Abstract
Mechanical forces have been proposed to modulate organ growth, but a molecular mechanism that links them to growth regulation in vivo has been lacking. We report that increasing tension within the cytoskeleton increases Drosophila wing growth, whereas decreasing cytoskeletal tension decreases wing growth. These changes in growth can be accounted for by changes in the activity of Yorkie, a transcription factor regulated by the Hippo pathway. The influence of myosin activity on Yorkie depends genetically on the Ajuba LIM protein Jub, a negative regulator of Warts within the Hippo pathway. We further show that Jub associates with α-catenin, and that its localization to adherens junctions and association with α-catenin are promoted by cytoskeletal tension. Jub recruits Warts to junctions in a tension-dependent manner. Our observations delineate a mechanism that links cytoskeletal tension to regulation of Hippo pathway activity, providing a molecular understanding of how mechanical forces can modulate organ growth.
INTRODUCTION
Elucidating the mechanisms that regulate growth to generate organs of correct size and proportion remains a fundamental goal of developmental biology. Much attention has focused over recent decades on intercellular signaling pathways that modulate growth, and many such pathways have been identified and characterized. However, mechanical forces can also influence proliferation of cultured cells (Curtis and Seehar, 1978; Huang and Ingber, 1999), and models incorporating a role for physical forces in modulating growth during development have been proposed (Aegerter-Wilmsen et al., 2007; 2012; Hufnagel et al., 2007; Shraiman, 2005). While it seems clear that cells in developing organs must experience mechanical forces, it has been difficult to assess the potential contribution of these forces to the regulation of growth, because a mechanistic understanding of how mechanical forces are connected to molecular processes that control growth has been lacking. Here, we describe a molecular mechanism that links tension in the apical actin cytoskeleton to the growth-regulatory Hippo signaling pathway.
The Hippo pathway was discovered through tumor suppressor mutations in Drosophila, but is now known to be a crucial regulator of both normal and oncogenic growth across a range of species, including humans (Harvey et al., 2013; Staley and Irvine, 2012; Yu and Guan, 2013). The central core of the pathway comprises the protein kinase Warts (Wts, LATS in mammals) and its substrate Yorkie (Yki, YAP and TAZ in mammals) (Figure 1A). Yki is a transcriptional co-activator protein, and Wts inhibits Yki by promoting its cytoplasmic localization (Oh and Irvine, 2010). Several distinct regulatory mechanisms can impinge on Wts, including pathways that influence the activity of kinases that promote (Hippo) or inhibit (Minibrain) Wts activity (Degoutin et al., 2013; Hamaratoglu et al., 2006; Yu et al., 2010), pathways that influence Wts stability (Fat) (Cho et al., 2006), pathways that influence Wts localization (Merlin) (Yin et al., 2013), pathways that act through Wts-binding proteins (Jub) (Thakur et al., 2010), and pathways that act through as yet ill-defined mechanisms. Several of these Wts-regulatory mechanisms have been linked to cell-cell junctions or intercellular signaling pathways, and these connections implicate Hippo signaling as an integrator of diverse inputs regulating organ growth (Enderle and Mcneill, 2013; Yu and Guan, 2013).
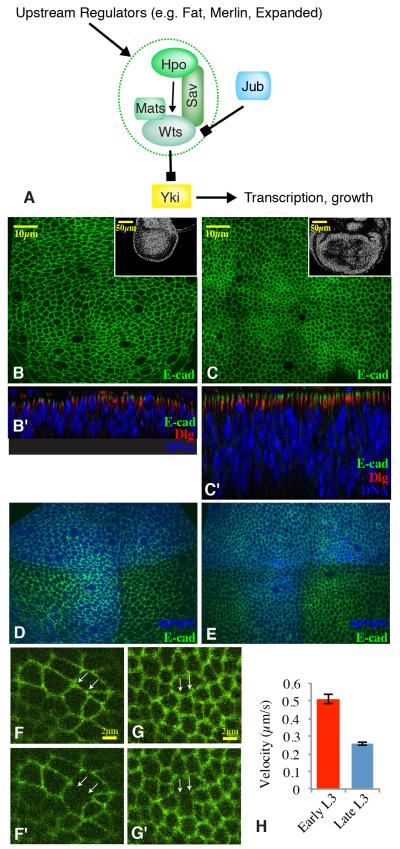
Figure 1. Cytoskeletal tension declines during wing development.
A) The central core of the Hippo pathway comprises the kinases Hpo and Wts, which negatively regulate the transcriptional co-activator Yki. B,C) Horizontal (upper) and vertical (lower, marked by prime symbols) confocal sections through younger (B) or older (C) third instar wing discs, stained for E-cad (green), DNA (blue), and Discs large (Dlg, red). Insets show lower magnification views of DNA staining (white) with the entire wing pouch visible. D,E) Confocal sections through live younger (D) or older (E) third instar wing discs, expressing for E-cad:GFP (green), and UAS-BFP (blue) marking dorsal and A-P boundary cells. F,G) High magnification views of the discs in D and E, 1 second before and 8 seconds after laser cutting of cell junctions between the white arrows. See Supplemental material for movies. H) Quantitation of mean displacement velocities of vertices adjacent to cut junctions within the first 300 ms after cutting. Averages are based on 36 (older) or 37 (younger) cuts, error bars show sem. See also supplemental movies.
