Abstract
The vagus nerve has an important role in regulation of metabolic homeostasis, and efferent vagus nerve-mediated cholinergic signalling controls immune function and proinflammatory responses via the inflammatory reflex. Dysregulation of metabolism and immune function in obesity are associated with chronic inflammation, a critical step in the pathogenesis of insulin resistance and type 2 diabetes mellitus. Cholinergic mechanisms within the inflammatory reflex have, in the past 2 years, been implicated in attenuating obesity-related inflammation and metabolic complications. This knowledge has led to the exploration of novel therapeutic approaches in the treatment of obesity-related disorders.
Introduction
The vagus nerve (cranial nerve X) is the main nerve of the parasympathetic division of the autonomic nervous system. The vagus nerve regulates metabolic homeostasis by controlling heart rate, gastrointestinal motility and secretion, pancreatic endocrine and exocrine secretion, hepatic glucose production, and other visceral functions. In addition, the vagus nerve is a major constituent of a neural reflex mechanism—the inflammatory reflex—that controls innate immune responses and inflammation during pathogen invasion and tissue injury.1–3
Innate immune responses are activated by pathogen-associated and danger-associated molecular patterns that are recognized by sensors on the immune cell surface or in intracellular compartments. These cellular sensors include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors (NLRs) and other pattern-recognition receptors (Figure 1).4–6 Activation of signalling cascades downstream of TLRs results in increased production and release of tumour necrosis factor (TNF), IL-6 and other proinflammatory cytokines.1,7 In addition, activation of NLRs is associated with the formation of multimeric protein complexes, termed inflammasomes, which regulate maturation and release of the proinflammatory cytokines IL-1β and IL-18.5 Proinflammatory cytokines, along with chemo-kines, reactive oxygen species, nitrogen inter mediates and other inflammatory molecules, are critically implicated in extracellular pathogen clearance, vasodilatation, neutrophil recruitment, increased vascular permeability and induction of acute-phase proteins, such as C-reactive protein (CRP), and coagulation molecules.1,5,7 Proinflammatory progression is balanced by the release of IL-10, TGF-β, soluble cytokine receptors and other anti-inflammatory molecules.
Figure 1.
The functional anatomy of the inflammatory reflex. Inflammatory mediators, such as cytokines, are released by activated macrophages and other immune cells when TLRs and NLRs are activated upon immune challenge. These mediators are detected by sensory components of the afferent arm of the inflammatory reflex (red). Neuronal interconnections between the NTS, A P, DMN, NA, and higher forebrain regions (not shown) integrate afferent signalling and efferent vagus nerve-mediated immunoregulatory output. Efferent vagus nerve cholinergic output to the spleen, liver and gastrointestinal tract (blue) regulates immune activation and suppresses proinflammatory cytokine release (dotted red lines). This efferent cholinergic arm of the inflammatory reflex can be activated in the brain through mAChR-mediated mechanisms triggered by mAChR ligands and AChE inhibitors, such as galantamine. Abbreviations: AChE, acetylcholinesterase; A P, area postrema; DMN, dorsal motor nucleus of the vagus nerve; LPS, lipopolysaccharide (endotoxin); mAChR, muscarinic acetylcholine receptor; NA, nucleus ambiguus; NLRs, nucleotide-binding oligomerization domain-like receptors; NTS, nucleus tractus solitarius; TLR4, toll-like receptor 4.
Inflammation is normally a local and temporary event and, upon its resolution, immune and physiological homeostasis is restored. However, disrupted innate immune regulation can result in continual pro-inflammatory cytokine activity and excessive or chronic inflammation. This state underlies the pathogenesis of a range of disease syndromes, including sepsis, rheumatoid arthritis, inflammatory bowel disease and other inflammatory and autoimmune disorders.8–10 Understanding endogenous mechanisms that prevent or neutralize excessive proinflammatory responses could lead to novel therapeutic options for diseases associated with an excessive or chronic inflammatory state.
Chronic inflammation as a result of immune and metabolic dysregulation is a characteristic feature in patients with obesity and is causally linked with insulin resistance and other metabolic complications.11–13 Decreased vagus nerve activity in the context of obesity has been reported.14–17 Selective cholinergic activation within the efferent vagus nerve-mediated arm of the inflammatory reflex can suppress obesity-associated inflammation and reverse metabolic complications.18–20 These findings raise the intriguing possibility that dysregulation of vagus nerve-mediated signalling might contribute to the pathogenes is of obesity and its related comorbidities.
In this Review, we provide a conceptual view of the inflammatory reflex as a physiological mechanism that functions on the path between immunity and metabolism and could be exploited in the treatment of obesity-associated inflammation and obesity-related disorders.
The inflammatory reflex
Communication between the immune system and the brain is vital for controlling inflammation. The inflammatory reflex is a centrally integrated physiological mechanism in which afferent vagus nerve signalling, activated by cytokines or pathogen-derived products, is functionally associated with efferent vagus nerve-mediated output to regulate proinflammatory cytokine production and inflammation (Figure 1). The absence of this inflammatory reflex—resulting from neural lesions or genetic ablation of essential components—results in excessive innate immune responses and cytokine toxicity.21,22 The inflammatory reflex has been comprehensively reviewed elsewhere.1–3,9,23
Afferent arm
Afferent vagus nerve fibres sense peripheral inflammatory molecules and convey signals to the brain and are, therefore, important for immune-to-brain communication (Figure 1).1,24 Afferent vagus neurons, residing in the nodose and jugular ganglia, terminate primarily in the nucleus tractus solitarius in the brainstem medulla oblongata (Figure 1).24 Afferent signalling is further communicated through neural contacts between brainstem nuclei, the hypothalamus and forebrain regions associated with integration of visceral sensory information as well as coordination of autonomic function and behavioural responses.23,24
Peripheral administration of bacterial lipopolysaccharide (also known as endotoxin) or IL-1β causes afferent vagus nerve activation, as determined by increased c-Fos expression and electrical activity.24,25 IL-1β receptors expressed on vagus nerve afferents and chemosensory (glomus) cells in paraganglia surrounding afferent vagus nerve endings have been implicated in the recognition of immune activation (Figure 1).24,26,27 Prostaglandin-dependent mechanisms have also been implicated in activating vagus nerve afferents by increasing levels of circulating IL-1β.26 Intraportal administration of IL-1β to rats increases afferent and, subsequently, efferent vagus nerve and splenic nerveactivity.25 Peripheral immune stimulation causes sickness behaviour, which is attenuated in rodents who have undergone surgical transection of the vagus nerve to prevent immune signalling at peripheral nerve endings.24 This attenuation is dependent on the magnitude of immune activation, because fairly large quantities of circulating IL-1β produce fever and sickness behaviour by bypassing the neural circuits, acting through brain circumventricular organs (including the area postrema) and via other humoral mechanisms (Figure 1).28,29 Accordingly, afferent vagus nerve endings appear to be important for relaying information about immune status to the brain when proinflamma tory cytokines are present at fairly low levels.24,28 However, TLR4 (which can be activated by lipopolysaccharide) is expressed in the nodose ganglion,30,31 which suggests a mechanism by which lipopolysaccharide and other inflammatory molecules can activate vagus nerve afferents above their visceral endings (Figure 1).
