Abstract
A representative series of structural analogs of the antimitotic tripeptides hemiasterlins have been designed and synthesized, as potential inhibitors of tubulin polymerization. Relying also on a computational approach, we aimed to explore unknown extensive changes at the C-fragment, by incorporating the conformationally required double bond into five- and six-membered rings. Key steps of the synthetic strategy are a dynamic resolution affording the A-fragment in 97 % ee and the preparation of six new cyclic C fragments, all potentially able to interact with tubulin by means of H bonds. Unexpectedly, biological evaluation of these analogs did not provide evidences neither for cytotoxic effect nor for inhibition of tubulin polymerization.
Keywords: Hemiasterlin, Antimitotic peptides, Tubulin, Cytotoxicity, Dynamic resolution
Introduction
The tubulin/microtubule system plays a key role during mitosis, and disturbing its dynamic equilibrium can prevent cell division and induce apoptosis. Moreover, this system has been recognized as an established target of many anticancer drugs [1–3]. However, in most cases, the clinical use of anti-tubulin drugs is associated with problems of significant toxicity, drug resistance [4], and bioavailability [5]. Because of these limitations in currently used agents interacting with tubulin, new drugs with better properties are needed.
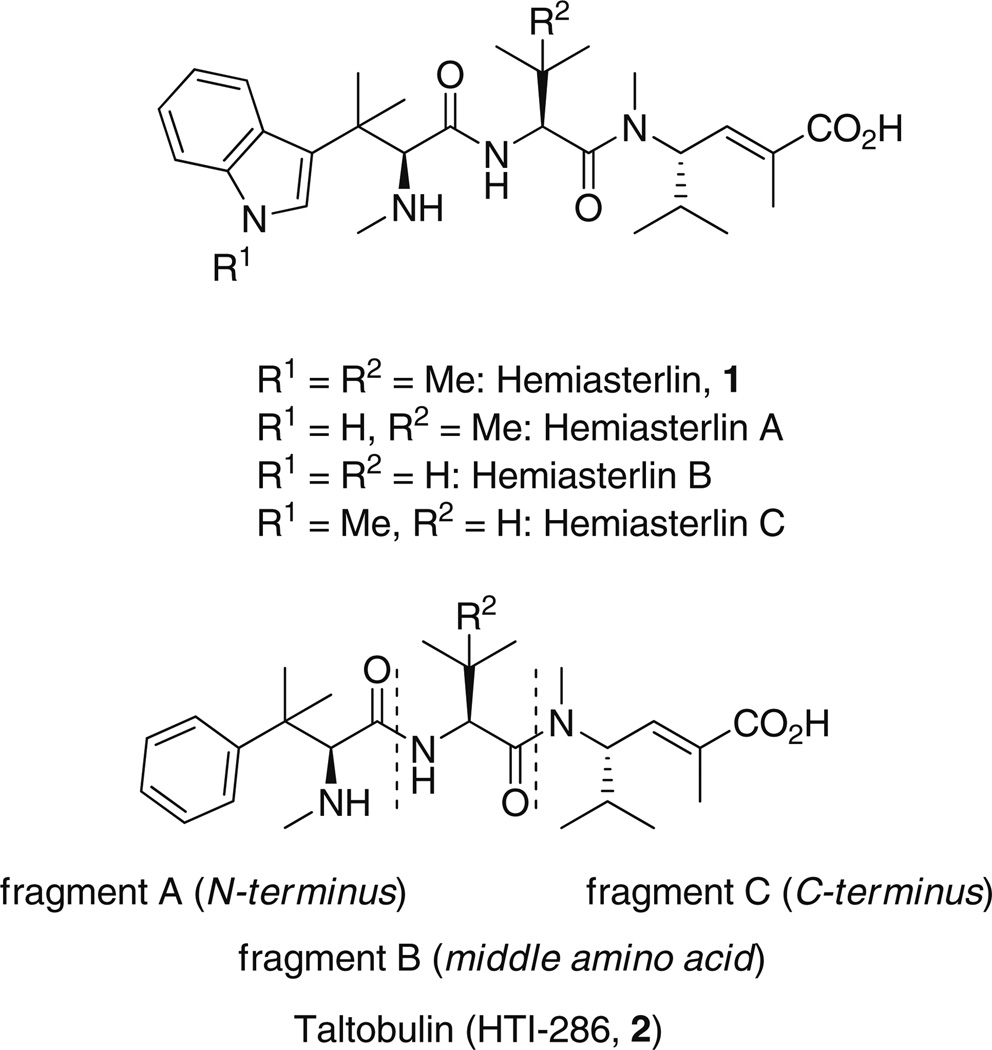
Searching for more promising active agents in this field, in particular, aiming to discover agents that are not substrates for drug efflux pumps such as P-glycoprotein [6,7], we became interested in hemiasterlins [8] (Fig. 1). Hemiasterlins are a family of natural tripeptides, discovered and isolated from the South African marine sponge Hemiastrella minor some years ago [9,10]. The most active members of the family show cytotoxicity in the nanomolar range and are highly potent inhibitors of microtubule polymerization and bind in the vinca domain of tubulin [11,12].
Fig. 1.
Tubulin polymerization inhibitors hemiasterlins and taltobulin
Relative to other known antimitotic agents, hemiasterlins possess an attractive combination of structural simplicity and potent antimitotic activity, making them ideal targets for synthetic modification [13,14]. A synthetic analog of hemiasterlin 1, taltobulin (HTI-286, 2) [15–17], wherein a phenyl group replaces the 3-substituted indole ring, advanced to clinical trials [18–22]. Unlike paclitaxel and vinblastine, taltobulin is a poor substrate for P-glycoprotein drug transporters and maintains toxicity toward cell lines with high expression of multidrug resistant (MDR) drug pumps. Hemiasterlins and taltobulin contain three highly modified amino acids that are responsible for compound stability and in vivo activity. For the sake of simplicity, they are named fragments A (N-terminus), B (middle amino acid), and C (C-terminus) [23,24].
Several studies have reported the synthesis of analogs, mostly modified at the N-terminus, to obtain more SAR information and possibly compounds with relative structural simplicity and potent activity [25–28]. With regards to fragment C, it is believed that the olefin bond plays a constitutive role during the biological interaction; it should serve as a center of conformational rigidity, presenting the carboxylic acid group in the proper orientation for binding to tubulin. Thus, owing to the fact that the C-terminus double bond is an essential requirement for efficient binding and high levels of cyto-toxicity [14], up to now most synthesized analogs bear this functional group unmodified [29].
These SAR conclusions intrigued us greatly, leading to an interest in exploring more extensive changes at the C-terminal. We wanted to preserve the double bond while incorporating it into five- and six-membered rings. This would allow us to explore new ways to confer rigidity on a hemiasterlin-like skeleton. In particular, we chose to replace the olefin with substituted benzene rings or five-membered heterocyclic moieties.
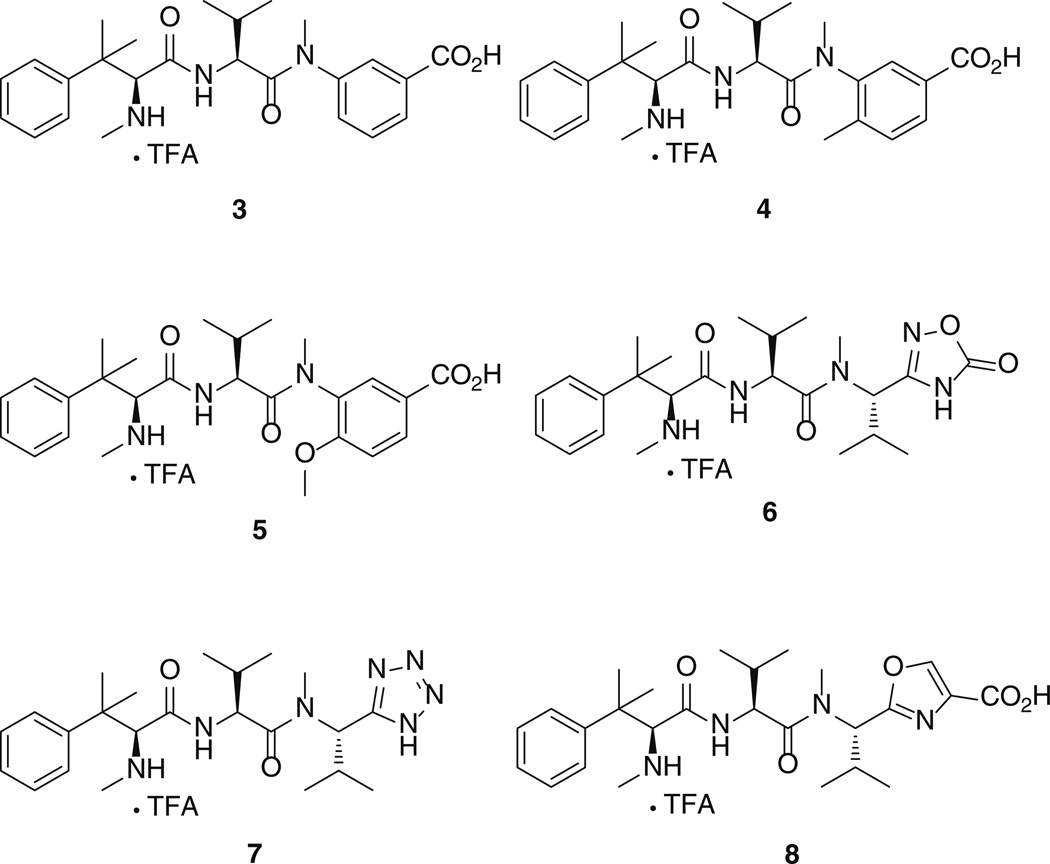
Six new hemiasterlin analogues were designed (Fig. 2 compounds 3–8) all contain potential cyclic bioisosteres of the fragment C double bond. It should be noted that two compounds (6, 7) were designed lacking the terminal carboxylic acid group and still be potentially able to interact with tubulin by means of H bonds. We describe their computational conformational analysis, their synthesis, and evaluation of both cytotoxic effects and ability to inhibit tubulin polymerization.
Fig. 2.
Hemiasterlin analogues 3–8 containing cyclic fragments C (C-terminus)
Results and discussion
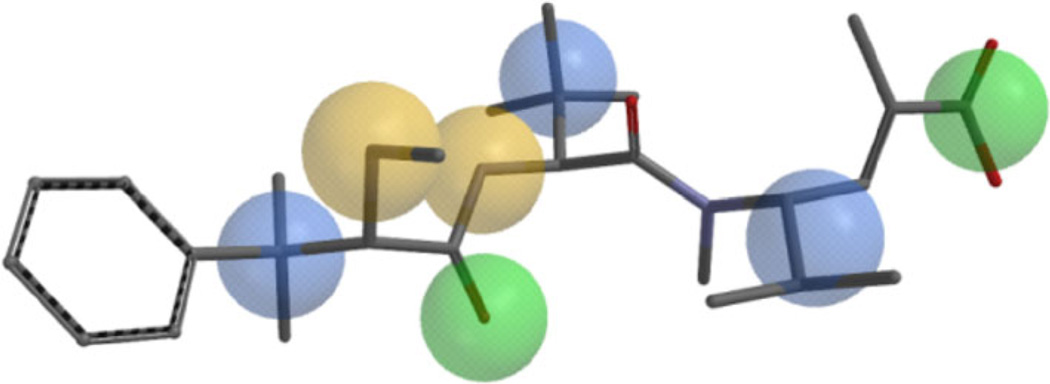
The binding mode for antimitotic peptides within tubulin is still unknown, but experimental evidence both from SAR and STD-NMR analysis [30] and computational studies established that taltobulin and other similar peptides (e.g., dolastatins, hemiasterlins and cryptophycins) bind in proximity of the vinca binding site [31,32]. From such studies some important geometrical features have been outlined as important for biological activity. In the docked pose the amide backbone of taltobulin adopts a bent conformation, and some contacts with the receptor are realized. There are hydrophobic interactions of the gem-dimethyl on the first residue and the tert-butyl group on the middle residue with lipophilic pockets in β-tubulin. Further, hydrogen bonds are observed between the two NH hydrogen atoms and β-Asp179, between the B-part CO oxygen and β-Ser174 and between β-Asn186 and the terminal carboxylic function. To investigate the effect of modification of the C part of hemiasterlin on conformational behavior, with regard to possible consequences on bioactivity, compounds 3–8 and taltobulin were subjected to computational conformational analysis. To evaluate the similarity of compounds 3–8 with the reference compound 2, the lowest energy conformations were superimposed accordingly to CFDs (chemical functionality descriptors), which were selected on the basis of the previously described geometrical requirements (three hydrophobic, two H bond donor, and two H bond acceptor CFDs, see Fig. 3).
Fig. 3.
Selected CFDs on taltobulin 2 for similarity analysis of compounds 3–8. The following colors are used: blue hydrophobic, yellow H bond donor, green H bond acceptor. Hydrogen atoms are omitted for clarity. (Color figure online)
Briefly, compounds 3–8 and taltobulin 2 were studied by means of a MC/EM (Monte Carlo/Energy Minimization) protocol using the MMFF94 force field with the software Spartan’08 [33]. Conformations up to 6 kcal/mol from the global minimum were kept. In terms of geometry, for the reference compound taltobulin 2, we obtained results that are in complete agreement with the previously reported ones [17].
