Abstract
Aim—To analyse the effect of sectioning on the assessment of karyotypic heterogeneity by interphase cytogenetics in paraffin wax embedded normal squamous epithelium and to apply the principles derived to invasive cervical carcinoma.
Methods—Normal male (n = 5) and female (n = 5) squamous epithelia were hybridised with peri-centromeric repeat probes specific for chromosomes X (DXZ1) and 17 (D17Z1) individually and in combination to assess the effect of sectioning on mono-, di-, tri-, and tetrasomic populations. Section thickness, interobserver variation and variation between different areas of the epithelium were evaluated. Invasive squamous carcinomas of the cervix (n = 5) were then hybridised with the DXZ1 probe and intratumoral heterogeneity was assessed by comparison of signal distributions obtained from different areas.
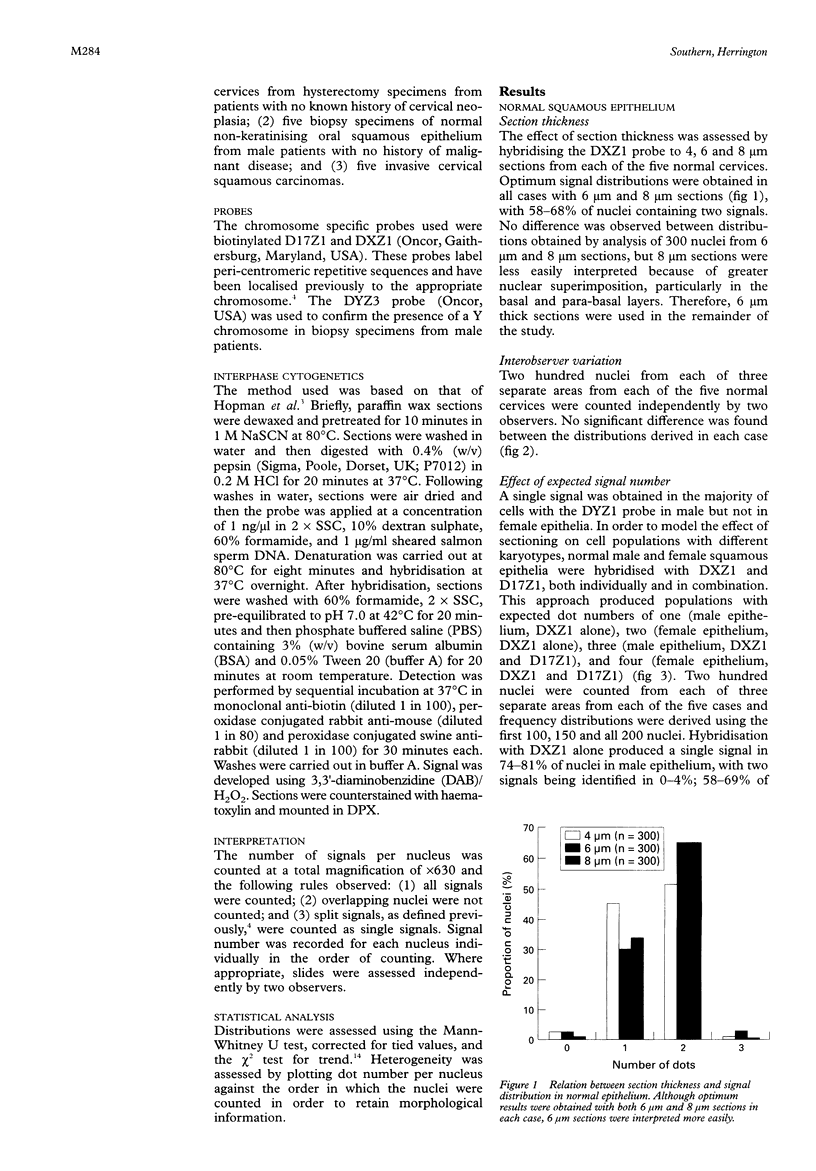
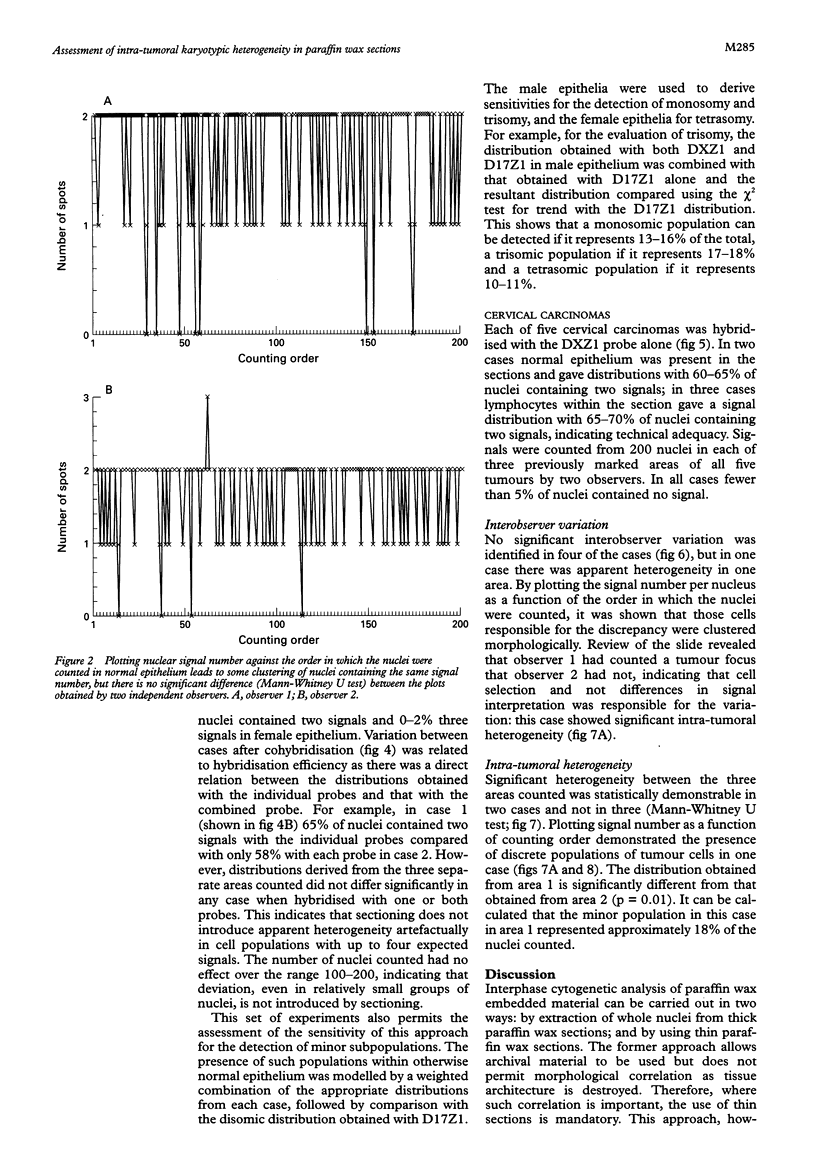
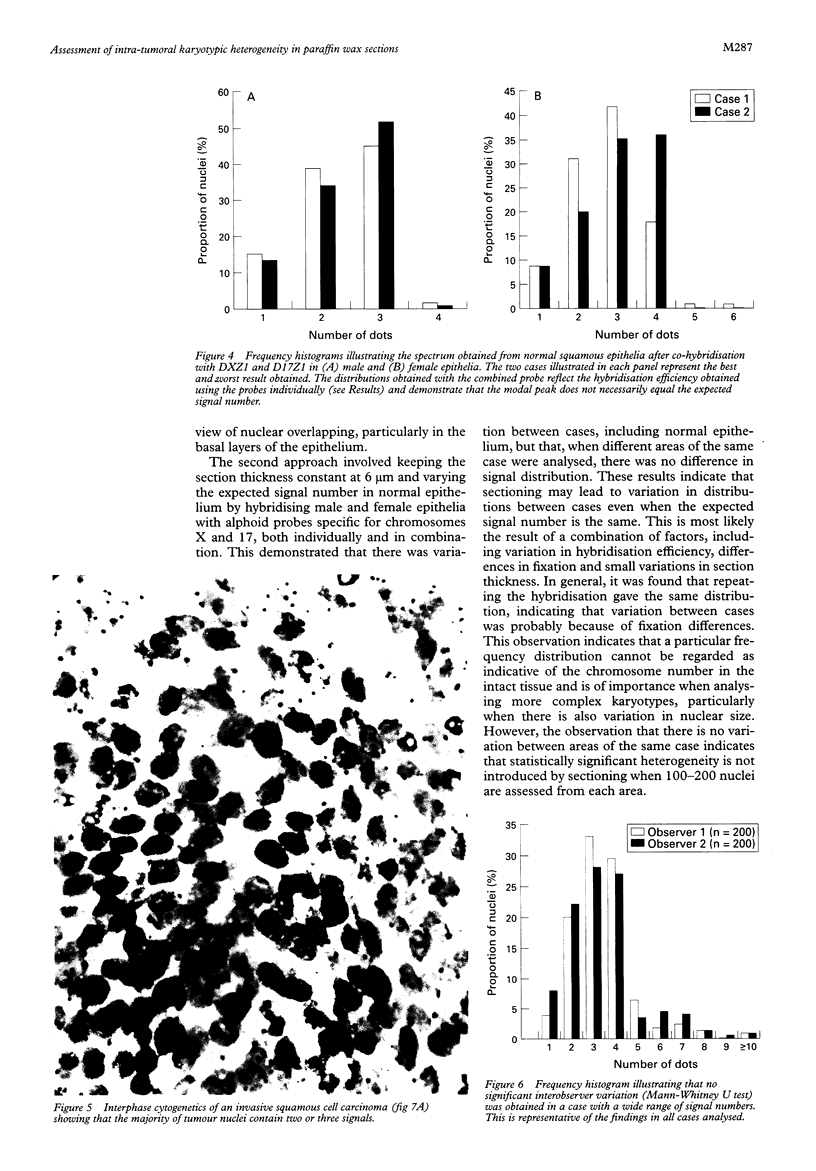
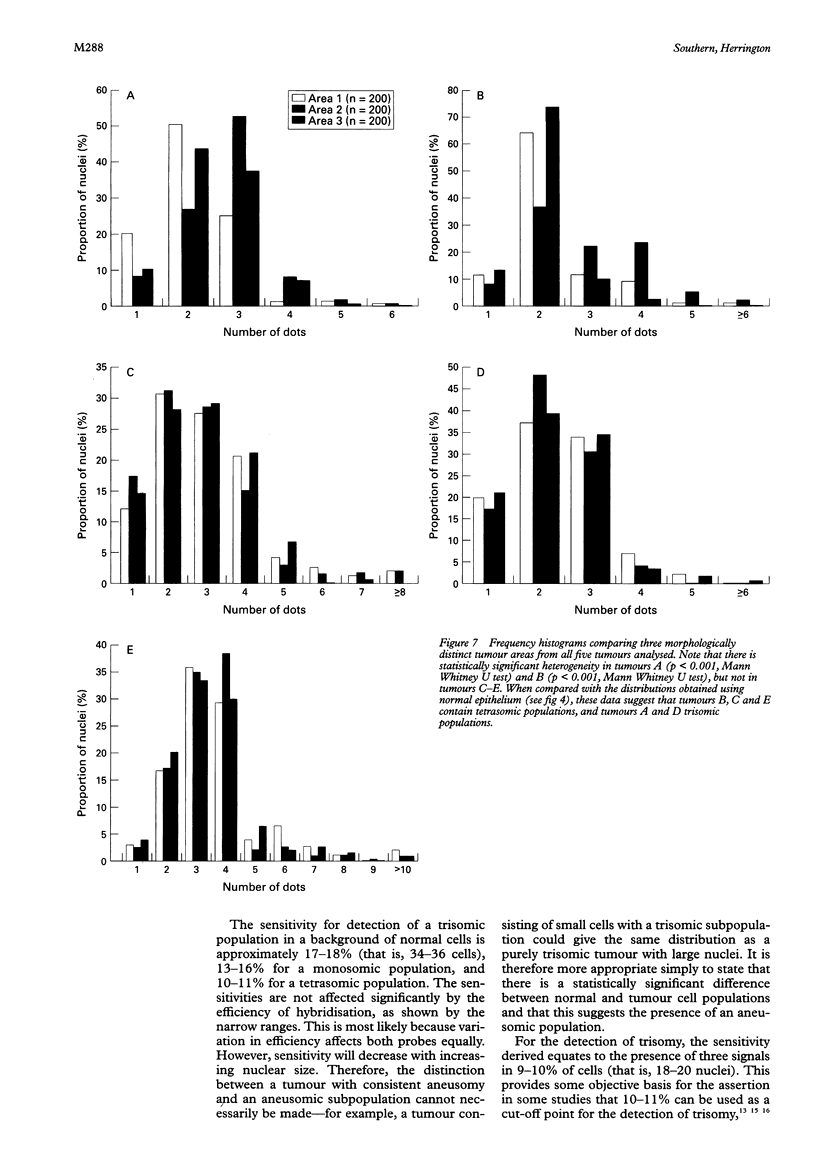
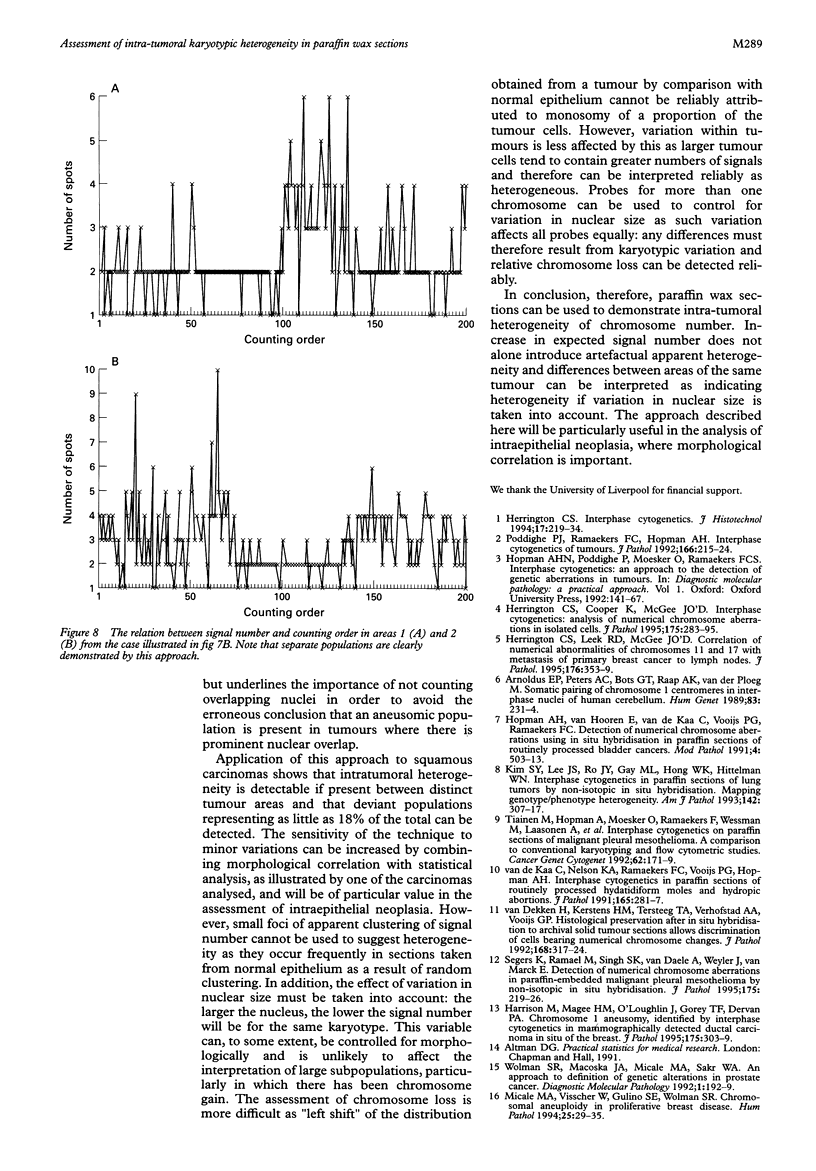
Results—The optimum section thickness for the assessment of normal epithelium was 6 μm. Variation in the expected signal number in the range 1-4 did not introduce artefactual heterogeneity at this section thickness. The sensitivity of this approach for the detection of minor subpopulations was calculated to be 13-16%, 17-18% and 10-11% for mono-, tri- and tetrasomic populations, respectively. Karyotypic heterogeneity was detected in two of the five tumours and, in one case where the populations where clustered morphologically, a minor population representing 18% was identified.