Recently, evidence for regulation of Yki, YAP, and TAZ by mechanical forces has been described. This includes differences in localization and transcriptional activity of YAP and TAZ when cultured cells are grown under conditions of varied attachment to the extracellular matrix or varied cell density, or after genetic or pharmacological perturbations of the actin cytoskeleton (Aragona et al., 2013; Dupont et al., 2011; Fernández et al., 2011; Sansores-Garcia et al., 2011; Wada et al., 2011; Zhao et al., 2012). Some studies of cultured cells indicated that YAP/TAZ activity could be influenced by mechanical perturbations in an actin-dependent fashion independently of LATS activity, with the actual mechanism remaining elusive (Aragona et al., 2013; Dupont et al., 2011). Other studies have identified influences on YAP/TAZ that depend on the actin cytoskeleton and are mediated by LATS, but how LATS was regulated is unknown (Wada et al., 2011; Zhao et al., 2012). Similarly, studies in Drosophila have reported that accumulation of F-actin can increase Yki activity through an unknown mechanism that is nonetheless sensitive to Wts (Fernández et al., 2011; Sansores-Garcia et al., 2011). Additionally, Zyxin, a protein known to be regulated by mechanical forces in mammalian cells (Hirata et al., 2008), was found to participate in Fat pathway regulation of Yki activity in Drosophila (Rauskolb et al., 2011), but so far no evidence for participation of Zyxin in mechanical regulation of Yki activity has been described. While these studies suggest that mechanical regulation of Yki could occur, absent an understanding of the molecular mechanism by which this is achieved, it has not been possible to assess its role and significance in vivo.
Here, we utilize the developing wing imaginal discs of Drosophila to investigate regulation of Yki activity by mechanical tension. Wing discs are clusters of undifferentiated epithelial cells that will give rise to the wing and notum of the fly. They have long been used as models for studies of growth and patterning during development, including investigations of the role and regulation of Hippo signaling. We describe a molecular mechanism that links tension in the actin cytoskeleton to organ growth through the regulation of Hippo signaling. We report that Yki activity is sensitive to cytoskeletal tension within the developing wing disc. This regulation of Yki requires Jub, a negative regulator of Wts activity within the Hippo pathway. We show that Jub localization is regulated by cytoskeletal tension, and implicate α-catenin as a mechanotransducer responsible for Jub localization. We also describe Wts localization in vivo, and show that Jub interacts with Wts in a tension-dependent fashion. Our studies thus delineate a molecular mechanism linking mechanical tension to the regulation of Yki activity through the Hippo pathway.
RESULTS
Cytoskeletal tension declines during wing development
A characteristic feature of Drosophila wing development is that as the wing disc grows, cells appear to become more compressed in the central region that will give rise to the wing blade (Aegerter-Wilmsen et al., 2012). This is visible through the transition to progressively more columnar cells within the wing pouch (Fig. 1B,C). This change in cell architecture is intriguing in light of models that have proposed that mechanical compression could contribute to reduced disc growth at the end of larval development (Aegerter-Wilmsen et al., 2007; 2012; Hufnagel et al., 2007; Shraiman, 2005). Relative cytoskeletal tension can be estimated by the recoil of intercellular vertices after laser cutting of cell junctions (Farhadifar et al., 2007). Recent applications of this technique in wing discs demonstrated that peripheral cells are more stretched than cells near the center of the wing pouch (Legoff et al., 2013; Mao et al., 2013). We applied this technique to compare cytoskeletal tension in younger (~84 h, mid-third instar) versus older (~120 h, late third instar) wing discs. Cell junctions were visualized using GFP-tagged E-cadherin (E-cad:GFP) (Fig. 1D,E). We estimated relative position and avoided cells at or near compartment boundaries, which are known to exhibit increased tension (Aliee et al., 2012; Landsberg et al., 2009; Major and Irvine, 2006), using BFP-expressing transgenes that mark compartment boundaries, and also restricted our analysis to cell junctions roughly perpendicular to the proximal distal axis. These studies revealed that from mid- to late third instar, during which growth rates decrease (Bryant and Levinson, 1985; Martín et al., 2009; Wartlick et al., 2011), there is a decrease in cytoskeletal tension (Fig. 1F-H, Supplemental movies). The results obtained by laser cutting of cell junctions, indicating a higher tension in younger versus older wing discs, and a higher tension in the periphery versus the center, are consistent with inferences of tissue compression by stress-birefringence in wing discs (Nienhaus et al., 2009).
