Abstract
Purpose
To develop a clinical eye-specific prediction model for advanced age-related macular degeneration (AMD).
Design
The Age-Related Eye Disease Study (AREDS) cohort followed for 8 years served as the training dataset and the Blue Mountains Eye Study (BMES) cohort followed for 10 years served as the validation dataset.
Participants
4,507 AREDS participants (contributing 1,185 affected vs. 6,992 unaffected eyes) and 2,169 BMES participants (69 affected vs. 3,694 unaffected eyes).
Methods
Employing Bayes' theorem in a logistic model, we used 8 baseline predictors: age, sex, education level, race, smoking status, and presence of pigment abnormality, soft drusen, and maximum drusen size, to devise and validate a macular risk scoring system (MRSS). We assessed the performance of the MRSS by calculating sensitivity, specificity, and the area under the receiver operating characteristic (ROC) curve (i.e. c-index).
Main Outcome Measures
Advanced AMD.
Results
The internally validated c-indexAREDS (0.88, 95% confidence interval (CI): 0.87 to 0.89) and the externally validated c-indexBMES (0.91, 95% CI: 0.88 to 0.95) suggested an excellent performance of the MRSS. The sensitivity and specificity at the optimal MR score cutoff point “0” were 87.6% and 73.6%, respectively. An application software (App) for iPhone/iPad was also developed as a practical tool for the MRSS.
Conclusion
The MRSS was developed and validated to provide satisfactory accuracy and generalizability. It may be used to screen patients at risk of developing advanced AMD.
Keywords: retina, retinal pigment epithelium, aging, risk assessment, epidemiology, composite score, blindness, prognosis, genetics, nutrition
INTRODUCTION
Age-related macular degeneration (AMD) is a progressive disease and its advanced forms account for over 50% of the legal blindness in the United States (US).1 Vision impairment due to advanced AMD also significantly reduces quality of life and consumes over 50% of eye care cost in Medicare budget.2 As the proportion of elderly is growing rapidly, AMD has become a major personal and global public health concern.3 In the US alone, it is estimated that the number of advanced AMD cases will reach almost 3 million in 2020.1 Unfortunately, the disease is often asymptomatic providing few warnings to alert that treatment should be sought. From a clinical point of view, early identification and close follow-up of those at high risk of developing advanced AMD are essential to allow for timely implementations of strategies to delay progression to vision compromising stages of the disease.4-7
This study aimed at developing a simple algorithm to allow clinicians to predict eye-specific risk for progression to advanced AMD. We derived such an algorithm on the basis of clinical risk factors from our analysis of two differently designed cohorts. An application software (App) for iPhone/iPad was also developed for the algorithm as a practical tool with satisfactory accuracy and generalizability.
METHODS
Study subjects
The two prospective cohort studies (Age-Related Eye Disease Study (AREDS) and Blue Mountains Eye Study (BMES), as described below) used in the current study have been conducted according to the principles expressed in the Declaration of Helsinki. This study is an analysis of pre-existing data from the two cohort studies and data were analyzed anonymously in the US. The Tufts Health Sciences Campus institutional review board (IRB) has certified the current study an exempt of IRB approval.
Training dataset-AREDS
The Age-Related Eye Disease Study (AREDS) of the National Eye Institute (NEI), National Institute of Health (NIH) is a long-term multicenter, prospective study. The objective of the AREDS was to assess the clinical course, prognosis, risk factors, and prevention strategy of AMD.8 A total of 4,757 participants, aged 55 to 80 y at recruitment, were enrolled by the AREDS investigators in 11 ophthalmic clinics throughout the US from November 1992 to January 1998. Prior to study initiation, the protocol was approved by an independent data and safety monitoring committee and by the IRB for each clinical center. Written informed consent was obtained from all participants before enrollment. All participants were required to have ≥1 eye with a visual acuity of 20/32 or better, and the lens and vitreous had to be sufficiently clear to allow good-quality retinal photographs. In addition, ≥1 eye of each participant was to be free of eye disease that could complicate assessment of AMD, and that eye could not have had previous ocular surgery (except cataract surgery and unilateral photocoagulation for AMD). Finally, potential participants were excluded for illness or disorders that would make long-term follow-up or compliance with the study protocol unlikely or difficult. The AREDS dataset is available through the database of Genotypes and Phenotypes (dbGaP) at http://www.ncbi.nlm.nih.gov/gap (last access date: April 03, 2013).
