Abstract
Little is known of the impact of Fc receptor polymorphism in macaques on the binding of human IgG (huIgG) and nothing is known of this interaction in the pigtail macaque (M. nemestrina) which is used in preclinical evaluation of vaccines and therapeutic antibodies. We defined the sequence and huIgG binding characteristics of the M. nemestrina activating FcγRIIa (mnFcγRIIa) and inhibitory FcγRIIb (mnFcγRIIb) and predicted their structures using the huIgGFc:huFcγRIIa crystal structure. Large differences were observed in the binding of huIgG by mnFcγRIIa and mnFcγRIIb compared to their human FcR counterparts. MnFcγRIIa has markedly impaired binding of huIgG1 and huIgG2 immune complexes compared to huFcγRIIa (His131). In contrast, mnFcγRIIb has enhanced binding of huIgG1 and broader specificity as, unlike huFcγRIIb, it avidly binds IgG2. Mutagenesis and molecular modelling of mnFcγRIIa showed that Pro159 and Tyr160 impair the critical FG loop interaction with huIgG. The enhanced binding of huIgG1 and huIgG2 by mnFcγRIIb was shown to be dependent on His131 and Met132. Significantly, both His131 and Met132 are conserved across FcγRIIb of rhesus and cynomolgus macaques. We identified functionally significant polymorphism of mnFcγRIIa, wherein Pro at position 131, also an important polymorphic site in huFcγRIIa, almost abolished binding of huIgG2 and huIgG1 and reduced binding of huIgG3 compared to mnFcγRIIa His131. These marked interspecies differences in IgG-binding between human and macaque FcRs and polymorphisms within species have implications for pre-clinical evaluation of antibodies and vaccines in macaques.
Introduction
Fc receptors (FcR) are cell surface molecules primarily expressed on effector leucocytes of the innate immune system and by binding immunoglobulins provide adaptive immunity with a cell-based effector system (1). There are three classes of leukocyte IgG FcRs (FcγRs) in humans, the high affinity IgG receptor FcγRI (CD64) and the low affinity IgG receptors, FcγRII (CD32) and FcγRIII (CD16).
The two major genes of the huFcγRII family encode FcγRIIa and FcγRIIb and its splice variants. These receptors play activating and inhibitory roles in normal immune responses and immune homeostasis (1, 2) and imbalance in these opposing roles is a key contributing factor to the development of pathological inflammation in several autoimmune diseases (3). FcγRIIa is one of several activating Fc receptors, but is the most widespread and abundant FcγR of humans present on all leukocytes except lymphocytes. Although a low affinity receptor, FcγRIIa avidly binds oligovalent antibody coated targets (immune complexes) to induce cytokine release from inflammatory leukocytes, respiratory burst, antibody dependent cell mediated killing, internalisation of complexes and platelet aggregation. FcγRIIb in humans is a powerful inhibitor of immune receptor signalling and is critical to the modulation of humoural immunity and antibody dependent immune functions (4-6). Its immunoreceptor tyrosine-based inhibitory motif (ITIM) dependent modulation of immunoreceptor tyrosine-based activation motif (ITAM) signalling cascades, regulates B cell antigen receptor signalling and consequent antibody responses. FcγRIIb also regulates signalling by the activating Fc receptors, FcγRI, FcγRIIa, FcγRIIIa, FcεRI and FcαRI. In a practical sense, the IgG-FcγR interaction is a key contributor to the effectiveness of many vaccines both at the level of immune regulation and induction of effector function. Indeed, it has been suggested that the IgG-FcγR interaction mediating antibody dependent cell mediated cytotoxicity may play a role in HIV vaccine-induced protective immunity of humans (7). Moreover the effectiveness of allergen immunotherapy (8) and many therapeutic monoclonal antibodies, particularly anti-cancer antibodies, has been attributed at least in part to successful engagement of Fc receptor dependent effector systems including FcγRIIa and FcγRIIb (1).
In humans, several polymorphisms of the activating IgG receptor, FcγRIIa, are known (9, 10). The most clinically significant polymorphism encodes amino acid position 131 where either a histidine or an arginine residue may be present resulting in profound effects on binding of hIgG2 (11).
Genetic polymorphisms of human Fc receptors that affect their capacity to bind IgG or alter the balance of activation over inhibition have been implicated in resistance to HIV and bacterial infection (12-18), susceptibility to autoimmunity (19, 20) and the effectiveness of therapeutic monoclonal antibodies mostly IgG1 but increasingly IgG2 (1). As a result of the success of monoclonal antibodies, considerable effort has been made to improve their FcR –dependent potency by engineering Fc portions for the purpose of selective engagement of either activating Fc receptors (1) including FcγRIIa (21) or inhibitory FcRIIb (22). Furthermore the most clinically significant polymorphism of the activating IgG receptor, FcγRIIa, (9, 10) encodes either a histidine or an arginine residue at amino acid position 131 and influences the clinical outcome of antibody therapy (23) and additionally has profound effects on binding of hIgG2 (11).
The genetic diversity of non-human primate (NHP) Fc receptors has not been extensively characterised, even though NHP are key animal models for many diseases, including HIV. Examination of functional Fc polymorphisms in NHP is, as found in humans, pertinent to understanding susceptibility to infectious and autoimmune diseases. Interspecies functional substitutions will likely substantially influence the evaluation of human antibodies in NHP. Indeed although evolutionarily conserved, the limited information available to date shows significant interspecies sequence differences are apparent between the human FcR and their orthologues in different NHP species (24-27) Some of this sequence diversity occurs at sites that are predicted by homology to be essential for the interaction with IgG and may influence the binding of IgG. These differences may result in alterations to the relative contributions that the different FcR classes make to antibody dependent effector function in vivo in macaques compared to humans. Correspondingly, differences in species IgG-FcR interactions may greatly complicate the interpretation of preclinical studies aimed at evaluating the functional activity of therapeutic antibodies or vaccines in NHP models such as macaques including M. nemestrina. In addition, use of out-bred populations of macaques, may further complicate interpretation due to non-synonymous polymorphisms in the macaque FcR genes.
Little is yet known of the impact of FcR polymorphism on the binding of human IgG in NHP, especially in the three commonly used macaque species used in medical research - rhesus, M. mulatta, cynomolgus, M. fascicularis and pigtail, M. nemestrina. Furthermore nothing is known of the activity of Fc receptors of the pigtail macaque M. nemestrina. Like cynomolgus and rhesus macaques, (28, 29), pigtailed macaques are used in the evaluation of antibody immunotherapy (30-32), humoral immune responses to infection and vaccine candidates including dengue virus (33, 34) and HIV/SHIV (35, 36). Furthermore species have been used interchangeably in models (30, 32).
In this study, we define the ligand binding properties of activating FcγRIIa and the inhibitory FcγRIIb of M. nemestrina (mnFcγRIIa and mnFcγRIIb). We identify a functionally significant polymorphism of FcγRIIa and define the molecular and structural basis of impaired binding to IgG by mnFcγRIIa and for the enhanced binding and broader specificity of mnFcγRIIb compared to their human orthologues (huFcγRIIa and huFcγRIIb). These data also have implications for the analysis of IgG function in other macaque species and NHP.
Materials and Methods
Animals
Outbred 3-5 year old pigtail macaques (Macaca nemestrina) were obtained from the Australian National Macaque Breeding Facility and studies approved by the University of Melbourne and CSIRO animal health institutional animal ethics committees. Whole venous blood was obtained from animals sedated with Ketamine as previously described (36) and peripheral blood mononuclear cells (PBMC) were isolated over ficoll-hypaque and stored in liquid nitrogen.
Cloning of Fcγ receptors (FcγR)
Gene transcripts for mnFcγRIIa and mnFcγRIIb were obtained by PCR of cDNA (AffinityScript QPCR cDNA synthesis kit, Agilent Technologies, Santa Clara, CA) produced from total RNA (RNeasy Mini Kit, Qiagen, Melbourne, Australia) from PBMC of unrelated animals. Restriction enzymes and DNA-modifying enzymes were all from New England Biolabs (Beverly, MA) except for PCR applications, which used the Accuprime Pfx DNA polymerase (Life Technologies, Melbourne, Australia). The primers to generate FcγR PCR fragments were synthesised by Sigma-Aldrich (Sydney, Australia).
Because of the absence of any DNA sequence information from M. nemestrina, the 5′-and 3′-amplifying primers for FcγRIIa or FcγRIIb were based on sequences of rhesus macaque M. mulatta (GenBank:NM_001257300) or cynomolgus macaque M. fasicularis (GenBank:AF485814.1) respectively. The FcγRIIa 5′ primer was complementary to the initiation codon and the following 5 codons and the 3′ primer was complementary to the last 7 codons and the termination codon. The FcγRIIb 5′ primer was complementary to the initation codon and the first 5 codons and the 3′ primer complementary to the final 6 codons and the termination codon. The primers also contained KpnI and EcoRV sites. The Kpn1/EcoRV digested PCR products were cloned into pENTR1A (Life Technologies, Melbourne, Australia) followed by Gateway LR cloning (Life Technologies, Melbourne, Australia) into a Gateway-adapted pMXI expression vector containing a neomycin resistance cassette (37).
