A single gene product mediates in the virulence activity of a bacterial effector.
Abstract
Enhanced disease susceptibility1 (EDS1) and phytoalexin deficient4 (PAD4) are well-known regulators of both basal and resistance (R) protein-mediated plant defense. We identified two EDS1-like (GmEDS1a/GmEDS1b) proteins and one PAD4-like (GmPAD4) protein that are required for resistance signaling in soybean (Glycine max). Consistent with their significant structural conservation to Arabidopsis (Arabidopsis thaliana) counterparts, constitutive expression of GmEDS1 or GmPAD4 complemented the pathogen resistance defects of Arabidopsis eds1 and pad4 mutants, respectively. Interestingly, however, the GmEDS1 and GmPAD4 did not complement pathogen-inducible salicylic acid accumulation in the eds1/pad4 mutants. Furthermore, the GmEDS1a/GmEDS1b proteins were unable to complement the turnip crinkle virus coat protein-mediated activation of the Arabidopsis R protein Hypersensitive reaction to Turnip crinkle virus (HRT), even though both interacted with HRT. Silencing GmEDS1a/GmEDS1b or GmPAD4 reduced basal and pathogen-inducible salicylic acid accumulation and enhanced soybean susceptibility to virulent pathogens. The GmEDS1a/GmEDS1b and GmPAD4 genes were also required for Resistance to Pseudomonas syringae pv glycinea2 (Rpg2)-mediated resistance to Pseudomonas syringae. Notably, the GmEDS1a/GmEDS1b proteins interacted with the cognate bacterial effector AvrA1 and were required for its virulence function in rpg2 plants. Together, these results show that despite significant structural similarities, conserved defense signaling components from diverse plants can differ in their functionalities. In addition, we demonstrate a role for GmEDS1 in regulating the virulence function of a bacterial effector.
Resistance (R) protein-mediated or effector-triggered immunity (ETI) is one of the several different types of defense mechanisms induced in response to pathogen infection in plants. ETI primarily protects against specific races of pathogens and involves the deployment of specific plant R proteins, which recognize cognate pathogen effectors, termed avirulence (Avr) proteins (gene-for-gene interactions; Flor, 1971). A majority of the known plant R proteins contain conserved structural domains, including N-terminal coiled-coil (CC) or Toll-interleukin1 receptor (TIR)-like domains, a central nucleotide-binding (NB) domain, and C-terminal leucine-rich repeat (LRR) domains (Kachroo et al., 2006; Martin et al., 2009. Plant R proteins can recognize cognate pathogen Avr effectors via direct interactions between the two proteins or indirectly by perceiving changes in other host proteins targeted by the pathogen effector (Chisholm et al., 2006; Jones and Dangl, 2006; Kachroo et al., 2006). Induction of R-mediated signaling is often accompanied by a hypersensitive response (HR), which is typified by localized cell death at the site of pathogen entry, early on during infection (Holliday et al., 1981). HR is usually considered to be one of the first visible manifestations of pathogen-induced defense responses. However, it is unclear whether HR is the cause or consequence of resistance signaling. Subsequent induced responses in the infected and uninfected (distal) parts of the plant involve an increase in endogenous levels of defense-related phytohormones, including salicylic acid (SA), and transcriptional up-regulation of a large set of defense genes, including those encoding pathogenesis-related (PR) proteins (Dempsey et al., 1999; Durrant and Dong, 2004).
SA-induced responses are a well-known essential feature of plant resistance signaling (Vlot et al., 2009). Mutations that impair pathogen-induced SA accumulation or SA responsiveness enhance susceptibility to several pathogens. Examples include SA induction-deficient2 (sid2; mutation in isochorishmate synthase1 [ICS1]), which impairs SA accumulation, and nonexpressor of PR1 (npr1), which impairs SA signaling (Cao et al., 1997; Ryals et al., 1997; Wildermuth et al., 2001). In addition, mutations in enhanced disease susceptibility1 (eds1), eds5, and phytoalexin deficient4 (pad4) also impair SA levels and/or responsiveness and thereby enhance susceptibility to pathogen infection (Falk et al., 1999; Jirage et al., 1999; Nawrath et al., 2002). The ICS1, NPR1, EDS1, EDS5, and PAD4 proteins also mediate basal or pathogen-associated molecular patterns-triggered immunity (PTI; Cao et al., 1997; Ryals et al., 1997; Falk et al., 1999; Jirage et al., 1999; Wildermuth et al., 2001; Nawrath et al., 2002). EDS1 and PAD4 also function independently of the SA-mediated pathway (Chandra-Shekara et al., 2004; Bartsch et al., 2006; Wang et al., 2008). EDS1 and PAD4 are structurally related to lipase/esterase-like proteins, although lipase-like (LP) biochemical activities have not been demonstrated for either protein (Falk et al., 1999; Feys et al., 2001, 2005). The Arabidopsis (Arabidopsis thaliana) EDS1 and PAD4 proteins interact with each other and are required for HR formation and pathogen resistance (Feys et al., 2001, 2005; He and Gan, 2002; Zhu et al., 2011). In addition to PAD4, EDS1 also interacts with senescence-associated protein101 (SAG101), another LP protein, which can form a ternary complex with EDS1 and PAD4, and functions in pathogen resistance (Feys et al., 2005; Zhu et al., 2011). EDS1 also interacts with suppressor of rps4-RLD1 (SRFR1), a tetratricopeptide repeat domain containing a negative regulator of ETI derived from TIR-NB-LRR proteins (Kim et al., 2009; Kwon et al., 2009).
EDS1 was thought to participate in resistance signaling mediated primarily by the TIR-NB-LRR type of R proteins (Aarts et al., 1998). However, recent findings show that EDS1 and SA function redundantly in signaling mediated by some CC-NB-LRR proteins, and it is likely that this redundancy masked the requirements for EDS1 by such R proteins (Venugopal et al., 2009). Thus, their requirement for EDS1 only became evident in plants lacking the ability to generate pathogen-responsive SA (Venugopal et al., 2009). Examples include HR to Turnip crinkle virus (HRT), which confers resistance to turnip crinkle virus (TCV; Cooley et al., 2000; Kachroo et al., 2000). EDS1 also interacts with the TIR-NB-LRR proteins RPS4 and RPS6, and these interactions are disrupted by the presence of the cognate Avr effectors AvrRps4 and HopA1, respectively (Bhattacharjee et al., 2011; Heidrich et al., 2011). EDS1 interacts with HRT and potentiates the HRT-mediated cell death response (Zhu et al., 2011). However, unlike EDS1-RPS4, the EDS1-HRT interaction is unaffected by the cognate avirulence effector, the coat protein (CP) of TCV (Zhu et al., 2011).
We isolated EDS1 (GmEDS1)- and PAD4 (GmPAD4)-like genes from soybean (Glycine max) and tested their functions in resistance derived from several R loci in soybean. We show that two EDS1 isoforms and one PAD4 protein are required for bacterial resistance derived from the soybean Resistance to Pseudomonas syringae pv glycinea2 (Rpg2) locus. The two GmEDS1 proteins directly associate with the cognate pathogen effector AvrA1 and mediate its virulence function in soybean. However, despite their structural similarities to AtEDS1, the GmEDS1 proteins are unable to substitute for all AtEDS1 functions.
RESULTS
Soybean Genome Encodes Two EDS1 Isoforms and One PAD4 Ortholog
The Arabidopsis genome encodes two EDS1 isoforms (At3g48080, designated AtEDS1-80, and At3g48090, designated AtEDS1-90), although the At3g48080 isoform is nonfunctional in many ecotypes, including Landsberg erecta, Wassilewskija (Ws), and Dijon-17 (Zhu et al., 2011). Likewise, the soybean genome contains two sequences that encode predicted proteins with similarities to AtEDS1 and one sequence encoding a predicted protein with similarity to Arabidopsis PAD4 (AtPAD4, At3g52430). The presence of two EDS1 isoforms in soybean is in line with more than one EDS1 isoform in several plant species. The soybean sequences were designated GmEDS1a (Glyma04g34800), GmEDS1b (Glyma06g19920), and GmPAD4 (Glyma08g00420). Alignment of predicted protein sequences (Supplemental Fig. S1) showed that GmEDS1a (ACT98433) and GmEDS1b (ADC45394) are approximately 86% identical to each other and approximately 43% to 44% identical to AtEDS1. Phylogenetic analysis indicates that pairs of EDS1 genes are the result of independent duplications that occurred after the separation of the soybean and Arabidopsis lineages (Fig. 1A). The predicted GmEDS1 protein sequences contained conserved domains of the lipase3 superfamily, including the active site/flap and the nucleophilic elbow (InterPro-Integrated Resource of Protein Domains and Functional Sites. © 2001 The InterPro Consortium. IPR002921). The GmEDS1 proteins also contain the conserved Ser, which lies within the nucleophilic elbow, and Asp residues but lack the conserved His comprising the catalytic triad of LP proteins (Supplemental Fig. S1). Furthermore, the predicted catalytic triad residues and the nucleophilic elbow of the GmEDS1 proteins lie within the region that is highly similar to the LP domain of AtEDS1 (residues 123–332 in GmEDS1a and 123–331 in GmEDS1b). In addition, the GmEDS1 proteins contain the EDS1-PAD4-like domain at the C-terminal end (residues 430–550 in GmEDS1a and 427–546 in GmEDS1b). Like the GmEDS1 proteins, GmPAD4 contains predicted LP and EDS1-PAD4-like domains at residues 131 to 203 and 416 to 548, respectively. The GmPAD4 contains the conserved active site for the lipase superfamily at the N terminus (Supplemental Fig. S2). In addition, the N-terminal region of GmPAD4 also contains the conserved nucleophilic elbow and the Ser and Asp residues of the catalytic triad, located within the predicted LP domain. GmPAD4 is most related to PAD4 from Populus trichocarpa (PtPAD4), sharing approximately 58% sequence identity with this protein (Fig. 1B), and is approximately 42% identical to AtPAD4 (Supplemental Fig. S2).
Figure 1.