Of the available 4,757 subjects, we first excluded 6 persons with missing data on demographic factors at baseline and 244 persons lost to follow up. Of the remaining 4,507 persons, 837 persons contributed only one eye, because the fellow eye was affected with advanced AMD at baseline and was excluded. Advanced AMD was defined as neovascularization or central geographic atrophy. This left 8,177 eyes at risk of progression to advanced AMD.
Data on risk factors for AMD was obtained from a detailed questionnaire on basic characteristics and demographic data at baseline. Stereoscopic fundus photographs of the macula were taken and graded at baseline, at the 2-y visit, and annually thereafter during the 8 y (mean: 6.3 y) of follow-up using the AREDS protocol and AMD Classification System. 8, 9 An “event” of advanced AMD was defined as the occurrence of the first advanced AMD in an eye at a single visit.10 During the follow-up period, 1,185 eyes of the 8,177 eligible eyes developed advanced AMD (i.e., 6,992 eyes remained free of advanced AMD).
Validation dataset-BMES
The Blue Mountains Eye Study (BMES) was the first large population-based assessment of visual impairment and common eye diseases of a representative older Australian community sample.11 The Study was approved by the Western Sydney Area Health Service Human Ethics Committee and signed informed consent was obtained from all participants. 3,654 residents aged 49-97 were examined during the period 1992-1994 (BMES-1, an overall response of 82.4% of non-institutionalised residents). During 1997-1999, all surviving participants were invited to attend a 5-year follow up examination, for which 2,334 persons returned (75% of survivors - BMES-2). A second census of the same postcode areas was conducted in 1999, identifying a further 1,510 residents now eligible to participate of which 1,206 were examined as part of an extension study during 1999-2000 (BMES-E). Overall, 2,395 participants (65.6% of the original cohort) had at least one eye at risk of advanced AMD at baseline. Persons who died were older and more likely to have other systemic chronic conditions and disabilities. Relative to participants who were observed, surviving participants who were lost to follow-up were more likely to have been slightly younger (mean ages, 62 vs. 64; both within our reference category) and to smoke (21% vs. 13%) at baseline.12 In 2002, participants were invited to return for 10-year follow-up exams. At each examination, 30° color stereoscopic retinal photographs of the macula and other retinal fields of both eyes were taken using an FF3 fundus camera (Zeiss, Oberkochen, Germany).12 The photograph grading closely followed the Wisconsin Age-Related Maculopathy Grading System.13
We excluded 226 participants with missing bilateral ophthalmic (n=94) or demographic (n=132) data. This remained to 2,169 participants, including 1,594 participants with both eyes and 575 participants with only one eye available for analysis at baseline. Of the 3,763 eyes examined at the 10-y follow-up (mean=10.5 y), 69 eyes were found to be affected with advanced AMD (i.e. 3,694 eyes remained free of advanced AMD).
Baseline predictors
In order to maximize the practicability of our model, we only included clinically readily available risk factors as our predictors. These predictors are among the most consistent risk factors across studies.14 The 5 baseline demographic predictors collected by the baseline questionnaires of the AREDS and BMES were age, sex, education level, race, and smoking status (Table 1). Although body mass index (BMI) was considered as a potential predictor, we decided not to include it in our model because in our exploratory analysis we found that BMI does not improve the accuracy of our model and it is a secondary index calculated from height and weight.
Table 1.
The eye-specific MRSS for the prediction of advanced AMD.*
| Baseline clinical risk factor | c-indexi ‡ | Category score (MRj) |
|---|---|---|
| Intercept | −6.26 | |
| Demographic factors | 0.658 | |
| Age (yrs) | 0.605 | |
| ≤64 (referent) | 0 | |
| 65-74 (X1 ) | 0.44 | |
| ≥75 (X 2) | 0.91 | |
| Sex | 0.507 | |
| Male (referent) | 0 | |
| Female (X3) | 0.19 | |
| Education level | 0.543 | |
| College or higher (referent) | 0 | |
| High school or lower (X4) | 0.13 | |
| Race | 0.521 | |
| Non-white (referent) | 0 | |
| white (X5) | 1.30 | |
| Smoking status | 0.546 | |
| Non-smokers (referent) | 0 | |
| Past smokers (X6) | 0.05 | |
| Current smokers (X7) | 0.61 | |
| Ophthalmic factors † | 0.860 | |
| Pigment abnormalities | 0.769 | |
| None (referent) | 0 | |
| Yes (X8) | 1.57 | |
| Maximum drusen | 0.779 | |
| None-<C1 (referent) | 0 | |
| ≥C1 (X9) | 1.09 | |
| Soft drusen | 0.757 | |
| None (referent) | 0 | |
| Yes (X 10) | 1.57 |
Abbreviation: AMD, age-related macular degeneration; MRSS, macular risk scoring system; AREDS, Age-Related Eye Disease Study; MR, macular risk.