Efferent arm
A little more than a decade ago, an important role of efferent vagus nerve cholinergic signalling in brain-to-immune communication was revealed by observations that vagus nerve stimulation suppresses local and serum proinflammatory cytokine levels in rodents with endo-toxaemia, and that acetylcholine inhibits the release of TNF, IL-1β and IL-18 from lipopolysaccharide-stimulated macrophages.21 These findings led to the definition of the cholinergic anti-inflammatory pathway as the efferent vagus nerve-based arm of the inflammatory reflex (Figure 1).21 This efferent arm can be centrally regulated, and muscarinic acetylcholine receptors in the brain have been implicated in this regulation (Figure 1).32–35 Galantamine is a centrally acting acetylcholinesterase inhibitor and activates the efferent cholinergic arm of the inflammatory reflex by through a muscarinic receptor-dependent mechanism in the brain.34 Defining a brain network that can be used to explore central mechanisms of inflammatory reflex control is the aim of ongoing studies.
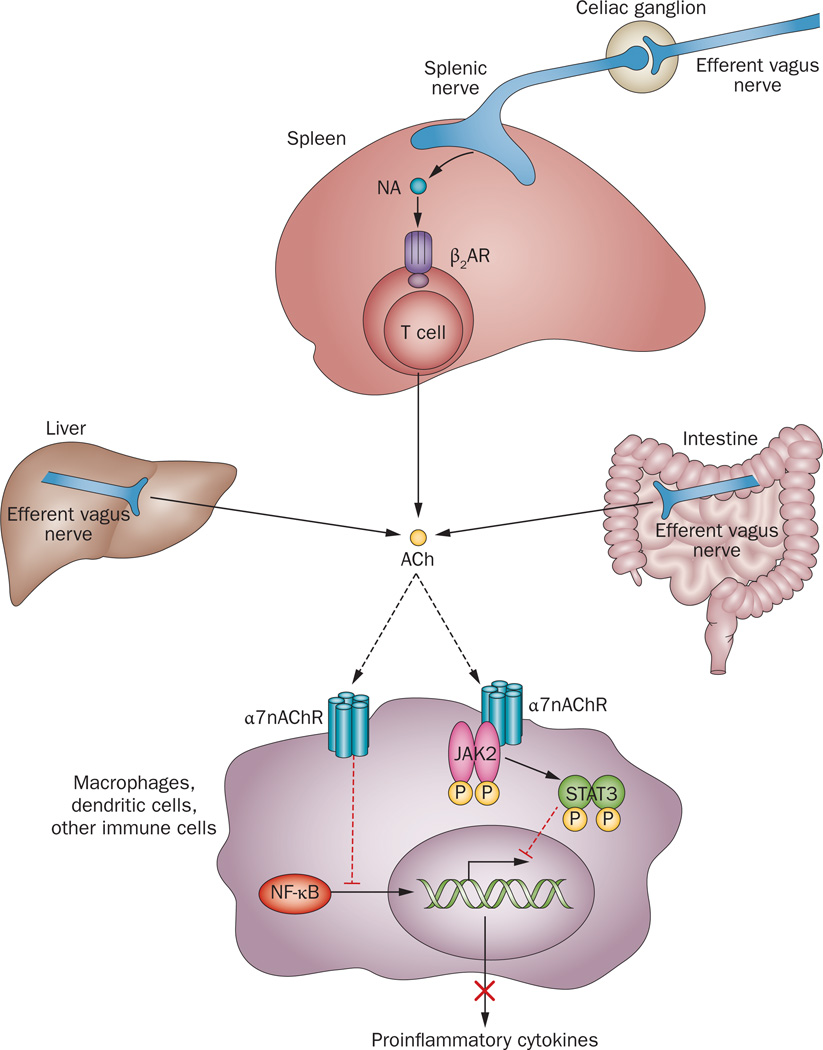
In peripheral tissues, the α7 nicotinic acetylcholine receptor (α7nAChR) is important for mediating anti-inflammatory signalling within the efferent arm of the inflammatory reflex (Figure 2).22 Accordingly, several α7nAChR agonists have been identified as experimental anti-inflammatory therapeutics with potential for clinical development.2,36–38 The α7 subunit of the receptor is expressed in macrophages, monocytes, dendritic cells, T cells, endothelial and other non-neuronal cells, and the structural and functional characterization of non-neuronal α7nAChRs is an area of ongoing study.2,38 The presence of α7nAChRs on bone marrow-derived cells is required for the functional integrity of the inflammatory reflex, but their presence on T cells and neuronal cells is not essential.39 The anti-inflammatory action of α7nAChR agonists or vagus nerve stimulation is associated with downregulation of CD14 and TLR4 expression in immune cells.40 Cholinergic inhibition of proinflammatory cytokine production is mediated through intracellular signal pathways downstream of α7nAChR that culminate in suppression of NF-κB nuclear translocation (Figure 2).36–38,40–42 The tyrosine protein kinase JAK2 might also be recruited and activated by α7nAChR upon cholinergic stimulation.43 Subsequent phosphorylation of the transcription factor STAT3 results in suppression of proinflammatory cytokine production in intestinal macrophages (Figure 2).43
Figure 2.
Molecular mechanisms of cholinergic control of inflammation. Efferent vagus nerve activity is translated into catecholamine-mediated activation of T-cell-derived acetylcholine release in the spleen and into direct acetylcholine release from efferent vagus nerve endings in other organs. Inhibition of NF-κB nuclear translocation and activation of a JAK2-STAT3-mediated signalling cascade in macrophages and other immune cells are implicated in cholinergic α7nAChR-mediated control of proinflammatory cytokine production. Abbreviations: ACh, acetylcholine; β2AR, β2 adrenergic receptor; JAK2, Janus kinase 2; α7nAChR, α 7 nicotinic acetylcholine receptor; NA, noradrenaline; NF-κB, nuclear factor κB; STAT3, signal transducer and activator of transcription 3.
The anti-inflammatory effects of vagus nerve stimulation in animals with endotoxaemia require neural signals along the adrenergic splenic nerve,44 which originates in the celiac ganglion that is innervated by the efferent vagus nerve (Figure 1, Figure 2). Somewhat unexpectedly, a specific subset of T cells (memory CD4+ T cells, which express adrenoreceptors), instead of neurons, provide an endogenous source of acetylcholine in the spleen (Figure 2).45 T cell production of acetylcholine in the spleen is critical for the inflammatory reflex. In nude mice, which lack T cells, vagus nerve stimulation fails to suppress TNF levels during endotoxaemia. However, transfer of acetylcholine-producing T cells, which repopulate the spleen in nude mice, restores the integrity of the neural circuit.45 In addition to this spleen-mediated mechanism in endotoxaemia, efferent vagus nerve endings might directly regulate the immune function by releasing acetylcholine, without the requirement for either signalling along the splenic nerve or T cells (Figure 1, Figure 2). Such a direct mechanism has been implicated in the suppression of inflammation in experimental settings of haemorrhagic shock, ileus, inflammatory bowel disease, autoimmune myocarditis and other inflammatory and autoimmune conditions.2,10,41,43,46–49 Importantly, many of these disorders are associated with autonomic dysfunction and decreased vagus nerve tone. Enhancement of vagus nerve output could, therefore, have therapeutic potential in these settings.50,51
Vagus nerve in metabolic regulation
Vagus nerve afferent and efferent signalling has an important role in the regulation of feeding behaviour and metabolic homeostasis. This finely tuned regulation is aimed at preserving energy balance and preventing fluctuations in body weight and metabolism that can be detrimental to the individual.
Dietary intake and metabolism
Vagus nerve afferents innervating the gastrointestinal tract and liver are major constituents of a sensory system that detects changes in micronutrient and metabolic molecules. These nerve fibres transmit information detected by associated mechanoreceptors, chemoreceptors and specific metabolite receptors in the gut and hepatic portal system concerning levels of lipids, cholecystokinin, leptin, peptide YY, insulin and glucose to the brain (Figure 3).52,53 Vagus nerve efferents, on the other hand, provide brain-derived output to the gastrointestinal tract, liver and pancreas (Figure 3).52 Vagus nerve innerva-tion of white adipose tissue has also been demonstrated using retrograde neuronal tracing.54 Other researchers, however, found limited retrograde labelling in the dorsal motor nucleus following injection of the tracer into white adipose tissue and a lack of some parasympathetic markers in adipose tissue.55 Therefore, the anatomy and a functional role of the vagus nerve in relation to adipose tissue is an area of active study.
