Galactolipids play an important role in plant salt tolerance through maintaining chloroplast structure and function.
Abstract
In plants, the galactolipids monogalactosyldiacylglycerol (MGDG) and digalactodiacylglycerol (DGDG) are major constituents of photosynthetic membranes in chloroplasts. One of the key enzymes for the biosynthesis of these galactolipids is MGDG synthase (MGD). To investigate the role of MGD in the plant’s response to salt stress, we cloned an MGD gene from rice (Oryza sativa) and generated tobacco (Nicotiana tabacum) plants overexpressing OsMGD. The MGD activity in OsMGD transgenic plants was confirmed to be higher than that in the wild-type tobacco cultivar SR1. Immunoblot analysis indicated that OsMGD was enriched in the outer envelope membrane of the tobacco chloroplast. Under salt stress, the transgenic plants exhibited rapid shoot growth and high photosynthetic rate as compared with the wild type. Transmission electron microscopy observation showed that the chloroplasts from salt-stressed transgenic plants had well-developed thylakoid membranes and properly stacked grana lamellae, whereas the chloroplasts from salt-stressed wild-type plants were fairly disorganized and had large membrane-free areas. Under salt stress, the transgenic plants also maintained higher chlorophyll levels. Lipid composition analysis showed that leaves of transgenic plants consistently contained significantly higher MGDG (including 18:3-16:3 and 18:3-18:3 species) and DGDG (including 18:3-16:3, 18:3-16:0, and 18:3-18:3 species) contents and higher DGDG-MGDG ratios than the wild type did under both control and salt stress conditions. These results show that overexpression of OsMGD improves salt tolerance in tobacco and that the galactolipids MGDG and DGDG play an important role in the regulation of chloroplast structure and function in the plant salt stress response.
Salt stress is a major environmental factor that poses a serious threat to crop yield and future food production (Møller and Tester, 2007). When plants are exposed to salinity, they suffer two primary obstacles: low external water potentials and high concentrations of toxic ions (Hirayama and Mihara, 1987). These obstacles generally lead to the disruption of various enzymatic processes, changes in membrane lipid composition, alteration in chloroplast structure and function, impairment of photosynthetic capacity, and inhibition of plant growth (Brown and Dupont, 1989; Elkahoui et al., 2004; Munns and Tester, 2008; Sui et al., 2010; Shu et al., 2012).
Membranes are the primary matrix for numerous physiological and biochemical activities, and plants easily change their membrane lipid compositions in response to environmental stresses (Harwood, 1996). A number of studies have proved that salt stress can induce changes in plant membrane lipids (Huflejt et al., 1990; Elkahoui et al., 2004; Sui et al., 2010). Sui et al. (2010) found that, in Suaeda salsa, salt stress increased the proportion of phosphatidylglycerol and reduced the proportion of galactolipids. Similar results were observed in Catharanthus roseus cultured cell suspensions, which showed an increase in phospholipid content and a decrease in galactolipid content that were more obvious under 100 mm NaCl than under 50 mm (Elkahoui et al., 2004). Meanwhile, it was shown that salt tolerance in plants is strongly linked with their membrane lipid composition and especially with their galactolipid content, which is positively related to salt tolerance (Hirayama and Mihara, 1987).
In plants, galactolipids are major constituents of the photosynthetic membrane, which is the most abundant membrane in nature (Lee, 2000). Two galactolipids, monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), are the predominant photosynthetic membrane lipid building blocks, accounting for about 52 mol % and 26 mol % of thylakoid membrane lipids, respectively (Block et al., 1983). These galactolipids are also the major lipids in cyanobacteria, suggesting that MGDG and DGDG are important for all oxygenic photosynthetic organisms (Hölzl and Dörmann, 2007). They are components of PSII and are the indispensable matrix for photosynthesis (Mizusawa and Wada, 2012). Their unique characteristics also give them distinctive packing properties that are important for the organization of thylakoid membranes (Lee, 2000). MGDG has a conical shape due to its smaller head group and a high content of unsaturated fatty acids, giving it nonbilayer-forming characteristics (Webb and Green, 1991; Aronsson et al., 2008); this feature is assumed to play an important structural role in the dense packing of proteins in the membrane (Williams, 1998; Garab et al., 2000). In contrast to MGDG, DGDG has a cylindrical shape that is typical for most plastid lipids and is considered a bilayer-prone lipid (Aronsson et al., 2008); this feature is involved in lipid-mediated contacts between adjacent trimers of light-harvesting complex II (LHCII) when they are packed into two-dimensional crystalline arrays (Lee, 2000). In addition to forming important membrane structures in the thylakoids, MGDG and DGDG are also present in extraplastidic membranes, including the plasma membrane, tonoplasts, endoplasmic reticulum, and Golgi membranes, indicating the crucial role of these galactoglycerolipids in higher plant membrane systems (Yoshida and Uemura, 1986; Brown and Dupont, 1989; Härtel et al., 2000).
Two enzymes are involved in the biosynthesis of these galactoglycerolipids: MGDG synthase (MGD), which transfers a galactosyl residue from UDP-Gal to diacylglycerol, and DGDG synthase, which catalyzes the further galactosylation of MGDG to form DGDG (Shimojima et al., 1997; Dörmann et al., 1999; Shimojima and Ohta, 2011). Thus, MGD is the key enzyme in the biosynthesis of both galactolipids and, consequently, also in the formation of photosynthetic membranes (Nakamura et al., 2010). A number of studies have revealed that MGD is vital for plant growth and development. The loss of MGD function in plants leads to a pale-green phenotype, defects in the chloroplast ultrastructure, disruption in the photosynthetic membranes, and complete impairment of photosynthetic ability and photoautotrophic growth, suggesting a unique role for MGD in chlorophyll formation, the structural organization of the plastidic membranes, and photosynthetic growth (Jarvis et al., 2000; Kobayashi et al., 2007; Botté et al., 2011; Myers et al., 2011). The crucial role of MGD under environmental stresses, including phosphorus deficiency and wounding, is also well studied (Kobayashi et al., 2004, 2009a, 2009b; Moellering and Benning, 2011). However, although a number of studies have proved that salt stress can induce changes in plant membrane lipids (Huflejt et al., 1990; Elkahoui et al., 2004; Sui et al., 2010), little is known about the role of MGD and the involvement of galactolipids in response to salt stress.
To investigate the function of MGD in plant salt tolerance, we cloned the relevant gene, which is called OsMGD (Qi et al., 2004), from rice (Oryza sativa ‘FR13A’). This gene has a high similarity to Arabidopsis (Arabidopsis thaliana) MGD2 and MGD3, and its expression is induced by several environmental stresses, including salt, drought, and submergence (Qi et al., 2004; Benning and Ohta, 2005). We then generated tobacco (Nicotiana tabacum) plants overexpressing OsMGD and investigated the salt tolerance ability in the transgenic lines and wild-type plants. The results of this study demonstrate that an increase in galactolipid content in leaves is beneficial for maintaining chloroplast structure and function and leads to enhanced salt tolerance in tobacco.
RESULTS
Overexpression of OsMGD in Transgenic Tobacco
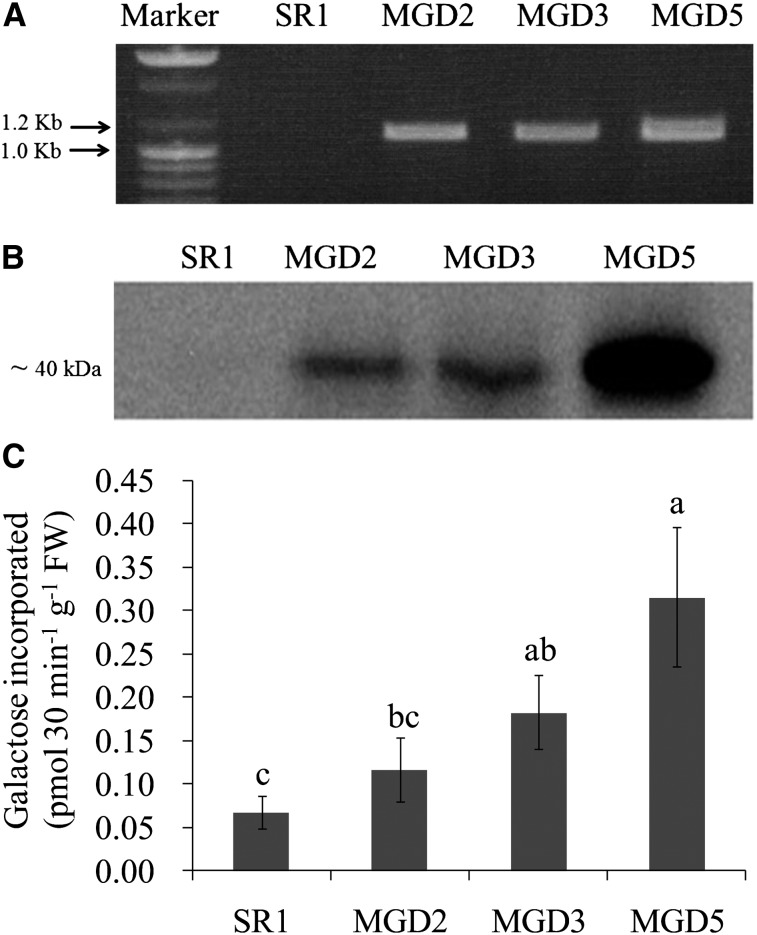
The gene encoding MGDG synthase in rice (OsMGD) was cloned into the plant expression vector pGWB2 under the control of the cauliflower mosaic virus 35S promoter and used to transform tobacco (cv SR1) leaves. Transformants were selected by genomic PCR using OsMGD gene-specific primers, and an expected approximately 1.1-kb band was found in the transgenic lines MGD2, MGD3, and MGD5 but not in the wild-type cv SR1 plant (Fig. 1A). The expression of OsMGD in these transgenic lines was further verified by western-blot analysis using antibodies raised against OsMGD (Fig. 1B). The antibody recognized a protein of about 40 kD from the transgenic leaf extracts, which corresponds to the mass of OsMGD, while it showed no reaction in cv SR1. Moreover, the activity of MGDG synthase was measured in leaves of cv SR1 and the three transgenic lines. The transgenic plants consistently had 1.7- to 4.7-fold higher levels of MGDG synthase activity than the cv SR1 plants had (Fig. 1C). These results clearly demonstrate that the OsMGD gene was successfully introduced into tobacco plants, which then had substantially and specifically increased activities of MGDG synthase.
