Background: CCR5 is a chemokine receptor and a coreceptor for HIV entry.
Results: A pool of spare CCR5 binds the potent anti-HIV chemokine analog PSC-RANTES but not native chemokines.
Conclusion: Targeting a large number of CCR5 improves the anti-HIV property of PSC-RANTES.
Significance: Spare receptors represent a target for inhibiting HIV and might constitute a viral escape route.
Keywords: Antiviral Agent, Endocytosis, G-protein-coupled Receptor (GPCR), HIV-1 Protease, Virus Entry, CCR5, Conformation
Abstract
CCR5 binds the chemokines CCL3, CCL4, and CCL5 and is the major coreceptor for HIV-1 entry into target cells. Chemokines are supposed to form a natural barrier against human immunodeficiency virus, type 1 (HIV-1) infection. However, we showed that their antiviral activity is limited by CCR5 adopting low-chemokine affinity conformations at the cell surface. Here, we investigated whether a pool of CCR5 that is not stabilized by chemokines could represent a target for inhibiting HIV infection. We exploited the characteristics of the chemokine analog PSC-RANTES (N-α-(n-nonanoyl)-des-Ser(1)-[l-thioprolyl(2), l-cyclohexylglycyl(3)]-RANTES(4-68)), which displays potent anti-HIV-1 activity. We show that native chemokines fail to prevent high-affinity binding of PSC-RANTES, analog-mediated calcium release (in desensitization assays), and analog-mediated CCR5 internalization. These results indicate that a pool of spare CCR5 may bind PSC-RANTES but not native chemokines. Improved recognition of CCR5 by PSC-RANTES may explain why the analog promotes higher amounts of β-arrestin 2·CCR5 complexes, thereby increasing CCR5 down-regulation and HIV-1 inhibition. Together, these results highlight that spare CCR5, which might permit HIV-1 to escape from chemokines, should be targeted for efficient viral blockade.
Introduction
CC chemokine receptor 5 (CCR5) is a G-protein-coupled receptor (GPCR)7 for the chemokines RANTES (regulated on activation, normal T cell expressed and secreted)/CCL5, MIP1α (macrophage inflammatory protein-1)/CCL3, and MIP1β/CCL4 (1). CCR5 is also a CD4-associated coreceptor required for human immunodeficiency virus, type 1 (HIV-1) entry into host cells (2). It plays a prominent role during transmission, progression of infection, and evolution to AIDS. Individuals that do not express a functional CCR5 are highly resistant to HIV-1 infection without major pathological consequences (3). Therefore, CCR5 is a suitable therapeutic target for HIV-1 blockade. A number of non-competitive and allosteric antagonists have been developed, among which maraviroc is the only one currently used in HIV treatment (4). However, viral resistance to this drug is a major drawback, highlighting the need for new inhibitors (5).
GPCRs can oscillate between various inactive and active conformations at the cell surface (6). Structurally different ligands can stabilize and/or create distinct conformations (7) that interact with specific effectors, including heterotrimeric G-proteins and β-arrestins, which are scaffolding proteins involved in receptor desensitization and endocytosis (8). Reciprocally, interaction of a particular receptor conformation with an effector can increase the affinity of the receptor for the ligand (6). CCR5 exists in multiple conformations, displaying distinct pharmacological and antigenic properties (9, 10). This conformational heterogeneity is exploited by HIV-1 to escape inhibition by native CCR5-binding chemokines and small-molecule CCR5 inhibitors (10–12). Therefore, taking into account the multiplicity of CCR5 conformations is essential for the development of new anti-HIV molecules.
In this study, we investigated whether a pool of CCR5 that is not accessible to native chemokines would be a target for inhibiting HIV-1 infection. For this purpose, we studied the molecular mechanisms whereby the N-terminally modified CCL5 analog PSC-RANTES shows improved antiviral activity compared with native chemokines (13, 14). This drug protects macaques from HIV-1 infection after vaginal application (15). The exceptional capacity of PSC-RANTES to inhibit infection is related to its ability to increase CCR5 down-regulation (11, 13). A superagonist activity of PSC-RANTES (16) and its ability to sequester CCR5 into the trans-Golgi network (17) have been suggested to play a role in this context. However, we report here that PSC-RANTES also displays the unique property of recognizing a larger array of CCR5 conformational states than native chemokines, making it more efficient in recruiting β-arrestins and down-regulating CCR5. These findings provide evidence for the existence of a set of CCR5 that is not stabilized by native chemokines at the cell surface and highlight the targeting of this spare CCR5 as an anti-HIV principle.
EXPERIMENTAL PROCEDURES
Plasmids, siRNA, and Chemokines
The dominant negative mutant of Eps15 GFP-EΔ95/295, described previously (18), was a gift from A. Benmerah (Institut Imagine, Paris, France). YFP-tagged CCR5, β-arrestin 2 RLuc (βarr2-Rluc), and the construct encoding for GFP fusions of wild-type β-arrestin 2 (βarr2-GFP) have been described previously (19, 20). The double-stranded 5′-ACCUGCGCCUUCCGCUAUG-3′ siRNA sequence (Dharmacon) was used to simultaneously target both βarr1 and βarr2, as described previously (21). The βarr1/2 siRNA and a scrambled siRNA (control, 5′-UGGUUUACAUGUCGACUAA-3′) (Dharmacon) were transfected by RNAimax (Invitrogen) according to the instructions of the manufacturer. The chemokines MIP-1α/CCL3 and RANTES/CCL5 were purchased from Peprotech (Tebu-bio). MIP1β/CCL4 was chemically synthesized by F. Baleux (Institut Pasteur, Paris, France). [125I]CCL3 was from PerkinElmer Life Sciences. The CCR5 antagonist TAK779 was obtained through the Centre for AIDS reagents, National Institute for Biological Standards and Control (NIBSC, UK), with permission of Takeda Chemical Industries Ltd. (22). The chemokine analog 5P12 with an antagonist feature has been described previously (23). The chemokine analog PSC-RANTES (N-α-(n-nonanoyl)-des-Ser1-[l-thioprolyl2,l-cyclohexylglycyl3]-RANTES(4-68)) was provided by the Centre for AIDS Reagents, NIBSC, UK. PSC-RANTES carrying a C-terminal biotin (PSC-RANTES-biot) was synthesized essentially as in Ref. 13, except that synthesis of the C-terminal fragment was carried out on a phenylacetamidomethyl (PAM) resin derivatized with N-α-tert-butyloxycarbonyl-glycine (Boc-Gly-PAM) resin, incorporating a Lys residue with an Fmoc-protected ϵ amino group. At the end of synthesis, the side chain of this Lys residue was deprotected and elongated successively with Fmoc-aminocaproic acid and Biotin-OSu. Biotinylated PSC-RANTES shows indistinguishable activity to non-biotinylated material in an R5-tropic, envelope-dependent cell fusion assay.8
Cell Lines and Primary CD4+ T Lymphocytes
HEK293 cells stably expressing FLAG-tagged CCR5 WT and CCR5–349 mutant have been described (24). The human A3.01 T cell line stably expressing FLAG-tagged CCR5 was generated by Amaxa nucleofector technology (Amaxa Biosystems) and cultured for several weeks in 1 mg/ml G418 (Geneticin, Invitrogen). Cell clones were screened and sorted by flow cytometry (on a MoFlo Astrios, Beckman Coulter) using the M1 anti-FLAG (Sigma) or the anti-CCR5 2D7 (BD Biosciences) monoclonal antibody. FLAG-CCR5 HEK293 cells stably expressing βarr2-GFP were generated by calcium phosphate coprecipitation and specific sorting. HEK293 cells were grown in DMEM supplemented with 10% FBS and antibiotics. T cell lines were grown in RPMI 1640 supplemented with 10% FBS and antibiotics. Human CD4+ T lymphocytes were purified from peripheral blood mononuclear cells (PBMCs) of healthy blood donors (Etablissement Français du Sang) by Ficoll centrifugation (PAA), followed by immunomagnetic selection (Miltenyi Biotec). They were maintained for 2 days in RPMI 1640 medium containing phytohemagglutinin (1 μg/ml) and interleukin 2 (300 IU/ml) and then in medium containing IL-2 before use.