MRSS = – In (incidence in target population/2) + (−6.26) + ΣMRj; c-index=0.875. X1, X2,..., X10 were indicator variables and coded as “1” for “presence” and “0” for “absence.”
Pigment abnormalities and maximum drusen were graded within an area equivalent to within the grid used in the AREDS AMD Classification System, which is approximately a circle centered on the fovea within the venous arcades and temporal to temporal disc margin. C1=125 μm, which is approximately the typical width of a retinal vein at the disc margin.
The c-indexi was derived from the model only including the corresponding predictor(s).
The three ocular predictors used in this study have been shown to be informative in the grading of AMD severity,9 and we set them at clinical scales. The 3 eye-specific ophthalmic predictors derived from the baseline fundus pictures were the presence of pigment abnormality (hyper-pigmentation and/or depigmentation), maximum drusen size (larger or smaller than C1=125 μm, which is approximately the typical width of a retinal vein at the disc margin), and the presence of soft drusen in the retina.
Statistical analyses
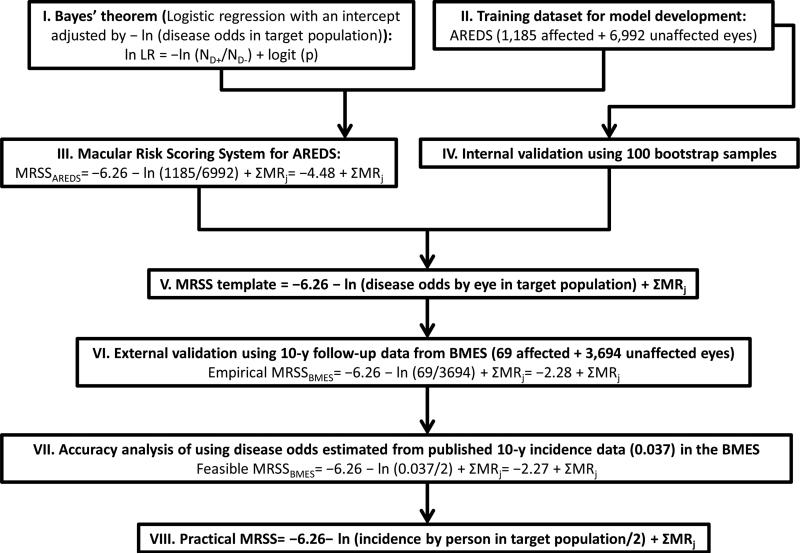
We stratified our analysis strategy into eight steps (Fig. 1). In brief, applying Bayes’ theorem in a logistic regression (I) we first used the AREDS dataset (II) to construct (III) and validate (IV) a prediction model-the macular risk scoring system for the AREDS (MRSSAREDS). We derived an MRSS template (V) from the above analysis. Next, we tested this template in the BMES dataset (VI). Since users may be less familiar with disease odds, we demonstrated the eligibility of using incidence data to estimate the disease odds in the MRSS (VII). Finally, we derived a practical form of MRSS (VIII).
Figure 1.
We stratified our analysis strategy into eight steps for the development of an eye-specific prediction model for advanced AMD.
Abbreviation: AMD, age-related macular degeneration; MRSS, macular risk scoring system; AREDS, Age-Related Eye Disease Study; BMES, Blue Mountains Eye Study.