Phylogenetic analysis and interactions between GmEDS1 and GmPAD4 proteins. A and B, Sequence alignment was carried out using ClustalW in the Megalign program of the DNASTAR package. C, In planta localization of GmEDS1a, GmEDS1b, and GmPAD4. C-terminal GFP-tagged proteins were transiently expressed in N. benthamiana using agroinfiltration. Transient expression of GFP alone is shown at right for reference. D, BiFC assay showing in planta interactions. Forty times magnification of micrographs at 48 h postinfiltration from leaves coexpressing NYFP-GmPAD4/AtPAD4/GST with the CYFP-GmEDS1a/GmEDS1b/AtEDS1 are shown. Images are representative of three separate infiltrations from two independent experiments for each interaction using both combinations of N/C-YFP-fused proteins. Bars = 10 µm. E, Relative expression of GmEDS1a, GmEDS1b, and GmPAD4 in Rpg1-b plants (cv Merit) at indicated dpi with Psg avrB as determined by qRT-PCR analysis. Error bars indicate sd (n = 3). Asterisk denotes significant difference from 0 dpi for the respective control (Student’s t test, P < 0.0001).
Consistent with the sequence conservation between the Arabidopsis and soybean proteins, GmEDS1 and GmPAD4 showed similar in planta localization as AtEDS1 and AtPAD4 (Fig. 1C; Zhu et al., 2011). Confocal microscopy of Nicotiana benthamiana leaves transiently expressing C-terminal GFP-tagged proteins showed the GmEDS1a/GmEDS1b and GmPAD4 proteins localized to the nucleus and cell periphery (Fig. 1C; Supplemental Fig. S3). Furthermore, the GmEDS1 and GmPAD4 proteins interacted with each other in bimolecular fluorescence complementation (BiFC) assays, where these proteins were tagged with reciprocal N- or C-terminal halves of enhanced yellow fluorescent protein (nEYFP and cEYFP); coexpression of GmEDS1a/GmEDS1b with GmPAD4 resulted in reconstitution of EYFP, indicating positive interaction (Fig. 1D). Likewise, GmEDS1a/GmEDS1b proteins also interacted with AtPAD4, and the GmPAD4 protein interacted with AtEDS1-90. In all cases, interaction was detected in both cytoplasm and nucleus. The sequence analysis suggests that soybean EDS1/PAD4 proteins are likely true orthologs of Arabidopsis proteins.
GmEDS1 and GmPAD4 Contribute to Defense Responses in Soybean
We tested if the GmEDS1 and GmPAD4 genes were induced in response to pathogen infection. Quantitative reverse transcription (qRT)-PCR as well as RNA gel-blot analysis showed that transcripts of both GmEDS1a and GmEDS1b were induced to high levels in soybean ‘Merit’ at 2 d postinoculation (dpi) with an avirulent strain of the bacterial pathogen Pseudomonas syringae pv glycinea (Psg) expressing AvrB (Psg avrB; Fig. 1E; Supplemental Fig. S4). GmPAD4 transcripts were not detected in RNA gel-blot analysis, but qRT-PCR analysis showed that the GmPAD4 transcripts were induced approximately 2-fold at 2 dpi with Psg avrB (Fig. 1E). These data indicated that the GmEDS1 and GmPAD4 genes were pathogen inducible. We next tested if the identified GmEDS1 and GmPAD4 genes contributed to pathogen resistance. For this, we silenced the GmEDS1a/GmEDS1b and GmPAD4 genes in soybean using the bean pod mottle virus (BPMV)-based vector (Zhang and Ghabrial, 2006; Kachroo and Ghabrial, 2012). We generated two vectors derived from GmEDS1a (SEDS1a) and GmEDS1b (SEDS1b; Supplemental Fig. S5), and each of the two GmEDS1 vectors was expected to silence both GmEDS1a and GmEDS1b simultaneously because the two genes share greater than 80% identity. This was also desirable because inhibition of defense responses in Arabidopsis is known to require mutations in both AtEDS1 isoforms (Feys et al., 2005; Zhu et al., 2011). We also generated a vector targeting GmPAD4 (SPAD4). Plants (cv Merit) infected with the various vectors were tested for silencing of the respective genes using qRT-PCR and semiquantitative reverse transcription (RT)-PCR analysis (Fig. 2A; Supplemental Fig. S6A). Expression of GmEDS1a/GmEDS1b and GmPAD4 genes in control plants infected with BPMV empty vector (V) was similar to that in mock-inoculated plants. This suggested that BPMV infection did not alter GmEDS1a/GmEDS1b or GmPAD4 expression. The SEDS1a and SEDS1b plants were each silenced for both GmEDS1a and GmEDS1b but were unaffected in the expression of GmPAD4, whereas the SPAD4 plants were silenced for GmPAD4 only (Supplemental Fig. S6A). Silencing GmEDS1a/GmEDS1b or GmPAD4 did not alter the morphology of soybean plants (Supplemental Fig. S6B).
Figure 2.
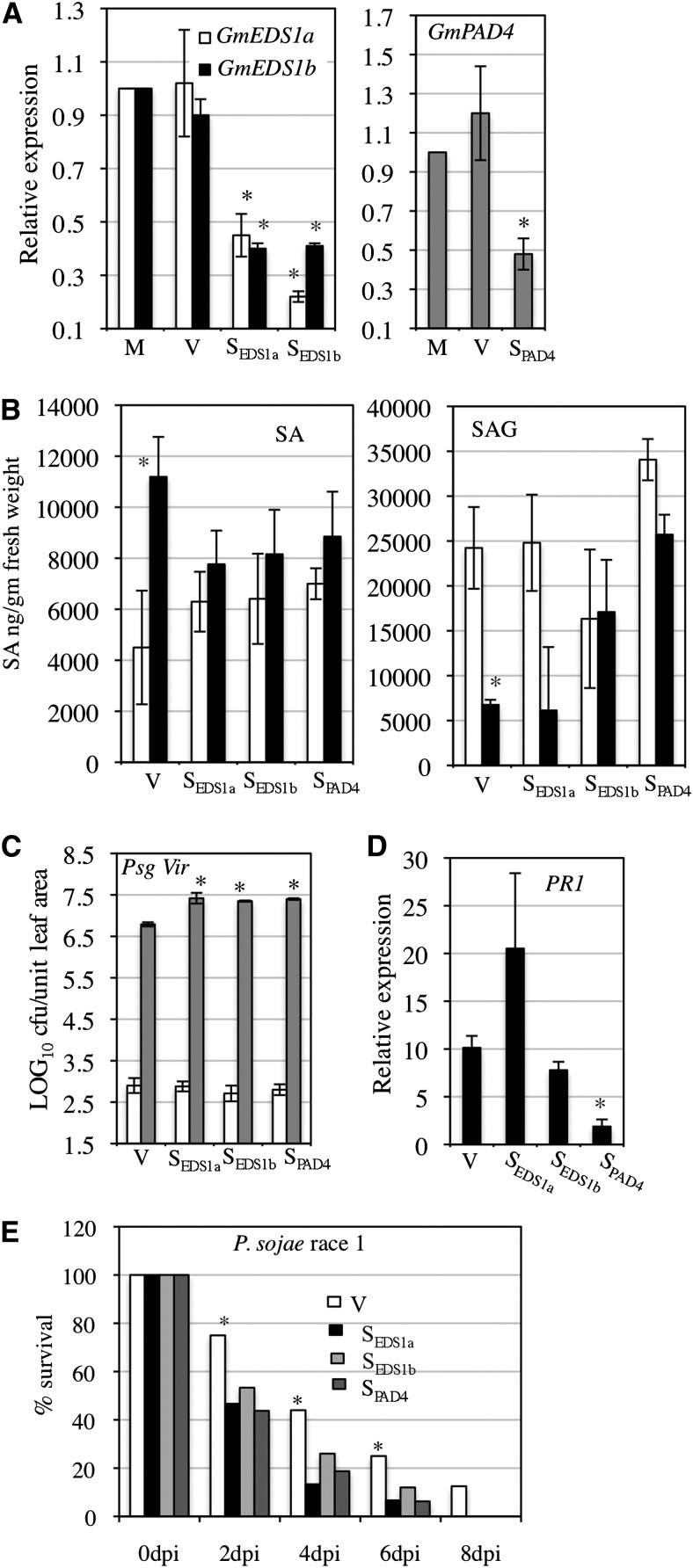
Silencing GmEDS1 and GmPAD4 inhibits defense responses in soybean. A, Relative expression of GmEDS1a, GmEDS1b, and GmPAD4 in mock-infected (M), empty vector-inoculated (V), or GmEDS1a/GmEDS1b (SEDS1a/EDS1b)- and GmPAD4 (SPAD4)-silenced plants as determined by qRT-PCR analysis. Error bars indicate sd (n = 3). Asterisks denote significant difference from mock infected (Student’s t test, P < 0.0001). B, SA and SAG levels in SEDS1a/EDS1b, SPAD4, or V plants at 0 (white bars) and 2 (black bars) dpi with P. sojae (race 1). Error bars indicate sd (n = 3). Asterisks denote significant difference from V plants (Student’s t test, P < 0.001). C, Psg Vir infection of V, SEDS1a/EDS1b, or SPAD4 plants. Log10 values of cfu per unit leaf disc from infected leaves at 0 (white bars) or 4 (gray) dpi are presented. Error bars indicate sd (n = 5). Asterisks denote statistically significant (Student’s t test) differences from V plants (P < 0.0005). D, Relative induction of PR1 expression in SEDS1a/EDS1b, SPAD4, and V plants at 2 dpi with P. syringae. Error bars indicate sd (n = 3). Asterisk denotes significant difference from V (Student’s t test, P < 0.001). E, Percentage survival of V, SEDS1a, SEDS1b, and SPAD4 plants at indicated dpi with race 1 of P. sojae. Asterisks denote significant difference from V plants (Student’s t test, P < 0.001). Results are representative of two to three independent experiments. The cultivar used is ‘Harosoy.’