Viral Production and HIV-1 Virion-based Fusion Assay
HIV-1 particles containing BlaM-Vpr were produced by cotransfection of HEK293T cells with a NL4.3Ren-based plasmid expressing the envelope glycoprotein gp160 of the HIV-1 R5-tropic strain Bx08 (11) and pCMV-BlaM-vpr encoding β-lactamase fused to the viral protein Vpr (25). After 48 h of culture at 37 °C, the virus-containing supernatant was centrifuged at low speed to remove cellular debris and then ultracentrifuged at 72,000 × g for 90 min at 4 °C to sediment viral particles. The virion-enriched pellet was resuspended in DMEM and aliquoted for storage at −80 °C. Following 30 min of incubation with chemokines, 1.5 × 105 primary CD4+ T cells were inoculated with the BlaM-Vpr-containing viruses (50 ng p24 Gag) by 1 h of spinoculation at 4 °C and incubated 2 h at 37 °C. Cells were then loaded with CCF2/AM, the BlaM-Vpr substrate (2 h at room temperature) and fixed. Enzymatic cleavage of CCF2/AM by β-lactamase (the readout of viral entry fusion) was measured by flow cytometry (FACSCanto, BD Biosciences), and data were analyzed with FlowJo software. The percentage of fusion corresponds to the percentage of cells displaying increased cleaved CCF2/AM fluorescence (447 nm).
Binding Assays on A3.01-FLAG-CCR5 Cell Lines
Displacement of [125I]CCL3 (0.5 nm) in the presence of CCL5, CCL4, CCL3, or PSC-RANTES was performed as in Ref. 11, except that incubations were done in Eppendorf tubes at 4 °C. To remove unbound [125I]CCL3, cells were pelleted at 4 °C (5 min) and then washed once with washing buffer (50 mm Hepes (pH 7.4), 5 mm MgCl2, 1 mm CaCl2, and 150 mm NaCl). Pellets were resuspended in the washing buffer, and radioactivity was counted in a Multi Crystal LB2111 (Berthold). Displacement of PSC-RANTES-biot (1 nm) in the presence of CCL5, CCL3, or PSC-RANTES was done in Eppendorf tubes at 4 °C for 90 min. Unbound PSC-RANTES-biot was remove by centrifugation, and pellets were incubated 30 min with PBS/BSA 0.2% containing streptavidin-phycoerythrin (BD Biosciences). Cells were analyzed by flow cytometry (Gallios flow cytometer, Beckman Coulter). Analysis of the binding data was made using Prism software (GraphPad).
[35S]GTPγS Binding
[35S]GTPγS binding to crude membrane preparations of CCR5-expressing HEK293T cells has been described previously (9). Briefly, membranes were incubated for 15 min at 30 °C in 20 mm HEPES (pH 7.4) containing 100 mm NaCl, 10 μg/ml saponin, 1 μm GDP, and 3 mm MgCl2 in the presence or absence (basal [35S]GTPγS binding) of chemokines. Then, 0.1 nm [35S]GTPγS (PerkinElmer Life Sciences) was added to membrane-containing mixtures, which were incubated for 30 min at 30 °C. The incubation was stopped by centrifugation (800 × g for 10 min) at 4 °C and removal of supernatants. Microplates were counted for 2 min/well in a Wallac 1450 Microbeta Trilux (PerkinElmer Life Sciences).
Calcium Measurements
FLAG-CCR5-expressing HEK293 cells or primary CD4+ T cells were incubated for 20 min at 37 °C with 1.5 μm Fura-2/AM (Molecular Probes). Calcium measurements by spectrofluorimetry were performed in mammalian saline as described previously (26) with a Cary Eclipse spectrofluorimeter (Varian) (excitation, 340 and 380 nm; emission, 510 nm). For measurements by microscopy, cells were analyzed with a Nikon TE2000 microscope (Nikon, Melville, NY) equipped with a cooled CCD camera (Cascade, Princeton Instruments) and a Matafluor imaging system (Roper Scientific), as described previously (27). Calcium levels are represented as a ratio of 340/380 fluorescence intensity.
Fluorescence Flow Cytometry Assays of Receptor Internalization and Recycling
Flow cytometry was used to quantitate the internalization and the recycling of CCR5 by measuring the levels of cell surface FLAG-tagged receptors as described previously (24). Mean values were used to compute the proportion of internalized receptors as indicated by a decrease of immunoreactive surface receptor with agonist compared with untreated cells. The percentage of receptor recycling was the proportion of receptors that was recovered at the cell surface out of the internalized receptors.
Cell Surface Biotinylation and Assay of Agonist-induced Proteolysis - Western Blot Analysis
The cell surface biotinylation assay has been described previously (24). Briefly, cells were incubated with 300 μg/ml Sulfo-NHS-Biotin (Pierce) in PBS at 4 °C for 30 min. Unreacted Sulfo-NHS-Biotin was quenched by washes with ice-cold Tris-buffered saline. Cells were incubated with medium at 37 °C for the indicated times in the absence or presence of agonists. They were chilled on ice, washed with PBS, and extracted with lysis buffer (0.5% TX-100, 10 mm Tris-HCl, 120 mm NaCl, 25 mm KCl, and protease inhibitors mixture). Extracts were centrifuged, and equal amounts of proteins (Bradford analysis) were incubated with 25 μl of streptavidin beads (Pierce) overnight. Eluted proteins were resolved by SDS-PAGE and transferred to a PVDF membrane for Western blot analysis. For receptor detection, blots were incubated with M1 anti-FLAG antibody (Sigma) and then with an HRP-coupled goat anti-mouse IgG for enzyme-linked chemiluminescence detection (ECL system, Amersham Biosciences Life Sciences). A control of the quantity of proteins per lane was performed after an acid strip and incubation of blots with an avidin-HRP complex (Sigma). Band intensities were quantified by densitometry of films.
For detection of β-arrestin after siRNA transfection, equal amount of proteins were resolved by SDS-PAGE and transferred to a PVDF membrane. Blots were incubated overnight with the anti-β-arrestin antibody D24H9 (Cell Signaling Technology). After washing, blots were incubated with an anti-rabbit-HRP antibody (Jackson ImmunoResearch Laboratories, Inc.) before detection by ECL. A control of the quantity of proteins per lane was performed after an acid strip and incubation of blots with anti-LDH5 (Biodesign) and anti-goat-HRP (Dako) antibodies.
siRNA Rescue
For recombinant protein re-expression in FLAG-CCR5–349-expressing HEK293 cells, βarr2-GFP was mutated by site-directed mutagenesis to ACC TGT GCC TTT AGG TAT G (the underlined bases show synonymous mutations introduced) to assure resistance to knockdown by βarr1/2 siRNA (res-βarr2-GFP). The siRNA (10 nm) and plasmid (50 ng) were cotransfected (with a 48-h interval between the two) using RNAimax and Lipofectamine 2000, respectively. Experiments of internalization were conducted 3 days after siRNA transfection. After staining, GFP-positive cells were analyzed for CCR5 cell surface expression by flow cytometry (Gallios flow cytometer, Beckman Coulter).
BRET Assays
BRET assays were performed as described previously (28). Briefly, 24 h post-transfection (with 30 ng βarr2-Rluc-encoding plasmid and increasing concentrations of plasmid encoding CCR5-YFP), cells were detached with DMEM without phenol red (Invitrogen) and distributed in white, 96-well Optiplates (PerkinElmer Life Sciences). 50 nm of chemokines was added and incubated at 37 °C for the indicated times. After incubation, Coelentherazine h (Interchim) was added to a final concentration of 5 μm and incubated for 3 min at 25 °C. BRET readings were collected using a Mithras LB 940 multilabel reader (Berthold Technologies). Substrate and light emissions were detected at 480 nm (Rluc) and 540 nm (YFP) for 1 s. The BRET signal corresponds to the ratio between the fluorescence emitted by YFP and the light emitted by Rluc (YFP/Rluc). The ratio values were corrected by subtracting background BRET signals detected when βarr2-Rluc was expressed alone. milli-BRET (mBRET) values were calculated by multiplying these ratios by 1000. For kinetics analysis, cells were transfected with 100 ng of βarr2-Rluc and 250 ng of CCR5-YFP. The day after, Coelenterazine h and the agonist were added simultaneously, and the BRET signal was measured over time.
