Abstract
Olfactory learning in blood-feeding insects, such as mosquitoes, could play an important role in host preference and disease transmission. However, standardised protocols allowing testing of their learning abilities are currently lacking, and how different olfactory stimuli are learned by these insects remains unknown. Using a Pavlovian conditioning paradigm, we trained individuals and groups of Aedes aegypti mosquitoes to associate an odorant conditioned stimulus (CS) with a blood-reinforced thermal stimulus (unconditioned stimulus; US). Results showed, first, that mosquitoes could learn the association between L-lactic acid and the US, and retained the association for at least 24 h. Second, the success of olfactory conditioning was dependent upon the CS – some odorants that elicited indifferent responses in naïve mosquitoes, such as L-lactic acid and 1-octen-3-ol, were readily learned, whereas others went from aversive to attractive after training (Z-3-hexen-1-ol) or were untrainable (β-myrcene and benzyl alcohol). Third, we examined whether mosquitoes' ability to learn could interfere with the action of the insect repellent DEET. Results demonstrated that pre-exposure and the presence of DEET in the CS reduced the aversive effects of DEET. Last, the nature of the formed memories was explored. Experiments using cold-shock treatments within the first 6 h post-training (for testing anaesthesia-resistant memory) and a protein synthesis inhibitor (cycloheximide; to disrupt the formation of long-term memory) both affected mosquitoes' performances. Together, these results show that learning is a crucial component in odour responses in A. aegypti, and provide the first evidence for the functional role of different memory traces in these responses.
KEY WORDS: Olfactory learning, Long-term memory, Appetitive conditioning, Disease vector, Aedes aegypti
INTRODUCTION
A variety of processes, both ecological and physiological, mediate the ability of mosquitoes to transmit vector-borne diseases to their vertebrate hosts. A key factor controlling the mosquito–host interaction is the ability for mosquitoes to accurately identify their hosts using several sensory modalities, one of which, olfaction, is a key mediator of host attraction in blood-feeding insects in general (Lehane, 2005; Lazzari, 2009; Guerenstein and Lazzari, 2009). The olfactory system can mediate innate responses to hosts (Bock and Cardew, 1996; Clements, 1999), but these responses can also be modulated by ecological factors such as vector density or host defensive behaviour (Kelly and Thompson, 2000), and physiological factors including internal- and circadian-state-dependent effects (e.g. Trpis et al., 1973; Klowden, 1990). In addition, it has been largely acknowledged that cognitive abilities, and more precisely the ability to learn and retain information, have an epidemiological impact by modulating the way disease vectors will respond to host-associated cues (McCall and Kelly, 2002; Alonso and Schuck-Paim, 2006).
In terms of disease transmission, one important step that learning and memory could affect is the process of host selection. Mosquitoes do not equally bite all hosts, even in the same population (Kelly, 2001). Differences in odour profiles between individuals could explain in part this heterogeneous biting behaviour, but would it be sufficient to state that, as suggested by Woolhouse et al. (Woolhouse et al., 1997), only 20% of a host population is responsible for 80% of the transmission potential? The ability to learn to select a subset of preferred hosts could also contribute to the heterogeneous distribution of vectors amongst host populations (Kelly and Thompson, 2000). One can expect that it would indeed confer an adaptive advantage to mosquitoes to be able to learn and remember the cues associated with the best hosts (e.g. the easiest to feed on) beause hosts play the double role of prey and predator (Lazzari, 2009). Mosquitoes could thus benefit from their past foraging experience to determine which signals to avoid, as well as which ones to follow, during subsequent host-seeking episodes (McCall and Kelly, 2002).
During the last decade or so, increasing attention has been devoted towards unravelling the learning abilities of haematophagous insects, including mosquitoes (Mwandawiro et al., 2000; McCall and Eaton, 2001; McCall et al., 2001; McCall and Kelly, 2002; Kaur et al., 2003; Alonso and Schuck-Paim, 2006; Tomberlin et al., 2006; Bouyer et al., 2007; Vinauger et al., 2011a; Vinauger et al., 2011b; Vinauger et al., 2012; Vinauger et al., 2013; Chilaka et al., 2012; Menda et al., 2013). Regarding mosquitoes, a handful of studies have provided direct or indirect evidence suggesting that learning abilities could be involved in host preference, choice of oviposition sites, and home range [for a detailed review, see references above and McCall and Kelly (McCall and Kelly, 2002) and Menda et al. (Menda et al., 2013)]. While indicative of the potential for learning, these studies lacked precise temporal and quantitative control of the conditioned and unconditioned stimuli when training groups of individuals, and therefore do not allow further investigation of the mechanisms underlying these cognitive abilities (Rescorla, 1988).
In this study, we examine the odour-learning abilities of the yellow fever and dengue vector mosquito, Aedes aegypti (Linnaeus 1762), under controlled laboratory conditions to provide insights into how their cognitive abilities could mediate host selection. Adapting an olfactory conditioning procedure developed in the blood-sucking bug Rhodnius prolixus (Vinauger et al., 2011a) and determining the time frame in which A. aegypti mosquitoes were in the proper physiological state and activity level for olfactory conditioning, we were able to ask: are individual mosquitoes able to associate a human-related odour (L-lactic acid) with a blood reward, and how do those responses compare with innately attractive stimuli (e.g. carbon dioxide); do all odorants (host- and non-host related) have the same valences in the mosquitoes' ability to learn their association with a blood meal; and can we manipulate the formation of this memory in order to characterise it?
RESULTS
Individual training
To train mosquitoes, the presentation of an odorant [conditioned stimulus (CS)] was paired with a blood reward (appetitive conditioning), using heat as unconditioned stimulus (US) to evoke attraction and blood-feeding responses (Fig. 1A). Twenty-four hours after training, the mosquitoes were tested in a Y-maze olfactometer in which they were given the choice between two stimuli (Fig. 1B) (see Materials and methods for details).
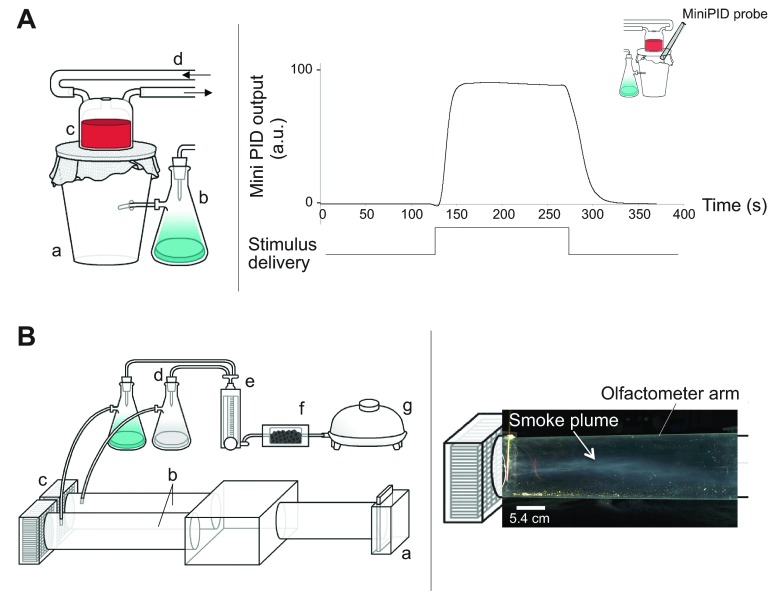
Fig. 1.
Illustration of methods for olfactory training and testing. (A) Left: artificial feeder used in the appetitive conditioning procedure. It allows the pairing of the presentation of an odorant [conditioned stimulus (CS)] with a heated [unconditioned stimulus (US)] blood reward (associative conditioning). a, Plastic insect container; b, glass bottle containing the CS, connected to an air pump via silicone tubing; c, glass artificial feeder; d, silicone tubing conducting warm water (37°C) from a water bath to the artificial feeder. Right: miniaturized photoionization detector (miniPID) characterisation of the stimulus delivery, using a low molecular weight volatile (ethanol) as a tracer. The probe of the miniPID was positioned at the junction between the central box and the olfactometer arms. (B) Left: olfactometer designed for the analysis of the olfactory orientation of mosquitoes. a, Releasing chamber; b, decision arms of the olfactometer; c, computer fans, filters and screens; d, glass bottles containing either the CS or the solvent-control solution; e, flowmeter equipped with a valve needle; f, charcoal filter; g, air pump. Right: visualisation of the stimulus plume structure using a smoke plume. The series of flow straighteners and mesh screens in the olfactometer provided a unidirectional airflow that rapidly created a filamentous plume when the odour left the nozzle from the odour line. For all experiments, the air speed of the stimulus line represented 25% of the air velocity generated by the olfactometer fans.
