Abstract
Objective
An increase in ectopic lipids in peripheral tissues has been implicated in attenuating insulin action. The botanical extract of Artemisia dracunculus L. (PMI-5011) improves insulin action, yet the precise mechanism is not known. We sought to determine whether the mechanism by which the bioactive compounds in PMI-5011 improve insulin signaling is through regulation of ceramide metabolism
Methods
L6 Myotubes were separately preincubated with 250uM palmitic acid with or without PMI-5011 or four bioactive compounds isolated from PMI-5011 and postulated to be responsible for the effect. The effects on insulin signaling, ceramide and glucosylceramide profiles were determined.
Results
Treatment of L6 myotubes with palmitic acid resulted in increased levels of total ceramides and glucosylceramides, and cell surface expression of gangliosides. Palmitic acid also inhibited insulin-stimulated phosphorylation of protein kinase B/Akt and reduced glycogen accumulation. Bioactives from PMI-5011 had no effect on ceramide formation but one active compound (DMC-2) and its synthetic analogue significantly reduced glucosylceramide accumulation and increased insulin sensitivity via restoration of Akt phosphorylation.
Conclusions
The observations suggest that insulin sensitization by PMI-5011 is partly mediated through moderation of glycosphingolipid accumulation.
Keywords: Glycosphingolipids, Akt phosphorylation, insulin resistance, botanicals
Introduction
Skeletal muscle insulin resistance is a major contributor to reduced whole body glucose disposal in obesity and type 2 Diabetes Mellitus. A strong correlation between insulin resistance and lipid accumulation in tissues is observed with weight gain, prolonged physical inactivity, and/or systemic hyperlipidemia [1]. Exposure of muscle to excessive lipids leads to accumulation of triacylglycerols (TAGs), diacylglycerols (DAGs), and ceramides which initiate pathways leading to the inactivation of various insulin signaling intermediates [1–3]. In a previous study using L6 myotubes exposed to high levels of free fatty acids (FFAs) we confirmed the role of ceramides but not DAGs and TAGs in attenuating insulin signaling by inhibiting protein kinase B (Akt-1 and Akt-2) phosphorylation [3]. In this regard, interventions that improve insulin sensitivity such as pharmaceutical agents i.e., thiazolidinediones and lifestyle interventions i.e., exercise, have been shown to lower muscle ceramide levels in humans and rodents [4,5].
Plants have traditionally been a rich source of medicinal compounds considered safe as complementary therapies for many indications, including diabetes. The ethanolic extract of the perennial herb Artemisia dracunculus L. or Russian tarragon (termed PMI-5011) has been shown to significantly decrease blood glucose levels in genetic and chemically induced murine models of diabetes and improve insulin sensitivity [3, 6]. In our previous study [3] we reported that this botanical modulated the role of ceramides on insulin signaling but the mechanism involved was not secondary to an effect on the formation or accumulation of ceramides. With PMI 5011 intervention, we observed increased insulin signaling via preservation of insulin-stimulated Akt-1 and Akt-2 phosphorylation despite the presence of high ceramide levels in cells.
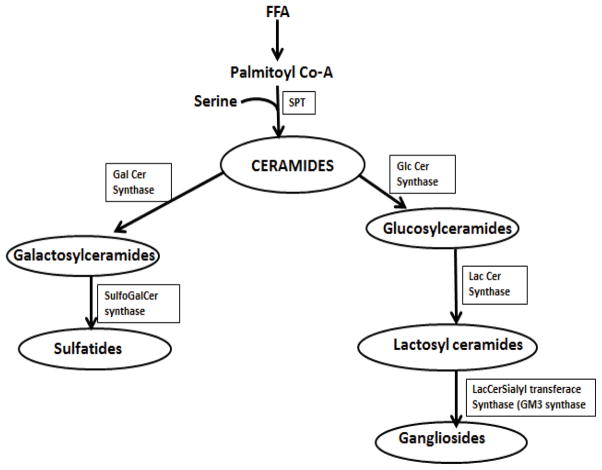
Sphingolipid metabolism is complex and involves hundreds of molecular species and metabolic pathways. Ceramide provides the platform for the synthesis of more complex sphingolipids and leads to the formation of sphingosine, sphingosine 1-phosphate, ceramide 1-phosphate, sphingomyelin and the glycosphingolipids [glucosylceramides, galactosylceramides, lactosylceramides, sulfatides and gangliosides] [7,8]. In this study we focused on the glycosphingolipid pathway and how perturbations in this pathway contribute to aberrant insulin resistance and the role of PMI-5011 bioactives in preventing accumulation of lipid intermediates. To explore the role PMI-5011 compounds on the glycosphingolipid pathway (Figure 1) in relation to insulin signaling, we used tandem mass spectrometry and flow cytometry with monoclonal antibodies to quantify the intracellular level of glucosylceramides and cell surface expression of ganglioside GM3, respectively, in rat skeletal muscle cells in the presence of excess free fatty acids and PMI-5011 bioactives. Our data demonstrates that despite significant ceramide accumulation, in the presence of excess free fatty acids, the bioactive compounds in PMI-5011 preserve insulin sensitivity. While ceramide levels remained unchanged, one specific bioactive compound i.e., 2′,4-dihydroxy-4′-methoxydihydrochalcone (DMC 2) and its synthetic analogue (Fig 2) downregulated the expression of glucosylceramide synthase (GCS) the enzyme that catalyses the transfer of the carbohydrate moiety from a sugar-nucleotide, uridine 5-diphosphate(UDP)-glucose to the ceramide resulting in lower glucosylceramide levels in the cells. Using an inhibitor of GCS (D threo PDMP) and interference RNA (RNAi) we show that lowering of glycosphingolipids, without significant reduction of ceramides, reverses insulin resistance. Based on these observations, we postulated that PMI 5011 inhibition of glycosphingolipid synthesis is one of the mechanisms by which this botanical in increases insulin sensitivity in vitro and in vivo.