Individual eyes were used for the development of the scoring system. To account for departures from the assumption of the independence of data points due to the correlated data resulting from repeated measurements (both eyes) on the same individual, we used non-parametric bootstrap techniques to assess the variability and internal validity of the c-index (see below for definition) by calculating its confidence intervals (CIs).15, 16 Each of our 100 bootstrap samples was obtained by random sampling with replacement from the original dataset and had the same sample size with the original dataset.16
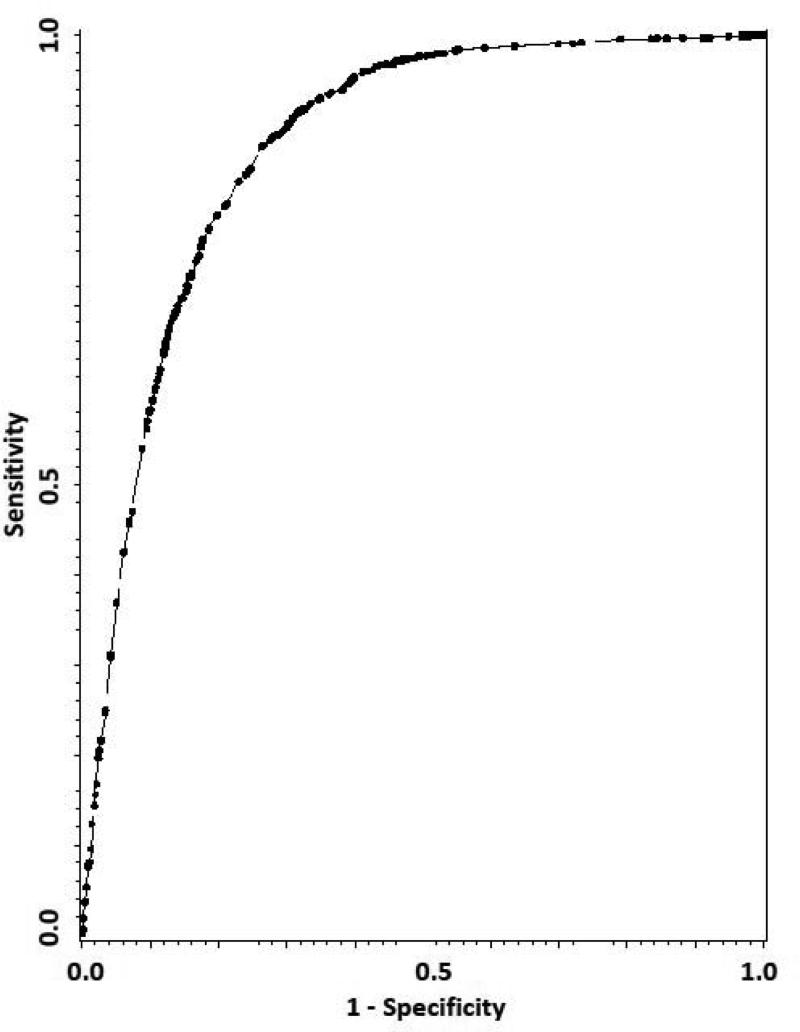
To measure how well our model correctly classifies subjects, we used the sensitivity to indicate the probability of correct classification of advanced AMD progressors, and the specificity to indicate the probability of correct classification of non-progressors at a specific macular risk (MR) score cutoff point (see step IV in RESULTS for details). While sensitivities and specificities measure the accuracy of the scoring system at various MR score cutoff points, the area under the receiver operating characteristic (ROC) curve (i.e., c-index) reflects the overall accuracy and discrimination of the prediction model. The ROC curve was graphed by plotting the sensitivity vs. (1 – specificity) at various MR score cutoff points (Fig. 2).17 From a regression point of view, the c-index also measures the predictability of the logistic regression model in Formula 1.18 In general, a c-index of over 0.85 can be rated as ‘very good.’19
Figure 2.
ROC curve of MRSS (c-index=0.88, 95% CI: 0.87 to 0.89) for the prediction of advanced AMD. Sensitivity: the probability of correct classification of advanced AMD progressors. Specificity: the probability of correct classification of non-progressors.
Abbreviation: ROC, receiver operating characteristic; MRSS, macular risk scoring system; AMD, age-related macular degeneration.
To assess calibration, we calculated the Brier score, which measures the difference between a predicted probability, which must be between zero and one, and the actual outcome, which can take on values of only 0 and 1.20 Therefore, the Brier score takes on a value between zero and one, and the lower the Brier score is, the better the model prediction is calibrated. For external validation analysis, we calculated the Hosmer-Lemeshow calibration statistic, which measures the significance of the deviation between predicted and observed outcomes.21
SAS (version 9.2; SAS Institute Inc, Cary, NC) software was used for statistical analyses.