Cytoskeletal tension modulates wing growth
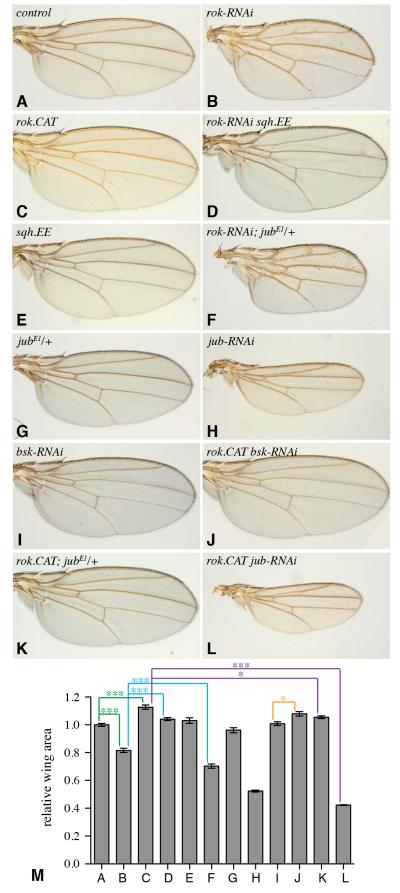
To examine the possibility that cytoskeletal tension could influence growth in vivo, we manipulated the activity of the major non-muscle myosin, Myosin II (Myo II), which controls tension within the actin cytoskeleton. Myo II activity is regulated by Rho-associated protein kinase (ROCK), encoded in Drosophila by the Rho-kinase gene (rok). ROCK phosphorylates the regulatory light chain of Myo II (Sqh, encoded in Drosophila by spaghetti squash, sqh) to increase myosin contractility (Amano et al., 1996; Winter et al., 2001). Although dynamic myosin activity is essential for multiple cellular processes, cells can tolerate modest global decreases or increases in myosin activity. We manipulated myosin activity by expressing UAS transgenes under the control of nub-Gal4, which drives the expression throughout the developing wing (Fig. S1F). Decreasing ROCK levels using RNAi resulted in flies with smaller wings (Fig. 2A,B,M). Conversely, increasing ROCK activity by expressing an activated form of ROCK comprising the catalytic domain (ROCK.CAT) resulted in flies with larger wings (Fig. 2C,M). To confirm that the effect of rok RNAi on wing size was mediated through the influence of ROCK on Sqh, we co-expressed an activated form of Sqh (Sqh.EE) containing phosphomimetic Ser to Glu mutations at regulatory sites normally phosphorylated by ROCK (Bertet et al., 2009; Winter et al., 2001). Expression of Sqh.EE reversed the reduced wing growth associated with rok RNAi (Fig. 2D,M). Our observations are consistent with a recent report that physically stretching wing discs could stimulate cell proliferation (Schluck et al., 2013).
Figure 2. Cytoskeletal tension modulates wing growth.
A-L) Adult wings from flies expressing nub-Gal4 UAS-dcr2 and A) control, B) UAS-rok-RNAi, C) UAS-rok.CAT, D) UAS-rok-RNAi UAS-sqh.EE, E) UAS-sqh.EE, F) UAS-rok-RNAi jubE1/+, G) jubE1/+, H) UAS-jub-RNAi, I) UAS-bsk-RNAi, J) UAS-rok.CAT UAS-bsk.RNAi, K) UAS-rok.CAT jubE1/+, L) UAS-rok.CAT UAS-jub.RNAi. M) Mean wing areas for the indicated genotypes (lettered according to genotypes displayed in figure panels above), calculated from 9 to 15 wings per genotype. Error bars indicate sem, statistical significance of selected pairwise combinations are indicated by colored lines. See also Fig. S1.
Cytoskeletal tension modulates Yki activity
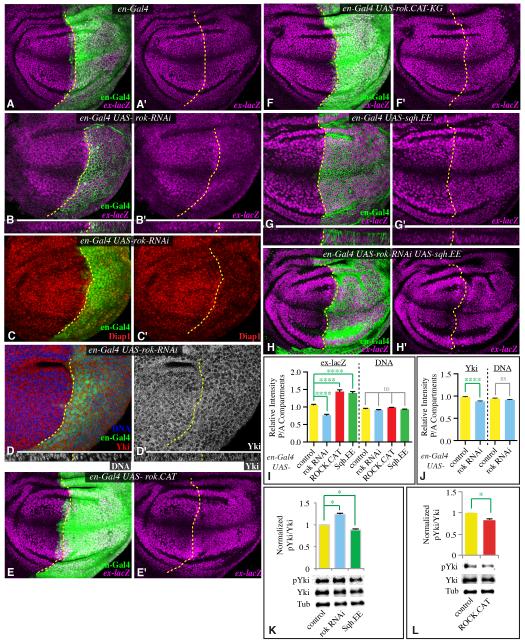
To investigate whether the modulation of wing growth caused by changes in ROCK activity is associated with altered Hippo signaling, we assayed markers of Yki activity in developing wing discs. For these experiments we expressed transgenes under the control of en-Gal4, which drives expression in posterior cells. In this situation, anterior cells, which do not express transgenes, serve as an internal control. Reduction of ROCK levels in posterior cells by RNAi reduced growth within wing discs (Fig. S1B,E), and reduced the expression of a direct transcriptional target of Yki, ex-lacZ (Fig. 3B, I). Another target of Yki activity, Diap1, was also reduced in posterior cells by rok RNAi (Fig. 3C). We also examined the subcellular localization of Yki protein, as activity of Yki is correlated with its nuclear localization. Reduction of ROCK was associated with a slight decrease in nuclear Yki (Fig. 3D, J). As a further test, we quantified phosphorylation of Yki at the major Wts site (Ser168) (Dong et al., 2007; Oh and Irvine, 2008) by Western blotting. In whole wing disc lysates with rok RNAi expressed under nub-Gal4 control, the relative fraction of phosphorylated Yki was increased (indicating decreased Yki activity) by 24% (Fig. 3K), which, as the nub domain comprises roughly half of the wing disc (Fig. S1F), understates the increased Yki phosphorylation within rok RNAi-expressing cells.
