Abstract
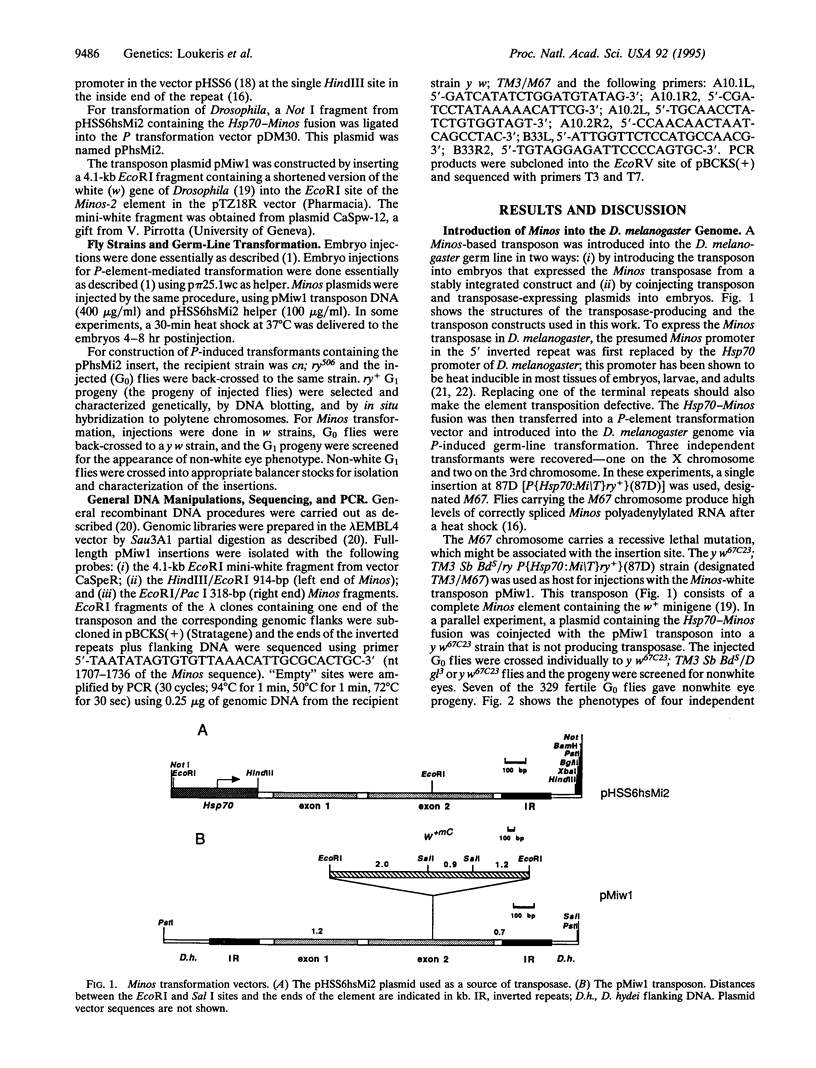
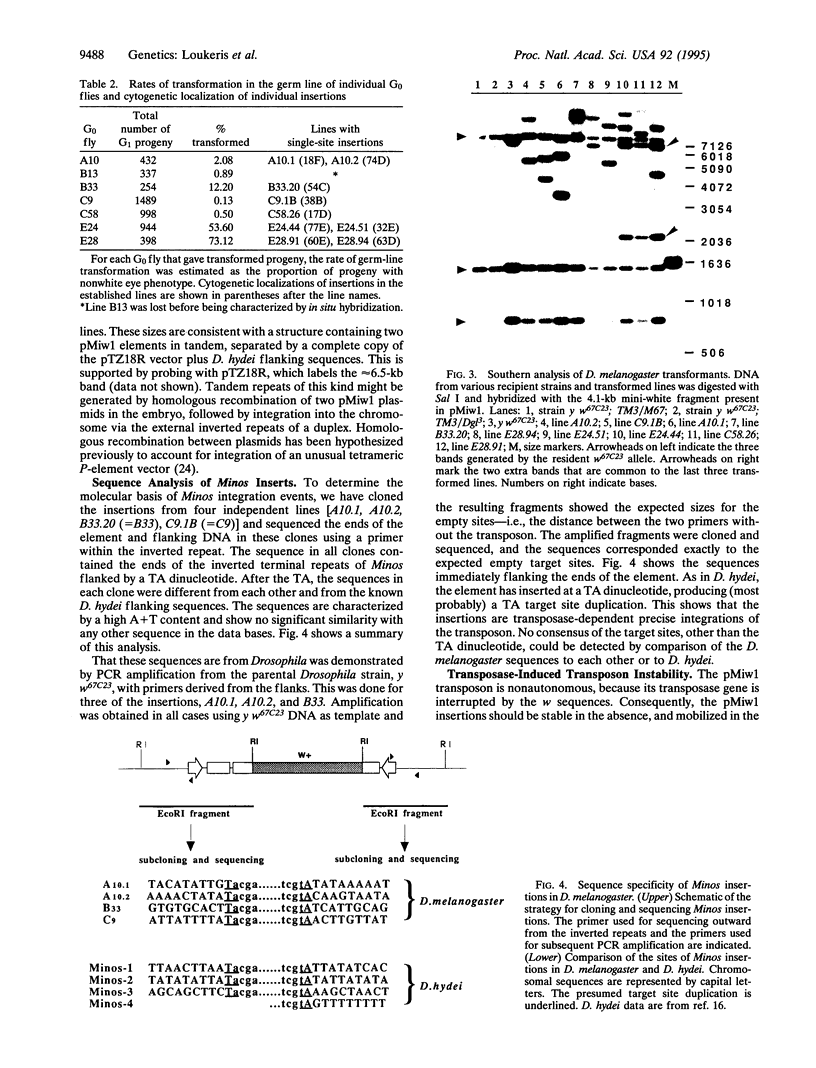
A transposon based on the transposable element Minos from Drosophila hydei was introduced into the genome of Drosophila melanogaster using transformation mediated by the Minos transposase. The transposon carries a wild-type version of the white gene (w) of Drosophila inserted into the second exon of Minos. Transformation was obtained by injecting the transposon into preblastoderm embryos that were expressing transposase either from a Hsp70-Minos fusion inserted into the genome via P-element-mediated transformation or from a coinjected plasmid carrying the Hsp70-Minos fusion. Between 1% and 6% of the fertile injected individuals gave transformed progeny. Four of the insertions were cloned and the DNA sequences flanking the transposon ends were determined. The "empty" sites corresponding to three of the insertions were amplified from the recipient strain by PCR, cloned, and sequenced. In all cases, the transposon has inserted into a TA dinucleotide and has created the characteristic TA target site duplication. In the absence of transposase, the insertions were stable in the soma and the germ line. However, in the presence of the Hsp70-Minos gene the Minos-w transposon excises, resulting in mosaic eyes and germ-line reversion to the white phenotype. Minos could be utilized as an alternative to existing systems for transposon tagging and enhancer trapping in Drosophila; it might also be of use as a germ-line transformation vector for non-Drosophila insects.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman R. K., Grimaila R., Koehler M. M., Gelbart W. M. Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell. 1987 May 22;49(4):497–505. doi: 10.1016/0092-8674(87)90452-1. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Koehler M. M., Grimaila R., Gelbart W. M. Identification of a fully-functional hobo transposable element and its use for germ-line transformation of Drosophila. EMBO J. 1989 Jan;8(1):211–217. doi: 10.1002/j.1460-2075.1989.tb03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. D., Rowan R. G., Dickinson W. J. Introduction of a functional P element into the germ-line of Drosophila hawaiiensis. Cell. 1984 Aug;38(1):147–151. doi: 10.1016/0092-8674(84)90535-x. [DOI] [PubMed] [Google Scholar]