Total Internal Reflection Fluorescence (TIRF) Microscopy
4 × 105 FLAG-CCR5/βarr2-GFP-expressing HEK293 cells plated on MatTek plates were incubated in the presence or absence of 2 nm CCL5 or PSC-RANTES in DMEM/1%BSA medium for the indicated time. Cells were put on ice and fixed with paraformaldehyde (PFA) 4% at 4 °C for 40 min before three washes in PBS. Experiments were performed using a TIRF microscope (IX81F-3, Olympus) equipped with a ×100 numerical aperture 1.45 Plan Apo TIRFM Objective (Olympus) and fully controlled by CellM (Olympus). Images were collected using an IxonEM+ camera (DU885, Andor). All TIRF images analyses were performed using ICY software. The number of spot detected per cell was normalized to the size of the cell surface (29).
RESULTS
Native Chemokines and PSC-RANTES Trigger Similar G Protein Signaling but Different CCR5 Intracellular Trafficking
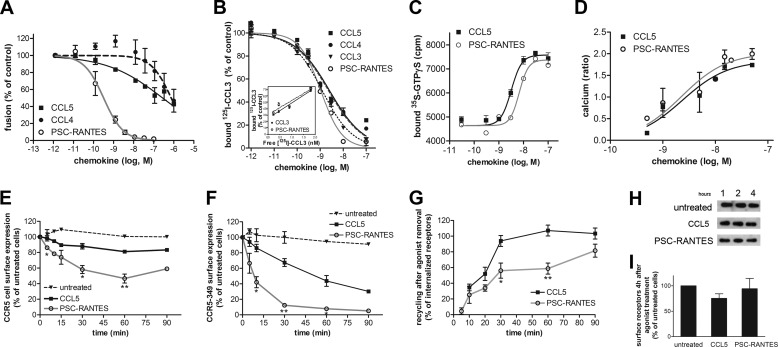
The antiviral activity of PSC-RANTES (IC50, 0.38 ± 0.14 nm), which is much more potent than that of CCL5 or CCL4 (IC50, 259 ± 26 nm and >1000 nm, respectively) (Fig. 1A), is attributed to its ability to induce a stronger CCR5 down-regulation (13). We investigated whether this effect could result from an increased binding affinity of PSC-RANTES for CCR5 or from an increased ability to activate G-proteins (16). Native chemokines and PSC-RANTES displaced [125I]CCL3 binding to CCR5-expressing A3.01 cells with comparable potencies (IC50, 2.6 ± 0.11, 2 ± 0.25, 1.1 ± 0.14 nm, and 1.2 ± 0.12 for CCL5, CCL4, CCL3, and PSC-RANTES, respectively) (Fig. 1B), in line with previous observations (11). Of note, the extent of [125I]CCL3 binding displacement by the unlabeled chemokines varied with the concentration of [125I]CCL3, indicating that a competitive inhibition of [125I]CCL3 binding occurred (30) (Fig. 1B, inset). These chemokines also displayed similar potencies and efficacies for activating G-proteins in [35S]GTPγS binding assays (EC50, 3.4 ± 0.27 and 7 ± 0.17 nm for CCL5 and PSC-RANTES, respectively) (Fig. 1C) and for calcium responses (EC50, 2.3 nm for CCL5 and PSC-RANTES) (Fig. 1D).
FIGURE 1.
Functional properties of PSC-RANTES and native chemokines. A, chemokine-mediated inhibition of fusion between Bx08Ren viruses containing BlaM-Vpr and CCF2/AM-loaded primary CD4+ T lymphocytes. B, displacement of 0.5 nm [125I]CCL3 binding to CCR5-expressing A3.01 cells by increasing concentrations of the indicated unlabeled chemokines. Inset, displacement of increasing concentrations of [125I]CCL3 binding to CCR5-expressing A3.01 cells by 1.5 nm CCL3 or PSC-RANTES. C, chemokine-induced [35S]GTPγS binding to CCR5-expressing HEK293T cell membranes. D, calcium responses elicited in CCR5 Fura-2-loaded HEK293 cells by the indicated chemokines and measured by spectrofluorimetry (ratio, F340/F380). A–D, data (mean ± S.E. of two or three independent experiments) were fitted according to a sigmoidal dose-response model with a variable slope. E and F, CCR5 internalization. Cell surface expression of FLAG-CCR5 (E) or FLAG-CCR5-349 (F) was monitored by flow cytometry in stable HEK293 cell clones after 50 nm chemokine stimulation for the indicated time. The percentage of total bound antibody was calculated from the mean fluorescence intensity relative to untreated cells (mean ± S.E. from at least two independent experiments). G, CCR5 recycling. FLAG-CCR5-expressing HEK293 cells fed with anti-FLAG antibody were treated with 200 nm CCL5 or 10 nm PSC-RANTES to obtain 40% of CCR5 internalization for both agonists. Receptor recovery at the cell surface, in the presence of an excess of TAK779, was monitored by flow cytometry (mean ± S.E., n = 3). E–G, *, p < 0.05; **, p < 0.01 compared with CCL5-treated cells in unpaired, two-tailed Student's t test. H, postendocytic sorting of CCR5. Shown is a biotin degradation assay of FLAG-CCR5 in cells treated with CCL5 (100 nm) or PSC-RANTES (10 nm) for 1, 2, or 4 h. One representative anti-FLAG blot of six is shown. I, quantification of blots from experiments performed in H. The amount of cell surface receptors was quantified by densitometry and plotted relative to the amount of receptor detected in untreated cells (mean ± S.E., n = 6). The amounts of cell surface receptors were not statistically different (p = 0.39 in unpaired, two-tailed Student's t test) in the presence of CCL5 or PSC-RANTES.
Despite the similar output of CCL5 and PSC-RANTES in terms of G-protein activation, the analog promoted stronger receptor sequestration than the native chemokine (Fig. 1, E and F), in line with observations on other cells (13). After 30 min of stimulation with a saturating concentration (50 nm) of PSC-RANTES or CCL5 (Fig. 1E), cell surface CCR5 was reduced by 50 and 20%, respectively. We investigated whether these differences could be explained by differences in the rates of CCR5 endocytosis or recycling to the cell surface (24). In cells expressing CCR5–349, a receptor mutant unable to recycle (24), PSC-RANTES-induced internalization reached 90% at 30 min, whereas CCL5-dependent internalization only reached 30% (Fig. 1F). Endocytosis half-life was 50 min for CCL5 and 7 min for PSC-RANTES, indicating that PSC-RANTES induced faster receptor down-regulation than the native chemokine. CCR5 recycling after PSC-RANTES-induced endocytosis was maintained, although at a slower rate compared with CCL5 (Fig. 1G). In addition, regardless of the agonist used, the receptor was not directed to the degradation pathway (Fig. 1, H and I), in agreement with previous data (17). In conclusion, both improved receptor endocytosis and slower recycling likely explain the strong CCR5 down-regulation and the potent antiviral activity of PSC-RANTES.