Figure 1.
Demonstrates ceramide metabolism pathway and cellular targets for PMI 50111 compounds
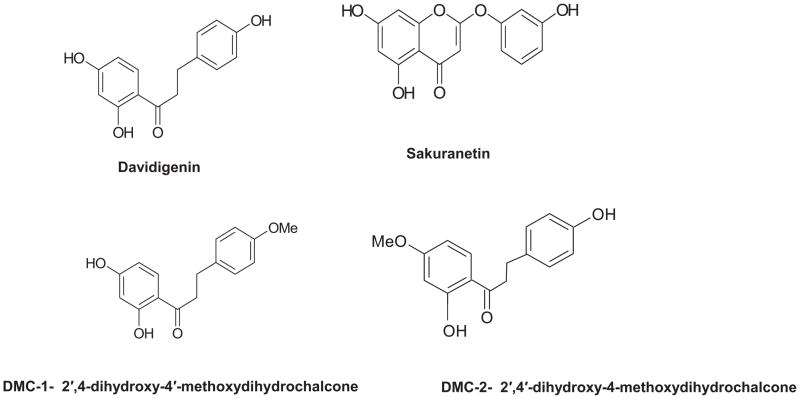
Figure 2.
Demonstrates structure of four proposed bioactive compounds isolated from PMI 5011 and evaluated in current study
Materials and Methods
Source and characterization of PMI-5011 and active compounds
The study used a well characterized ethanolic extract of Artemisia dracunculus L., called PMI-5011, as well as four bioactive compounds previously identified and isolated from PMI-5011 using bioactivity guided fractionation: davidigenin, sakuranetin, 2′,4′-dihydroxy-4-methoxydihydrochalcone (DMC-2) and 2′,4-dihydroxy-4′-methoxydihydrochalcone (DMC-1) as previously reported [6,9–11]. A chemically synthesized version of DMC-2 was also tested.
Standards and reagents
Ceramide and glucosylceramide standards were purchased from Avanti Polar Lipids (Alabaster, AL). (±)–threo-1-phenyl-2 decanoylamino-3-morpholino-1-propanol hydrochloride (PDMP) and palmitic acid were obtained from Sigma-Aldrich. The predesigned RNAi for UDP-glucose ceramide glucosyltransferase (GCS) and its forward and backward primers were obtained from Ambion ‘Life Technologies (Carlsbad, CA).
Cell Culture and treatments
L6 myoblasts were obtained from the American Type Culture Collection and maintained at 37°C, 95% air, and 5% CO2 in low glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% CBS serum and antibiotics. For individual experiments, myoblasts were subcultured onto 6 or 12 well plates, grown to 80–90% confluence, and differentiated into fused myotubes for 5 days by switching to media with 2% horse serum. All cells used were within five passages.
Cultures were exposed to 250 μM palmitic acid conjugated with 1% BSA. PMI-5011 extract and each of the five test compounds were added separately at a final dose of 10ug/ml. For insulin signaling studies, myotubes were serum-starved in DMEM containing 0.2% BSA. Insulin was added at a final concentration of 100 nmol/L for 10 min before subsequent analysis. The GCS inhibitor PDMP at doses of 10 and 20uM and RNAi targeting GCS expression were used as positive controls to assess the effect of GCS expression and activity on Akt phosphorylation. Cells in the process of differentiation after 1 day (90–95% confluence) were transfected with 2.5 μg DNA and Lipofectamine 2000 at a ratio of 1:2.5 in 6-well plates in reduced serum media for 48 hours. Media was then switched to that with 2% horse serum for 4 days before treating the cells for 16 h with FFA, with or without PMI-5011 compounds.
Insulin signaling parameters and protein expression
Insulin sensitivity was evaluated by determining glycogen accumulation in basal and insulin stimulated states with use of glycogen hydrolysis and glucose determination by the method described by Gomez et al. [12]. Akt-1, Akt-2, GCS and GM3 synthase protein contents and phosphorylation were determined by standard Western blotting procedures. Specific antibodies used were anti–AKt-1 and -2, AKt-1 phos Ser 473 (Millipore, Temecula, CA), anti AKt-2 phos Ser 474 (Bioworld, St Louis MN), anti GCS (Abbiotec, San Diego, CA) and anti β-actin and GM3 synthase both from Santa Cruz Biotechnology, Santa Cruz, CA. In all cases, Western blot results were verified in three separate experiments.
Gene expression for GCS
For gene expression of GCS, the same treatments above were performed. Total RNA was purified using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA total content and quality was determined spectrophotometrically by absorbance measurements at 260 and 280 nm using the NanoDrop system (NanoDrop Technologies). cDNAs were synthesized for each sample by reverse transcription following manufacturer’s protocol (Qiagen) and were used for PCR reactions and amplification. A gene-specific commercial primer for GCS (Ambion ‘Life technologies (Carlsbad, CA) and SYBR Green PCR master mix (Qiagen) were used. Real time PCR was analyzed by the Sequence Detection System (Rotor Gene 6000). Amounts of the GCS were normalized to the amount of β-actin mRNA as an internal control.
Lipid extraction and Quantitative analysis of ceramides and glucosylceramides
After 24 h incubation with treatments, cells grown in 6 well plates were collected with 100 μL ice-cold deionized water and subjected to a double extraction for lipids and processed as previously shown [3]. Liquid chromatography-electrospray ionization tandem-mass spectrometry (LC-MSMS) was used to measure intracellular levels of ceramide and glucosylceramides as previously described [3]. According to the retention times of standards, common product ion and ions reflecting fatty acid substituents, all target compounds were quantified (Supplementary data, Table 2).
