and CD44 are specifically endowed with virtually all detectable endothelial selectin ligand activity, and that no other glycoproteins or glycolipids can compensate for their deficiency.
E-SELECTIN SIGNALS INTO ROLLING LEUKOCYTES THROUGH CD44
Previous studies have shown that the ligation of E-selectin ligands can trigger the polarization of L-selectin and PSGL-1 on human neutrophils in vitro (Green et al., 2004; Simon et al., 2000). To determine whether this phenomenon also takes place in vivo, we assessed in real time L-selectin distribution on rolling leukocytes using high-speed fluorescence videomicroscopy (Supplementary Figure 6 and Videos 4 & 5). In these experiments, L-selectin distribution on leukocytes was imaged in real time using a single injection of low dose (0.02 mg / Kg) fluorescence-conjugated anti-L-selectin antibody in mice treated with TNF-α. This dose of antibody resulted in only 15% occupancy of surface receptors as determined by FACS analysis (data not shown), but allowed clear real time evaluation of L-selectin distribution. Unexpectedly, we observed that a large fraction of rolling wild-type leukocytes exhibited L-selectin polarization (Fig. 6A). This fraction progressively increased over time to reach a plateau of ~60% polarized leukocytes 200 min after TNF-α injection (Fig. 6B). Interestingly, both L-selectin and PSGL-1 clusters co-localized on rolling leukocytes (76/76 analyzed leukocytes from 3 experiments; Supplementary Fig. 7). To assess whether this phenomenon depends on E-selectin, we evaluated L-selectin distribution in Sele−/− mice. We found that much fewer rolling leukocytes (23.5 ± 1.5%) displayed such polarized distribution of L-selectin in Sele−/− mice (Fig. 6B), indicating that ligation of E-selectin ligands in vivo induces the redistribution of adhesion molecules on rolling leukocytes.
Figure 6. CD44 mediates E-selectin-induced clustering of L-selectin on rolling leukocytes.
(A) Sequence of images showing L-selectin distribution on rolling leukocytes (see Supplementary Videos 4 & 5), obtained from recordings of cremasteric venules from mice previously treated with TNF-α and injected with 0.02 mg/kg of a PE-conjugated anti-L-selectin antibody. Fluorescence images (Cy3 channel; top panels) and fluorescence intensity plots (lower panels) show two rolling leukocytes with clustered Lselectin (red clusters). Real time (s : ms) is displayed in the upper left corner and the arrow indicates the direction of flow. Bar = 10 μm. (B) L-selectin clustering is Eselectin- dependent. The number of rolling leukocytes displaying clustered L-selectin was determined in wild-type and Sele−/− mice from 160 to 230 min after TNF-α administration. * p < 0.05. (C) E-selectin-dependent L-selectin clustering on rolling leukocytes requires CD44-mediated signaling. The redistribution of L-selectin into the polar cap was determined 160 to 230 min after TNF-α administration in a total of 4,320 rolling leukocytes from 17-30 venules (3-5 mice) per group. Mice used for these analyses were transplanted at least 4 weeks prior with untransduced BM cells from Cd44−/− or Selplg−/− donor mice or BM cells from wild-type donors transduced with the shESL-1 vector. Only GFP+ leukocytes were analyzed in this last group. Untransplanted wild-type and Sele−/− mice were used for the first two groups. ** p<0.0001 for Sele−/− and Cd44−/− compared to all other groups. (D) L-selectin clustering in rolling leukocytes is p38 dependent. Wild-type mice were injected i.v. with vehicle (DMSO) or 100 μg of SB203580 2h before analysis of L-selectin redistribution by intravital microscopy. Results are derived from the analysis of 954 rolling cells; n=14- 16 venules (3-4 mice) per group. * p<0.0001. (E) Crosslinking of CD44 activates p38 MAPK. Purified PMNs from wild-type or Cd44−/− mice were incubated with control rat IgG or anti-CD44 antibodies at 37°C and analyzed for the presence of phospho-p38 by Western blotting. Shown is a representative experiment of two. (F) Crosslinking of CD44 induces p38-mediated clustering of PSGL-1. Purified C57BL/6 PMNs were treated or not with SB203580, CD44 was engaged by crosslinking, and surface PSGL-1 stained. Cells were fixed, cytospun onto slides and analyzed for PSGL-1 distribution (PSGL-1 in red and DAPI in blue; top panels). The frequency of polarized neutrophils was determined according to the distribution of PSGL-1 on the cell surface (bottom bar graph). Data from 3 independent experiments, * p<0.001. Bar = 10 μm. (G) Differential distribution of PSGL-1 and CD44 on rolling leukocytes. Lethally irradiated Cd44−/− mice were transplanted with wild-type bone marrow. One month later, mice were treated with TNF-α and 150 min later injected with 0.02 mg/kg of APC-conjugated anti-CD44 and PE-conjugated anti-PSGL-1 antibodies before image acquisition. Fluorescence images (Cy5 channel in green, and Cy3 channel in red; left panels) and fluorescence intensity plots (right panels) show two leukocytes with CD44 evenly distributed or concentrated on the leukocyte-endothelium interface (arrowheads), while PSGL-1 is clustered to the luminal side (red areas in right-bottom panel). The bottom panel shows blended images from fluorescence and brightfield channels to allow the visualization of the leukocyteendothelial interface. The arrow indicates the direction of flow.