The huFcγRIIb2 construct was obtained from PBMC-derived cDNA as above using 5′- and 3′-primers based on the sequence of FcγRIIb (38) and cloned into pMXI as above.
The nucleotide sequences of mnFcγR were determined from both PBMC-derived PCR amplified cDNA and from six FcγR clones for each receptor from each individual animal using Big Dye Version 3.1 terminator cycle sequencing (Applied Biosystems, Melbourne, Australia) and separated at the Australian Genome Research Facility (Melbourne, Australia). All cDNA sequences have been submitted to GenBank, submission numbers 1637005 and 1655933.
Alignments of FcγRII sequences were generated using CLC Sequence Viewer version 6.4 (CLC bio, Denmark).
Isolation and expression of the human FcγRIIa-H131 and FcγRIIa-R131 have been described previously (39-41).
Site-Directed Mutagenesis
Mutated FcγR were constructed by in-vitro site-directed mutagenesis using a series of mutation primers and the thermostable polymerase Pfx (Life Technologies, Melbourne, Australia) to obtain the following for mnFcγRIIa-allele1 (Mutant 1: N135T(N→T), Mutant 2: P159L, Y160F (PY→LF)), mnFcγRIIa-allele2 (Mutant 3: P131H, M132L, N133D (PMN→HLD)), the double mutants of mnFcγRIIa-allele2 (Mutant 4: PMN→HLD with N→T, and Mutant 5: PMN→HLD with PY→LF), and for mnFcγRIIb1 (Mutant 6:H131R, M132S (HM→RS).
Stable expression of FcγRII
A pMXI retroviral expression system was used to introduce FcγR DNA into the FcR deficient IIA1.6 cells. Phoenix packaging cells were maintained in RPMI containing glutamine and 5% heat-inactivated fetal-bovine serum and were transfected with FcγRII plasmids using Lipofectamine 2000 (Life Technologies) to generate retrovirus. The retroviral suspension was overlaid onto IIA1.6 cells as described (39). Transduced IIA1.6 cells were selected for resistance to 0.4 mg/ml geneticin (Life Technologies,)
Antibodies
Purified human myeloma proteins - IgG1κ, IgG2κ and IgG3κ were purchased from both Sigma-Aldrich (Castle Hill, Australia) and The Binding Site (Birmingham, United Kingdom). Phycoerythrin conjugated F(ab')2 goat anti-human IgG F(ab')2 specific polyclonal was from Jackson Immunoresearch (West Grove, PA). Human IgG in the form of intravenous immunoglobulin Sandogobulin was obtained from Novartis (Sydney, Australia).
Antibodies used to identify populations of peripheral blood leukocytes were anti-CD 20, clone L27, BD biosciences, Sydney) anti-CD 14 (clone m5E2, BD biosciences) anti-CD56 (clone NCAM16.2 BD biosciences) anti-CD159a (NKG2) (clone Z199, Beckman Coulter, Melbourne) anti-FcγRIII clone 3G8 (BD Biosciences) and anti-CD41a (clone HIP8, BD Biosciences).
The anti-FcγRIIa monoclonal antibodies 8.7, 8.2, IV.3 and the anti-FcγRIIb monoclonal X63-21/7.2 have been previously described (40, 42, 43). Fab fragments of IV-3 and F(ab')2 fragments of 8.7, 8.2 and X63 monoclonal antibodies were generated as described (42) and then biotinylated with EZ-link Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL).
IgG Ligands
Two separate methods were used to evaluate the binding of human IgG to cell surface expressed FcR of M. nemestrina and human.
First, Anti-Fab complexes: Complexes of IgG were generated by crosslinking IgG with F(ab')2 fragments of fluorochrome-conjugated anti-human Fab as previously described (44). Briefly, individual human IgG1, 2 or 3 subclasses was incubated with F(ab')2 fragments of PE-conjugated goat anti-human IgG F(ab)2 at a 2:1 ratio for 30min at 37°C, and then ice for 5min.
Second, IgG-dimer/trimer: Biotinylated IgG-dimers/trimers complexes of pooled human IgG were generated using the crosslinker, tris-succinimidyl aminotriacetate (TSAT). TSAT, (Thermo Scientific, Rockford, IL) 5mg in 0.5ml of anhydrous DMSO, was mixed with 3.85mg of biotin-X-hydrazide (Sigma Aldrich, Castle Hill, Australia) in 0.5ml of anhydrous DMSO for 2hr at room temperature to generate the intermediate biotinyl bis-succinimidyl aminotriacetate (BBSAT) without purification. Human IgG (4ml at 25 mg/ml in PBS) was mixed with an 11-fold excess of BBSAT (0.7ml) and allowed to react for 1hr at room temperature. The resulting IgG-dimer/trimer were purified from the reaction mixture and separated from monomer and multimers by gel filtration on a Sephacryl-S300 column (1.5cm × 100cm). Fractions were analysed by SDS-PAGE and those corresponding to IgG-dimer/trimer pooled and stored in aliquots at -80°C.
Flow cytometric detection of immune complex binding and receptor expression
The binding of IgG complexes to allelic forms of receptors and mutants thereof, was determined as described (40). Briefly, anti-F(ab')2 PE:IgG, complexes at the indicated concentrations, were incubated with 1.2×105 cells in 50μl for 1hr on ice then washed twice and resuspended in 200μl of PBS/0.5% BSA. Similarly, aggregated huIgG or biotinylated IgG-dimer/trimer binding was detected by indirect fluorescence following incubation with cells on ice for 1 hour. Cells were then washed and incubated with PE-labelled goat anti-human Ab or APC-streptavidin respectively, for 30min on ice, washed twice and then resuspended in 200μl of PBS/BSA. Background binding controls included non-specific binding of IgG to untransfected IIA1.6 cells and non-specific binding of conjugate only to FcR expressing cells.
Expression levels of FcγR on the transfected cells were determined in each experiment by flow cytometry using the following biotinylated anti-FcR mAbs: F(ab')2 of 8.7, 8.2, and Fab of IV.3 or the anti-FcγRIIb X63-21/7.2 F(ab')2 (40). Cells were then washed and binding detected using APC-streptavidin as described above. Gating was set to record fluorescence of 10,000 viable single cells. The analysis of human IgG binding to each human or macaque FcγR and mutants thereof was performed on at least five independent occasions using the anti Fab complexes and at least three independent occasions using the IgG-dimer/trimers.
Flow cytometric analysis of Fc receptor expression on blood cells
Whole blood was collected from four M. nemestrina as above and from healthy human volunteers after informed consent as approved by the Alfred Health Human Ethics Committee or the Monash University Standing Committee on Ethics in Research Involving Humans. Peripheral blood mononuclear cells were isolated on a ficoll density gradient. Total leukocytes were obtained from buffy coat then erythrocytes lysed with hypotonic, Red Blood Cell Lysis Solution according to the manufactures instructions (Miltenyi Biotech, Sydney). Expression of surface markers was determined using flow cytometry where human and M. nemestrina B lymphocytes were identified with anti-CD 20, and monocytes with anti-CD 14 respectively. Human NK cells were defined with anti-CD56 and macaque NK cells with anti-CD159a (NKG2)(45). Neutrophils were identified by forward and side scatter profiles, Platelet-rich plasma was isolated by low speed centrifugation and platelets identified by expression of CD41a. The expression of FcγRIII was defined using clone 3G8 and FcγRII identified with mAb 8.7 as described above.
Homology modelling of the macaque FcγRIIa:huIgG1-Fc interaction
Protein homology models were prepared using the Discovery Studio suite, version 3.0 (Accelrys, San Diego, CA). The co-crystal structure of huFcγRIIa:huIgG1-Fc (PDB ID: 3RY6) (40) was used as a template to generate a homology model of the macaque FcγRIIa:huIgG1-Fc complex using the amino acid sequence of mnFcγRIIa-allele1 (Fig. 1). The 3D model of the macaque FcγRIIa:huIgG1-Fc complex was optimized by conjugate-gradient energy minimization against spatial restraints extracted from the 3RY6 template and a probability density function using the Modeller algorithm (46). The protein interface between FcγRIIa and huIgG1-Fc was analysed using the protein interfaces, surfaces and assemblies (PISA) server at the European Bioinformatics Institute (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) (47). The buried surface area of residues at the interface of FcγRIIa and the bound huIgG1-Fc were plotted for comparison with the total buried surface area between huFcγRIIa and mnFcγRIIa-allele1. Since mnFcγRIIa-allele1 has one additional N-linked glycosylation site compared to huFcγRIIa, in silico glycosylation of this extra site was performed using the GlyProt web-based server (http://www.glycosciences.de/modeling/glyprot/php/main.php) (48).