After being released in the starting chamber of the olfactometer (Fig. 1B), individual mosquitoes displayed different behavioural responses according to their respective training experience (Figs 2, 3). In the absence of odours, naïve untrained females chose randomly between the two choice arms of the olfactometer [clean air group: 52.95% in one arm and 47.05% in the other; preference index (PI)=0.06; binomial exact test, P=0.86], revealing no bias in the maze or in the experimental room. But when confronted with a clean air current versus a current loaded with CO2, naïve mosquitoes preferred the arm delivering the CO2 (CO2 group: 70.27%; PI=0.41; binomial exact test, P=0.02). However, naïve mosquitoes did not display any preference when confronted with a clean air current versus an air current loaded with L-(+)-lactic acid (LA) (LA group: 54.29% towards LA and 45.71% towards clean air; PI=0.08; binomial exact test, P=0.74; Fig. 3). This contrasts with the previously shown slight attraction of mosquitoes to LA (Geier and Boeckh, 1999) but not with the observations made in Anopheles gambiae (Dekker et al., 2002) and more recently in A. aegypti (McMeniman et al., 2014), where LA had a synergistic effect on the attractiveness of CO2, skin odours and skin-rubbing extracts from humans and other vertebrates.
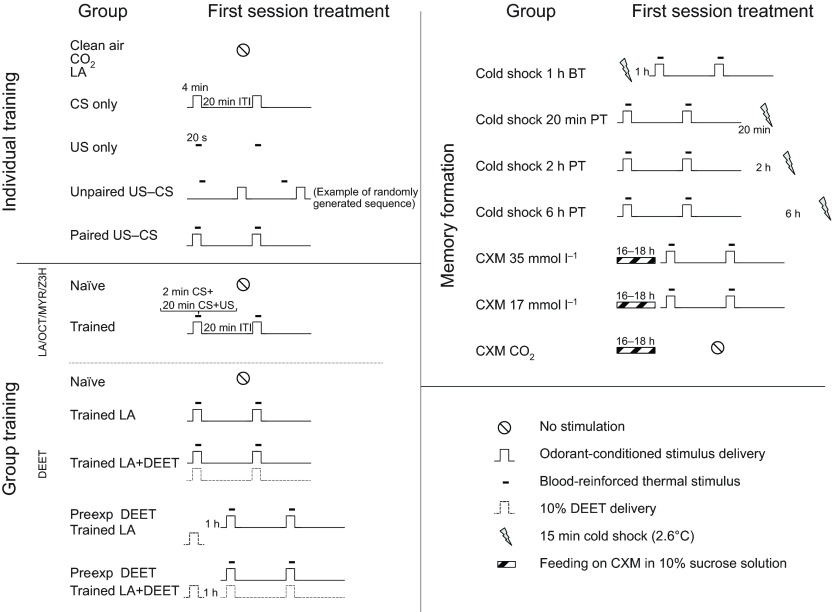
Fig. 2.
Sequence of event delivery [i.e. US, CS and inter-trial interval (ITI)] during training sessions of the different experimental groups. BT, before training; CXM, cycloheximide; DEET, N,N-diethyl-meta-toluamide; LA, l-lactic acid; MYR, myrcene; OCT, 1-octen-3-ol; Pre-exp, pre-exposed; PT, post training; Z3H, Z-3hexen-1-ol.
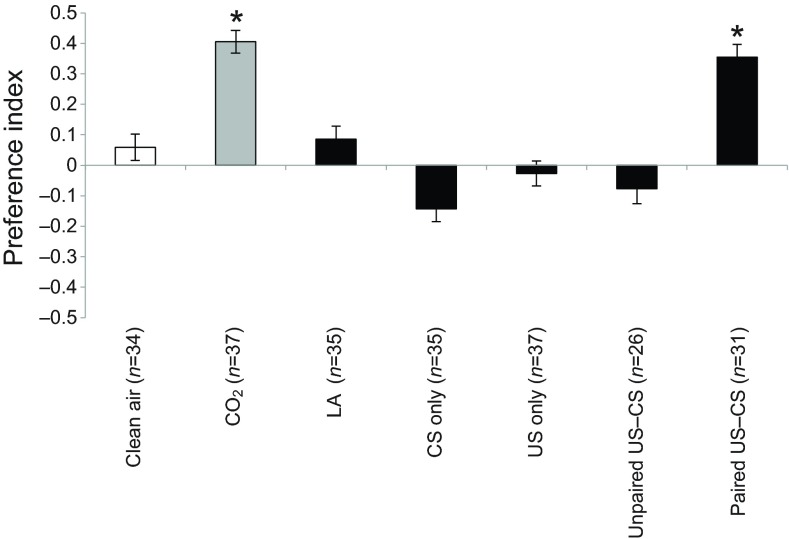
Fig. 3.
Preference of individually trained Aedes aegypti females. Female mosquitoes were individually tested in the olfactometer and given a choice between two stimuli: clean air versus air loaded with either clean air (white bar), CO2 (2300 ppm above ambient level; grey bar) or l-lactic acid (black bars). Preference is represented as the preference index computed from the distribution of insects in the olfactometer, and error bars represent the standard errors of the binary distribution. Each bar represents an experimental group: clean air, neutral control group; CO2, positive control group; LA, l-lactic acid naïve control group; CS only, CS-only group; US only, US-only group; unpaired US–CS, unpaired US–CS group; paired US–CS, appetitive-conditioning group. Asterisks indicate distributions that are significantly different from random (P<0.05).
Two control groups were pre-exposed to the CS or the US during the first session. The CS-only group was pre-exposed to LA and to the same manipulations and experimental context (i.e. setup, containers, etc.) as trained insects except that they were not fed and not exposed to heat (US). When tested in the olfactometer, females of the CS-only group did not display a preference for either arm of the olfactometer (LA, 42.86%; clean air, 57.14%; PI=−0.14; binomial exact test, P=0.49). In other words, pre-exposure to LA was not responsible for any significant change in subsequent behavioural response to LA. The mosquitoes of the US-only group were pre-exposed to heat, fed on two blood meals and were manipulated in the same way as trained insects, except that they were not exposed to LA during the first session. When tested in the olfactometer, they displayed a random distribution as well (LA, 48.65%; clean air, 51.35%; PI=−0.03; binomial exact test, P=0.62), revealing no effect of blood ingestion on the behavioural response to LA.
In an additional control group, the unpaired US–CS group, the US and the CS were delivered in a random order and without contingency during the training session (Rescorla, 1988). Mosquitoes submitted to this training procedure also displayed a random distribution during the test (LA, 46.15%; clean air, 53.85%; PI=−0.08; binomial exact test, P=0.84). Thus no cumulative effect of US and CS presentations was observed in absence of contingency.
For the last group of mosquitoes, females were individually exposed to the contingency of LA and heat-induced blood meal. The majority of mosquitoes belonging to this group chose the arm delivering the LA-loaded current (67.74%; PI=0.35; binomial exact test, P=0.03), revealing a clear learned preference for LA, 24 h after the training procedure.
Group training
If individual training provided control on the experimental conditions, group training experiments could offer a more rapid way to train mosquitoes to different odorants, allowing the assessment of their valences as CS. Thus, in addition to the individual-trained experiments, we adapted the conditioning procedure to groups of individuals, which allowed rapid training and efficient testing of different olfactory stimuli as CS. When trained in groups and tested individually to the same concentration of LA, mosquitoes displayed similar learning performances as individually trained insects, and significantly preferred LA (LA: 67.8%; PI=0.35; binomial exact test: P=0.04; Fig. 4). In addition, naïve insects that experienced a group situation displayed indifference to LA (LA: 44%; PI=-0.11; binomial exact test: P=0.70), similarly to individually maintained animals. However, when trained to other odorants, their performances were dependent on the nature of the CS. For example, mosquitoes trained to 1-octen-3-ol (OCT) – an odorant emitted from human skin and plants and flowers (Cork and Park, 1996) – were able to develop a positive attraction to the odorant, as demonstrated by their preference for the odorant side of the olfactometer during tests (OCT: 65%; PI=0.30; binomial exact test: P=0.04). The corresponding naïve control group showed no preference for the odour (OCT: 56%; PI=0.13; binomial exact test: P=0.67; Fig. 4), suggesting that mosquitoes showed no innate preference for this odorant.