Figure 1.
Confirmation of OsMGD transgenic tobacco plants by genome PCR (A), western-blot analysis (B), and the activity of MGD (C). The gene-specific primer and antibody were used for genome PCR and western-blot analysis, respectively, and MGD activity was measured as described in “Materials and Methods.” Data are means ± sd (n = 6). Values followed by the same letter are not significantly different according to lsd test (P < 0.05). FW, Fresh weight.
Localization of OsMGD in Tobacco Chloroplast
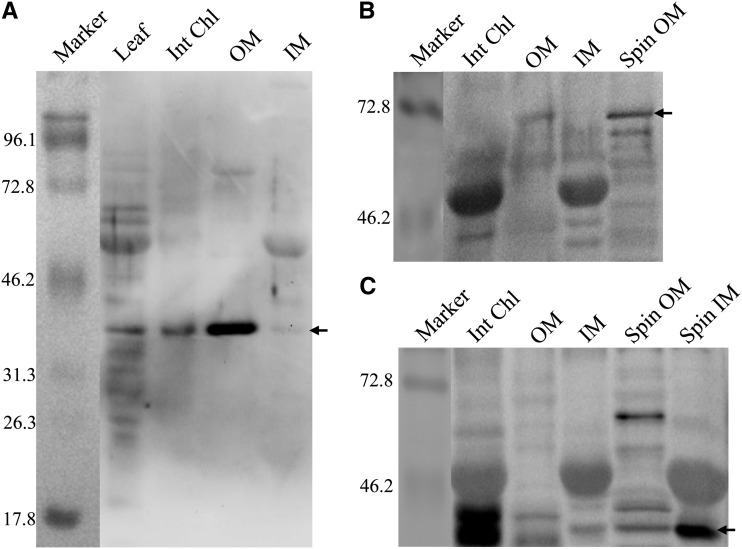
The MGD in higher plants has been classified into two groups, type A and type B, which are located in the inner and outer chloroplast envelope membranes, respectively (Shimojima and Ohta, 2011). To determine the localization of OsMGD, chloroplasts from tobacco leaf were isolated and inner and outer membrane fractions were prepared. As markers for the inner and outer envelope membranes, Tic40 and Toc75 were used, respectively, and the spinach (Spinacia oleracea) inner and outer envelope membranes were used as the positive controls. Immmunoblot analysis with the OsMGD-specific antibody showed that a large amount of OsMGD protein was detected in the outer envelope membrane, and comparatively, this protein was evidently not enriched in the inner envelope membrane of the chloroplast (Fig. 2). Moreover, we could not detect the Toc75 band in the intact chloroplast fraction of tobacco (Fig. 2B), which may be due to the low amount of outer membrane protein in this fraction.
Figure 2.
Localization of OsMGD in isolated tobacco chloroplasts by immunodetection. Proteins extracted from MGD5 tobacco leaf, intact chloroplasts (Int Chl), outer chloroplast envelope membrane (OM), and inner chloroplast envelope membrane (IM), as well as spinach outer and inner chloroplast envelope (Spin OM and Spin IM, used as positive controls) were separated by SDS-PAGE and immunoblotted with antibodies against OsMGD (A), Toc75 (a chloroplast outer envelope protein; B), and Tic40 (a chloroplast inner envelope protein; C) antibodies as described in “Materials and Methods.” For leaf samples, 25 μg of protein was loaded, and for other samples, 12 μg of protein was loaded. The molecular masses of standard proteins (in kD) are indicated at the left, and the specific bands are indicated by arrows at the right.
OsMGD-Overexpressing Lines Are Tolerant of Salt Stress
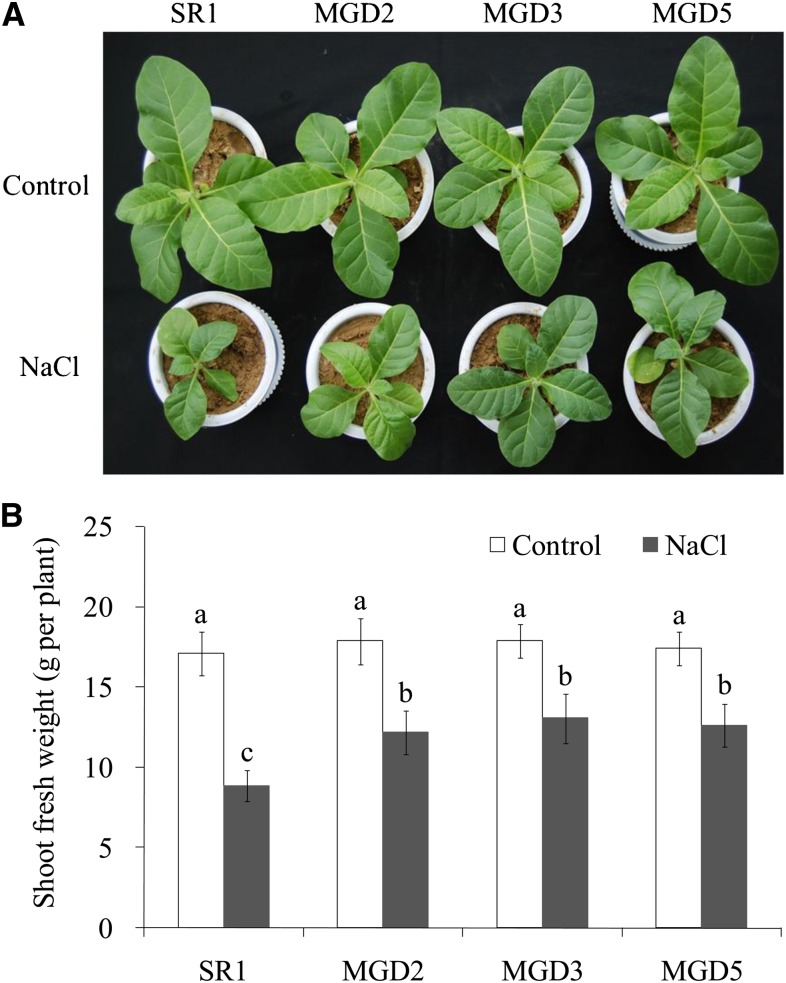
Plant growth was observed after 10 d of salt treatment. There was no difference in growth between the wild-type cv SR1 and transgenic plants under control conditions (Fig. 3). Under salt stress conditions, however, shoot growth was reduced by 48% in cv SR1 but only by 32%, 27%, and 28%, respectively, in MGD2, MGD3, and MGD5. These results show that the OsMGD-overexpressing lines had higher growth rates than cv SR1 did under salt stress.
Figure 3.
Effects of salt stress on plant growth of wild-type cv SR1 and transgenic lines MGD2, MGD3, and MGD5. Photographs were taken after 10 d of salt treatment (A), and shoot fresh weights were measured (B). Data are means ± sd (n = 6). Values followed by the same letter are not significantly different according to the Tukey-Kramer test (P < 0.05).