To determine which glycoprotein ligand mediates E-selectin-dependent activation of leukocytes in vivo, we analyzed L-selectin clustering on rolling leukocytes from mice transplanted with BM cells from wild-type, Cd44−/− or Selplg−/− mice, or wild-type BM cells transduced with the shESL-1 lentiviral vector (see Supplementary Table 3 for venular hemodynamic parameters). We found that a high proportion of rolling leukocytes deficient in PSGL-1 or ESL-1 exhibited L-selectin clustering (63 ± 4% for Selplg−/− and 71 ± 5% for shESL-1; Fig. 6C). In sharp contrast, the number of rolling leukocytes with clustered L-selectin was markedly reduced (20 ± 2%; Fig. 6C), to the levels of Sele−/− mice, when CD44 was absent. These results indicate that CD44 is the signaling receptor mediating E-selectin-dependent redistribution of L-selectin on rolling leukocytes in vivo. To determine whether p38 MAPK signaling was involved in CD44- mediated L-selectin redistribution, we treated wild-type mice with the inhibitor SB203580 prior to intravital microscopy observation. Inhibition of p38 activation significantly reduced L-selectin redistribution on rolling leukocytes (Fig. 6D), suggesting that this pathway is involved in downstream signals from CD44. Furthermore, the engagement of CD44 on primary neutrophils by Mab crosslinking strongly induced p38 phosphorylation (Fig. 6E). p38 activation was specific since it did not occur when Cd44- /- PMNs were tested in parallel (Fig.6E). CD44 crosslinking also induced a strong polarization of PSGL-1 on the neutrophil surface, which was completely inhibited by preincubation of PMNs with SB203580 (Fig. 6F). These experiments thus demonstrate that the engagement of CD44 on neutrophils triggers p38-mediated clustering of surface glycoproteins.
Since CD44 mediates steady slow rolling and PSGL-1 does not play a major role once the leukocyte has engaged into steady rolling, we hypothesized that these two glycoproteins are differentially distributed on slow rolling leukocytes. To investigate this issue, we imaged the distribution of PSGL-1 and CD44 on leukocytes in TNF-α-treated mice. To avoid undesired anti-CD44 antibody binding to the endothelium, these experiments were carried out in CD44-deficient mice reconstituted with wild-type bone marrow. We found that PSGL-1 was clustered in a manner similar to L-selectin on the majority of slow rolling leukocytes (Fig. 6G). In contrast, on rolling leukocytes displaying polarized PSGL-1, CD44 remained evenly distributed on most rolling cells (~88%), and in some instances was more densely distributed in areas of contact with the endothelium (Fig. 6G). Thus these results suggest that the surface distributions of CD44 and PSGL-1 in slowly rolling leukocytes reflect their distinct functions.
DISCUSSION
In these studies, we have identified virtually all endothelial selectin ligand activity mediating leukocyte recruitment to inflammatory sites. Our results suggest that PSGL- 1, ESL-1 and CD44 are specifically targeted by ligand-synthesizing glycosyltransferases, and that no other neutrophil glycoprotein or glycolipid can compensate for their absence. We show that these ligands closely collaborate with each other to facilitate leukocyte recruitment. Moreover, our results suggest a highly dynamic collaboration in which, for instance, the engagement of E-selectin through one ligand (e.g. CD44) can induce signals culminating in the redistribution of another (e.g. PSGL-1) in actively rolling leukocytes. While each ESL exerts remarkably distinct functions, ESL-1 appears to stand out as the most powerful and versatile.
Solid biochemical studies have implicated ESL-1 as a ligand for E-selectin (Huang et al., 2000; Steegmaier et al., 1995; Wild et al., 2001). However, the wide expression of the Glg1 gene in multiple tissues (Burrus et al., 1992; Gonatas et al., 1989; Steegmaier et al., 1995), cellular compartments (Gonatas et al., 1998; Kohl et al., 2000; Steegmaier et al., 1997), and extracellular milieux (Olofsson et al., 1997), has puzzled researchers in this field for more than a decade. Glg1−/− mice have a skeletal phenotype characterized by severe growth retardation but no defect in leukocyte rolling behavior was noted by intravital microscopy (Drs. Brendan Lee, Klaus Ley, and Arthur Beaudet, unpublished data, personal communication). The present studies, however, using specific downregulation of Glg1 gene expression in hematopoietic cells, clearly demonstrate that ESL-1 is indeed a major ESL on leukocytes. The possible difference between the knockdown and the genetic knockout may result from compensation mechanisms during development in mice genetically deficient from the embryonic stage. It is possible that such compensation mechanisms are not operative when the expression of a gene is altered in the adult. Knockdown by lentiviral delivery of shRNA in hematopoietic stem and progenitor cells presents several potential advantages to study ESL-1. First, it circumvents the possible influences of the Glg1 gene from nonhematopoietic expression since it can be targeted specifically to postnatal bone marrow Lin- cells. Second, the generation of chimeric animals by bone marrow transplantation provides the means to track specifically GFP+ leukocyte progeny in the vasculature or in tissues. Third, the presence of both deficient (transduced) and sufficient (nontransduced) leukocytes simultaneously in the same animal, gives rise to a competitive environment that is likely more sensitive than classical genetic knockout within entire hematopoietic compartment. Finally, the incomplete downregulation of gene expression at the cellular level may have allowed the maintenance of normal homeostatic cellular functions in transduced cells. Indeed, the residual ~15% cellular expression of ESL-1 appears sufficient for transduced stem cells to engraft, survive, and proliferate normally in the bone marrow of recipient mice. Thus, our results do not exclude other functions of ESL-1, for instance in hematopoietic cell proliferation and survival. It is noteworthy that the surface expression of ESL-1 was negligible on the cell surface of shESL-1- transduced cells where it appears dispensable for vital cellular functions. Therefore, shRNA downregulation of Glg1 gene expression has predominantly depleted surface ESL-1 while maintaining low levels of expression in the Golgi apparatus.