Conclusions—Interphase cytogenetic analysis of sections from paraffin wax embedded material can be used for the detection of minor subpopulations in tumours. This approach will be of particular value in the assessment of the relation between human papillomavirus infection and tumour karyotype and in the analysis of intraepithelial neoplasia.
Keywords: interphase cytogenetics
Keywords: cervix
Keywords: chromosome
Keywords: heterogeneity
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harrison M., Magee H. M., O'Loughlin J., Gorey T. F., Dervan P. A. Chromosome 1 aneusomy, identified by interphase cytogenetics, in mammographically detected ductal carcinoma in situ of the breast. J Pathol. 1995 Mar;175(3):303–309. doi: 10.1002/path.1711750308. [DOI] [PubMed] [Google Scholar]
- Herrington C. S., Cooper K., McGee J. O. Interphase cytogenetics: analysis of numerical chromosome aberrations in isolated cells. J Pathol. 1995 Mar;175(3):283–295. doi: 10.1002/path.1711750306. [DOI] [PubMed] [Google Scholar]
- Herrington C. S., Leek R. D., McGee J. O. Correlation of numerical chromosome 11 and 17 imbalance with metastasis of primary breast cancer to lymph nodes. J Pathol. 1995 Aug;176(4):353–359. doi: 10.1002/path.1711760406. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Lee J. S., Ro J. Y., Gay M. L., Hong W. K., Hittelman W. N. Interphase cytogenetics in paraffin sections of lung tumors by non-isotopic in situ hybridization. Mapping genotype/phenotype heterogeneity. Am J Pathol. 1993 Jan;142(1):307–317. [PMC free article] [PubMed] [Google Scholar]
- Micale M. A., Visscher D. W., Gulino S. E., Wolman S. R. Chromosomal aneuploidy in proliferative breast disease. Hum Pathol. 1994 Jan;25(1):29–35. doi: 10.1016/0046-8177(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Poddighe P. J., Ramaekers F. C., Hopman A. H. Interphase cytogenetics of tumours. J Pathol. 1992 Mar;166(3):215–224. doi: 10.1002/path.1711660303. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Hopman A., Moesker O., Ramaekers F., Wessman M., Laasonen A., Pyrhönen S., Tammilehto L., Mattson K., Knuutila S. Interphase cytogenetics on paraffin sections of malignant pleural mesothelioma. A comparison to conventional karyotyping and flow cytometric studies. Cancer Genet Cytogenet. 1992 Sep;62(2):171–179. doi: 10.1016/0165-4608(92)90258-a. [DOI] [PubMed] [Google Scholar]
- Wolman S. R., Macoska J. A., Micale M. A., Sakr W. A. An approach to definition of genetic alterations in prostate cancer. Diagn Mol Pathol. 1992 Sep;1(3):192–199. [PubMed] [Google Scholar]
- van Dekken H., Kerstens H. M., Tersteeg T. A., Verhofstad A. A., Vooijs G. P. Histological preservation after in situ hybridization to archival solid tumour sections allows discrimination of cells bearing numerical chromosome changes. J Pathol. 1992 Nov;168(3):317–324. doi: 10.1002/path.1711680312. [DOI] [PubMed] [Google Scholar]
- van de Kaa C. A., Nelson K. A., Ramaekers F. C., Vooijs P. G., Hopman A. H. Interphase cytogenetics in paraffin sections of routinely processed hydatidiform moles and hydropic abortions. J Pathol. 1991 Dec;165(4):281–287. doi: 10.1002/path.1711650403. [DOI] [PubMed] [Google Scholar]