RESULTS
Below, we describe our results by the eight steps listed in Figure 1.
- Based on Bayes' theorem, we proposed a prediction model which models the natural log (ln) likelihood ratios (LR) of developing advanced AMD by a logistic regression with an adjustment of −ln (disease odds in a target population): 22-25
We defined this ln LR as a MR score and modeled the logistic regression using our predictors (see Baseline predictors and Table 1). Therefore, the MR score can be expressed as:
where X1, X2,..., X10 were indicator variables and coded as “1” for “presence” and “0” for “absence.” Using the AREDS dataset (1,185 affected and 6,992 unaffected eyes during 8-y follow-up, i.e. ND+=1,185 and ND-=6,992), we estimated the regression intercept (MR0) and coefficients (MR1, MR2,..., MR10).
- Using the regression intercept (=−6.26) and coefficients (MR1, MR2,.., MR10 as listed in Table 1) estimated from II, the macular risk scoring system for the AREDS (MRSSAREDS) can be expressed as:
Next, to evaluate the accuracy of the MRSSAREDS, we calculated the corresponding sensitivity and specificity at various cutoff points (Table 2). The sensitivity and specificity at the theoretical optimal MR score cutoff point (i.e. where the maximal [sensitivity+specificity] appears) “0” were 87.6% and 73.6%, respectively. The area under the ROC curve (c-indexAREDS) was calculated to be 0.88 (Fig. 2). For internal validity analysis, we calculated the 100-time bootstrap estimate and its 95% CI for the c-indexAREDS to be 0.876 and (0.866 to 0.885), respectively. The Brier score for this model is 0.09. These data suggest an excellent performance and internal validity of the MRSSAREDS when applied in similar samples with the AREDS.
- Based on the data above (steps I-IV), we used the regression intercept (=−6.26) and coefficients (MRj) estimated from II to construct an MRSS template as:
The performance of prediction models when applied in samples different from the training sample is called ‘transportability’ or external validity. We used the BMES dataset (disease odds=69/3694) to evaluate the external validity of the MRSS template (Formula 2). In other words, we tested if the regression intercept (−6.26) and coefficients (MRj) estimated from the AREDS dataset are valid for advanced AMD prediction in the BMES cohort. Using the MRSS template, we constructed the MRSSBMES (=−6.26 − ln (69/3694) + ΣMRj=−2.28 + ΣMRj). We used this formula to calculate a MR score for every eligible eye in the BMES cohort according to the participant's baseline predictor information. The corresponding sensitivity/specificity for each MR score cutoff point and cindexBMES were calculated to demonstrate the overall accuracy and discriminatory ability of the MRSSBMES (Table 2). The high c-indexBMES (0.91) indicates a very good overall performance of the MRSSBMES and implies an excellent predictability of the logistic regression model developed from the AREDS dataset.
Based on both internal (step IV) and external (step VI) validation analyses, we concluded that the MRSS template (Formula 2) performs very well for the prediction of advanced AMD. However, since disease odds is less popular, we further assessed the feasibility of using “incidence by person” to estimate “disease odds by eye”. Using a previously published 10-y incidence data (=0.037) for advanced AMD in the BMES,12 we estimated the disease odds by eye to be 0.0185 (=incidence by person/2=0.037/2) and constructed a feasible MRSSBMES (=−6.26 − ln (0.0185) + ΣMRj)=−2.27 + ΣMRj, which is very close to the empirical MRSSBMES (=−2.28 + ΣMRj). Our analysis also shows that replacing “disease odds by eye” with “incidence by person/2” in the MRSS does not result in a loss in accuracy at the theoretical optimal MR score cutoff point “0” (Table 2). The Hosmer-Lemeshow statistic was 7.37 (P = 0.39) for both feasible and empirical models using the BMES data set, indicating good calibration.
- Summarizing the data above, we concluded that the most practical form of the MRSS would be:
Table 2.