Figure 3.
The role of the vagus nerve in metabolic regulation. Gastrointestinal and hepatic vagus nerve afferents (red) communicate alterations in peripheral levels of micronutrients and metabolic molecules to the brain. Neural interaction between the interconnected NTS, DMN and A P, within the dorsal vagal complex, and reciprocal projections between this brainstem region and several hypothalamic areas (arcuate and paraventricular nuclei and mediobasal and lateral areas), underlie brain integration of visceral information and the modulation of efferent motor vagus nerve output, leading to regulation of metabolic homeostasis. Efferent vagus nerve signalling (blue) can be triggered by sensing metabolic alterations in the brainstem and the hypothalamus. Complex communication between hypothalamic nuclei and other forebrain structures (such as the insula and premotor cortex, amygdala, nucleus accumbens, parabrachial nucleus and thalamus) mediate hedonic, motivational and rewarding aspects of feeding behaviour and their interaction with vagus nerve-mediated homeostatic mechanisms. Abbreviations: A P, area postrema; CCK, cholecystokinin; DMN, dorsal motor nucleus of the vagus nerve; GLP-1, glucagon-like peptide-1; NA, nucleus ambiguus; NTS, nucleus tractus solitarius; PYY, peptide YY.
Hepatic and gastrointestinal vagus nerve afferents are involved in the regulation of short-term feeding behaviour.52,53 Afferent vagus nerve signalling mediates gastric cholecystokinin-induced satiety and meal termination.52 Synergistic activation of vagus nerve afferents by both cholecystokinin and leptin mediates their short-term inhibitory effects on food intake.53 Lipid accumulation in the upper intestine, and consequent intestinal cholecystokinin release, also triggers vagus nerve-mediated and brain-integrated suppression of hepatic glucose production.56,57 Additionally, the efferent arm of this reflex mechanism can be stimulated by direct activation of neurons in the dorsal vagal complex.58
Glucose-induced pancreatic insulin secretion can be stimulated by treatment with a truncated form of glucagon-like peptide-1, which activates hepatic vagus nerve afferents and, subsequently, vagus nerve efferents innervating the pancreas.59,60 In addition to its intrinsic effects on the enteric nervous system, glucose in the lumen of the gastrointestinal tract causes neuronal activation in vagus nerve afferents, the nucleus tractus solitarius, the arcuate hypothalamic nucleus and the dorsal motor nucleus, thus highlighting the role of brain circuitry in vagus nerve regulation of gastrointestinal function and pancreatic secretion.61
Additionally, metabolic molecules can act directly in the brain to trigger efferent output that regulates metabolic homeostasis (Figure 3). Insulin signalling in the mediobasal hypothalamus is mediated through modulation of ATP-sensitive potassium channels and has been implicated in the suppression of hepatic glucose production.62,63 Efferent vagus nerve signalling to the liver is essential for this regulation.63 Hypothalamic sensing of fluctuations in circulatory lipid levels triggers efferent vagus nerve-mediated output that regulates hepatic glucose production and glucose homeostasis.64 In the hypothalamus, cholinergic activation dependent on muscarinic receptors increases hepatic glycogen synthesis through a mechanism mediated by the vagus nerve.65,66 Vagus nerve-derived cholinergic signalling through a mechanism mediated by M3 muscarinic receptors has also been implicated in stimulating insulin release in the pancreas.67
The dorsal vagal complex and different hypothalamic regions are important constituents of a brain network associated with vagus nerve-mediated regulation of peripheral metabolic functions (Figure 3). Interconnections between these and other forebrain regions also have a role in the integration of afferent and efferent signalling in terms of the control of metabolic homeostasis and the regulation of hedonic and motivational aspects of feeding behaviour (Figure 3).68–70
Postprandial inflammation
Serum lipopolysaccharide levels in healthy individuals are increased after consuming high-fat, or high-fat and high-carbohydrate meals.71,72 Endotoxaemia following high-fat diet ingestion or lipid intake has been implicated in postprandial inflammation as plasma levels of IL-6 and the expression of TLR4, TLR2 and SOCS3 in mononuclear cells increases.72–74 Postprandial endotoxaemia and inflammation are transient, however, and data from several studies suggest that the vagus nerve and the inflammatory reflex might have a role in suppressing postprandial inflammation. Afferent vagus nerve signalling has a primary role in informing the brain about the presence of peripheral inflammation during exposure to low levels of proinflammatory stimuli.28 TLR4, which is expressed by vagus nerve afferents,30,31 provides a molecular sensory component for neural detection of lipopolysaccharide in postprandial inflammation.
Dietary lipid infusion causes the release of chole-cystokinin, which acts both via vagus nerve afferents and directly in the brain to trigger activation of efferent vagus nerve signalling, which in turn suppresses the release of proinflammatory cytokines.75 Activation of vagus nerve afferents by cholecystokinin or leptin is potentiated by IL-1β.76,77 Furthermore, in rats, truncal vagotomy is associated with increased bacterial trans location across the intestinal mucosa,78 which suggests a tonic vagus nerve control of intestinal permeability and postprandial endotoxaemia.
The inflammatory reflex and obesity
Disruption in metabolic and immune homeostasis in obesity is associated with hyperglycaemia, insulin resistance, dyslipidaemia and hypertension. This cluster of conditions characterizes the metabolic syndrome.79 Moreover, levels of proinflammatory cytokines and acute-phase proteins such as CRP are increased in individuals with obesity, indicating chronic inflammation.11,80 This inflammatory state is considered to be an essential pathophysiological constituent in obesity, underlying its adverse consequences and linking it to the other components of the metabolic syndrome.80,81 Several lines of evidence indicate that vagus nerve activity could be impaired in obesity, and enhancing cholinergic signalling within the inflammatory reflex can suppress obesity-associated inflammation and its adverse implications.