Analysis of SA levels in the SEDS1a, SEDS1b, and SPAD4 plants showed that these plants were defective in pathogen-inducible SA accumulation (Fig. 2B). Infection with Phytophthora sojae resulted in approximately 3-fold increase in SA levels in the control plants (V) but not in the SEDS1a, SEDS1b, or SPAD4 plants (Fig. 2B, left). Conversely, P. sojae infection resulted in a reduction in SAG levels in the V plants. Some reduction in salicylic acid glucoside (SAG) levels was also observed in the SEDS1a plants but not SEDS1b or SPAD4 plants (Fig. 2B, right). Consistent with their reduced SA levels, the SEDS1a/EDS1b and SPAD4 plants (cv Harosoy) showed enhanced susceptibility to virulent strains of Psg (Psg Vir) or P. sojae (race 1); SEDS1a/EDS1b and SPAD4 plants consistently accumulated approximately 8-fold more Psg Vir (P < 0.001) than V plants (Fig. 2C). The enhanced susceptibility of SPAD4 plants correlated with reduced induction of pathogen-responsive PR expression in these plants (Fig. 2D). PR1 induction was not affected in the SEDS1a/EDS1b plants (Fig. 2D). The SEDS1a/EDS1b and SPAD4 plants were also significantly more susceptible to P. sojae (race 1) than V plants; at 4 dpi, only 10% to 20% of the SEDS1a/EDS1b or SPAD4 plants survived infection compared with approximately 42% of control plants (Fig. 2E). By 8 dpi, none of the SEDS1a/EDS1b or SPAD4 plants survived P. sojae infection compared with approximately 15% of V plants. Silencing the GmEDS1a/GmEDS1b and GmPAD4 genes also affected basal resistance to soybean mosaic virus (SMV). The V, SEDS1a/EDS1b, and SPAD4 plants (cv Essex) were inoculated with the G5 isolate of SMV, and virus accumulation in systemic tissues was monitored over time. Western-blot analysis of total protein extracts from infected plants showed that systemic tissues of SEDS1a/EDS1b and SPAD4 plants contained more SMV at 4 dpi compared with V plants (Supplemental Fig. S7A), and the silenced plants showed more severe SMV-associated disease symptoms at 10 dpi (Supplemental Fig. S7B). Together, these results suggest that GmEDS1a/GmEDS1b and GmPAD4 contribute to basal resistance to virulent bacterial, oomycete, and viral pathogens in soybean.
GmEDS1 and GmPAD4 Are Required for Rpg2-Mediated Resistance in Soybean
To test GmEDS1a/GmEDS1b and GmPAD4 functions in R-mediated resistance, we silenced these genes in different cultivars containing R loci with specificities against different avr strains of Psg. Four known soybean R loci mediate race-specific resistance to specific strains of Psg. These include Rpg1-b, Rpg2, Rpg3, and Rpg4, which specify resistance against Psg expressing AvrB1Pgyrace4 (commonly designated AvrB), AvrA1Pgyrace6, AvrB2Pgyrace4 (original designation, AvrC), and AvrD1PtoPT23, respectively (Staskawicz et al., 1984, 1987; Keen et al., 1988; Tamaki et al., 1988; Keen and Buzzell, 1991; Bisgrove et al., 1994; Ashfield et al., 2003, 2004; Lindeberg et al., 2005).
The GmEDS1a/GmEDS1b and GmPAD4 genes were silenced in the Rpg1-b Rpg2 (cv Merit) and Rpg3 Rpg4 (cv Flambeau) backgrounds (Supplemental Fig. S6A). The various silenced and control plants were then inoculated with respective Psg avr strains, Psg avrB and Psg avrA1 on Rpg1-b Rpg2 plants and Psg avrB2 and Psg avrD1 on Rpg3 Rpg4 plants. Analysis of pathogen growth over time showed that the SEDS1a/EDS1b and SPAD4 plants (Rpg1-b Rpg2) accumulated significantly more Psg avrA1 compared with V plants; the SEDS1a/EDS1b and SPAD4 plants each contained approximately 10-fold more Psg avrA1 than V plants (Fig. 3A). Furthermore, the levels of Psg avrA1 bacteria in the SEDS1a/EDS1b and SPAD4 plants were similar to levels of Psg Vir (virulent strain of Psg) accumulation in V plants. By contrast, the SEDS1a/EDS1b and SPAD4 plants (Rpg1-b Rpg2) accumulated comparable levels of Psg avrB as V plants (Fig. 3A). Likewise, the SEDS1a/EDS1b and SPAD4 plants in Rpg3 Rpg4 background accumulated similar levels of Psg avrB2 or Psg avrD1 as V plants (Fig. 3B). These results indicated that silencing GmEDS1a/GmEDS1b or GmPAD4 completely abolished Rpg2-mediated resistance against Psg avrA1 but did not affect resistance derived from the Rpg1-b, Rpg3, or Rpg4 loci. We next monitored ion leakage to determine if GmEDS1a/GmEDS1b or GmPAD4 also contributed to the pathogen-induced cell death phenotype. The Psg avrA1-infected SEDS1a/EDS1b and SPAD4 plants in Rpg1-b Rpg2 background showed reduced ion leakage compared with V plants (Rpg1-b Rpg2). Notably, the SEDS1a/EDS1b plants were more severely affected in ion leakage than the SPAD4 plants (Fig. 3C). This correlated well with the Psg avrA1-induced expression of GmEDS1a, GmEDS1b, or GmPAD4 (Supplemental Fig. S8) in wild-type Rpg1-b Rpg2 plants (cv Merit). As expected, SEDS1a/EDS1b and SPAD4 plants (Rpg1-b Rpg2) showed similar ion leakage as V plants (Rpg1-b Rpg2) in response to Psg avrB infection (Fig. 3D). Together, these results indicate that GmEDS1a/GmEDS1b and GmPAD4 are required for Rpg2 function but not for Rpg1-b, Rpg3, or Rpg4.
Figure 3.
GmEDS1 and GmPAD4 are required for resistance derived from Rpg2. A and B, Bacterial counts in Rpg1-b Rpg2 (cv Merit; A) or Rpg3 Rpg4 (cv Flambeau; B) plants silenced for GmEDS1a/GmEDS1b (SEDS1a/EDS1b), GmPAD4 (SPAD4), or those infected with the empty BPMV vector (V). Plants were infiltrated with Psg Vir, avrB, avrA1, avrB2, or avrD1 (105 cfu mL–1). Log10 values of cfu per unit leaf disc from infected leaves at 0 (white bars) or 4 (gray/black bars) dpi are presented. Error bars indicate sd (n = 5). Asterisks denote significant difference from avr-infected V plants in the respective cultivars (Student’s t test, P < 0.0001). C and D, Electrolyte leakage in V, SEDS1a, SEDS1b, or SPAD4 plants at 6 h postinfection with Psg avrA1 (C) or Psg avrB (D). Error bars indicate sd (n = 5). Results are representative of two to three independent experiments.
Rpg2-Mediated Resistance Also Requires GmRAR1, GmSGT1, and GmHsp90
Recently, we showed that Rpg1-b, Rpg3, or Rpg4 but not Rpg2 require the soybean non race specific disease resistance1 (GmNDR1) proteins for resistance signaling (Selote et al., 2013). This, together with the above results related to the requirements of EDS1 and PAD4 for Rpg2-mediated resistance prompted us to determine the requirements for other known signaling components for this R locus. We tested the requirements for required for Mla12-mediated resistance1 (GmRAR1), suppressor of the G2 allele of skp1 (GmSGT1-1), GmSGT1-2, and heat shock protein90 (GmHsp90) using gene-specific silencing constructs that were described earlier (Fu et al., 2009). The GmRAR1, GmSGT1-1, GmSGT1-2, and GmHsp90 genes were silenced in the Rpg1-b Rpg2 (cv Merit) and Rpg3 Rpg4 (cv Flambeau) backgrounds. Silencing was confirmed (Supplemental Fig. S9), and the various silenced plants were inoculated with the respective avirulent Psg strain, Psg avrB and Psg avrA1 on Rpg1-b Rpg2 plants and Psg avrB2 and Psg avrD1 on Rpg3 Rpg4 plants. Analysis of pathogen growth over time showed that the SRAR1 (silenced for GmRAR1), SSGT1 (silenced for GmSGT1-1), SSGT2 (silenced for GmSGT1-2), and SHsp90 (silenced for GmHsp90) plants in the Rpg1-b Rpg2 background accumulated significantly more Psg avrA1 compared with V plants; the SRAR1, SSGT1, SSGT2, and SHsp90 plants each contained approximately 5- to 8-fold more Psg avrA1 than V plants (Fig. 4A). Furthermore, levels of Psg avrA1 in these plants were not significantly different from growth of Psg Vir on V plants. By contrast, the SSGT1 and SHsp90 or SRAR1 and SSGT2 plants accumulated comparable or slightly more (P < 0.005) Psg avrB than V plants, respectively. In the Rpg3 Rpg4 background, resistance to Psg avrB2 was not altered in the SSGT1, SSGT2, and SHsp90 plants, whereas the SRAR1 plants contained slightly more Psg avrB2 (P < 0.005). No change in resistance to Psg avrD1 was observed in any of the silenced plants compared with V in the Rpg3 Rpg4 background (Fig. 4B). Together, these results indicated that GmRAR1, GmSGT1-1, GmSGT1-2, and GmHsp90 are required for Rpg2-mediated resistance but not Rpg4. Furthermore, GmRAR1 partially affected resistance derived from Rpg1-b and Rpg3.
Figure 4.
GmRAR1, GmSGT1, and GmHsp90 are required for resistance derived from Rpg2. A and B, Bacterial counts in Rpg1-b Rpg2 (cv Merit) or Rpg3 Rpg4 (cv Flambeau) plants silenced for GmRAR1 (SRAR1), GmSGT1-1 (SSGT1), GmSGT1-2 (SSGT2), GmHsp90 (SHSP90), or those infected with the empty BPMV vector (V) infected with Psg avrB or Psg avrA1 (A) and Psg avrB2 or Psg avrD1 (B). Plants were infiltrated with Psg Vir, avrA1, avrB, avrB2, or avrD1 (105 cfu mL–1). Log10 values of cfu per unit leaf disc from infected leaves at 0 (white bars) or 4 (gray/black bars) dpi are presented. Error bars indicate sd (n = 5). Asterisks denote significant difference from avr-infected V plants in the respective cultivars (Student’s t test, P < 0.0001 for Psg avrA1 and P < 0.005 for Psg avrB and avrB2). Significant difference from V plants of corresponding cultivar infected with Vir strain is indicated by the letter a (P < 0.0005). Results are representative of two independent experiments.