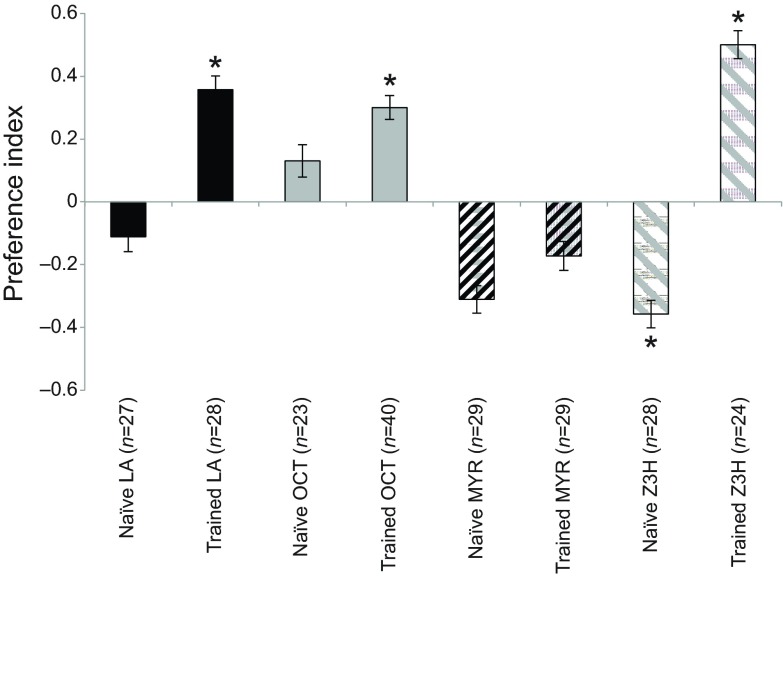
Fig. 4.
Preference of group-trained and individually tested A. aegypti females to different odorants. Once placed in the olfactometer, mosquitoes were given a choice between two stimuli: clean air versus air loaded with: LA (black bars), OCT (grey bars), MYR (black hatching on grey bars) or Z3H (grey hatching on white bars). Preference is represented as the preference index computed from the distribution of insects in the olfactometer, and error bars represent the standard errors of the binary distribution. Each bar represents an experimental group: naïve, untrained groups; trained, appetitive-conditioning groups. Asterisks indicate distributions that are significantly different from random (P<0.05).
However, other odorants elicited different behavioural responses. For example, groups of mosquitoes were unable to learn to associate β-myrcene (MYR) with the prospect of obtaining a blood meal (trained MYR: 41.3%; PI=−0.17; binomial exact test: P=0.22; naïve MYR: 34.4%; PI=−0.31; binomial exact test: P=0.07). This volatile can be found in several fruits (Andrade et al., 2000), including Zanthoxylum piperitum fruit oil, which tends to be a repellent for naïve A. aegypti (naïve group: 34.5% MYR; PI=−0.31; binomial exact test: P=0.06) (see also Choochote et al., 2007). In other group-trained/group-tested assays, similar results were found when testing other plant-emitted odorants (benzyl alcohol and nonanol; supplementary material Fig. S2). For these odorants, the group-trained mosquitoes did not show a preference, or the odorants tended to elicit a seeming aversive response (supplementary material Fig. S2).
For the last group-trained/individually tested experiment, mosquitoes were trained with Z-3hexen-1-ol (Z3H), an odorant emitted from leaves and flowers of some plants (Reddy et al., 2002). We observed that mosquitoes were able to associate Z3H with the blood reward (Z3H: 75%; PI=0.50; binomial exact test: P=0.01), despite the fact that this odorant was also repellent for mosquitoes of the corresponding naïve control group (Z3H: 32%; PI=−0.35; binomial exact test: P=0.04).
Effect of learning on DEET repellency
If group-trained mosquitoes can associate aversive odours with a blood meal and thus be attracted to this odour during subsequent encounters, one can wonder whether mosquitoes can learn to respond positively to commonly used insect repellents. DEET (N,N-diethyl-meta-toluamide) is a strong aversive odorant for mosquitoes, but the presence of DEET in combination with a learned odorant may alter the percept of the CS, or mask the odorant (Syed and Leal, 2008). Moreover, recent work has shown that repeated DEET exposure lessens the aversion (Stanczyk et al., 2013). We thus performed a series of experiments testing the effects of DEET using pre-exposed, DEET-trained and DEET-naïve animals (Fig. 2).
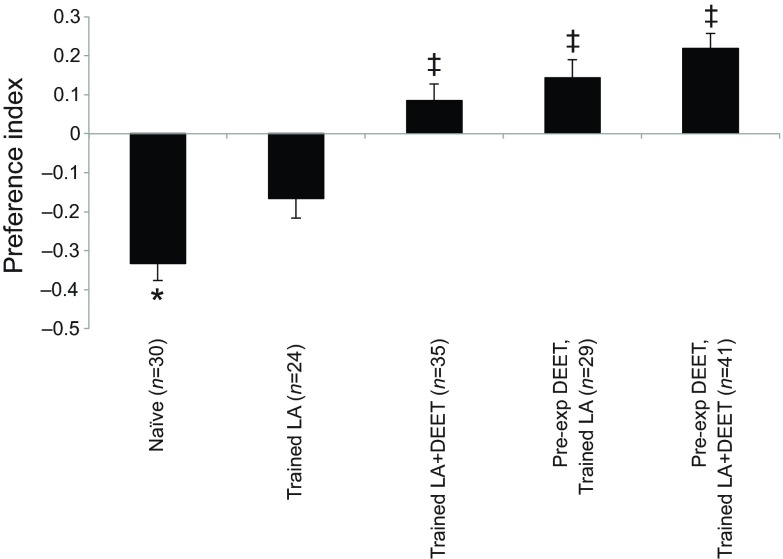
Given the choice between a clean air stream and an air stream loaded with LA+DEET, naïve mosquitoes significantly preferred the arm delivering clean air over the LA+DEET arm (LA+DEET: 33%; PI=−0.33; binomial exact test: P=0.049; Fig. 5). When group-trained with LA only, mosquitoes did not display a preference for LA in the presence of DEET (LA+DEET: 41.6%; PI=−0.16; binomial exact test: P=0.84). Similarly, training mosquitoes to the mixture of LA+DEET did not result in a distribution significantly different from a random one (LA+DEET: 54.2%; PI=0.08; binomial exact test: P=0.36).
Fig. 5.
Preference of group-trained and individually tested A. aegypti females to LA and DEET. Once placed in the olfactometer, mosquitoes were given a choice between two stimuli: clean air versus air loaded with a mixture of LA and 10% DEET. Preference is represented as the preference index computed from the distribution of insects in the olfactometer, and error bars represent the standard errors of the binary distribution. Each bar represents an experimental group: naïve, untrained groups; trained LA, trained with LA only; trained LA+DEET, trained with LA plus DEET; pre-exp DEET, trained LA, pre-exposed to DEET 1 h before training and then trained to LA only; pre-exp DEET, trained LA+DEET, pre-exposed to DEET 1 h before training and then trained to LA+DEET. Asterisks indicate distributions that are significantly different from random (P<0.05), and double daggers indicate distributions that are significantly different from the distribution of naïve, untrained insects when tested for their response to LA+DEET (P<0.05).
When pre-exposed to DEET 1 h before being trained to LA only, insects still displayed a random distribution in the olfactometer during the test (LA+DEET: 58.6%; PI=0.14; binomial exact test: P=0.22; Fig. 5). Similarly, the distribution of mosquitoes pre-exposed to DEET 1 h before being trained to LA+DEET was not significantly different from random (LA+DEET: 60.9%; PI=0.21; binomial exact test: P=0.11).
Pre-exposure and training to DEET did not elicit distributions significantly different from random; nonetheless, these treatment groups exhibited responses that were markedly different from the naïve mosquitoes. We thus compared the distribution of the different treatment groups with the distribution of naïve females tested for their preference between LA+DEET and clean air. The analysis revealed an effect of training on mosquitoes' responses when they were trained to LA+DEET (binomial exact test: P=0.01), pre-exposed to DEET and trained to LA (binomial exact test: P=0.005), or pre-exposed to DEET and trained to LA+DEET (binomial exact test: P=0.0003). Only the group trained to LA alone displayed a distribution that was not significantly different from the naïve group (binomial exact test: P=0.24). Thus, pre-exposure and the presence of DEET in the CS significantly reduced the aversive effects of DEET even if no significant attraction was induced by the training procedure.
Memory characterisation
The olfactory memory exhibited by mosquitoes 24 h after training may be due to two types of longer-term memory, including anaesthesia-resistant memory (ARM) and long-term memory (LTM). ARM is formed after multiple consecutive training trials, or massed training (repeated training sessions without a rest interval), and is not disrupted by cold-induced anaesthesia but insensitive to the protein synthesis inhibitor cycloheximide (CXM). Conversely, LTM is formed after spaced training and is sensitive to CXM (for a review, see Tully et al., 1994). In order to assess the nature of the formed memory, individual mosquitoes were subjected to either a cold-shock treatment (for testing ARM) or fed on CXM (for testing LTM) (Fig. 2).