Compared with Native Chemokines, PSC-RANTES Induces Increased β-Arrestin 2 Recruitment to CCR5
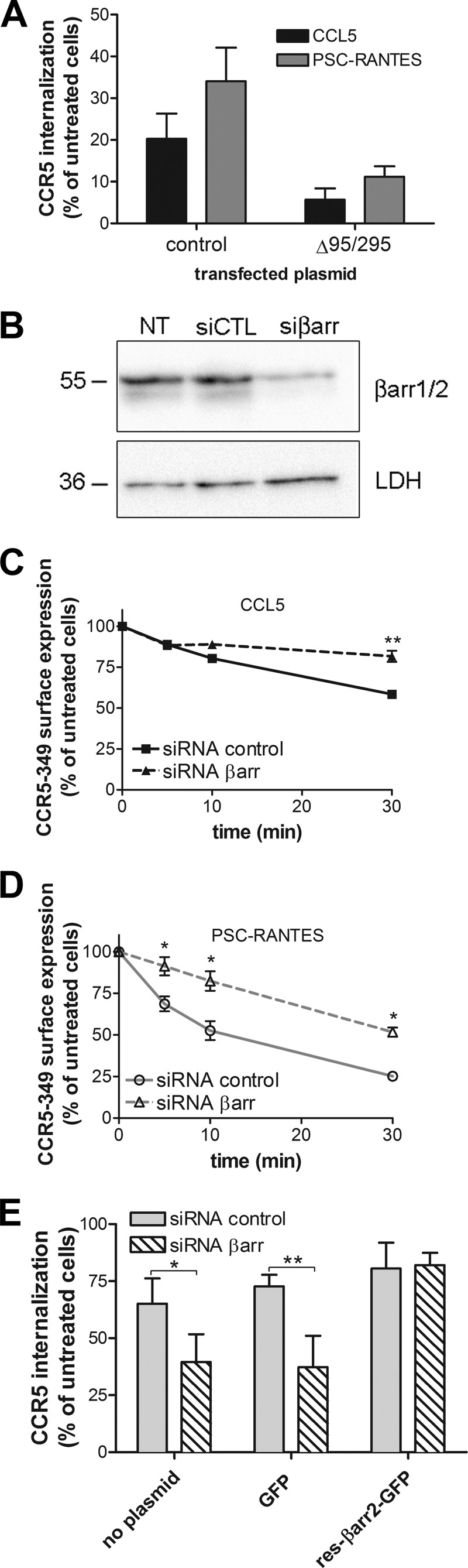
We next investigated whether PSC-RANTES-dependent CCR5 trafficking would involve a distinct endocytic pathway. Both CCL5- and PSC-RANTES-induced internalization were impaired in the presence of the Δ95/295 dominant negative mutant of Eps15 (Fig. 2A), a component of clathrin-coated pits (18). Thus, both agonists induced CCR5 internalization via the clathrin-driven endocytic pathway. As a control, Δ95/295 also inhibited the internalization of transferrin (data not shown).
FIGURE 2.
PSC-RANTES internalization follows a clathrin- and β-arrestin-dependent pathway. A, The Eps15 dominant negative mutant GFP-Δ95/295 inhibits CCR5 internalization. Cell surface expression of CCR5 in GFP-Δ95/295-transfected or GFP-transfected (control) HEK293 cells was monitored after 30 min of 50 nm chemokine stimulation. GFP-positive cells were analyzed by flow cytometry (mean ± S.D. of two independent experiments). B–D, β-arrestins 1/2 are involved in CCR5 internalization. B, Western blot analysis of total cellular levels of βarr1/2 in CCR5-349-expressing HEK293 cells transfected (72 h) with either nonsilencing siRNA (siCTL) or βarr1/2 siRNA (siβarr) (10 nm). Lysates were also blotted for LDH5 (LDH) to test the specificity of knockdown. One representative blot of three independent experiments is shown. NT, non-transfected cells. C and D, cell surface expression of CCR5-349 was monitored by flow cytometry after stimulation with 100 nm CCL5 (C) or PSC-RANTES (D) of siRNA-treated cells for the indicated time. E, rescue of siRNA-mediated β-arrestin knockdown. CCR5-349-expressing HEK293 cells were cotransfected or not (no plasmid) with βarr1/2 siRNA (10 nm) and a plasmid encoding GFP (50 ng) or a siRNA-resistant βarr2-GFP construct (res-βarr2-GFP) (50 ng) and stimulated for 20 min with 100 nm PSC-RANTES (n = 4). *, p < 0.05; **, p < 0.01 compared with siRNA control in unpaired, two-tailed Student's t test.
GPCR endocytosis most often requires β-arrestins, which bridge the receptor cargo to AP2 and clathrin (31). Silencing (70% reduction) β-arrestin 1 and β-arrestin 2 (βarr1 and βarr2, respectively) endogenous expressions with siRNA similarly decreased CCR5–349 internalization regardless of the agonist used (Fig. 2, B–D). To test specificity, we rescued CCR5 internalization by cotransfecting a βarr2-GFP mutant resistant to knockdown by βarr1/2 siRNA (Fig. 2E). These experiments indicate that β-arrestins contributed to CCR5 internalization whatever the agonist. These results are consistent with recent data showing intracellular clustering of βarr2 after CCR5 stimulation with PSC-RANTES (32).
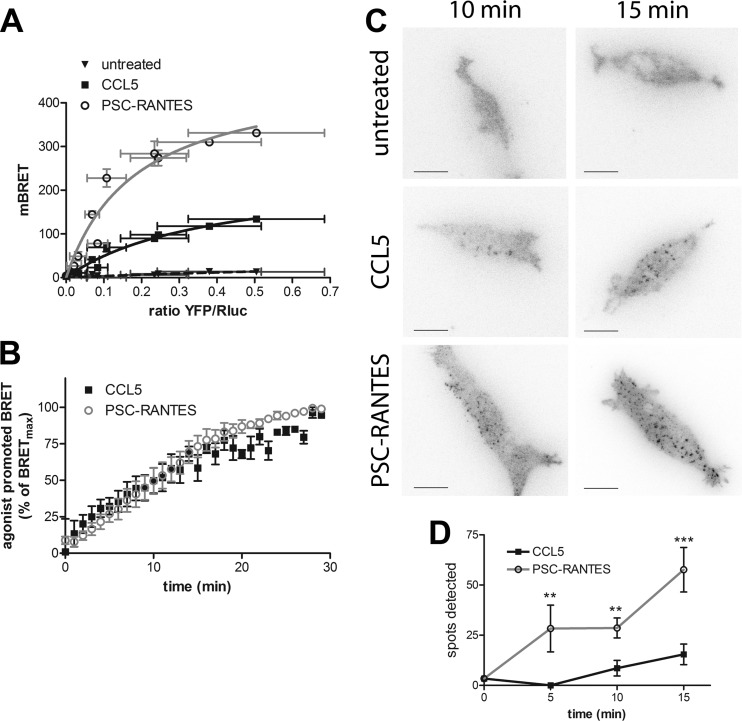
We examined whether the improved CCR5 sequestration and slow recycling induced by PSC-RANTES were due to increased affinity of βarr2 for the PSC-RANTES-occupied receptor. βarr2 translocation to agonist-bound GPCRs can be monitored using bioluminescence resonance energy transfer (BRET) (28). We conducted BRET saturation assays in living HEK293 cells coexpressing plasmids encoding βarr2-Rluc as the BRET donor and CCR5-YFP as the BRET acceptor in the presence or absence of 50 nm PSC-RANTES or CCL5. We obtained hyperbolic saturation curves in the presence of both agonists, indicating a specific recruitment of βarr2 to agonist-bound CCR5 (Fig. 3A). BRET50 values (YFP/Rluc value for half-maximal BRET), which reflect the propensity of βarr2-Rluc to associate with CCR5-YFP, were not significantly different after CCL5 and PSC-RANTES stimulation (0.58 ± 0.27 versus 0.22 ± 0.05, p = 0.24 in unpaired, two-tailed Student's t test), ruling out the hypothesis of an increased affinity of βarr2 for PSC-RANTES-occupied receptors compared with CCL5-occupied receptors. Accordingly, a kinetic analysis revealed similar half-time values for the association of βarr2 to CCL5- and PSC-RANTES-bound CCR5 (16.2 ± 2.7 versus 11.2 ± 2.7 min, p = 0.1 in unpaired, two-tailed Student's t test) (Fig. 3B).
FIGURE 3.
PSC-RANTES triggers a massive recruitment of β-arrestin 2 to CCR5. A, saturation BRET experiments in cells expressing βarr2-Rluc and increasing amounts of CCR5-YFP under basal conditions (untreated) or after 10 min chemokine (50 nm) stimulation (fitted according to a one-site binding model, four independent experiments). Results are expressed in milli-BRET units (mBRET). B, kinetics of BRET after chemokine (50 nm) stimulation (fitted according to a one-phase association model). Values correspond to the mean ± S.E. of four independent experiments. C and D, TIRF microscopy on HEK293 cells stably expressing βarr2-GFP and FLAG-CCR5 obtained 5, 10, and 15 min after stimulation with 2 nm CCL5 or PSC-RANTES. C, βarr2 spots were detected on TIRF images. Scale bars = 5 μm. D, quantification of the spots over time using ICY software (mean ± S.D, n ≥ 10 cells). **, p < 0.01; ***, p < 0.001 compared with CCL5-treated cells in unpaired, two-tailed Student's t test.