Figure 3. Cytoskeletal tension modulates Yki activity.
A-H) Third instar wing imaginal discs expressing en-Gal4 UAS-dcr2 UAS-GFP or UAS-RFP (green) and A) control B-D) UAS-rok-RNAi, E) UAS-rok.CAT, G) UAS-rok.CAT-KG, H) UAS-sqh.EE, I) UAS-sqh.EE UAS-rok-RNAi, stained for expression of ex-lacZ (magenta), Diap1 (red), DNA (blue/white) or Yki (red/white), as indicated. Lower panels for B, D, and G show vertical sections through the same discs. Dashed yellow line marks A-P compartment boundary. Panels marked prime show individual stains of discs to the left. I) Quantitation of ex-lacZ expression in discs of the genotypes depicted here in panels A, B, E, and G, represented as the mean intensity of nuclear ß-gal staining in P compartment regions divided by mean intensity of nuclear ß-gal staining in A compartment regions; mean DNA staining intensity per nuclear volume is also shown for comparison (N=22 (wild type), 22 (rok RNAi), 10 (ROCK.CAT), 30 (Sqh.EE)). J) Quantitation of Yki in en-Gal4 UAS-dcr2 UAS-rok-RNAi UAS-GFP discs, represented as the mean intensity of nuclear Yki staining in P compartment regions divided by mean intensity of nuclear Yki staining in A compartment regions; mean DNA staining intensity per nuclear volume is also shown for comparison (N=22 (wild type), 19 (rok RNAi)). K,L) Representative western blots and results of quantitation of four independent blots performed on wing disc lysates (20 discs per lane) from nub-Gal4 UAS-dcr2 (labeled as control) and (K) nub-Gal4 UAS-dcr2 with UAS-rok-RNAi (labeled as rok RNAi) or UAS-sqh.EE (labeled as Sqh.EE) or (L) nub-Gal4 UAS-dcr2 UAS-rok.CAT (labeled as ROCK.CAT). The phospho-Yki (pYki) to Yki ratio was normalized to the average ratio in wild-type lysates, using ß-tubulin (Tub) as a loading control. See also Fig. S2.
In complementary experiments, ROCK activity was increased in posterior cells by expression of ROCK.CAT. This increased growth within wing discs and increased ex-lacZ expression (Figs 3E,I, S1C,E). Quantification of Yki Ser168 phosphorylation in discs expressing ROCK.CAT under nub-Gal4 revealed that the relative fraction of phosphorylated Yki was reduced (indicating increased Yki activity) by 17% (Fig. 3L). As for technical reasons these experiments were again done using nub-Gal4, this understates the decreased Yki phosphorylation within ROCK.CAT-expressing cells. An inactive version of ROCK, ROCK.CAT.KG (Winter et al., 2001), had no affect on ex-lacZ (Fig. 3F). Direct activation of Myo II activity by expression of Sqh.EE could also increase disc growth (Fig S1D,E), induce ex-lacZ expression (Fig. 3G, I), and decrease Yki phosphorylation (Fig. 3K). Moreover, Sqh.EE was epistatic to rok RNAi for ex-lacZ regulation (Fig. 3H). We note that adult wings from flies expressing Sqh.EE under nub-Gal4 control were not significantly larger than control wings, even though Sqh.EE could suppress rok RNAi (Fig. 2E, M). As Sqh.EE has stronger effects than ROCK.CAT in other respects (eg myosin accumulation and JNK activation, see below), we speculate that the influence of constituitive high Sqh activity on other cellular and morphogenetic processes partially obscures our ability to detect its influence on Yki activity by examination of wing size in adult flies. Nonetheless, taken together our observations on ROCK and Sqh establish that Myo II activity promotes Yki activity in vivo. Moreover, the observation that phosphorylation of Ser168 is affected implies that Yki activity is regulated by Myo II through the Hippo pathway (ie, by affecting Wts activity).
The induction of ex-lacZ associated with expression of sqh.EE or ROCK.CAT was not uniform. Instead, it was most evident near the center of the wing disc (except along the D-V boundary, where ex-lacZ can not be activated by Yki), where endogenous levels of ex-lacZ are generally lower. Intriguingly, this also appears to be where tension is lower (Legoff et al., 2013; Mao et al., 2013; Nienhaus et al., 2009), which might explain why the consequences of increased Myo II activity appear greater here.