Accuracy analysis demonstrates that replacing “disease odds by eye” with “incidence by person/2” in the MRSS template does not result in a loss in accuracy at the theoretical optimal MR score cutoff point “0”.*
| MR score cutoff | −0.15 | 0 | 0.15 | |
|---|---|---|---|---|
| MRSSAREDS =−4.48 + ΣMRj | Sensitivity | 88.4 | 87.6 | 81.3 |
| c-index (95% CI) = 0.88 (0.87 to 0.89) | Specificity | 72.3 | 73.6 | 78.8 |
| Empirical MRSSBMES =−2.28 + ΣMRj | Sensitivity | 89.9 | 89.9 | 87.0 |
| c-index (95% CI) = 0.91 (0.88 to 0.95) | Specificity | 69.3 | 72.9 | 80.4 |
| Feasible MRSSBMES =−2.27 + ΣMRj | Sensitivity | 89.9 | 89.9 | 87.0 |
| c-index (95% CI) = 0.91 (0.88 to 0.95) | Specificity | 69.3 | 72.9 | 80.4 |
Abbreviation: MRSS, macular risk scoring system; MR, macular risk; AREDS, Age-Related Eye Disease Study; BMES, Blue Mountains Eye Study; CI, confidence interval.
The MRSSAREDS (disease odds=1185/6992) and Empirical MRSSBMES (disease odds=69/3694) are derived from the MRSS template (=−6.26 −ln (disease odds by eye in a target population) + ΣMRj). The Feasible MRSSBMES is derived from replacing “disease odds by eye” with previously published “incidence 23 by person” divided by 2 (=0.037/2)12 in the MRSS template.
DISCUSSION
Currently only a few studies provided risk assessment for AMD but, unlike our study, none of them provided eye-specific prediction and external validation in different settings from the training cohort.26-29 Although AMD usually discordantly develops in the two eyes of a patient, all of previously published prediction models only provide risk prediction by person.26-29 The accuracy of such a model is limited by misclassification when applied in patients with only one eye affected. In a recently published study,28 which used two American trial cohorts in their model development and validation and thus may have a limited generalizability.30 However, because of the differences in study design (by-person vs. by-eye) and predictor formats, it is difficult to statistically compare different models in parallel. Using predictors that are similar to ours plus BMI and advanced AMD in one eye, Klein et al proposed a 5-year model with a c-index of 0.865.28 Seddon et al developed a 5-year model with a c-index of 0.873, which used similar predictors to ours plus BMI and antioxidant treatment.26, 29 These data indicate that with fewer predictors our model (c-index=0.876) has a similar performance to previous models. In our model development, we purposely selected clinical risk factors as our predictors which have been consistently related to AMD risk across studies. Although a model summarizing all AMD risk factors can also be used to predict the risk of advanced AMD and may be of research interest,26, 29 it has limited clinical value, because in some case collecting some of risk factor information used for the model requires additional procedures, such as administering a food frequency questionnaire for nutritional factors or genotyping for collecting genetic factors. These procedures are not routinely performed in clinics. Some of the models relay on genetic information to improve their prediction accuracy.26, 29 However, the report of the American Academy of Ophthalmology task force on genetic testing also recommends avoiding routine genetic testing for AMD and confining the genotyping of AMD patients to research studies until specific treatment or surveillance strategies have been proved to be of benefit to patients with specific AMD-associated genotypes.31 Here we show that, even without genetic information, a combination of readily available, non-invasive, clinical risk factor information provided in the patient history and eye examinations allows clinicians to do eye-specific risk prediction for advanced AMD at a comparable performance to previous models.
Our MRSS provides several additional useful features. First, by assigning a specific incidence data in the practical form of the MRSS (Formula 3), our MRSS offers the flexibility to predict the risk at different time points up to 10 years. Second, because the MR score is proportional to the hazard of developing advanced AMD for an eye (Formula 1), it allows eye-specific prediction and risk comparison between eyes from the same or different individuals. Third, using our MRSS will have utility in identifying people at high risk (i.e. those with high MR scores) for early intervention.24 Fourth, because the MR score represents the combined impact from the 8 established risk factors, it can be used to identify a homogeneous group (i.e. with a same MR score) in clinical trials or studies for new potential risk factors of AMD. Other less quantitative approaches were used for this purpose to increase statistical power and save research cost in some studies. For example, in a genome-wide association study (GWAS), the authors deliberately chose clearly defined ocular phenotypes, recruited only white individuals, and kept the proportions of males/females and smokers/nonsmokersthe same in cases and controls.32 Mainly due to this effort, the study derived a conclusive association between complement factor H (CFH) and AMD from only 96 cases and 50 controls. Finally, our software development team has developed the MRSS Apps for iPhone/iPad use in the AREDS and BMES (see Appendix 1, available at http://aaojournal.org). Using the practical MRSS (Formula 3), the App program is easily adaptable to other populations as well.