These data suggest that PSC-RANTES and CCL5 stabilize CCR5 in a similar βarr2-interacting conformation. In this context, the 3-fold increase of the BRETmax values (the BRET values corresponding to the plateau of the hyperbola) in the presence of PSC-RANTES might indicate that a larger fraction of βarr2 (the BRET donor) would be associated with CCR5 (the acceptor) in the presence of PSC-RANTES compared with CCL5 (Fig. 3A). However, because BRETmax values also depend on the distance and on the relative orientation of the BRET donor and acceptor (28), different BRETmax values could also correspond to different conformations of βarr2-CCR5 complexes elicited by CCL5 and PSC-RANTES. To discriminate between these two possibilities, we examined the amount of βarr2 recruited at the plasma membrane using TIRF microscopy, which restricts the observation to the first 100–200 nm from the coverslip (33). TIRF acquisitions were performed in fixed cells stably expressing βarr2-GFP and FLAG-CCR5 (Fig. 3, C and D). βarr2-GFP fluorescence was not enriched at the plasma membrane in untreated cells (Fig. 3C). After receptor stimulation with PSC-RANTES or CCL5, βarr2-GFP was recruited into spots close to the plasma membrane, likely corresponding to CCR5-containing clathrin-coated pits (Fig. 3C). The number of spots increased over time after agonist exposure. At all time points, the number of βarr2-GFP spots in PSC-RANTES-treated cells was at least three times higher than in CCL5-treated cells (Fig. 3D). Note that the surface of spots was not significantly different between both treatments at 10 min (surfaceCCL5, 5 ± 0.8 pixels; surfacePSC-RANTES, 6.25 ± 0.6 pixels) and increased slightly for PSC-RANTES-treated cells at 15 min (surfaceCCL5, 4.7 ± 0.6 pixels; surfacePSC-RANTES, 7.5 ± 0.6 pixels). This indicates that the difference in the amount of βarr2-GFP spots detected after PSC-RANTES treatment compared with CCL5 is mainly due to an increase in the quantity of βarr2 recruited to the plasma membrane rather than to a difference in the size distribution of βarr2-GFP.
These results indicate that PSC-RANTES may trigger the formation of a larger number of βarr2-CCR5 complexes compared with CCL5. This may explain why, after stimulation with CCL5, only a fraction of βarr2-Rluc molecules interacted with CCR5-YFP in saturation BRET experiments (Fig. 3A). The increased recruitment of βarr2 to CCR5 after PSC-RANTES exposure likely contributed to the increased internalization of CCR5 (Fig. 1) compared with that observed after CCL5 stimulation.
PSC-RANTES Stabilizes a Pool of CCR5 That Is Not Accessible to Native Chemokines
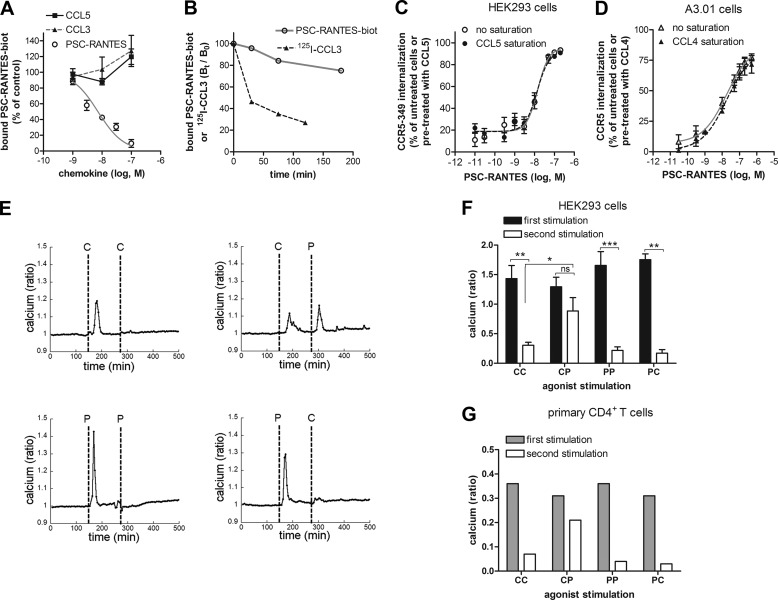
The data above are consistent with a model where PSC-RANTES can activate a larger pool of CCR5 than native chemokines. To explore the hypothesis that some CCR5 conformations would bind PSC-RANTES but not native chemokines, we conducted competition experiments in which increasing concentrations of unlabeled chemokines (CCL5, CCL3, and PSC-RANTES) were used to inhibit the binding of biotinylated PSC-RANTES (PSC-RANTES-biot) to CCR5 (Fig. 4A). Although PSC-RANTES competed with PSC-RANTES-biot for binding to the receptor (IC50, 7 ± 0.67 nm), native chemokines, even at micromolar concentration, could not (Fig. 4A). This result, showing that PSC-RANTES-biot prevents the binding of native chemokines to CCR5, contrasts with the competitive inhibition of [125I]CCL3 binding by unlabeled chemokines shown Fig. 1B and suggests that the receptors binding PSC-RANTES-biot and those binding [125I]CCL3 represent two different receptor populations. We measured a slow dissociation rate of PSC-RANTES-biot from CCR5 (Fig. 4B), which could contribute to this result. Alternatively, but not exclusively, native chemokines might display a marginal affinity for this particular fraction of CCR5, therefore explaining why they failed to displace PSC-RANTES-biot. Considered together, the results from Figs. 1B and 4A suggest that CCR5 exists in two distinct pools, one with a high affinity for both PSC-RANTES and native chemokines and another, the larger one, with a high affinity for PSC-RANTES and a minimal affinity for native chemokines.
FIGURE 4.
PSC-RANTES stabilizes spare CCR5 conformations. A, displacement of 1 nm PSC-RANTES-biot binding to CCR5-expressing A3.01 cells by increasing concentrations of the indicated chemokines. Data with unlabeled PSC-RANTES used as a competitor were fitted according to a one-site competitive binding model (mean ± S.E., n = 4). B, PSC-RANTES-biot or [125I]CCL3 dissociation from CCR5. CCR5-expressing A3.01 cells were incubated for 90 min with 1 nm PSC-RANTES-biot or for 120 min with 0.5 nm [125I]CCL3. Dissociation was initiated by addition of 100 nm 5P12 (a high-affinity CCR5 antagonist). PSC-RANTES-biot or [125I]CCL3 remaining bound to CCR5 was measured over time and analyzed as in Figs. 4A and 1B (one representative experiment of two). C and D, dose-dependent CCR5 internalization in CCR5-349-expressing HEK293 cells (C) or in CCR5-expresssing A3.01 cells (D) mediated by 20 min (C) or 5 min (D) of stimulation with PSC-RANTES at 37 °C. C, receptor internalization was measured after incubation, or no incubation, of CCR5-349-expressing HEK293 cells with 100 nm CCL5 at 4 °C for 90 min (data were fitted according to a sigmoidal dose-response model with a variable slope) (mean ± S.E., n = 4). D, receptor internalization was measured after incubation, or no incubation, of A3.01 cells with 300 nm CCL4 at 4 °C for 90 min (data were fitted according to a sigmoidal dose-response model with a variable slope) (mean ± S.E., n = 3). E–G, calcium responses measured in Fura-2-loaded CCR5-expressing HEK293 cells (E and F) or primary T cells (G) stimulated twice (2-min intervals between the first and the second stimulation) with 15 nm chemokines. E, results were obtained by imaging cells coated on a glass coverslip. Each trace is the mean of at least 25 single-cell calcium responses. F and G, results were obtained by spectrofluorimetry on the entire resuspended HEK293 cell population (F) (n = 3 independent experiments) or primary T cell population (G) (one representative experiment of two performed on two different donors). *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant; unpaired, two-tailed Student's t test. C, CCL5; P, PSC-RANTES.