Figure 1.
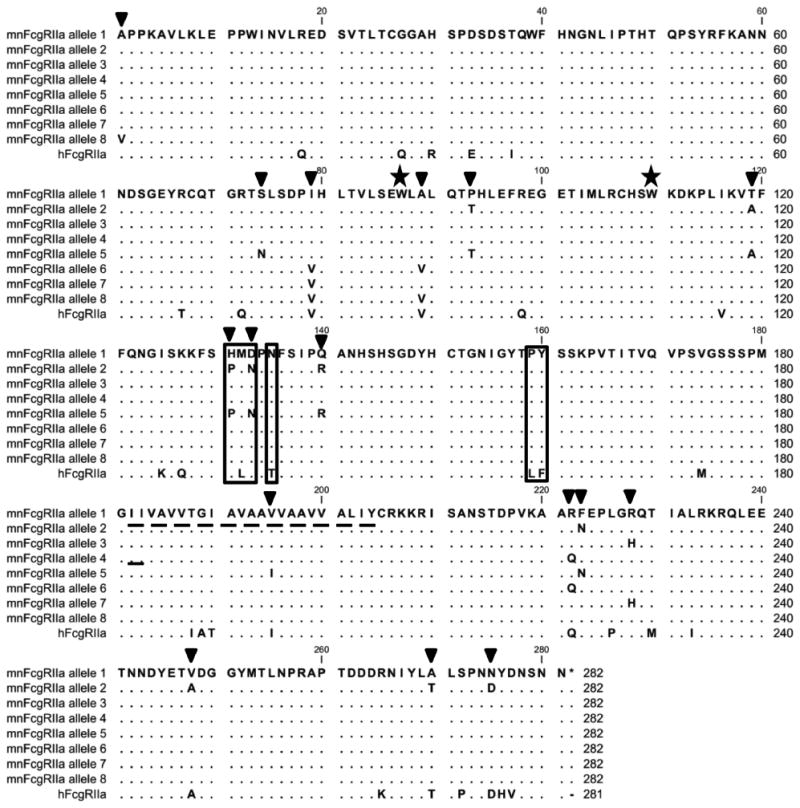
Alignment of the eight allelic forms of FcγRIIa of M. nemestrina with their human counterpart (huFcγRIIa- His131, Uniprot: P12318.4) (41). Amino acids identical to M. nemestrina allele1 encoded receptor are shown as dots and positions of polymorphism indicated by inverted triangles; the transmembrane region is shown as a dashed underline. Critical residues analysed in this paper are boxed. Trp87 and Trp110 are indicated with stars. These sequences were derived from ten animals. The M. nemestrina sequences are available in Genbank [www.ncbi.nlm.nih.gov/genbank] as follows: mnFcγRIIa allele 1: accession number KF234399; mnFcγRIIa allele 2: accession number KF234400; mnFcγRIIa allele 3: accession number KF234401; mnFcγRIIa allele 4: accession number KF234402; mnFcγRIIa allele 5: accession number KF562260; mnFcγRIIa allele 6: accession number KF562261; mnFcγRIIa allele 7: accession number KF562262 and mnFcγRIIa allele 8: accession number KF562263.
Results
Sequence comparison of huFcγRIIa and mnFcγRIIa
The mnFcγRIIa sequences were determined from cDNA isolated from 10 animals of an outbred colony of M. nemestrina. Replicate sequence analysis of PCR products and of multiple clones from independently derived, duplicate PBMC samples were used to establish and then confirm FcR sequences.
Sequence analysis comparing M. nemestrina sequences to the human FcγRIIa revealed amino acid differences between macaque FcγRIIa and humans that were conserved in all animals and also identified polymorphic sequence variation between the FcγRIIa of individual macaques (Fig. 1). Thus 26 amino acids that differed from human FcγRIIa were conserved in all individuals. Sixteen of these amino acid differences mapped to the extracellular region, three to the transmembrane region and seven to the cytoplasmic tail of mnFcγRIIa (Fig.1).
Further sequence analysis among the ten animals revealed extensive polymorphism and identified eight allelic products of M. nemestrina FcγRIIa (Fig. 1). These allelic forms were distinguished from each other by 16 polymorphic residues of which nine were located in the extracellular domains (at positions 1, 74, 79, 89, 93, 119, 131, 133, 140), one was located in the transmembrane region and six were found in the cytoplasmic tail. Interestingly, the identity of the 9 polymorphic amino acids within the extracellular region suggests that FcγRIIa alleles 1,3,4,6,7,8 are closely related as they contain the human-equivalent residue in many of the polymorphic positions. In contrast, FcγRIIa alleles 2 and 5, found in two of the ten animals investigated, are closely related to each other but are the most different to human FcγRIIa with 7 or 8 of the nine polymorphic positions respectively, being distinct from human FcγRIIa (Fig. 1).
In M. nemestrina, the inhibitory FcγRIIb has 23 non-polymorphic amino acid differences from huFcγRIIb and 21 of these occur in the extracellular domains. A single polymorphism resulting in amino acid substitution Met/Val 254 was apparent in the cytoplasmic tail (Fig. 2).
Figure 2.

Alignment of the allotypic form of FcγRIIb of M. nemestrina with the splice variants of its human counterpart (huFcγRIIb1, GenBank: NM_001002275.2; huFcγRIIb2, GenBank: NM_001002273.2) (38). Amino acids identical to M. nemestrina are shown as dots and positions of polymorphism indicated by inverted triangles; the transmembrane region is shown as underlined dashed line and the deletions within the cytoplasmic tail of FcγRIIb2 resulting from mRNA splicing or within the membrane stalk are indicated by solid lines. Critical residues analysed in this paper are boxed. Trp87 and Trp110 are indicated with stars. M. nemestrina sequences are available in Genbank [www.ncbi.nlm.nih.gov/genbank] as follows: mnFcγRIIb1: accession number KF234403; mnFcγRIIb2: accession number KF234404.
Binding of Human IgG to M. nemestrina FcγRIIa
A comparative analysis of the binding of human IgG subclasses to allelic forms of mnFcγRIIa and to huFcγRIIa was undertaken in transfected cells where their equal expression was confirmed by binding of the anti-FcγRIIa mAb F(ab')2 fragments (Fig. 3A), which detects an epitope in the extracellular domains in FcγRIIa and FcγRIIb (40).
Figure 3.
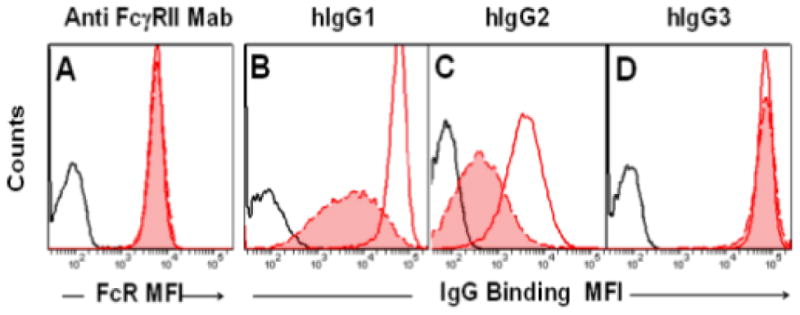
Comparative binding of human IgG subclasses to mnFcγRIIa-allele1 (red filled histogram with dashed red line) or the huFcγRIIa His 131 (unfilled histogram, solid red line). Cell surface expression of receptor protein was determined using the anti-FcγRIImAb 8.7, which recognises huFcγRIIa and mnFcγRIIa. Background binding is shown as a solid black line. The binding of each IgG subclass to each receptor was tested on at least five occasions.
The binding of human IgG3 complexes to mnFcγRIIa-allele1 and huFcγRIIa-His 131 was analysed by flow cytometry and was shown to be essentially identical with relative cell surface staining of mean fluorescence intensity (MFI) of 70,000 and 71,000, respectively (Fig. 3D). In contrast, mnFcγRIIa-alelle1 bound IgG1 or IgG2 complexes with MFIs of 5000 and 360 respectively, which were 8-10 times lower than their binding to huFcγRIIa-His 131 (Fig. 3B-C). Thus in comparison to human FcγRIIa, the mnFcγRIIa-allele1 has equivalent binding of complexes of huIgG3, but greatly diminished binding of complexes of huIgG1 and huIgG2.