Inflammation and obesity pathogenesis
The characteristic inflammatory state in obesity has been extensively discussed elsewhere.11,13,80,82,83 White adipose tissue in individuals with obesity is expanded and infiltrated with T cells, bone-marrow-derived macrophages, mast cells and B cells.83 As such, this tissue becomes a major source of proinflammatory factors.80,83–86 Adipocyte enlargement as a result of increased lipid deposition leads to metabolic alterations associated with dysregulated secretion of adipokines, including leptin, resistin, adiponectin and visfatin.80,84 Additionally, cell death and hypoxia occur in adipose tissue, leading to increased macrophage recruitment and generation of reactive oxygen species, which promote inflammation.85,87 Increased production and release of TNF, IL-6, CCL2 and other proinflammatory mediators from adipocytes, as well as from activated M1 macrophages and other immune cells, also drives inflammation.80,82,84,85 In addition to adipose tissue, activation of inflammatory pathways also occurs in the liver, skeletal muscle and brain.12,82
Adipocyte and macrophage expression of TLR4 and TLR2 provides a mechanism for transforming metabolic overload (high levels of saturated free fatty acids and glucose) into proinflammatory responses, as free fatty acids and glucose can be ligands for TLRs. Subsequent intracellular signalling mediated through JNK, IKK and PKR leads to stimulation of the transcription factors NF-κB and AP-1, and increased production of pro-inflammatory mediators.82,84,85,88 Metabolic overload also results in endoplasmic reticulum stress, which is associated with JNK and IKK signalling and generation of reactive oxygen species, triggering inflammation.13,82 In addition, activation of macrophages and T cells in the adipose tissue of patients with obesity can be mediated by inflammasomes.89 Pancreatic islet inflammation, as occurs in patients with type 2 diabetes mellitus, can also be mediated by these protein complexes.90
The intestinal microflora also contribute to the development of inflammation in individuals with obesity.91,92 Chronic ingestion of a high-fat diet increases the proportion of lipopolysaccharide-containing microbiota in the gut and this increase is associated with low-grade metabolic endotoxaemia in both mice and humans.92,93 In mice, mimicking metabolic endotoxaemia by chronic administration of a low dose of lipopoly-saccharide results in inflammation, increased adiposity, weight gain and metabolic complications.93 These changes result from activation of CD14–TLR4 signalling, which triggers increased production of proinflammatory cytokines by adipocytes and macrophages.85,93
The role of specific cytokines and adipokines in mediating obesity-associated complications (Table 1) has been the subject of several reviews.83,84,88,94–96 Inflammation is causally linked with impaired insulin signalling in peripheral target tissues and insulin resistance, a major complication in obesity and type 2 diabetes mellitus.12,84,85,95 Activation of inflammatory pathways in the brain interferes with insulin and leptin signalling in the brain and also contributes to insulin resistance.82,97 Increased lipolysis in insulin-resistant adipose tissue leads to enhanced release of free fatty acids, which stimulate inflammatory responses and reinforce insulin resistance. Proinflammatory cytokines and adipokines can inhibit insulin signalling by targeting IRS proteins or the insulin receptor. Specifically, TNF induces inhibition of insulin-activated tyrosine phosphorylation of the insulin receptor and serine phosphorylation of IRS-1, which leads to inactivation of insulin signalling.85,95 TNF and other proinflammatory cytokines act in a paracrine manner to stimulate JNK, IKK and possibly other kinases, which mediate serine phosphorylation of IRS proteins.82 Additionally, proinflammatory cytokines such as TNF, IL-6 and the adipokine resistin induce the expression of SOCS proteins, which block insulin signalling through inhibition of insulin receptor tyrosine kinase activity or IRS-1 ubiquitination and degradation.95,98 The net result is a negative influence of proinflammatory cytokines on insulin signalling.
Table 1.
Contributions of key adipokines and cytokines to obesity-related disease
| Cytokines and adipokines | Cell source | Role in obesity pathogenesis |
|---|---|---|
| Leptin (adipokine) | Adipocytes Gastric mucosa cells |
Regulates food intake and energy balance136* Increased release in parallel with weight gain* Activates anorexigenic pathways in the hypothalamic arcuate nucleus and other brain regions69,136* Acts through leptin receptors on vagus nerve afferents and on brain neurons and via intracellular JAK2–STAT3-mediated signalling31,69,136* Positive regulator of glucose homeostasis and insulin sensitivity in muscle and liver136* Leptin resistance in obesity associated with intracellular SOCS3-mediated suppression of STAT3 phosphorylation98,136 Hyperleptinaemia has proinfammatory effects in obesity84,137,138 |
| TNF (proinfammatory cytokine) | Macrophages Other immune cells Adipocytes |
Major contributor to local and systemic insulin resistance80,82,85,139 Increased expression in adipose tissue139 Stimulates resistin expression in human macrophages and suppresses adiponectin expression in human adipocytes83,88 Implicated in pathogenesis of fatty liver disease94 |
| IL-6 (proinfammatory cytokine) | Macrophages Other immune cells Adipocytes |
Mediates local and systemic insulin resistance80,82,85 Suppresses adiponectin expression in human adipocytes Implicated in pathogenesis of fatty liver disease94 Inducer of acute-phase proteins, including C-reactive protein in liver, which is linked to an increased risk of cardiovascular disease80,96,140 Increased expression in adipose tissue and increased circulating levels in obesity13,80 |
| CCL2 (chemokine) | Adipocytes Endothelial cells Macrophages |
Mediates macrophage and other immune cell infltration into adipose tissue141 Linked to insulin resistance and hepatosteatosis in rodents141 Increased levels in obesity85 |
| Resistin (adipokine) | Adipocytes (mice) Macrophages (humans) |
Induces insulin resistance in mice, and might have a similar role in humans85,142 Stimulates TNF and IL-6 production84 Increased circulating and adipose tissue levels in rodent models of obesity11 |
| Adiponectin (adipokine) | Adipocytes | Anti-infammatory and insulin-sensitizing functions95 Suppresses macrophage activation and proinfammatory cytokine release84 Inversely correlated with insulin resistance, type 2 diabetes mellitus, fatty liver disease and atherosclerosis84,94 Decreased circulating levels in obesity84 |
Physiological functions under normal conditions.
Abbreviations: CCL2, CC-motif chemokine 2 (also known as monocyte chemotactic protein 1 or MCP-1); TNF, tumour necrosis factor.
Vagus nerve signalling in obesity
Obesity and obesity-related type 2 diabetes mellitus are associated with attenuation of the afferent and efferent vagus nerve signalling that is implicated in metabolic regulation. Obesity in rodents fed a high-fat diet results in leptin resistance (indicated by decreased leptin-mediated STAT3 phosphorylation) in afferent vagus neurons.31 This decrease occurs in parallel with an increase in expression of SOCS3 (an inhibitor of STAT3 phosphorylation) in nodose ganglia.31 SOCS3 expression is also induced by lipopolysaccharide. Consequently, endotoxaemia, resulting from impaired gut permeability,99 could also contribute to leptin resistance in vagus nerve afferents through activation of TLR4.31 In rodents, the progression of obesity-related type 2 diabetes mellitus is associated with attenuation of glucose-induced afferent vagus nerve signalling.100
Experimental evidence indicates that vagus nerve signalling regulating hepatic glucose production, a major determinant of blood glucose levels, is decreased in obesity. For instance, a high-fat diet causes impairment of a cholecystokinin-triggered vagus nerve reflex circuit that suppresses hepatic glucose production.57 Additionally, the fatty acid-triggered hypothalamic suppression of hepaticglucose production, which is vagus nerve-mediated,101 is attenuated by voluntary overeating.102 This attenuation has been suggested to contribute to the rapid onset of weight gain and hepatic insulin resistance,102 and has been postulated as a feature of obesity-related type 2 diabetes mellitus.101
Insulin resistance in sucrose-fed rats is associated with impaired muscarinic receptor-mediated hepatic vagus nerve signalling,103 as well as impaired baroreflex sensitivity resulting from decreased vagus nerve activity104 Vagus nerve cholinergic and muscarinic receptor-dependent signalling controls glucose-stimulated insulin secretion by pancreatic β cells, and mice with selective deficiency of the M3 muscarinic acetylcholine receptor in pancreatic β cells have suppressed insulin secretion and impaired glucose tolerance.105 Furthermore, a high-fat diet fails to induce glucose intolerance and hyperglycaemia in transgenic mice with selective overexpression of the M3 muscarinic receptor on pancreatic β cells.105
Autonomic dysfunction and diminished vagus nerve activity occur frequently in individuals with obesity and type 2 diabetes mellitus. 14–16 A 15-year follow-up study has revealed a strong relationship between autonomic dysfunction and insufficient vagus nerve activity (revealed by impaired heart rate recovery following exercise cessation), impaired glucose homeostasis and development of type 2 diabetes mellitus.17 Together, these preclinical and clinical findings support the hypothesis that diminished vagus nerve signalling in obesity could lead to enhanced inflammation and metabolic complications.
Cholinergic alleviation of obesity
Targeting cholinergic mechanisms in the inflammatory reflex using α7nAChR agonists or a centrally-acting acetylcholinesterase inhibitor could alleviate in flammation and metabolic complications in obesity.