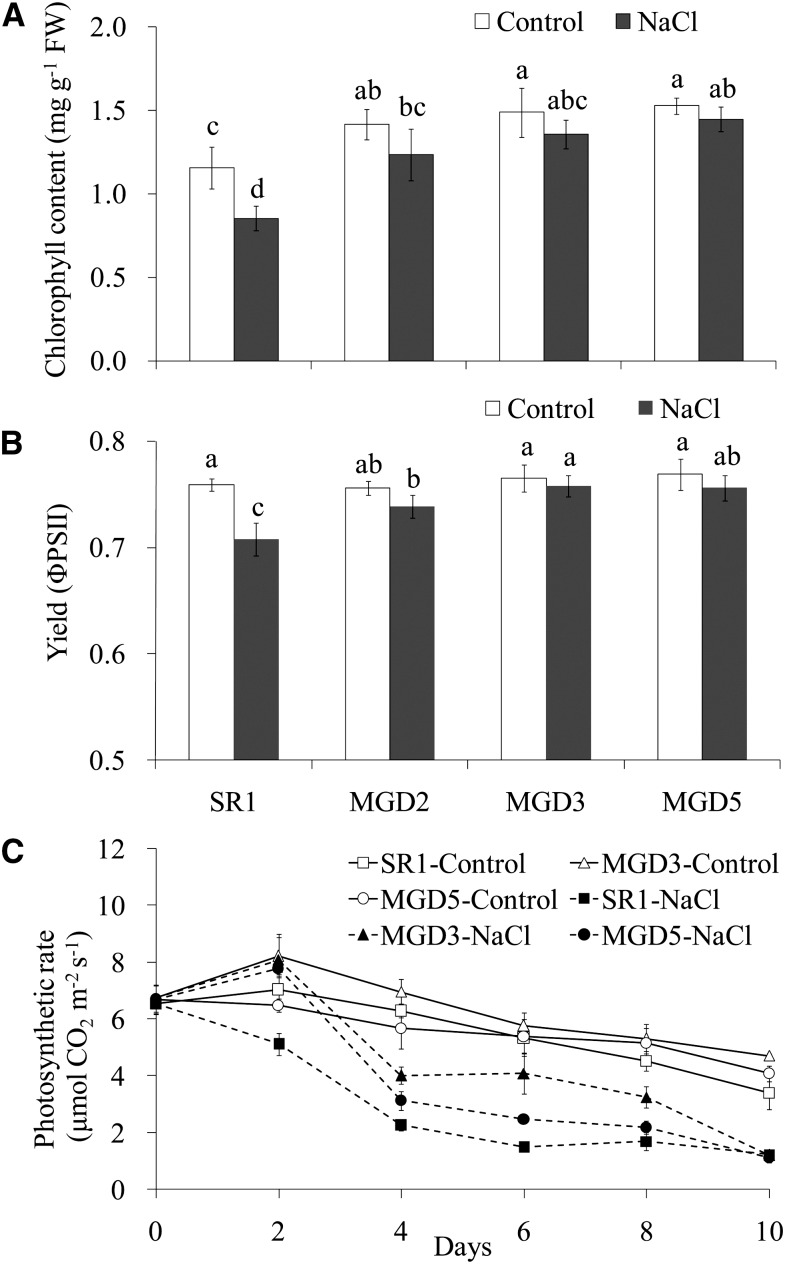
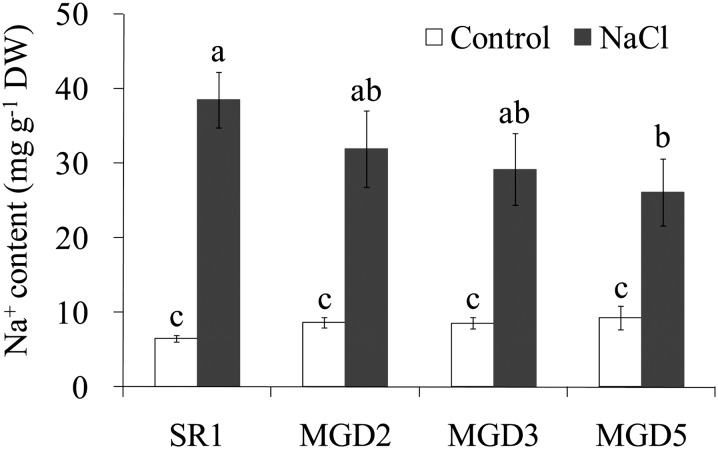
To further investigate the responses of cv SR1 and transgenic plants to salt stress, chlorophyll contents and photosynthetic parameters were measured. When plants were exposed to salt stress, plants from the transgenic lines had higher chlorophyll contents than cv SR1 plants had. This was observed even under control conditions (Fig. 4A). Regarding the effective quantum yield of PSII, salt stress caused a significant decrease in the quantum yield of PSII in cv SR1 but no change in transgenic plants (Fig. 4B). The photosynthetic rate was higher in transgenic lines than in cv SR1, with reductions of 40% and 53% in MGD3 and MGD5, respectively, compared with a 65% reduction in cv SR1, after 4 d of salt treatment (Fig. 4C). In addition, although Na+ content in leaf was increased with salt treatment applied, there was no difference between the wild type and the transgenic plants, except a lower level in MGD5 under salt stress (Fig. 5).
Figure 4.
Effects of salt stress on chlorophyll content (A), effective quantum yield (B), and photosynthetic rate (C) of wild-type cv SR1 and transgenic lines MGD2, MGD3, and MGD5. The chlorophyll content and effective quantum yield were determined after 10 d of salt treatment, and the photosynthetic rate was measured by time course. Data are means ± sd (n = 6). Values followed by the same letter are not significantly different according to the Tukey-Kramer test (P < 0.05). FW, Fresh weight.
Figure 5.
Effects of salt stress on Na+ contents in the leaves of wild-type cv SR1 and transgenic lines MGD2, MGD3, and MGD5. Leaves were sampled from control or 10-d salt-treated tobacco plants. Na+ contents were analyzed by atomic absorption spectrophotometer as described in “Materials and Methods.” Data are means ± sd (n = 4). Values followed by the same letter are not significantly different according to the Tukey-Kramer test (P < 0.05). DW, Dry weight.
Chloroplast Ultrastructure Observation
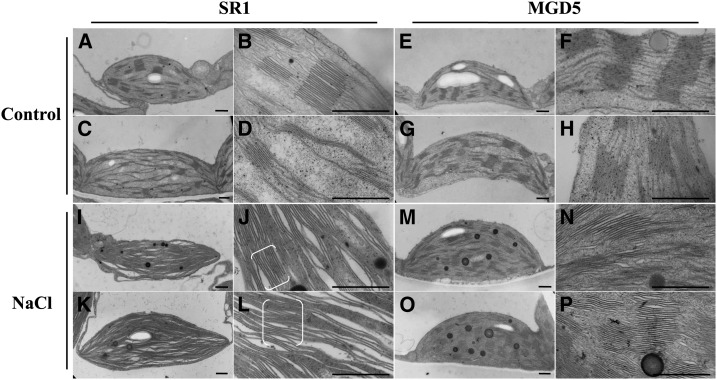
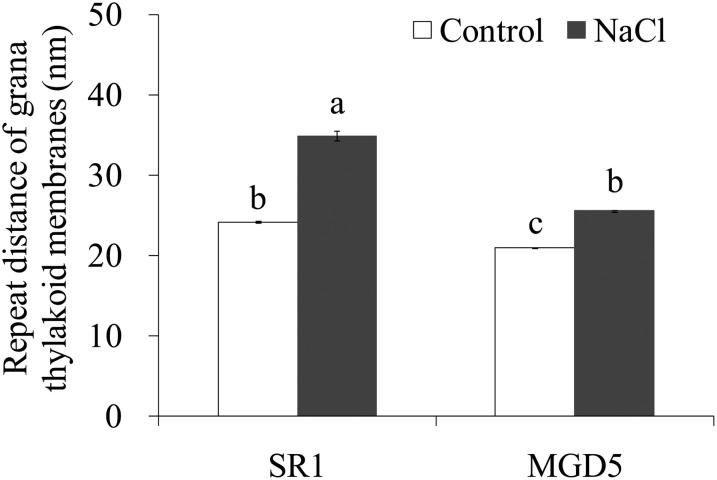
To assess the function of OsMGD in chloroplast biogenesis, plastid morphologies in the wild type and the typical transgenic line MGD5 were analyzed by transmission electron microscopy (Fig. 6). Plastids from both wild-type and transgenic plants growing under control conditions have a well-developed thylakoid membrane system composed of well-organized granal stacks and connected by stroma lamellae (Fig. 6, A–H). Compared with cv SR1, MGD5 chloroplasts showed tight stacking and fewer thylakoid-free areas. As shown in Figure 7, the repeat distance of grana thylakoid membranes was estimated to be 24.14 ± 0.14 nm in cv SR1 chloroplasts and 20.94 ± 0.09 nm in MGD5 chloroplasts. The more significant differences in plastid morphology between cv SR1 and transgenic plants were observed under salt stress. The distortions of plastids occurred under salt stress in both cv SR1 and MGD5 plants, but the chloroplast ultrastructure in cv SR1 was much more severely destroyed, and the membrane system was sparser and far less organized compared with that in MGD5 (Fig. 6, I–P). The repeat distance of grana thylakoid membranes was estimated to be 34.91 ± 0.64 nm in cv SR1 and 25.52 ± 0.16 nm in MGD5 chloroplasts, which reflected a larger interthylakoidal space in cv SR1 thylakoids compared with MGD5 thylakoids (Fig. 7). Furthermore, it is clear that under salt stress conditions, the chloroplasts contained large amounts of osmiophilic lipid droplets, which were big and round as well as present in great numbers, in MGD5 chloroplasts compared with cv SR1 chloroplasts.
Figure 6.
Ultrastructure of chloroplasts from wild-type cv SR1 and transgenic line MGD5. Electron micrographs of representative chloroplasts (two each) from wild-type cv SR1 (A–D) and transgenic line MGD5 (E–H) growing under control conditions and wild-type cv SR1 (I–L) and transgenic line MGD5 (M–P) growing under salt treatment for 10 d are shown. In each pair of photographs (e.g. A and B, C and D, etc.), the right image shows the same chloroplast shown on the left, but at a higher magnification. The brackets in J and L indicate the grana. Bars = 0.5 μm.
Figure 7.
Ultrastructural analysis of the stacking repeat distance in thylakoids of wild-type cv SR1 and the transgenic line MGD5. The repeat distance of grana thylakoid membranes was analyzed from transmission electron microscopy images, and data are means ± se (n = 100 from 10 different chloroplasts). Values followed by the same letter are not significantly different according to the Tukey-Kramer test (P < 0.05).