Our results highlight the specialized contributions of PSGL-1, ESL-1, and CD44 in various aspects of E-selectin-mediated leukocyte recruitment, including the initial capture (tethering), transition to steady rolling, slow rolling, firm adhesion, and signaling (Fig. 7). PSGL-1 mediates the capture of free-flowing leukocytes to the endothelium through its interaction with both P-and E-selectins. This function is supported by fast association and dissociation rates (Mehta et al., 1998) and its location on the very tip of leukocyte microvilli (Moore et al., 1995). ESL-1 has also been reported to be concentrated on microvilli but largely excluded from the very tip (Steegmaier et al., 1997), while CD44 is exclusively distributed on the planar surface of the leukocyte (von Andrian et al., 1995). The present studies indicate that ESL-1 may act as a bridge between PSGL-1 and CD44 in that it contributes to the initial capture with PSGL-1, and on the other hand, it controls with CD44 the velocity of rolling leukocytes (Fig. 3) and leukocyte arrest on the endothelium (Fig. 5). Therefore ESL-1 appears to be the most versatile ESL, but its unique contribution to leukocyte recruitment is arguably in the transition to steady slow rolling (Fig. 4). In contrast to PSGL-1 and ESL-1, the contribution of CD44 becomes apparent only at the later stages of the leukocyte recruitment cascade. It is tempting to speculate that microvilli likely recede as the leukocyte integrates activation signals, allowing CD44, buried in the planar interface, to interact with E-selectin and support steady slow rolling.
Figure 7. Model for selectin ligand-mediated leukocyte tethering, rolling and activation.
Free flowing leukocytes tether on selectins expressed on the inflamed endothelium through high-affinity interactions largely mediated by PSGL-1 and ESL-1, both of which are present on microvilli. P-selectin interacts exclusively with PSGL-1 while E-selectin can engage both PSGL-1 and ESL-1. ESL-1, present on the lateral aspects of microvilli, is critical for stabilization and the transition to steady rolling on E-selectin. Microvilli likely recede in stably rolling leukocytes, exposing CD44 that controls rolling velocities together with ESL-1. Engagement of leukocyte CD44, present on the planar surface but excluded from microvilli, initiates signaling events activating the p38 MAPK, and resulting in L-selectin and PSGL-1 redistribution in a major pole on rolling leukocytes. We speculate that the E-selectin-CD44-dependent clustering of PSGL-1 and L-selectin, promote secondary tethering of leukocytes with each other. Gradient bars at the bottom indicate the range of action of PSGL-1, ESL-1 and CD44 in this sequence of events.
It is remarkable that CD44-mediated signals resulting from its interaction with Eselectin, can induce the redistribution and clustering of adhesion molecules (e.g. Lselectin and PSGL-1) expressed on the tip of microvilli. Leukocyte polarization, unexpectedly, was observed in the majority of slow rolling leukocytes ≥ 190 min after TNF-α administration, required E-selectin signals transmitted exclusively by CD44, and involved p38. These results are consistent with recent in vitro studies revealing that the engagement of soluble E-selectin on human neutrophils could trigger capping of Lselectin and PSGL-1 in a p38-dependent manner (Green et al., 2004). Why would PSGL-1 and L-selectin redistribute on one major pole of slow rolling leukocytes? The redistribution of receptors specialized for leukocyte tethering fits the notion that these receptors no longer play important roles on microvilli of leukocytes engaged in slow rolling. It is likely that clustered L-selectin and PSGL-1 provide an additional platform to capture circulating leukocytes and enhance further leukocyte recruitment through leukocyte-leukocyte interactions. A phenomenon of string formation downstream of rolling cells has been well documented in flow chambers in vitro (Alon et al., 1996; Bargatze et al., 1994). Although such strings have not been clearly demonstrated in vivo, brightfield intravital microscopy analyses have revealed that leukocyte-leukocyte interactions contribute to leukocyte recruitment through the binding of PSGL-1 to Lselectin (Sperandio et al., 2003). The individual roles of slow rolling and firm leukocyte adhesion in this activity have not been evaluated. Interestingly, we have observed using high-speed intravital digital videomicroscopy that the vast majority (~95%) of adherent leukocytes in TNF-α-inflamed venules exhibits polarization of PSGL-1 and Lselectin at the trailing edge (Chiang et al., 2007).