Attenuation of inflammation
Murine adipocytes express α7nAChR, and treatment with the receptor agonist nicotine suppresses adipocyte TNF levels.19,106 This receptor is also expressed in human adipocytes and has a role in suppressing proinflammatory gene expression.107 Oral administration of the selective α7nAChR agonist TC-7020 to genetically obese Leprdb/db mice (which lack functional leptin receptors) significantly suppresses serum TNF levels. This effect is abolished by co-administration of the selective α7nAChR antagonist methyllycaconitine.18 Administration of a selective α4/α2nAChR agonist failed to suppress serum TNF levels in Leprdb/dbmice, which indicates the α7nAChR-specific nature of this effect18 Administration of nicotine to Leprdb/db mice and mice with high-fat-diet-induced obesity—in doses that did not affect food intake and body weight— significantly ameliorated inflammation in adipose tissue and the systemic proinflammatory state that is characteristic of both groups of mice.19 Nicotine administration significantly reduced levels of TNF in serum and abdominal adipose tissue, and decreased adipose tissue levels of TNF, IL-6 and IL-1β mRNAs. Nicotine also inhibited adipose tissue expression of CCL2 and the macrophage marker F4/80, indicating that macrophage infiltration and inflammation were both suppressed.19
Mice lacking α7nAChR that were fed a high-fat diet had greater M1 macrophage infiltration and higher expression of TNF and CCL2 in adipose tissue than was observed in wild-type mice.19 Additionally, expression of proinflammatory genes following exposure to free fatty acids or TNF was higher in macrophages from mice lacking α7nAChR than in macrophages from wild-type mice.19 Nicotine treatment failed to suppress this increased expression of proinflammatory genes in macro-phages from mice lacking the receptor.19 These results provide a mechanistic link between the inflammatory reflex and signalling through α7nAChR in the inhibition of obesity-associated inflammation.
One pharmacological strategy to activate the efferent cholinergic arm of the inflammatory reflex is administration of the centrally-acting acetylcholinesterase inhibitor galantamine.34 This compound enhances cholinergic muscarinic receptor-mediated signalling in the brain and stimulates efferent vagus nerve activity.34,108 Galantamine treatment of mice with high-fat-diet-induced obesity and hyperglycaemia suppressed plasma IL-6, CCL2, leptin and resistin levels.20 Importantly, galantamine treatment of obese mice lowered plasma IL-6 and CCL2 expression to levels detected in lean control mice.20 Collectively, these findings highlight that activation of components of the inflammatory reflex results in inhibition of obesity-associated inflammation.
Alleviation of metabolic complications
Nicotine administration to mice fed a high-fat diet significantly improves glucose homeostasis and insulin signalling by restoring tyrosine phosphorylation of the insulin receptor and IRS-1 in skeletal muscle, adipose tissue and liver. 19 Administration of the α7nAChR agonist TC-7020 to Leprdb/dbmice reduces weight gain, food intake and levels of blood glucose, HbA1c and triglycerides.18 When this treatment is combined with administration of the selective α7nAChR antagonist methyllycaconitine, these effects are abolished.18 In contrast to the effects of TC-7020 administration, treatment with a selective agonist targeting α4/β2nAChR, which is predominantly expressed on brain neurons, suppresses only weight gain and food intake.18 Administration of an inhibitor of JAK2 counters the effects of TC-7020 treatment on body weight, food intake and blood glucose levels, thus indicating a mechanistic link between α7nAChR and JAK2-mediated signalling. Mice lacking α7nAChR that are fed a high-fat diet have greater impairment in glucose homeostasis, insulin sensitivity, and insulin signalling in muscle and liver than do wild-type mice fed the same diet.19 In addition, the expression of human adipocyte α7nAChR (which suppresses proinflammatory responses) is significantly decreased in individuals with obesity; however, weight loss partially restores expression of α7nAChR.107 These findings further suggest a critical role for α7nAChR in maintaining metabolic homeostasis.
Galantamine treatment of mice with high-fat-diet-induced obesity reduces body weight and food intake, and selectively suppresses abdominal adiposity.20 Galantamine also lowers fasting blood glucose, plasma insulin and cholesterol levels, improves glucose homeostasis and insulin resistance, and decreases serum alanine transaminase levels, liver weight and hepatosteatosis in these mice.20 The role of brain and peripheral cholinergic activation by galantamine (acting as an acetylcholinesterase inhibitor and a positive allosteric modulator of nicotinic receptors, including α7nAChRs)109 in mediating these beneficial metabolic alterations remains to be further elucidated.
Cholinergic signalling, including via α7nAChR-mediated mechanisms in brain regions such as the lateral hypothalamus, is implicated in the complex regulation of appetite and feeding behaviour.110 Suppression of food intake following nicotine administration is thought to involve activation of α3/β4nAChR in pro-opiomelanocortin neurons of the hypothalamic arcuate nucleus, which induces activation of melanocortin 4 receptors in the hypothalamic paraventricular nucleus.111 Thus, several cholinergic receptors, including α7nAChR, α4/β2nAChR and α3/β4nAChR, have been implicated in suppressing food intake and weight gain. However, α7nAChR is unique in having been specifically linked to controlling obesity-associated metabolic complications through suppression of inflammation.
Therapeutic implications
Obesity is directly linked with the epidemic of type 2 diabetes mellitus and cardiovascular disease.112,113 Although weight loss through increased physical activity and dietary alterations can be beneficial in counteracting obesity and its adverse consequences,81 these lifestyle modifications are difficult to implement and can be problematic to sustain. Permanent weight loss and attenuation of dyslipidaemia and type 2 diabetes mellitus can be achieved through bariatric surgery, but these operations are associated with considerable risks and are currently recommended only for individuals with morbid obesity. Current pharmacological options for treatment of the metabolic syndrome are limited to targeting its individual components separately and, therefore, multiple medications are required.81,114 Accordingly, an unmet need remains for new treatments for the metabolic syndrome and type 2 diabetes mellitus, especially for drugs that target common steps in their pathogenesis.
The chronic inflammatory state associated with obesity is one such common step that could be targeted. Some anti-inflammatory approaches have already been explored in the treatment of obesity-linked disorders in preclinical and clinical scenarios.82,95 For example, patients with type 2 diabetes mellitus who were treated with a recombinant human IL-1 receptor antagonist (anakinra) experienced reductions in levels of IL-6 and CRP. Additionally, HbA1c levels in these patients were reduced and their pancreatic β-cell secretory function improved.115 Administration of salicylate—a known IKK inhibitor in rodents, which propagates proinflammatory signals—significantly improved glucose homeostasis, reduced free fatty acid levels and increased adiponectin levels in patients with type 2 diabetes mellitus.116
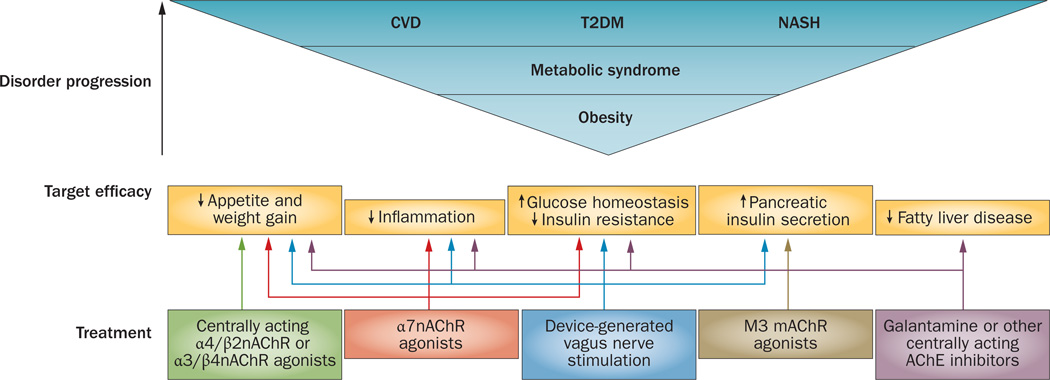
The importance of the vagus nerve in the regulation of metabolic and immune homeostasis and the efficacy of selective cholinergic modalities in alleviation of inflammation and metabolic complications indicate that specific drugs or devices that stimulate neural circuits directly to activate the inflammatory reflex could be used in the treatment of obesity-related complications (Figure 4).