Effect of Salt Stress on MGDG and DGDG Contents
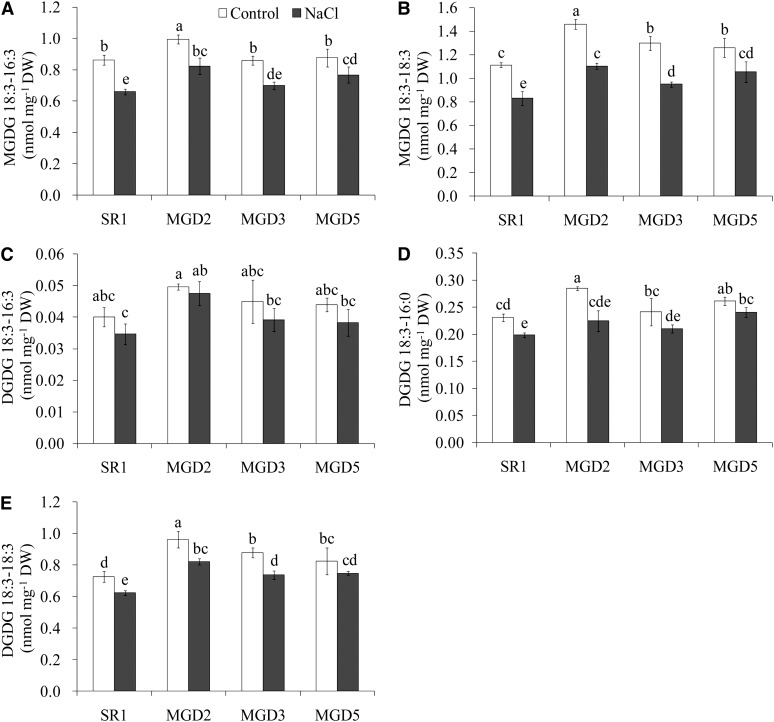
To verify the effect of OsMGD on salt tolerance, MGDG and DGDG contents were measured. Before the MGDG and DGDG analysis, we confirmed that tobacco leaf contains no MGDG or DGDG with two fully saturated acyl chains. Thus, hydrogenated MGDG and hydrogenated DGDG (purchased from Matreya) can serve as internal standards for the quantification of galactolipids in tobacco leaf. In this study, two classes of MGDG, 18:3-16:3 (carbon number:double bond number) and 18:3-18:3, and three classes of DGDG, 18:3-16:3, 18:3-16:0, and 18:3-18:3, were detected in tobacco leaves (Fig. 8). In nontreated cv SR1, the 18:3-16:3 MGDG accounted for approximately 44% and 18:3-18:3 accounted for approximately 56% of the total MGDG. Overexpression of OsMGD did not affect 18:3-16:3 MGDG levels except for an increase in MGD2, but it did lead to a 13% to 31% increase in 18:3-18:3 MGDG content as compared with that in cv SR1 (Fig. 8, A and B). Salt treatment caused a significant decrease in both classes of MGDG, but the transgenic plants still maintained higher MGDG levels, especially 18:3-18:3 MGDG, which was 14% to 33% more abundant in the transgenic plants than in the wild type.
Figure 8.
Effects of salt stress on the contents of MGDG (A and B) and DGDG (C–E) species in the leaves of wild-type cv SR1 and transgenic lines MGD2, MGD3, and MGD5. Leaves were sampled from control or 10-d salt-treated tobacco plants. MGDG and DGDG contents were analyzed by ESI MS/MS as described in “Materials and Methods.” Data are means ± sd (n = 4). Values followed by the same letter are not significantly different according to the Tukey-Kramer test (P < 0.05). DW, Dry weight.
As for DGDG, the 18:3-18:3 type was the main type, accounting for approximately 72% of total DGDG in cv SR1 leaves. The transgenic plants consistently had higher levels of 18:3-16:0 and 18:3-18:3 DGDG than cv SR1 plants had under control conditions (Fig. 8, C–E). Salt treatment caused a decrease in DGDG content, but DGDG levels were still higher in the transgenic lines than in cv SR1 plants, especially for 18:3-18:3 DGDG.
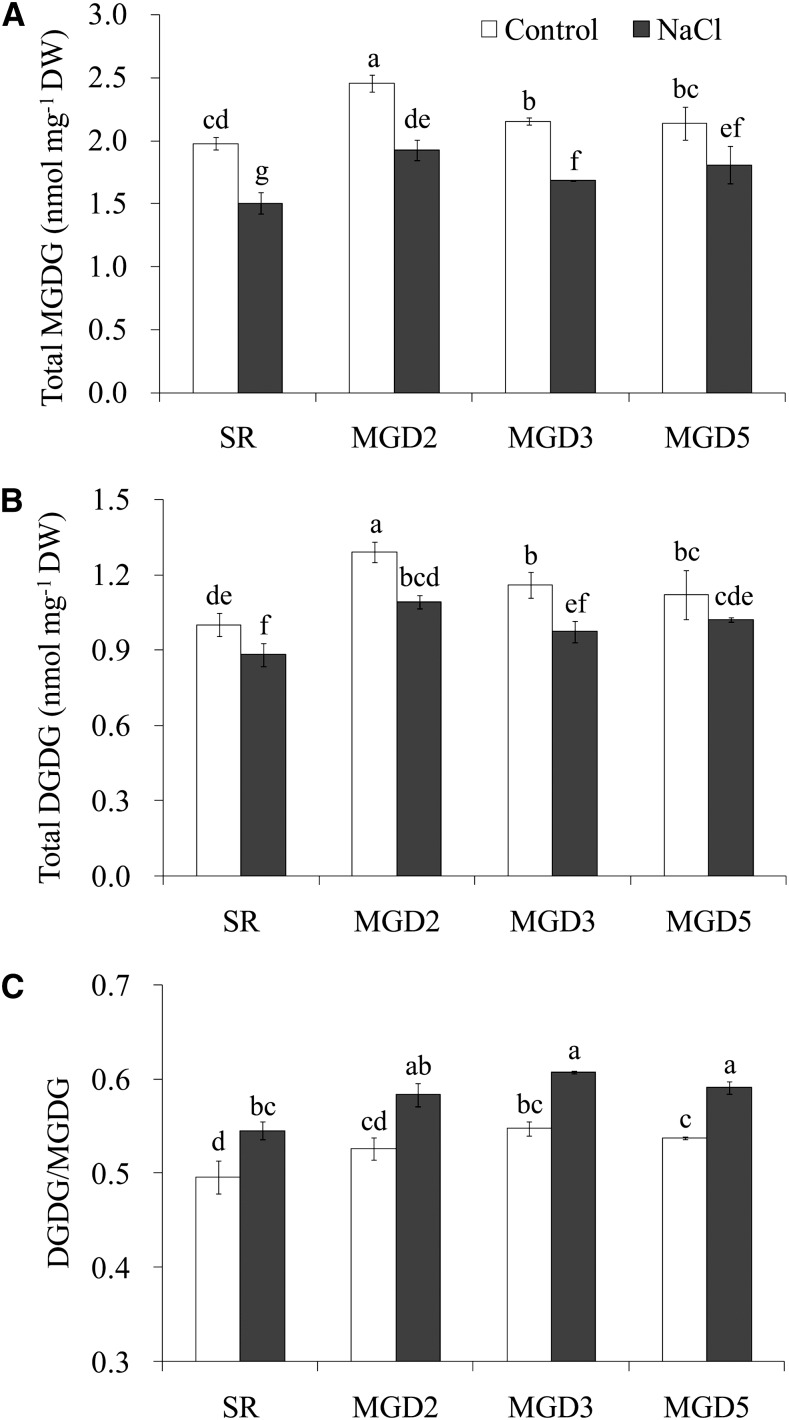
The total MGDG and DGDG levels in each line were also compared. Regardless of the presence or absence of salt treatment, the transgenic plants constantly contained a higher level of total MGDG and DGDG than the wild-type plants did (Fig. 9, A and B). After the salt treatment, the total MGDG was reduced by 24%, 22%, 22%, and 15% in cv SR1 and MGD2, MGD3, and MGD5, respectively, and the total DGDG was reduced by 12%, 15%, 16%, and 9%, which indicates that MGDG is more susceptible to salt stress than DGDG is. As a result, the ratio of DGDG to MGDG was enhanced by salt treatment, but the ratio was higher in the transgenic lines than in the cv SR1 plants under both control conditions and salt treatment (Fig. 9C). Moreover, the lipid-to-chlorophyll ratio was increased by salt treatment in all tested lines, while under salt stress, the ratios of both MGDG to chlorophyll and DGDG to chlorophyll were significantly higher in SR than in the transgenic lines (Table I).
Figure 9.
Effects of salt stress on the total contents of MGDG and DGDG (A and B) and the ratio of DGDG to MGDG (C) in the leaves of wild-type cv SR1 and transgenic lines MGD2, MGD3, and MGD5. Leaves were sampled from control or 10-d salt-treated tobacco plants. MGDG and DGDG contents were analyzed by ESI MS/MS as described in “Materials and Methods.” Data are means ± sd (n = 4). Values followed by the same letter are not significantly different according to the Tukey-Kramer test (P < 0.05). DW, Dry weight.
Table I. Lipid-to-chlorophyll ratio in wild-type cv SR1 and transgenic lines MGD2, MGD3, and MGD5 under control and salt stress conditions.