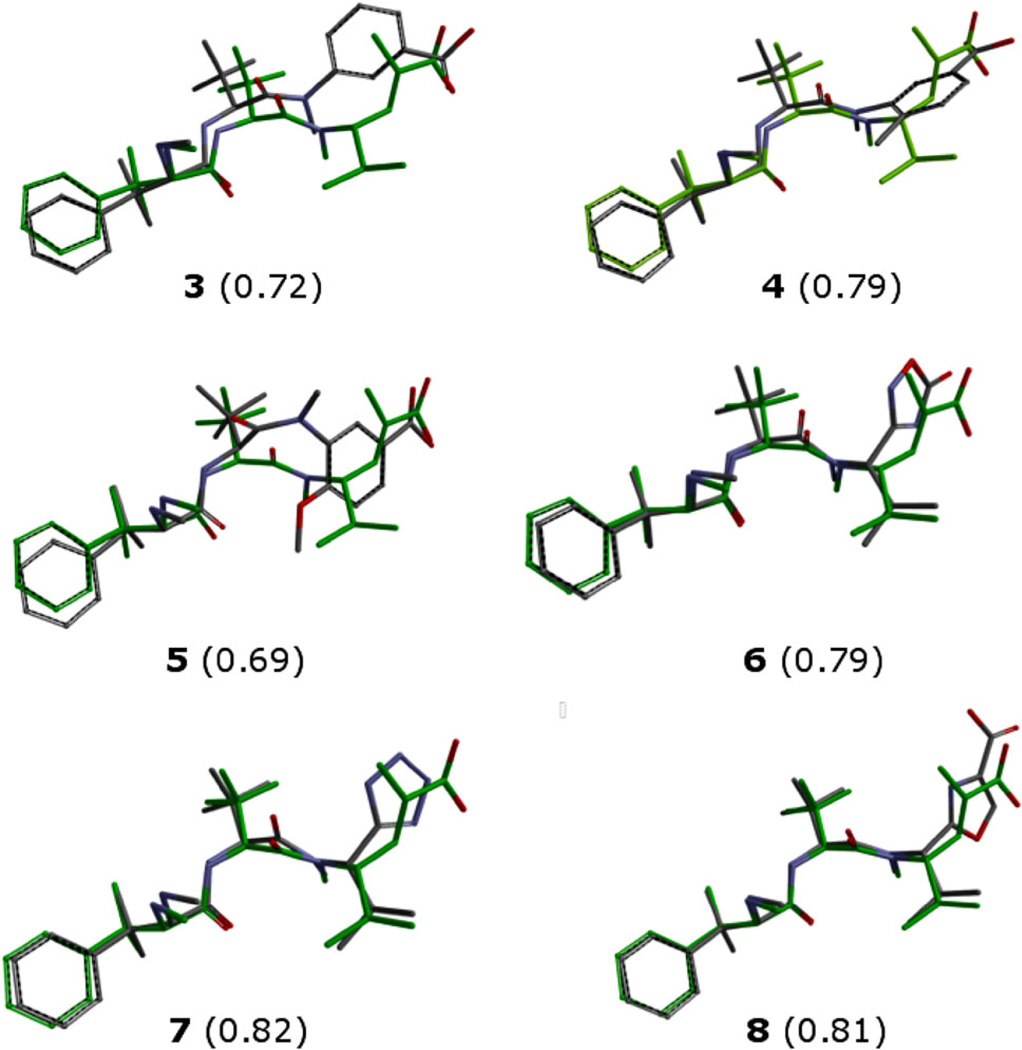
The structures obtained from conformational analysis of 3–8 were then superimposed on the global minimum of taltobulin 2 according to the selected CFDs. The success of the superimposition was measured with the score function of the software1 (Fig. 4). As expected, for all compounds the unmodified AB portion generally showed good superimposition on taltobulin, while more evident differences were observed for the C part, for which two CFDs were described (hydrophobic on the isopropyl and H bond acceptor on the carboxylic moiety). Compounds 3–5 showed very different behaviors, depending on the substituent on the phenyl ring, and the best superimposition was obtained with 4, in which the methyl substituent on the phenyl superimposed well on the isopropyl of taltobulin 2. The presence of a methoxy group in 5 produced a severe displacement of the amide backbone, leading to the lowest superimposition score versus taltobulin 2. Compounds 6–8 overall showed good superimposition scores; in these cases, the hydrophobic isopropyl group is retained, and the differences are due to the orientation of the terminal heterocyclic H bond acceptor moiety. For 6 and 8 the H bond acceptor is an oxygen, while for 7 it is the tetrazole nitrogen. The best score was obtained for 7, in which the presence of four nitrogen atoms allowed for a good orientation of the terminal heterocycle as an efficient H bond acceptor.
Fig. 4.
Superimpositions of compounds 3–8 with taltobulin 2 (green). Hydrogen atoms are omitted for clarity. Scores from CFDs superimposition with taltobulin are indicated in brackets. (Color figure online)
To summarize, computational studies revealed a good similarity between compounds 3–8 and taltobulin. Compounds 3–5 are valuable in positioning the terminal H bond acceptor carboxylate moiety, while the phenyl ring in 4 and 5 is less effective in mimicking the hydrophobic portion of the C part. Compounds 6–8 seem to be more similar to taltobulin 2, and, in particular, the tetrazole ring of compound 7 matched well with the terminal H bond acceptor moiety.
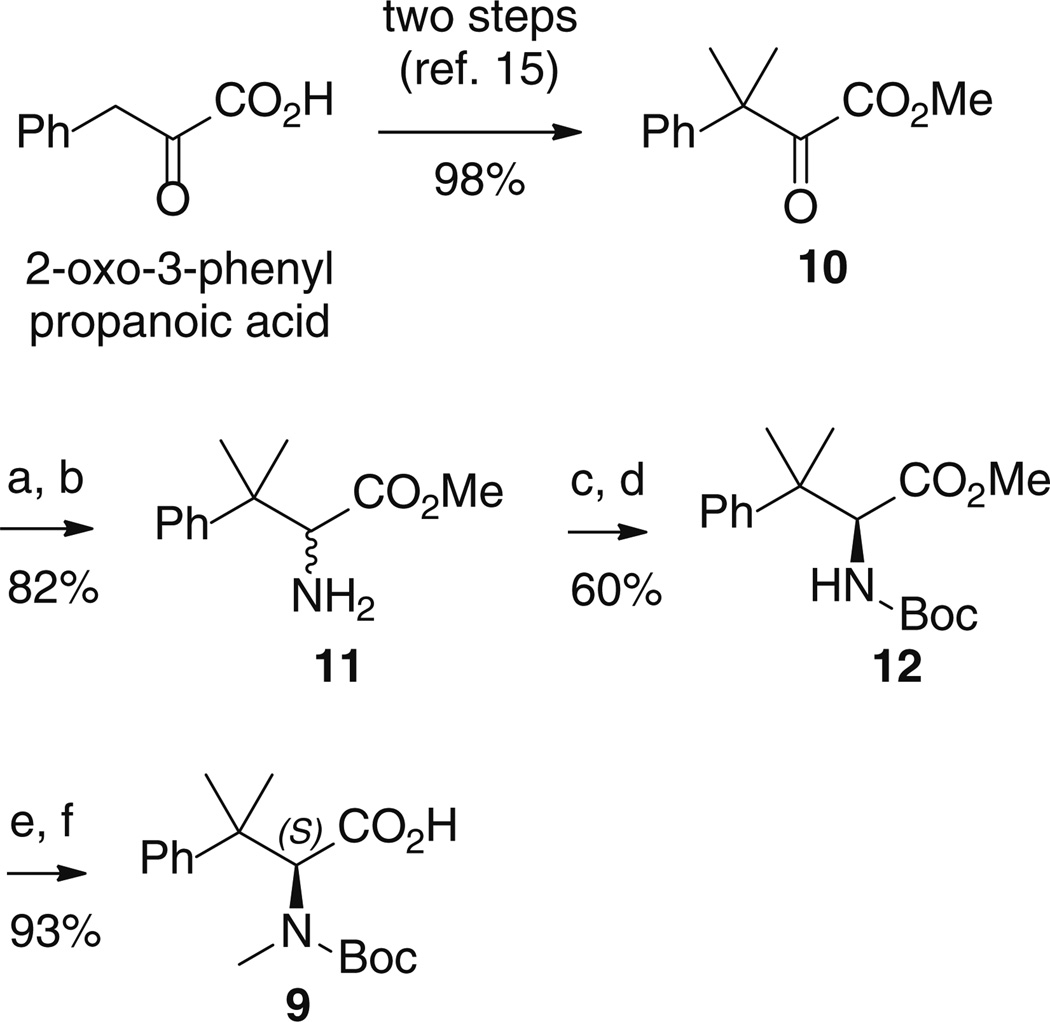
For the synthesis of the N-protected A-fragment amino acid 9, we modified a literature procedure [15], which starts from 2-oxo-3-phenylpropanoic acid to give keto ester 10 in two steps (Scheme 1). From 10, reaction with hydroxylamine hydrochloride, followed by oxime reduction with Zn metal in H2SO4/AcOH afforded racemic amino ester 11 in high yield. In order to obtain enantiopure 11 in multigram scale, thus avoiding diastereoisomer separation after the condensation step of the A-fragment with the remaining chiral portion of the molecule, we developed a dynamic resolution protocol. It uses slightly less than one equivalent of (S)-mandelic acid as the resolving agent with 5 mol% 5-nitrosalicylaldehyde, as racemizing agent [34]. The mandelate salt of (S)-11 was obtained in 74 % yield and directly converted to the N-Boc derivative 12 (97 % ee, from chiral HPLC, see Supporting Information).
Scheme 1.
Reaction conditions: a NH2OH·HCl, MeOH, 65 °C, 16 h. b Zn, 30 % H2SO4aq, AcOH, rt, 16 h. c (S)-mandelic acid, 5 mol% 5-nitrosalicylaldehyde, 7:3 isopropyl acetate/2-propanol mixture, 45 °C, 12 h. d Boc2O, Et3N, CH2Cl2, r.t., 16 h. e NaH, MeI, DMF, r.t., 18 h. f 1 M LiOH aq, MeOH, H2O, r.t., 16 h
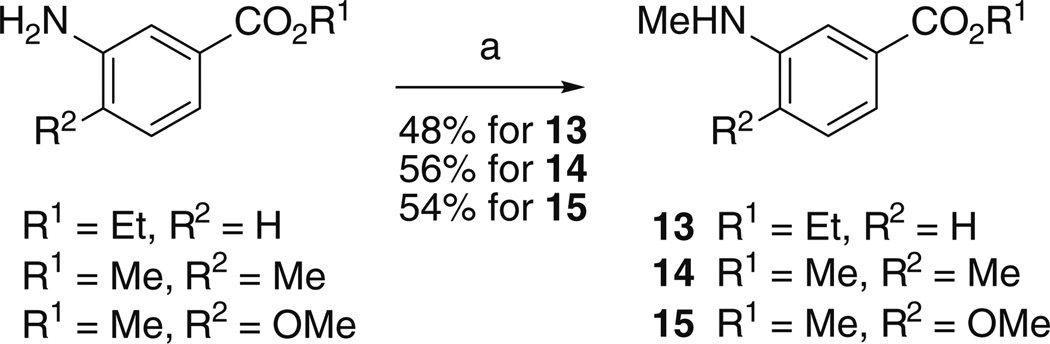
From 12, N-Boc-amino acid 9 was obtained by N-methylation, followed by ester hydrolysis. As fragment B, we employed (l)-valine, in place of (l)-tert-leucine, as it represents a known variation allowing substantial bioequivalence [14]. From the appropriately substituted 3-amino benzoic esters, reductive amination with formaldehyde afforded without problems the aromatic fragments C 13–15 of the target compounds 3, 4, and 5 (Scheme 2).
Scheme 2.
Reaction conditions: a 37 % HCHO aq, Na(AcO)3BH, MgSO4, CH2Cl2, r.t., 16 h
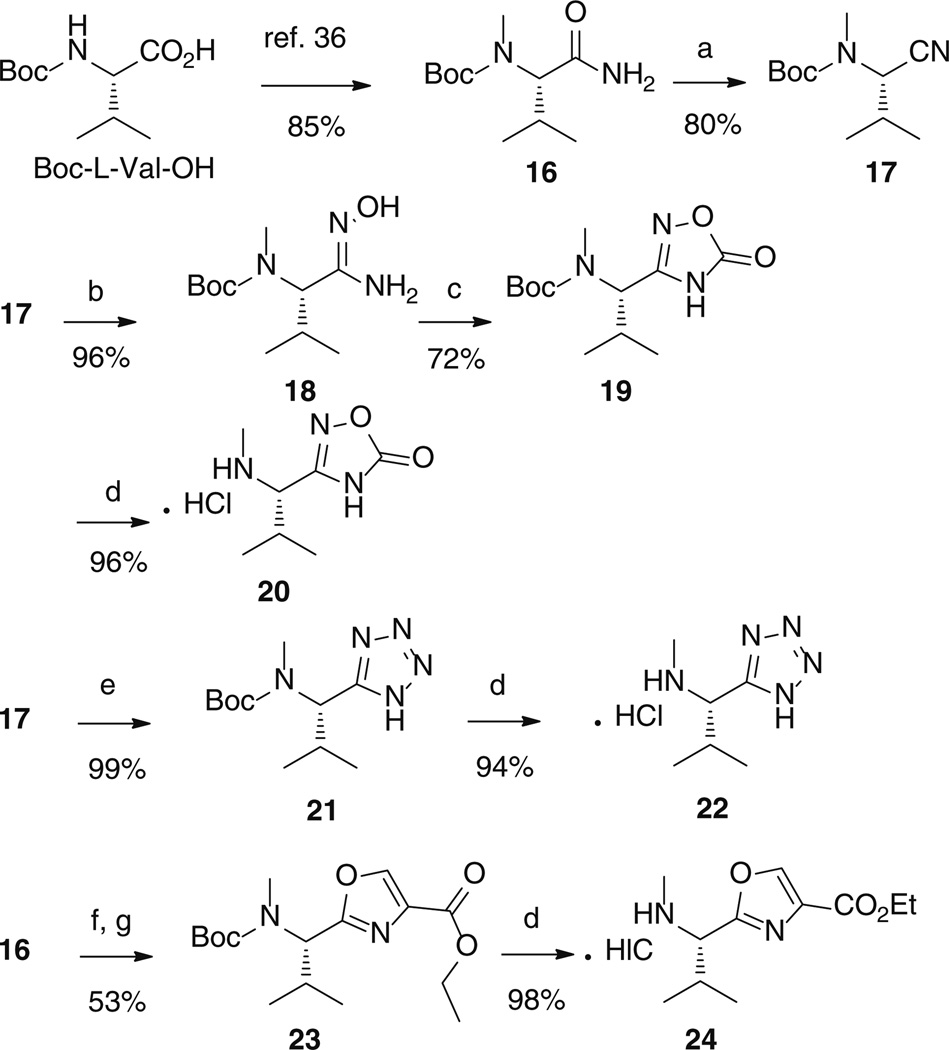
For the synthesis of the heterocycle-based fragments C (Scheme 3), we started with commercially available Boc-l-Val-OH, which was easily converted to the known [35,36] amide 16. Careful amide dehydration by means of cyanuric chloride in DMF afforded the corresponding nitrile 17, avoiding possible racemisation [37].
Scheme 3.
Reaction conditions: a Cyanuric chloride, DMF, 0 °C, 1 h. b NH2OH.HCl, Na2CO3, EtOH, H2O, 90 °C, 16 h. c CDI, THF, 65 °C, 18 h. d 3 M HCl in dioxane, r.t., 3 h. e NaN3, NH4Cl, DMF, 130 °C, 6 h. f Ethyl bromopyruvate, NaHCO3, THF, 65°C, 16 h. g TFAA, Py, THF, r.t., 16 h.