GmEDS1a and GmEDS1b Interact with AvrA1 and Are Required for the Virulence Activity of This Psg Effector in Soybean
AtEDS1, which is required for resistance derived from RPS4 and RPS6, was shown to interact with their cognate P. syringae effectors AvrRps4 and HopA1, respectively (Gassmann et al., 1999; Gassmann, 2005; Kim et al., 2009; Bhattacharjee et al., 2011). Based on these reports and our findings related to the requirement of GmEDS1a/GmEDS1b in Rpg2-derived resistance against Psg avrA1, we examined binding between GmEDS1 proteins and AvrA1. BiFC assays showed that AvrA1 interacted with both GmEDS1a and GmEDS1b in planta; YFP fluorescence was detected in plants coinfiltrated with AvrA1 and GmEDS1a/GmEDS1b but not glutathione S-transferase (GST; Fig. 5A). Interestingly, YFP fluorescence in leaves coexpressing AvrA1 with GmEDS1 was detected along the cell periphery as well as in punctate structures within the cell periphery. In contrast to the AvrA1-GmEDS1 interaction and as reported recently (Selote et al., 2014), the AvrA1-soybean RPM1-interacting protein4 like (GmRIN4b) interaction was detected along the cell periphery (Fig. 5A). Similar punctate fluorescence was also reported for the AtEDS1-SRFR1 interaction and shown to be associated with the cytoplasmic microsomal fraction (Bhattacharjee et al., 2011). To test if the punctate fluorescence in the AvrA1-GmEDS1 BiFC assays was due to the peculiar localization of AvrA1, we transiently expressed full-length YFP-tagged (N-terminal) AvrA1 in N. benthamiana. Confocal imaging showed that AvrA1-YFP was detected in similar punctate structures as in the BiFC assays and this did not change in the presence of MYC-tagged GmEDS1a or GmEDS1b (Fig. 5B; Supplemental Fig. S10A). The AvrA1-YFP fluorescence did not colocalize with the red fluorescent protein-tagged Golgi-localized protein endo-β-mannanase1 (GmMan1; Nebenführ et al., 1999; Supplemental Fig. S10B). Furthermore, unlike the AtEDS1-SRFR1 complex, AvrA1 was detected in the soluble fraction of total leaf (N. benthamiana) extracts (Fig. 5C). This suggested that AvrA1 interacted with the cytoplasmic fraction of the GmEDS1 proteins. The AvrA1-GmEDS1a/GmEDS1b interaction was further confirmed using coimmunoprecipitation assays. Western-blot analysis showed that MYC-tagged GmEDS1a and GmEDS1b immunoprecipitated with FLAG-tagged AvrA1 (Fig. 5D). By contrast, AvrA1 did not immunoprecipitate with GmPAD4 (Fig. 5E).
Figure 5.
GmEDS1 binds AvrA1. A, BiFC assay showing in planta interaction between AvrA1 and GmEDS1 proteins in transgenic N. benthamiana expressing cyan florescent protein (CFP)-H2B. CFP and YFP overlay images (40× magnification) of micrographs at 48 h postinfiltration from leaves coexpressing NYFP-AvrA1 with the CYFP-GmEDS1a/GmEDS1/GmRIN4b/GST are shown. Images are representative of three separate infiltrations from two independent experiments for each interaction using both combinations of N/C-YFP fused proteins. B, In planta localization of AvrA1. N-terminal YFP-tagged AvrA1 was transiently expressed in N. benthamiana via agroinfiltration by itself or together with MYC-tagged GmEDS1a/GmEDS1b. Bars = 10 µm. C, Western-blot analysis showing localization of AvrA1 in the soluble (S) fraction of total (T) extracts from N. benthamiana leaves transiently expressing MYC-tagged AvrA1. The letter M indicates membrane fraction. TIP- and Hsc70-specific antibodies were used to test purity of the membrane and soluble fractions, respectively. D and E, Immunoprecipitation assays using proteins coexpressed in N. benthamiana. D, MYC-tagged GmEDS1a or GmEDS1b were coexpressed with FLAG-tagged AvrA1. E, MYC-tagged AvrA1 was coexpressed with FLAG-tagged GmPAD4. Proteins were immunoprecipitated (IP-FLAG) from total (T) extracts using α-FLAG antibodies and visualized using tag-specific antibodies. F, Log10 values of cfu per unit leaf disc from leaves of plants silenced for GmEDS1a/GmEDS1b (SEDS1a/EDS1b), GmPAD4 (SPAD4), or those infected with the empty BPMV vector (V) at 0 (white bars) or 4 (gray/black bars) dpi with Psg Vir or Psg avrA1. Error bars indicate sd (n = 5). Asterisks denote significant difference from Vir-infected V plants (Student’s t test, P < 0.001). Results are representative of two independent experiments.
Avr effectors are thought to target host defense components to enhance pathogen virulence in the absence of cognate R protein (Alfano and Collmer, 2004). Based on the direct interaction of GmEDS1 proteins with AvrA1 and their involvement in basal defense responses, we tested if the GmEDS1a/GmEDS1b proteins contributed to the virulence function of AvrA1 in soybean. The GmEDS1a/GmEDS1b genes were silenced in the rpg2 background (cv Flambeau), and plants were inoculated with Psg avrA1. The GmPAD4-silenced plants were used as control because GmPAD4 does not interact with AvrA1. As expected, AvrA1 enhanced Psg virulence on rpg2 plants; Psg avrA1 accumulated at significantly (P < 0.002) higher levels than Psg Vir (Fig. 5F). Like rpg2 V plants, the rpg2 SPAD4 plants also accumulated more Psg AvrA1. By contrast, the rpg2 SEDS1a and rpg2 SEDS1b plants contained less Psg avrA1 than V or SPAD4. Notably, levels of Psg avrA1 in rpg2 SEDS1a or rpg2 SEDS1b plants were similar to levels of Psg Vir in rpg2 V plants (Fig. 5F). Together, these results suggest that GmEDS1a/GmEDS1b are required for the virulence function of AvrA1 on rpg2 plants.
GmEDS1a and GmEDS1b Partially Complement the Arabidopsis eds1-1 Mutation
To test the functionality of the GmEDS1 genes, we expressed C-terminal MYC-tagged GmEDS1a and GmEDS1b constitutively in the Arabidopsis eds1-1 mutant. Two independent transgenic lines were analyzed for GmEDS1a (line nos. 22 and 24) and GmEDS1b (line nos. 37 and 47) each. T2 plants containing GmEDS1a (35S::GmEDS1a) or GmEDS1b (35S::GmEDS1b) expressed the respective transgenes constitutively (Fig. 6A). Consistent with transgene expression, the eds1-1 35S::GmEDS1a/GmEDS1b plants showed constitutive expression of the respective transprotein, as detected by western-blot analysis using MYC-specific antibodies (Fig. 6B). We next tested whether the GmEDS1 genes complemented the SA-related defects of the eds1-1 mutant. Levels of SA and its glucoside SAG were measured from healthy and pathogen-infected P. syringae pv tomato (Pst) wild-type (ecotype Ws), eds1-1, and eds1-1 35S::GmEDS1a/GmEDS1b plants. Basal SA and SAG levels in the eds1-1 and eds1-1 35S::GmEDS1a plants were similar to wild-type plants (Fig. 6C, white bars). By contrast and unlike the eds1-1 plants, both the eds1-1 35S::GmEDS1a/GmEDS1b lines accumulated significantly (P < 0.0001) higher SA in response to Pst-avrRps4 infection, and these were comparable to levels of SA in wild-type plants (Fig. 6C, left, black bars). SAG levels in Pst avrRps4 infected eds1-1 35S::GmEDS1 plants were higher than eds1-1 plants but lower than wild-type plants (Fig. 6C, right, gray bars). Consistent with their basal and induced SA levels, the eds1-1 35S::GmEDS1a/GmEDS1b plants were also able to induce PR1 expression in response to infection by Pst avrRps4 (Fig. 6D), albeit at later time points post-Pst infection than wild-type plants (Supplemental Fig. S11). Furthermore, unlike the eds1-1 mutant, which was significantly more susceptible to both virulent (DC3000) and avirulent (avrRps4) strains of Pst, both the eds1-1 35S::GmEDS1a and eds1-1 35S::GmEDS1b plants accumulated wild-type-like levels of Pst avrRps4 or DC3000 (Fig. 6E). Together, these results indicated that the GmEDS1 genes only partially rescued the defect in pathogen-responsive SA accumulation but fully complemented the eds1-1 defect in bacterial resistance and SA responsiveness.
Figure 6.
Complementation of the Arabidopsis eds1-1 mutation by GmEDS1. A, Northern-blot analysis showing expression of GmEDS1a or GmEDS1b in wild-type (Ws), eds1-1, and transgenic eds1-1 plants expressing C-terminal MYC-tagged GmEDS1a (35S::1a, 22 and 24) or GmEDS1b (35S::1b, 37 and 47) via 35S promoter. Ethidium bromide staining of ribosomal RNA (rRNA) was used as a loading control. B, Western-blot analysis showing expression of GmEDS1a/GmEDS1b proteins in wild-type, mutant, and transgenic plants. Proteins were visualized using MYC-specific antibodies. Ponceau S staining of the membrane was used as loading control. C, Basal (white bars) and pathogen-induced (2 dpi with Pst avrRps4) levels of SA (black bars) and SAG (gray bars) in plants of indicated genotype. Error bars indicate sd (n = 3). Asterisks denote data significantly different (Student’s t test, P < 0.0001) from the wild type. D, Relative induction of PR1 expression in Ws, eds1-1, and 35S::1a (no. 24) or 35S::1b (no. 47) transgenic plants at 2 dpi with P. syringae. Error bars indicate sd (n = 3). Asterisk denotes significant difference from V (Student’s t test, P < 0.0001). E, Response to Pst avrRps4 (black bars) and Pst DC3000 (gray bars) in indicated plants. Bacterial numbers at 0 and 3 dpi are presented as log10 values of cfu per unit leaf disc. Error bars indicate sd (n = 5). Asterisks denote data significantly different from the wild type (Student’s t test, P < 0.0001). Results are representative of two independent experiments.