In addition to the differences in huIgG binding between human and M. nemestrina FcγRIIa observed above, even greater differences were found in huIgG binding to the allelic forms of mnFcγRIIa (Fig. 4). The binding of all human IgG subclasses to mnFcγRIIa-allele2 receptor was reduced greatly (Fig. 4B-D). Compared to binding by mnFcγRIIa-allele1(MFI=5000), IgG1 binding to mnFcγRIIa-allele2 was reduced 80-fold (MFI=90) and was barely detectable above background (MFI=30, Fig. 4B). Similarly, IgG2 binding of mnFcγRIIa-allele2 was also barely detectable above background levels (Fig. 4C). The binding of IgG3 complexes (MFI=11,600) was also reduced 6-fold compared to mnFcγRIIa-allele1 (MFI=70,300). Thus, not only do interspecies differences of FcR affect human IgG binding, but polymorphisms (intraspecies differences) in M. nemestrina FcγRIIa also influence the binding of human IgG. The mnFcγRIIa-allele2 is greatly impaired in ligand binding of human IgG1, IgG2 and IgG3.
Figure 4.

Comparative binding of human IgG subclasses to allelic forms and mutants of mnFcγRIIa. Binding of anti-receptor antibody mAb8.2 (A) or IgG (B-D) to mnFcγRIIa-allele2 (blue filled histogram, solid line) or the mnFcγRIIa-allele1 (red filled histogram, dashed line) and background binding (black line). Binding of anti-FcγRIImAb 8.7 (E) or IgG (F-H) to mnFcγRIIa-allele2 HLD where sequence PMN(131-133) was replaced by human sequence HLD(131-133) (blue filled histogram, dashed line) or mnFcγRIIa-allele2 HLD +LF where both PMN(131-133) and PY(159-160) were replaced by human HLD(131-133) and LF(159-160) (unfilled histogram, solid blue line). Binding of anti-receptor mAb 8.7 (I) or IgG (J-L) to mnFcγRIIa-allele1 (red filled histogram, dashed line as in panels B-D) or mnFcγRIIa- allele1 LF where PY (159-160) was replaced with human LF(159-160) (unfilled histogram, solid red line). The binding of each IgG subclass to each allelic receptor was tested on at least five occasions and the binding to mutated receptors was tested on at least three occasions.
Next we defined the sequence substitutions responsible for the low ligand binding activity of mnFcγRIIa-allele2. Analysis of the polymorphic amino acid differences in the mnFcγRIIa in the context of known functional regions of huFcγRIIa showed that position 131 is part of the major contact surface in the huFcγRIIa:IgG interaction (40, 49, 50). Moreover in humans, polymorphic FcγRIIa (high and low responder allelic products that differ at position 131) have very different interactions with mouse IgG1 and human IgG2 (44). Thus, it seemed possible that the large differences in IgG binding between the two M. nemestrina allelic receptors results from the sequence differences in this segment, namely, HMD(131-133) of the functional receptor encoded by mnFcγRIIa-allele1 compared to the PMN(131-133) of the poorly functional receptor encoded by mnFcγRIIa-allele2 (Fig. 1).
Consequently, we attempted to reconstitute binding of human IgG by replacing PMN(131-133) of mnFcγRIIa-allele2 with HLD(131-133) from huFcγRIIa. Note that His131 and Asp133 of mnFcγRIIa-allele1 are identical in huFcγRIIa (Fig. 1). Weak binding of IgG1 and IgG2 to mnFcγRIIa-allele2 was demonstrated (Fig. 4B,C) and the HLD(131-133) substitution substantially rescued binding with a MFI of 2900 and a MFI of 300 for IgG1 and IgG2, respectively (Fig. 4F,G; blue filled, dashed line) that was comparable to that of mnFcγRIIa-allele1 (MFI=5000 for IgG1 and 360 for IgG2) (Fig. 4B, C; red filled, dashed line). Similarly, the binding of IgG3 was also improved from a MFI of 11,600 to a MFI of 53,400 (Fig. 4D, H). However, this increased binding of IgG was still markedly lower than the binding of IgG1 and IgG2 complexes to the huFcγRIIa-His131 - MFI of 57,200 and 3600 respectively. Binding of IgG3 by the mnFcγRIIa-allele2 with HLD(131-133) remained slightly reduced compared to the human receptor (53,400 for mnFcγRIIa-His131 compared to 70,100 for huFcγRIIa-His131; compare to Fig. 3). The failure to fully enable IgG binding to levels observed for the human receptor implied other amino acid residue differences between mnFcγRIIa and huFcγRIIa contribute to ligand binding. Of particular interest in this context were residues Pro159 and Tyr160 in mnFcγRIIa and Leu159 and Phe160 in huFcγRIIa, which are located in the G strand of the second domain adjacent to the critical FG loop which contacts IgG.
Thus, allele2-encoded mnFcγRIIa which we had mutated to contain the HLD(131-133) of huFcγRIIa His131 (Fig. 4E-H dashed line) was further modified by replacing Pro159 and Tyr160 with the equivalent Leu159 and Phe160 from huFcγRIIa (Fig. 4E-H). This substitution increased IgG1 binding 5-fold to levels similar to huFcγRIIa-His131 and increased the binding of IgG2 almost 10-fold. Interestingly, there was no further improvement in the IgG3 binding beyond that already achieved by the substitution of PMN(131-133) for HLD(131-133).
The role of Pro159 and Tyr160 in macaque receptor binding of IgG1 and IgG2 was further examined in a similar analysis of the mnFcγRIIa-allele1, which contained the HMD(131-133) sequence. The replacement of Pro159 and Tyr160 with Leu159 and Phe160 from huFcγRIIa also improved IgG1 and IgG2 binding to levels similar to that of huFcγRIIa binding (Fig. 4I-L). Thus, the presence of PY (159-160) in mnFcγRIIa results in a significant impairment of binding of the human IgG1 and IgG2 subclasses.
Binding of Human IgG to mnFcγRIIb
The interaction of human IgG subclasses with the inhibitory FcγRIIb of M. nemestrina was investigated and substantial differences observed in specificity and binding compared to huFcγRIIb (Fig. 5). Indeed, unlike the activating FcγRIIa of M. nemestrina, the mnFcγRIIb had a10-fold greater capacity to bind IgG1 complexes than huFcγRIIb (Fig. 5B). The greatest functional divergence was in the binding of human IgG2, which failed to bind to huFcγRIIb as expected, but surprisingly was strongly bound by mnFcγRIIb (Fig. 5C). FcγRIIb from both species bound IgG3 at similar levels (Fig. 5D).
Figure 5.

Comparative binding of human IgG subclasses (B-D) to mnFcγRIIb (solid blue line) or huFcγRIIb (solid red line) compared to a mutant of mnFcγRIIb (blue filled histogram) wherein sequence HMD(131-133) was replaced with the human derived sequence RSD(131-133). Expression of receptor protein was determined using the huFcγRIIa mAb 8.7 (A). The binding of IgG to each receptor or mutated receptor was tested on at least five occasions.
This difference in the capacity of the inhibitory mnFcγRIIb to bind IgG2 was surprising since in humans IgG2 essentially binds only to the activating FcγRIIa-His131, which is the consequence of the Arg/His131 polymorphism dictating specificity for IgG2 in this activating receptor.
Comparison of the amino acid sequence of the IgG binding regions of human and M. nemestrina FcγRIIb shows the principal sequence differences occur around position 131 wherein mnFcγRIIb encodes His131 and Met132 whereas huFcγRIIb has Arg131 and Ser132 (Fig. 2). Thus we replaced the HM(131-132) of mnFcγRIIb with the RS(131-132) of huFcγRIIb and measured the impact on IgG1 and IgG2 binding (Fig. 5B, 5C). The substitution of the human derived RS(131-132) into mnFcγRIIb resulted in a marked loss in IgG1 binding, decreasing to levels equivalent to huFcγRIIb (Fig. 5B). Moreover IgG2 binding was completely lost (Fig. 5C) which is entirely consistent with the essential role of His131 in dictating the binding of IgG2.
Binding of human IgG dimers to FcγRII
The binding measurements above were obtained using complexes generated by oligomerisation of IgG with an anti-Fab antibody, a technique commonly used for the detection of immune complex binding to low affinity receptors (44). To exclude the possibility that the anti-Fab antibody may influence FcR binding of the IgG complexes we used a second method where dimers/trimers of human IgG were generated by cross-linking of pooled human IVIG with a biotinylated cross-linker (Fig. 6).
Figure 6.

The binding of human IgG dimers/trimers to mnFcγRIIa and mnFcγRIIb. Biotinylated human IgG dimers/trimers were titrated on transfectants expressing (A) huFcγRIIa (▪) or mnFcγRIIa- allele2 (◆) and mutants thereof wherein PMN(131-133) was replaced by HLD(131-133) of huFcγRIIa (−) then further modified by additional replacement of PY(159-160) with LF(150-160) of huFcγRIIa respectively (▲) or N135 replaced by T of huFcγRIIa (•); (B) huFcγRIIa (▪) or mnFcγRIIa-allele1 (−) and mutants thereof wherein PY(150-160) was replaced by with LF(159-160) of huFcγRIIa (Δ) or where N135 was replaced with T135 of huFcγRIIa (○) or binding to untransfected cells only (×); (C) huFcγRIIb1 (+) or mnFcγRIIb2 (○) or mnFcγRIIb1 (□), and mutants thereof wherein HM(131-132) was replaced with RS(131-132) of huFcγRIIb (◊). The binding of IgG to each receptor or mutated receptor was tested on at least three occasions.