Cellular consequences of altered ROCK activity
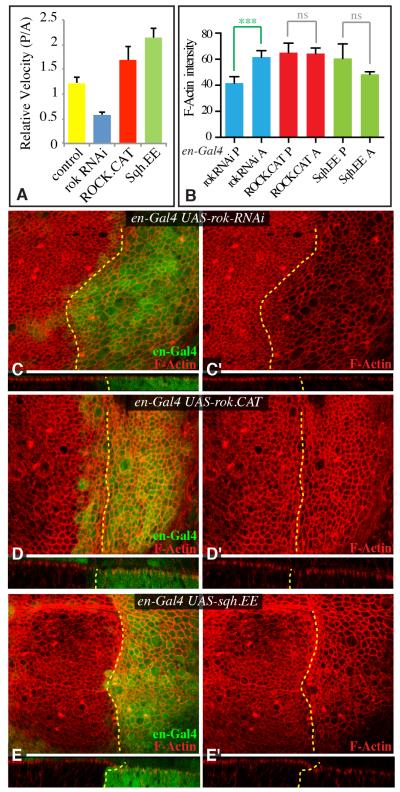
To confirm the influence of altered ROCK activity on Myo II under our conditions, we examined the accumulation of Myo II using Sqh:GFP and Zip:GFP (for Myo II heavy chain, encoded in Drosophila by zipper (zip)). As expected, expression of rok RNAi in posterior cells reduced junctional accumulation of Myo II, whereas expression of ROCK.CAT or Sqh.EE in posterior cells increased junctional accumulation of Myo II (Supplementary Fig S1G-J), consistent with observations that Myo II activity correlates with Myo II accumulation (Fernandez-Gonzalez et al., 2009). As a further test, we assessed relative tension using laser ablation of cell junctions. Comparing pairs of cells with corresponding proximal-distal locations in the posterior versus anterior compartments of discs with altered ROCK or Sqh activity in posterior cells revealed consistent differences in cytoskeletal tension (Fig 4A).
Figure 4. Cellular consequences of altered ROCK activity.
A) Quantitation of relative retraction velocity between junctions in the posterior versus anterior compartment in control wing discs, or wing discs expressing UAS-rok-RNAi, UAS-rok.CAT or UAS-sqh.EE, as indicated, in posterior cells under en-Gal4 control. Junctions of similar orientation and location in A and P compartments were cut in the same disc, and the relative velocities of matched pairs determined (N=12(wild type), 21 (rok RNAi), 10 (ROCK.CAT), 25 (Sqh.EE)). B) Quantitation of relative F-actin levels between P and A compartments in discs of the genotypes depicted in panels C to E (N=8 for all genotypes). C-E) Third instar wing imaginal discs expressing en-Gal4 UAS-dcr2 UAS-GFP or UAS-RFP (green) and C) UAS-rok-RNAi, D) UAS-rok.CAT, or E) UAS-sqh.EE, stained for F-actin using phalloidin (red). Lower panels show vertical sections through the same discs. Dashed yellow line marks A-P compartment boundary. Error bars indicate sem. See also Fig. S3.
Strong accumulation of F-actin, as induced for example, by mutation or down regulation of actin capping proteins, can be associated with activation of Yki (Fernández et al., 2011; Sansores-Garcia et al., 2011). The decreased tension associated with rok RNAi reduced F-actin accumulation (Fig. 4B,C), but the increased tension associated with expression of ROCK.CAT or Sqh.EE had little effect on F-actin levels (Fig 4B,D,E), suggesting that their influence on Yki could be distinct from the influence of mutations that directly and strongly modulate F-actin.
It has been reported that strong activation of Myo II can lead to activation of JNK (Warner et al., 2010). As JNK can promote Yki activation (Grusche et al., 2011; Shaw et al., 2010; Staley and Irvine, 2010; Sun and Irvine, 2011; 2013), we considered the possibility that JNK might be responsible for the Yki activation induced by ROCK.CAT or Sqh.EE. However, under our experimental conditions, activation of JNK was limited, and not distributed in a manner consistent with the observed Yki activation (Fig. S2A-D). Moreover, inhibition of JNK activity by expressing a dominant negative form of Drosophila JNK (Basket, Bsk), or Bsk RNAi, did not prevent ex-lacZ induction or increased wing growth induced by Sqh.EE or ROCK.CAT (Figs 2, S2). Thus, increased cytoskeletal tension can elevate Yki activity in wing discs through a JNK-independent mechanism.