The ratio (µmol g−1) was calculated from the total MGDG or DGDG content and the chlorophyll content. Data are means ± se (n = 4). Values followed by the same letter within a column are not significantly different according to the Tukey-Kramer test (P < 0.05).
| Plant | Lipid-to-Chlorophyll Ratio |
|||
|---|---|---|---|---|
|
MGDG-Chlorophyll |
DGDG-Chlorophyll |
|||
| Control | NaCl | Control | NaCl | |
| cv SR1 | 102.5 ± 1.4 a | 137.1 ± 4.4 a | 51.9 ± 1.4 a | 80.3 ± 2.4 a |
| MGD2 | 97.0 ± 1.6 a,b | 120.1 ± 2.9 b | 51.0 ± 1.0 a | 68.1 ± 1.0 b |
| MGD3 | 93.9 ± 0.7 b | 99.2 ± 0.7 c | 50.6 ± 1.3 a | 57.3 ± 1.5 c |
| MGD5 | 92.3 ± 3.3 b | 103.6 ± 4.9 c | 48.4 ± 2.4 a | 58.5 ± 0.3 c |
DISCUSSION
Salt stress severely hampers plant growth and highly reduces crop productivity and yield. Under salt stress, various physiological and biochemical changes take place within plant cells that can lead to severe damage in the structure and function of the plant cells. Our results here revealed that transgenic plants overexpressing OsMGD complementary DNA (cDNA) showed significantly higher shoot growth rate and photosynthetic capacity than the wild type did under salt stress, indicating that salt tolerance in the transgenic plants was significantly elevated (Figs. 3 and 4). Furthermore, even under long-term salt stress (more than 10 d), no difference in Na+ accumulation was found between the wild-type and transgenic plants (Fig. 5). Therefore, these results indicate that the difference in salt tolerance between wild-type and transgenic plants is independent of the degree of Na+ accumulation but may be related to the enhanced levels of galactolipids.
In higher plants, two classes of MGD have been distinguished (type A, which is targeted to the inner envelope membrane, and type B, which is localized to the outer envelope membrane), and the distinct functions of the two types of MGD have been established. In Arabidopsis, type A (AtMGD A) is responsible for the bulk of galactolipid biosynthesis in photosynthetic tissues, while type B (AtMGD B) has no important role in chloroplast biogenesis under normal conditions (Kobayashi et al., 2009b). The amino acid sequence analysis showed that OsMGD has a higher homology to AtMGD B (74%) than to AtMGD A (59.5%; Qi et al., 2004). To confirm the localization of OsMGD, immunoblot analysis was performed using tobacco chloroplasts. It is clear that OsMGD was enriched in the outer envelope membrane of tobacco chloroplast (Fig. 2). Different from AtMGD B, which does not contribute to galactolipid synthesis under normal conditions, the overexpression of OsMGD resulted in increased galactolipid content (Fig. 8). While similar to AtMGD B, both of them were located in the outer envelope membrane. Furthermore, under salt stress, the transcript level of OsMGD was increased; in contrast, AtMGD2 and AtMGD3 were not affected (Qi et al., 2004; Kobayashi et al., 2009b). Thus, it is assumed that OsMGD may represent a type of MGD that is not totally the same as the type B in Arabidopsis, as suggested by Qi et al. (2004) and Shimojima and Ohta (2011).
Increased levels of galactolipids could contribute to the maintenance of organization and function of photosynthetic membranes, especially under stress conditions. As the most major lipid constituents of the chloroplast membrane, the nonbilayer lipid MGDG is quite crucial for maintaining the stability of membrane structure and function. The conical shape of MGDG is proposed to provide high lateral pressure on the proteins embedded in the membrane and thus facilitates the dense packing of proteins in the membrane (Garab et al., 2000). An in vitro assembly study revealed that MGDG is responsible for the lamellar organization of the thylakoid membranes through its association with LHCII (Simidjiev et al., 2000). Thus, the decrease in MGDG level caused by salt stress could lead to the serious disorder and dysfunction of photosynthetic membranes. Moreover, it has been shown that the nonbilayer lipid is quite important for the self-regulation of the lipid content of the thylakoid membrane through the ability of the nonbilayer lipids to be segregated, and this self-regulation is thought to be of special physiological importance in plants, especially under extreme environmental conditions (Garab et al., 2000). In this study, compared with cv SR1, the transgenic plants exhibited a large number and big osmiophilic lipid droplets in the chloroplast, possibly due to the high contents of nonbilayer lipid in the transgenic plants, which leads to easy segregation of lipids from the membrane. It is assumed that these sequestered nonbilayer lipids can serve directly as a reservoir or can be available for metabolic transformation (Garab et al., 2000). In addition, mutant studies have definitely confirmed the essential effect of MGDG on chloroplast structure, organization, and biogenesis (Jarvis et al., 2000; Kobayashi et al., 2007; Aronsson et al., 2008). Taken together, we hypothesize that the elevated contents of MGDG may play an important role in membrane structural and functional stability as well as membrane assembly in chloroplasts of the transgenic plants under salt stress.
In addition to MGDG, DGDG also has indispensable and unique functions in chloroplast membrane configuration. The plastid morphological defects are found in an Arabidopsis dgd1 mutant that contains low DGDG levels compared with the wild type (Dörmann et al., 1995; Härtel et al., 1997). As a bilayer-forming lipid, DGDG is important for the lamellar structure stability of chloroplast membranes. It has been proved that the decrease in DGDG level caused the disassembly of LHCII PSII-containing macrodomains, the degradation of PSI complexes, and the shortening of chlorophyll fluorescence lifetime, which resulted in lowered stability of the thylakoid membrane, especially at elevated temperature (Krumova et al., 2010). A similar mechanism can be assumed in the case of salt stress, where the reduced level of DGDG may be responsible for the disordered chloroplast membrane under salt stress. Observation of chloroplasts by transmission electron microscopy has shown that the thylakoid membranes become fewer and seriously disorganized after salt treatment in wild-type tobacco (Figs. 6 and 7) and also in rice and cucumber (Cucumis sativus), with a large decrease in chlorophyll concentration simultaneously (Yamane et al., 2004; Shu et al., 2012). Besides, it is suggested that the lipid-to-chlorophyll ratio is a good estimate for the protein-packing density in thylakoids, and the high lipid-to-chlorophyll ratio reflects a low protein-packing density (Haferkamp and Kirchhoff, 2008; Kirchhoff et al., 2013). Under stress conditions such as nitrogen deficiency, the lipid-to-chlorophyll ratio is markedly increased, indicating that the protein-packing density in thylakoids tends to be decreased under stresses (Gaude et al., 2007). In this study, the damage to the chloroplast membrane and the decrease in chlorophyll contents were less in transgenic plants, while the protein-packing density was significantly high (lower lipid-to-chlorophyll ratio) in the transgenic plants compared with the wild type under salt stress (Table I); all those changes may be ascribed to their higher MGDG and DGDG contents than in the wild type (Fig. 8). Furthermore, under control conditions, the transgenic plants also exhibited higher chlorophyll contents than the wild type (Fig. 4A), while the lipid-to-chlorophyll ratio was similar; thus, it is possible that the transgenic plants have more chloroplasts per cell or more thylakoid membranes per chloroplast than the wild type.
The balance between the levels of the lamella-forming lipid DGDG and the non-lamella-forming lipid MGDG affects the stability of thylakoid membranes (Williams, 1998; Shimojima and Ohta, 2011). It is known that MGDG and DGDG are readily interconvertible (Harwood, 1998). Under stress conditions, the relatively high level of bilayer lipid could facilitate lamellar membrane stability. In this study, the ratio of DGDG to MGDG was increased under salt stress (Fig. 9); similar results were found in cyanobacteria, Catharanthus roseus cultured cells, and Suaeda salsa (Huflejt et al., 1990; Elkahoui et al., 2004; Sui et al., 2010). These results indicate that plants apparently avoid lipid compositions that could result in excessive membrane destabilization or in the formation of nonlamellar phases within the bilayer under salt stress. Also, the increase of the DGDG-MGDG ratio reflects the tendency to compensate for the effect of high salt (Na+) concentration on the membrane surface by the synthesis of a stabilizing lipid species (Huflejt et al., 1990). Modulation of the DGDG-MGDG ratio also occurs in response to other environmental stresses, such as drought and high temperature. It is suggested that the increase in DGDG-MGDG ratio could be an adaptive strategy of the tolerant cultivar to resist drought stress by maintaining the membrane in a physical state compatible with the normal functioning of membrane proteins (Torres-Franklin et al., 2007). The increased DGDG-MGDG ratio is also widely believed to enhance the stability of the thylakoid membrane at elevated temperatures (Süss and Yordanov, 1986; Chen et al., 2006). The relative amount of DGDG and/or the DGDG-MGDG ratio also play critical roles in other essential cellular processes, such as protein folding, insertion, and intracellular protein trafficking in chloroplast (Bruce, 1998; Chen et al., 2006). On the contrary, the reduced DGDG level and the ratio of DGDG to MGDG could block the accumulation of chlorophyll and decrease the ability of the plants to acquire thermotolerance to high-temperature stress (Chen et al., 2006). In addition, it has also been demonstrated that the MGDG produced by type B MGD was thought to be used directly for subsequent DGDG synthesis in Arabidopsis under phosphate starvation (Kobayashi et al., 2009a; Shimojima and Ohta, 2011). In this study, the transgenic plants maintained a higher ratio of DGDG to MGDG than the wild type did, giving them a high capability to stabilize and maintain their membrane structures and functions, and also maintained a high content of chlorophyll; this could explain how these transgenic plants maintained high photosynthetic rates under salt stress.