GmEDS1a or GmEDS1b Does Not Promote Activation of the Arabidopsis R Protein HRT
Previously, we showed that AtEDS1 interacts with the Arabidopsis R protein HRT and potentiates TCV-CP-mediated activation of HRT (Zhu et al., 2011). To test if the GmEDS1 proteins could substitute for AtEDS1 in HRT activation, we used the previously developed transient system based on reconstitution of HR in N. benthamiana. This assay is facilitated because coinfiltration of HRT and its cognate Avr effector TCV-CP induces only delayed and weak HR in N. benthamiana. However, coinfiltration of AtEDS1-80 or AtEDS1-90 along with HRT and TCV-CP promotes rapid HR formation (Zhu et al., 2011). We transiently coexpressed HRT and TCV-CP in N. benthamiana, by themselves or together with AtEDS1-90, GmEDS1a, or GmEDS1b. As expected, presence of AtEDS1-90 resulted in rapid induction of cell death in HRT + TCV-CP-expressing leaves (Fig. 7A). This was not the case in leaves expressing HRT + TCV-CP alone. Interestingly, and in contrast to AtEDS1-90, coexpression of either GmEDS1a or GmEDS1b did not promote HRT + TCV-CP-derived cell death. This was not due to problems with expression of EDS1, HRT, and/or TCV-CP (Fig. 7B). Analysis of ion leakage in the various leaves further confirmed the visual cell death phenotype. Rapid ion leakage was only observed when HRT + TCV-CP was coexpressed with AtEDS1-90 but not GmEDS1a or GmEDS1b (Fig. 7C).
Figure 7.
GmEDS1 proteins interact with but do not potentiate activation of the Arabidopsis R protein HRT. A, Visual cell death in N. benthamiana leaves transiently expressing indicated combinations of proteins. Images were taken at indicated h post-agroinfiltration. MYC-HRT, MYC-At/GmEDS1, and CP of TCV were used. HRT and CP were coexpressed (HRT + CP) by themselves or together with GmEDS1a (+Gm1a) and/or GmEDS1b (+Gm1b). B, Western-blot analysis showing respective protein levels in the N. benthamiana leaves from A. Proteins were visualized using α-MYC for HRT, AtEDS1, and GmEDS1 or TCV-CP-specific antibodies. C, Electrolyte leakage in N. benthamiana leaves transiently expressing indicated proteins at 48 h post-agroinfiltration. Error bars represent sd (n = 5). Some small error bars are not visible in the graphs. D, Immunoprecipitation assays using proteins coexpressed in N. benthamiana. MYC-tagged GmEDS1a/GmEDS1b were coexpressed with FLAG-tagged HRT. Proteins were immunoprecipitated (IP-FLAG) from total (T) extracts using α-FLAG antibodies and visualized using tag-specific antibodies. Results are representative of two independent experiments. [See online article for color version of this figure.]
The inability of GmEDS1 to promote HRT activation could be associated with their inability to interact with HRT. Therefore, we tested binding between GmEDS1a/GmEDS1b and HRT. MYC-tagged GmEDS1a or GmEDS1b were transiently coexpressed with FLAG-tagged HRT, and proteins were immunoprecipitated from total extracts using FLAG-specific antibodies. Surprisingly, protein-blot analysis showed that both GmEDS1a and GmEDS1b immunoprecipitated with HRT (Fig. 7D). These data indicated that interaction between HRT and GmEDS1a/GmEDS1b proteins, and, by extension, AtEDS1, was perhaps not the only factor contributing to TCV-CP-mediated activation of HRT.
GmPAD4 Complements the Arabidopsis pad4 Mutation
To test the functionality of the GmPAD4 gene, we expressed C-terminal FLAG-tagged GmPAD4 constitutively in the Arabidopsis pad4 mutant. Two independent transgenic lines (nos. 5 and 8) were analyzed. T2 plants containing GmPAD4 (35S::GmPAD4) expressed the GmPAD4 transcript constitutively (Fig. 8A). Consistent with transgene expression, the 35S::GmPAD4 plants showed constitutive expression of the transprotein, as detected by western-blot analysis using FLAG-specific antibodies (Fig. 8B). We next tested whether GmPAD4 expression complemented the pad4 mutation by analyzing SA levels, PR gene expression, and pathogen resistance. As expected, the pad4 mutant plants contained significantly (P < 0.0005) reduced basal (white bars) as well as pathogen-induced (black bars) levels of SA compared with wild-type plants (Fig. 8C, left). This was also true for both of the pad4 35S::GmPAD4 lines. Basal and pathogen-induced levels of SAG in the pad4 and pad4 35S::GmPAD4 plants were comparable to wild-type levels (Fig. 8C, right). This suggested that expression of the GmPAD4 gene did not rescue the defects in SA accumulation of the pad4 mutant. However, unlike the pad4 mutant, the pad4 35S::GmPAD4 plants were able to induce PR1 expression in response to Pst avrRps4 infection (Fig. 8D), albeit at a later time point than wild-type plants (Supplemental Fig. S13). Notably, unlike the pad4 mutant, which was significantly more susceptible to both virulent (DC3000) and avirulent (avrRps4) strains of Pst, the pad4 35S::GmPAD4 plants accumulated wild-type-like levels of Pst avrRps4 and DC3000 (Fig. 8E). Together, these results suggested that even though the GmPAD4 gene was unable to complement the defect in SA accumulation, it fully complemented the defect in bacterial resistance and SA-responsive PR1 expression.
Figure 8.
GmPAD4 rescues the pad4 mutation in Arabidopsis. A, Northern-blot analysis showing expression of GmPAD4 in wild-type (ecotype Columbia [Col-0]), pad4, and transgenic pad4 plants expressing C-terminal FLAG-tagged GmPAD4 (5 and 8) via 35S promoter. Ethidium bromide staining of ribosomal RNA (rRNA) was used as a loading control. B, Western-blot analysis showing expression of GmPAD4 protein in wild-type (ecotype Columbia), pad4, and and transgenic plants. Proteins were visualized using FLAG-specific antibodies. Ponceau S staining of the membrane was used as loading control. C, Basal (white bars) and pathogen-induced (2 dpi with Pst avrRps4) levels of SA (black bars) and SAG (gray bars) in indicated plants. Error bars indicate sd (n = 3). Asterisks denote data significantly different (Student’s t test, P < 0.0005) from the wild type. D, Relative induction of PR1 expression in ecotype Columbia, pad4, and 35S::GmPAD4 number 8 transgenic plants at 2 dpi with P. syringae. Error bars indicate sd (n = 3). Asterisk denotes significant difference from V (Student’s t test, P < 0.0001). E, Response to Pst avrRps4 (black bars) or Pst DC3000 (gray bars) in wild-type (ecotype Columbia), pad4, and transgenic plants. Bacterial numbers at 0 and 3 dpi are presented as log10 values of cfu per unit leaf area. Error bars indicate sd (n = 5). Asterisks denote data significantly different (Student’s t test, P < 0.0001) from the wild type. Results are representative of two independent experiments.
DISCUSSION
The EDS1 and PAD4 proteins, which contain eukaryotic LP-conserved domains, are well-known regulators of basal defense against bacterial, fungal, and oomycete pathogens (Parker et al., 1996; Aarts et al., 1998; Falk et al., 1999; Jirage et al., 1999; Xiao et al., 2005). EDS1 and PAD4 interact with each other and also function in race-specific resistance derived from multiple R proteins from diverse plants (Parker et al., 1996; Aarts et al., 1998; Feys et al., 2001; Peart et al., 2002; Liu et al., 2002; Hu et al., 2005; Gao et al., 2010). We show that soybean contains two EDS1-like genes and one PAD4-like gene, which encode proteins with significant structural similarities to the AtEDS1 and AtPAD4 proteins, respectively. Consistent with this similarity, the GmEDS1 and GmPAD4 proteins interact with each other and the AtPAD4 and AtEDS1 proteins, respectively. Moreover, like their Arabidopsis counterparts, the GmEDS1 and GmPAD4 genes are required for both basal resistance and ETI in soybean. For instance, silencing the GmEDS1 or GmPAD4 genes affected pathogen-induced SA accumulation and PR gene expression in soybean. Moreover, soybean plants silenced for either of these genes exhibited enhanced susceptibility to virulent strains/isolates of P. syringae, P. sojae, and SMV.
EDS1 and PAD4 have been more extensively studied for their roles in R-mediated defense. EDS1 is thought to primarily mediate signaling derived from the TIR-NB-LRR type of R proteins (Parker et al., 1996; Aarts et al., 1998), whereas signaling derived from most CC-NB-LRR proteins often involves the NDR1 protein (Century et al., 1995; Aarts et al., 1998). Based on the requirement for GmEDS1 and GmPAD4 (this study), and the nonrequirement for GmNDR1 (Selote et al., 2013) by Rpg2, we predict that the Rpg2 locus likely encodes a TIR-NB-LRR protein. On the other hand, Rpg3 and Rpg4 are likely to encode CC-NB-LRR proteins similar to Rpg1-b because these require GmNDR1 (Selote et al., 2013) but not GmEDS1/GmPAD4 for resistance signaling. However, this delineation of signaling requirements is not strictly applicable to all R proteins, and many CC-NB-LRR proteins not only function independent of the NDR1 pathway, but also recruit EDS1 and/or PAD4 for resistance signaling (Kachroo et al., 2000; Bittner-Eddy and Beynon et al., 2001; Chandra-Shekara et al., 2004; McDowell et al., 2000). Furthermore, EDS1 can function redundantly with SA, such that the requirement for EDS1 by some CC-NB-LRR proteins becomes evident only in SA-deficient backgrounds (Venugopal et al., 2009). The Arabidopsis R protein HRT, which induces HR and resistance against TCV, is one such example where a CC-NB-LRR protein functions independent of NDR1 but requires both EDS1 (redundantly with SA) and PAD4 for resistance signaling (Kachroo et al., 2000; Chandra-Shekara et al., 2004; Zhu et al., 2011). HRT-mediated signaling is activated in the presence of TCV-CP (Cooley et al., 2000; Kachroo et al., 2000; Zhao et al., 2000), but direct interactions between HRT and CP have not been detected (Zhu et al., 2011). AtEDS1 interacts with HRT and potentiates TCV-CP-mediated activation of HRT, resulting in the induction of cell death (Zhu et al., 2011). Interestingly, even though the GmEDS1 proteins interact with HRT, they are unable to reconstitute HRT-CP-mediated HR in N. benthamiana. This suggests that despite their considerable sequence identity, the GmEDS1 isoforms are partly functionally distinct from AtEDS1. This could also be the reason why endogenous N. benthamiana orthologs of EDS1 cannot facilitate HRT-CP-mediated cell death. It is possible that additional proteins in the HRT-EDS1 complex contribute to the TCV-CP-dependent activation of HRT, and the GmEDS1 (or NbEDS1) proteins might differ in their abilities to associate with such proteins. The indirect association of HRT and EDS1 is supported by the fact that this interaction cannot be detected in BiFC assays (Zhu et al., 2011). Functional differences between the AtEDS1 and GmEDS1 proteins are further emphasized by the fact that constitutive expression of GmEDS1a/GmEDS1b does not fully restore basal or pathogen-responsive SA accumulation in the eds1-1 mutant. However, despite these differences, GmEDS1a/GmEDS1b is able to complement resistance to virulent and avirulent pathogens, suggesting that SA and pathogen responses might be governed by different aspects of EDS1 or that the reduced amount of SA accumulating in the transgenic plants is sufficient to restore pathogen resistance. Like AtEDS1, overexpression of GmEDS1a/GmEDS1b does not confer increased pathogen resistance or constitutive PR-1 expression (Venugopal et al., 2009; García et al., 2010).