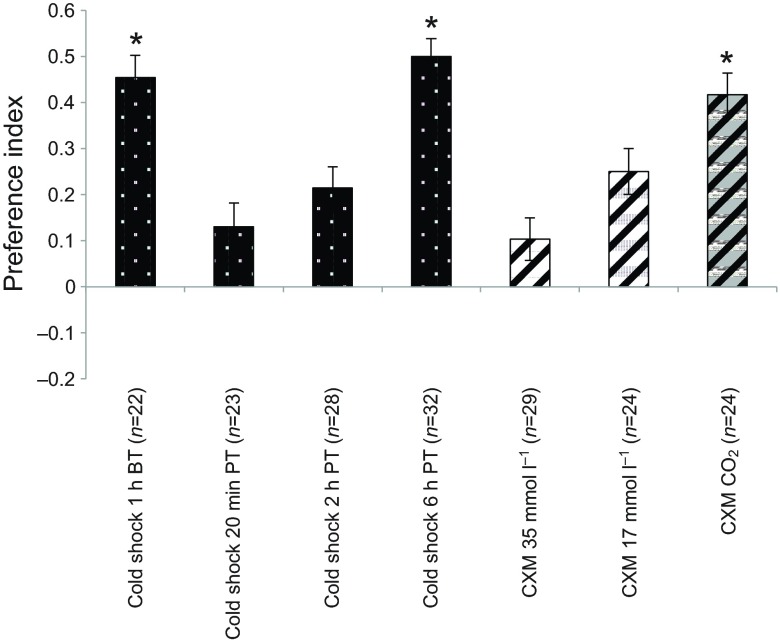
Individual mosquitoes were submitted to cold shock anaesthesia either 1 h before training (a control for the cold-shock treatment) or 20 min, 2 h or 6 h post-training and tested 24 h post-training in all cases (Fig. 6). When submitted to the cold shock 1 h before training, mosquitoes preferred LA (72.2%; PI=0.45; binomial exact test, P=0.02), indicating that prior hypothermic exposure did not affect their ability to learn the association between the CS and the US. However, when submitted to hypothermic anaesthesia either 20 min or 2 h post-training, their distribution was no longer significantly different from random (LA arm: 56.5%, PI=0.13 and 60.7%, PI=0.21, respectively; binomial exact test, P=0.33 and P=0.17, respectively). By contrast, when cold anaesthetised 6 h post-training, trained females clearly chose LA during the test (LA: 75%; PI=0.5; binomial exact test: P=0.003), suggesting the late formation of an anaesthesia-resistant form of memory.
Fig. 6.
Preference of individually trained A. aegypti females that were individually tested in the olfactometer and given a choice between two stimuli. Insects exposed to cold shock (white circles on black bars) were tested for their preference between clean air and air loaded with LA. Insects fed on CXM were tested for their preference between clean air and LA (black hatching on white bars) or between clean air and CO2 (2300 ppm above ambient level; black hatching on grey bar). Preference is represented as the preference index computed from the distribution of insects in the olfactometer, and error bars represent the standard errors of the binary distribution. Each bar represents an experimental group: cold shock 1 h BT, trained insects exposed to a cold shock 1 h before training; cold shock 20 min PT, trained insects exposed to a cold shock 20 min post training; cold shock 2 h PT, trained insects exposed to a cold shock 2 h post training; cold shock 6 h PT, trained insects exposed to a cold shock 6 h post training; CXM 35 mmol l−1, trained insects fed on 35 mmol l−1 CXM in 10% sucrose solution during 16–18 h previous to the training session; CXM 17 mmol l−1, trained insects fed on 17 mmol l−1 CXM in 10% sucrose solution during 16–18 h previous to the training session; 35 mmol l−1 CXM CO2; naïve insects fed on 35 mmol l−1 CXM in 10% sucrose solution, and tested for their response to CO2 as a positive control. Asterisks indicate distributions that are significantly different from random (P<0.05).
Prior to determining the effects of CXM on the memory of mosquitoes, we performed a series of experiments to determine whether CXM had non-memory-related effects on mosquitoes, such as altering their innate attraction to carbon dioxide or their flight behaviour. Mosquitoes that were fed the CXM and subsequently tested in the olfactometer for their preference for carbon dioxide or clean air showed a clear preference for the CO2 (CO2: 70.8%; PI=0.42; binomial exact test: P=0.03), thus revealing that CXM-treated mosquitoes were still able to perceive odours and display an oriented response when stimulated with an innately attractive odorant. To determine whether CXM also affected mosquitoes' normal flight behaviours and odour-evoked responses, additional groups were tested in the olfactometer while their flight behaviour was recorded (supplementary material Fig. S3). The analysis revealed that sucrose-fed and CXM-fed trained mosquitoes had similar flight speeds [24.84±1.22 and 21.14±1.45 cm s−1 for sucrose- and CXM-fed mosquitoes, respectively (means ± s.e.m.); Student's t-test: P=0.064; supplementary material Fig. S4). Moreover, no significant differences existed in the flight speeds of trained versus naïve untreated mosquitoes (24.84±1.22 and 25.35±2.58 cm s−1, respectively; Student's t-test: P=0.85). Finally, the activity level and motivation to leave the starting chamber and choose between the two choice arms of CXM-treated females was similar to those of untreated mosquitoes (on average 52.3% for CXM treated and 51.8% for untreated females).
To assess the LTM formation of trained mosquitoes, females were fed on 10% sucrose supplemented with CXM at 35 mmol l−1 and subsequently trained to LA (Fig. 6). Results from these experiments showed that performance was impaired when tested 24 h post-training (LA: 55.2%; PI=0.10; binomial exact test: P=0.35). When the concentration of CXM was reduced by half (17 mmol l−1) to reduce its potential non-memory-specific effects, performance of trained mosquitoes was also impaired when tested 24 h post-training (LA: 62.5%; PI=0.25; binomial exact test: P=0.15).
DISCUSSION
In this study, we investigated the ability of A. aegypti mosquitoes to learn to associate different olfactory stimuli with the prospect of obtaining a blood meal. Three distinct questions were addressed in the present work: at the individual scale, are mosquitoes able to perform true Pavlovian conditioning; are different odorants equivalent in terms of CS and, in that context, are mosquitoes able to associate repellent odorants with appetitive cues; and finally, what forms of memory contribute to the performance of trained mosquitoes?
In the first part of the present work, we showed that classical, or Pavlovian, conditioning procedures previously adapted from classical models to haematophagous insects (Vinauger et al., 2011a) could be used to train individual mosquitoes to respond positively to a previously neutral odour (LA). Different control treatments allowed us to discard alternative hypotheses (e.g. sensory adaptation, motor fatigue and sensitisation), by controlling for any potential effect of the CS or US acting alone or together but in the absence of contingency and thus revealing true associative learning. In addition, individual training experiments revealed that only two feeding experiences are sufficient for A. aegypti to form new memories that persist for at least 24 h. In their natural environment, some individuals will fully engorge in a single blood meal and only return to feeding after developing and laying their eggs, while others will only partially feed as a result from host defensive behaviour (Klowden and Lea, 1978). In this context, forming memory of what have been successful or unsuccessful feeding events appears to be highly adaptive for mosquitoes.
In the second part of this work, we adapted the conditioning procedure to groups of individuals. While individual training offers better control of the experimental conditions, results of group training experiments to LA showed that individually tested but group-trained mosquitoes performed similarly to individually trained subjects. In contrast to previously published studies (e.g. Chilaka et al., 2012; Menda et al., 2013) where all the individuals of trained groups were tested simultaneously and without distinction of trained versus untrained animals in the same cohort, our experimental procedures allowed us to discard unfed females, thus only testing individuals that were actually subjected to the temporal contingency between US and CS during training.
Results of these experiments also revealed that different odorants did not have the same value to be used as potential CSs. Some odours that were not innately attractant (LA, OCT) could be associated with the possibility of obtaining a blood meal, while an innately aversive odour (MYR) could not. Interestingly, mosquitoes learnt the innately aversive odorant Z3H as a food predictor in spite of its repulsive nature for naïve individuals. This result raised the following question: because aversive volatiles, such as DEET, are commonly used as insect repellents, to what extent would their efficacy be affected by the learning abilities of mosquitoes? The efficiency of DEET, the most commonly employed insect repellent (Fradin, 1998), relies on its ability to either ‘jam’ an insect's olfactory perception (Bohbot and Dickens, 2010; Pellegrino et al., 2011) or trigger aversive behavioural responses (Syed and Leal, 2008; Liu et al., 2010). Its efficiency in repelling mosquitoes was recently shown to be reduced in A. aegypti and Rhodnius prolixus shortly after previous exposure (Sfara et al., 2011; Stanczyk et al., 2013). If non-associative phenomena can impair DEET efficacy, it seems legitimate to wonder whether mosquitoes' abilities to perform associative learning could also interfere with DEET efficiency.