Another fact is that MGDG and DGDG are not only the essential lipid constituents of photosynthetic membranes but are also, as revealed by crystallization studies, part of the photosynthetic complex. MGDG is associated with the core of the reaction center of PSI, PSII, and the cytochrome b6f complex, and DGDG is a component of PSI, PSII, and LHCII (Jordan et al., 2001; Stroebel et al., 2003; Liu et al., 2004; Loll et al., 2005; Mizusawa and Wada, 2012). A decrease in MGDG and/or DGDG content may lead to a negative effect on a number of photosynthetic processes. A study by Aronsson et al. (2008) showed that an Arabidopsis mgd1 mutant has low CO2-saturated assimilation capacity due to a reduced capacity of nonphotochemical quenching and an impairment of thylakoid membrane energization and photoprotection in leaves. Other evidence revealing the pivotal role of galactolipids in the photosynthetic performance of plants comes from the dgd1 mutant, which contains less than 10% of the DGDG content and shows stunted growth, pale green leaf color, and reduced photosynthetic capacity due to the reduced stability of LHCII, which is the most abundant pigment-protein complex (Dörmann et al., 1995). These results clearly indicate that MGDG and DGDG do not simply provide a membrane environment to physically support the photosynthetic complex; rather, they also contribute directly to various photosynthesis-related processes. Moreover, the DGDG-MGDG ratio is also crucial for maintaining the structure and function of the photosynthetic apparatus (Dörmann and Benning, 2002). For example, x-ray crystallographic analysis showed that the cyanobacterial PSII has four DGDG and six MGDG molecules, which are structurally and functionally important for the flexibility and assembly of PSII (Loll et al., 2005; Mizusawa and Wada, 2012). In this study, the lower degradation of galactolipid contents and increased DGDG-MGDG ratio in transgenic plants could contribute to the reduction of damage to the photosynthetic apparatus and maintain chlorophyll content and photosynthetic capability under salt stress. Vice versa, in wild-type cv SR1 plants, the reduced photosynthetic quantum yield may be a direct consequence of the disassembly of photosynthetic complexes and the reduction in chlorophyll content. Meanwhile, the decrease in photosynthetic efficiency seems to be a likely cause for the inhibition of plant growth under salt stress.
It is worth noting that the transgenic lines possessed high galactolipid levels under control conditions and also high amounts of chlorophyll under the same conditions compared with the wild type, but no growth difference was observed between these transgenic and wild-type plants. These results suggest that the oversynthesized galactolipids tended to accumulate in chloroplasts and that this accumulation has no effect on plant growth under nonstress conditions. One of the reasons to consider this possibility is that tobacco is a plant with a low light saturation point; thus, the increased chlorophyll content will be less effective at increasing the photosynthetic and growth rates, especially under the low-light growth conditions used in these experiments (200 μmol photons m−2 s−1).
It is known that tobacco is a typical 16:3 plant, possessing both prokaryotic and eukaryotic pathways for MGDG biosynthesis (Heemskerk et al., 1990). Here, we observed that both 18:3-16:3 and 18:3-18:3 MGDG levels were significantly decreased by salt treatment (Fig. 8), indicating that both the prokaryotic pathway and the eukaryotic pathway leading to MGDG synthesis were strongly affected by salt stress. The prokaryotic pathway takes place exclusively in the chloroplastic compartment, and the eukaryotic pathway involves the chloroplastic compartment and the endoplasmic reticulum (Benhassaine-Kesri et al., 2002). Thus, these results suggest that salt stress possibly interferes with the function of both the chloroplastic compartment and the endoplasmic reticulum. Unlike salt stress, drought stress resulted in a decrease only in the prokaryotic molecular species of MGDG but had no effect on the eukaryotic molecular species in rape (Brassica napus), which has also been identified as a 16:3 plant (Benhassaine-Kesri et al., 2002). In addition, it is obvious that under normal conditions, the transgenic plants had significantly higher 18:3-18:3 MGDG contents than the wild-type did (Fig. 8). The localization study showed that OsMGD is located in the outer envelope membrane, where the type B MGD-catalyzed synthesis of MGDG takes place. Since type B MGD is more specialized for diacylglycerol derived from the eukaryotic pathway, the overexpression of OsMGD may facilitate the promotion of the eukaryotic pathway. Another consideration is that rice is regarded as an 18:3 plant, which only possesses the eukaryotic pathway, and genes from 18:3 plants are thought to prefer 18:2-18:2 diacylglycerol. However, there is a lack of evidence, since the properties of type B from 18:3 plants are largely unknown (Shimojima and Ohta, 2011).
In conclusion, overexpression of the OsMGD gene in tobacco plants alleviates the effects of salt stress on growth inhibition; this alleviating effect could be ascribed to a high galactolipid synthesis ability, which would enable plants to maintain high chlorophyll content, high protein-packing density in thylakoids, stable chloroplast ultrastructure, and less impairment of photosynthetic capability under salt stress. Here, we report a clear role of the OsMGD gene in plant salt tolerance and prove that increased galactolipid levels and DGDG-MGDG ratios through the overexpression of the OsMGD gene conferred improved salt tolerance. This could be a promising avenue for research into possible improvements in salt stress tolerance in crop species.
MATERIALS AND METHODS
Construction of the Plant Expression Vector and Plant Transformation
The cDNA containing the OsMGD gene (accession no. AB112060) was amplified by PCR using the Advantage 2 PCR Enzyme System (Clontech) with gene-specific primers (5′-ACCATGGAGAGCTCGTACAAGTT-3′) and (5′-CATAAGTTGTATAGGTGTGGTTTC-3′). The amplified coding sequence of OsMGD was cloned into the pENTR TOPO vector using pENTR Directional TOPO Cloning Kits (Invitrogen) according to standard procedures. The OsMGD gene sequence was then transferred from pENTR into the plant expression vector pGWB2 containing the cauliflower mosaic virus 35S promoter using the Gateway LR Clonase II enzyme kits (Invitrogen) according to standard procedures. The pGWB2-OsMGD construct was subsequently transformed into the C58C1 Agrobacterium tumefaciens strain by electroporation (Gene Pulser, model 1652098; Bio-Rad Laboratories) set at 600 Ω, 25 μF, and 2.5 kV. A. tumefaciens transformation of tobacco (Nicotiana tabacum ‘SR1’) was done according to the method of Badawi et al. (2004).
Molecular Analysis of Transgenic Tobacco
To verify the presence of the OsMGD transgene, genomic DNA was isolated from transgenic and wild-type cv SR1 tobacco leaves using the ISOPLANT II kit (Nippon Gene). Gene-specific primers were used to conduct the PCR. For immunodetection of OsMGD protein in transgenic plants, full-length OsMGD cDNA was first cloned into pBAD TOPO TA vector (Invitrogen). The 6×His-tagged recombinant OsMGD protein was purified by nickel-nitrilotriacetic acid agarose (Qiagen) according to the manufacturer’s instructions. The protein was injected into guinea pigs to raise antibodies against the purified recombinant OsMGD protein. Protein was extracted after leaves were homogenized in 50 mm Tris-HCl (pH 7.5) buffer containing 100 mm NaCl, 1 mm EDTA, 0.1% (w/v) SDS, and 1% (v/v) protease inhibitor (Sigma-Aldrich). Total protein concentration was determined using a Bio-Rad protein assay reagent with bovine serum albumin as the standard. Proteins (20 μg) were separated by 10% (w/v) SDS-PAGE and transferred to nitrocellulose membrane (Amersham Biosciences). Immunoblot analysis was performed using diluted (1:2,000) guinea pig antibodies raised against His-tagged OsMGD protein as the primary antibody and a diluted (1:5,000) horseradish peroxidase-conjugated anti-guinea pig IgG (Sigma-Aldrich) as the secondary antibody. The protein was visualized using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Measurement of MGDG Synthase Activity
Leaves were frozen with liquid nitrogen, ground to a powder, and extracted with 50 mm MOPS-KOH (pH 7.8) containing 1 mm dithiothreitol, 6 mm CHAPS, 25 mm KCl, 25 mm KH2PO4, 2% (v/v) glycerol, and 150 mm NaCl according to the method of Dubots et al. (2010). Ten volumes of buffer (to the weight of tissues) was used for homogenization. The reaction mixture consisted of 50 mg L−1 diolein, 12 mg L−1 phosphatidic acid, and 10 or 50 μL of enzyme in a total volume of 400 μL (adjusted with homogenized buffer). The reaction was started by adding 5 μL of UDP-Gal [6-3H] (20 kBq per 13.5 pmol). After incubation at 25°C for 30 min, lipids were extracted (Bligh and Dyer, 1959), and the chloroform layer containing lipids was washed once with water. The layer of lipids was taken and mixed with a liquid scintillator, and radioactivity was measured.