Flow cytometry analysis of the binding of the cross-linked IgG dimers/trimers to FcγRIIa and FcγRIIb and receptor mutants entirely agreed with the binding observed using the complexes generated with the anti-Fab antibody (Fig. 3-5). The mnFcγRIIa-allele2 failed to bind the IgG dimers/trimers, but the replacement of PMN(131-133) with HLD(131-133) enabled binding similar to that of allele1 (Fig. 6A). Further mutation of this construct wherein PY(159-160) was replaced with the LF(159-160) of huFcγRIIa resulted in further increase of IgG binding to levels approaching that of huFcγRIIa-His131 (Fig. 6A).
One other significant structural difference between human and macaque in this region is a potential N-glycosylation site at position 135 in mnFcγRIIa, which is absent from huFcγRIIa, but found in both human and macaque FcγRIIb. Since this site sits adjacent to the critical His131 we replaced N135 in mnFcγRIIa with the human equivalent T135, however this mutation had little effect on the binding of any human IgG subclass (Fig 6A).
Similarly, the same modifications of mnFcγRIIa-allele1 (Fig. 6B) were made and resulted in similar effects on IgG binding. Replacement of PY(159-160) with the human LF(159-160) residues greatly improved IgG binding, but as seen in mnFcγRIIa-allele2 the removal of the 135 glycosylation site had little effect.
In the case of FcγRIIb (Fig. 6C), as was observed with the anti-Fab antibody complexes, the M. nemestrina receptors bound IgG dimers/trimers more strongly than huFcγRIIb and as expected the substitution of the M. nemestrina FcγRIIa sequence HM(131-132) with the human equivalent RS(131-132) profoundly diminished binding to levels, which were even lower than observed with huFcγRIIb.
Molecular Modelling of mnFcγRIIa
The human FcγRIIa:IgG interaction has been well characterised by extensive mutagenesis (42, 51, 52), X-ray structure studies of huFcγRIIa alone (50) and in complex with human IgG1-Fc (40) which have collectively identified four, structurally contiguous, regions that form the IgG binding surface in the second domain of FcγRIIa. To understand the key interspecies sequence differences between macaque and human receptors, we generated a molecular model of mnFcγRIIa-allele1 interaction with human IgG1 using the X-ray structure of the huFcγRIIa:huIgG1-Fc complex (40). This model (Fig. 7) predicts that key contacts defined in the huFcγRIIa:huIgG1-Fc interface are altered in the interaction of mnFcγRIIa and human IgG.
Figure 7.
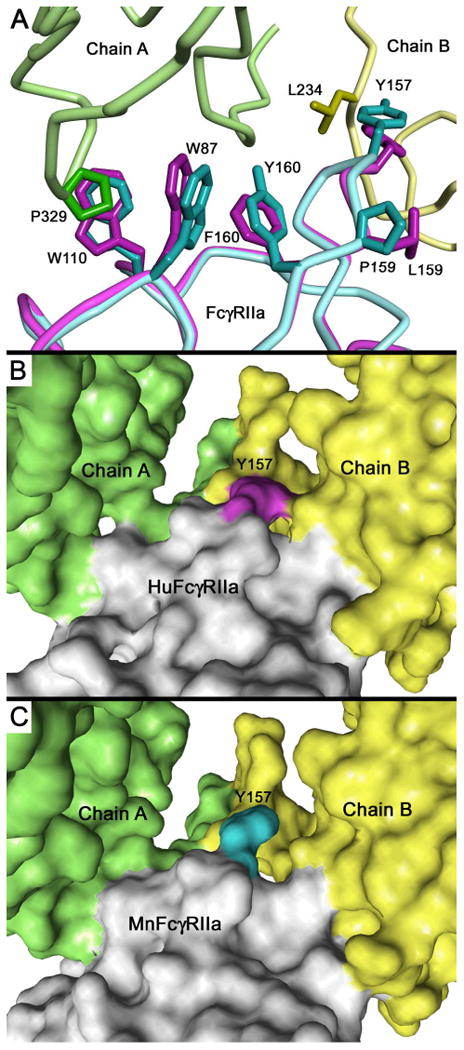
Homology based model of mnFcγRIIa complex with human IgG1-Fc. (A) Comparison of the FG loop and Trp residues of the “trp sandwich” of mnFcγRIIa (cyan wire) or huFcγRIIa (magenta wire) interacting with human IgG1-Fc B chain (yellow wire) or A chain (green wire). (B) Solvent-accessible surface view of human FcγRIIa complex with huIgG1-Fc and (C) solvent-accessible surface view of macaque FcγRIIa complex with huIgG1-Fc. The comparison of B and C indicates the critical Tyr157 of the FG loop has a reduced contact with human IgG1 in mnFcγRIIa. The Tyr157 residues of huFcγRIIa and mnFcγRIIa are shown in magenta and cyan, respectively. The IgG1-Fc chain A is shown in green, chain B in yellow and FcγRIIa molecules in light grey. Models were prepared based on the co-crystal structure of huFcγRIIa:huIgG1-Fc (PDB ID:3RY6) (40).
The FG loop of the second domain of FcγRIIa is one major contact between receptor and IgG-Fc where Tyr157, conserved in all macaque species and humans, makes critical contacts with Leu234 and Gly236 in the lower hinge sequence LLGG(234-237), on the IgG-Fc B chain (Fig. 7A). Compared to huFcγRIIa:Fc complex, the contacts between the Tyr157 of mnFcγRIIaFG loop and the IgG-Fc are reduced in the modelled interface, from a buried surface area of 100 Å2 to 69 Å2, including the loss of a potential hydrogen bond (Fig. 7B, C). The interface contact is further diminished at Trp87 of mnFcγRIIa (68 Å2 buried surface) compared to the complexed huFcγRIIa (82 Å2 buried surface) which together with Trp110 forms the so called “Trp-sandwich” that binds Pro329 of the Fc A chain (Fig. 7A). These alterations to contact residues (Tyr157 and Trp87) can be attributed to the influence of the interspecies substituted residues Pro159 and Tyr160 in the G strand of mnFcγRIIa. The model predicts that the mnFcγRIIa Pro159 kinks the G strand and repositions Tyr157 reducing its contact with the Fc (Fig 7A). Furthermore, the Tyr160 side-chain packs against Trp87 reducing contact in the “Trp sandwich” of Trp87 with Pro329 of the IgG-Fc A chain (Fig. 7A). The structural effect of these residues PY(159-160) on the critical contact residues agrees with the mutagenesis data (Figs. 4, 6) which showed that the replacement of the PY(159-160) of mnFcγRIIa with LF(159-160) of huFcγRIIa optimised the interaction with human IgG1 and IgG2.
Importantly, previous studies of human FcγRIIa support our binding and modelling data and are consistent with a role for Tyr160 in the macaque receptor in modulating interactions with IgG. Varying the sequence at position 160 of the human receptor by replacement of Phe160 with Ala substantially increased binding of both human IgG1 and IgG2 (50, 52). Conversely, the replacement of Phe160 with Tyr reduced binding of IgG (53); note that Tyr occupies this position in mnFcγRIIa (Fig. 1). Thus, it is likely that the improved binding of human IgG by the mutated mnFcγRIIa following our replacement of Pro159-Tyr160 with Leu159-Phe160 of huFcγRIIa results from a more favourable orientation of the contact Tyr157 and of the Trp87/Trp110 sandwich in the interaction with human IgG-Fc (Fig. 7).
Another major contact of FcγRIIa in complex with Fc is made by the C'E loop, especially residue 131, which determines the functional differences of high/low responder polymorphism of FcγRIIa in humans. Arg131 is accommodated in a shallow depression in the Fc and is somewhat “crowded” but the His131 is more readily accommodated (40) and in the mnFcγRIIa-allele1 and mnFcγRIIb which also contain His 131, this also is likely to be the case. In the case of mnFcγRIIa-allele2, the near complete loss of binding caused by the presence of proline in position 131 is likely due to a major alteration of the C'Eloop structure leading to the disruption of the interface with IgG.