In all of the performed experiments, mosquitoes' avoidance for DEET could not be switched to attraction. However, when compared with the innate responses of naïve insects, the analysis revealed a significant effect of the treatment for insects that were trained, pre-exposed or both trained and pre-exposed to DEET. For these groups, the innate aversion switched to random behavioural responses, i.e. indifference, 24 h post training. These results suggest that pre-exposure to DEET indeed induces a decreased repellence of A. aegypti, thus confirming results obtained by Stanczyk et al. (Stanczyk et al., 2013), and seem to indicate that learning can also play a role in decreasing DEET repellence (see LA+DEET trained group; Fig. 5).
In all of these experiments, the observed memory was retained for at least 24 h. In fruit flies, the memory observed 24 h post training is from the combination of genetically distinct and functionally independent memory components: ARM and LTM (Tully et al., 1994; Tully et al., 2003; Margulies et al., 2005). When focusing on memory in fruit flies, Tully et al. (Tully et al., 1994) showed that: (1) anaesthesia-sensitive memory could be disrupted by cold anaesthesia, (2) ARM was resistant to hypothermic disruption and insensitive to protein synthesis inhibitor and (3) LTM could be disrupted by ingestion of CXM. By applying similar memory-disrupting treatments (cold shock and CXM) at different times before and after training, we were able to shed light on the consolidated nature of the formed mnesic trace in mosquitoes subjected to a two-trial training procedure. Results revealed the progressive consolidation of a form of memory that is resistant to cold-induced anaesthesia. Similar results have been observed in fruit flies, where a 2 min cold shock delivered immediately after training significantly impaired flies' performances in a 3 h memory retention test, whereas shock delivered later after training (from 30 min to 2 h) indicated the progressive consolidation of this ARM (Tully et al., 1994). In the present work, mosquitoes that were treated with CXM also showed disrupted performances 24 h post-training. These results could suggest the formation of a CXM-sensitive long-term memory, but future work is needed to definitely characterise the different memory forms on which mosquitoes' performances relied after training.
In addition, we found that two training trials were sufficient for mosquitoes to form a consolidated form of LTM trace. This would actually appear highly adaptive for mosquitoes to be able to remember an experience with a defensive host (or a successful feeding experience) after a limited number of encounters, and to remember these unique experiences in a reliable way, i.e. in the long term and in a consolidated stable way, would then directly contribute to their fitness.
Consequences of learning
In nature, mosquitoes rely on their innate responses to CO2 (e.g. Acree et al., 1968; Dekker et al., 2005) in combination with other host-related odours such as LA and OCT (Takken et al., 1997; Geier and Boeckh, 1999; Dekker et al., 2002). These responses can be modulated by their individual experience, but mosquitoes can also associate odorants that were initially neutral, or non-attractive, with a successful feeding event. As a consequence, host–vector interactions can be modulated by learning, which may underlie the observed heterogeneous distribution of vectors in host populations (Kelly and Thompson, 2000; McCall and Kelly, 2002), leading to high reproductive rates and increases in the transmission of disease in these subpopulations (Dye and Hasibeder, 1986; Hasibeder and Dye, 1988).
Taken together, results from this study provide the first characterisation of consolidated olfactory memory in mosquitoes, and demonstrate that certain odorants have differing valences in their ability to be associated with a blood reward. In addition, as discussed above, these results suggest that mosquitoes' learning ability might also play a crucial role in the efficiency of odorant-based insect repellents. Finally, it is worth mentioning that this is only the third study to investigate associative learning in A. aegypti (Alonso et al., 2003; Menda et al., 2013), despite its potential vectorial importance. This work also sets the bases for future work on mosquitoes that would unravel what stimuli are adequate as CS or US, characterise their learning abilities, and determine the effects of physiological state (i.e. age-, appetitive-, reproductive- and circadian-related effects) on mosquito learning abilities. Last, these methods and results provide strong impetus for identifying the neurobiological substrates and mechanisms of blood host-odour learning.
MATERIALS AND METHODS
Insects
Wild-type A. aegypti (line F21 MRA-726, MR4, ATCC®, Manassas, VA, USA) were used in all of the experiments. Groups of 200 larvae were reared on a diet of Hikari Tropic First Bites (Petco, San Diego, CA, USA) in a 26×35×4cm covered pan containing 1 cm of water, at 25°C, 60±10% relative humidity, and under a photoperiod of 12 h:12 h (light:dark). Adults were transfer into mating cages, maintained on 10% sucrose and blood-fed on weekdays on bovine heparinised blood (Lampire Biological Laboratories, Pipersville, PA, USA), using an artificial feeder (D. E. Lillie Glassblowers, Atlanta, GA, USA; 2.5 cm internal diameter).
For the mosquitoes used in learning bioassays, ~200 same-age animals (both males and females) were separated from the colony at pupation and maintained on 10% sucrose after emergence. Emerged males and females were kept in a collapsible cage (20×20×20 cm; BioQuip Products, Rancho Dominguez, CA, USA) for 6 days to allow for mating to occur (random dissection of females revealed that 95% of them had oocytes present). After this time period, female mosquitoes were either captured individually using a mouth aspirator for individual training, or chilled until immobile at 10°C for group training, and transferred to either individual or group containers (300 ml clear plastic cups, Solo Cup Company, Lake Forest, IL, USA), the tops of which were covered by a piece of fabric mesh. Females were used in experiments the day following their isolation.
Preliminary experiments
In A. aegypti, it has been shown that various behavioural activities follow cyclic patterns (Haddow and Gillett, 1957; McClelland, 1959; Boorman, 1961; Gillett et al., 1962; Jones et al., 1972; Trpis et al., 1973). In order to train mosquitoes during periods of the day at which they are responsive to host-associated cues, and determine the best amount of blood meal that maintained motivational states, a series of preliminary experiments was performed. First, behavioural responses to artificial feeding of 6-day-old starved females were tested at four different times of the mosquitoes' subjective day. The results of these preliminary experiments revealed that mosquitoes displayed higher levels of responses a few hours after the onset of the lights and a few hours before the offset of lights (see supplementary material Fig. S1). Consequently, the experiments presented here were performed during the two activity peaks displayed by female A. aegypti (Trpis et al., 1973). In addition, the volume of blood provided during training is an important component for the learning paradigm – a large enough volume is necessary to provide a reward, but the volume needs to be small enough to maintain a high motivational state in the behaving insect. To assess the ingested volume, we exposed mosquitoes to blood for short durations during training and then compared the mass of females with that of unfed and fully engorged females. For individually and group-trained mosquitoes, the amount of blood ingested during training represents ~38.57% and 72.51% of a full meal, respectively. For cycloheximide (CXM)-treated mosquitoes, the amount of blood ingested during training represents 16.38% of a complete blood meal (i.e. 2.82±0.57 mg; n=20 females).
Experimental apparatus
Artificial feeder
Female mosquitoes were handled in the plastic containers described above and trained 24 h after their isolation. The tops of the containers were covered with a fabric mesh, allowing the insects to fly and access the artificial feeder by landing on the surface of the mesh. The artificial feeder (Fig. 1A) was composed of a glass feeder (D. E. Lillie Glassblowers; 3.8 cm internal diameter, 6 cm height), the bottom of which was sealed with Parafilm®, through which mosquitoes were able to bite. The feeder was filled with 10 ml of bovine heparinized blood (Lampire Biological Laboratories) and connected to a water bath maintaining the temperature of the blood at 36±1°C, which roughly corresponds to human blood temperature. A volatile-delivery system, via a constant, charcoal-filtered airstream (5 cm s−1), could be connected to insect containers to deliver the CS (Fig. 1A). Similar to the stimulus delivery system of the olfactometer (detailed below), the airflow was split into two circuits, each circuit being made of Teflon® tubing (3 mm internal diameter), conducting the air flow through 20 ml glass bottles filled with 8 ml of either the test solution or the same volume of the corresponding solvent. During training, the choice of the circuit was controlled manually by connecting the tubes into the individual container; this enabled us to subject the mosquitoes to streams of either clean ambient air or air loaded with the CS at the same temperature, flow rate and relative humidity.