Nitrile 17 in the presence of hydroxylamine hydrochloride at 90 °C gave the N′-hydroxy-imidamide derivative 18, which was easily converted to the oxadiazolone 19 upon treatment with carbonyldiimidazole. From nitrile 17, the tetrazole derivative 21 was easily obtained by a click reaction with sodium azide at 130 °C. Finally, from amide 16, reaction with ethyl bromo pyruvate, followed by cyclization promoted by trifluoroacetic anhydride, cleanly afforded the oxazole derivative 23. Treatment with HCl in dioxane on 19, 21, and 23 yielded quantitatively N-deprotected heterocyclic derivatives 20, 22, and 24 as hydrochloride salts.
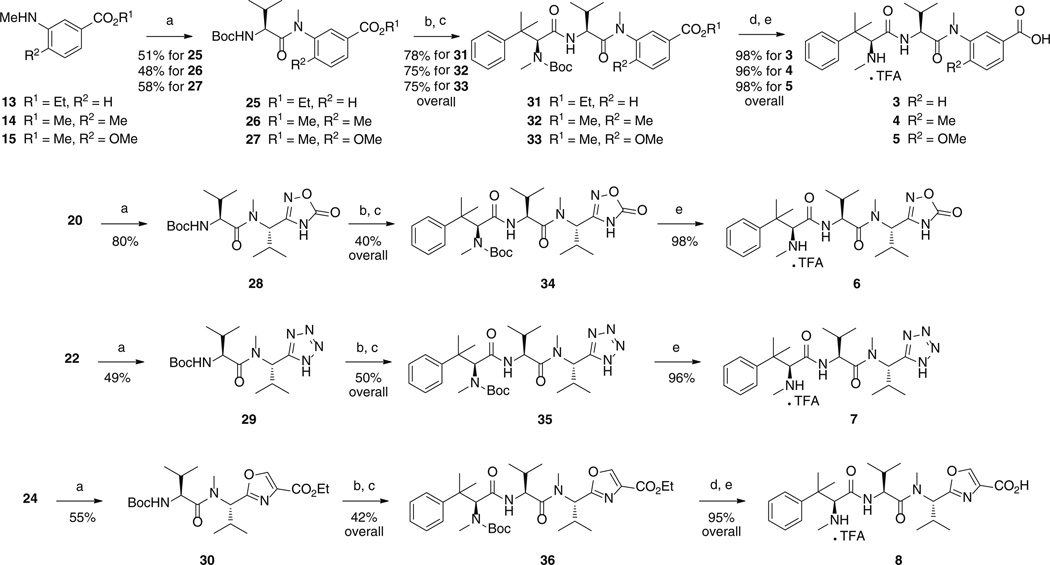
With all fragment C analogs (13–15, 20, 22, 24) in hand, we then pursued their coupling with N-Boc-valine and, subsequently, with the A-fragment 9. The first coupling proved to be difficult with low yield under different reaction conditions (HATU, HOAt, NMM, DMF; PivCl, DIPEA, THF; oxalyl chloride, Py, DCM; DCC, HOBt, NMM, THF), especially in the cases of methyl anilines 13–15. In the end, we found that activating N-Boc-valine with cyanuric fluoride was the best choice to deliver satisfactory yields of the requisite protected dipeptides 25–30, in reactions with both anilines 13–15 and heterocycle derivatives 20, 22 and 24 (Scheme 4).
Scheme 4.
Reaction conditions: a Boc-Val-F (from Boc-Val-OH, pyridine, cyanuric fluoride, CH2Cl2, −10 °C, 1 h), CH2Cl2, r.t., 24 h. b 3 M HCl in dioxane, r.t., 3 h. c Acid 9, PyBOP, DIPEA, CH2Cl2, r.t., 24 h. d 1 M LiOH aq, MeOH, H2O, 60 °C, 2 h. e 50 % TFA in CH2Cl2, r.t., 30 min
After N-Boc deprotection, dipeptides 25–30 were condensed with acid 9 to give tripeptides 31–36 in acceptable yields. Compounds 31–33 and 36 were then submitted to ester hydrolysis by means of LiOH in aqueous methanol. Deprotected targets 3–8 were finally obtained as trifluoroacetate salts by treatment with 50 % TFA in CH2Cl2.
The cytotoxicity on HeLa, HT29, SEM, and Jurkat tumor cells and the antitubulin activities of the various analogues synthesized are shown in Tables 1 and 2.
Table 1.
In vitro cytotoxic activity of compounds 2–8
| Compd | IC50 (µM) |
|||
|---|---|---|---|---|
| HeLa | HT29 | SEM | Jurkat | |
| 2 | 0.0002 ± 0.0001 | 0.0003 ± 0.0001 | 0.0001 ± 0.00005 | 0.0002 ± 0.0005 |
| 3 | 4.7 ± 0.5 | 1.9 ± 0.5 | >10 | 0.15 ± 0.02 |
| 4 | 5.3 ± 0.6 | 2.7 ± 0.2 | >10 | 0.29 ± 0.05 |
| 5 | >10 | >10 | >10 | >10 |
| 6 | >10 | >10 | >10 | >10 |
| 7 | >10 | >10 | >10 | >10 |
| 8 | >10 | >10 | >10 | >10 |
Table 2.
Inhibition of tubulin assembly and the binding of [3H] Vinblastine [3H] Dolastatin 10 and [3H] Halicondrin B by compounds 1–5
| Compd | Inhibition of tubulin assembly IC50 (µM) ± SD |
Inhibition of bindinga of |
|||||
|---|---|---|---|---|---|---|---|
| [3H]vinblastine |
[3H]dolastatin 10 |
[3H]halichondrin B |
|||||
| 5 µM | 20 µM | 5 µM | 20 µM | 5 µM | 20 µM | ||
| 1 | 1.3 ± 0.05 | 14 ± 4 | 66 ± 4 | 19 ± 7 | 89 ± 1 | 38 ± 4 | 92 ± 2 |
| 2 | 1.2 ± 0.01 | 29 ± 9 | 60 ± 2 | 0 ± 0 | 30 ± 1 | 38 ± 6 | 63 ± 1 |
| 3 | >20 | 2 ± 1 | 7 ± 3 | 0 ± 0 | |||
| 4 | >20 | 9 ± 1 | 0 ± 0 | 3 ± 1 | |||
| 5 | >20 | 0 ± 0 | 8 ± 2 | 0 ± 0 | |||
SD Standard deviation
Ligand binding studies were performed in 0.1 M 4-morpholinethanesulfonate (pH 6.9 in 1 M stock solution adjusted with NaOH)-0.5 mM MgCl2 containing 10 µ M tubulin (1.0 mg/mL), 10 µ M radiolabeled ligand, and inhibitors as indicated. At least two experiments performed for each condition. Data are presented as % inhibition ± SD versus a control reaction without hemiasterlin or hemiasterlin analog
Despite their structural similarity with taltobulin 2, we were surprised to find that compounds 3–8 were devoid of any appreciable cytotoxic activity. Our synthesized compound 2 (taltobulin), tested in parallel as a reference compound, showed nanomolar IC50 values in line with the literature [17]. Compounds 3–5 were also inactive as inhibitors of tubulin assembly and of the binding of [3H]vinblastine, [3H]dolastatin 10, and [3H]halichondrin B, while hemiasterlin 1 [9] and 2 were active in all assays. This indicates that a better orientation of the C-terminus H bond acceptor is required for affinity for tubulin, despite of the good overall conformational similarity of our compounds with taltobulin.
Conclusion
In summary, we developed an original approach to the synthesis of hemiasterlin analogs, aimed at modification of fragment C of the lead compound taltobulin. On the basis of recent SAR data and of modeling studies, some aromatic and heterocyclic type fragments C were introduced in place of the C-terminal double bond of taltobulin, with the aim of modulating the conformational rigidity of the entire molecule. Contrary to our expectations, biological evaluation of compounds 3–8 did not afford active analogs. Taking these results into account and using docking studies, a new structure-based approach is now underway to evaluate structural modifications, including introduction of additional potential recognition features for binding to tubulin.
Experimental section
General methods
All solvents were distilled and properly dried, when necessary, prior to use. All chemicals were purchased from commercial sources and used directly, unless indicated otherwise. All reactions were run under N2, unless otherwise indicated. All reactions were monitored by thin layer chromatography (TLC) on precoated silica gel 60 F254; spots were visualized with UV light or by treatment with 1 % KMnO4 aqueous solution. Products were purified by flash chromatography on silica gel 60 (230–400 mesh).
All NMR spectra were acquired on a Bruker 400 Avance spectrometer equipped with a z gradient coil probe operating at a proton frequency of 400.10 MHz. Data are presented as follows: chemical shift (multiplicity, integration, coupling constant). Chemical shifts (δ) are expressed in ppm relative to TMS at δ = 0 ppm for 1H NMR and relative to CDCl3 at δ = 77.16 ppm for 13C NMR. Abbreviations of multiplicity are as follows; s: singlet, d: doublet, t: triplet, q: quartet, oct: octet, m: multiplet, br: broad. Coupling constant (J) are reported in hertz (Hz). 13C NMR spectra were recorded using the APT pulse sequence; the signals of CH and CH3 are positive, while CH2 and quaternary carbons are negative. High-resolution MS spectra were recorded with a Waters Micromass Q-ToF micro™ mass spectrometer, equipped with an ESI source.
Synthetic procedures and characterization data for compounds 3–36
Methyl 3-methyl-2-oxo-3-phenylbutanoate (10)
To a solution of 3-phenylpyruvic acid (5.0 g, 0.03 mol) in tetrahydrofuran (40 mL) and water (10 mL), methyl iodide (5.7 mL, 0.091 mol), and 5 M aqueous sodium hydroxide solution (18.2 mL, 0.091 mol) were added, under nitrogen atmosphere with cooling with an ice–water bath. The cooling bath was removed, and the resulting mixture was heated at reflux for 6 h. The reaction mixture was allowed to cool to room temperature, and methyl iodide (1.9 mL, 0.03 mol) was added, followed by 5 M aqueous sodium hydroxide solution (12.2 mL, 0.061 mol). Stirring was continued overnight at room temperature. The volatiles were removed under reduced pressure, and the residual aqueous solution was extracted with ethyl acetate to remove non-acidic components. The residue was cooled and acidified with 1 M aqueous hydrogen chloride to pH 1.0, and the resulting aqueous layer was extracted with ethyl acetate (3 × 40 mL). The combined organic extracts were washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford 3-methyl-2-oxo-3-phenylbutanoic acid (5.8 g, 99 %), as a brown oil. This product was used in the following step without further purification. 1H NMR (300 MHz, CDCl3) δ 8.52 (s, br, 1H), 7.45-7.17 (m, 5H), 1.67 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 197.8, 161.8, 141.5, 129.0-126.2 (5C), 50.2, 25.3 (2C).
To a solution of 3-methyl-2-oxo-3-phenylbutanoic acid (3.0 g, 0.016 mol) in Et2O (12 mL) and CH3OH (18 mL) at 0 °C, trimethylsilyldiazomethane (2.0 M solution in hexanes, 14 mL, 0.028 mol) was added dropwise. The solvents were removed under reduced pressure, and the residue was taken up in Et2O/hexane (1:1, 50 mL) and washed with 2 % aqueous H3PO4 (3 × 50 mL), saturated aqueous NaHCO3 (3 × 50 mL) and brine (50 mL). The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give methyl 3-methyl-2-oxo-3-phenylbutanoate 10 (3.2 g, 99 %), as a straw-colored liquid. This product was used in the following step without further purification. 1H NMR (400 MHz, CDCl3) δ 7.44-7.22 (m, 5H), 3.64 (s, 3H), 1.66 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 197.9, 162.6, 141.8, 128.8-126.2, 52.2, 50.5, 25.2 (2C). HRMS (ESI) calcd for C12H15O3 (MH+) 207.1021, found 207.1023.
Methyl 2-amino-3-methyl-3-phenylbutanoate (11)
A mixture of methyl 3-methyl-2-oxo-3-phenylbutanoate (3.22 g, 0.016 mol), hydroxylamine hydrochloride (3.34 g, 0.048 mol), and dry methanol (60 mL) was refluxed for 16 h and then evaporated to dryness. The residue was taken up in saturated aqueous NaCl (50 mL), extracted with ethyl acetate (3 × 50 mL), dried over anhydrous Na2SO4, and evaporated to give methyl 2-(hydroxyimino)-3-methyl-3-phenylbutanoate (3.42 g, 99 %). This product was used in the following step without further purification. 1H NMR (400 MHz, CDCl3) δ 8.16 (s, br, 1H), 7.44-7.21 (m, 5H), 3.66 (s, 3H), 1.62 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 164.1, 158.9, 143.9, 128.4-126.4 (5C), 52.0, 42.9, 27.1 (2C).
To a solution of methyl 2-(hydroxyimino)-3-methyl-3-phenylbutanoate (3.9 g, 0.018 mol) in acetic acid (40 mL), 30 % H2SO4 aqueous solution was added with cooling with an ice-saltwater bath. Powdered zinc (3.5 g, 0.054 mol) was added to this cooled solution portion wise. After the addition of the zinc was complete, the reaction mixture was allowed to stir at room temperature for 16 h. The volatiles were removed under reduced pressure, and the residual aqueous solution was extracted with ethyl acetate (50 mL). The residue was cooled and basified with NaHCO3 saturated aqueous solution, and the resulting aqueous layer was extracted with ethyl acetate (3 × 50 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford pure methyl 2-amino-3-methyl-3-phenylbutanoate 11 (3.03 g, 83 %), as a foam. 1H NMR (300 MHz, CDCl3) δ 7.42-7.17 (m, 5H), 3.67 (s, 1H), 3.59 (s, 3H), 2.00 (s, br, 2H), 1.40 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 174.3, 146.1, 128.2-126.3 (5C), 64.0, 51.4, 41.8, 25.8, 23.0. HRMS (ESI) calcd for C12H18NO2 (MH+) 208.1338, found 208.1341.
(S)-Methyl 2-((tert-butoxycarbonyl)amino)-3-methyl-3-phenylbutanoate (12)
To a solution of methyl 2-amino-3-methyl-3-phenylbutanoate (3.92 g, 0.019 mol), isopropyl acetate (70 mL), and isopropyl alcohol (30 mL), (S)-mandelic acid (2.86 g, 0.019 mol) was added. The resulting mixture was heated to 45 °C for 4 h, before the addition of 5-nitrosalicylaldehyde (158 mg, 0.95 mmol). The resulting reaction mixture was then stirred at 45 °C for 12 h and cooled at room temperature. The resulting crystalline mandelate salt was collected by filtration, and the crystals washed with cold isopropyl alcohol and dried under reduced pressure to afford (S)-1-methoxy-3-methyl-1-oxo-3-phenylbutan-2-aminium (S)-2-hydroxy-2-phenylacetate (4.35 g, 64 %). (c 1.0, MeOH). 1H NMR (300 MHz, DMSO-d6) δ 7.46-7.13 (m, 10H), 4.95 (s, 1H), 3.81-3.00 (m, 4H), 3.53 (s, 1H), 3.41 (s, 3H), 1.32 (s, 3H), 1.29 (s, 3H). 13C NMR (75 MHz, D2O) δ 181.8, 171.9, 145.3, 143.0, 131.7-128.8 (10C), 77.4, 64.6, 55.8, 42.4, 28.1, 25.1.