Expression of FcγRII in M. nemestrina blood cells
The distribution of FcγRII on leukocyte types has been determined for rhesus and cynomologus macaques (26, 27, 45) and we analysed expression in M. nemestrina. Flow cytometry analysis of peripheral blood leukocytes from four macaques confirmed the expression of FcγRII on CD20+ B cells, CD14+ monocytes (Fig. 8) and platelets (not shown) and as expected was absent from macaque CD159a+NK cells and human CD56bright NK cells which is in agreement with cell distribution in rhesus and cynomolgous macaques (26, 27, 45) and humans, reviewed in (1, 2) Considerable differences in FcγR expression were observed on neutrophils wherein FcγRII and FcγRIII were both expressed on human neutrophils (Fig. 9) but only FcγRII was present on M. nemestrina neutrophils and also at a level five-fold greater than detected on human cells. The absence of FcγRIII from M. nemestrina neutrophils has also been reported for rhesus and cynomolgus macaques (26, 45) and increased expression of FcγRII has also been shown in cynomologus macaques (27).
Figure 8.

Expression of FcγRII on mononuclear leukocytes from humans and M. nemestrina. Mononuclear cells were stained with anti-FcγRIIa mAb 8.7 and populations identified by costaining with anti-CD20 (B cells), anti-CD14 (monocytes), anti-CD159a (NKG2) (M. nemestrina NK cells) or anti-CD56 (human NK cells). Plots are representative of three individual experiments involving four different animals or human volunteers.
Figure 9.

Macaque neutrophils express elevated levels of FcγRII but lack FcγRIII. Flow cytometry analysis of blood neutrophils from human or M. nemestrina stained for FcγRII with mAb 8.7 or FcγRIII mAb 3G8. Plots are representive of three individual experiments.
Discussion
In this study, we demonstrate that human and M. Nemestrina FcγRIIa and FcγRIIb have distinct hierarchies of binding of human IgG1 and IgG2. Whilst the FcγRIIa of both species bind IgG3 essentially equivalently, IgG1 and IgG2 binding to mnFcγRIIa is impaired by comparison to its human ortholog. Remarkably, the converse is the case for FcγRIIb where it is the human receptor that exhibits impaired binding of IgG1 relative to mnFcγRIIb and IgG2 is not detectably bound. Indeed, mnFcγRIIb avidly binds IgG2 and thereby has a broader specificity for human IgG subclasses than huFcγRIIb. These interspecies differences of IgG binding and specificity to M. nemestrina FcγR that we describe herein were determined in the physiological context of the cell surface. Similar results were observed in cell free surface plasmon resonance analysis of recombinant ectodomains of cynomolgus FcγR, which showed lower affinity of huIgG1 and huIgG2 for cynomolgus FcγRIIa than huFcγRIIa and conversely showed increased affinity for IgG1 and a greatly increased affinity of IgG2 for cynomolgus FcγRIIb compared to its human orthologue (27).
To identify key residues that contribute substantially to the observed interspecies IgG binding differences, we exchanged equivalent residues between the human and macaque FcR receptors in site-directed mutagenesis studies (Figs. 4, 5, 6). In short, Pro159 and Tyr160 contribute to the lower activity of the mnFcγRIIa, while His131 and Met132 are key to the higher activity of mnFcγRIIb compared to huFcγRIIb. Furthermore we have also described the profound influence of polymorphism of mnFcγRIIa on IgG binding and identified FcγRIIa as highly polymorphic with eight alleles being detected in only 10 individuals.
The studies described herein have implications for the structural and functional analysis of IgG FcR interactions, not only in M. nemestrina but also in the widely used rhesus and cynomolgus macaques and other NHP. A comparison FcγRIIa and FcγRIIb sequences of other NHPs shows that critical residues in the FG and C'E loops identified here as affecting the mnFcγRII interaction with human IgG are preserved in some but not all NHP (Figs. 10, 11, 12).
Figure 10.

Structural location of human and macaque interspecies sequence diversity and macaque polymorphism of FcγRIIa. The locations of polymorphic or non-polymorphic amino acids are shown on the alpha carbon trace of residues 4 -170 of human FcγRIIa. Cyan spheres show the location of non-polymorphic amino acids that are identical to both M. nemestrina (A) and M. mulatta (B) but differ from human FcγRIIa. The red spheres indicate the location of residues where polymorphism is unique to one macaque species. The yellow spheres indicate location of residues where polymorphism has been identified in both M. nemestrina and M. mulatta. Sequence numbering follows that of Fig. 1.
Figure 11.

Alignment of the extracellular domains of NHP including rhesus allelic forms and huFcγRIIa.; for additional allelic forms of M. nemestrina see Fig1. Amino acids identities are shown as dots. Critical residues analysed in this paper are boxed. Species shown are pigtail macaque (Macaca nemestrina), rhesus macaque (Macaca mulatta) (24), cynomologus macaque (Macaca fasicularis, GenBank: AF485813) (27), baboon (Papio anubis, GenBank: XM_003892972.1), chimpanzee (Pan troglodytes, Uniprot: Q8SPV8.1), bonobo (Pan paniscus, GenBank: XM_003804397.1), orangutan (Pongo abelii, GenBank: XM_002809886.2), squirrel monkey (Samiri boliviensis, GenBank: XM_003943372.1), and marmoset (Callithrix jacchus, GenBank: XM_003735165.1). The known allelic forms with polymorphisms in the extracellular region are shown for M. nemestrina and M. mulatta.
Figure 12.
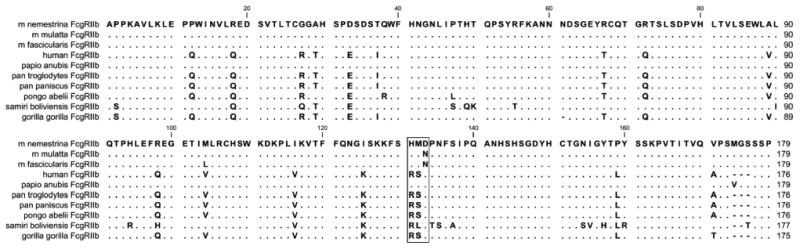
Alignment of the extracellular domains of NHP and huFcγRIIb. Amino acids identities are shown as dots. Critical residues analysed in this paper are boxed. Pigtail macaque (Macaca nemestrina), rhesus macaque (Macaca mulatta) (24), cynomologus macaque (Macaca fasicularis) (27), baboon (Papio anubis, GenBank: XM_003892974.1), chimpanzee (Pan troglodytes, GenBank: XM_ 001153863.2) bonobo, (Pan paniscus, GenBank: XM_003824761.1) orangutan (Pongo abelii, GenBank: XM_003892974.1), squirrel monkey (Samiri boliviensis, GenBank: XM_003943370.1) and gorilla (Gorilla gorilla, GenBank: XM_004027772).
The Pro159, Tyr160 residues of the FG loop that adversely affect IgG binding by mnFcγRIIa are conserved in rhesus (M. mulatta) (Fig. 10, Fig. 11) and cynomolgus (M. fasicularis) macaque species (Fig. 11) (24, 27, 54) suggesting that these residues could be a key interspecies difference that modulates the engagement of human IgG in FcγRIIa of other macaque species such as cynomolgus FcγRIIa (27). Notably PY(159-160) are not conserved in other NHP (Fig. 11) which raises the possibility that the diminished human IgG binding may be only a feature of macaque FcγRIIa.
In the case of position 131 which forms a major contact with huIgG several, but importantly not all, NHPs also have His at this position in FcγRIIa, which could favour huIgG2 binding. His131 is also found in FcγRIIa of rhesus (M. mulatta), and cynomologus (M. fasicularis) macaques, chimpanzee (Pan troglodytes) and bonobo, (Pan paniscus), baboon (Papio anubis) and squirrel monkey (Samiri boliviensis) thus, it is likely that they also bind huIgG2. Indeed rhesus and cynomolgus macaque FcγRIIa do bind huIgG2 as measured by SPR or immune complex binding to cells (27) However, orangutan (Pongo abelii) and marmoset (Callithrix jacchus) have Tyr131 and Arg131 respectively and therefore, may have altered human IgG binding compared to macaque FcγRIIa, especially with respect to huIgG2.
Our data also describe the profound influence of polymorphism in mnFcγRIIa on IgG binding which adds additional complexity to analysis of human IgG interaction with macacque FcR. In M. nemestrina and interestingly in rhesus macaque, FcγRIIa is the most polymorphic of the FcγR (24, 54) (Figs. 1, 2) However, only M. nemestrina showed sequence variation at position 131 wherein proline in this position in allele 2, also present in allele 5, results in ablation of IgG1 and IgG2 binding. This is the equivalent position to the clinically relevant high and low responder polymorphism in huFcγRIIa (11, 23) wherein allotypic receptor with Arg131 is hypofunctional with respect to IgG2 binding.
While the functional importance of FcR polymorphism on IgG binding in other macaque species has not been investigated it is noteworthy that in rhesus macaques, polymorphism in the C'E loop at position 128 results in the presence/absence of a possible N-glycosylation site that replaces the Lys128 adjacent to Phe129, which in human FcγRIIa is a critical IgG contact (Fig. 10) (40). Interestingly the same site is found in baboon and a unique site of possible N-glycosylation is present at position 133 in marmoset (Fig. 11) but whether these positions are polymorphic or functionally important in these species is unknown.