If PSC-RANTES might stabilize an additional pool of CCR5, a significant fraction of receptors internalized in response to PSC-RANTES would not be accessible to native chemokines. This would explain why PSC-RANTES induces a stronger CCR5 down-regulation than native chemokines. To investigate this possibility, we tested, both in HEK293 cells (Fig. 4C) and in the A3.01 T cell line (Fig. 4D), whether a saturating concentration of native chemokines could prevent subsequent PSC-RANTES-induced internalization. Preincubating cells for 90 min at 4 °C with 100 nm CCL5 (or 300 nm CCL4) influenced neither the potency nor the efficacy of PSC-RANTES to induce CCR5 internalization (Fig. 4, C and D), demonstrating that PSC-RANTES can induce the internalization of receptors that are not targeted by native chemokines.
The binding of PSC-RANTES to a pool of spare receptors was also supported by experiments of agonist-induced CCR5 desensitization. A first, stimulation with CCL5 (C) promoted a CCR5-dependent calcium release, whereas a second challenge with the same chemokine failed to induce any further response (Fig. 4, E and F, CC on graphs). In contrast, a second challenge by PSC-RANTES (P) could elicit a calcium response (Fig. 4, E and F, CP), suggesting that a proportion of CCR5, not desensitized by the first stimulation with CCL5, could still be activated by PSC-RANTES. In a parallel experiment, the initial stimulation by PSC-RANTES desensitized all receptors because no further calcium signal could be elicited by challenge with PSC-RANTES or the native chemokine (Fig. 4, E and F, PP and PC). These data were obtained both at a single cell level (Fig. 4E) and in assays on cell populations (Fig. 4F). These results were confirmed in primary CD4+ T cells (Fig. 4G).
DISCUSSION
In this study, we investigated whether a pool of CCR5 that is not occupied by native chemokines could represent a target for inhibiting HIV-1 infection. Our results are consistent with the existence of spare forms of CCR5 on the cell surface that bind PSC-RANTES with high affinity but not native chemokines. These results support the concept that CCR5 exists in multiple conformations that are differentially stabilized by functionally different ligands. They are also relevant in light of our recent findings showing that HIV-1 can escape inhibition by native chemokines by exploiting low-chemokine affinity conformations of CCR5 (11). PSC-RANTES would bypass this limitation by targeting spare CCR5. This would lead to a larger number of βarr2-CCR5 complexes, stronger CCR5 down-regulation, and potent HIV-1 inhibition compared with native chemokines.
Spare CCR5 Receptors
GPCRs are dynamic proteins adopting an array of inactive and active conformations (6, 7). Recent NMR experiments suggest that agonists turn the receptor into heterogeneous ligand-bound intermediate states that are stabilized into fully active conformations when bound to downstream effectors (6). In this context, our BRET experiments (showing a similar affinity of βarr2 for CCL5-bound and PSC-RANTES-bound CCR5) suggest that PSC-RANTES and native chemokines stabilize CCR5 into a similar intermediate conformational state likely to interact with βarr2. Consequently, the higher amount of βarr2-CCR5 complexes formed by PSC-RANTES could result from a higher affinity of PSC-RANTES with this intermediate conformation compared with native chemokines. Alternatively, the higher amount of βarr2-CCR5 complexes could depend on the selection by PSC-RANTES of additional resting receptor states. Consistent with these two hypotheses, we found that native chemokines failed to prevent biotinylated PSC-RANTES binding to CCR5 (Fig. 4A), whereas they were as potent as unlabeled PSC-RANTES in displacing [125I]CCL3 binding (Fig. 1B). This suggests that CCR5 may exist in at least two different conformational states, one with a high affinity for both PSC-RANTES and native chemokines and the other, the larger one, preferentially binding the analog. We showed previously that CCR5 coupling to nucleotide-free forms of G-proteins (NFG-proteins) is necessary for high-affinity binding of native chemokines (11). Thus, it is possible that the pool of CCR5 that binds PSC-RANTES but not native chemokines corresponds to NFG-protein-uncoupled receptors, whereas the pool of CCR5 capable of binding both native chemokines and PSC-RANTES would correspond to NFG-protein-coupled receptors. In agreement with previous experiments showing that the recruitment of βarr2 to CCR5 and CCR5 endocytosis is independent of G-proteins (9, 11), G-protein-uncoupled and PSC-RANTES-bound CCR5 would maintain the capacity of recruiting β-arrestins.
The above results imply that, in the range of concentrations at which native chemokines and PSC-RANTES produced comparable effects on G-protein activation (Fig. 1), native chemokines occupy a smaller fraction of receptors than PSC-RANTES. In other words, this indicates that not all CCR5 are occupied by native chemokines to produce full G-protein activation. This property of an agonist is also observed in other receptor systems (34) where reserve receptors (also named spare receptors) exist and are not needed for the agonists to evoke a maximal functional response. Here we provide evidence indicating that a population of CCR5 is poorly accessible to native chemokines while being targeted by PSC-RANTES, supporting the concept of spare CCR5. First, we observed that saturating concentrations of native chemokines failed to prevent β-arrestin-dependent, PSC-RANTES-mediated CCR5 internalization (Fig. 4, C and D). Second, although PSC-RANTES fully desensitized further CCR5 activation mediated by native chemokines, the reverse situation was not observed (Fig. 4, E–G). This latter observation also suggests that a fraction of spare receptors that is targeted by PSC-RANTES would represent receptors capable of interacting with G-proteins. This result might appear at odds with the observation that PSC-RANTES and native chemokines activate G-protein dependent signals with comparable efficacy (Fig. 1). However, PSC-RANTES might stabilize intermediate CCR5 conformations likely to couple to G-proteins less efficiently than those induced by native chemokines. This suggests that PSC-RANTES-occupied CCR5 displays a bias toward β-arrestin recruitment over G-protein coupling compared with native chemokines. This hypothesis is in line with recent data showing that biased agonism, which results in differential activation of different signaling pathways, is a common property of chemokines (35).
Molecular Determinants and Mechanisms Featuring PSC-RANTES Functionality
The classical model for chemokine binding to CCR5 and receptor activation proposes that the chemokine core binds extracellular regions of the receptor, whereas the N-terminal tail of the chemokine interacts with the transmembrane domains (36). The length and the amino acid composition of the N-terminal tail of CCR5-binding chemokines affect their binding and signaling properties as well as their anti-HIV potency (37, 38). Moreover, different classes of N-terminal-modified CCL5 analogs displaying improved anti-HIV properties have different pharmacological profiles (23), suggesting that they may stabilize different conformations of CCR5. Mutations in CCR5 transmembrane domains differentially impact the antiviral activities of these chemokine analogs, including PSC-RANTES (39). Interestingly, these mutations did not alter the ability of the analogs to displace [125I]CCL3 binding to the receptor (39). We can hypothesize that the mutations may change the relative proportions of receptors likely to establish high-affinity interactions with the analogs. In line with this hypothesis, recent computational docking approaches showed that mutations in the transmembrane domains stabilize different sets of CCR5 conformations, each of which differs in the ability to bind different ligands and/or different effectors (40).
Overall, our results are consistent with the view that the robust CCR5 down-regulation following PSC-RANTES stimulation results from the stabilization by the N-terminal tail of PSC-RANTES of a higher amount of βarr2-CCR5 complexes but not from faster CCR5 internalization. Indeed, we observed similar kinetics of CCR5 internalization (Fig. 1E) and of βarr2 recruitment (Fig. 3B) regardless of the agonist used, in agreement with previous studies (11, 17). We also showed different rates of CCR5 recycling after PSC-RANTES- and CCL5-induced endocytosis (Fig. 1G) (17). Although the strength of βarr2-receptor interactions appears to control the extent of receptor recycling and the postendocytic fate of receptors (41), our results showing similar βarr2 affinities for PSC-RANTES-bound and CCL5-bound CCR5 (Fig. 3A) do not support this hypothesis in the case of CCR5.