Jub is required for the influence of cytoskeletal tension on Yki activity
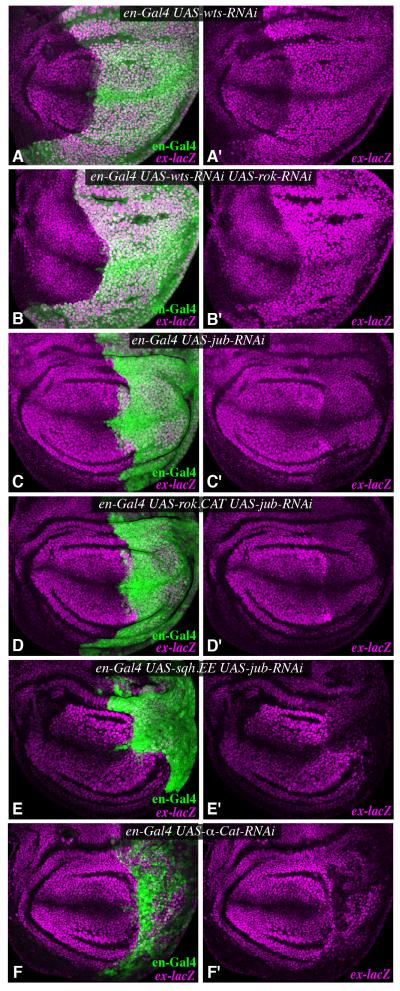
To gain further insight into how Myo II activity influences Yki, we examined genetic interactions of rok and sqh with Hippo pathway components. The key direct negative regulator of Yki activity within the Hippo pathway is Wts (Fig. 1A). Downregulation of Wts in posterior cells by RNAi leads to strong upregulation of Yki activity, readily visualized by increased ex-lacZ expression (Fig. 5A). This activation of Yki was not impaired by rok RNAi, nor was the obvious overgrowth of the posterior compartment suppressed by rok RNAi (Fig. 5B). These observations suggest that rok acts at or upstream of wts. In complementary experiments, we examined whether activation of Yki triggered by ROCK.CAT or Sqh.EE could be suppressed by reduction of Ajuba LIM protein (Jub). Jub is a negative regulator of Wts activity that binds directly to Wts (Fig. 1A) (Thakur et al., 2010). ROCK.CAT or Sqh.EE were unable to increase wing growth or Yki activity in the presence of jub RNAi (Figs 2L,M, 5C-E), consistent with the inference that cytoskeletal tension influences Yki activity through the Hippo pathway, and implying that it does so upstream of, or in parallel to, Jub. A significant connection between jub and rok was further supported by genetic interactions. Heterozygosity for jub normally has little effect on wing size, but significantly enhanced the reduced wing size observed in rok RNAi, and slightly suppressed the increased size of ROCK.CAT-expressing wings (Fig. 2). Similarly, heterozygosity for yki enhances the reduced wing growth of flies expressing rok RNAi (Fig. S3).
Figure 5. Jub is genetically required for the influence of cytoskeletal tension on Yki activity.
Third instar wing imaginal discs expressing en-Gal4 UAS-dcr2 with or without UAS-GFP and A) UAS-wts-RNAi, B) UAS-wts-RNAi UAS-rok-RNAi C) UAS-jub-RNAi, D) UAS-jub-RNAi UAS-rok.CAT, E) UAS-jub-RNAi UAS-sqh.EE, F) UAS-α-cat-RNAi, stained for expression of ex-lacZ (magenta). Posterior cells were marked by expression of Dcr2 or GFP (green). Panels marked prime show individual stains of discs to the left. See also Fig. S4.
Apical localization of Jub is promoted by cytoskeletal tension
The genetic requirement for jub in tension-dependent regulation of Yki prompted us to re-examine Jub localization in vivo. Jub has been reported to localize apically, near cell junctions, in disc epithelial cells (Sabino et al., 2011; Thakur et al., 2010). Moreover, a mammalian homologue of Jub, Ajuba, can localize to cell junctions in cultured cells, and can bind α-catenin, which links the actin cytoskeleton to adherens junctions (Marie et al., 2003).
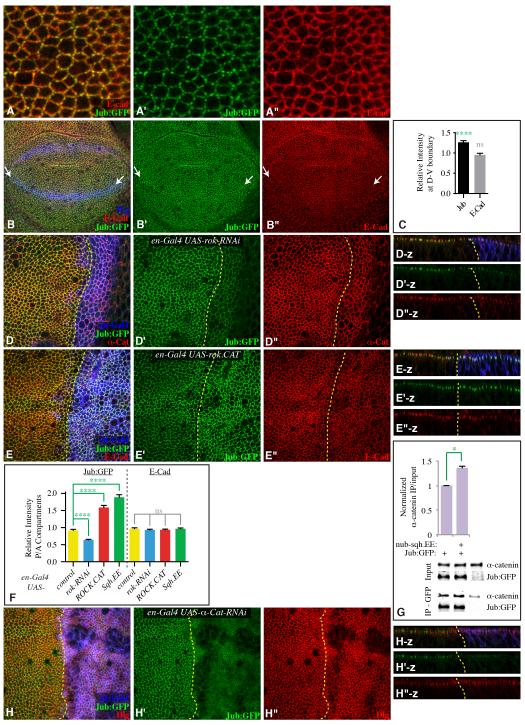
Jub localization was examined using a genomic construct encoding a GFP-tagged Jub protein, which rescues jub mutants (Sabino et al., 2011). Close examination of Jub:GFP in wild-type wing discs revealed two striking features. First, while Jub:GFP overlaps E-cadherin and α-catenin staining along cell junctions, Jub:GFP localization is less even, as it accumulates in bright puncta, whereas other areas exhibit low Jub:GFP (Figs 6, S4). Second, elevated Jub:GFP accumulation is evident along compartment boundaries (Figs 6B,C, S4A,B). As compartment boundaries are sites of increased cytoskeletal tension (Aliee et al., 2012; Landsberg et al., 2009; Major and Irvine, 2005; 2006), this elevated Jub:GFP suggests that Jub localization to junctions is promoted by tension. Indeed, decreasing Myo II activity by expression of rok RNAi decreased apical Jub (Figs 6D,F, S4E), whereas increasing Myo II activity by expression of ROCK.CAT or Sqh.EE increased apical Jub (Figs 6E,F, S4C,F). At higher magnification, this increase was evident as brighter and more numerous puncta of Jub:GFP along cell junctions (Fig. S4C). Increased apical localization of Jub was not suppressed by reduction of JNK activity (Fig. S4H). expanded (ex), an upstream regulator of the Hippo pathway, had little affect on Jub:GFP (Fig. S4G), suggesting that altered Jub localization in response to increased Myo II activity does not occur as a consequence of altered Hippo pathway activity, but rather reflects a response to altered tension. As an independent test of this, we used a ROCK inhibitor, Y-27632, known to decrease myosin activity in wing discs (Legoff et al., 2013). Jub:GFP localization to apical junctions was visibly decreased after a 90 minute incubation with Y-27632 (Fig. S5A-E). Together, our observations identify Jub as a protein whose subcellular localization is regulated by cytoskeletal tension.