It is important to emphasize that the critical role of CD44 to signal clustering of Lselectin and PSGL-1 does not exclude a role for other ESLs in cell signaling. Previous reports have suggested that soluble E-selectin (Ruchaud-Sparagano et al., 1998), crosslinking of neutrophil PSGL-1 (Green et al., 2004), or the engagement of P-selectin to PSGL-1 (Evangelista et al., 1999) may induce activation of β2 integrins. However, intravital microscopy studies have indicated that PSGL-1 is not the ESL that cooperates with the chemokine receptor CXCR2 to induce leukocyte arrest in vivo (Smith et al., 2004). Although we show here that CD44 can clearly signal the redistribution of other adhesion molecules, it is not clear whether CD44 or another ESL (e.g. ESL-1) mediates β2 integrin activation. The present studies in fact suggest that ESL-1 may represent the leading candidate since the depletion of surface ESL-1 appears sufficient to alter significantly the in vivo arrest of wild-type leukocytes (Fig. 5).
In summary, we have shown that virtually all endothelial selectin ligand activity is provided by three glycoproteins on mouse neutrophils. PSGL-1, ESL-1 and CD44 exhibit remarkably distinct, highly dynamic functions to optimize leukocyte capture to the venular endothelium and recruitment into inflamed tissues (Fig. 7). Taken in the context of recent literature, these data suggest that the interplay of selectin ligands’ physical attributes, including spatial cellular distribution, unique structure allowing allosteric modulation of binding (Phan et al., 2006) and specific intracellular signaling, may dictate their tightly regulated functions in the inflammatory response
EXPERIMENTAL PROCEDURES
MICE
Wild-type C57BL/6 male mice were purchased from Charles River (Wilmington, MA). Sele−/− mice were backcrossed into the C57BL/6 background (Frenette et al., 1996). Cd44−/− animals were purchased from the Jackson Laboratory (Bar Harbor, ME) (Protin et al., 1999). Selplg−/− mouse colony was derived from breeding pairs given by Dr. Bruce Furie (Harvard Medical School, Boston, MA) (Yang et al., 1999). Cd44−/− / Selplg−/− DKO mice were generated in our laboratory (Katayama et al., 2005). All animals were housed at the Mount Sinai School of Medicine barrier facility. Experimental procedures performed on the animals were approved by the Animal Care and Use Committee of Mount Sinai.
DESIGN AND CLONING OF THE shRNA LENTIVIRUS SYSTEM
The LentiLox 3.7 (pLL3.7) vector and the three packaging vectors (pVSV-G, pRSV-REV and pMDLg/pRRE) required for lentiviral production have been described (Rubinson et al., 2003). The vector was engineered by introducing the mouse U6 promoter upstream of a CMVEGFP expression cassette to create a vector that simultaneously produces shRNAs and the GFP reporter gene. We selected two shRNA sequences using a computer software (http://www.dharmacon.com), which were ligated into HpaI and XhoI sites downstream of the U6 promoter. These sequences derived from the intraluminal domain of ESL-1, and were entirely specific to ESL-1 as determined by a genome wide comparison. We verified the integrity of the inserted oligonucleotides by sequencing. One of the vectors resulted in greater reduction of ESL-1 protein as determined by Western blotting and was selected for our experiments (Supplementary Fig.1). The oligonucleotide sequence of this shESL-1 is for the sense strand: TGT GTA GAG AAG CAC TAA CTT TCA AGA GAA GTT AGT GCT TCT CTA CAC TTT TTT C and for the antisense strand: TCG AGA AAA AAG TGT AGA GAA GCA CTA ACT TCT CTT GAA AGT TAG TGC TTC TCT ACA CA. In some experiments, a vector containing a scrambled version of the shESL-1 sequence was used. The oligonucleotide sequence of the scrambled shESL-1 for the sense strand is: TGA ACA GTT AGG AAT CAC GTT TCA AGA GAA CGT GAT TCC TAA CTG TTC TTT TTT C, and for the antisense strand: TCG AGA AAA AAG AAC AGT TAG GAA TCA CGT TCT CTT GAA ACG TGA TTC CTA ACT GTT CA. This sequence does not target any known mRNAs in the mouse genome. Analyses of mice transplanted with Linneg cells derived from Selplg−/− mice and transduced with the scrambled vector revealed a leukocyte behavior identical to that found in the Selplg−/− group transduced with the GFP control vector in all parameters analyzed (Supplementary Fig. 8).
GENERATION OF LENTIVIRUS
Lentiviral particles were produced by co-transfection of the lentiviral and three packaging vectors into 293T cells. Viral-containing supernatants were collected after 36h and concentrated by ultracentrifugation for 90 min at 25,000 rpm in a Beckman SW28 rotor. Viral pellets were resuspended in cold PBS and stored at -80°C until use. Titers were determined by infecting 293T cells with serial dilutions of concentrated lentiviral particles in the presence of 8 μg / mL polybrene (Fluka, Steimheim, Switzerland). Titers ranged from 2 – 6 × 108 infectious particles per mL. Percentages of GFP+ infected cells were measured by flow cytometry 48h after infection and absolute numbers calculated.