Cell surface expression of ganglioside GM3
After treatments with FFA and PMI-5011 compounds, cell surface expression of GM3 was examined by flow cytometry (BD FACSCalibur flow cytometer) after indirect staining. Cells were collected in PBS containing 2% BSA and incubated with GM3 monoclonal antibody (Santa Cruz Bio. Dallas, TX) at a concentration of 1:100 for 1 h at 4°C. After washing twice, cells were incubated with Fluorescein isothiocyanate labelled goat anti rabbit IgG-FITC secondary antibody (Santa Cruz Bio. Dallas, TX) for 30 minutes at 4°C washed twice, and resuspended in 0.5 ml PBS. Cell suspensions were used for flow cytometry.
Statistical analysis
All data are presented as means ± SE. Difference between means was determined by ANOVA with subsequent Fisher’s protected least significant difference tests or Student’s t test. P < 0.05 was considered significant. All results are an average of three experiments.
RESULTS
Effect of FFA and PMI 5011 compounds on glycogen content and insulin signaling
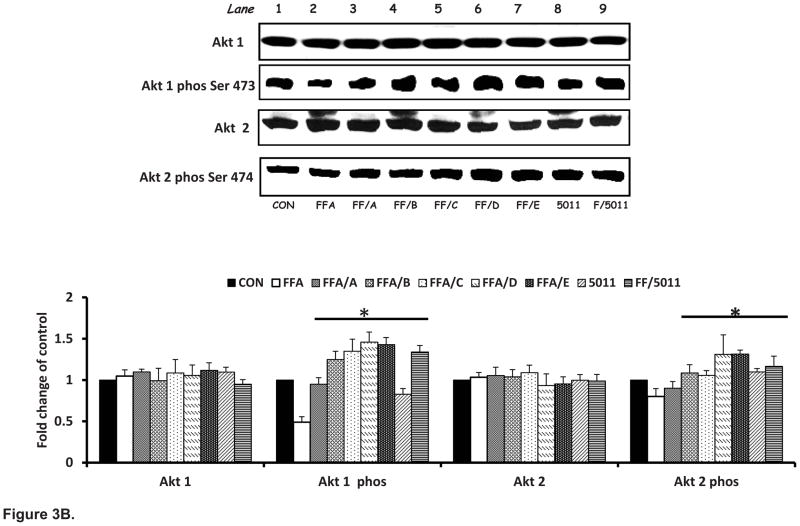
Insulin stimulation resulted in a threefold increase in glycogen content compared with basal conditions. Palmitic acid resulted in a 30% decrease in glycogen content (Figure 3A). Incubation with PMI-5011 and the bioactive compounds DMC-1 (C) and DMC-2 (D) and synthetic DMC-2 (E) in the presence of FFA resulted in significant increased glycogen content when compared with incubation of FFA only treatment (P < 0.05; Fig 3A). The effect of PMI-5011 and the five different compounds on insulin stimulated Akt-1 and -2 phosphorylation in the presence of FFAs is shown in Figure 3B. As demonstrated, isolated (D) and synthetic DMC-2 (E) showed the highest stimulation of Akt 1 and Akt 2 phosphorylation.
Figure 3. PMI 5011 and isolated compounds enhance glycogen synthesis (A) and Akt Phosphorylation (B) in presence of excess FFAs.
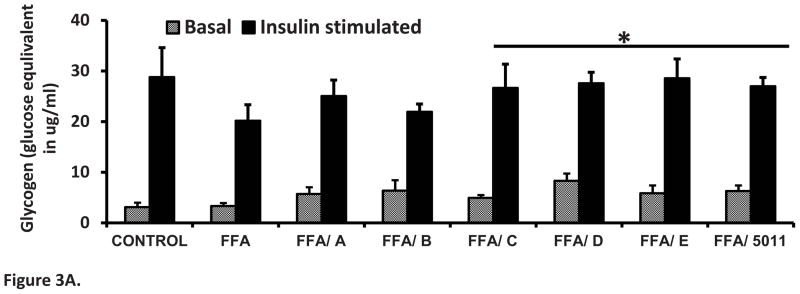
Figure 3A: Rat skeletal muscle cells were treated with 250uM FFAs with or without 10 μg/ml of PMI 5011 or each of the five isolated compounds for 16 hours. Glycogen content was measured by the method of Gomez et al. [12]. PMI 5011 and bioactives C, D and E enhanced insulin stimulated glycogen synthesis to levels not different from that of the control treatment but significantly different from that of the FFA only treatment. Bioactives studied were: davidigenin (A), sakuranetin (B), DMC-1 (C), DMC-2 (D) and synthetic DMC-2 (E). * P < 0.05 vs. FFA only treatment. All experiments were conducted in triplicate.
Figure 3B: Rat skeletal muscle cells were treated with 250uM palmitic acid with or without 10 μg/ml of whole PMI 5011 or each of the isolated compounds for 16 hours before insulin stimulation for 10 minutes. Western blotting showed that palmitic acid attenuated the phosphorylation of Akt-1 and -2 post insulin stimulation, whereas incubation with PMI 5011 or isolated compounds increased Akt phosphorylation significantly when compared to incubation FFA alone (see lane 2 vs. lanes 3 to 9). A) davidigenin, (B) sakuranetin. (C) DMC-1, (D) DMC-2 and E synthetic DMC-2. The bar graphs summarize the results of quantification of gel plots from three experiments. *P < 0.05 vs. FFA only treatment. All experiments were conducted in triplicate.