To a stirred solution of (S)-1-methoxy-3-methyl-1-oxo-3-phenylbutan-2-aminium (S)-2-hydroxy-2-phenylacetate (1.86 g, 0.0052 mol) in CH2Cl2 (40 mL), triethylamine (3.6 mL, 0.026 mol) and a solution of di-tert-butyl dicarbonate (2.49 g, 0.011 mol) in CH2Cl2 (20 mL) were added. The reaction mixture was stirred at room temperature for 16 h. The solvent was removed under reduced pressure, and the residue was taken up in ethyl acetate (60 mL) and washed with 1 % aqueous H3PO4 (3 × 50 mL), saturated aqueous NaHCO3 (3 × 50 mL), and brine (50 mL). The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified using silica gel column flash chromatography (9:1, n-hexane:EtOAc) to give (S)-methyl 2-((tert-butoxycarbonyl)amino)-3-methyl-3-phenylbutanoate 12 (1.5 g, 94 %, 97.1 % ee), as a foam. (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.44-7.15 (m, 5H), 5.00 (d, J = 8.8 Hz, 1H), 4.51 (d, J = 8.8 Hz, 1H), 3.49 (s, 3H), 1.42 (s, 3H), 1.39-1.38 (br, s, 12H). 13C NMR (100 MHz, CDCl3,) δ 171.8, 155.4, 144.8, 131.0-122.8 (5C), 79.8, 62.1, 51.6, 41.6, 28.2 and 27.4 (3C, 2 conformers), 25.6, 24.7. HRMS (ESI) calcd for C17H26NO4 (MH+) 308.1862, found 308.1870.
(S)-2-((it Tert-butoxycarbonyl)(methyl)amino)-3-methyl-3-phenylbutanoic acid (9)
To a vigorously stirred suspension of sodium hydride (0.29 g, 0.012 mol) in dry DMF (20 mL) cooled with an ice-salt water bath, a solution of (S)-methyl 2-((tert-butoxycarbonyl)amino)-3-methyl-3-phenylbutanoate (1.5 g, 0.0049 mol) in dry DMF (20 mL) was slowly added. After the bubbling had ceased, methyl iodide (1.5 mL, 0.024 mol) was added, and the resulting gray suspension was stirred for 18 h at room temperature. The excess sodium hydride was quenched by cautious addition of saturated aqueous NH4Cl (120 mL), and the resulting aqueous layer was extracted with ethyl acetate (3 × 80 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford (S)-methyl 2-((tert-butoxycarbonyl)(methyl)amino)-3-methyl-3-phenylbutanoate (1.49 g, 95 %). (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of two conformers) δ 7.68-6.73 (m, 5H), 5.30 and 4.92 (m, 1H), 3.60 and 3.59 (m, 3H), 2.81 and 2.75 (m, 3H), 1.69-1.14 (m, 15H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 170.9, 156.6, 146.5, 133.5-117.7 (5C), 80.5 and 79.9 (1C), 66.9 and 65.3 (1C), 51.4, 42.4, 33.8 and 33.4 (1C), 28.3 (3C), 27.6, 22.6.
(S)-Methyl - 2-((tert-butoxycarbonyl)(methyl)amino)-3-methyl-3-phenylbutanoate (1.38 g, 0.0043 mol) was dissolved in methanol (100 mL). While stirring, first H2O (35 mL) and then an 1 M aqueous solution of lithium hydroxide (35 mL, 0.035 mol) were added. The resulting mixture was stirred at room temperature for 16 h. The solvent was removed under reduced pressure, and the residue was washed with CH2Cl2 (50 mL). The aqueous layer was acidified to pH 3 by addition of 5 % aqueous H3PO4 and extracted with ethyl acetate (3 × 60 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford (S)-2-((tert-butoxycarbonyl)(methyl)amino)-3-methyl-3-phenylbutanoic acid 9 (1.29 g, 98 %), as a foam. (c 7.0, CHCl3). 1H NMR (300 MHz, CDCl3, mixture of two conformers) δ 7.65-6.86 (m, 5H), 5.12 and 4.94 (m, 1H), 2.75 and 2.61 (m, 3H), 1.70-1.30 (m, 15H). HRMS (ESI) calcd for C17H26NO4 (MH+) 308.1862, found 308.1866.
Ethyl 3-(methylamino)benzoate (13)
To a stirred solution of ethyl 3-aminobenzoate (3 g, 0.018 mol) in dry CH2Cl2, anhydrous MgSO4 (5 g) and 37 % formaldehyde aqueous solution (1.6 mL, 0.022 mol) were added. The resulting suspension was stirred for 2 h, and then sodium triacetoxyborohydride (4.6 g, 0.022 mol) was added. The reaction mixture was stirred at room temperature for 16 h and filtered to remove the magnesium sulfate. A saturated aqueous NaHCO3 solution (50 mL) was added to the filtrate, and the two layers were separated, and then the aqueous layer was extracted with ethyl acetate (3 × 40 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The residue was purified using silica gel column chromatography (85:15, n-hexane:EtOAc) to give ethyl 3-(methylamino)benzoate 13 (1.57 g, 48 %), as a foam. 1H NMR (300 MHz, CDCl3) δ 7.59-7.18 (m, 3H), 7.00-6.75 (m, 1H), 4.35 (q, J = 7.2 Hz, 2H), 2.87 (s, 3H), 1.37 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 167.1, 149.3, 131.3, 129.0, 119.2, 116.8, 112.8, 60.8, 30.6, 14.3. HRMS (ESI) calcd for C10H14NO2 (MH+) 180.1025, found 180.1006.
Methyl 4-methyl-3-(methylamino)benzoate (14)
Starting from methyl 4-methyl-3-aminobenzoate, the same procedure, as for compound 13, was followed. After the reaction, the residue was purified using silica gel column chromatography (8:2, n-hexane:EtOAc) to give methyl 4-methyl-3-(methylamino)benzoate 14 (1.83 g, 56 %), as a foam. 1H NMR (300 MHz, CDCl3) δ 7.40 (dd, J = 7.7 and 1.6 Hz, 1H), 7.34 (s, br, 1H), 7.11 (d, J = 7.7 Hz, 1H), 4.96 (s, br, 1H), 3.89 (s, 3H), 2.94 (s, 3H), 2.21 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 167.8, 147.1, 129.7, 129.0, 127.3, 118.3, 109.6, 51.8, 30.7, 17.6. HRMS (ESI) calcd for C10H14NO2 (MH+) 180.1025, found 180.1031.
Methyl 4-methoxy-3-(methylamino)benzoate (15)
Starting from methyl 4-methoxy-3-aminobenzoate, the same procedure, as for compound 13, was followed. After the reaction, the residue was purified using silica gel column chromatography (8:2, n-hexane:EtOAc) to give methyl 4-methyl-3-(methylamino)benzoate 15 (1.76 g, 54 %), as a foam. 1H NMR (300 MHz, CDCl3) δ 7.45 (dd, J = 8.3 and 2.1 Hz, 1H), 7.27 (d, J = 2.1 Hz, 1H), 6.76 (d, J = 8.3 Hz, 1H), 4.87 (s, br, 1H), 3.89 (s, 3H), 3.87 (s, 3H), 2.90 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 167.6, 150.6, 138.8, 123.1, 119.3, 109.9, 108.2, 55.5, 51.7, 30.3. HRMS (ESI) calcd for C10H14NO3 (MH+) 196.0974, found 196.0972.
(S)-Tert-butyl (1-amino-3-methyl-1-oxobutan-2-yl)(methyl)carbamate (16)
Sodium hydride as 80 % suspension in mineral oil (8.6 mL, 0.14 mol) was added slowly in portions over a period of 2 h to a cooled (0 °C) solution of N-Boc-valine (3 g, 0.014 mol) and iodomethane (8.6 mL, 0.14 mol) in anhydrous THF (40 mL). The reaction mixture was stirred at room temperature for 16 h. The solvent was removed under reduced pressure, and the residue was taken up in diethyl ether (50 mL). The excess sodium hydride was quenched by cautious addition of H2O (50 mL), and then the two layers were separated, and the aqueous layer was washed with diethyl ether (2 × 50 mL). The aqueous layer was acidified to pH 3 by addition of 5 % aqueous H3PO4 and extracted with ethyl acetate (3 × 100 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford (S)-2-((tert-butoxycarbonyl)(methyl)amino)-3-methylbutanoic acid (3.1 g, 97 %). (c 1.1, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of two conformers) δ 4.14 (m, 0.36H, minor conformer), 3.98 (d, J = 10.1 Hz, 0.64H, major conformer), 2.91 (s, 3H), 2.40 (m, 0.64H, major conformer), 2.24 (m, 0.36H, minor conformer), 1.50 (s, 9H), 1.06 (d, J = 6.6 Hz, 3H), 0.95 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.3, 175.3, 157.0, 155.7, 80.9, 80.7, 65.4, 65.1, 28.4, 27.8, 27.5, 20.1, 19.8, 19.1, 19.0.
A solution of (S)-2-((tert-butoxycarbonyl)(methyl)amino)-3-methylbutanoic acid (3.1 g, 0.013 mol), N-methylmorpholine (1.6 mL, 0.015 mol), and ethyl chloroformate (1.4 mL, 0.015 mol) in THF (70 mL) was cooled to −10 °C (ice–saltwater bath). After stirring for 20 min at the same temperature, 30 % NH3 aqueous solution (6 mL, 0.045 mol) was added, and stirring was continued for another 5 h. The solvent was removed in vacuo, and the residue was dissolved in ethyl acetate (60 mL). The organic layer was washed with 1 % aqueous H3PO4 (3 × 50 mL), saturated aqueous NaHCO3 (3 × 50 mL), and brine (50 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford (S)-tert-butyl (1-amino-3-methyl-1-oxobutan-2-yl)(methyl)carbamate 16 (2.7 g, 88 %), as a foam. (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 6.19 (s, br, 1H), 5.38 (s, br, 1H), 4.07 (d, J = 11.1 Hz, 1H), 2.79 (s, 3H), 2.24 (m, 1H), 1.46 (s, 9H), 0.96 (d, J = 6.6 Hz, 3H), 0.86 (d, J = 6.6 Hz, 3H). HRMS (ESI) calcd for C11H23N2O3 (MH+) 231.1709, found 231.1722.
(S)-Tert-butyl (1-cyano-2-methylpropyl)(methyl)carbamate (17)
To a solution of (S)-tert-butyl (1-amino-3-methyl-1-oxobutan-2-yl)(methyl)carbamate (1.5 g, 0.0065 mol) in DMF (30 mL) at 0 °C, cyanuric chloride (1.68 g, 9.1 mmol, 1.4 equiv) was added. After stirring for 1 h at 0 °C, the reaction mixture was quenched with a cold 0.5 M sodium hydroxide solution (70 mL), and the mixture extracted with ethyl acetate (3 × 40 mL). The organic layers were combined and washed with water and brine, dried over anhydrous Na2SO4, filtered, and concentrated. The crude residue was purified by silica gel chromatography (9:1, n-hexane:EtOAc) to afford (S)-tert-butyl (1-cyano-2-methylpropyl)(methyl)carbamate 17 (1.11 g, 80 %), as a foam. (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3, mixture of two conformers) δ 4.82 (s, br, 0.63H, major conformer), 4.51 (s, br, 0.37H, minor conformer), 2.88 (s, 3H), 2.07 (m, 1H), 1.46 (s, 9H), 1.13 (d, J = 6.6 Hz, 3H), 0.90 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 117.5, 81.2, 54.0, 30.5, 30.3, 28.2 (3C), 19.3 (2C), 18.1. HRMS (ESI) calcd for C11H21N2O2 (MH+) 213.1603, found 213.1607.
(S)-Tert-butyl (1-amino-1-(hydroxyimino)-3-methylbutan-2-yl)(methyl)carbamate (18)
To a solution of (S)-tert-butyl (1-cyano-2-methylpropyl) (methyl)carbamate (0.4 g, 0.0019 mol) in ethanol (4 mL), H2O (2 mL), hydroxylamine hydrochloride (0.24 g, 0.0037 mol), and sodium carbonate (0.4 g, 0.0038 mol) were added. The reaction mixture was stirred at 90 °C for 16 h and then diluted with ethyl acetate (30 mL). The organic layer was washed with 1 % aqueous H3PO4 (3 × 30 mL), saturated aqueous NaHCO3 (3 × 30 mL), and brine (30 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford (S)-tert-butyl (1 -amino - 1 -(hydroxyimino) -3- methylbutan-2-yl)(methyl)carbamate 18 (0.44 g, 96 %), as a foam. (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 6.48 (s, br, 1H), 5.64 (s, br, 1H), 4.66 (s, br, 1H), 3.74 (d, J = 10.4 Hz, 1H), 3.00-2.70 (m, 3H), 2.50 (m, 1H), 1.45 (s, 9H), 0.97 (d, J = 6.6 Hz, 3H), 0.88 (d, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 157.0, 152.5, 80.1, 64.8 and 63.9 (1C), 32.6, 28.4 (3C), 26.4, 20.1, 19.1.HRMS (ESI) calcd for C11H24N3O3 (MH+) 246.1818, found 246.1831.
(S)-Tert-butyl methyl(2-methyl-1-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)propyl)carbamate (19)
To a solution of (S)-tert-butyl (1-amino-1-(hydroxyimino)-3-methylbutan-2-yl)(methyl)carbamate (0.4 g, 0.0016 mol) in tetrahydrofuran (20 mL), 1, 1′-carbonyldiimidazole (0.4 g, 0.0025 mol) was added, and the mixture was heated at 65 °C for 18 h. The reaction mixture was cooled and concentrated. The residue was dissolved in CH2Cl2 (25 mL) and extracted with a 1 M sodium hydroxide aqueous solution (25 mL). The aqueous layer was carefully acidified with 1 M hydrochloric acid aqueous solution to pH 3 and extracted with CH2Cl2 (3 × 50 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford (S)-tert-butyl methyl(2-methyl-1-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)propyl)carbamate 19 (0.31 g, 72 %), as a foam. (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 9.60 (s, br, 1H), 3.85 (d, J = 10.9 Hz, 1H), 2.87 (s, 3H), 2.62 (m, 1H), 1.46 (s, 9H), 1.19-0.69 (m, 6H). 13C NMR (75 MHz, CDCl3) δ 159.1, 157.5, 156.6, 81.7, 60.9, 34.2, 28.2 (3C), 27.2, 19.9, 18.9. HRMS (ESI) calcd for C12H22N3O4 (MH+) 272.1610, found 272.1609.