HEMATOPOIETIC STEM CELL INFECTION AND BONE MARROW TRANSPLANT
Donor BM cells from the appropriate genotype (wild-type, Cd44−/−, Selplg−/− or Cd44−/− / Selplg−/− DKO) were harvested by flushing both tibiae and femora into PBS containing 0.5% FBS and 2 mM EDTA (PEB buffer). Low-density mononuclear cells (MNC) were collected after centrifugation at 250 g over a 65% percoll solution and washed in PEB buffer. Contaminating erythrocytes were lysed in a 0.8% NH4Cl solution. MNCs were incubated for 20 min at 4°C with a cocktail of lineage antibodies comprising rat antimouse Gr-1, CD45R/B220 and TER119 (from BD PharMingen, San Diego, CA), CD3ε (Southern Biotech, Birmingham, Alabama,) and CD11b (M1/70 clone, purified from hybridoma supernatants). Cells were washed and incubated for 30 min at 4°C with sheep anti-rat Dynabeads (Dynal, Olso, Norway) following the manufacturer's instructions. Lin- cells were separated using a magnet, washed and counted. Cells were then resuspended in IMDM media containing 10% FBS (Stem Cell Technologies, Vancouver, BC, Canada), 20mM HEPES, 2mM L-glutamine, 50μM 2-Mercaptoethanol, 20 ng/mL Flt-3, 100 ng/mL mouse SCF, 10 ng/mL IL-11 (all cytokines from R&D Systems, Minneapolis, MN), 8 μg/mL polybrene and 75 μg/ml verapamil (MP Biomedicals, Solon, OH). Cells were mixed with viral concentrates (multiplicity of infection: 10-20) and centrifuged for 90 min at 2500 rpm at room temperature (RT), after which they were maintained in culture at 37°C for 3-12 hours before transplant. Recipient wild-type C57BL/6 mice were lethally irradiated (1200 cGy, split doses 3h apart) prior to receiving 2 - 4 × 105 transduced Lin- cells by retroorbital injection. Engraftment of recipient animals was assessed at least three weeks following transplantation by retroorbital bleeding and analysis of GFP+ leukocytes by flow cytometry.
CELL LINE AND E-SELECTIN / IgM CHIMERIC PROTEIN
The 32D mouse myeloid progenitor and bEnd.3 brain endothelial cell lines (ATCC, Manassas, VA) were cultured as previously described (Katayama et al., 2005). Parental 32D cells were spin-infected with either GFP control or shESL-vectors, expanded in culture and sorted using a FACS Vantage SE cell sorter (Becton Dickinson) on the basis of GFP expression. The Eselectin/ IgM chimera was produced and used as described in (Katayama et al., 2005). 293T cells were cultured in DMEM media (Cellgro, Herndon, VA) containing 5% FBS (Hyclone, Logan, UT).
FLOW CYTOMETRY AND E-SELECTIN BINDING ASSAY
Primary blood leukocytes were stained by incubation with 10 μg/mL of biotin-labeled anti-CD44 (clone IM7, BD Biosciences) or anti-PSGL-1 (clone 4RA10), or control antibody followed by incubation with Cy5-conjugated streptavidin (Jackson ImmunoResearch Laboratories). Fluidphase binding of the E-selectin/IgM chimera to blood leukocytes was performed as described previously (Katayama et al., 2005). Neutrophils were gated on the basis of low forward-scatter and high side-scatter. Samples were acquired using a FACSCalibur flow cytometer and analyzed using the CellQuest software (Becton Dickinson).
THIOGLYCOLLATE-INDUCED PERITONITIS
To assess neutrophil recruitment efficiency, we generated cohorts of chimeric mice by transplantation with BM cells of all four genotypes (wild-type, Cd44−/−, Selplg−/− or Cd44−/− / Selplg−/− DKO) transduced with control or shESL-1 vectors. At least 4 weeks after transplantation, mice were injected i.p. with 1 mL of thioglycollate as previously described (Katayama et al., 2005). After 8 h, venous blood and peritoneal exudates were collected and aliquots stained with APCconjugated anti-Gr-1 antibody (Pharmingen, BD Biosciences). Samples were analyzed by flow cytometry as indicated above. To estimate the efficiency of extravasation into the peritoneum, the ratio of GFP+ neutrophils between the blood and the exudates was determined. The absolute number of neutrophil recruitment in the four different genotypes was determined in mice that were transplanted with uninfected BM cells from all four genotypes. The ratios of blood vs peritoneum GFP+ neutrophils were then applied to the total number of neutrophils in each group to estimate the absolute recruitment efficiencies (further details in Supplementary Fig. 5 and Table 2).