Effect of PMI-5011 compounds on Glucosylceramide synthase (GCS) and GM3 synthase expression
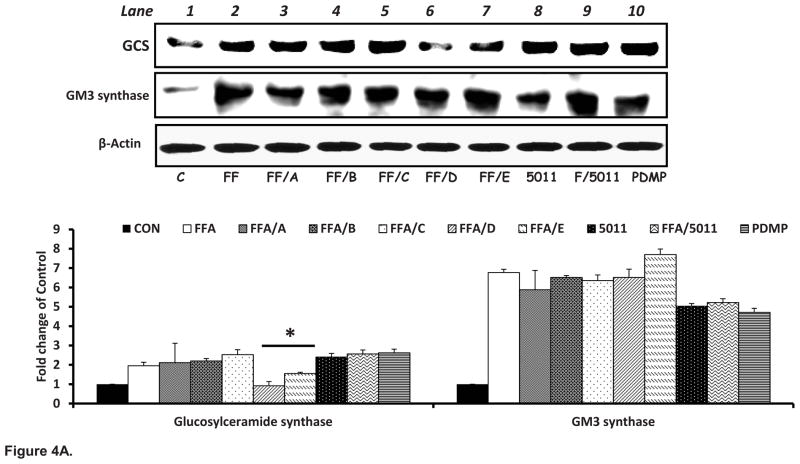
Compared to control, palmitic acid significantly increased the protein content of GCS two fold and increased protein content of GM3 synthase 6 fold (Fig 4A lane 1 vs 2). Both the isolated DMC-2 (D) and synthetic DMC-2 (E) decreased the protein content of GCS in presence of free fatty acids (Fig 4A, lanes 6 & 7). PMI-5011 and all isolated compounds had no effect on expression of GM3 synthase in presence of elevated FFAs.
Figure 4. PMI 5011 compounds downregulate the protein content of Glucosylceramide synthase (A) and mRNA expression (B) in presence of excess lipids.
Figure 4A: Rat skeletal muscle cells were treated with 250uM palmitic acid with or without 10 μg/ml of whole PMI 5011 or each of the isolated compounds for 16 hours. Separate wells were treated with PDMP the inhibitor of GCS. Palmitic acid significantly increased the expression of GCS and GM3 synthase (see lane 1 vs lane 2). DMC-2 isolated from PMI 5011 and synthetic DMC-2 (Compounds D and E) downregulated the expression of GCS but not GM3 synthase in the presence of excess FFAs (see lanes 6 and 7 vs lane 2). The bar graphs summarize the results of quantification of gel plots from three experiments. *P < 0.05 vs. FFA only treatment. All experiments were conducted in triplicate.
Figure 4B: Rat skeletal muscle cells were treated with 250uM palmitic acid with or without 10 μg/ml of whole PMI 5011 or each of the isolated compounds for 24 hours. Separate wells were treated with 20uM PDMP the inhibitor of GCS. Real time PCR on extracted RNA showed that palmitic acid upregulated GCS expression by one fold while PDMP resulted in increased GCS expression by 0.5 fold. DMC-2 (compounds D and E) downregulated the mRNA of GCS despite presence of excess lipids. The bar graphs summarize the results of from three experiments. * P < 0.05 vs. FFA only treatment.
Effect of PMI-5011 Compounds and D-threo PDMP on GCS mRNA gene expression
FFA increased GCS mRNA expression 1.25 fold (P<0.05) compared to control cells (Fig 4B). In presence of excess FFAs, DMC-2 (compounds D and E) each reduced GCS gene expression to levels significantly different from those of FFA only treated cells (p<0.05). Although PMI 5011 reduced GCS mRNA in FFA treated cells the expression level was not significantly different from that of FFA only treated cells (p=ns). D threo PDMP, the inhibitor of GCS activity, was used as a positive control and it significantly increased GCS mRNA by 0.5 fold in the absence of FFAs compared to the control but PMI-5011 did not.
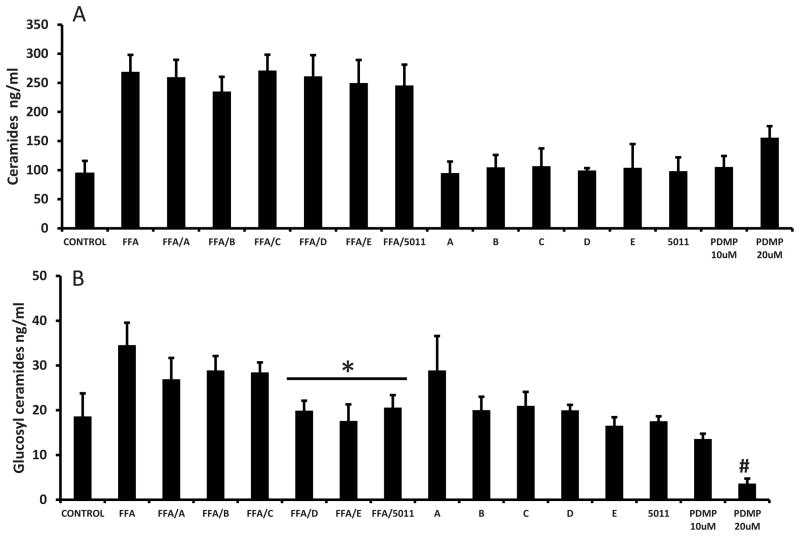
Effect of FFA and PMI-5011 compounds on ceramide and ceramide metabolites
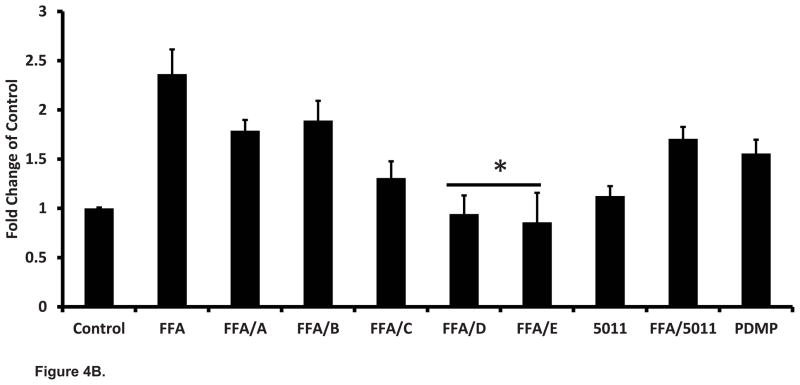
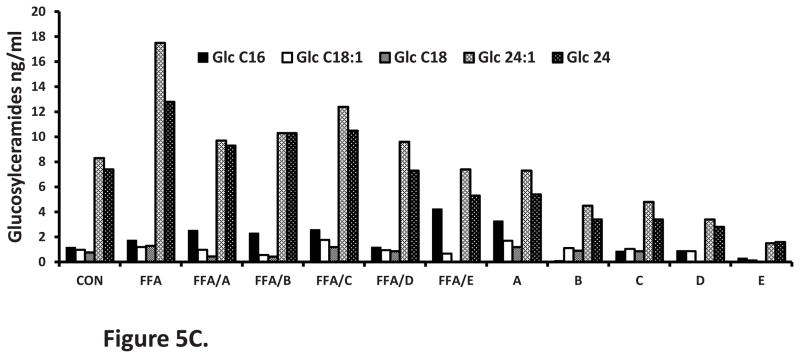