Olfactometer
To compare the responses of controlled, untrained and trained mosquitoes to different odours, an olfactometer was used. It consisted of an enclosed Y-maze made of Plexiglas® (Cooperband et al., 2008) (110 cm long, 10 cm internal diameter; Fig. 1B). Fans (Rosewill, Los Angeles, CA, USA) were connected to two of the arms of the olfactometer (choice arms) to generate airflows (air speed ~20 cm s−1). Airflow generated by the fans first went through an air filter (to remove odour contaminants; C16x48, Complete Filtration Services, Greenville, NC, USA) and a series of mesh screens and a honeycomb (10 cm long) to create a laminar flow before entering the Y-maze (Fig. 1B). As for the artificial feeder, odour delivery was achieved via a charcoal-filtered air stream that was split into two circuits and adjusted via flowmeters equipped with needle valves. Each circuit was made of Teflon® tubing (3 mm internal diameter) conducting the airflow (5 cm s−1) through a 20 ml glass bottle containing 8 ml of either the test odour or the control solution (i.e. MilliQ water). To avoid contamination, tubing and bottles were cleaned with ethanol and changed for each odour. Ends of the tubes were placed in the arms of the olfactometer, 4 cm from the fans, and in the centre of the olfactometer's arm. The bottles containing the test and control stimuli were replaced every 15 to 30 min to control for any change in odorant intensity.
To prevent the accumulation of odours in the experimental room, both artificial feeding and olfactometer experiments were conducted in a well-ventilated environmental chamber (Environmental Structures, Colorado Springs, CO, USA), at a temperature (25±2°C) and relative humidity (40–50%) that remained constant throughout all experiments.
In order to avoid environmental biases, the stimulus and control treatments were randomly exchanged in the olfactometer arms between experiments. In addition, the positions of the different parts of the olfactometer (i.e. choice tubes and fans) were also randomised. Data analysis did not reveal a preference for the left or right side of the olfactometer (P=0.86). After each experiment, the olfactometer was cleaned with ethanol (50%, 70% and 95% ethanol) to remove odorant contamination.
Training protocols
Two conditioning paradigms were adapted in the present work to assess the ability of mosquitoes to learn the association between an olfactory stimulus and a blood reward. The first involved training individual mosquitoes using conditioning protocols adapted from classical insect models for haematophagous insects (Vinauger et al., 2011a). This permitted detailed control of the experimental treatments to determine whether mosquitoes learned the association under Pavlovian conditioning and to investigate the nature of the involved memory form. In a second set of experiments, group training allowed rapid training and efficient testing of different olfactory stimuli as CS.
Individual training
For individual training experiments, single mosquitoes were exposed to L-(+)-lactic acid (LA; Sigma, ≥98% purity) at a concentration of 22 mmol l−1 in MilliQ water. This concentration is similar to that emitted by human skin (Eiras and Jepson, 1991; Cork and Park, 1996; Geier et al., 1996).
Before the training session began, and before each trial, mosquitoes were allowed to acclimate for 1 min in the absence of stimulation, except for the delivery of a clean air current. After this time, a trial begun when the airflow loaded with LA was delivered for 2 min. The artificial feeder was then placed over the containers for two further minutes, during which LA stimulation was maintained.
From this moment, mosquitoes that did not feed during this period of time were considered as not motivated and discarded from further analysis. Those that landed on the mesh and started biting through the membrane of the feeder were allowed to feed for 20 s before the artificial feeder was removed from the individual container. Only females that fed during the two trials were kept for the analysis. Trials were separated by 20 min. During this inter-trial interval (ITI), mosquitoes were maintained in the same experimental room, and only exposed to a clean air current. Conditioned mosquitoes were submitted to two trials before being tested in the olfactometer, 24 h after the end of the training session.
In order to discard potential effects of CS or US on the performance of mosquitoes during the test, specific control groups were performed where mosquitoes were exposed only to the CS or the US during the first session and tested 24 h later. Another group was exposed to both the CS and the US in an unpaired way during the first session, i.e. in the absence of contingency (Rescorla, 1988), and then tested 24 h later. Additional untrained mosquitoes were tested in the olfactometer while having to choose between two clean air currents, a clean air current versus CO2 (positive control, [CO2]=2300 ppm above ambient level) or a clean air current versus LA (Barrozo and Lazzari, 2004).
Group training
For group training experiments, the following odorants were tested: LA, 1-octen-3-ol [OCT; Aldrich, ≥98% purity; enantiomeric ratio: ≥99:1 (GC)], Z-3-hexen-1-ol (Z3H; Sigma, >98% purity; 92% of the Z isomer) and β-myrcene (MYR; Fluka, 95% purity). LA was dissolved in MilliQ water at the same concentration as for individual training experiments (22 mmol l−1). In order to provide a similar level of humidity – a strong activator of behaviour in mosquitoes – OCT (14 mmol l−1) and Z3H (91 mmol l−1) were also diluted in MilliQ water. These concentrations were chosen to match the same volatility as odours used successfully in behaviour assessment paradigms similar to our own (Cooperband et al., 2008). For MYR treatments, we used a 1/10,000 odorant dilution (0.58 mmol l−1) as higher concentrations caused avoidance behaviours, resulting in no insects leaving the starting chamber.
In a preliminary experiment, solid phase micro-extraction fibres were placed in the two arms of the olfactometer while delivering Z3H in water in one arm and Z3H in mineral oil in the other arm. This allowed us to quantify (via GCMS) the emission rate of Z3H diluted in water or mineral oil. The analysis revealed that emission rates between the two arms were not statistically different (0.98±0.33 ng min−1 for mineral oil; 1.09±0.13 ng min−1 for water).
Similarly to individual training, the air-delivery system was connected to insects' containers to deliver the odorant or the ‘no odour’ (MilliQ water) control (Fig. 1A). The training session began when the odorant was perfused into the container for 2 min. The group was then exposed to the blood feeder for 20 min while still delivering the odorant. This succession of events represented one training trial. As for individually trained mosquitoes, groups were submitted to two training trials spaced by a 20 min ITI.
One hour before testing, groups were chilled and females were transferred into individual containers. Mosquitoes that did not feed during the training session (determined by the absence of blood in the abdomen or by the absence of abdominal distension) were considered as not motivated and thus discarded from analysis. Tests were then performed similarly to the individual training experiments, one mosquito being tested at a time. The testing session was performed 24 h post-training. To determine whether the mosquitoes exhibited any innate preference for the test odorants, control groups were also tested. Insects of these groups were naïve and had not been exposed to the odorant prior to the test.
Effect of learning on DEET repellency
We performed an additional series of training experiments in order to test whether mosquitoes' ability to learn the association between odorants and blood feeding could interfere with the action of insect repellents such as DEET (N,N-diethyl-meta-toluamide; Supelco, ≥95% purity). We thus performed a series of experiments using pre-exposed, DEET-trained and DEET-naïve animals. In these experiments, 100 μl of either 10% DEET/90% ethanol (DEET) or 100% ethanol (solvent) was loaded on to a filter paper (Whatman) that was placed in a 20 ml scintillation vial (DeGennaro et al., 2013).
Different procedures were tested: (a) females trained to LA were tested for their response to LA+DEET versus control (clean air + solvent); (b) females trained to LA+DEET were tested for their response to LA+DEET versus control (clean air + solvent); (c) females pre-exposed to DEET 1 h before training were then trained to LA and tested for their responses to LA+DEET versus control (clean air + solvent); and (d) females pre-exposed to DEET 1 h before training were trained to LA+DEET and tested for their response to LA+DEET versus control (clean air + solvent).
Memory characterisation
The observed performance of trained animals relies on the formation of a mnesic trace that can last for different durations, depending on its consolidated nature (Tully et al., 1994; Tully et al., 2003). Because short-term (STM) and intermediate-term (ITM) memories last from a few to several hours (Tully et al., 2003), we expect the memory that we observed 24 h post-training to belong to longer lasting forms of memory. Among them, the anaesthesia-resistant memory (ARM) formed after massed training and spaced training is not disrupted by cold-induced anaesthesia but insensitive to the protein synthesis inhibitor CXM, while the long-term memory (LTM) is formed after spaced training only and is sensitive to CXM (for a review, see Tully et al., 1994).