Thus polymorphism, at least in FcγRIIa, is extensive, eight alleles in ten animals and it is likely that more alleles exist in M. nemestrina. Importantly the presence of null or hypofunctional allotypic receptors, is sufficiently frequent in M. nemestrina, two of eight allotypic receptors among ten individuals, and potentially other species to warrant the exercise of caution when interpreting results of studies involving models of antibody-based effects.
The inhibitory FcγRIIb also contains His131 in all major macaque species widely used in research, pig-tail (M. nemestrina), rhesus (M. mulatta), and cynomologus (M. fasicularis) as well as the baboon (Papio anubis) (Fig. 12). Based on the M. nemestrina data herein the FcγRIIb of these species is likely to bind human IgG1, and importantly IgG2, more avidly than human FcγRIIb. Indeed, avid binding of IgG2 has been observed in cynomologus FcγRIIb (27). In all other NHP species, arginine is preserved at this position, which suggests FcγRIIb of these species may behave more like human FcγRIIb with reduced immune complex binding and little IgG2 binding, making the FcγRIIb of macaques unique in this regard.
In humans subtle affinity differences have indeed been shown to be critically important in the respective functions of human FcγRIIa and FcγRIIb (53). Because of the altered specificity of mnFcγRIIb and/or the reversed hierarchy of IgG1 and IgG2 binding to mnFcγRIIa and mnFcγRIIb it is conceivable that antibody therapeutics, especially IgG2, may not behave in M. nemestina or other macaque species as they may be expected to in humans. The increased binding to mnFcγRIIb may obscure potent and desirable effector function or alternatively obscure adverse reactions that may otherwise be manifest in humans where affinity for the inhibitory FcγRIIb is lower. Furthermore with the success of monoclonal antibody therapy (1) efforts have been made to alter the potency of useful therapeutic monoclonal antibodies which include specific engineered changes to the IgG-Fc region to optimise the interaction with human FcγRs (21, 22, 55). However the FcγRIIa or FcγRIIb selectivity of such engineered antibodies may not necessarily exhibit improved binding to macaque FcγR (56).
Thus our data highlights that the activities of mAbs designed to alter interactions between human antibodies and human Fc receptors may not be faithfully recapitulated in preclinical studies in nonhuman primates, or at least in macaques.
Similar caveats may apply to viral pathogenesis studies in macaques of human infections where FcγRIIa or FcγRIIb are involved in significant clinical or biological aspects of the natural history of the infection in humans e.g. antibody dependant enhancement (ADE)(57) in dengue infection or skewing of the FcγRIIa:FcγRIIb expression ratio in HIV infection (12) or resistance to HIV (13, 14).
Thus the interspecies and polymorphic differences we describe herein may translate to alterations of antibody induced inflammatory outcomes in vivo in NHP that are distinct from those in humans.
In considering the impact of differences in FcγR binding function in vivo in NHP it is important to consider whether any differences in cell distribution may also confound the interpretation of in vivo studies of antibody function. The cell distribution of FcγR is for the most part similar to humans in M. nemestrina (Figs. 8, 9) and in rhesus (26, 45) and cynomolgus macaques (27) with the notable exception of neutrophils where FcγRIII is lacking but FcγRII is elevated. Thus the implication is that differences in cell distribution in M. nemestrina or other macaques species, may not affect the evaluation of mAbs except where effector function is dependent on the FcγRs of neutrophils.
Whilst the macaque is a valuable model of human immune function, clear differences exist between species. Cautious interpretation of responses involving antibody induced responses is prudent. Clearly analysis of NHP FcγR genotype and binding profiles of therapeutic IgG to receptors may be useful in the design of experimental or preclinical studies. Thus the use of NHP as a model of human immunity or preclinical model for the evaluation of antibody FcR interactions will be greatly assisted by identifying and understanding the basis for the differences in interaction of macaque and human FcR with human IgG.
Abbreviations
- huFcγR
human FcγR
- huIgG
human IgG
- IVIG
intravenous immunoglobulin
- MFI
median fluorescence intensity
- mnFcγR
Macaca nemestrina FcγR
- NCBI
National Centre for Biotechnology Information
- NHP
non-human primate
- PDB ID
Protein Data Bank Identification code
- PISA
protein interfaces, surfaces and assemblies
Footnotes
Supported by NHMRC Fellowship and Project Grants (1002270) NHMRC program grant (510448), and by the Victorian Operational Infrastructure Support Program.
References
- 1.Hogarth PM, Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nature reviews Drug discovery. 2012;11:311–331. doi: 10.1038/nrd2909. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 3.Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol. 2002;14:798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- 4.Cady CT, Rice JS, Ott VL, Cambier JC. Regulation of hematopoietic cell function by inhibitory immunoglobulin G receptors and their inositol lipid phosphatase effectors. Immunol Rev. 2008;224:44–57. doi: 10.1111/j.1600-065X.2008.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 6.Daeron M, Lesourne R. Negative signaling in Fc receptor complexes. Adv Immunol. 2006;89:39–86. doi: 10.1016/S0065-2776(05)89002-9. [DOI] [PubMed] [Google Scholar]
- 7.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cady CT, Powell MS, Harbeck RJ, Giclas PC, Murphy JR, Katial RK, Weber RW, Hogarth PM, Johnson S, Bonvini E, Koenig S, Cambier JC. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcgammaRIIA and FcgammaRIIB. Immunol Lett. 2010;130:57–65. doi: 10.1016/j.imlet.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tate BJ, Witort E, McKenzie IF, Hogarth PM. Expression of the high responder/non-responder human Fc gamma RII. Analysis by PCR and transfection into FcR-COS cells. Immunol Cell Biol. 1992;70(Pt 2):79–87. doi: 10.1038/icb.1992.12. [DOI] [PubMed] [Google Scholar]
- 10.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J Exp Med. 1990;172:19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–1343. [PubMed] [Google Scholar]
- 12.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwer KC, Lal RB, Mirel LB, Yang C, van Eijk AM, Ayisi J, Otieno J, Nahlen BL, Steketee R, Lal AA, Shi YP. Polymorphism of Fc receptor IIa for IgG in infants is associated with susceptibility to perinatal HIV-1 infection. AIDS. 2004;18:1187–1194. doi: 10.1097/00002030-200405210-00012. [DOI] [PubMed] [Google Scholar]
- 14.Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- 15.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. 2009;4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French Ma, Tanaskovic S, Law MG, Lim A, Fernandez S, Ward LD, Kelleher AD, Emery S. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a ‘high-affinity’ FcgammaRIIa genotype. AIDS. 2010;24:1983–1990. doi: 10.1097/QAD.0b013e32833c1ce0. [DOI] [PubMed] [Google Scholar]
- 17.Sanders LA, van de Winkel JG, Rijkers GT, Voorhorst-Ogink MM, de Haas M, Capel PJ, Zegers BJ. Fc gamma receptor IIa (CD32) heterogeneity in patients with recurrent bacterial respiratory tract infections. The Journal of infectious diseases. 1994;170:854–861. doi: 10.1093/infdis/170.4.854. [DOI] [PubMed] [Google Scholar]
- 18.Solé-Violán J, García-Laorden MI, Marcos-Ramos JA, de Castro FR, Rajas O, Borderías L, Briones ML, Herrera-Ramos E, Blanquer J, Aspa J, Florido Y, García-Bello MA, Ferrer-Agüero JM, Sologuren I, Rodriguez-Gallego C. The Fcγ receptor IIA-H/H131 genotype is associated with bacteremia in pneumococcal community-acquired pneumonia. Crit Care Med. 2011;39:1388–1393. doi: 10.1097/CCM.0b013e31820eda74. [DOI] [PubMed] [Google Scholar]
- 19.Duits AJ, Bootsma H, Derksen RH, Spronk PE, Kater L, Kallenberg CG, Capel PJ, Westerdaal NA, Spierenburg GT, Gmelig-Meyling FH. Skewed distribution of IgG Fc receptor IIa (CD32) polymorphism is associated with renal disease in systemic lupus erythematosus patients. Arthritis Rheum. 1995;38:1832–1836. doi: 10.1002/art.1780381217. [DOI] [PubMed] [Google Scholar]