It is noteworthy that an eye-specific model may not show its advantage when applied to a low-risk population and it is most appropriate for our MRSS to replace the incidence of advanced AMD per eye with a half of the population-based disease incidence per person (Formula 3) when applied to such target populations. However, the advantage of an eye-specific model will be profound when applied to a high-risk cohort in which many patients develop bilateral advanced AMD over the prediction period. Therefore, our MRSS will also benefit from the development of spatial (geographical) epidemiology, which will provide a more accurate incidence data for advanced AMD in our MRSS.
Our MRSS also provides some simple guidelines that may be useful for making a treatment plan. For example, according to the prediction for an AREDS patient with a high risk profile in demographic characteristics (i.e. over 75-year-old, white female/male smoker with an education level of high school or less), her/his eye will not develop advanced AMD in 6 years (sensitivity=88%, specificity=74%) as long as the eye does not have pigment abnormality or soft drusen no matter if a large drusen exists or not. For a patient with the same high risk profile except never smoking or having quit smoking, having only one of the three ocular lesions in her/his eye will not render the eye to develop advanced AMD in 6 years (sensitivity=88%, specificity=74%).
As shown in Table 2, the higher the cutoff points of the MR score, the lower the sensitivity and the higher the specificity. Therefore, instead of using “0” as the cutoff point to gain the maximal (sensitivity+specificity), users may choose an appropriate cutoff point according to the purpose and situation of using the scoring system. For example, we may set a higher cutoff point for screening of a community with low advanced AMD prevalence to obtain a higher positive predictive value (the proportion of positive test results that are true positives) and fewer false positives. On the other hand, we may set a lower cutoff point for screening a high-risk group to reach a higher negative predictive value (the proportion of negative test results that are true negatives) and fewer false negatives.
Another potential concern with this study is that for our MRSS development the information for the three ophthalmic predictors was derived from standard classification systems. However, this should not hinder the utility of the MRSS since we have reduced the three ophthalmic predictors into clinical scales (Table 1). Furthermore, although different classification systems were used in the AREDS9 and BMES13, our findings that the MRSS template developed from the AREDS dataset performs very well in the BMES cohort provides an evidence that the MRSS is robust to the error. Unlike the AREDS in which the participants consisted of high risk patients, the BMES was composed of elders from a general population. Despite of the smaller case number in the BMES than in the AREDS and the difference in study design (clinic-based AREDS vs. population-based BMES) between the two cohorts, the overlap between the two 95% CIs for c-index indicates that there is no significant difference in the performance of the MRSS when applied in the two cohorts. However, although we included baseline fundus data as our predictors and treated every eye as the same by using statistical methods to address the issue of correlated eye data from a same individual, biologically or clinically speaking a disease-free eye in one person who already has AMD in the other eye is not necessarily equivalent to a disease-free eye in a second person whose both eyes are still free of AMD. Furthermore, due to the small case number in the BMES, the validity of replacing “eye-specific AMD incidence” by “person-specific incidence/2” in our MRSS needs to be further confirmed in other populations.
In conclusion, we devised a MRSS algorithm that provides a composite score summarizing clinical information for predicting the risk of advanced AMD. It may be useful to clinicians to inform patients of risk of developing advanced AMD and to guide treatment plans to prevent the disease from occurring, and to researchers for increasing study power while reducing cost.
Supplementary Material
Acknowledgments
Financial support for this project has been provided by the R01EY021826 (C-JC) from the National Institutes of Health and the USDA under agreements, 1950-5100-060-01A (C-JC, AT).