Figure 4.
Possible therapies based on cholinergic-based approaches for the treatment of obesity-driven disorders. Obesity progression and the metabolic syndrome are closely associated with debilitating diseases, including T2DM, CVD and NASH. Dysregulated immune and metabolic homeostasis is associated with inflammation underlying obesity-related disease pathogenesis, which mediates insulin resistance and other complications. Efficacy of cholinergic agents in activating regulatory mechanisms in the inflammatory reflex in animals indicates a rationale for developing novel treatments for humans. Cholinergic therapeutics and devices for vagus nerve stimulation could selectively control the complex interplay between immune and metabolic pathways. Abbreviations: CVD, cardiovascular disease; M3 mAChR, M3 muscarinic acetylcholine receptor; α7nAChR, α7 nicotinic acetylcholine receptor; α4/β2nAChR, α4/β2 nicotinic acetylcholine receptor; α3/β4nAChR, α3/β4 nicotinic acetylcholine receptor; NASH, nonalcoholic steatohepatitis; T2DM, type 2 diabetes mellitus; VNS, vagus nerve stimulation.
Pharmacological approaches
The efficacy of galantamine in suppressing inflammation, insulin resistance and development of fatty liver in preclinical settings provides a rationale for further clinical studies.20 Galantamine is approved by the FDA as a treatment for Alzheimer disease, and this drug has also been used in Europe for decades in the clinical management of myasthenia gravis as well as other diseases. The availability of data related to the previous clinical use of galantamine might facilitate its utilization in obesity-driven disorders, particularly in subgroups of individuals who have limited capacity to benefit from lifestyle interventions, such as elderly people. The efficacy of galantamine in alleviating liver damage and inhibiting hepatosteatosis in rodents with obesity is a rationale for clinical studies to investigate the utility of this drug in the treatment of fatty liver diseases (Figure 4), such as nonalcoholic steato-hepatitis, which is considered to be the hepatic manifestation of the metabolic syndrome. Current treatments for nonalcoholic steatohepatitis are not efficient and the disease can progress to cirrhosis and liver cancer—new therapies are clearly needed.117
The preclinical efficacy of nAChR agonists in obesity models identify nAChRs, including α7nAChR, as molecular targets for drug development for the treatment of the metabolic syndrome and type 2 diabetes mellitus (Figure 4). Several α7nAChR agonists, including TC-7020 and GTS-21, have proven anti-inflammatory properties and the latter had good tolerability in human volunteers.118 Currently, a phase II clinical trial of the selective α7nAChR agonist TC-6987 is ongoing in patients with type 2 diabetes mellitus.119
Obesity and the metabolic syndrome are closely associated with atherosclerosis and impaired cardiovascular regulation. Donepezil, another centrally acting and FDA-approved acetylcholinesterase inhibitor, suppresses CCL2 and TNF expression, attenuates athero-sclerosis and improves long-term survival following chronic heart failure in rodents.120,121 These findings suggest that centrally acting acetylcholinesterase inhibitors could potentially be developed for the treatment of atherosclerosis.
Efferent vagus nerve-derived cholinergic activation of pancreatic insulin secretion is well documented. In addition, human pancreatic α cells secrete acetylcholine, which might act through a M3 muscarinic receptor-mediated mechanism as a paracrine stimulator of glucose-induced β-cell insulin release.122 An interesting consideration is the hypothesis that this local acetyl-choline release in the pancreas could also exert a regulatory influence on immune cells. Future studies might test the capacity of M3 muscarinic receptor agonists to promote pancreatic insulin secretion and of α7nAChR agonists to suppress pancreatic inflammation in patients with type 2 diabetes mellitus (Figure 4).
Patients taking antipsychotic medication, particularly clozapine or olanzapine, frequently develop obesity, insulin resistance and other metabolic complications. These adverse effects represent a challenging clinical problem.123 Adipose tissue inflammation could contribute to the metabolic derangements associated with antipsychotic treatment, as chronic administration of olanzapine to rodents even at a dose that does not induce significant body weight gain results in enhanced adiposity and increased adipose tissue macrophage infiltration and TNF expression.124 Furthermore, the adverse metabolic effects of antipsychotic treatments are postulated to be related to their antagonism of peripheral and central M3 muscarinic receptors and their consequent suppression of positive cholinergic effects on insulin signalling and glucose homeostasis.125,126 Using galantamine treatment to alleviate the metabolic syndrome in patients taking antipsychotics is an interesting possibility to explore. Galantamine and other centrally-acting acetylcholinesterase inhibitors have already been tested in the context of schizophrenia and shown to ameliorate selective cognitive deficits in patients with the disease.127 Intriguingly, progression of type 2 diabetes mellitus often correlates with cognitive deterioration, and new therapeutic agents for type 2 diabetes mellitus that prevent or reduce this decline in cognitive function would be advantageous.128 Treatment of patients with type 2 diabetes mellitus with galantamine, which has proven efficacy in ameliorating cognitive deficiency, could represent a promising intervention targeting this pathophysiological relationship.127
Devices for vagus nerve stimulation
Vagus nerve activity is decreased in chronic inflammatory conditions, including obesity. Weight loss resulting from increased physical activity, dieting or bariatric surgery is accompanied by reductions in levels of inflammatory markers, amelioration of metabolic complications and an increase in the vagus nerve-activity component of heart rate variability.15,129 A role for vagus nerve signalling in achieving and maintaining weight loss after bariatric surgery has also been reported.130 In view of these findings and the importance of the vagus nerve in immune and metabolic regulation, restoring or augmenting vagus nerve activity could attenuate inflammation and other conditions associated with obesity (Figure 4).
Stimulation of the vagus nerve using surgically implanted devices is in clinical use for epilepsy and depression and, as a result, the safety profile for this approach is well described.131 In patients with obesity and treatment-resistant depression, vagus nerve stimulation is associated with significant weight loss, which was positively correlated with the degree of obesity.132 These clinical findings are in agreement with those of several studies in obese rodents and minipigs, in which vagus nerve stimulation suppressed food intake and weight gain.133,134 The devices used in these studies predominantly stimulated afferent vagus nerve fibres; however, afferent and efferent vagus nerve signals converge within the dorsal vagal complex and higher brain regions, which also provide neurophysiological circuitry to activate efferent vagus nerve activity that regulates metabolic homeostasis and immune function. Thus, stimulation of afferent and efferent vagus nerve signalling has the potential for controlling the inflammatory state in obesity and re storing metabolic regulation and insulin signalling (Figure 4).
Unpublished data from our group indicate that vagus nerve stimulation suppresses insulin resistance in rodents. Vagus nerve activities regulating hepatic glucose metabolism and cardiac function are both impaired in obesity, which suggests that augmentation of efferent vagus nerve activity could be beneficial in patients with hyper glycaemia, insulin resistance and cardiovascular diseases (Figure 4). Vagus nerve stimulation is also being studied as a method to suppress cardiac inflammation in patients with heart failure.135 Additionally, this approach could be studied in patients with obesity-associated disorders, such as the metabolic syndrome and type 2 diabetes mellitus. Carefully designed clinical studies are needed to assess the potential for treatment with selective cholinergic agonists or neurostimulating devices in such patients.