Figure 6. Apical localization of Jub is promoted by cytoskeletal tension.
A,B,D,E,H) Third instar wing discs expressing Jub:GFP (green), and stained for expression of E-cad, α-cat, or Dlg (red), as indicated. A) High magnification view showing Jub:GFP puncta along cell junctions. B) Mid third instar disc, with D-V compartment boundary marked by arrows. Wg (blue) marks the D-V boundary and proximal wing. C) Quantitation of Jub:GFP and E-cad along the D-V compartment boundary in early to mid third instar, presented as the ratio of junctional staining along the boundary compared to random cell junctions (N=13 pairs of measurements). D, E) en-Gal4 UAS-dcr2 UAS-RFP (blue) and D) UAS-rok-RNAi or E) UAS-rok.CAT. Panels to the right (-z) show vertical sections. F) Quantitation of Jub:GFP and E-cad at adherens junctions in discs of en-Gal4 UAS-dcr2 UAS-RFP plus, where indicated, UAS-rok-RNAi, UAS-rok.CAT or UAS-sqh.EE, represented as the mean intensity of junctional fluorescence in P compartment regions divided by the mean intensity in A compartment regions (N=12(wild type), 32(rok RNAi), 30(ROCK.CAT), 25(Sqh.EE)). G) Western blots and quantitation of co-precipitation of α-catenin with Jub:GFP from third instar wing disc lysates (200 discs per lane). Lysates were made on discs dissected from nub-Gal4 Jub:GFP, nub-Gal4 Jub:GFP UAS-sqh.EE, or w-. Upper two panels show amounts in lysates (Input), lower two panels show amounts precipitated by GFP-TrapA beads. Some non-specific precipitation of α-catenin occurs, but precipitation from animals expressing Jub:GFP is consistently greater. Histogram shows the average ratio of α-catenin in IP over that in input after background subtraction from three biological replicates, normalized to the ratio in nub-Gal4 Jub:GFP. For both input and IP blots, the level of α-catenin was normalized to the Jub:GFP level. H) en-Gal4 UAS-dcr2 UAS-RFP (blue) UAS-α-cat-RNAi. Panels to the right (-z) show vertical sections. Yellow dashed lines mark the A-P compartment boundary. Error bars show sem. See also Fig. S5.
One of the mammalian homologues of Jub, Ajuba, has been reported to bind α-catenin (Marie et al., 2003). Jub localization to apical junctions in wing discs requires α-catenin, as it was suppressed by RNAi-mediated reduction of α-catenin (Figs 6H, S5F,G). α-catenin is also required for retention of E-Cadherin and ß-catenin at adherens junctions, but not other junctional proteins, including Discs large, Echinoid, and Bazooka (Figs 6H, S5G) (Sarpal et al., 2012), thus the α-catenin dependence of Jub localization indicates that it requires adherens junctions. We confirmed that Jub could physically associate in a complex with α-catenin in vivo through co-immunoprecipitation experiments. Although the signal in terms of specifically co-precipitated α-catenin was low, this likely stems from the small amount of starting material, which comprised lysates of hand-dissected Drosophila wing imaginal discs. Jub:GFP-specific precipitation of α-catenin was statistically significant (Figs 6G, S5H).