Additional treatments were thus performed to investigate the nature of the memory formed during conditioning experiments. Four groups of individually trained mosquitoes were submitted to a 15 min cold shock (2.6°C) 1 h before training, 20 min after training, 2 h after training or 6 h after training. The cold shock was delivered by placing the individual container in an ice-filled Styrofoam box (30×35×20 cm). Two additional groups were constituted and individuals from these groups were starved for 14–18 h and then allowed to feed with either 17 or 35 mmol l−1 of CXM (Tully et al., 1994) in a 10% sucrose solution for 16–18 h before the training session. All groups were tested 24 h post-training. An additional group was fed on 35 mmol l−1 CXM before its response to CO2 was tested (CXM positive control group). For this group, insects were fed on 35 mmol l−1 CXM in 10% sucrose for the same duration as the experimental groups and tested for their response to CO2 (positive control, [CO2]=2300 ppm above ambient level) versus ambient air.
It is worth mentioning that the CXM-treated groups displayed a higher mortality rate than the sugar-fed groups (34.6% of mortality being observed 8 days after training compared with 12% for sugar-fed females). Interestingly, the increased mortality induced by the ingestion of CXM appears to be due to the ingestion of a warm blood meal, as CXM-fed mosquitoes that were not blood-fed had mortality rates similar to those of sucrose-fed (control) mosquitoes (16% of mortality). These results suggest that the drug might also impair their ability to deal with the heat stress generated by the ingestion of warm blood (Benoit et al., 2011).
To make sure that CXM effects were not affecting the mosquitoes' flight motor responses or olfactory perception, tracking of flight pathways was performed by video recording (Logitech Quickcam pro, 2MPixel; Newark, CA, USA) of the experiments in the olfactometer and subsequent analysis of the data in MATLAB (v7.02, The MathWorks, Natwick, MA, USA) using the DLT toolbox (DLT DigitizingTools) (Hedrick, 2008). Three treatment groups were tested: naïve mosquitoes fed on 10% sucrose solution (naïve control); trained mosquitoes fed on 10% sucrose solution (trained treatment); and trained mosquitoes fed on 35 mmol l−1 of CXM in 10% sucrose solution (CXM treatment).
Testing protocols
The testing sessions began when a single mosquito was placed in the starting chamber located at the extremity of the starting arm of the olfactometer and closed in by a transparent Plexiglas® door (Fig. 1B). After a 30 s familiarisation period, the door was opened. Led by its positive anemotaxis and optomotor responses (Kennedy, 1940; Takken and Knols, 1999), the insect flew along the starting arm and, at the bifurcation, could choose to follow one of the olfactometer arms, one bearing the stimulus and the other only clean air (plus the associated solvent), by entering into one of the two choice arms. We considered the first choice made by mosquitoes when they crossed the entry of an arm. Females that did not choose or did not leave the starting chamber were considered as not responding and discarded from the preference analyses. On average, 42% of females were motivated to leave the starting chamber of the olfactometer and chose between the two choice arms. For both individually trained and group-trained experiments, more females were active in the trained groups (52% on average) than in the corresponding control groups (33%) (χ2 test: P<0.001).
Data analysis
For both individual and group experiments, binary data collected in the olfactometer were analysed and all statistical tests were computed using R software (R Development Core Team, 2013). Comparisons were performed by means of the exact binomial test (α=0.05). For each treatment, the choice of the mosquitoes in the olfactometer was compared either with a random distribution of 50% on each arm of the maze or with the distribution of the corresponding control when appropriate. For binary data, the standard errors (s.e.m.) were calculated as (Le, 2003):
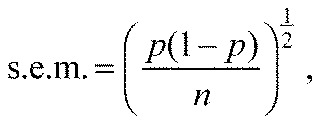 |
(1) |
where p is the observed proportion and n is the number of observations.
For each experimental group, a preference index (PI) was computed the following way: PI=[(number of females in the test arm – number of females in the control arm)/(number of females in the control arm + number of females in the test arm)]. A PI of +1 indicates that all the motivated insects chose the test arm, a PI of 0 indicates that 50% of insects chose the test arm and 50% the control arm, and a PI of −1 indicates that all insects chose the control arm of the olfactometer (adapted from Schwaerzel et al., 2003). Means of instantaneous flight speeds were analysed in Excel and flight speed comparisons were made in R, by means of Student's t-test (α=0.05).
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Nguyen for mosquito care, and P. Chhabra for assistance in behavioural experiments. J. Z. Parrish, C. R. Lazzari and C. Lahondère provided helpful suggestions and critical reading of the manuscript, and comments from three anonymous reviewers substantially improved the manuscript.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by a National Institutes of Health grant to J.A.R. (01R01DC013693), and the University of Washington's Royalty Research Fund. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.101279/-/DC1
References
- Acree F., Jr, Turner R. B., Gouck H. K., Beroza M., Smith N. (1968). l-Lactic acid: a mosquito attractant isolated from humans. Science 161, 1346-1347. [DOI] [PubMed] [Google Scholar]
- Alonso W. J., Schuck-Paim C. (2006). The ‘ghosts’ that pester studies on learning in mosquitoes: guidelines to chase them off. Med. Vet. Entomol. 20, 157-165. [DOI] [PubMed] [Google Scholar]
- Alonso W. J., Wyatt T. D., Kelly D. W. (2003). Are vectors able to learn about their hosts? A case study with Aedes aegypti mosquitoes. Mem. Inst. Oswaldo Cruz 98, 665-672. [DOI] [PubMed] [Google Scholar]
- Andrade E. H. A., Maia J. G. S., Zoghbi M., d. G. B. (2000). Aroma volatile constituents of Brazilian varieties of mango fruit. J. Food Compost. Anal. 13, 27-33. [Google Scholar]
- Barrozo R. B., Lazzari C. R. (2004). Orientation behaviour of the blood-sucking bug Triatoma infestans to short-chain fatty acids: synergistic effect of l-lactic acid and carbon dioxide. Chem. Senses 29, 833-841. [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Lopez-Martinez G., Patrick K. R., Phillips Z. P., Krause T. B., Denlinger D. L. (2011). Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc. Natl. Acad. Sci. USA 108, 8026-8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock G. R., Cardew G. (1996). Olfaction in Mosquito–Host Interactions. Ciba Foundations 200. New York, NY: John Wiley and Sons Ltd. [Google Scholar]
- Bohbot J. D., Dickens J. C. (2010). Insect repellents: modulators of mosquito odorant receptor activity. PLoS ONE 5, e12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman J. P. T. (1961). Observations on the habits of mosquitos of Plateau Province, Northern Nigeria, with particular reference to Aedes (Stegomyia) vittatus (Bigot). Bull. Entomol. Res. 52, 709-725. [Google Scholar]
- Bouyer J., Pruvot M., Bengaly Z., Guerin P. M., Lancelot R. (2007). Learning influences host choice in tsetse. Biol. Lett. 3, 113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilaka N., Perkins E., Tripet F. (2012). Visual and olfactory associative learning in the malaria vector Anopheles gambiae sensu stricto. Malar. J. 11, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choochote W., Chaithong U., Kamsuk K., Jitpakdi A., Tippawangkosol P., Tuetun B., Champakaew D., Pitasawat B. (2007). Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia 78, 359-364. [DOI] [PubMed] [Google Scholar]
- Clements A. N. (1999). The Biology of Mosquitoes: Sensory, Reception and Behavior, Vol. 2 London: Chapman and Hall. [Google Scholar]
- Cooperband M. F., McElfresh J. S., Millar J. G., Cardé R. T. (2008). Attraction of female Culex quinquefasciatus Say (Diptera: Culicidae) to odors from chicken feces. J. Insect Physiol. 54, 1184-1192. [DOI] [PubMed] [Google Scholar]
- Cork A., Park K. C. (1996). Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 10, 269-276. [DOI] [PubMed] [Google Scholar]
- DeGennaro M., McBride C. S., Seeholzer L., Nakagawa T., Dennis E. J., Goldman C., Jasinskiene N., James A. A., Vosshall L. B. (2013). orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T., Steib B., Cardé R. T., Geier M. (2002). l-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med. Vet. Entomol. 16, 91-98. [DOI] [PubMed] [Google Scholar]