Conclusions
The inflammatory reflex mediated by the vagus nerve has been successfully exploited therapeutically in preclinical models of diseases with aetiologies characterized by excessive inflammatory responses. Insufficient efferent vagus nerve cholinergic output might have a causative role in the dysfunctional immune and metabolic regulation observed in obesity, as selective activation of the efferent cholinergic arm of the inflammatory reflex attenuates both inflammation and metabolic derangements. Although cholinergic suppression of inflammation can contribute specifically to alleviating metabolic complications, direct cholinergic effects on metabolic pathways could also have a role in alleviating symptoms associated with the metabolic syndrome and type 2 diabetes mellitus. These complex interactions and the contribution of central and peripheral mechanisms in this regulation are topics of ongoing study. Additionally, intracellular mechanisms by which cholinergic signals control obesity-associated inflammation and modulate insulin signalling are under investigation. α7nAChR agonists, centrally acting acetylcholinesterase inhibitors and direct electrical stimulation of the vagus nerve offer potential therapeutic strategies for treating obesity, the metabolic syndrome, type 2 diabetes mellitus and other disorders associated with obesity. The use of cholinergic modalities in combination with existing or new therapeutic approaches to target neural, endocrine and immune functions for therapeutic benefit in patients with obesity-related disorders should also be considered.
Key points.
-
▪
The inflammatory reflex is a physiological mechanism through which the vagus nerve regulates immune function and inhibits excessive proinflammatory cytokine production
-
▪
Vagus nerve signalling has an important role in the regulation of feeding behaviour and metabolic homeostasis
-
▪
Disruption of metabolic and immune regulation in obesity results in inflammation, which mediates insulin resistance and the development of type 2 diabetes mellitus as well as other debilitating and life-threatening conditions
-
▪
Activation of cholinergic signalling in the efferent arm of the inflammatory reflex alleviates obesity-associated inflammation and metabolic derangements
-
▪
The inflammatory reflex can potentially be exploited for treatment of the metabolic syndrome, type 2 diabetes mellitus and other obesity-driven disorders
Review criteria.
Original research papers and reviews (in English) were considered for inclusion in this manuscript on the basis of the authors’ knowledge of the field and the results of PubMed and Google Scholar searches using the following keywords: “vagus”, “feeding behaviour”, “metabolism”, “obesity”, “inflammation”, “metabolic syndrome”, “type 2 diabetes mellitus”, and “cholinergic”, alone and in combination. The authors have also reviewed the reference lists of key manuscripts to identify additional relevant papers. Paper selection was limited to the past 35 years (1967–2012).
Acknowledgements
The authors’ research work is supported in part by grants from the National Institute of General Medical Sciences, NIH. The authors thank Peder S. for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare that they are inventors on patents related to the content of this manuscript. K. J. Tracey also declares an association with the following company: SetPoint Medical. See the article online for the full details of the relationships.
Author contributions
Both authors contributed equally to researching data for the article, writing the manuscript, discussions of the content, and review or editing of the manuscript before submission.
References
- 1.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ. Reflex control of immunity. Nat. Rev. Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2011;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baccala R, et al. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 5.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlov VA. Cholinergic modulation of inflammation. Int. J. Clin. Exp. Med. 2008;1:203–212. [PMC free article] [PubMed] [Google Scholar]
- 11.Bastard JP, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 12.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 14.Richter WO, Geiss HC, Aleksic S, Schwandt P. Cardiac autonomic nerve function and insulin sensitivity in obese subjects. Int. J. Obes. Relat. Metab. Disord. 1996;20:966–969. [PubMed] [Google Scholar]
- 15.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am. J. Cardiol. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler D, et al. Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp. Clin. Endocrinol. Diabetes. 2006;114:153–159. doi: 10.1055/s-2006-924083. [DOI] [PubMed] [Google Scholar]
- 17.Carnethon MR, Jacobs DR, Jr, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care. 2003;26:3035–3041. doi: 10.2337/diacare.26.11.3035. [DOI] [PubMed] [Google Scholar]
- 18.Marrero MB, et al. An α7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J. Pharmacol. Exp. Ther. 2010;332:173–180. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yang Z, Xue B, Shi H. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152:836–846. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satapathy SK, et al. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol. Med. 2011;17:599–606. doi: 10.2119/molmed.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 23.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 24.Goehler LE, et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 25.Niijima A. The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 26.Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1β: role of endogenous prostaglandins. J. Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goehler LE, et al. Interleukin-1β in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1β-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 29.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell. Mol. Life Sci. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton. Neurosci. 2005;120:104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 31.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab. 2011;301:E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guarini S, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc. Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Pavlov VA, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlov VA, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee ST, et al. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164–171. doi: 10.1016/j.brainres.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov VA, et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 37.Parrish WR, et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallowitsch-Puerta M, Pavlov VA. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;80:2325–2329. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olofsson PS, et al. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med. 2012;18:539–543. doi: 10.2119/molmed.2011.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamano R, et al. Stimulation of α7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 41.Guarini S, et al. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 42.Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 2006;34:1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 43.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 44.Rosas-Ballina M, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Bonaz B. The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology. 2007;133:1370–1373. doi: 10.1053/j.gastro.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 48.Leib C, et al. Role of the cholinergic antiinflammatory pathway in murine autoimmune myocarditis. Circ. Res. 2011;109:130–140. doi: 10.1161/CIRCRESAHA.111.245563. [DOI] [PubMed] [Google Scholar]
- 49.van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5:229–232. doi: 10.1038/nrrheum.2009.31. [DOI] [PubMed] [Google Scholar]
- 50.Koopman FA, et al. Restoring the balance of the autonomic nervous system as an innovative approach to the treatment of rheumatoid arthritis. Mol. Med. 2011;17:937–948. doi: 10.2119/molmed.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern. Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta. 2010;1802:416–431. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Owyang C, Heldsinger A. Vagal control of satiety and hormonal regulation of appetite. J. Neurogastroenterol. Motil. 2011;17:338–348. doi: 10.5056/jnm.2011.17.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kreier F, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat—functional implications. J. Clin. Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giordano A, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 56.Wang PY, et al. Upper intestinal lipids trigger a gut–brain–liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 57.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Lam CK, et al. Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J. Biol. Chem. 2010;285:21913–21921. doi: 10.1074/jbc.M109.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am. J. Physiol. 1996;271:E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 60.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1449–R1454. doi: 10.1152/ajpregu.2000.279.4.R1449. [DOI] [PubMed] [Google Scholar]
- 61.Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium–calmodulin kinase signaling in the gut–brain axis in awake rats. Neurogastroenterol. Motil. 2011;23:e282–e293. doi: 10.1111/j.1365-2982.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 63.Pocai A, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 64.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain–liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Shimazu T, Matsushita H, Ishikawa K. Cholinergic stimulation of the rat hypothalamus: effects of liver glycogen synthesis. Science. 1976;194:535–536. doi: 10.1126/science.9692. [DOI] [PubMed] [Google Scholar]