EDS1 and PAD4 are considered essential mediators of SA signaling (Falk et al., 1999; Jirage et al., 1999; Chandra-Shekara et al., 2004; Wiermer et al., 2005). Furthermore, SA and EDS1 are considered to function in a positive feedback regulatory loop. Even the SA-independent branch of EDS1 signaling requires SA for complete manifestation of pathogen resistance (Bartsch et al., 2006). Our results with the GmEDS1a/GmEDS1b-expressing eds1-1 and GmPAD4-expressing pad4 plants discount this to a certain extent. This is because both the GmEDS1a/GmEDS1b-expressing eds1-1 plants and the GmPAD4-expressing pad4 plants are restored in pathogen resistance despite their inability to accumulate wild-type levels of SA. This suggests that either SA accumulation is not an absolute requirement for pathogen resistance signaling via EDS1 and PAD4 or that the lower than wild-type levels of SA in the transgenic eds1-1 and pad4 plants are sufficient for pathogen resistance in the presence of GmEDS1 and GmPAD4 proteins, respectively. Notably, PR-1 expression, which is considered an SA marker, is also restored in pathogen-infected GmEDS1a/GmEDS1b-expressing eds1-1 and GmPAD4-expressing pad4 plants. Testing the functionality of the GmEDS1 and GmPAD4 proteins in the SA-deficient sid2 background might help resolve whether a threshold level of SA is sufficient for their function in the respective mutant backgrounds. Alternatively, the partial induction of PR-1 expression in the GmEDS1a/GmEDS1b or GmPAD4 transgenic plants could be the result of SA-independent signaling, as reported recently (Tsuda et al., 2013).
AtEDS1 interacts with the TIR-NB-LRR proteins RPS4 and RPS6 and their respective cognate bacterial effectors AvrRps4 and HopA1 (Bhattacharjee et al., 2011). Furthermore, AvrRps4 and HopA1 inhibit the AtEDS1-RPS4 and AtEDS1-RPS6 interactions, respectively (Bhattacharjee et al., 2011). This could also be the case for the GmEDS1-interacting AvrA1, although AvrA1 is not structurally related to AvrRps4 or HopA1. We are unable to test the GmEDS1-Rpg2 interaction or its disruption by AvrA1 due to the unknown identity of Rpg2. Clearly though, the GmEDS1 proteins are essential for the virulence function of AvrA1 because Psg avrA1 lose virulence in rpg2 plants silenced for the GmEDS1 genes. This is further supported by the fact that Psg avrA1 is not hypervirulent on Rpg2 plants silenced for GmEDS1. To our knowledge, this is the first report identifying a positive regulatory role for EDS1 in the virulence activity of a pathogen effector. Interestingly, the AvrA1-GmEDS1 interaction was detected in localized regions within the cytoplasm. This is unlike the EDS1-AvrRps4 or EDS1-HopA1 interactions, which were detected in the cytoplasm and nucleus or cytoplasm alone, respectively (Bhattacharjee et al., 2011). This is also unlike the AvrA1-GmRIN4 interaction, which was detected along the cell periphery (Selote et al., 2014). EDS1 is present in the nucleus as well as cytoplasm, although preferential localization can occur depending upon the relative levels of its interacting partners PAD4 and SAG101 (Zhu et al., 2011), and both the cytosolic and nuclear fractions of EDS1 are considered essential for resistance signaling (García et al., 2010; Heidrich et al., 2011). The presence of transiently expressed AvrA1 in the soluble fraction of plant protein extracts and the detection of the GmEDS1-AvrA1 interaction in unknown cytosolic sublocation poses the possibility that the cytosolic EDS1 fraction might be more important for its function in mediating effector virulence. Alternatively, the virulence function of AvrA1 may be associated with the altered localization of EDS1 because nuclear localization of EDS1 was shown to be important for defense-related transcriptional changes during ETI and PTI (García et al., 2010; Heidrich et al., 2011). It is also possible that the punctate cytosolic sublocalization of AvrA1 is associated with the EDS1-interacting SRFR1 protein, which shows a similar localization pattern (Kwon et al., 2009). Perhaps AvrA1 targets the EDS1-SRFR1 complex to function as a virulence effector. Determining the intracellular location of pathogen-delivered AvrA1 and testing the virulence of Psg avrA1 on soybean plants expressing nuclear- or cytosol-targeted GmEDS1, as well as plants lacking SRFR1 ortholog(s), could help resolve this.
MATERIALS AND METHODS
Plant Growth Conditions and Generation of Transgenic Plants
Soybean (Glycine max) ‘Merit’ (Rpg1-b Rpg2), ‘Flambeau’ (Rpg3 Rpg4), ‘Essex’ (Resistance to SMV1 [rsv1]), ‘Essex-Rsv1’ (Rsv1), ‘Williams’ (rps1), and ‘Williams82’ (Rps1-k) were grown in the greenhouse with day and night temperatures of 25°C and 20°C, respectively. Arabidopsis (Arabidopsis thaliana) plants were grown in MTPS 144 Conviron walk-in chambers at 22°C, 65% relative humidity, and 14-h photoperiod.
The full-length complementary DNAs corresponding to GmEDS1a, GmEDS1b, and GmPAD4 genes were cloned into binary vector pGWB with 35S promoter. These recombinant vectors were moved into Agrobacterium tumefaciens strain GV3101 (MP90) by electroporation and were used to transform Arabidopsis via the floral dip method. Selection of transformants was carried out on medium containing kanamycin or hygromycin.
Construction of Viral Vectors, in Vitro Transcription, and Plant Inoculation
For silencing experiments, construction of silencing vectors, in vitro transcription, rub inoculation of recombinant BPMV vectors on soybean leaves, and confirmation of silencing was carried out as described before (Kachroo et al., 2008; Kachroo and Ghabrial, 2012). Briefly, target gene sequences (see amino acid residue coordinates below) were cloned into pGG7R2V, which is the cloned RNA2 of BPMV (Zhang and Ghabrial, 2006), in vitro transcribed, and inoculated along with in vitro transcribed RNA1 of a mild (Hancock) BPMV strain. Once infectious virus was established on the plant and silencing of target gene was confirmed, infected tissue was freeze dried and used as inoculum for subsequent inoculations. For each new inoculation, silencing was confirmed before performing the specific experiment. Virus/transcripts were inoculated on the first true leaves at VC stage. Pathogen infection and other assays were performed at the V2 to V3 stages. The 183-bp fragment (Q352–S412) of GmEDS1a, the 156-bp fragment (A246–N297) of GmEDS1b, and the 189-bp fragment (W223–V285) of GmPAD4 were used to generate vector targeting GmEDS1a, GmEDS1b, and GmPAD4, respectively.
BiFC Assays
BiFC assays were carried out as described before (Selote and Kachroo, 2010). Briefly, the various proteins were fused to the N/C-terminal halves of EYFP using the pSITE-n/cEYFP vectors (Martin et al., 2009) and introduced in A. tumefaciens strain LBA4404. A. tumefaciens strains expressing proteins fused to reciprocal halves of EYFP were coinfiltrated into CFP-nuclear-localized histone2B (H2B)-tagged Nicotiana benthamiana plants (transgenic plants expressing nuclear-localized CFP). Forty-eight hours later, water-mounted sections of leaf tissue were examined by confocal microscopy using a water immersion PLAPO60XWLSM (numerical aperture 1.0) objective. Positive interactions were detected as yellow fluorescence upon reconstitution of the complete EYFP. CFP and YFP overlay images (40× magnification) are shown. All interactions were confirmed using both combinations of reciprocal nEYFP/cEYFP fusion proteins in two separate experiments (three replicates per experiment).
Pathogen Strains and Inoculations
Pseudomonas syringae pv glycinea (Psg) race 4 (Staskawicz et al., 1984) expressing AvrB or AvrB2 (Tamaki et al., 1988) via the broad host range plasmid pDSK519 (Keen et al., 1988) and AvrA1 or AvrD1 (Staskawicz et al., 1984; Kobayashi et al., 1990) via the broad host range plasmid pDSK600 (Murillo et al., 1994) were used. Psg strains were grown on King’s B medium at 29°C, supplemented with 50 μg mL–1 rifampicin plus 50 μg mL–1 kanamycin (for strains carrying pDSK519) or 50 μg mL–1 streptomycin and 50 μg mL–1 spectinomycin (for strains carrying pDSK600). Psg inoculation of soybean and monitoring of bacterial proliferation were carried out as described before (Fu et al., 2009). Mock inoculations were carried out with 10 mm MgCl2 in 0.01% (w/v) Silwett L-77. Results are representative of three to four independent repeats, unless noted otherwise. Race 1 of Pseudomonas sojae was grown on V8 agar at 25°C in the dark. P. sojae and SMV inoculations were carried out as described before (Kachroo et al., 2008; Fu et al., 2009). Results are representative of a minimum of three independent experiments with 15 to 20 plants tested per silenced line per experiment.
The bacterial strains Pseudomonas syringae pv tomato (Pst) DC3000 (containing pVSP61, empty vector) and Pst avrRps4 (pVSP61-expressing AvrRps4) were grown overnight in King’s B medium containing rifampicin and kanamycin (Sigma). The bacterial cells were harvested, washed, and suspended in 10 mm MgCl2. The cells were diluted to a final density of 1 × 105 colony forming units (cfu) mL–1 (A600) and used for infiltration. The bacterial suspension was injected into the abaxial surface of the leaf using a needleless syringe. Three leaf discs from the inoculated leaves were collected at 0 and 3 dpi. The leaf discs were homogenized in 10 mm MgCl2 diluted 103- or 104-fold and plated on King’s B medium.