INTRAVITAL MICROSCOPY (IVM)
Mice were injected intrascrotally with 0.5 μg recombinant mouse TNF-α (R&D Systems) and anesthetized with 2% α-chloralose and 10% urethane (Sigma-Aldrich, St. Louis, MO) in PBS, and tracheotomized with PE-160 polyethylene tubing (Becton Dickinson). The cremaster muscle was carefully exteriorized, opened with cautery, and secured across a Plexiglas platform with sutures. Exposed tissues were continuously superfused with endotoxin-free bicarbonatebuffered saline, equilibrated with 95% N2 and 5% CO2, and warmed to 37oC. Immediately before recording, 1 μg of an AlexaFluor647-conjugated rat IgG antibody (prepared using the AlexaFluor647 antibody labeling kit from Molecular Probes, Eugene, OR) was injected to delineate the vasculature. All movies were acquired with an Olympus BX61WI workstation mounted on a motorized X,Y stage (Applied Scientific Instrumentation, Eugene, OR) allowing precise computer-controlled lateral movement between XY positions and a Z focusing drive to allow the focal plane to be rapidly changed. A LUMPlanFI 60× NA 0.90 ∞ water-immersion objective was used for all recordings (Olympus, Melville, NY). The microscope is equipped with a Lambda DG-4 high-speed wavelength switcher based on dual scanning galvanometers (Sutter Instrument, Novato, CA) from a 175-Watt xenon light source with excitation filters (360 nm, 480 nm and 590 nm) matched to a triple-band filter for DAPI, FITC and Cy3, and individual filter sets for DAPI, FITC, Cy3, TexasRed and Cy5 (Chroma, Brattleboro, VT) in the body of the microscope. Transmitted brightfield light was delivered through a shutter (Uniblitz VS25, Vincent Associates, Rochester, NY) allowing the acquisition of both brightfield and fluorescence images without interruption through computer control. Images were collected with a SensiCam camera (9.9 μm2 pixel, 640 × 480 pixel format; Cooke, Auburn Hills, MI) mounted on a Videoscope image intensifer (Sterling, VA). The SlideBook software (Intelligent Imaging Innovations, Denver, CO), run on a Dell Precision 650 computer system (Dell, Inc, Round Rock, TX) with dual Xeon 3.06GHz microprocessors and 3.0 GB of RAM, coordinated image acquisition and allowed offline data analysis.
Six to ten venules per mouse were analyzed 150 to 210 min after TNF-α treatment by acquisition of fluorescence (FITC channel for GFP) and brightfield images with 2 × 2 binning for 2 min. To obtain centerline blood velocities, a 7-10 s movie was acquired for each venule, at 40 frame-per-second (fps) using the FITC channel, 4 × 4 binning, to allow detection of free flowing cells.
For analyses of L-selectin distribution on the surface of rolling cells, we prepared mice for IVM as indicated above except that they were injected i.v. immediately before recording with 0.02 mg / Kg of PE-conjugated anti-L-selectin antibody (clone MEL-14, BD Biosciences) as well as the AlexaFluor647-conjugated rat IgG antibody. Movies were recorded for 1 to 2 min by acquisition of fluorescence and brightfield images, or fluorescence only (FITC and Cy3 channels) at 5 fps, 2 × 2 binning. Centerline velocities were estimated as above except the Cy3 fluorescence channel was used to detect free flowing cells. In some experiments, 0.02 mg / Kg PE-conjugated anti-PSGL-1 and APCconjugated anti-CD44 antibodies (clones 2PH1 and IM7, respectively, BD Biosciences) were injected and analyzed as indicated above.
ANALYSIS OF IVM EXPERIMENTS
Wall shear rates (γ) were calculated based on Poiseuille's Law for a Newtonian fluid = [γ 8 (Vmean / Dv), where Dv is the diameter of the venule and Vmean is estimated form the centerline RBC velocities (VRBC), using the empirical correlation Vmean = VRBC / 1.6]. VRBC was measured for each venule using high-speed (40 fps) recordings by dividing the distance traveled by the fastest free flowing cells per frame by 0.026 s (40 fps = 26 ms / frame). Each rolling leukocyte passing through the field of view (150 μm long) was counted and leukocyte rolling flux was calculated by dividing leukocyte rolling flux by the total leukocyte flux, which was estimated as [GFP-WBC] × Vmean / π / (d/2)2, where GFP-WBC is the number of GFP+ WBC per μl of blood and d is the venular diameter. An automated cell counter was used to determine absolute WBC counts from blood samples obtained from retroorbital bleeding at the end of the experiment, and the percentage of GFP+ leukocytes determined by flow cytometry. Leukocyte rolling velocities were measured for each GFP+ cell by measuring the distance traveled during 2 s. Leukocytes were considered “arrested” if they remained static for ≥ 30 s.
For analysis of L-selectin clustering, movies were converted into 2D timelapse intensity plots using the SlideBook software. Rolling cells that presented intensity levels of at least 2-fold over the rest of the cell body in a discrete region (5-30% of the cell surface) were considered “polarized” (see Supplementary Fig. 6).
RABBIT ANTISERUM AGAISNT ESL-1 AND IMMUNOSTAINING
A recombinant ESL-1 protein (ESL-1myc) consisting of the extracellular part of ESL-1 (Steegmaier et al., 1995) and a C-terminal myc-tag was obtained as described before (Wild et al., 2001), and used to immunize a rabbit giving rise to anti-ESL-1 serum VE12. For immunostaining, blood samples from mice transplanted with GFP control or shESL-1-transduced Lin- cells were depleted of RBCs by lysis in 0.8% NH4Cl buffer and the remaining leukocytes cytospun onto slides. Cells were fixed with 4% paraformaldehyde, washed with PEB buffer and blocked with 1:500 dilution of goat serum in PEB buffer. After washing, cells were incubated with PBS containing 0.1% Triton X100 for 15 min, and washed again before incubation with a 1:1000 dilution of an anti-ESL-1 for 1 h at RT. Cells were washed again and incubated with a Cy5-conjugated anti-rabbit antibody for 1 h at RT. After extensive washing, samples were mounted with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA).