Treatment with palmitic acid resulted in a more than two fold increase in total ceramides (Fig 5A), a one fold increase in glucosylceramides (Fig 5B), and more than two fold increase in the surface expression of ganglioside GM3 (Table 1). As expected neither PMI-5011 or any of the isolated compounds altered total ceramide content when incubated with palmitic acid compared to palmitic acid alone (P = ns, Figure 5A). All ceramide species remained elevated (data for specific species not shown). In the absence of palmitic acid, PDMP (20uM) the inhibitor of GCS, increased the quantity of ceramides by 48%. Treatment of cells with PMI 5011 and both the isolated and synthetic DMC-2 (compounds D and E), resulted in significantly lower levels of total glucosylceramides (P<0.05) (Fig 5B) and ganglioside GM3 surface expression (Table 1) despite the presence of excess lipids. Glucosylceramides C24 and C24:1 were the most dominant species (Fig 5C) in all samples. PDMP (20uM) decreased glucosylceramide content by 80.6% (Figure 5B) and ganglioside surface expression decreased by almost 60% (Table 1).
Figure 5. PMI 5011 and ceramide profiles.
Figure 5A: PMI 5011 compounds do not prevent the accumulation of ceramides in presence of excesss lipids. Rat skeletal muscle cells were treated with 250uM palmitic acid with or without 10 μg/ml of whole PMI 5011 or each of the isolated compounds for 24 hours. Separate wells were treated with 20uM PDMP the inhibitor of GCS. Liquid chromatography/tandem mass spectrometry (LCMS/MS) showed that therewas no statistically significant difference in total ceramide levels between FFA only treated cells and those treated with FFA together with any of the five compounds (P=ns vs FFA only treatment). In absence of FFAs, the PMI 5011 compounds and PDMP did not significantly change ceramide levels
Figure 5B: PMI 5011 compounds reduce the accumulation of glucosylceramides in presence of excesss lipids.. PMI 5011 and DMC 2 (compounds D, and E resulted in statistically lower amounts of glucosylceramides as compared to the FFA only treated cells. *P < 0.05 vs. FFA only treatment.
Figure 5C: Glucosylceramide profile. Glucosylceramide C24:1 and C24 were the most predominant species. C18 was the lowest and was below detectable limits in some treatments. *P < 0.05 vs. FFA only treatment. A) davidigenin) (B) sakuranetin (C) DMC-1 and (D) DMC-2 and E synthetic DMC-2.
Table 1.
Effect of PMI-5011 compounds on surface expression of ganglioside GM3 in L6 cells pretreated with palmitic acid using flow cytometry (fluorescence activated cell sorter analysis).
| GM3 Fluorescence Relative intensity | |
|---|---|
| Control | 1.00 |
| FFA | 2.34 |
| FFA/A | 2.18 |
| FFA/B | 1.98 |
| FFA/C | 2.25 |
| FFA/D | *1.56 |
| FFA/E | *1.67 |
| FFA/PMI 5011 | *1.69 |
| D-threo PDMP 10uM | 0.41 |
| D-threo PDMP 20uM | 0.39 |
Data are presented as mean ± SEM.
P < 0.05 vs. FFA only treated; N = 3 assessments for each treatment.