RNA Extraction, Northern Blot, and qRT-PCR Analysis
RNA from leaf tissues of soybean plants at V2/V3 growth stage was extracted using the TRIzol reagent (Invitrogen), per manufacturer’s instructions. Northern-blot analysis and synthesis of random-primed probes were carried out as described before (Kachroo et al., 2008). The probes used for detecting GmEDS1a and GmEDS1b correspond to the region comprising Q352 to S412 of GmEDS1a and A246 to N297 of GmEDS1b. RT and first strand complementary DNA synthesis were carried out using Superscript II (Invitrogen). Two to three independent RNA preparations were analyzed by semiquantitative RT-PCR to evaluate relative differences in transcript levels.
Gene-specific primers to generate 140- to 150-bp PCR products were designed using the OligoPerfect (Invitrogen) software. qRT-PCR was carried out in a 96-well plate using SYBR Green PCR Master Mix (Applied Biosystems), and the reactions were run on an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems) with the following conditions: 95°C for 1 min, 40 cycles of 95°C for 10 s, and 60°C for 30 s. At the end of each run, a dissociation stage was added (95°C, 2 min) to check PCR specificity. A negative control reaction without template was also included for each primer combination. Transcript levels for each of the target genes were normalized to the endogenous actin transcripts from Arabidopsis or soybean as appropriate. The qRT-PCR data acquired through RQ manager 1.2 software (Applied Biosystems) were exported to Excel and analyzed with the Δ-Δ threshold cycle method as previously described (Livak and Schmittgen, 2001). Each sample or treatment was tested in at least three biological repeats, and the same experiment was performed twice.
Protein Extraction and Immunoblot Analysis
Proteins were extracted in buffer containing 50 mm Tris-HCl, pH 7.5, 10% (v/v) glycerol, 150 mm NaCl, 10 mm MgCl2, 5mm EDTA, 5 mm dithiothreitol, and 1× protease inhibitor cocktail (Sigma-Aldrich). Crude lysate was filtered through four layers of cheesecloth and centrifuged at 10,000g for 10 min at 4°C. The clear supernatant constitutes the total protein fraction. Total protein extract was centrifuged at 10,000g followed by a second centrifugation at 125,000g for 1 h at 4°C to obtain supernatant comprising the soluble fraction and pellet comprising the microsomal fraction. Protein concentration was measured by the Bio-Rad protein assay. For Ponceau S staining, polyvinylidene difluoride membranes were incubated in Ponceau S solution (40% [v/v] methanol, 15% [v/v] acetic acid, and 0.25% [w/v] Ponceau S). The membranes were destained using deionized water.
Proteins (10–50 μg) were fractionated on a 7% to 10% (w/v) SDS-PAGE gel and subjected to immunoblot analysis using α-MYC, α-FLAG (Sigma-Aldrich), or α-CP. Antibodies specific to tonoplast intrinsic protein (TIP) and cytosolic heat shock protein70 (Hsc70; Enzo Lifesciences) were used for testing purity of soluble and membrane fractions, respectively. Immunoblots were developed using enhanced chemiluminescence detection kit (Roche) or alkaline phosphatase-based color detection.
Ion Leakage Experiments
For ion leakage in response to Psg avrA1 and Psg avrB, soybean leaves were infiltrated with 1 × 105 cfu mL–1 cells of the respective strain. Six hours later, six leaf discs per treatment (diameter = 0.7 cm) were collected with a cork borer, washed in distilled water for 30 min, and subsequently transferred to tubes containing 10 mL of distilled water. Conductivity of the solution was determined with a traceable digital conductivity meter (Fisher Scientific) at the indicated time points. In the beginning, ion leakage was measured every 2 h until 12 h postinoculation (hpi) and then again at 24 hpi. sd was calculated from three replicate measurements per treatment per experiment. Results are representative of three independent experiments.
HRT-TCV-CP-Mediated Cell Death
Agrobacterium spp. cells (LBA4404) expressing HRT, TCV-CP, AtEDS1-90, GmEDS1a, and GmEDS1b were grown overnight in Luria-Bertani medium. The cells were harvested, washed, and suspended in 10 mm MgCl2 with 10 mm MES to 0.6 optical density at 600 nm. One hundred millimolar acetosyringone (1.5 μL mL–1) was added to the cells and incubated at room temperature for 3 h. Bacteria were injected into the abaxial surface of N. benthamiana leaves using a needleless syringe. Visual cell death on leaves was recorded between 12 and 72 hpi. For conductivity assays, six leaf discs per treatment (d = 0.7 cm) were collected at 24 hpi. For immunoblot analysis and coimmunoprecipitation assays, leaves were sampled at 48 hpi.
Primers, Sequence Accessions, and Phylogenetic Analysis
Primers for the various silencing constructs as well as for full-length sequences are as listed in Supplemental Table S1. Primers were designed based on the full-length coding sequences available in the database for PR1 (AI930866) and β-tubulin (M21297). Sequence alignment and phylogenetic analysis of various genes were carried out using the Megalign program in the DNASTAR package (Swofford, 2000). Accession numbers for sequences used are as follows: AtEDS1-80 (AEE78365 and AT3G48080), AtEDS1-90 (AAD20950 and AT3G48090), GmEDS1a (ACT98433 and Glyma04G34800), GmEDS1b (ADC45394 and Glyma06G19920), NbEDS1 (AAL85347, Nicotiana tabacum NtEDS1, and AM62411), Oryza sativa OsEDS1 (BAF25000), PtEDS1-1 (EEE96846), PtEDS1-2 (EEF06269), Solanum lycopersicum SlEDS1 (AAX73302), Solanum tuberosum StEDS1 (AAT84084), Vicia villosa VvEDS1-1 (AEM75096), VvEDS1-2 (ABU43059), Zea mays ZmEDS1 (ACG29690), AtPAD4 (AEE78945 and AT3G52430), GmPAD4 (ACQ57001 and Glyma08G00420), PtPAD4 (EEE94047), Arabidopsis lyrata AlPAD4-1 (ABR46062), and AlPAD4-1 (ABR46057).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sequence conservation between soybean and Arabidopsis EDS1 proteins.
Supplemental Figure S2. Sequence conservation between soybean and Arabidopsis PAD4 proteins.
Supplemental Figure S3. Western-blot analysis of protein extracts from N. benthamiana plants.
Supplemental Figure S4. GmEDS1a/b expression in Rpg1-b plants.
Supplemental Figure S5. Sequence of the regions in GmEDS1a and GmEDS1b genes used for generating silencing vectors.
Supplemental Figure S6. Silencing GmEDS1a, GmEDS1b, and GmPAD4 in different cultivars.
Supplemental Figure S7. PR expression in GmEDS1- and GmPAD4-silenced plants.
Supplemental Figure S8. Silencing GmEDS1a, GmEDS1b, and GmPAD4 enhances susceptibility to SMV.
Supplemental Figure S9. Psg avrA1 infection induces GmEDS1 or GmPAD4 expression.
Supplemental Figure S10. Silencing GmRAR1, GmSGT1, GmSGT1, and GmHsp90 in soybean.
Supplemental Figure S11. In planta localization of AvrA1.
Supplemental Figure S12. PR1 expression in 35S:GmEDS1a and 35S:GmEDS1b plants.
Supplemental Figure S13. PR1 expression in 35S:GmPAD4 plants.
Supplemental Table S1. List of primers used.
Supplementary Material
Acknowledgments
We thank Said Ghabrial for the BPMV vector, Adam Bogdanove and Massimo Delledone for the Psg strains, Shifeng Zhu for help with HRT cell death assays, Michael Goodin for the red fluorescent protein-tagged Golgi marker and TIP antibodies, Pradeep Kachroo for critical review of the manuscript and valuable suggestions, Todd Pfeiffer for amplifying soybean cultivar seeds, and Amy Crume for management of plant growth facilities.
Glossary
- ETI
effector-triggered immunity
- LRR
leucine-rich repeat
- CC
coiled-coil
- NB
nucleotide-binding
- HR
hypersensitive response
- SA
salicylic acid
- TCV
turnip crinkle virus
- CP
coat protein
- WS
Wassilewskija
- LP
lipase-like
- BiFC
bimolecular fluorescence complementation
- qRT
quantitative reverse transcription
- dpi
days post infection
- BPMV
bean pod mottle virus
- RT
reverse transcription
- V
bean pod mottle virus empty vector
- Psg
Pseudomonas syringae pv glycinea
- SMV
soybean mosaic virus
- GST
glutathione S-transferase
- Pst
Pseudomonas syringae pv tomato
- TIP
tonoplast intrinsic protein
- cfu
colony forming units
Footnotes
This work was supported by the United Soybean Board (project no. 1291 to A.K.), the Earmarked Fund for Modern Agroindustry Technology Research System (grant no. CARS–04–02A), the Transgenic Special Fund of Ministry of Agriculture (grant no. 2011ZX08004–001–06 to G.H.), and the China Scholarship Council (fellowship to J.W.). The information reported in this article (no. 14–12–045) is part of a project of the Kentucky Agricultural Experiment Station and is published with the approval of the Director.