(S)-3-(2-Methyl-1-(methylamino)propyl)-1,2,4-oxadiazol-5(4H)-one hydrochloride (20)
(S)-Tert-butyl methyl(2-methyl-1-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)propyl)carbamate (0.32 g, 0.0012 mol) was dissolved in 5 mL of a 3 M HCl/dioxane solution. The mixture was stirred at room temperature for 3 h. The solvent was removed in vacuo, and the residue was dried under reduced pressure to afford (S)-3-(2-methyl-1-(methylamino)propyl)-1,2,4-oxadiazol-5(4H)-one hydrochloride 20 (0.28 g, 96%). (c 1.0, MeOH). 1H NMR (300 MHz, DMSO-d6) δ 10.09 (s, br, 2H), 4.24 (d, J = 6.4 Hz, 1H), 3.61 (s, br, 1H), 2.62 (s, 3H), 2.44 (m, 1H), 1.04 (d, J = 6.8 Hz, 3H), 0.97 (d, J = 6.8 Hz, 3H). 13C NMR (75 MHz, D2O) δ 162.1, 155.4, 60.6, 32.7, 30.0, 18.2, 17.1. HRMS (ESI) calcd for C7H14N3O2 (M+) 172.1086, found 172.1099.
(S)-Tert-butyl methyl(2-methyl-1-(1H-tetrazol-5-yl)propyl)carbamate (21)
(S)-Tert-butyl (1-cyano-2-methylpropyl)(methyl)carbamate (0.4 g, 0.0019 mol), ammonium chloride (0.8 g, 0.015 mol), and sodium azide (0.85 g, 0.013 mol) were dissolved in dry DMF (3 mL). The mixture was stirred at 130 °C for 6 h. The mixture was acidified to pH 3 by addition of 1 % aqueous H3PO4 and extracted with ethyl acetate (3 × 20 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford (S)-tert-butyl methyl(2-methyl-1-(1H-tetrazol-5-yl)propyl)carbamate 21 (0.48 g, 99 %), as a foam. (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 4.90 (d, J = 11.3 Hz, 1H), 2.89 (s, br, 3H), 2.68 (m, 1H), 1.47 (s, 9H), 1.02 (d, J = 6.6 Hz, 3H), 0.78 (d, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 156.8, 154.6, 81.9, 56.7, 31.3, 29.1, 28.5 (3C), 19.9, 19.3. HRMS (ESI) calcd for C11H22N5O2 (MH+) 256.1773, found 256.1772.
(S)-N,2-Dimethyl-1-(1H-tetrazol-5-yl)propan-1-amine hydrochloride (22)
Starting from (S)-Tert-butyl methyl(2-methyl-1-(1H-tetrazol-5-yl)propyl)carbamate, the same procedure, as for compound 20, was followed, affording (S)-N,2-dimethyl-1-(1H-tetrazol-5-yl)propan-1-amine hydrochloride 22 (0.34 g, 94 %). (c 1.0, MeOH). 1H NMR (300 MHz, DMSO-d6) δ 9.96 (s, br, 1H), 9.75 (s, br, 1H), 4.71 (d, J = 5.6 Hz, 1H), 2.67-2.50 (m, 1H), 2.48 (s, 3H), 0.95 (d, J = 6.8 Hz, 3H), 0.84 (d, J = 6.8 Hz, 3H). 13C NMR (75 MHz, D2O) δ 154.7, 60.2, 32.3, 30.8, 18.7, 17.0. HRMS (ESI) calcd for C6H14N5 (M+) 156.1249, found 156.1256.
(S)-Ethyl 2-(1-((tert-butoxycarbonyl)(methyl)amino)-2-methylpropyl)oxazole-4-carboxylate (23)
To a solution of (S)-tert-butyl (1-amino-3-methyl-1-oxobutan-2-yl)(methyl)carbamate (0.3 g, 0.0013 mol) in THF (15 mL) NaHCO3 (1.65 g, 0.02 mol) and ethyl bromopyruvate (1.3 mL, 0.01 mol) were added. The reaction was heated at 65 °C for 16 h. The resulting orange suspension was filtered through celite and concentrated under reduced pressure. The resulting orange oil was dissolved in THF (2 mL) and to this solution, pyridine (1.6 mL, 0.02 mol) and tri-fluoroacetic anhydride (1.1 mL, 0.008 mol) were added. The mixture was stirred at room temperature for 16 h. The reaction mixture was diluted with ethyl acetate (25 mL) and washed with 1 % aqueous H3PO4 (3 × 20 mL), saturated aqueous NaHCO3 (3 × 20 mL), and brine (20 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified using silica gel column chromatography (85:15, n-hexane:EtOAc) to give (S)-ethyl 2-(1-((tert-butoxycarbonyl)(methyl)amino)-2-methylpropyl)oxazole-4-carboxylate 23 (0.22 g, 53 %), as a foam. (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of two conformers) δ 8.16 (s, 1H), 5.14 (d, J = 10.7 Hz, 0.55 H, major conformer), 4.89 (d, J = 10.7 Hz, 0.45 H, minor conformer), 4.38 (q, J = 7.1 Hz, 2H), 2.76 (s, br, 3H), 2.63-2.38 (m, 1H), 1.46 (s, 9H), 1.37 (t, J = 7.1 Hz, 3H), 0.96 (d, J = 6.1 Hz, 3H), 0.90 (s, br, 3H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 163.7, 161.2, 156.0, 143.7, 133.4, 80.5 and 80.1 (1C), 61.1, 59.9 and 58.2 (1C), 29.4, 28.3 (4C), 20.7, 18.0, 14.3. HRMS (ESI) calcd for C16H27 N2O5 (MH+) 327.1920, found 327.1900.
(S)-Ethyl 2-(2-methyl-1-(methylamino)propyl)oxazole-4-carboxylate hydrochloride (24)
Starting from (S)-Ethyl 2-(1-((tert-butoxycarbonyl)(methyl)amino)-2-methylpropyl)oxazole-4-carboxylate, the same procedure, as for compound 20, was followed, affording (S)-ethyl 2-(2-methyl-1-(methylamino)propyl)oxazole-4-carboxylate hydrochloride 24(156 mg, 98 %). (c 1.0, MeOH). 1H NMR (300 MHz, DMSO-d6) δ 8.16 (s, 1H), 4.70 (d, J = 7.3 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H), 2.51 (s, 3H), 2.48 (m, 1H), 1.37 (t, J = 7.1 Hz, 3H), 0.97 (d, J = 6.4 Hz, 3H), 0.92 (d, J = 6.4 Hz, 3H). 13C NMR (75 MHz, D2O) δ 165.1, 160.4, 143.0, 136.3, 64.8, 60.2, 30.8, 29.9, 19.1, 18.8, 15.0. HRMS (ESI) calcd for C11H19N2O3 (M+) 227.1396, found 227.1398.
(S)-Ethyl 3-(2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido)benzoate (25)
Boc-Val-OH (934 mg, 4.3 mmol) was dissolved in CH2Cl2dry (12 mL), pyridine (0.3 mL, 4.3 mmol) was added, and the solution was cooled to −20 °C. Cyanuric fluoride (0.8 mL, 9.46 mmol) was added, and the reaction was left for 1 h at −10 °C. Cold water and CH2Cl2 were added directly in the reaction flask. The two phases were separated, and the aqueous phase was extracted 3 times with CH2Cl2. The organic phases were reunited and washed with cold water. The organic phase was then dried on Na2SO4 and evaporated under reduced pressure. The obtained product was dissolved in CH2Cl2, and compound 13 (1 equiv) was added. The reaction was left stirring for 24 h under N2. Once the reaction was complete, it was washed with aq NaHCO3, H3PO4 5 % aq solution, H2O, and brine. The organic phase was then dried with Na2SO4 and evaporated under reduced pressure. The residue was purified using silica gel column chromatography (9:1, CH2Cl2:EtOAc) to give (S)-ethyl 3-(2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido) benzoate 25 (829 mg, 51 %), as a foam. (c 0.5, CHCl3). 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.5 Hz, 1H), 7.91 (s, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 5.14 (d, J = 9.4 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 4.25-4.07 (m, 1H), 3.31 (s, 3H), 1.81 (m, 1H), 1.43 (s, 9H), 1.42 (t, J = 7.1 Hz, 3H), 0.80 (d, J = 6.7 Hz, 3H), 0.74 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.4, 165.5, 155.3, 143.2, 132.3, 132.1, 129.9, 129.1, 128.5, 79.3, 61.3, 55.6, 37.7, 31.6, 28.3 (3C), 19.5, 17.3, 14.3. HRMS (ESI) calcd for C20H31N2O5 (MH+) 379.2233, found 379.2241.
(S)-Methyl 3-(2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido)-4-methylbenzoate (26)
Starting from compound 14, the same procedure, as for 25, was followed. The residue was purified using silica gel column chromatography (9:1, CH2Cl2:EtOAc) to give (S)-methyl 3-(2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido)-4-methylbenzoate 26 (167 mg, 48 %), as a foam. (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of two conformers) δ 7.96 (d, J = 7.8 Hz, 1H), 7.84 (s, 1H), 7.42 (d, J = 7.8 Hz, 1H), 5.18 (d, J = 9.8 Hz, 0.7H, major conformer), 5.07 (d, J = 9.8 Hz, 0.3H, minor conformer), 3.94 (s, 2.1H, major conformer), 3.92-3.82 (m, 1H), 3.89 (s, 0.9H, minor conformer), 3.24 (s, 2.1H, major conformer), 3.19 (s, 0.9H, minor conformer), 2.34 (s, 0.9H, minor conformer), 2.27 (s, 2.1H, major conformer), 1.94-1.69 (m, 1H), 1.40 (s, 9H), 0.82 (d, J = 6.8 Hz, 3H), 0.76 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 172.5, 166.1, 155.3, 142.0, 141.5, 133.5-126.4 (3C), 129.4, 79.2, 56.1 and 55.5 (1C), 52.2 and 52.1 (1C), 36.4, 31.8 and 31.0 (1C), 28.2 (3C), 19.6, 18.8, 15.4. HRMS (ESI) calcd for C20H31N2O5(MH+) 379.2233, found 379.2220.
(S)-Methyl 3-(2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido)-4-methoxybenzoate (27)
Starting from compound 15, the same procedure, as for 25, was followed. The residue was purified using silica gel column chromatography (9:1, CH2Cl2:EtOAc) to give (S)-ethyl 3-(2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido) benzoate 27 (210 mg, 58 %), as a foam. (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of two conformers) δ 8.21-8.01 (m, 1H), 7.89 (d, J = 1.7 Hz, 1H), 7.02 (d, J = 8.7 Hz, 1H), 5.25 (d, J = 9.7 Hz, 0.7H, major conformer), 5.10 (d, J = 9.7 Hz, 0.3H, minor conformer), 4.09-3.71 (m, 7H), 3.22 (s, 2.1H, major conformer), 3.18 (s, 0.9H, minor conformer), 1.86-1.70 (m, 1H), 1.42 (s, 9H), 0.93-0.66 (m, 6H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 173.7, 166.0, 158.8, 155.3, 131.8-130.5 (3C), 122.9, 111.6, 78.8, 57.3, 54.9, 52.1 and 52.0 (1C), 36.6 and 35.9 (1C), 32.5, 28.3 (3C), 19.4, 17.3. HRMS (ESI) calcd for C20H31N2O6 (MH+) 395.2182, found 395.2185.
Ethyl 3((S)-2-((S)-2-(tert-butoxycarbonyl(methyl)amino)-3-methyl-3-phenylbutanamido)-N,3-dimethylbutanamido) benzoate (31)
Compound 25 (154 mg, 0.5 mmol) was dissolved in 2 mL of a 3 M HCl/dioxane solution. The mixture was stirred at room temperature for 3 h. The solvent was removed in vacuo, and the residue was dried under reduced pressure. Then it was dissolved in CH2Cl2 (3 mL), and it was added to a stirring solution of compound 9 (139 mg, 0.5 mmol) in CH2Cl2 (3 mL). The mixture was then cooled to 0 °C, and PyBop (1.1 equiv), and DIPEA (3 equiv) were added. The reaction was left to react at room temperature for 24 h. Once the reaction is completed, the solvent was evaporated, and the residue was dissolved in AcOEt and washed with 1 % H3PO4 aqueous solution, sat NaHCO3, water, and finally with brine. The organic phases are reunited and dried with Na2SO4. The solvent is then evaporated under reduced pressure. Purification by chromatographic column (7:3, n-hexane:EtOAc) afforded product 31 (221 mg, 78 %), as a foam. (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of two conformers in 7:3 ratio) δ 8.03 (m, 1H), 7.84 (s, br, 1H), 7.64-7.11 (m, 7H), 6.01 (m, 0.3H), 5.88 (m, 0.7H), 5.20 (s, br, 0.7H), 4.86 (s, br, 0.3H), 4.42 (q, J = 7.1 Hz, 1.4H), 4.41 (q, J = 7.1 Hz, 0.6H), 4.30 (m, 1H, 0.3H), 4.23 (t, br, J = 7.8 Hz, 0.7H), 3.25 (s, br, 3H), 2.92 (s, 3H), 1.76-1.67 (m, 1H), 1.65 (s, br, 5.1H), 1.62 (s, 0.9H), 1.55-1.40 (m, 12H), 0.67-0.46 (m, 6H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 171.5 and 171.4 (1C), 169.8 and 168.8 (1C), 165.6, 157.0, 147.3, 143.1, 127.6, 132.3-126.3 (9C), 80.7 and 80.1 (1C), 66.7 and 65.3 (1C), 61.4, 54.0, 42.7, 37.6, 33.5 and 33.1(1C), 31.4, 28.4-27.3 (3C), 25.4, 24.8, 19.4 and 17.4 and 17.1 (2C), 14.3. HRMS (ESI) calcd for C32H46N3O6 (MH+) 568.3381, found 568.3387.