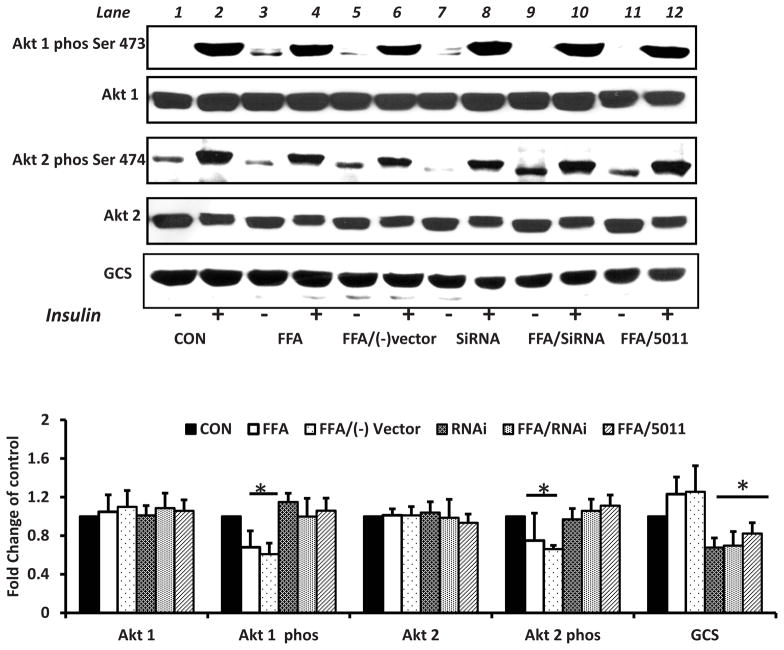
Effect of GCS RNAi on insulin sensitivity
To assess effect of GCS expression levels on AKt phosphorylation, RNAi was used to down regulate GCS expression. When compared with L6 myotubes incubated with FFA alone, RNAi transfection lowered GCS levels in L6 myotubes incubated with FFA and increased insulin stimulated Akt 1 and Akt 2 phosphorylation (see lanes 4 and 6 vs. lane 10 Fig 6). The application of PMI-5011 to cells treated with FFA also lowered GCS protein content and increased insulin stimulated Akt1 and Akt 2 phosphorylation (see lane 4 vs lane 12 Fig 6).
Figure 6. Downregulating the expression of GCS using RNAi or PMI 5011 enhances Akt phosphorylation in presence of excess FFAs.
Rat skeletal muscle cells were treated with 250uM palmitic acid with or without 10 μg/ml of whole PMI 5011 for 16h before insulin stimulation. Replicate wells were treated with FFAs after transfection with RNAi for glucosylceramide synthase (GCS) or the negative vector.. FFAs downregulated Akt phosphorylation (lane 2 vs lane 4). Cells transfected with RNAi had enhanced Akt phosphorylation despite presence of excess FFAs (lane 4 vs lane 10). PMI 5011 downregulated GCS expression and resulted in enhanced Akt phosphorylation in the prescence of excess FFAs (lane 4 vs lane 12). The bar graphs summarize the results of quantification of gel plots from three experiments. *P < 0.05 indicates a significant difference for each parameter compared with the FFA only treated cells.
DISCUSSION
Ceramide is the precursor for the synthesis of complex sphingolipids. Aside from the formation of sphingosine, sphingosine 1-phosphate and ceramide 1-phosphate, ceramide leads to the synthesis of sphingomyelin and the glycosphingolipids which include gangliosides [8]. This study focused on the role of glycosphingolipids in regulating insulin stimulated glucose metabolism and the role of the bioactive compounds of PMI-5011 on the accumulation of these metabolites in rat skeletal muscle cells. It was previously that shown that PMI-5011 intervention, in presence of excess ceramides, increased insulin sensitivity via increased insulin-stimulated Akt-1 and Akt-2 phosphorylation without a significant reduction of ceramide formation or accumulation [3]. However, those results could not preclude that PMI-5011 may have some effects on downstream metabolism of ceramides into forms that are less or more deleterious to insulin signaling, while still maintaining high overall ceramide levels. Although the bioactives do not prevent ceramide formation we hypothesized that they preserve insulin sensitivity in presence of elevated ceramides by preventing their metabolism into complex glycosphingolipids that are also lipotoxic in insulin sensitive tissues. This could occur if PMI-5011 down regulates glucosylceramide synthase and/or GM3 synthase, hence reducing the accumulation of glycosphingolipids downstream. Our results show that a specific bioactive from PMI-5011 i.e DMC-2 significantly down-regulated the protein content of GCS but not GM3 synthase in presence of excess FFAs and resulted in overall lower levels of glucosylceramides in the cells without a significant change in ceramide levels. All seven ceramide species remained elevated in the presence of free fatty acids and PMI-5011 compounds. Using the GCS inhibitor PDMP and GCS RNAi as positive controls, we further show that when glucosylceramide levels are low, the cells remain insulin sensitive despite the presence of elevated ceramides.
The sphingolipid pathway is complex and its impact on insulin resistance and type 2 diabetes remain unresolved. Recent evidence suggests a key role for excessive glycosphingolipids in the etiology of obesity-induced insulin resistance [13–17]. This study focused on the effect of PMI-5011 bioactives on glucosylceramide synthase (GCS) and GM3 synthase. The transfer of glucose from UDP-glucose to ceramide by GCS is the first step in glycosphingolipid synthesis [17, 18]. GM3 is a downstream product of glucosylceramides and the simplest ganglioside. Its synthesis by GM3 synthase is the first major step in the formation of most other gangliosides. GM3 has been shown to induce insulin resistance and is a potential therapeutic target for type 2 diabetes [8,19]. While ceramides and glucosylceramides interfere with insulin signaling at the level of Akt phosphorylation, gangliosides modulate the activity of some receptor tyrosine kinases, including the insulin receptor [8,19]. A strategy for sphingolipid metabolism-based interventions for the treatment of Insulin resistance would be to develop drugs that suppress the excessive accumulation of metabolites that induce insulin resistance, an approach used for other metabolic disorders [16].