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA 95: 10306–10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano JR, Collmer A. (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42: 385–414 [DOI] [PubMed] [Google Scholar]
- Ashfield T, Bocian A, Held D, Henk AD, Marek LF, Danesh D, Peñuela S, Meksem K, Lightfoot DA, Young ND, Shoemaker RC, Innes RW. (2003) Genetic and physical localization of the soybean Rpg1-b disease resistance gene reveals a complex locus containing several tightly linked families of NBS-LRR genes. Mol Plant Microbe Interact 16: 817–826 [DOI] [PubMed] [Google Scholar]
- Ashfield T, Ong LE, Nobuta K, Schneider CM, Innes RW. (2004) Convergent volution of disease resistance gene specificity in two flowering plant families. Plant Cell 16: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Halane MK, Kim SH, Gassmann W. (2011) Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 334: 1405–1408 [DOI] [PubMed] [Google Scholar]
- Bisgrove SR, Simonich MT, Smith NM, Sattler A, Innes R. (1994) A disease resistance gene in Arabidopsis with specificity for two different pathogen avirulence genes. Plant Cell 6: 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner-Eddy PD, Beynon JL. (2001) The Arabidopsis downy mildew resistance gene, RPP13-Nd, functions independently of NDR1 and EDS1 and does not require the accumulation of salicylic acid. Mol Plant Microbe Interact 14: 416–421 [DOI] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Century KS, Holub EB, Staskawicz BJ. (1995) NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci USA 92: 6597–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Navarre D, Kachroo A, Kang HG, Klessig D, Kachroo P. (2004) Signaling requirements and role of salicylic acid in HRT- and rrt-mediated resistance to turnip crinkle virus in Arabidopsis. Plant J 40: 647–659 [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814 [DOI] [PubMed] [Google Scholar]
- Cooley MB, Pathirana S, Wu HJ, Kachroo P, Klessig DF. (2000) Members of the Arabidopsis HRT/RPP8 family of resistance genes confer resistance to both viral and oomycete pathogens. Plant Cell 12: 663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D, Shah J, Klessig DF. (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18: 547–575 [Google Scholar]
- Durrant WE, Dong X. (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Falk A, Feys BJ, Frost LN, Jones JDG, Daniels MJ, Parker JE. (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA 96: 3292–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Moisan LJ, Newman MA, Parker JE. (2001) Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20: 5400–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Wiermer M, Bhat RA, Moisan LJ, Medina-Escobar N, Neu C, Cabral A, Parker JE. (2005) Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. (1971) Current status of gene-for-gene concept. Annu Rev Phytopathol 9: 275–296 [Google Scholar]
- Fu DQ, Ghabrial S, Kachroo A. (2009) GmRAR1 and GmSGT1 are required for basal, R gene-mediated and systemic acquired resistance in soybean. Mol Plant Microbe Interact 22: 86–95 [DOI] [PubMed] [Google Scholar]
- Gao F, Shu X, Ali MB, Howard S, Li N, Winterhagen P, Qiu W, Gassmann W. (2010) A functional EDS1 ortholog is differentially regulated in powdery mildew resistant and susceptible grapevines and complements an Arabidopsis eds1 mutant. Planta 231: 1037–1047 [DOI] [PubMed] [Google Scholar]
- García AV, Blanvillain-Baufumé S, Huibers RP, Wiermer M, Li G, Gobbato E, Rietz S, Parker JE. (2010) Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. PLoS Pathog 6: e1000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W. (2005) Natural variation in the Arabidopsis response to the avirulence gene hopPsyA uncouples the hypersensitive response from disease resistance. Mol Plant Microbe Interact 18: 1054–1060 [DOI] [PubMed] [Google Scholar]
- Gassmann W, Hinsch ME, Staskawicz BJ. (1999) The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR-NBS-LRR family of disease-resistance genes. Plant J 20: 265–277 [DOI] [PubMed] [Google Scholar]
- He Y, Gan S. (2002) A gene encoding an acyl hydrolase is involved in leaf senescence in Arabidopsis. Plant Cell 14: 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich K, Wirthmueller L, Tasset C, Pouzet C, Deslandes L, Parker JE. (2011) Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334: 1401–1404 [DOI] [PubMed] [Google Scholar]
- Hu G, deHart AK, Li Y, Ustach C, Handley V, Navarre R, Hwang CF, Aegerter BJ, Williamson VM, Baker B. (2005) EDS1 in tomato is required for resistance mediated by TIR-class R genes and the receptor-like R gene Ve. Plant J 42: 376–391 [DOI] [PubMed] [Google Scholar]
- Holliday MJ, Keen NT, Long M. (1981) Cell death patterns and accumulation of fluorescent material in the hypersensitive response of soybean leaves to Pseudomonas syringae pv. glycinea. Physiolo Plant Pathol 18: 279–287 [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. (1999) Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA 96: 13583–13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Fu DQ, Havens W, Navarre D, Kachroo P, Ghabrial SA. (2008) An oleic acid-mediated pathway induces constitutive defense signaling and enhanced resistance to multiple pathogens in soybean. Mol Plant Microbe Interact 21: 564–575 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Ghabrial S. (2012) Virus-induced gene silencing in soybean. Methods Mol Biol 894: 287–297 [DOI] [PubMed] [Google Scholar]
- Kachroo P, Chandra-Shekara AC, Klessig DF. (2006) Plant signal transduction and defense against viral pathogens. Adv Virus Res 66: 161–191 [DOI] [PubMed] [Google Scholar]
- Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF. (2000) Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen NT, Buzzell RI. (1991) New resistance genes in soybean against Pseudomonas syringae pv. glycinea: evidence that one of them interacts with a bacterial elicitor. Theor Appl Genet 81: 133–138 [DOI] [PubMed] [Google Scholar]
- Keen NT, Tamaki S, Kobayashi D, Trollinger D. (1988) Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70: 191–197 [DOI] [PubMed] [Google Scholar]
- Kim SH, Kwon SI, Saha D, Anyanwu NC, Gassmann W. (2009) Resistance to the Pseudomonas syringae effector HopA1 is governed by the TIR-NBS-LRR protein RPS6 and is enhanced by mutations in SRFR1. Plant Physiol 150: 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi DY, Tamaki SJ, Trollinger DJ, Gold S, Keen NT. (1990) A gene from Pseudomonas syringae pv. glycinea with homology to avirulence gene D from P. s. pv. tomato but devoid of the avirulence phenotype. Mol Plant Microbe Interact 3: 103–111 [DOI] [PubMed] [Google Scholar]
- Kwon SI, Kim SH, Bhattacharjee S, Noh JJ, Gassmann W. (2009) SRFR1, a suppressor of effector-triggered immunity, encodes a conserved tetratricopeptide repeat protein with similarity to transcriptional repressors. Plant J 57: 109–119 [DOI] [PubMed] [Google Scholar]
- Lindeberg M, Stavrinides J, Chang JH, Alfano JR, Collmer A, Dangl JL, Greenberg JT, Mansfield JW, Guttman DS. (2005) Proposed guidelines for a unified nomenclature and phylogenetic analysis of type III Hop effector proteins in the plant pathogen Pseudomonas syringae. Mol Plant Microbe Interact 18: 275–282 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP. (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30: 415–429 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Martin K, Kopperud K, Chakrabarty R, Banerjee R, Brooks R, Goodin MM. (2009) Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J 59: 150–162 [DOI] [PubMed] [Google Scholar]
- McDowell JM, Cuzick A, Can C, Beynon J, Dangl JL, Holub EB. (2000) Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. Plant J 22: 523–529 [DOI] [PubMed] [Google Scholar]
- Murillo J, Shen H, Gerhold D, Sharma A, Cooksey DA, Keen NT. (1994) Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid 31: 275–287 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux JP. (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA. (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ. (1996) Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by RPP genes. Plant Cell 8: 2033–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart JR, Lu R, Sadanandom A, Malcuit I, Moffett P, Brice DC, Schauser L, Jaggard DA, Xiao S, Coleman MJ, et al (2002) Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc Natl Acad Sci USA 99: 10865–10869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, et al. (1997) The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I κ B. Plant Cell 9: 425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote D, Kachroo A. (2010) RPG1-B-derived resistance to AvrB-expressing Pseudomonas syringae requires RIN4-like proteins in soybean. Plant Physiol 153: 1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote D, Robin GP, Kachroo A. (2013) GmRIN4 protein family members function nonredundantly in soybean race-specific resistance against Pseudomonas syringae. New Phytol 197: 1225–1235 [DOI] [PubMed] [Google Scholar]
- Selote D, Shine MB, Robin GP, Kachroo A. (2014) Soybean NDR1-like proteins bind pathogen effectors and regulate resistance signaling. New Phytol 202: 485–498 [DOI] [PubMed] [Google Scholar]
- Staskawicz BJ, Dahlbeck D, Keen NT. (1984) Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc Natl Acad Sci USA 81: 6024–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B, Dahlbeck D, Keen N, Napoli C. (1987) Molecular characterization of genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol 169: 5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL (2000) PAUP: Phylogenetic Analysis Using Parsimony and Other Methods (Software Version 4.0 b10. 4.0b10). Sinaur Associates, Sunderland, MA [Google Scholar]
- Tamaki S, Dahlbeck D, Staskawicz B, Keen NT. (1988) Characterization and expression of two avirulence genes cloned from Pseudomonas syringae pv. glycinea. J Bacteriol 170: 4846–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F. (2013) Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet 9: e1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal SC, Jeong RD, Mandal M, Zhu S, Chandra-Shekara AC. (2009) ENHANCED DISEASE SUSCEPTIBILITY 1 and SALICYLIC ACID act redundantly to regulate resistance gene expression and low oleate-induced defense signaling. PLoS Genet 5: e1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Wang L, Mitra RM, Hasselmann KD, Sato M, Lenarz-Wyatt L, Cohen JD, Katagiri F, Glazebrook J. (2008) The genetic network controlling the Arabidopsis transcriptional response to Pseudomonas syringae pv. maculicola: roles of major regulators and the phytotoxin coronatine. Mol Plant Microbe Interact 21: 1408–1420 [DOI] [PubMed] [Google Scholar]
- Wiermer M, Feys BJ, Parker JE. (2005) Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8: 383–389 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Xiao S, Calis O, Patrick E, Zhang G, Charoenwattana P, Muskett P, Parker JE, Turner JG. (2005) The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J 42: 95–110 [DOI] [PubMed] [Google Scholar]
- Zhang C, Ghabrial SA. (2006) Development of bean pod mottle virus-based vectors for stable protein expression and sequence-specific virus-induced gene silencing in soybean. Virology 344: 401–411 [DOI] [PubMed] [Google Scholar]
- Zhao Y, DelGrosso L, Yigit E, Dempsey DA, Klessig DF, Wobbe KK. (2000) The amino terminus of the coat protein of Turnip crinkle virus is the AVR factor recognized by resistant Arabidopsis. Mol Plant Microbe Interact 13: 1015–1018 [DOI] [PubMed] [Google Scholar]
- Zhu S, Jeong RD, Venugopal SC, Lapchyk L, Navarre D, Kachroo A, Kachroo P. (2011) SAG101 forms a ternary complex with EDS1 and PAD4 and is required for resistance signaling against turnip crinkle virus. PLoS Pathog 7: e1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.