For the detection of PSGL-1 redistribution on neutrophils, bone marrow PMNs were purified on a discontinuous Percoll gradient (65%). PMNs (106) were pretreated with 10 mM SB203580 for 45 min at 37°C, and then incubated with 1 μg of control rat IgG or anti-CD44 antibody (clone IM7) for 20 min at 4°C. PMNs were washed, incubated with 1 μg anti-rat IgG antibody for 10 min at 37°C, and then stained at 4°C with 5 μg/ mL of a PE-conjugated anti-PSGL-1 antibody (Pharmingen, BD Biosciences). After washing, cells were fixed in 2% paraformaldehyde and cytospun onto slides for fluorescence microscopy analysis.
WESTERN BLOTTING
Cells were biotinylated by incubation with 0.5 mg / mL of EZ-Linksulfo- NHS-LC-biotin (Pierce) for 30 min at 4°C. After washing with PEB buffer, cells were lysed in a buffer containing 10 mM Tris-HCl pH 7.5, 1% Triton-X100, 150 mM NaCl, a protease inhibitor cocktail (Sigma) and 1 mM PMSF. Lysates were depleted of cell debris by centrifugation at 16,000 g for 15 min at 4°C. An aliquot of lysates was kept for estimation of total ESL-1. Lysates were then incubated overnight with streptavidin-conjugated agarose (Sigma) at 4°C, and unbound proteins removed by extensive washing of the beads in lysis buffer. Beads and total lysates were boiled for 4 min at 100°C in the presence of sample loading buffer containing 2% SDS and 10 mM DTT, and proteins resolved in a 7% SDS-PAGE gel. Proteins were transferred onto PVDF membranes (Millipore, Bedford, MA), and then blotted with the anti-ESL-1 serum (1:1000 dilution). Bands were detected with the ECL method (West Dura Extended Duration, Pierce). Because the levels of GFP protein may affect total protein estimations in transduced versus untransduced groups, extracts from the same cell counts rather than protein content were loaded per lane.
For detection of phospho-p38, purified BM PMNs from wild-type or Cd44−/− mice were incubated with control or anti-CD44 antibodies (clone IM7) as indicated above, incubated in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 2 mM Na3VO4, 1 mM EDTA, 1 mM 4-(2-aminoethyl)- benzene sulfonylfluoride, 10 mM NaF, 5 mM NaPPi, 10 mM β-glycerophosphate) containing protease inhibitors. Proteins from 2 × 105 cells were resolved by SDS-PAGE on a 10% polyacrylamide gel. Gels were blotted onto PVDF membranes and incubated with an anti-phospho-p38 antibody (Cell Signalling Technology; Danvers, MA) followed by a peroxidase-conjugated anti-rabbit antibody. Bands were detected with the ECL method (Pierce).
QUANTITATIVE REAL-TIME RT-PCR
Total RNA was extracted from cells using the RNeasy mini kit (Qiagen). Potential contaminating DNA was removed using the RNase- Free DNase Set (Qiagen). Total RNA (1 μg) was reverse-transcribed using first strand cDNA synthesis with random primers (Promega, Madison, WI). Quantitative real time RT-PCR was performed as previously described (Katayama et al., 2006). The specific primers used to amplify cDNA fragments corresponding to ESL-1 were CAA GAT GAC GGC CAT CAT TTT CA (forward) and TTC CCC AAG ACG AAT GCT GC (reverse), and the primers corresponding to the housekeeping gene GAPDH were TTG GCA TTG TGG AAG GGC TCA T (forward) and GAT GAC CTT GCC CAC AGC CTT (reverse). The PCR conditions were 95°C for 2 min; 40 cycles of 95°C for 15 s, 55°C for 15 s, 72°C for 30 s; 1 cycle of 95°C for 15 s, 60°C for 15 s, 95°C for 15 s. All experiments were done in triplicate and normalized to GAPDH.