- Caizzi R., Caggese C., Pimpinelli S. Bari-1, a new transposon-like family in Drosophila melanogaster with a unique heterochromatic organization. Genetics. 1993 Feb;133(2):335–345. doi: 10.1093/genetics/133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G., Loukeris T. G., Dialektaki G., Thompson C. R., Savakis C. Mobile Minos elements from Drosophila hydei encode a two-exon transposase with similarity to the paired DNA-binding domain. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4746–4750. doi: 10.1073/pnas.91.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G., Savakis C. Minos, a new transposable element from Drosophila hydei, is a member of the Tc1-like family of transposons. Nucleic Acids Res. 1991 Dec 11;19(23):6646–6646. doi: 10.1093/nar/19.23.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza D., Medhora M., Koga A., Hartl D. L. Introduction of the transposable element mariner into the germline of Drosophila melanogaster. Genetics. 1991 Jun;128(2):303–310. doi: 10.1093/genetics/128.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Medhora M., Maruyama K., Hartl D. L. Molecular and functional analysis of the mariner mutator element Mos1 in Drosophila. Genetics. 1991 Jun;128(2):311–318. doi: 10.1093/genetics/128.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M., MacLeod E. G. Five major subfamilies of mariner transposable elements in insects, including the Mediterranean fruit fly, and related arthropods. Insect Mol Biol. 1993;2(3):125–139. doi: 10.1111/j.1365-2583.1993.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Robertson H. M. The mariner transposable element is widespread in insects. Nature. 1993 Mar 18;362(6417):241–245. doi: 10.1038/362241a0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. Regulated expression of genes injected into early Drosophila embryos. EMBO J. 1984 Jan;3(1):165–173. doi: 10.1002/j.1460-2075.1984.tb01778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannopoulos G., Stamatis N., Monastirioti M., Hatzopoulos P., Louis C. hobo is responsible for the induction of hybrid dysgenesis by strains of Drosophila melanogaster bearing the male recombination factor 23.5MRF. Cell. 1987 May 22;49(4):487–495. doi: 10.1016/0092-8674(87)90451-x. [DOI] [PubMed] [Google Scholar]