Methyl 3-((S)-2-((S)-2-(tert-butoxycarbonyl(methyl)amino)-3-methyl-3-phenylbutanamido)-N,3-dimethylbutanamido)-4-methylbenzoate (32)
Starting from compound 26, the same procedure, as for 31, was followed. Purification by chromatographic column (7:3, n-hexane:EtOAc) afforded product 32 (140 mg, 75 %), as a foam. (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of 4 conformers in 0.5:0.2:0.15:0.15 ratio) δ 8.01-7.14 (m, 8H), 6.15 (d, br, J = 9.6 Hz, 0.5H), 6.11-6.01 (m, 0.35H), 5.93 (d, br, J = 8.8 Hz, 0.15H), 5.20 (s, 0.5H), 5.15 (s, 0.15H), 4.87 (s, 0.2H), 4.83 (s, 0.15H), 4.37-4.24 (m, 0.3H), 4.15-4.05 (m, 0.7H), 3.96 (s, 0.6H), 3.93 (s, 1.95H), 3.91 (s, 0.45H), 3.22-3.11 (m, 3H), 2.91-2.82 (m, 3H), 2.30 (s, 0.6H), 2.17 (s, 0.45H), 1.97 (s, 1.95), 1.75 (m, 1H), 1.64 (s, br, 2H), 1.60 (s, br, 4H), 1.53-1.38 (m, 9H), 0.68-0.43 (m, 6H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 172.3 and 172.1 (1C), 169.6 and 169.1 (1C), 166.8, 157.7 and 156.3 (1C), 147.8, 142.7, 141.0, 130.0, 132.7-126.8 (8C), 81.4 and 80.8 (1C), 67.3 and 66.0 (1C), 54.2 and 54.0 (1C), 53.0, 43.3 and 43.0 (1C), 37.3 and 37.1 (1C), 34.1 and 33.8 (1C), 32.3 and 31.3 (1C), 29.0 (3C), 25.9, 25.7, 20.4 and 17.4 (3C). HRMS (ESI) calcd for C32H46N3O6(MH+) 568.3387, found 568.3366.
Ethyl 3-((S)-2-((2S,3R)-4-tert-butoxy-3-methyl-4-oxo-2-(2-phenylpropan-2-yl)butanamido)-N,3-dimethylbutanamido)-4-methoxybenzoate (33)
Starting from compound 27, the same procedure, as for n31, was followed. Purification by chromatographic column (8:2, n-hexane:EtOAc) afforded product 33 (198 mg, 75 %), as a foam. (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3, mixture of 4 conformers in 0.45:0.25:0.2:0.1 ratio) δ 8.17-6.86 (m, 8H), 6.30 (d, br, J = 9.1 Hz, 0.45H), 6.22 (d, br, J = 9.1 Hz, 0.2H), 6.01 (d, br, J = 9.1 Hz, 0.1H), 5.92 (d, br, J = 9.1 Hz, 0.25H), 5.28 (s, 0.45H), 5.10 (s, 0.2H), 4.93 (s, 0.25H), 4.78 (s, 0.1H), 4.32-4.15 (m, 1H), 3.95 (s, 0.8H), 3.92 (s, 2.3H), 3.89 (s, 0.8H), 3.77 (s, 0.3H), 3.52 (s, 0.7H), 3.49 (s, 1.1H), 3.19 (s, 0.75H), 3.17 (s, 1.35H), 3.13 (s, 0.3H), 3.11 (s, 0.6H), 2.92 (s, 0.3H), 2.90 (s, 0.6H), 2.87 (s, 0.75H), 2.82 (s, 1.35H), 1.80-1.39 (m, 16H), 0.73-0.47 (m, 6H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 172.7 and 171.5 (1C), 168.8, 166.0, 158.7 and 158.5 (1C), 157.0, 147.7, 147.0, 131.9-125.9 (7C), 123.1 and 122.9 (1C), 111.8 and 111.3 (1C), 80.8 and 80.2 (1C), 66.8 and 65.3 (1C), 55.8, 54.0 and 53.5 (1C), 52.1, 42.6 and 42.3 (1C), 36.7 and 35.9 (1C), 33.6 and 32.9 (1C), 32.2 and 32.1 (1C), 28.4-27.0 (3C), 25.7, 25.4, 19.6 and 19.2 (1C), 17.3 and 16.9 (1C). HRMS (ESI) calcd for C32H46N3O7 (MH+) 584.3336, found 584.3345.
3-((S)-N,3 Dimethyl-2-((S)-3-methyl-2-(methylamino)-3-phenylbutanamido)butanamido) benzoic acid trifluoroacetate (3)
Compound 31 (155 mg, 0.27 mmol) was dissolved in MeOH (2 mL) and H2O (1 mL). 1 M LiOH aqueous solution (0.81 mL, 0.81 mmol) was added, and the reaction was left at 60 °C for 2 h. Then, MeOH was evaporated, and the remaining aqueous solution was extracted with CH2Cl2. The aqueous phase was then acidified to pH 3 with 5% H3PO4 aqueous solution and then extracted with AcOEt. The organic phase was dried with Na2SO4, and the solvent was then evaporated under reduced pressure. The residue was dissolved in CH2Cl2 (1 mL), and TFA was added (1 mL). The reaction was left at room temperature for 30 min, and then it was evaporated under vacuum, to afford compound 3 (146 mg, 98 %), as a foam. (c 0.7, MeOH). 1H NMR (400 MHz, CD3OD) δ 8.10 (d, br, J = 7.6 Hz, 1H), 7.93 (s, br, 1H), 7.63 (t, J = 7.6 Hz, 1H), 7.60-7.52 (m, 3H), 7.48 (t, J = 7.6 Hz, 2H), 7.36 (t, J = 7.3 Hz, 1H), 4.29 (d, J = 7.9 Hz, 1H), 4.19 (s, 1H), 3.27 (s, 3H), 2.52 (s, 3H), 1.95 (oct, J = 7.0 Hz, 1H), 1.58 (s, 3H), 1.55 (s, 3H), 0.84 (d, J = 6.7 Hz, 3H), 0.83 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CD3OD) δ 171.4, 167.7, 166.8 (q, J = 27.0 Hz, 1C), 165.6, 144.2, 143.6, 133.2, 132.6, 130.4, 129.8, 129.6 (3C), 128.1, 126.7 (2C), 116.0 (q, J = 288.0 Hz, 1C), 71.1, 55.9, 41.2, 37.3, 33.4, 31.9, 26.0, 23.7, 19.1, 17.9. HRMS (ESI) calcd for C25H34N3O4 (M+) 440.2549, found 440.2556.
3-((S)-N,3-Dimethyl-2-((S)-3-methyl-2-(methylamino)-3-phenylbutanamido)butanamido)-4-methylbenzoic acid trifluoroacetate (4)
Starting from compound 32, the same procedure, as for 3, was followed, affording compound 4 (136 mg, 96 %), as a foam. (c 0.8, MeOH). 1H NMR (400 MHz, CD3OD, mixture of two conformers in 0.55:0.45 ratio) δ 8.05-7.34 (m, 8H), 4.37-4.33 (m, 1H), 4.32 (s, 0.55H), 4.23 (s, 0.45H), 3.24 (s, 1.65H), 3.16 (s, 1.35H), 2.54 (s, 1.35H), 2.48 (s, 1.65H), 2.37 (s, 1.35H), 2.33 (s, 1.65H), 2.04 (m, 0.55H), 1.83 (oct, J = 6.7 Hz, 0.45H), 1.60 (s, 1.35H), 1.59 (s, 1.35H), 1.53 (s, 1.65H), 1.49 (s, 1.65H), 0.95 (d, J = 6.7 Hz, 1.65H), 0.85 (d, J = 6.7 Hz, 1.35H), 0.80-074 (m, 3H). 13C NMR (100 MHz, CD3OD, mixture of two conformers) δ 171.1 and 171.0 (1C), 167.1, 166.8 (q, J = 25.6 Hz, 1C), 165.3 and 165.0 (1C), 143.6 and 143.4 (1C), 141.5, 140.6, 130.3 and 130.2 (1C), 131.7-126.0 (8C), 116.0 (q, J = 288.4 Hz, 1C), 70.7 and 70.4 (1C), 54.9, 40.5, 35.8 and 35.6 (1C), 32.8, 30.9 and 30.6 (1C), 25.0, 23.2, 19.0 and 16.5 (1C), 16.80, 18.8 and 15.8 (1C). HRMS (ESI) calcd for C26H36N3O4 (M+) 454.2706, found 454.2711.
3-((S)-N,3-Dimethyl-2-((S)-3-methyl-2-(methylamino)-3-phenylbutanamido)butanamido)-4-methoxybenzoic acid trifluoroacetate (5)
Starting from compound 33, the same procedure, as for 3, was followed, affording compound 5 (80 mg, 98 %), as a foam. (c 0.5, MeOH). 1H NMR (400 MHz, CD3OD, mixture of two conformers in 0.55:0.45 ratio) δ 8.16 (dd, J = 8.7 and 2.1 Hz, 0.45H), 8.14 (dd, J = 8.7 and 2.1 Hz, 0.55H), 8.00 (d, J = 2.1 Hz, 0.45H), 7.87 (d, J = 2.1 Hz, 0.55H), 7.62-7.55 (m, 2H), 7.52-7.45 (m, 2H), 7.41-7.34 (m, 1H), 7.30 (d, J = 8.7 Hz, 0.45H), 7.26 (d, J = 8.7 Hz, 0.55H), 4.49 (d, J = 5.9 Hz, 0.45H), 4.29 (s, 0.45H), 4.25 (d, J = 7.9 Hz, 0.55H), 4.17 (s, 0.55H), 3.96 (s, 1.35H), 3.95 (s, 1.65H), 3.21 (s, 1.35H), 3.13 (s, 1.65H), 2.53 (s, 1.65H), 2.45 (s, 1.35H), 2.37 (s, 1.35H), 2.33 (s, 1.65H), 2.04 (oct, J = 6.9 Hz, 0.45H), 1.88 (oct, J = 7.2 Hz, 0.55H), 1.60-1.54 (m, 6H), 0.93 (d, J = 7.0 Hz, 1.35H), 0.85 (d, J = 6.7 Hz, 1.35H), 0.81 (d, J = 6.7 Hz, 1.65H), 075 (d, J = 6.7 Hz, 1.65H). 13C NMR (100 MHz, CD3OD, mixture of two conformers) δ 172.1 and 171.7(1C), 167.8, 166.7 (q, J = 24.8 Hz, 1C), 165.3 and 165.2 (1C), 143.3, 144.2, 132.6-126.6 (8C), 124.2 and 124.1 (1C), 116.0 (q, J = 288.0 Hz, 1C), 112.8 and 112.4 (1C), 71.2 and 71.1 (1C), 56.2, 55.3, 41.2, 36.2 and 35.8 (1C), 33.4, 32.0 and 31.4 (1C), 25.6, 20.9, 19.7 and 16.9 (1C), 18.9 and 17.8 (1C). HRMS (ESI) calcd for C26H36N3O5 (M+) 470.2655, found 470.2640.
Tert-butyl (S)-3-methyl-1-(methyl((S)-2-methyl-1-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)propyl)amino)-1-oxobutan-2-yl carbamate (28)
Boc-Val-OH (154 mg, 0.70 mmol) was dissolved in CH2Cl2dry (2 mL), pyridine (0.056 mL, 0.70 mmol) was added, and the solution was cooled to −20 °C. Cyanuric fluoride (0.13 mL, 1.54 mmol) was added, and the reaction was left for 1 h at −10 °C. Cold water and CH2Cl2 were added directly in the reaction flask. The two phases were separated, and the aqueous phase was extracted 3 times with CH2Cl2. The organic phases were reunited and washed with cold water. The organic phase was then dried on Na2SO4 and evaporated under reduced pressure. The obtained product was dissolved in CH2Cl2, and salt 20 (1 equiv) was added, together with Et3N (2 equiv). The reaction was left stirring for 24 h under N2. Once the reaction was complete, the solvent was evaporated, and the residue was purified using silica gel column chromatography (1:1, n-hexane:EtOAc) to give pure compound 28 (207 mg, 80 %), as a foam. (c 0.9, MeOH). 1H NMR (400 MHz, CDCl3) δ 11.9-11.5 (m, 1H), 6.21 (d, br, J = 8.0 Hz, 1H), 5.11 (d, br, J = 8.8 Hz, 1H), 4.36 (t, J = 8.0 Hz, 1H), 3.20 (s, 3H), 2.53 (m, 1H), 1.89 (m, 1H), 1.43 (s, 9H), 1.09 (d, J = 6.6 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H), 0.98 (d, J = 6.9 Hz, 3H), 0.87 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.3, 160.2, 156.6, 156.3, 79,7, 56.7 (2C), 31.9, 30.4, 28.3 (3C), 26.4, 19.9, 19.4, 18.8, 18.4. HRMS (ESI) calcd for C17H31N4O5 (MH+) 371.2294, found 371.2278.
Tert-butyl methyl((S)-3-methyl-1-((S)-3-methyl-1-(methyl((S)-2-methyl-1-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)propyl)amino) -1-oxobutan-2-ylamino)-1-oxo-3-phenylbutan-2-yl)carbamate (34)
Starting from compound 28, the same procedure, as for 31, was followed. Purification by chromatographic column (3:2, n-hexane:EtOAc) afforded pure 34 (141 mg, 40 %), as a foam. (c 0.9, MeOH). 1H NMR (300 MHz, 100 °C, DMSO-d6, mixture of two conformers in 4:1 ratio) δ 7.47-7.37 (m, 4H), 7.28 (t, br, J = 7.8 Hz, 2H), 7.21-7.14 (m, 1H), 5.16 (d, br, J = 10.5 Hz, 2H), 5.02 (s, 1H), 4.51 (t, J = 7.8 Hz, 1H), 3.01 (s, br, 3H), 2.77 (s, 3H), 2.35 (m, 1H), 2.01 (m, 1H), 1,53 (s, 0.6H), 1.46 (s, 2.6H), 1.45 (s, 2.6H), 1.44 (s, 0.6H), 1.38 (s, 1.8H), 1.37 (s, 7.2H), 0.98 (d, J = 6.8 Hz, 2.4H), 0.95 (d, J = 6.8 Hz, 0.6H), 0.89-0.82 (m, 6H), 0.80 (d, J = 6.8 Hz, 2.4H), 0.73 (d, J = 6.8 Hz, 0.6H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 169.9 (2C), 156.3 (2C), 146.9, 146.5, 128.2-125.9 (5C), 80.6, 64.8 and 64.6 (1C), 55.2, 55.0, 42.4, 33.5, 32.4, 30.4, 30.0, 28.4-28.2 (3C), 26.2, 25.6, 19.9-18.6 (4 C). HRMS (ESI) calcd for C29H46N5O6 (MH+) 560.3448, found 560.3456.