Isolation of Chloroplast Inner and Outer Envelope Membranes and Immunoblot Analysis
Intact chloroplast inner and outer envelope membranes were isolated from leaf homogenates according to the method of Miyao (2009). Freshly collected leaves were homogenized in ice-cold 0.33 m sorbitol, 50 mm MES-NaOH (pH 6.1), 2 mm EDTA, 1 mm MnCl2, 1 mm MgCl2, 20 mm NaCl, 2 mm isoascorbic acid, and 1% (w/v) polyvinylpyrrolidone-40. Chloroplasts were sedimented for 70 s at 2,500g and then purified on a 40% (v/v) and 90% (v/v) Percoll gradient in buffer A (0.33 m sorbitol, 50 mm HEPES-NaOH [pH 6.8], 2 mm EDTA, 1 mm MnCl2, 1 mm MgCl2, 1 mm sodium pyrophosphate, 5 mm isoascorbic acid, and 5 mm glutathione) with a swing rotor at 2,500g for 20 min. The intact chloroplasts were recovered in the lower green layer (Supplemental Fig. S1A), washed with buffer A at 2,500g for 70 s, and suspended in 0.6 m Suc in Tris-EDTA (TE) buffer (pH 7.5), and the chlorophyll concentration was adjusted to 2 mg mL−1. After being kept on ice for 10 min, the chloroplasts were ruptured by three freeze-thaw cycles in which the suspension was placed in a −20°C freezer for 1.5 h and then at room temperature until thawed. The suspension of broken chloroplasts was adjusted to 0.2 m Suc by TE buffer and centrifuged at 4,500g for 15 min to remove most of the thylakoids and residual intact chloroplasts, whereas the envelope membranes remained in the supernatant fraction. Then the supernatant was centrifuged at 40,000g for 30 min to gather the envelope membranes, which were subsequently suspended in 0.2 m Suc in TE buffer. The envelope suspension was placed on a linear gradient of 0.6 to 1.2 m Suc in TE buffer and centrifuged at 113,000g for 14 h at 4°C in a swing rotor (RPS50-2; Hitachi). The fraction of inner envelope membranes was recovered from the lower and the fraction of outer envelope membranes from the upper Suc/Suc interface of the gradients (Supplemental Fig. S1B). Immunoblotting was performed using standard techniques, as mentioned above. Polyclonal antibodies against the chloroplast outer and inner envelope membrane proteins Tic40 and Toc75 were purchased from Agrisera (AS 10709 and AS06 150), and the peroxidase-conjugated goat anti-rabbit secondary antibody was purchased from Sigma-Aldrich (A 6154).
Plant Growth Conditions and Stress Treatments
Three transgenic lines (MGD2, MGD3, and MGD5) and wild-type cv SR1 plants were used in this study. Seeds were sterilized with 1% (w/v) NaClO for 20 min and sown on the surfaces of one-half-strength Murashige and Skoog (Murashige and Skoog, 1962) agar plates containing 3% (w/v) Suc and 0 (for wild-type cv SR1) or 50 mg L−1 (for transgenic lines) hygromycin for 45 d. The seedlings were then transplanted into one-quarter-strength Hoagland solution (pH 5.5) for further culture, and the culture solution was changed every 3 d. Twenty days after hydroponic culture, the uniform seedlings were transplanted into pots each containing 1 kg of soil. Salt treatment was performed at the five- to six-leaf stage by irrigating plants with 70 mL of a 0 or 200 mm NaCl solution every day for 10 d. All experiments were carried out in a growth chamber kept at 25°C with a 14/10-h photoperiod at 200 μmol photons m−2 s−1.
Chlorophyll Content and Photosynthesis Measurement
Fresh leaves (0.2 g) were homogenized with 80% (v/v) acetone. After centrifugation, the extract was subjected to spectrophotometric measurements at 645, 663, and 652 nm. The amount of chlorophyll was calculated according to the method described by Knudson et al. (1977).
Photosynthetic rate was measured in a growth chamber using a portable photosynthesis system (Li-6400; LI-COR). A leaf was placed in a 6-cm2 chamber at a photon flux density of 500 µmol photons m−2 s−1, a flow rate through the chamber of 500 mL s−1, and a leaf temperature of 28°C. The effective quantum yield was determined with a PAM Chlorophyll Fluorometer (PAM-2100; Walz) according to the manufacturer’s instructions.
Ion Content Measurement
Dried leaf material was milled to a powder for ion analysis. The powder was weighed and digested in nitric acid in a glass tube at 200°C on a hot stove, and hydrogen peroxide (20 µL) was added two to three times during the digestion. When digestion was finished, only a dry white residue was left. This residue was dissolved in 10% (v/v) nitric acid. Sodium content was measured with an atomic absorption spectrophotometer (5100; PerkinElmer). The ion content was expressed as mg kg−1 dry weight.
Transmission Electron Microscopy for Chloroplast Ultrastructure
Leaf tissues were trimmed with a razor blade, fixed in 2% (v/v) glutaraldehyde in 0.1 m phosphate-buffered saline (pH 7.0) by infiltration under vacuum, and treated for 2 h. The fixed samples were washed in phosphate-buffered saline and postfixed in 2% (w/v) osmium tetroxide in the same buffer for 2 h. They were then dehydrated in an ethanol series, transferred to propylene oxide, and embedded in Quetol 812 resin. Thin sections (70–100 nm) were cut with a diamond knife on an ultramicrotome (MT-7000; RMC), and the sections were stained with 2% (w/v) uranyl acetate for 40 min and then with 3% (w/v) lead citrate for 2 to 3 min. The samples were observed using a transmission electron microscope (JEM-100CX II; JEOL) at 80 kV. The repeat distance of grana thylakoid membranes was measured from transmission electron microscopy images. In short, the distance of five layers of grana thylakoid membranes (from the middle of the first membrane to the middle of the fifth membrane) was measured, and the value was divided by 4 to calculate the repeat distance of grana thylakoid membranes.
Lipid Analysis
After treatment, 1 g of fresh leaves was boiled in distilled water for 10 min to inactivate any endogenous lipolytic enzymes. MGDG and DGDG were analyzed by means of electrospray ionization tandem mass spectrometry (ESI MS/MS; 3200 Q TRAP; AB SCIEX) according to the method of Devaiah et al. (2006). Leaves were placed in 15 mL of isopropanol with 0.01% (w/v) butylated hydroxytoluene and heated at 75°C for 15 min. Then, 7.5 mL of chloroform and 3 mL of water were added, and the mixture was agitated at 37°C for 1 h. After removal of the extract, the leaves were reextracted twice more with 5 mL of chloroform:methanol (2:1, v/v) with 0.01% (w/v) butylated hydroxytoluene until the remaining leaves turned white (30 min of agitation each time). The combined extract for each sample was evaporated, and the residue was dissolved in 10 mL of chloroform. For MGDG and DGDG analysis, 200 μL of extract was added to a mixture with a final composition of chloroform:methanol:50 mm aqueous sodium acetate at a ratio of 300:665:35, containing 20 nmol of hydrogenated MGDG and 10 nmol of hydrogenated DGDG (Matreya) as internal standards. The collision gas pressure was set at 1 (arbitrary units), and the collision energies were 84 eV for MGDG and 116 eV for DGDG. The source temperature was 100°C, the interface heater was on, the electrospray capillary was 5,500 V, and the curtain gas was set at 20 (arbitrary units). The flow rate was set at 5 μL min−1, and scans of the precursor of mass-to-charge ratio 243 in the mass range 750 to 850 (for MGDG) and 900 to 1,000 (for DGDG) were recorded. In each spectrum, the data were smoothed, and the peak area was integrated by Analyst 1.5.1 software. Corrections for the overlap of isotopic variants (A + two peaks) in higher mass lipids were applied. The lipids in each class were quantified in comparison with the internal standards of that class. In addition, in order to further investigate changes in the chloroplast, the lipid-to-chlorophyll ratio was calculated from the total MGDG or DGDG contents and the chlorophyll contents.
Statistical Analyses
Each experiment contained four or six independent replicates. Data were subjected to ANOVA using Statistical Analysis System (SAS version 8.0) software. Differences between means were compared by lsd test at P < 0.05 (for MGDG synthase activity data) or Tukey-Kramer test at P < 0.05 (for all other data).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AB112060.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Purification of intact chloroplasts and isolation of chloroplast inner and outer envelope membranes.
Supplementary Material
Acknowledgments
We thank Dr. Tsuyoshi Nakagawa (Shimane University) for providing the Gateway Plant Expression Vector pGWB2.
Glossary
- MGDG
monogalactosyldiacylglycerol
- DGDG
digalactosyldiacylglycerol
- LHCII
light-harvesting complex II
- cDNA
complementary DNA
- ESI MS/MS
electrospray ionization tandem mass spectrometry
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31200206), the West Light Foundation of the Chinese Academy of Sciences, the Chinese Universities Scientific Fund (grant no. ZD2012023), and the 111 Project of Chinese Education Ministry (grant no. B12007).
The online version of this article contains Web-only data.