STATISTICAL ANALYSES
Data are presented as mean ± SEM. Different genotypes of the same group (GFP vector or shESL-1) were analyzed by one-way ANOVA with Bonferroni/Dunn post-hoc test for multiple group comparisons using the StatView software. P values <0.0083 (5% risk level) were considered statistically significant for four-group comparisons, and p<0.005 (5% risk level) for five-group comparisons (Figure 6). Within each graph, groups of the same genotype but different lentiviral transduction were compared using two-tailed Student's t-test, with significance set at p<0.05 (5% risk level).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Bruce Furie for providing the founders of our Selplg−/− mouse colony. We also thank Stéphanie Dahan for advice on the p38 studies and Anna Prats for excellent artistic input (Fig. 7). This study was supported by the National Institutes of Health R01 grants DK56638 and HL69438 to P.S.F., and a grant from the Charles Revson Foundation to A.H. P.S.F. is an Established Investigator of the American Heart Association. The authors have no conflict of interest with the content of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon R, Feizi T, Yuen CT, Fuhlbrigge RC, Springer TA. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J Immunol. 1995;154:5356–5366. [PubMed] [Google Scholar]
- Alon R, Fuhlbrigge RC, Finger EB, Springer TA. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol. 1996;135:849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neut rophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrus LW, Zuber ME, Lueddecke BA, Olwin BB. Identification of a cysteine-rich receptor for fibroblast growth factors. Mol Cell Biol. 1992;12:5600–5609. doi: 10.1128/mcb.12.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnutt BC, Smith DF, Raffler NA, Smith ML, White EJ, Ley K. Induction of LFA-1-dependent neutrophil rolling on ICAM-1 by engagement of E-selectin. Microcirculation. 2006;13:99–109. doi: 10.1080/10739680500466376. [DOI] [PubMed] [Google Scholar]
- Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods. 2007 doi: 10.1038/nmeth1018. In press. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, Totani L, Piccardoni P, Vestweber D, de Gaetano G, Cerletti C. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN,H,R, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas JO, Chen YJ, Stieber A, Mourelatos Z, Gonatas NK. Truncations of the C-terminal cytoplasmic domain of MG160, a medial Golgi sialoglycoprotein, result in its partial transport to the plasma membrane and filopodia. J Cell Sci. 1998;111(Pt 2):249–260. doi: 10.1242/jcs.111.2.249. [DOI] [PubMed] [Google Scholar]
- Gonatas JO, Mezitis SG, Stieber A, Fleischer B, Gonatas NK. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus. J Biol Chem. 1989;264:646–653. published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264. [PubMed] [Google Scholar]
- Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol. 2004;172:7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Zollner O, Moll T, Maly P, Thall AD, Lowe JB, Vestweber D. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- Jones WM, Watts GM, Robinson MK, Vestweber D, Jutila MA. Comparison of E-selectin-binding glycoprotein ligands on human lymphocytes, neutrophils, and bovine gamma delta T cells. J Immunol. 1997;159:3574–3583. [PubMed] [Google Scholar]
- Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl R, Antoine M, Olwin BB, Dickson C, Kiefer P. Cysteine-rich fibroblast growth factor receptor alters secretion and intracellular routing of fibroblast growth factor 3. J Biol Chem. 2000;275:15741–15748. doi: 10.1074/jbc.M903271199. [DOI] [PubMed] [Google Scholar]
- Kotovuori P, Tontti E, Pigott R, Shepherd M, Kiso M, Hasegawa A, Ren konen R, Nortamo P, Altieri DC, Gahmberg CG. The vascular E-selectin binds to the leukocyte integrins CD11/CD18. Glycobiology. 1993;3:131–136. doi: 10.1093/glycob/3.2.131. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, et al. Charac terization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- Lo SK, Lee S, Ramos RA, Lobb R, Rosa M, Chi-Rosso G, Wright SD. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. J Exp Med. 1991;173:1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- Mehta P, Cummings RD, McEver RP. Affinity and kinetic analysis of P-selectin binding to P-selectin glycoprotein ligand-1. J Biol Chem. 1998;273:32506–32513. doi: 10.1074/jbc.273.49.32506. [DOI] [PubMed] [Google Scholar]
- Montoya MC, Holtmann K, Snapp KR, Borges E, Sanchez-Madrid F, Luscinskas FW, Kansas G, Vestweber D, de Landazuri MO. Memory B lymphocytes from secondary lymphoid organs interact with E-selectin through a novel glycoprotein ligand. J Clin Invest. 1999;103:1317–1327. doi: 10.1172/JCI4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyll actosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson A, Hellman U, Ten Dijke P, Grimsby S, Ichijo H, Moren A, Miyazono K, Heldin CH. Latent transforming growth factor-beta complex in Chinese hamster ovary cells contains the multifunctional cysteine-rich fibroblast growth factor receptor, also termed E-selectin-ligand or MG-160. Biochem J. 1997;324(Pt 2):427–434. doi: 10.1042/bj3240427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol. 2006;7:883–889. doi: 10.1038/ni1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Ruchaud-Sparagano MH, Drost EM, Donnelly SC, Bird MI, Haslett C, Dransfield I. Potential pro-inflammatory effects of soluble E-selectin upon neutrophil function. Eur J Immunol. 1998;28:80–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<80::AID-IMMU80>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M, Borges E, Berger J, Schwarz H, Vestweber D. The E-selectin-ligand ESL-1 is located in the Golgi as well as on microvilli on the cell surface. J Cell Sci. 1997;110(Pt 6):687–694. doi: 10.1242/jcs.110.6.687. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Hasslen SR, Nelso n RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Wild MK, Huang MC, Schulze-Horsel U, van der Merwe PA, Vestweber D. Affinity, kinetics, and thermodynamics of E-selectin binding to E-selectin ligand-1. J Biol Chem. 2001;276:31602–31612. doi: 10.1074/jbc.M104844200. [DOI] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner O, Lenter MC, Blanks JE, Borg es E, Steegmaier M, Zerwes HG, Vestweber D. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol. 1997;136:707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.