In contrast to WT CCR5, results regarding the recycling-defective mutant CCR5-349 show different rates of internalization, depending on PSC-RANTES or CCL5 (Fig. 1F). CCL5 caused a slower rate of CCR5 internalization compared with PSC-RANTES. Note that all of the CCR5-349 will finally be removed from the cell surface, which is not the case with the WT receptor, where a plateau was observed. This suggests that CCR5-349 internalization involves both high-chemokine affinity receptors and spare receptors (which have a high affinity for PSC-RANTES but bind native chemokines poorly). In this context, the low affinity of CCL5 for spare CCR5-349 and/or a slow isomerization rate between low CCL5 affinity and high CCL5 affinity conformations of CCR5-349 may explain why the native chemokine induces a slower receptor internalization compared with PSC-RANTES. Conversely, similar kinetics of internalization of WT CCR5 by PSC-RANTES and CCL5 suggest that both ligands engage the same high-affinity population of WT CCR5.
Role of Spare Receptors in HIV-1 Infection and Inhibition
The observation that chemokines display high-affinity binding to CCR5 and anti-HIV-1 activity in vitro led to the proposal that they act as a natural barrier against HIV-1 in vivo, but their role in HIV-1 transmission and progression of infection remains debatable (42). CCR5 chemokines show antiviral potencies lower than would be expected on the basis of their CCR5 binding affinity constants, indicating that HIV-1 could escape inhibition by chemokines (16, 38, 43). The existence of a set of CCR5 that is poorly accessible to chemokines, but could be exploited by HIV-1, might contribute to the low efficiency of CCR5 chemokines as a HIV-1 barrier and constitute a viral escape route. We demonstrated previously that different cell surface conformations of CCR5, which depend on whether the receptor interacts or does not interact with heterotrimeric G-proteins, are used differentially by native CCR5 chemokines and HIV envelope glycoproteins (11). In particular, we revealed that G-protein-uncoupled CCR5 represent low-chemokine affinity receptors while constituting a portal for HIV-1 entry. Our current findings show that the pool of CCR5 with high chemokine affinity probably represents only a minor fraction of the total receptor pool. This is in agreement with the small amount of nucleotide-free forms of G-proteins present in a cell, which confer to the receptor a high affinity for agonists (44).
Finally, our results are consistent with the fact that targeting spare receptors allows PSC-RANTES to form an increased amount of βarr2-CCR5 complexes, leading to a stronger down-regulation of CCR5 and potent HIV entry inhibition. Thus, spare CCR5 might represent a pool of coreceptors capable of supporting HIV-1 entry. This is in line with previous studies showing that HIV-1 entry is a cooperative process that requires the recruitment of several CD4 and coreceptor molecules into a fusion complex (45). In this context, HIV-1 could use these spare receptors in addition to high chemokine affinity receptors to enter into host cells more efficiently. Furthermore, targeting spare receptors would allow PSC-RANTES to bypass CCR5 populations with a higher affinity for the viral envelope glycoprotein (11). Thus, the example of PSC-RANTES illustrates a new principle to inhibit HIV-1 infection and provides a basis for future strategies regarding the development of new inhibitors.
Acknowledgments
We thank the flow cytometry and “Imagerie Dynamique” platforms of the Institut Pasteur for technical help.
This work was supported by Agence Nationale de Recherche sur le SIDA (ANRS), Fondation ARC, Fondation pour la Recherche Médicale (FRM), SIDACTION, INSERM, Institut Pasteur, the Swiss National Science Foundation, and by Grant ANR-10-LabEx-62-IBEID.
O. Hartley, G. Künzi, and H. Gaertner, unpublished results.
- GPCR
- G-protein-coupled receptor
- HIV-1
- human immunodeficiency virus, type 1
- RANTES
- regulated on activation, normal T cell expressed and secreted
- PSC-RANTES
- N(α)(n-nonanoyl)-des-Ser(1)-[l-thioprolyl(2), l-cyclohexylglycyl(3)]-RANTES(4–68)
- Fmoc
- N-(9-fluorenyl)methoxycarbonyl
- TIRF
- total internal reflection fluorescence
- BRET
- bioluminescence resonance energy transfer
- Rluc
- Renilla luciferase
- biot
- biotin
- β-arr
- β-arrestin.
REFERENCES
- 1. Mueller A., Mahmoud N. G., Strange P. G. (2006) Diverse signalling by different chemokines through the chemokine receptor CCR5. Biochem. Pharmacol. 72, 739–748 [DOI] [PubMed] [Google Scholar]
- 2. Alkhatib G., Combadiere C., Broder C. C., Feng Y., Kennedy P. E., Murphy P. M., Berger E. A. (1996) CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 3. Samson M., Libert F., Doranz B. J., Rucker J., Liesnard C., Farber C.-M., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C., Muyldermans G., Verhofstede C., Burtonboy G., Georges M., Imai T., Rana S., Yi Y., Smyth R. J., Collman R. G., Doms R. W., Vassart G., Parmentier M. (1996) Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 [DOI] [PubMed] [Google Scholar]
- 4. Ray N. (2009) Maraviroc in the treatment of HIV infection. Drug Des. Devel. Ther. 2, 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haqqani A. A., Tilton J. C. (2013) Entry inhibitors and their use in the treatment of HIV-1 infection. Antiviral Res. 98, 158–170 [DOI] [PubMed] [Google Scholar]
- 6. Nygaard R., Zou Y., Dror R. O., Mildorf T. J., Arlow D. H., Manglik A., Pan A. C., Liu C. W., Fung J. J., Bokoch M. P., Thian F. S., Kobilka T. S., Shaw D. E., Mueller L., Prosser R. S., Kobilka B. K. (2013) The dynamic process of β(2)-adrenergic receptor activation. Cell 152, 532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahsai A. W., Xiao K., Rajagopal S., Ahn S., Shukla A. K., Sun J., Oas T. G., Lefkowitz R. J. (2011) Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 7, 692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oppermann M. (2004) Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal 16, 1201–1210 [DOI] [PubMed] [Google Scholar]
- 9. Lagane B., Ballet S., Planchenault T., Balabanian K., Le Poul E., Blanpain C., Percherancier Y., Staropoli I., Vassart G., Oppermann M., Parmentier M., Bachelerie F. (2005) Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol. Pharmacol. 67, 1966–1976 [DOI] [PubMed] [Google Scholar]
- 10. Berro R., Klasse P. J., Lascano D., Flegler A., Nagashima K. A., Sanders R. W., Sakmar T. P., Hope T. J., Moore J. P. (2011) Multiple CCR5 conformations on the cell surface are used differentially by human immunodeficiency viruses resistant or sensitive to CCR5 inhibitors. J. Virol. 85, 8227–8240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colin P., Bénureau Y., Staropoli I., Wang Y., Gonzalez N., Alcami J., Hartley O., Brelot A., Arenzana-Seisdedos F., Lagane B. (2013) HIV-1 exploits CCR5 conformational heterogeneity to escape inhibition by chemokines. Proc. Natl. Acad. Sci. U.S.A. 110, 9475–9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berro R., Yasmeen A., Abrol R., Trzaskowski B., Abi-Habib S., Grunbeck A., Lascano D., Goddard W. A., 3rd, Klasse P. J., Sakmar T. P., Moore J. P. (2013) Use of G-protein-coupled and -uncoupled CCR5 receptors by CCR5 inhibitor-resistant and -sensitive human immunodeficiency virus type 1 variants. J. Virol. 87, 6569–6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartley O., Gaertner H., Wilken J., Thompson D., Fish R., Ramos A., Pastore C., Dufour B., Cerini F., Melotti A., Heveker N., Picard L., Alizon M., Mosier D., Kent S., Offord R. (2004) Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc. Natl. Acad. Sci. U.S.A. 101, 16460–16465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pastore C., Picchio G. R., Galimi F., Fish R., Hartley O., Offord R. E., Mosier D. E. (2003) Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES. Antimicrob. Agents Chemother. 47, 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lederman M. M., Veazey R. S., Offord R., Mosier D. E., Dufour J., Mefford M., Piatak M., Jr., Lifson J. D., Salkowitz J. R., Rodriguez B., Blauvelt A., Hartley O. (2004) Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306, 485–487 [DOI] [PubMed] [Google Scholar]