References
- Aronsson H, Schöttler MA, Kelly AA, Sundqvist C, Dörmann P, Karim S, Jarvis P. (2008) Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiol 148: 580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K. (2004) Enhanced tolerance to salt stress and water deficit by overexpression superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166: 919–928 [Google Scholar]
- Benhassaine-Kesri G, Aid F, Demandre C, Kader JC, Mazliak P. (2002) Drought stress affects chloroplast lipid metabolism in rape (Brassica napus) leaves. Physiol Plant 115: 221–227 [DOI] [PubMed] [Google Scholar]
- Benning C, Ohta H. (2005) Three enzyme systems for galactoglycerolipid biosynthesis are coordinately regulated in plants. J Biol Chem 280: 2397–2400 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Block MA, Dorne AJ, Joyard J, Douce R. (1983) Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem 258: 13281–13286 [PubMed] [Google Scholar]
- Botté CY, Deligny M, Roccia A, Bonneau AL, Saïdani N, Hardré H, Aci S, Yamaryo-Botté Y, Jouhet J, Dubots E, et al. (2011) Chemical inhibitors of monogalactosyldiacylglycerol synthases in Arabidopsis thaliana. Nat Chem Biol 7: 834–842 [DOI] [PubMed] [Google Scholar]
- Brown DJ, Dupont FM. (1989) Lipid composition of plasma membranes and endomembranes prepared from roots of barley (Hordeum vulgare L.): effects of salt. Plant Physiol 90: 955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce BD. (1998) The role of lipids in plastid protein transport. Plant Mol Biol 38: 223–246 [PubMed] [Google Scholar]
- Chen J, Burke JJ, Xin Z, Xu C, Velten J. (2006) Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant Cell Environ 29: 1437–1448 [DOI] [PubMed] [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. (2006) Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant. Phytochemistry 67: 1907–1924 [DOI] [PubMed] [Google Scholar]
- Dörmann P, Balbo I, Benning C. (1999) Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 284: 2181–2184 [DOI] [PubMed] [Google Scholar]
- Dörmann P, Benning C. (2002) Galactolipids rile in seed plants. Trends Plant Sci 7: 112–118 [DOI] [PubMed] [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C. (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7: 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubots E, Audry M, Yamaryo Y, Bastien O, Ohta H, Breton C, Maréchal E, Block MA. (2010) Activation of the chloroplast monogalactosyldiacylglycerol synthase MGD1 by phosphatidic acid and phosphatidylglycerol. J Biol Chem 285: 6003–6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahoui S, Smaoui A, Zarrouk M, Ghrir R, Limam F. (2004) Salt-induced lipid changes in Catharanthus roseus cultured cell suspensions. Phytochemistry 65: 1911–1917 [DOI] [PubMed] [Google Scholar]
- Garab G, Lohner K, Laggner P, Farkas T. (2000) Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis. Trends Plant Sci 5: 489–494 [DOI] [PubMed] [Google Scholar]
- Gaude N, Bréhélin C, Tischendorf G, Kessler F, Dörmann P. (2007) Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J 49: 729–739 [DOI] [PubMed] [Google Scholar]
- Haferkamp S, Kirchhoff H. (2008) Significance of molecular crowding in grana membranes of higher plants for light harvesting by photosystem II. Photosynth Res 95: 129–134 [DOI] [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C. (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97: 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Grimm B, Benning C. (1997) Changes in the composition of the photosynthetic apparatus in the galactolipid-deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol 115: 1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood JL. (1996) Recent advances in the biosynthesis of plant fatty acids. Biochim Biophys Acta 1301: 7–56 [DOI] [PubMed] [Google Scholar]
- Harwood JL (1998) Involvement of chloroplast lipids in the reaction of plants submitted to stress. In PA Siegenthaler, N Murata, eds, Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 287–302 [Google Scholar]
- Heemskerk JWM, Storz T, Schmidt RR, Heinz E. (1990) Biosynthesis of digalactosyldiacylglycerol in plastids from 16:3 and 18:3 plants. Plant Physiol 93: 1286–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama O, Mihara M. (1987) Characterization of membrane lipids of higher plants different in salt-tolerance. Agric Biol Chem 51: 3215–3221 [Google Scholar]
- Hölzl G, Dörmann P. (2007) Structure and function of glycoglycerolipids in plants and bacteria. Prog Lipid Res 46: 225–243 [DOI] [PubMed] [Google Scholar]
- Huflejt ME, Tremolieres A, Pineau B, Lang JK, Hatheway J, Packer L. (1990) Changes in membrane lipid composition during saline growth of the fresh water cyanobacterium Synechococcus 6311. Plant Physiol 94: 1512–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P, Dörmann P, Peto CA, Lutes J, Benning C, Chory J. (2000) Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc Natl Acad Sci USA 97: 8175–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411: 909–917 [DOI] [PubMed] [Google Scholar]
- Kirchhoff H, Sharpe RM, Herbstova M, Yarbrough R, Edwards GE. (2013) Differential mobility of pigment-protein complexes in granal and agranal thylakoid membranes of C3 and C4 plants. Plant Physiol 161: 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson LL, Tibbitts TW, Edwards GE. (1977) Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol 60: 606–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Awai K, Nakamura M, Nagatani A, Masuda T, Ohta H. (2009a) Type-B monogalactosyldiacylglycerol synthases are involved in phosphate starvation-induced lipid remodeling, and are crucial for low-phosphate adaptation. Plant J 57: 322–331 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Awai K, Takamiya K, Ohta H. (2004) Arabidopsis type B monogalactosyldiacylglycerol synthase genes are expressed during pollen tube growth and induced by phosphate starvation. Plant Physiol 134: 640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kondo M, Fukuda H, Nishimura M, Ohta H. (2007) Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proc Natl Acad Sci USA 104: 17216–17221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Nakamura Y, Ohta H. (2009b) Type A and type B monogalactosyldiacylglycerol synthases are spatially and functionally separated in the plastids of higher plants. Plant Physiol Biochem 47: 518–525 [DOI] [PubMed] [Google Scholar]
- Krumova SB, Laptenok SP, Kovács L, Tóth T, van Hoek A, Garab G, van Amerongen H. (2010) Digalactosyl-diacylglycerol-deficiency lowers the thermal stability of thylakoid membranes. Photosynth Res 105: 229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG. (2000) Membrane lipids: it’s only a phase. Curr Biol 10: R377–R380 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W. (2004) Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. (2005) Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II. Nature 438: 1040–1044 [DOI] [PubMed] [Google Scholar]
- Miyao M. (2009) Isolation of intact chloroplasts, thylakoids and photosystem II membranes from higher plants. Low Temp Sci 67: 197–203 [Google Scholar]
- Mizusawa N, Wada H. (2012) The role of lipids in photosystem II. Biochim Biophys Acta 1817: 194–208 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Benning C. (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16: 98–107 [DOI] [PubMed] [Google Scholar]
- Møller IS, Tester M. (2007) Salinity tolerance of Arabidopsis: a good model for cereals? Trends Plant Sci 12: 534–540 [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Myers AM, James MG, Lin Q, Yi G, Stinard PS, Hennen-Bierwagen TA, Becraft PW. (2011) Maize opaque5 encodes monogalactosyldiacylglycerol synthase and specifically affects galactolipids necessary for amyloplast and chloroplast function. Plant Cell 23: 2331–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Shimojima M, Ohta H, Kobayashi K (2010) Biosynthesis and function of monogalactosyldiacylglycerol (MGDG), the signature lipid of chloroplasts. In CA Rebeiz, C Benning, H Bohnert, H Daniell, JK Hoober, HK Lichtenthaler, AR Portis, BC Tripathy, eds, The Chloroplasts: Basics and Applications, Springer, Dordrecht, The Netherlands, pp 185–202 [Google Scholar]
- Qi Y, Yamauchi Y, Ling JQ, Kawano N, Li DB, Tanaka K. (2004) Cloning of a putative monogalactosyldiacylglycerol synthase gene from rice (Oryza sativa L.) plants and its expression in response to submergence and other stresses. Planta 219: 450–458 [DOI] [PubMed] [Google Scholar]
- Shimojima M, Ohta H. (2011) Critical regulation of galactolipid synthesis controls membrane differentiation and remodeling in distinct plant organs and following environmental changes. Prog Lipid Res 50: 258–266 [DOI] [PubMed] [Google Scholar]
- Shimojima M, Ohta H, Iwamatsu A, Masuda T, Shioi Y, Takamiya K. (1997) Cloning of the gene for monogalactosyldiacylglycerol synthase and its evolutionary origin. Proc Natl Acad Sci USA 94: 333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu S, Guo SR, Sun J, Yuan LY. (2012) Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol Plant 146: 285–296 [DOI] [PubMed] [Google Scholar]
- Simidjiev I, Stoylova S, Amenitsch H, Jávorfi T, Mustárdy L, Laggner P, Holzenburg A, Garab G. (2000) Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci USA 97: 1473–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, Choquet Y, Popot JL, Picot D. (2003) An atypical haem in the cytochrome b6f complex. Nature 426: 413–418 [DOI] [PubMed] [Google Scholar]
- Sui N, Li M, Li K, Son J, Wang BS. (2010) Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica 48: 623–629 [Google Scholar]
- Süss KH, Yordanov IT. (1986) Biosynthetic cause of in vivo acquired thermotolerance of photosynthetic light reactions and metabolic responses of chloroplasts to heat stress. Plant Physiol 81: 192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Franklin ML, Gigon A, de Melo DF, Zuily-Fodil Y, Pham-Thi AT. (2007) Drought stress and rehydration affect the balance between MGDG and DGDG synthesis in cowpea leaves. Physiol Plant 131: 201–210 [DOI] [PubMed] [Google Scholar]
- Webb MS, Green BR. (1991) Biochemical and biophysical properties of thylakoid acyl lipids. Biochim Biophys Acta 1060: 133–158 [Google Scholar]
- Williams WP (1998) The physical properties of thylakoid membrane lipids and their relation to photosynthesis. In PA Siegenthaler, N Murata, eds, Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 103–118 [Google Scholar]
- Yamane K, Rahman MS, Kawasaki M, Taniguchi M, Miyake H. (2004) Pretreatment with a low concentration of methyl viologen decreases the effects of salt stress on chloroplast ultrastructure in rice leaves (Oryza sativa L.). Plant Prod Sci 7: 435–441 [Google Scholar]
- Yoshida S, Uemura M. (1986) Lipid composition of plasma membranes and tonoplasts isolated from etiolated seedlings of mung bean (Vigna radiata L.). Plant Physiol 82: 807–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.