- 66.Matsushita H, Ishikawa K, Shimazu T. Chemical coding of the hypothalamic neurones in metabolic control. I. Acetylcholine-sensitive neurones and glycogen synthesis in liver. Brain Res. 1979;163:253–261. doi: 10.1016/0006-8993(79)90353-6. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz dAI, Gautam D, Guettier JM, Wess J. Novel insights into the function of β-cell M3 muscarinic acetylcholine receptors: therapeutic implications. Trends Endocrinol. Metab. 2011;22:74–80. doi: 10.1016/j.tem.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kampe J, Tschop MH, Hollis JH, Oldfield BJ. An anatomic basis for the communication of hypothalamic, cortical and mesolimbic circuitry in the regulation of energy balance. Eur. J. Neurosci. 2009;30:415–430. doi: 10.1111/j.1460-9568.2009.06818.x. [DOI] [PubMed] [Google Scholar]
- 69.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 70.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 71.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 72.Ghanim H, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundman P, et al. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutr. Metab. Cardiovasc. Dis. 2007;17:195–202. doi: 10.1016/j.numecd.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Laugerette F, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011;22:53–59. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Luyer MD, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bucinskaite V, Kurosawa M, Miyasaka K, Funakoshi A, Lundeberg T. Interleukin-1β sensitizes the response of the gastric vagal afferent to cholecystokinin in rat. Neurosci. Lett. 1997;229:33–36. doi: 10.1016/s0304-3940(97)00406-0. [DOI] [PubMed] [Google Scholar]
- 77.Gaige S, Abou E, Abysique A, Bouvier M. Effects of interactions between interleukin-1β and leptin on cat intestinal vagal mechanoreceptors. J. Physiol. 2004;555:297–310. doi: 10.1113/jphysiol.2003.054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doganay M, et al. The effects of vagotomy on bacterial translocation: an experimental study. J. Surg. Res. 1997;71:166–171. doi: 10.1006/jsre.1997.5157. [DOI] [PubMed] [Google Scholar]
- 79.Eckel RH. Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc. Nutr. Soc. 2007;66:82–95. doi: 10.1017/S0029665107005320. [DOI] [PubMed] [Google Scholar]
- 80.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 81.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab. Syndr. Relat. Disord. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 82.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2010;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 83.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 85.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 86.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 87.Nathan C. Epidemic inflammation: pondering obesity. Mol. Med. 2008;14:485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu. Rev. Med. 2011;62:361–380. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 92.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 93.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 94.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig. Dis. 2010;28:179–185. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 95.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 97.Scherer T, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol. Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 99.de La Serre CB, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J, et al. Glucose-sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;302:R657–R666. doi: 10.1152/ajpregu.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lam TK, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 102.Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J. Biol. Chem. 2004;279:31139–31148. doi: 10.1074/jbc.M400458200. [DOI] [PubMed] [Google Scholar]
- 103.Ribeiro RT, Lautt WW, Legare DJ, Macedo MP. Insulin resistance induced by sucrose feeding in rats is due to an impairment of the hepatic parasympathetic nerves. Diabetologia. 2005;48:976–983. doi: 10.1007/s00125-005-1714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller AW, Sims JJ, Canavan A, Hsu T, Ujhelyi MR. Impaired vagal reflex activity in insulin-resistant rats. J. Cardiovasc. Pharmacol. 1999;33:698–702. doi: 10.1097/00005344-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 105.Gautam D, et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo . Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 106.Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J. Pharmacol. Exp. Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- 107.Cancello R, et al. The nicotinic acetylcholine receptor α7 in subcutaneous mature adipocytes: downregulation in human obesity and modulation by diet-induced weight loss. Int. J. Obes. (Lond.) doi: 10.1038/ijo.2011.275. http://dx.doi.org/10.1038/ijo.2011.275. [DOI] [PubMed] [Google Scholar]
- 108.Waldburger JM, et al. Spinal p38 MAP kinase regulates peripheral cholinergic outflow. Arthritis Rheum. 2008;58:2919–2921. doi: 10.1002/art.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Albuquerque EX, Santos MD, Alkondon M, Pereira EF, Maelicke A. Modulation of nicotinic receptor activity in the central nervous system: a novel approach to the treatment of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2001;15(Suppl. 1):S19–S25. doi: 10.1097/00002093-200108001-00004. [DOI] [PubMed] [Google Scholar]
- 110.Jo YH, Wiedl D, Role LW. Cholinergic modulation of appetite-related synapses in mouse lateral hypothalamic slice. J. Neurosci. 2005;25:11133–11144. doi: 10.1523/JNEUROSCI.3638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mineur YS, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grundy SM. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 113.James WP. The epidemiology of obesity: the size of the problem. J. Intern. Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 114.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat. Rev. Endocrinol. 2010;6:578–588. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 116.Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin. Chem. 2011;57:162–167. doi: 10.1373/clinchem.2010.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kitagawa H, et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 119.US National Library of Medicine. 2012 doi: 10.1080/15360280801989377. ClinicalTrials.gov [online], http://clinicaltrials.gov/ct2/show/NCT01293669?term=Targacept&rank=11. [DOI] [PubMed]
- 120.Inanaga K, et al. Acetylcholinesterase inhibitors attenuate atherogenesis in apolipoprotein E-knockout mice. Atherosclerosis. 2010;213:52–58. doi: 10.1016/j.atherosclerosis.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 121.Handa T, et al. Anti-Alzheimer’s drug, donepezil, markedly improves long-term survival after chronic heart failure in mice. J. Card. Fail. 2009;15:805–811. doi: 10.1016/j.cardfail.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez-Diaz R, et al. α cells secrete acetylcholine as a non-neuronal paracrine signal priming β cell function in humans. Nat. Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Citrome L, Volavka J. Consensus development conference on antipsychotic drugs and obesity and diabetes: response to consensus statement. J. Clin. Psychiatry. 2005;66:1073–1074. doi: 10.4088/jcp.v66n0818c. [DOI] [PubMed] [Google Scholar]
- 124.Victoriano M, et al. Olanzapine-induced accumulation of adipose tissue is associated with an inflammatory state. Brain Res. 2010;1350:167–175. doi: 10.1016/j.brainres.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 125.Teff KL, Kim SF. Atypical antipsychotics and the neural regulation of food intake and peripheral metabolism. Physiol. Behav. 2011;104:590–598. doi: 10.1016/j.physbeh.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weston-Green K, Huang XF, Lian J, Deng C. Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur. Neuropsychopharmacol. 2011;22:364–373. doi: 10.1016/j.euroneuro.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Ribeiz SR, et al. Cholinesterase inhibitors as adjunctive therapy in patients with schizophrenia and schizoaffective disorder: a review and meta-analysis of the literature. CNS Drugs. 2010;24:303–317. doi: 10.2165/11530260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 128.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat. Rev. Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 129.Maser RE, Lenhard MJ, Irgau I, Wynn GM. Impact of surgically induced weight loss on cardiovascular autonomic function: one-year follow-up. Obesity (Silver Spring) 2007;15:364–369. doi: 10.1038/oby.2007.554. [DOI] [PubMed] [Google Scholar]
- 130.Bueter M, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes. Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clancy JA, Deuchars SA, Deuchars J. GL Brown Lecture ‘The Wonders of the Wanderer’. Exp. Physiol. 2012 doi: 10.1113/expphysiol.2012.064543. http://dx.doi.org/10.1113/expphysiol.2012.064543. [DOI] [PubMed] [Google Scholar]
- 132.Pardo JV, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int. J. Obes. (Lond.) 2007;31:1756–1759. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bugajski AJ, et al. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J. Physiol. Pharmacol. 2007;58(Suppl. 1):5–12. [PubMed] [Google Scholar]
- 134.Val-Laillet D, Biraben A, Randuineau G, Malbert CH. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite. 2010;55:245–252. doi: 10.1016/j.appet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 135.Schwartz PJ, et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur. J. Heart Fail. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 136.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 137.La Cava A, Matarese G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 138.Maedler K, et al. Leptin modulates β cell expression of IL-1 receptor antagonist and release of IL-1β in human islets. Proc. Natl Acad. Sci. USA. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leinonen E, et al. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166:387–394. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 141.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]