- 20.Rascu A, Repp R, Westerdaal Na, Kalden JR, van de Winkel JG. Clinical relevance of Fc gamma receptor polymorphisms. Ann N Y Acad Sci. 1997;815:282–295. doi: 10.1111/j.1749-6632.1997.tb52070.x. [DOI] [PubMed] [Google Scholar]
- 21.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–2527. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 22.Chu SY, Horton HM, Pong E, Leung IW, Chen H, Nguyen DH, Bautista C, Muchhal US, Bernett MJ, Moore GL, Szymkowski DE, Desjarlais JR. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcgammaRIIb with Fc-engineered antibody. J Allergy Clin Immunol. 2012;129:1102–1115. doi: 10.1016/j.jaci.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics. 2011;63:351–362. doi: 10.1007/s00251-011-0514-z. [DOI] [PubMed] [Google Scholar]
- 25.Rogers KA, Scinicariello F, Attanasio R. Identification and characterization of macaque CD89 (immunoglobulin A Fc receptor) Immunology. 2004;113:178–186. doi: 10.1111/j.1365-2567.2004.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers KA, Scinicariello F, Attanasio R. IgG Fc receptor III homologues in nonhuman primate species: genetic characterization and ligand interactions. J Immunol. 2006;177:3848–3856. doi: 10.4049/jimmunol.177.6.3848. [DOI] [PubMed] [Google Scholar]
- 27.Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol. 2012;188:4405–4411. doi: 10.4049/jimmunol.1200090. [DOI] [PubMed] [Google Scholar]
- 28.Chapman K, Pullen N, Graham M, Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat Rev Drug Discov. 2007;6:120–126. doi: 10.1038/nrd2242. [DOI] [PubMed] [Google Scholar]
- 29.Chapman KL, Andrews L, Bajramovic JJ, Baldrick P, Black LE, Bowman CJ, Buckley LA, Coney LA, Couch J, Maggie Dempster A, de Haan L, Jones K, Pullen N, de Boer AS, Sims J, Ian Ragan C. The design of chronic toxicology studies of monoclonal antibodies: implications for the reduction in use of non-human primates. Regul Toxicol Pharmacol. 2012;62:347–354. doi: 10.1016/j.yrtph.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Jaworski JP, Kobie J, Brower Z, Malherbe DC, Landucci G, Sutton WF, Guo B, Reed JS, Leon EJ, Engelmann F, Zheng B, Legasse A, Park B, Dickerson M, Lewis AD, Colgin LM, Axthelm M, Messaoudi I, Sacha JB, Burton DR, Forthal DN, Hessell AJ, Haigwood NL. Neutralizing Polyclonal IgG Present during Acute Infection Prevents Rapid Disease Onset in Simian-Human Immunodeficiency Virus SHIVSF162P3-Infected Infant Rhesus Macaques. J Virol. 2013;87:10447–10459. doi: 10.1128/JVI.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joag SV, Li Z, Wang C, Foresman L, Jia F, Stephens EB, Zhuge W, Narayan O. Passively administered neutralizing serum that protected macaques against infection with parenterally inoculated pathogenic simian-human immunodeficiency virus failed to protect against mucosally inoculated virus. AIDS Res Hum Retroviruses. 1999;15:391–394. doi: 10.1089/088922299311367. [DOI] [PubMed] [Google Scholar]
- 32.Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenchley JM. Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunol. 2013;00:1–9. doi: 10.1038/mi.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widjaja S, Winoto I, Sturgis J, Maroef CN, Listiyaningsih E, Tan R, Pamungkas J, Blair PJ, Sajuthi D, Porter KR. Macaca nemestrina and Dengue Virus Infectivity: a Potential Model for Evaluating Dengue Vaccine Candidates. Micobiol Indones. 2010;4:49–54. [Google Scholar]
- 35.Reece JC, Alcantara S, Gooneratne S, Jegaskanda S, Amaresena T, Fernandez CS, Laurie K, Hurt A, O'Connor SL, Harris M, Petravic J, Martyushev A, Grimm A, Davenport MP, Stambas J, De Rose R, Kent SJ. Trivalent live attenuated influenza-simian immunodeficiency virus vaccines: efficacy and evolution of cytotoxic T lymphocyte escape in macaques. J Virol. 2013;87:4146–4160. doi: 10.1128/JVI.02645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reece JC, Loh L, Alcantara S, Fernandez CS, Stambas J, Sexton A, De Rose R, Petravic J, Davenport MP, Kent SJ. Timing of immune escape linked to success or failure of vaccination. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wines BD, Trist HM, Monteiro RC, Van Kooten C, Hogarth PM. Fc receptor gamma chain residues at the interface of the cytoplasmic and transmembrane domains affect association with FcalphaRI, surface expression, and function. J Biol Chem. 2004;279:26339–26345. doi: 10.1074/jbc.M403684200. [DOI] [PubMed] [Google Scholar]
- 38.Stuart SG, Simister NE, Clarkson SB, Kacinski BM, Shapiro M, Mellman I. Human IgG Fc receptor (hFcRII; CD32) exists as multiple isoforms in macrophages, lymphocytes and IgG-transporting placental epithelium. The EMBO journal. 1989;8:3657–3666. doi: 10.1002/j.1460-2075.1989.tb08540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell MS, Barnes NC, Bradford TM, Musgrave IF, Wines BD, Cambier JC, Hogarth PM. Alteration of the Fc gamma RIIa dimer interface affects receptor signaling but not ligand binding. J Immunol. 2006;176:7489–7494. doi: 10.4049/jimmunol.176.12.7489. [DOI] [PubMed] [Google Scholar]
- 40.Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, Scott AM, Hogarth PM. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol. 2011;187:3208–3217. doi: 10.4049/jimmunol.1101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hibbs ML, Bonadonna L, Scott BM, McKenzie IF, Hogarth PM. Molecular cloning of a human immunoglobulin G Fc receptor. Proc Natl Acad Sci U S A. 1988;85:2240–2244. doi: 10.1073/pnas.85.7.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ierino FL, Hulett MD, McKenzie IF, Hogarth PM. Mapping epitopes of human Fc gamma RII (CDw32) with monoclonal antibodies and recombinant receptors. J Immunol. 1993;150:1794–1803. [PubMed] [Google Scholar]
- 43.Looney RJ, Abraham GN, Anderson CL. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986;136:1641–1647. [PubMed] [Google Scholar]
- 44.Bruhns P, Iannascoli B, England P, Mancardi Da, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 45.Choi EI, Wang R, Peterson L, Letvin NL, Reimann KA. Use of an anti-CD16 antibody for in vivo depletion of natural killer cells in rhesus macaques. Immunology. 2008;124:215–222. doi: 10.1111/j.1365-2567.2007.02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 47.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Bohne-Lang A, von der Lieth CW. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res. 2005;33:W214–219. doi: 10.1093/nar/gki385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hulett MD, Witort E, Brinkworth RI, McKenzie IF, Hogarth PM. Multiple regions of human Fc gamma RII (CD32) contribute to the binding of IgG. J Biol Chem. 1995;270:21188–21194. doi: 10.1074/jbc.270.36.21188. [DOI] [PubMed] [Google Scholar]
- 50.Maxwell KF, Powell MS, Hulett MD, Barton PA, McKenzie IF, Garrett TP, Hogarth PM. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat Struct Biol. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 51.Hulett MD, McKenzie IF, Hogarth PM. Chimeric Fc receptors identify immunoglobulin-binding regions in human Fc gamma RII and Fc epsilon RI. Eur J Immunol. 1993;23:640–645. doi: 10.1002/eji.1830230310. [DOI] [PubMed] [Google Scholar]
- 52.Hulett MD, Witort E, Brinkworth RI, McKenzie IF, Hogarth PM. Identification of the IgG binding site of the human low affinity receptor for IgG Fc gamma RII. Enhancement and ablation of binding by site-directed mutagenesis. J Biol Chem. 1994;269:15287–15293. [PubMed] [Google Scholar]
- 53.Syam S, Mero P, Pham T, McIntosh CA, Bruhns P, Booth JW. Differential recruitment of activating and inhibitory Fc gamma RII during phagocytosis. J Immunol. 2010;184:2966–2973. doi: 10.4049/jimmunol.0900016. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen FW, Padaki R, Morris AE, Aldrich TL, Armitage RJ, Allen MJ, Lavallee JC, Arora T. Molecular and functional characterization of cynomolgus monkey IgG subclasses. J Immunol. 2011;186:341–349. doi: 10.4049/jimmunol.1001685. [DOI] [PubMed] [Google Scholar]
- 55.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Vijh S, Johnson S, Bonvini E, Koenig S. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 56.Chu SY, Desjarlais JohnR, Karki SherBahadur, Lazar GregoryAlan, Moore GregoryL, Vostiar Igor. Methods and compositions for inhibiting CD32B expressing cells. Xencor, Inc; (Monrovia, CA, US), United States: 2011. [Google Scholar]
- 57.Boonnak K, Slike BM, Donofrio GC, Marovich MA. Human FcgammaRII cytoplasmic domains differentially influence antibody-mediated dengue virus infection. J Immunol. 2013;190:5659–5665. doi: 10.4049/jimmunol.1203052. [DOI] [PMC free article] [PubMed] [Google Scholar]