(S)-N,3-Dimethyl-N-((S))-2-methyl-1-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)propyl-2-((S)-3-methyl-2-(methylamino)-3-phenylbutanamido) butanamide trifluoroacetate (6)
Compound 34 (135 mg, 0.24 mmol) was dissolved in CH2Cl2 (1 mL), and TFA was added (1 mL). The reaction was left at room temperature for 30 min, and then it was evaporated under vacuum, to afford compound 6 (135 mg, 98 %), as a foam. (c 1.1, MeOH). 1H NMR (400 MHz, CD3OD, mixture of two conformers in 4:1 ratio) δ 7.56-7.30 (m, 5H), 5.21 (d, J = 11.4 Hz, 0.8H), 5.18 (d, J = 11.1 Hz, 0.2H), 4.79-4.71 (m, 1H), 4.25 (s, 0.8H), 4.18 (s, 0,2H), 3.23 (s, 2.4H), 3.22 (s, 0.6H), 2.52 (s, 2.4H), 2.51 (s, 0.6H), 2.51-2.39 (m, 1H), 2.22-2.11 (m, 0.8H), 2.06-1.94 (m, 0.2H), 1.57 (s, 0.6H), 1.50 (s, 0.6H), 1.49 (s, 2.4H), 1.40 (s, 2.4H), 1.10-0.83 (m, 12H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 174.1 and 173.4 (1C), 167.0 and 166.3 (1C), 161.0 (q, J = 35 Hz, 1C), 158.1, 144.1 (2C), 129.6-126.6 (5C), 116.2 (q, J = 293.9 Hz, 1C), 70.8 and 70.7 (1C), 56.7, 56.2, 41.3 and 40.7 (1C), 33.4 and 33.3 (1C), 31.6 and 31.4 (1C), 31.0, 28.8 and 26.1 (1C), 27.2, 23.5 and 21.0 (1C), 19.4-18.1 (4C). HRMS (ESI) calcd for C24H38N5O4 (M+) 460.2924, found 460.2923.
Tert-butyl(S)-3-methyl-1-methyl-1-(1h-tetrazol-5-yl)propyl)amino-1-oxobutan-2-ylcarbamate (29)
Starting from salt 22, the same procedure, as for 28, was followed. The residue was purified using silica gel column chromatography (1:1, n-hexane:EtOAc) to give pure compound 29 (121 mg, 49 %), as a foam. (c 0.9, MeOH). 1H NMR (400 MHz, DMSO-d6) δ 7.09 (d, J = 8.2 Hz, 1H), 5.71 (d, J = 11.3 Hz, 1H), 4.05 (t, J = 8.8 Hz, 1H), 3.02 (s, 3H), 2.47 (m, 1H), 1.88 (m, 1H), 1.37 (s, 9H), 0.89 (d, J = 6.5 Hz, 3H), 0.86 (d, J = 6.8 Hz, 3H), 0.73 (d, J = 6.5 Hz, 3H), 0.66 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 175.8 and 172.7 (1C), 158.1 and 156.0 (1C), 153.6 and 153.2 (1C), 82.4 and 80.1 (1C), 58.5, 55.4, 31.5, 31.0 and 30.5 (1C), 28.7 and 28.4 (1C), 28.2-28.1 (3C), 20.3-17.6 (4C). HRMS (ESI) calcd for C16H31N6O3 (MH+) 355.2458, found 355.2435.
Tert-butyl methyl((S)-3-methyl-1-(methyl((S)-2-methyl-1-((1h-tetrazol-5-yl)propyl)amino)-1-oxobutan-2-ylamino)-1-oxo-3-phenylbutan-2-yl)carbamate (35)
Starting from compound 29, the same procedure, as for 31, was followed. Purification by chromatographic column (3:2, n-hexane:EtOAc) afforded pure 35 (125 mg, 50 %), as a foam. (c 0.5 MeOH). 1H NMR (400 MHz CDCl3) δ 9.00-8.60 (s, br, 1H), 7.42-7.34 (m, 3H), 7.09-7.03 (m, 3H), 5.92 (m, 1H), 5.17 (s, 1H), 4.77 (t, J = 9.8 Hz, 1H), 3.07 (s, 3H), 2.98 (s, 3H), 2.83 (m, 1H), 1.97 (m, 1H), 1.57 (s, 3H), 1.53 (s, 3H), 1.41 (s, 9H), 1.07 (d, J = 6.5 Hz, 3H), 0.99 (d, J = 6.7 Hz, 3H), 0.82 (d, J = 6.7 Hz, 3H), 0.67 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 173.4, 169.2, 156.7, 153.0, 146.0, 127.8-126.3 (5C), 81.2, 64.1, 53.8 (2C), 42.3, 33.2, 31.6, 30.8, 28.5 (3C), 27.9, 26.4, 25.7, 20.0, 19.0, 18.9, 17.7. HRMS (ESI) calcd for C28H46N7O4 (MH+) 544.3611, found 544.3619.
Tert-butyl methyl((S)-3-methyl-1-((methyl((S)-2-methyl-1-(1h-tetrazol-5-yl)propyl)amino)-1-oxobutan-2-ylamino)-1-oxo-3-phenylbutan-2-yl)carbamate trifluoroacetate (7)
Starting from compound 35, the same procedure, as for 6, was followed, affording compound 7 (53 mg, 96 %), as a foam. (c 0.9, MeOH). 1H NMR (400 MHz, CD3OD, mixture of two conformers in 9:1 ratio) δ 7.54 (d, br, J = 7.5 Hz, 2H), 7.47 (t, J = 7.5 Hz, 2H), 7.37 (t, br, J = 7.3 Hz, 1H), 5.79 (d, J = 11.3 Hz, 0.1H), 5.73 (d, J = 11.3 Hz, 0.9H), 4.73 (d, J = 8.3 Hz, 0.9H), 4.72 (d, J = 8.6 Hz, 0.1H), 4.23 (s, 0.9H), 4.17 (s, 0.1H), 3.23 (s, 2.7H), 3.18 (s, 0.3H), 2.66 (m, 1H), 2.53 (s, 3H), 2.10 (m, 1H), 1.52 (s, 0.3H), 1.50 (s, 2.7H), 1.44 (s, 0.3H), 1.41 (s, 2.7H), 1.05 (d, J = 6.7 Hz, 3H), 1.03 (d, J = 6.7 Hz, 3H), 0.87 (d, J = 6.7 Hz, 3H), 0.86 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CD3OD) δ 172.4, 165.6, 160.2 (q, J = 39.0 Hz, 1C), 143.5 (2C), 129.0-126.0 (5C), 116.2 (q, J = 291.0Hz, 1C), 70.2, 55.4, 54.2, 40.7, 32.8, 30.5 and 30.4 (1C, 2 conformers), 28.2, 28.1, 20.3, 18.5, 18.4, 17.7, 17.6, 17.5. HRMS (ESI) calcd for C23H38N7O2 (M+) 444.3087, found 444.3088.
Ethyl 2-((S)-1-((S)-2-(tert-butoxycarbonylamino)-N,3-dimethylbutanamido)-2-methylpropyl)oxazole-4-carboxylate (30)
Starting from salt 24, the same procedure, as for 28, was followed. The residue was purified using silica gel column chromatography (85:15, n-hexane:EtOAc) to give pure compound 30 (117 mg, 55 %), as a foam. (c 0.5 MeOH). 1H NMR (400 MHz CDCl3, mixture of two conformers in 0.55:0.45 ratio) δ 7.29 (s, 1H), 5.29 (d, J = 10.5 Hz, 0.55H), 5.27 (d, J = 8.9 Hz, 0.45H), 4.78 (d, J = 10.3 Hz, 0.45H), 4.74 (d, J = 10.5 Hz, 0.55H), 4.44 (q, J = 7.1 Hz, 0.9H), 4.38 (q, J = 7.1 Hz, 1.1H), 3.06 (s, 1.35H), 3.1 (s, 1.45H), 2.83-2.67 (m, 1H), 2.24-2.02 (m, 1H), 1.65 (s, 4.95H), 1.63 (s, 4.05H), 1.44 (t, J = 7.1 Hz, 1.35H), 1.40 (t, J = 7.1 Hz, 1.65H), 1.23-0.82 (m, 12H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 169.1, 168.1, 160.6 and 160.4 (1C), 158.6 and 157.6 (1C), 150.3, 133.7, 87.3 and 86.9 (1C), 62.5 and 61.9 (1C), 61.7 and 61.2 (1C), 52.5 and 52.4 (1C), 31.6 and 31.4 (1C), 29.8, 29.4 and 29.3 (1C), 27.3 (3C), 20.5-18.0 (4C), 14.2. HRMS (ESI) calcd for C21H36N3O6 (MH+) 426.2604, found 426.2613.
Ethyl 2-((6S,9S,12S)-9-isopropyl-2,2,5,11,13-pentamethyl 4,7,10-trioxo-6-(2-phenylpropan-2-yl)-3-oxa-5,8,11-triazatetradecan-12-yl)-oxazole-4-carboxylate (36)
Starting from compound 30, the same procedure, as for 31, was followed. Purification by chromatographic column (3:2, n-hexane:EtOAc) afforded pure 36 (70 mg, 42 %), as a foam. (c 0.5, MeOH). 1H NMR (400 MHz, CDCl3, mixture of two conformers in 1.5:1 ratio) δ 8.12 (s, 1H), 7.48-7.29 (m, 5H), 7.12 (m, 0.6H), 7.05 (m, 0.4H), 5.25 (m, 1H), 4.92 (dd, J = 8.8 and 4.7 Hz, 0.6H), 4.82 (dd, J = 8.4 and 7.0 Hz, 0.4H), 4.44 (q, J = 7.1 Hz, 1.2H), 4.37 (q, J = 7.1 Hz, 0.8H), 4.33 (s, 1H), 3.20 (s, 1.2H), 3.19 (s, 1.8H), 2.30 (s, 3H), 2.23-2.07 (m, 2H), 1.62 and 1.47 (2s, 15H), 1.44 (t, J = 7.1 Hz, 1.8H), 1.40 (t, J = 7.1 Hz, 1.2H), 1.25-1.18 (m, 3H), 1.06 (d, J = 6.7 Hz, 3H), 0.98 (d, J = 6.7 Hz, 1.2H), 0.96-0.86 (m, 4.8H). 13C NMR (100 MHz, CDCl3, mixture of two conformers) δ 169.2 and 169.0 (1C), 167.7, 161.6 and 161.3 (1C), 158.3, 153.5, 151.0, 144.8, 131.3, 129.6-126.6 (5C), 85.7, 71.4, 62.4 and 61.8 (1C), 55.2 and 55.0 (1C), 53.0 and 52.9 (1C), 42.6, 32.6 and 32.3 (1C), 32.2, 32.0, 30.6, 27.7 (3C), 21.5 (2C), 20.1-17.1 (4C), 14.9. HRMS (ESI) calcd for C33H51N4O7 (MH+) 615.3758, found 615.3761.
2-((S)-1-((S)-N,3-Dimethyl-2-((S)-3-methyl-2-(methylamino)-3-phenylbutanamido)butanamido)-2-methylpropyl)oxazole-4-carboxylic acid trifluoroacetate (8)
Starting from compound 36, the same procedure, as for 3, was followed, affording compound 8 (63 mg, 95 %), as a foam. (c 0.5, MeOH). 1H NMR (400 MHz, CD3OD) δ 8.37 (s, 1H), 7.54-7.49 (m, 2H), 7.45 (t, br, J = 7.3 Hz, 2H), 7.35 (t, br, J = 7.3 Hz, 1H), 5.75 (d, br, J = 11.8 Hz, 1H), 5.11 (d, br, J = 9.4 Hz, 1H), 4.33 (s, 1H), 3.04 (s, 3H), 2.63 (s, 3H), 2.38-2.22 (m, 2H), 1.55 (s, 3H), 1.54 (s, 3H), 1.06 (m, 6H), 0.87 (m, 6H). 13C NMR (100 MHz, CD3OD) δ 169.6 (2C), 162.2, 160.0 (q, J = 37.2 Hz, 1C), 158.9, 151.2, 143.0, 130.5, 128.9-126.0 (5C), 116.1 (q, J = 288.0 Hz, 1C), 70.0, 55.7, 52.2, 42.5, 33.2, 30.6, 28.1, 28.0, 22.8, 22.0, 18.7, 18.6, 17.6, 17.2. HRMS (ESI) calcd for C26H39N4O5 (M+) 487.2920, found 487.2922.
Biological evaluation
Antiproliferative assays
Human T-cell leukemia (Jurkat) and human B-cell leukemia (SEM) were grown in RPMI-1640 medium, (Gibco, Milano, Italy). Human cervix carcinoma (HeLa) and human colon adenocarcinoma (HT-29) cells were grown in DMEM medium (Gibco, Milano, Italy). Both media were supplemented with 115 units/mL of penicillin G (Gibco, Milano, Italy), 115 µg/mL of streptomycin, (Invitrogen, Milano, Italy) and 10% fetal bovine serum (Invitrogen, Milano, Italy). All these cell lines were purchased from ATCC. Stock solutions (10 mM) of the different compounds were obtained by dissolving them in DMSO. Individual wells of a 96-well tissue culture microtiter plates were inoculated with 100 µL of complete medium containing 8 × 103 cells. The plates were incubated at 37 °C in a humidified 5 % CO2 incubator for 18 h prior to the experiments. After medium removal, 100 µL of fresh medium containing the test compound at different concentrations was added to each well and incubated at 37 °C for 72 h. Cell viability was assayed by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide test, and absorbance was measured at 560 nm using Victor3 TM 1420 Multilabel Counter (PerkinElmer, Waltham, MA, USA). The IC50 was defined as the compound concentration required to inhibit cell proliferation by 50 %.
Effects on tubulin polymerization and on colchicine binding to tubulin
To evaluate the effect of the compounds on tubulin assembly in vitro [38], varying concentrations of compounds were preincubated with 10 µM bovine brain tubulin in glutamate buffer at 30 °C and then cooled to 0 °C. After addition of 0.4 mM GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C. Tubulin assembly was followed turbidimetrically at 350 nm. The IC50 was defined as the compound concentration that inhibited the extent of assembly by 50 % after a 20 min incubation. The ability of the test compounds to inhibit vinblastine, dolastatin, and halicondrin B binding to tubulin was measured as described [11]. Briefly, experimentswere performed in 0.1 M 4-morpholinethanesulfonate (pH 6.9 in 1 M stock solution adjusted with NaOH)-0.5 mM MgCl2 containing 10 µM tubulin (1.0 mg/mL), 10 uM radiolabeled ligand, and inhibitors at different concentrations. Reaction volume was 0.3 mL, incubation time 15 min at rt (around 20 °C). Ligands were mixed prior to tubulin addition. Duplicate aliquots of each reaction mixture were applied to syringe columns of Sephadex G-50 (superfine) swollen in 0.1 M Mes-0.5 mM MgCl2(pH = 6.9).