- Dekker T., Geier M., Cardé R. T. (2005). Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 208, 2963-2972. [DOI] [PubMed] [Google Scholar]
- Dye C., Hasibeder G. (1986). Population dynamics of mosquito-borne disease: effects of flies which bite some people more frequently than others. Trans. R. Soc. Trop. Med. Hyg. 80, 69-77. [DOI] [PubMed] [Google Scholar]
- Eiras A. E., Jepson P. C. (1991). Host location by Aedes aegypti (Diptera, Culicidae) – a wind-tunnel study of chemical cues. Bull. Entomol. Res. 81, 151-160. [Google Scholar]
- Fradin M. S. (1998). Mosquitoes and mosquito repellents: a clinician's guide. Ann. Intern. Med. 128, 931-940. [DOI] [PubMed] [Google Scholar]
- Geier M., Boeckh J. (1999). A new Y-tube olfactometer for mosquitoes to measure the attractiveness of host odours. Entomol. Exp. Appl. 92, 9-19. [Google Scholar]
- Geier M., Sass H., Boeckh J. (1996). A search for components in human body odour that attract females of Aedes aegypti. In Mosquito Olfaction and Olfactory-Mediated Mosquito–Host Interactions. Ciba Foundation Symposium 200 (ed. Cardew G., Goode J.), pp. 132-148. New York, NY: John Wiley and Sons Ltd. [DOI] [PubMed] [Google Scholar]
- Gillett J. D., Haddow A. J., Corbet P. S. (1962). The sugar-feeding-cycle in a cage-population of mosquitoes. Entomol. Exp. Appl. 5, 223-232. [Google Scholar]
- Guerenstein P. G., Lazzari C. R. (2009). Host-seeking: how triatomines acquire and make use of information to find blood. Acta Trop. 110, 148-158. [DOI] [PubMed] [Google Scholar]
- Haddow A. J., Gillett J. D. (1957). Observations on the oviposition-cycle of Aëdes (Stegomyia) aegypti (Linnaeus). Ann. Trop. Med. Parasitol. 51, 159-169. [DOI] [PubMed] [Google Scholar]
- Hasibeder G., Dye C. (1988). Population dynamics of mosquito-borne disease: persistence in a completely heterogeneous environment. Theor. Popul. Biol. 33, 31-53. [DOI] [PubMed] [Google Scholar]
- Hedrick T. L. (2008). Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001. [DOI] [PubMed] [Google Scholar]
- Jones M. D. R., Cubbin C. M., Marsh D. (1972). The circadian rhythm of flight activity of the mosquito Anopheles gambiae: the light-response rhythm. J. Exp. Biol. 57, 337-346. [Google Scholar]
- Kaur J. S., Lai Y. L., Giger A. D. (2003). Learning and memory in the mosquito Aedes aegypti shown by conditioning against oviposition deterrence. Med. Vet. Entomol. 17, 457-460. [DOI] [PubMed] [Google Scholar]
- Kelly D. W. (2001). Why are some people bitten more than others? Trends Parasitol. 17, 578-581. [DOI] [PubMed] [Google Scholar]
- Kelly D. W., Thompson C. E. (2000). Epidemiology and optimal foraging: modelling the ideal free distribution of insect vectors. Parasitology 120, 319-327. [DOI] [PubMed] [Google Scholar]
- Kennedy J. S. (1940). The visual responses of flying mosquitoes. J. Zool. 109, 221-242. [Google Scholar]
- Klowden M. J. (1990). The endogenous regulation of mosquito reproductive behavior. Experientia 46, 660-670. [DOI] [PubMed] [Google Scholar]
- Klowden M. J., Lea A. O. (1978). Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.). Am. J. Trop. Med. Hyg. 27, 827-831. [DOI] [PubMed] [Google Scholar]
- Lazzari C. R. (2009). Orientation towards hosts in haematophagous insects: an integrative perspective. Adv. In Insect Phys. 37, 1-58. [Google Scholar]
- Le C. T. (2003). Introductory Biostatistics. Hoboken, NJ: John Wiley and Sons Ltd. [Google Scholar]
- Lehane M. J. (2005). The Biology of Blood-Sucking in Insects, pp. 321 New York, NY: Cambridge University Press. [Google Scholar]
- Liu C., Pitts R. J., Bohbot J. D., Jones P. L., Wang G., Zwiebel L. J. (2010). Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 8, e1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies C., Tully T., Dubnau J. (2005). Deconstructing memory in Drosophila. Curr. Biol. 15, R700-R713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall P. J., Eaton G. (2001). Olfactory memory in the mosquito Culex quinquefasciatus. Med. Vet. Entomol. 15, 197-203. [DOI] [PubMed] [Google Scholar]
- McCall P. J., Kelly D. W. (2002). Learning and memory in disease vectors. Trends Parasitol. 18, 429-433. [DOI] [PubMed] [Google Scholar]
- McCall P. J., Mosha F. W., Njunwa K. J., Sherlock K. (2001). Evidence for memorized site-fidelity in Anopheles arabiensis. Trans. R. Soc. Trop. Med. Hyg. 95, 587-590. [DOI] [PubMed] [Google Scholar]
- McClelland G. A. H. (1959). Observations on the mosquito, Aëdes (Stegomyia) aegypti (L.), in East Africa. I. The biting cycle in an outdoor population at Entebbe, Uganda. Bull. Entomol. Res. 50, 227-235. [Google Scholar]
- McMeniman C. J., Corfas R. A., Matthews B. J., Ritchie S. A., Vosshall L. B. (2014). Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menda G., Uhr J. H., Wyttenbach R. A., Vermeylen F. M., Smith D. M., Harrington L. C., Hoy R. R. (2013). Associative learning in the dengue vector mosquito, Aedes aegypti: avoidance of a previously attractive odor or surface color that is paired with an aversive stimulus. J. Exp. Biol. 216, 218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwandawiro C., Boots M., Tuno N., Suwonkerd W., Tsuda Y., Takagi M. (2000). Heterogeneity in the host preference of Japanese encephalitis vectors in Chiang Mai, northern Thailand. Trans. R. Soc. Trop. Med. Hyg. 94, 238-242. [DOI] [PubMed] [Google Scholar]
- Pellegrino M., Steinbach N., Stensmyr M. C., Hansson B. S., Vosshall L. B. (2011). A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature 478, 511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: Available at: http://www.R-project.org. [Google Scholar]
- Reddy G. V. P., Holopainen J. K., Guerrero A. (2002). Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J. Chem. Ecol. 28, 131-143. [DOI] [PubMed] [Google Scholar]
- Rescorla R. A. (1988). Behavioral studies of Pavlovian conditioning. Annu. Rev. Neurosci. 11, 329-352. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. (2003). Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfara V., Mougabure-Cueto G., Zerba E. N., Alzogaray R. A. (2011). Adaptation of the repellency response to DEET in Rhodnius prolixus. J. Insect Physiol. 57, 1431-1436. [DOI] [PubMed] [Google Scholar]
- Stanczyk N. M., Brookfield J. F., Field L. M., Logan J. G. (2013). Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PLoS ONE 8, e54438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z., Leal W. S. (2008). Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. USA 105, 13598-13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W., Knols B. G. (1999). Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 44, 131-157. [DOI] [PubMed] [Google Scholar]
- Takken W., Dekker T., Wijnholds Y. G. (1997). Odor-mediated flight behavior of Anopheles gambiae (Giles) sensu stricto and An. stephensi (Liston) in response to CO2, acetone, and 1-octen-3-ol (Diptera: Culicidae). J. Insect Behav. 10, 395-407. [Google Scholar]
- Tomberlin J. K., Rains G. C., Allan S. A., Sanford M. R., Lewis W. J. (2006). Associative learning of odor with food- or blood-meal by Culex quinquefasciatus Say (Diptera: Culicidae). Naturwissenschaften 93, 551-556. [DOI] [PubMed] [Google Scholar]
- Trpis M., McClelland G. A. H., Gillett J. D., Teesdale C., Rao T. R. (1973). Diel periodicity in the landing of Aedes aegypti on man. Bull. World Health Organ. 48, 623-629. [PMC free article] [PubMed] [Google Scholar]
- Tully T., Preat T., Boynton S. C., Del Vecchio M. (1994). Genetic dissection of consolidated memory in Drosophila. Cell 79, 35-47. [DOI] [PubMed] [Google Scholar]
- Tully T., Bourtchouladze R., Scott R., Tallman J. (2003). Targeting the CREB pathway for memory enhancers. Nat. Rev. Drug Discov. 2, 267-277. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Buratti L., Lazzari C. R. (2011a). Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. I. Appetitive learning. J. Exp. Biol. 214, 3032-3038. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Buratti L., Lazzari C. R. (2011b). Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. II. Aversive learning. J. Exp. Biol. 214, 3039-3045. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Pereira M. H., Lazzari C. R. (2012). Learned host preference in a Chagas disease vector, Rhodnius prolixus. Acta Trop. 122, 24-28. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Lallement H., Lazzari C. R. (2013). Learning and memory in Rhodnius prolixus: habituation and aversive operant conditioning of the proboscis extension response. J. Exp. Biol. 216, 892-900. [DOI] [PubMed] [Google Scholar]
- Woolhouse M. E., Dye C., Etard J. F., Smith T., Charlwood J. D., Garnett G. P., Hagan P., Hii J. L., Ndhlovu P. D., Quinnell R. J., et al. (1997). Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. USA 94, 338-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.