- 16. Gaertner H., Lebeau O., Borlat I., Cerini F., Dufour B., Kuenzi G., Melotti A., Fish R. J., Offord R., Springael J. Y., Parmentier M., Hartley O. (2008) Highly potent HIV inhibition: engineering a key anti-HIV structure from PSC-RANTES into MIP-1 β/CCL4. Protein Eng. Des. Sel. 21, 65–72 [DOI] [PubMed] [Google Scholar]
- 17. Escola J. M., Kuenzi G., Gaertner H., Foti M., Hartley O. (2010) CC chemokine receptor 5 (CCR5) desensitization: cycling receptors accumulate in the trans-Golgi network. J. Biol. Chem. 285, 41772–41780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. (1999) Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303–1311 [DOI] [PubMed] [Google Scholar]
- 19. Issafras H., Angers S., Bulenger S., Blanpain C., Parmentier M., Labbé-Jullié C., Bouvier M., Marullo S. (2002) Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J. Biol. Chem. 277, 34666–34673 [DOI] [PubMed] [Google Scholar]
- 20. Storez H., Scott M. G., Issafras H., Burtey A., Benmerah A., Muntaner O., Piolot T., Tramier M., Coppey-Moisan M., Bouvier M., Labbé-Jullié C., Marullo S. (2005) Homo- and hetero-oligomerization of β-arrestins in living cells. J. Biol. Chem. 280, 40210–40215 [DOI] [PubMed] [Google Scholar]
- 21. Molla-Herman A., Boularan C., Ghossoub R., Scott M. G., Burtey A., Zarka M., Saunier S., Concordet J. P., Marullo S., Benmerah A. (2008) Targeting of β-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS ONE 3, e3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baba M., Nishimura O., Kanzaki N., Okamoto M., Sawada H., Iizawa Y., Shiraishi M., Aramaki Y., Okonogi K., Ogawa Y., Meguro K., Fujino M. (1999) A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. U.S.A. 96, 5698–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaertner H., Cerini F., Escola J. M., Kuenzi G., Melotti A., Offord R., Rossitto-Borlat I., Nedellec R., Salkowitz J., Gorochov G., Mosier D., Hartley O. (2008) Highly potent, fully recombinant anti-HIV chemokines: reengineering a low-cost microbicide. Proc. Natl. Acad. Sci. U.S.A. 105, 17706–17711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Delhaye M., Gravot A., Ayinde D., Niedergang F., Alizon M., Brelot A. (2007) Identification of a postendocytic sorting sequence in CCR5. Mol. Pharmacol. 72, 1497–1507 [DOI] [PubMed] [Google Scholar]
- 25. Cavrois M., De Noronha C., Greene W. C. (2002) A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20, 1151–1154 [DOI] [PubMed] [Google Scholar]
- 26. Randriamampita C., Boulla G., Revy P., Lemaitre F., Trautmann A. (2003) T cell adhesion lowers the threshold for antigen detection. Eur. J. Immunol. 33, 1215–1223 [DOI] [PubMed] [Google Scholar]
- 27. Conche C., Boulla G., Trautmann A., Randriamampita C. (2009) T cell adhesion primes antigen receptor-induced calcium responses through a transient rise in adenosine 3′,5′-cyclic monophosphate. Immunity 30, 33–43 [DOI] [PubMed] [Google Scholar]
- 28. Achour L., Kamal M., Jockers R., Marullo S. (2011) Using quantitative BRET to assess G protein-coupled receptor homo- and heterodimerization. Methods Mol. Biol. 756, 183–200 [DOI] [PubMed] [Google Scholar]
- 29. Basquin C., Malardé V., Mellor P., Anderson D. H., Meas-Yedid V., Olivo-Marin J. C., Dautry-Varsat A., Sauvonnet N. (2013) The signalling factor PI3K is a specific regulator of the clathrin-independent dynamin-dependent endocytosis of IL-2 receptors. J. Cell Sci. 126, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 30. Watson C., Jenkinson S., Kazmierski W., Kenakin T. (2005) The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol. Pharmacol. 67, 1268–1282 [DOI] [PubMed] [Google Scholar]
- 31. Goodman O. B., Jr., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. (1996) β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383, 447–450 [DOI] [PubMed] [Google Scholar]
- 32. Tarancon Diez L., Bonsch C., Malkusch S., Truan Z., Munteanu M., Heilemann M., Hartley O., Endesfelder U., Furstenberg A. (2014) Coordinate-based co-localization-mediated analysis of arrestin clustering upon stimulation of the C-C chemokine receptor 5 with RANTES/CCL5 analogues. Histochem. Cell Biol., in press [DOI] [PubMed] [Google Scholar]
- 33. Fish K. N. (2009) Total internal reflection fluorescence (TIRF) microscopy. Curr. Protoc. Cytom. Chapter 12, Unit12.18, 10.1002/0471142956.cy1218s50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacquin L., Franceschi F., By Y., Durand-Gorde J. M., Condo J., Deharo J. C., Michelet P., Fenouillet E., Guieu R., Ruf J. (2013) Search for adenosine A2A spare receptors on peripheral human lymphocytes. FEBS Open Bio 3, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajagopal S., Bassoni D. L., Campbell J. J., Gerard N. P., Gerard C., Wehrman T. S. (2013) Biased agonism as a mechanism for differential signaling by chemokine receptors. J. Biol. Chem. 288, 35039–35048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blanpain C., Doranz B. J., Bondue A., Govaerts C., De Leener A., Vassart G., Doms R. W., Proudfoot A., Parmentier M. (2003) The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J. Biol. Chem. 278, 5179–5187 [DOI] [PubMed] [Google Scholar]
- 37. Nakajima T., Kaur G., Mehra N., Kimura A. (2008) HIV-1/AIDS susceptibility and copy number variation in CCL3L1, a gene encoding a natural ligand for HIV-1 co-receptor CCR5. Cytogenet. Genome Res. 123, 156–160 [DOI] [PubMed] [Google Scholar]
- 38. Schols D., Proost P., Struyf S., Wuyts A., De Meester I., Scharpé S., Van Damme J., De Clercq E. (1998) CD26-processed RANTES(3–68), but not intact RANTES, has potent anti-HIV-1 activity. Antiviral Res. 39, 175–187 [DOI] [PubMed] [Google Scholar]
- 39. Choi W. T., Nedellec R., Coetzer M., Colin P., Lagane B., Offord R. E., Hartley O., Mosier D. E. (2012) CCR5 mutations distinguish N-terminal modifications of RANTES (CCL5) with agonist versus antagonist activity. J. Virol. 86, 10218–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abrol R., Kim S. K., Bray J. K., Trzaskowski B., Goddard W. A., 3rd (2013) Conformational ensemble view of G protein-coupled receptors and the effect of mutations and ligand binding. Methods Enzymol. 520, 31–48 [DOI] [PubMed] [Google Scholar]
- 41. Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. (2000) Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 275, 17201–17210 [DOI] [PubMed] [Google Scholar]
- 42. Barretina J., Blanco J., Gutiérrez A., Puig L., Altisent C., Espanol T., Caragol I., Clotet B., Esté J. A. (2000) Evaluation of the putative role of C-C chemokines as protective factors of HIV-1 infection in seronegative hemophiliacs exposed to contaminated hemoderivatives. Transfusion 40, 461–467 [DOI] [PubMed] [Google Scholar]
- 43. Simmons G., Clapham P. R., Picard L., Offord R. E., Rosenkilde M. M., Schwartz T. W., Buser R., Wells T. N., Proudfoot A. E. (1997) Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276, 276–279 [DOI] [PubMed] [Google Scholar]
- 44. Chabre M., Deterre P., Antonny B. (2009) The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol. Sci. 30, 182–187 [DOI] [PubMed] [Google Scholar]
- 45. Kuhmann S. E., Platt E. J., Kozak S. L., Kabat D. (2000) Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74, 7005–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]