Computational details
Conformational analysis was performed with the software Spartan’08 [33] by means of the “conformer distribution” function, using the default search method (“Systematic” or “Monte-Carlo” is automatically chosen as that which leads to the smaller number of moves, depending on the number of rotatable bonds in the molecule). The MMFF force field was used for the energy minimization of the found structures. The structures were then clustered according to the default setting of the software (which consists in pruning out higher energy conformers, and keeping a diverse set of the low energy conformers using the RMS-torsion definition of nearness). Superimposition of the global minimum of compounds 3-8 with HTI-286 was made with the alignment tool of the software (according to selected CFDs) and measured with the alignment score function.
Supplementary Material
Footnotes
Scores are reported as obtained by the similarity analysis function implemented in the Spartan’06 software. The score is defined as [(1 − R2)/N], where R2 is the rms distance between template and molecule centers, and N is the number of similarity centers.
Electronic Supplementary Information (ESI) available
1H NMR and 13C NMR of all new compounds are available.
Contributor Information
Giordano Lesma, Dipartimento di Chimica, Università di Milano, via Golgi 19, Milan 20133, Italy.
Alessandro Sacchetti, Email: alessandro.sacchetti@polimi.it, Dipartimento di Chimica, Materiali ed Ing.Chimica ‘Giulio Natta’, Politecnico di Milano, p.zza Leonardo da Vinci 32, Milan 20133, Italy.
Rouli Bai, Screening Technologies Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Frederick National Laboratory for Cancer Research, National Institutes of Health, Frederick, MD 21702, USA.
Giuseppe Basso, Dipartimento di Salute della Donna e del Bambino, Università degli Studi di Padova, via Giustiniani 2, Padau 35128, Italy.
Roberta Bortolozzi, Dipartimento di Salute della Donna e del Bambino, Università degli Studi di Padova, via Giustiniani 2, Padau 35128, Italy.
Ernest Hamel, Email: hamele@dc37a.nci.nih.gov, Screening Technologies Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Frederick National Laboratory for Cancer Research, National Institutes of Health, Frederick, MD 21702, USA.
Alessandra Silvani, Email: alessandra.silvani@unimi.it, Dipartimento di Chimica, Università di Milano, via Golgi 19, Milan 20133, Italy.
Nadia Vaiana, Dipartimento di Chimica, Università di Milano, via Golgi 19, Milan 20133, Italy.
Giampietro Viola, Email: giampietro.viola.1@unipd.it, Dipartimento di Salute della Donna e del Bambino, Università degli Studi di Padova, via Giustiniani 2, Padau 35128, Italy.
References
- 1.Kingston DGI. Tubulin-interactive natural products as anticancer agents. J Nat Prod. 2009;72:507–515. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckers T, Mahboobi S. Natural, semisynthetic and synthetic microtubule inhibitors for cancer therapy. Drugs Fut. 2003;28:767. [Google Scholar]
- 3.Jordan A, Hadfield JA, Lawrence NJ, McGown AT. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev. 1998;18:259–296. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Ganguly A, Cabral F. New insights into mechanisms of resistance to microtubule inhibitors. Biochim Biophys Acta. 2011;1816:164–171. doi: 10.1016/j.bbcan.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mania S, Macapinlac M, Goel S, Verdier-Pinard D, Fojo T, Rothenberg M, Colevas D. The clinical development of new mitotic inhibitors that stabilize the microtubule. Anti-Cancer Drugs. 2004;15:553–558. doi: 10.1097/01.cad.0000131681.21637.b2. [DOI] [PubMed] [Google Scholar]
- 6.Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 8.Coleman JE, de Silva ED, Fangming K, Andersen RJ, Allen TM. Cytotoxic peptides from the marine sponge Cymbastela sp. Tetrahedron. 1995;51:10653–10662. [Google Scholar]
- 9.Gamble WR, Durso NA, Fuller RW, Westergaard CK, Johnson TR, Sackett DL, Hamel E, Cardellina JH, 2nd, Boyd MR. Cytotoxic and tubulin-interactive hemiasterlins from Auletta sp. and Siphonochalina spp. sponges. Bioorg Med Chem. 1999;7:1611–1615. doi: 10.1016/s0968-0896(99)00089-9. [DOI] [PubMed] [Google Scholar]
- 10.Talpir R, Benayahu Y, Kashman Y, Pannell L, Schleyer M. Hemiasterlin and geodiamolide TA; two new cytotoxic peptides from the marine sponge Hemiasterella minor (Kirkpatrick) Tetrahedron Lett. 1994;35:4453–4456. [Google Scholar]
- 11.Anderson HJ, Coleman JE, Andersen RJ, Roberge M. Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother Pharmacol. 1997;39:223–226. doi: 10.1007/s002800050564. [DOI] [PubMed] [Google Scholar]
- 12.Bai R, Durso NA, Sackett DL, Hamel E. Interactions of the sponge-derived antimitotic tripeptide hemiasterlin with tubulin: comparison with dolastatin 10 and cryptophycin 1. Biochemistry. 1999;38:14302–14310. doi: 10.1021/bi991323e. [DOI] [PubMed] [Google Scholar]
- 13.Vedejs E, Kongkittingam C. A total synthesis of (−)- hemiasterlin using N-Bts methodology. J Org Chem. 2001;66:7355–7364. doi: 10.1021/jo0104882. [DOI] [PubMed] [Google Scholar]
- 14.Kuznetsov G, TenDyke K, Towle MJ, Cheng H, Liu J, Marsh JP, Schiller SER, Spyvee MR, Yang H, Seletsky BM, Shaffer CJ, Marceau V, Yao Y, Suh EM, Campagna S, Fang FG, Kowalczyk JJ, Littlefield BA. Tubulin-based antimitotic mechanism of E7974, a novel analogue of the marine sponge natural product hemiasterlin. Mol Cancer Ther. 2009;8:2852–2860. doi: 10.1158/1535-7163.MCT-09-0301. [DOI] [PubMed] [Google Scholar]
- 15.Zask A, Birnberg G, Cheung K, Kaplan J, Niu C, Norton E, Suayan R, Yamashita A, Cole D, Tang Z, Krishnamurthy G, Williamson R, Khafizova G, Musto S, Hernandez R, Annable T, Yang X, Discafani C, Beyer C, Greenberger LM, Loganzo F, Ayral-Kaloustian S. Synthesis and biological activity of analogues of the antimicrotubule agent N,β, β-Trimethyl-l-phenylalanyl-N1-[(1S,2E)-3-carboxy-1-isopropylbut-2-enyl]-N1,3-dimethyl-l-valinamide (HTI-286) J Med Chem. 2004;47:4774–4786. doi: 10.1021/jm040056u. [DOI] [PubMed] [Google Scholar]
- 16.Niu C, Ho DM, Zask A, Ayral-Kaloustian S. Absolute configurations of tubulin inhibitors taltobulin (HTI-286) and HTI-042 characterized by X-ray diffraction analysis and NMR studies. Bioorg Med Chem Lett. 2010;20:1535–1538. doi: 10.1016/j.bmcl.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Zask A, Kaplan J, Musto S, Loganzo F. Hybrids of the hemi-asterlin analogue taltobulin and the dolastatins are potent antimicrotubule agents. J Am Chem Soc. 2005;127:17667–17671. doi: 10.1021/ja053663v. [DOI] [PubMed] [Google Scholar]
- 18.Hadaschik BA, Adomat H, Fazli L, Fradet Y, Andersen RJ, Gleave ME, So A. Intravesical chemotherapy of high-grade bladder cancer with HTI-286, a synthetic analogue of the marine sponge product hemiasterlin. Clin Cancer Res. 2008;14:1510–1518. doi: 10.1158/1078-0432.CCR-07-4475. [DOI] [PubMed] [Google Scholar]
- 19.Hadaschik BA, Ettinger S, Sowery RD, Zoubeidi A, Andersen RJ, Roberge M, Gleave ME. Targeting prostate cancer with HTI-286, a synthetic analog of the marine sponge product hemiasterlin. Int J Cancer. 2008;122:2368–2376. doi: 10.1002/ijc.23406. [DOI] [PubMed] [Google Scholar]
- 20.Ayral-Kaloustian S, Zask A. Taltobulin. Drugs Fut. 2005;30:254. [Google Scholar]
- 21.Loganzo F, Hari M, Annable T, Tan X, Morilla DB, Musto S, Zask A, Kaplan J, Minnick AA, Jr, May MK, Ayral-Kaloustian S, Poruchynsky MS, Fojo T, Greenberger LM. Cells resistant to HTI-286 do not overexpress P-glycoprotein but have reduced drug accumulation and a point mutation in α-tubulin. Mol Cancer Ther. 2004;3:1319–1327. [PubMed] [Google Scholar]
- 22.Loganzo F, Discafani CM, Annable T, Beyer C, Musto S, Hari M, Tan X, Hardy C, Hernandez R, Baxter M, Singanallore T, Khafizova G, Poruchynsky MS, Fojo T, Nieman JA, Ayral-Kaloustian S, Zask A, Andersen RJ, Greenberger LM. HTI-286, A synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res. 2003;63:1838–1845. [PubMed] [Google Scholar]
- 23.Nieman JA, Coleman JE, Wallace DJ, Piers E, Lim LY, Roberge M, Andersen RJ. Synthesis and antimitotic/cytotoxic activity of hemiasterlin analogues. J Nat Prod. 2003;66:183–199. doi: 10.1021/np020375t. [DOI] [PubMed] [Google Scholar]
- 24.Andersen RJ, Coleman JE, Piers E, Wallace DJ. Total synthesis of (−)-hemiasterlin, a structurally novel tripeptide that exhibits potent cytotoxic activity. Tetrahedron Lett. 1997;38:317–320. [Google Scholar]
- 25.Hsu L-C, Durrant DE, Huang C-C, Chi N-W, Baruchello R, Rondanin R, Rullo C, Marchetti P, Grisolia G, Simoni D, Lee RM. Development of hemiasterlin derivatives as potential anti-cancer agents that inhibit tubulin polymerization and synergize with a stilbene tubulin inhibitor. Invest New Drugs. 2012;30:1379–1388. doi: 10.1007/s10637-011-9702-9. [DOI] [PubMed] [Google Scholar]
- 26.Simoni D, Lee RM, Durrant DE, Chi N-W, Baruchello R, Rondanin R, Rullo C, Marchetti P. Versatile synthesis of new cytotoxic agents structurally related to hemiasterlins. Bioorg Med Chem Lett. 2010;20:3431–3435. doi: 10.1016/j.bmcl.2010.03.098. [DOI] [PubMed] [Google Scholar]
- 27.Qie J, Zhou W, Zhao X, He J, Zhang Y, Liu K. Hemiasterlin analogues with unnatural amino acids at the N-terminal and their inhibitory activity on tumor cells. Int J Pept Res Ther. 2009;15:187–194. [Google Scholar]
- 28.Yamashita A, Norton EB, Kaplan JA, Niu C, Loganzo F, Hernandez R, Beyer CF, Annable T, Musto S, Discafani C, Zask A, Ayral-Kaloustian S. Synthesis and activity of novel analogs of hemiasterlin as inhibitors of tubulin polymerization: modification of the A segment. Bioorg Med Chem Lett. 2004;14:5317–5322. doi: 10.1016/j.bmcl.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Zask A, Birnberg G, Cheung K, Kaplan J, Niu C, Norton E, Yamashita A, Beyer C, Krishnamurthy G, Greenberger LM, Loganzo F, Ayral-Kaloustian S. D-Piece modifications of the hemiasterlin analog HTI-286 produce potent tubulin inhibitors. Bioorg Med Chem Lett. 2004;14:4353–4358. doi: 10.1016/j.bmcl.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Milton MJ, Thomas Williamson R. Mapping the bound conformation and protein interactions of microtubule destabilizing peptides by STD-NMR spectroscopy. Bioorg Med Chem Lett. 2006;16:4279–4282. doi: 10.1016/j.bmcl.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 31.Ravi M, Zask A, Rush TS. Structure-based identification of the binding site for the hemiasterlin analogue HTI-286 on tubulin. Biochemistry. 2005;44:15871–15879. doi: 10.1021/bi051268b. [DOI] [PubMed] [Google Scholar]
- 32.Mitra A, Sept D. Localization of the antimitotic peptide and depsipeptide binding site on β-tubulin. Biochemistry. 2004;43:13955–13962. doi: 10.1021/bi0487387. [DOI] [PubMed] [Google Scholar]
- 33.Spartan’08, Wavefunction Inc, Irvine, CA. Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, Slipchenko LV, Levchenko SV, O’Neill DP, DiStasio RA, Jr, Lochan RC, Wang T, Beran GJO, Besley NA, Herbert JM, Lin CY, Van Voorhis T, Chien SH, Sodt A, Steele RP, Rassolov VA, Maslen PE, Korambath PP, Adamson RD, Austin B, Baker J, Byrd EFC, Dachsel H, Doerksen RJ, Dreuw A, Dunietz BD, Dutoi AD, Furlani TR, Gwaltney SR, Heyden A, Hirata S, Hsu C-P, Kedziora G, Khalliulin RZ, Klunzinger P, Lee AM, Lee MS, Liang WZ, Lotan I, Nair N, Peters B, Proynov EI, Pieniazek PA, Rhee YM, Ritchie J, Rosta E, Sherrill CD, Simmonett AC, Subotnik JE, Woodcock HL, III, Zhang W, Bell AT, Chakraborty AK, Chipman DM, Keil FJ, Warshel A, Hehre WJ, Schaefer HF, Kong J, Krylov AI, Gill PMW, Head-Gordon M. Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys. 2006;8:3172–3191. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell D, Hay LA, Koenig TM, McDaniel S, Nissen JS, Audia JE. Classical and dynamic resolution of 1-amino-3-methyl-1,3,4,5-tetrahydrobenzo[d]azepin-2-one. Tetrahedron: Asymmetry. 2005;16:3814–3819. [Google Scholar]
- 35.Lim HJ, Gallucci JC, RajanBabu TV. Annulated dike-topiperazines from dipeptides or Schöllkopf reagents via tandem cyclization-intramolecular N-arylation. Org Lett. 2010;12:2162–2165. doi: 10.1021/ol100663y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malkov AV, Vranková K, Černý M, Kočovský P. On the selective N-methylation of BOC-protected amino acids. J Org Chem. 2009;74:8425–8427. doi: 10.1021/jo9016293. [DOI] [PubMed] [Google Scholar]
- 37.Mangette JE, Johnson MR, Le V-D, Shenoy RA, Roark H, Stier M, Belliotti T, Capiris T, Guzzo PR. The preparation of optically active α-amino 4H-[1,2,4]oxadiazol-5-ones from optically active α-amino acids. Tetrahedron. 2009;65:9536–9541. [Google Scholar]
- 38.Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys. 2003;38:1–22. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.