Glucosylceramides and gangliosides are constituents of micro domains (lipid rafts) which are necessary for correct insulin signaling by facilitating and regulating protein–protein interactions between ligands, receptors and kinases. A perturbed lipid raft composition impairs insulin signaling and overall signal transduction at the cell surface [18]. Lipid rafts are highly enriched in glycosphingolipids and cholesterol which fills the space between the hydrocarbon chains of the sphingolipids. Changes in glucosylceramide levels or ganglioside levels impair the functionality of rafts in insulin target cells, thereby promoting insulin resistance [20–23]. It has been shown that lipid microdomains play a crucial role in triggering the phosphatidylinositol-3 kinase/Akt signaling pathway by facilitating Akt recruitment and activation [24]. Upregulation of glucosylceramide offers cellular protection and primes certain cells for proliferation but, prolonged overabundance is detrimental [10].
While there are several studies that have focused on role of botanical compounds on insulin resistance and Type 2 diabetes, there is no research focusing on plant compounds that inhibit accumulation of ceramide metabolites in cells and tissues. PMI-5011 decreases blood glucose in vivo and enhances insulin sensitivity as a mode of action [6, 25]. It has also been shown to increase glucose uptake, restore glycogen accumulation and mitigate inflammation in insulin sensitive cells [3, 9]. While the cellular mechanisms by which PMI-5011 improves insulin sensitivity and mitigates inflammation are not yet fully elucidated, past studies have shown that the activity is the combined result of at least 4 different bioactive compounds that demonstrate at least 3 different activities. For the active compounds used in this study, davidigen was shown to inhibit aldose reductase, sakuranetin to inhibit protein tyrosine phosphatase 1B activity while the chalcone derivatives, DMC-1 and DMC-2, inhibited the expression of phosphoenolpyruvate carboxykinase overexpression [9,10,25].
The previous study exploring the role of PMI-5011 intervention on the sphingolipid pathway, showed no effect on ceramide accumulation but, did not explore the effect of PMI- 5011 on ceramide metabolite accumulation. In this study we explore if the mechanism of this botanical involves the metabolism of ceramides into other lipotoxic metabolites. To accomplish our objectives, we used tandem mass spectrometry and flow cytometry to quantify ceramides and glucosylceramides and GM3 surface expression on L6 cells. In agreement with other reports [15], we demonstrated a consistent increase in lipid metabolites, i.e., ceramide, glucosylceramide, and cell surface expression of ganglioside GM3 with FFA incubation. We report that one of the mechanisms by which PMI 5011 improves insulin action was secondary to an effect on the formation or accumulation of glucosylceramides. While ceramides levels remained significantly unchanged, glucosylceramides and the surface expression of ganglioside GM3 were significantly lowered in cells treated with both FFA and the specific bioactive DMC-2 obtained PMI 5011. The resultant formation of lower amounts of GM3 on the surface of cells probably resulted from a lower level of glucosylceramides which are required for GM3 synthesis. Consistent with previous studies [14], PDMP prevented glucosylceramide accumulation and increased insulin sensitivity. Our results also agree with a report using animal models to show a link between altered levels of gangliosides and insulin resistance [15]. Other studies showed that preventing GM3 formation increased insulin sensitivity and prevented TNF-α from attenuating insulin signaling [17,19]. It is important to note that although the other three active compounds did not reduce GCS expression, they enhanced insulin signaling as evidenced by an increase in AKt phosphorylation and in some cases, glycogen synthesis. This is likely because these compounds enhance insulin action through other pathways as previously shown [10,11]. Lower glucosylceramides and ganglioside GM3 surface expression resulted in higher insulin sensitivity in the cells as shown by higher Akt 1 and Akt 2 phosphorylation levels and glycogen synthesis.
Using the GCS inhibitor PDMP and RNAi targeting GCS expression as the positive controls, we show that despite presence of high ceramide levels after FFA treatment, insulin sensitivity increases when the formation of glucosylceramides is inhibited. Based on these observations, we postulated that the inhibition of glycosphingolipid synthesis by bioactives of PMI-5011 is one of the mechanisms employed by this botanical for increasing insulin sensitivity. The presence of relatively high ceramide levels in cells that remain insulin sensitive has prompted some researchers to question whether ceramide is a contributor to insulin resistance or not [26, 27]. Skovbro et al [26] found that skeletal muscle ceramide content was similar between individuals showing marked differences in insulin sensitivity suggesting that ceramides alone are not a major factor in muscle insulin resistance. Our data suggests that ceramide metabolites and not ceramides themselves are responsible for insulin resistance.
CONCLUSION
Perturbations in sphingolipid metabolism contribute to the pathogenesis of insulin resistance. A growing body of literature supports the contention that the bioactive compounds from Artemisia dracunculus L. restore insulin sensitivity in type 2 diabetes. Although the mechanism of action is not fully defined in molecular terms, this study showed that the bioactive compound DMC-2 from PMI-5011 alters glycosphingolipid composition in muscle cells by down-regulating the expression of the enzyme responsible for glucosylceramide synthesis. Not just ceramide, but rather its glycosphingolipid metabolites are instrumental in the development of insulin resistance. Here we show that lowering of glycosphingolipids, without a significant reduction of ceramide, reverses insulin resistance in vitro. PMI-5011 is a promising botanical that we show has an effect on expression of proteins involved in sphingolipid metabolism and the levels of metabolites responsible for initiating insulin resistance. Glucosylceramide synthase regulates the activity of nearly all glycosphingolipids and it inhibition by botanicals, such as PMI-5011, may be useful strategy for treatment of insulin resistance.
Supplementary Material
Acknowledgments
Funding source: This work was supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, which funds the Botanical Research Center of Pennington Biomedical Research Center and the Biotech Center of Rutgers University.
List of Abbreviations and synonyms
- PMI-5011
Artemisia dracunculus L.