The funding sources had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
ABBREVIATIONS
- AMD
age-related macular degeneration
- App
application software
- AREDS
Age-Related Eye Disease Study
- BMES
Blue Mountains Eye Study
- BMI
body mass index
- CFH
complement factor H
- CI
confidence interval
- c-index
area under the ROC curve
- GWAS
genome-wide association study
- LR
likelihood ratio
- MR score
macular risk score
- MRSS
macular risk scoring system
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare that we have no conflict of interest. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Chung-Jung Chiu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The development of the Apps for the MRSS algorithm is an independent work from the MRSS research itself, and hence the distribution of the Apps in App Store at apple.com along with its credit should be considered as an independent interest from the MRSS research and its journal publication. The Apps are for research or investigational use only but not intended for diagnosis use.
REFERENCES
- 1.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Day S, Acquah K, Lee PP, et al. Medicare costs for neovascular age-related macular degeneration, 1994-2007. Am J Ophthalmol. 2011;152:1014–20. doi: 10.1016/j.ajo.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Dana T, Bougatsos C. Screening older adults for impaired visual acuity: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:44–58, W11-20. doi: 10.7326/0003-4819-151-1-200907070-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011;30:18–53. doi: 10.1016/j.preteyeres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu CJ, Klein R, Milton RC, et al. Does eating particular diets alter risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009;93:1241–6. doi: 10.1136/bjo.2008.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu CJ, Milton RC, Klein R, et al. Dietary compound score and risk of age-related macular degeneration in the Age-Related Eye Disease Study. Ophthalmology. 2009;116:939–46. doi: 10.1016/j.ophtha.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report number 6. Am J Ophthalmol. 2001;132:668–81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CJ, Milton RC, Klein R, et al. Dietary carbohydrate and progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2007;86:1210–8. doi: 10.1093/ajcn/86.4.1210. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia: the Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2007;114:92–8. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 14.Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007;14:184–7. doi: 10.1080/09286580701344381. [DOI] [PubMed] [Google Scholar]
- 15.Efron B. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika. 1981;68:589–99. [Google Scholar]
- 16.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 18.Royston P, Altman DG. Visualizing and assessing discrimination in the logistic regression model. Stat Med. 2010;29:2508–20. doi: 10.1002/sim.3994. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 20.Brier GW. Verification of forecasts expressed in terms of probability. Mon Weather Rev. 1950;78:1–3. [Google Scholar]
- 21.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 22.Chan SF, Deeks JJ, Macaskill P, Irwig L. Three methods to construct predictive models using logistic regression and likelihood ratios to facilitate adjustment for pretest probability give similar results. J Clin Epidemiol. 2008;61:52–63. doi: 10.1016/j.jclinepi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Irwig L. Modelling result-specific likelihood ratios [letter]. J Clin Epidemiol. 1992;45:1335–8. doi: 10.1016/0895-4356(92)90174-l. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CJ, Lee WC, Chiang CP, et al. A scoring system for the early detection of oral submucous fibrosis based on a self-administered questionnaire. J Public Health Dent. 2002;62:28–31. doi: 10.1111/j.1752-7325.2002.tb03417.x. [DOI] [PubMed] [Google Scholar]
- 25.Morise AP, Diamond GA, Detrano R, et al. The effect of disease- prevalence adjustments on the accuracy of a logistic prediction model. Med Decis Making. 1996;16:133–42. doi: 10.1177/0272989X9601600205. [DOI] [PubMed] [Google Scholar]
- 26.Seddon JM, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–53. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying GS, Maguire MG. Complications of Age-related Macular Degeneration Prevention Trial Research Group. Development of a risk score for geographic atrophy in Complications of the Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2011;118:332–8. doi: 10.1016/j.ophtha.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein ML, Francis PJ, Ferris FL, III, et al. Risk assessment model for development of advanced age-related macular degeneration. Arch Ophthalmol. 2011;129:1543–50. doi: 10.1001/archophthalmol.2011.216. [DOI] [PubMed] [Google Scholar]
- 29.Seddon JM, Reynolds R, Yu Y, et al. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011;118:2203–11. doi: 10.1016/j.ophtha.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein R, Klein BE, Myers CE. Risk assessment models for late age-related macular degeneration. Arch Ophthalmol. 2011;129:1605–6. doi: 10.1001/archophthalmol.2011.372. [DOI] [PubMed] [Google Scholar]
- 31.Stone EM, Aldave AJ, Drack AV, et al. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology Task Force on Genetic Testing. Ophthalmology. 2012;119:2408–10. doi: 10.1016/j.ophtha.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 32.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.