- FFA
Free Fatty acids
- TAGs
Triglycerides
- DAGs
Diglycerides
- CER
Ceramide
- DMC-1
2′,4-dihydroxy-4′, methoxydihydrochalcone
- DMC-2
2′,4′-dihydroxy-4-methoxydihydrochalcone
- SPT
Serine Palmitoyl tranferase
- GCS
Glucosyl ceramide synthase
- GM3
Ganglioside monosialosyllactosylceramide
- PBS
Phosphate buffered saline
- BSA
Bovine serum albumin
- RNAi
interference RNA
- PDMP
(±) –threo-1-phenyl-2 decanoylamino-3-morpholino-1-propanaol hydrochloride
- cDNA
complementary DNA
- PCR
Polymerase chain reaction
Footnotes
Author’s contributions: D.N.O designed the study and performed experiments and wrote a draft of this manuscript. D.R cultivated the plant, obtained the extract and isolated and identified the active compounds and reviewed the manuscript, I. R was involved in botanical identification and preparation, W.T.C was involved in study design, data analysis and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Precursor ions for sphingolipids are shown in Table 3. Ceramides, glucosylceramides all had the common product ion 264.2. According to the retention times, the individual long-chain fatty acids were quantified.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Obanda DN, Cefalu T. Regulation of Insulin signaling in Rat skeletal muscle cells by lipid metabolites and the role of PTP1B Activity. J Nutr Biochem. 2013;24 (8):1529–37. doi: 10.1016/j.jnutbio.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obanda DN, Hernandez A, Ribnicky D, Yu Y, Zhang X, Wang ZQ, Cefalu T. Bioactives of Artemisia dracunculus L. mitigate the Role of Ceramides in Attenuating Insulin Signaling in Rat Skeletal Muscle Cells. Diabetes. 2012;61:597–605. doi: 10.2337/db11-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planavila A, Alegret M, Sánchez RM, Rodríguez-Calvo R, Laguna JC, Vázquez-Carrera M. Increased Akt protein expression is associated with decreased ceramide content in skeletal muscle of troglitazone-treated mice. Biochem Pharmacol. 2005;69:1195–1204. doi: 10.1016/j.bcp.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Helge JW, Dobrzyn A, Saltin B, Gorski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol. 2004;89:119–127. doi: 10.1113/expphysiol.2003.002605. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZQ, Ribnicky D, Zhang XH, et al. An Extract of Artemisia dracunculus L. enhances insulin receptor signalling and modulates gene expression in skeletal muscle in KKay mice. J Nutr Biochem. 2011;22(1):71–8. doi: 10.1016/j.jnutbio.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kabayama K, Kabayama K, Sato T, Kitamura F, Uemura S, Kang BW, Igarashi Y, Inokuchi J. TNF alpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology. 2004;15:21–29. doi: 10.1093/glycob/cwh135. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZQ, Ribnicky D, Zhang XH, Raskin I, Yu Y, Cefalu WT. Bioactives of Artemisia dracunculus L enhance cellular insulin signaling in primary human skeletal muscle culture. Metabolism Clinical and Experimental. 2008;57(Suppl 1):S58–S64. doi: 10.1016/j.metabol.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logendra S, Ribnicky DM, Yang H, Poulev A, Ma J, Kennelly EJ, Raskin I. 2006. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry. 2006;67(14):1539–46. doi: 10.1016/j.phytochem.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. 2006. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550–7. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Lechon MJ, Ponsoda X, Castell JV. A microassay for measuring glycogen in 96-well-cultured cells. Anal Biochem. 1996 May 1;236:296–301. doi: 10.1006/abio.1996.0170. [DOI] [PubMed] [Google Scholar]
- 13.Langeveld M, Aerts JMFG. Glycosphingolipids and insulin resistance. Review. Prog Lipid Res. 2009;88:196–205. doi: 10.1016/j.plipres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst van Ejik AM, Dubbelhuis PF, Aten J, Kuipers F, Serlie MJ, Wennekes T, Sethi JK, O’Rahilly S, Overkleeft HS. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Przybylska M, Wu I-H, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH. Inhibiting Glycosphingolipid synthesis Improves Glycemic Control and Insulin Sensitivity in animal Models of Type 2 Diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 16.Cox T, Lachmann R, Hollak C, Aerts J, van Weely S, Hrebícek M, et al. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355(9214):1481–5. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 17.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 18.Fuller M. Sphingolipids: the nexus between Gaucher disease and insulin resistance. Lipids Health Dis. 2010;9:113. doi: 10.1186/1476-511X-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 21.Kasahara K, Sanai Y. Functional roles of glycosphingolipids in signal transduction via lipid rafts. Glycoconj J. 17(3–4):153–62. doi: 10.1023/a:1026576804247. [DOI] [PubMed] [Google Scholar]
- 22.Brown D. Structure and function of membrane rafts. Int J Med Microbiol. 2002;291 (6–7):433–7. doi: 10.1078/1438-4221-00150. [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and Regulation of Akt/PKB by the rector mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 24.Lasserre R, Guo X-J, Conchonaud F, Hamon Y, Hawchar O, et al. Rafts contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Biol. 2008;4(9):538–547. doi: 10.1038/nchembio.103. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt B, Ribnick DM, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism Clinical and Experimental. 2008;57(Suppl 1):S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skovbro M, Baranowski M, Skov-Jensen C, Flint A, Dela F, Gorski J, Helge JW. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia. 2008;51:1253–1260. doi: 10.1007/s00125-008-1014-z. [DOI] [PubMed] [Google Scholar]
- 27.Boden G. Ceramide: a contributor to insulin resistance or an innocent bystander? Diabetologia. 2008;51:1095–1096. doi: 10.1007/s00125-008-1015-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.