Notably, α-catenin at junctions in cultured mammalian cells appears to undergo a force-dependent conformational change, mediated by pulling from the actin cytoskeleton, such that α-catenin at junctions can bind Vinculin under conditions of high tension, but not under conditions of low tension (Yonemura et al., 2010). We hypothesized that the tension-dependent recruitment of Jub to adherens junctions that we observe in vivo (Figs 6, S4, S5) might similarly be mediated by a tension-dependent modulation of Drosophila α-catenin. In support of this, we found that co-precipitation of α-catenin with Jub:GFP was elevated in lysates of wing discs in which half the cells have elevated cytoskeletal tension (nub-Gal4 UAS-Sqh.EE) (Fig. 6G). Quantitative Western blotting also revealed that total levels of Jub were not affected by this increase in myosin activity (Fig. S4D). Thus, we propose that the tension-dependent localization of Jub to cell junctions in vivo could be mediated by a tension-dependent conformational change in α-catenin that enhances its binding to Jub.
Jub recruits Wts to adherens junctions
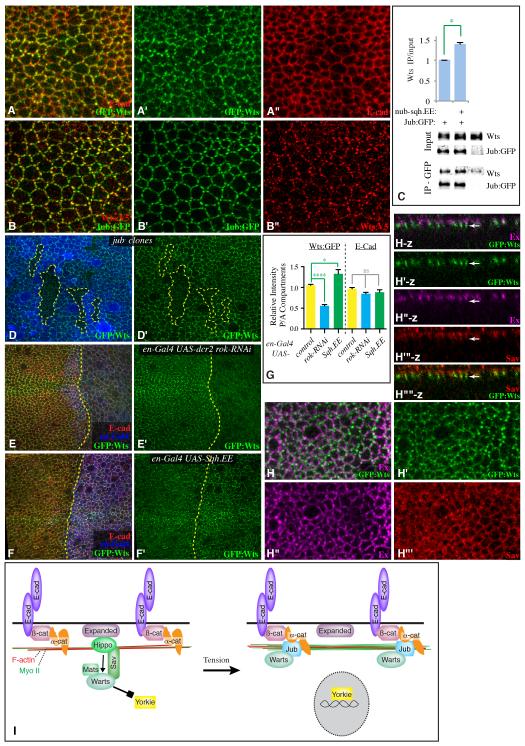
The role of Jub within the Hippo pathway is to bind and inhibit Wts (Thakur et al., 2010). To investigate the relationship between tension-dependent localization of Jub and its influence on Hippo signaling, we sought to examine Wts localization in vivo. Endogenous Wts localization has not been described previously, as available Wts antisera do not work for immunostaining. We used a V5-tagged wts genomic construct within a Bac clone that was transformed into Drosophila, and also used a MIMIC insertion (Venken et al., 2011) to GFP-tag wts at its normal cytological location (Fig. S6). Based on complementation tests with wts mutant alleles, both of these provide normal wts activity. Moreover, both Wts:V5 and GFP:Wts exhibit similar localization profiles, except that Wts:V5 staining is weaker. Strikingly, both Wts:V5 and GFP:Wts accumulate at adherns junctions of wing disc cells in a punctate distribution, reminiscent of Jub:GFP localization (Figs 7, S6). Indeed, when examined together, Jub:GFP and Wts:V5 apical puncta frequently co-localize (Figs 7B, S6F), and significant co-localization was confirmed calculation of Pearson’s Correlation Coefficient (Fig. S6G). Co-immunoprecipitation of endogenous Wts with Jub:GFP from wing disc lysates confirmed that this co-localization is reflective of physical association (Figs 7C, S6E). Junctional Wts was lost from jub mutant clones, indicating that Jub is required to recruit Wts into these apical complexes (Fig. 7D). Moreover, under conditions of elevated cytoskeletal tension, localization of junctional Wts was increased (Fig. 7F,G), and co-precipitation of Wts with Jub:GFP was enhanced (Fig. 7C). In contrast, junctional Wts was decreased when ROCK levels were reduced by RNAi (Fig. 7E,G). Together, these results demonstrate that Wts is recruited into a complex with the Wts-inhibitor Jub in a tension-dependent manner.
Figure 7. Jub recruits Wts to adherens junctions.
A) Wing disc expressing GFP:Wts (green), and stained for E-cad (red). B) Wing disc expressing Jub:GFP (green), and stained for Wts:V5 (red). C) Western blots and quantitation of co-immunoprecipitation of Wts with Jub:GFP from third instar wing disc lysates (200 discs per lane). Lysates were made on discs dissected from nub-Gal4 Jub:GFP, nub-Gal4 Jub:GFP UAS-sqh.EE, or w-. Upper two panels show amounts in lysates (Input), lower two panels show the amounts precipitated by GFP-TrapA beads. Some non-specific precipitation of Wts occurs, but precipitation from animals expressing Jub:GFP is consistently greater. Histogram shows the average ratio of Wts in input over that in IP from three biological replicates, normalized to the ratio in nub-Gal4 Jub:GFP,. For both input and IP blots, the level of Wts was normalized to the Jub:GFP level. D) Wing disc expressing GFP:Wts (green), with jub mutant clones marked by absence of arm-lacZ (blue). E,F) Wing discs expressing GFP:Wts (green) and en-Gal4 UAS-dcr2 UAS-RFP (blue) and E) UAS-rok-RNAi or F) UAS-sqh.EE, and stained for E-cad (red). G) Quantitation of Wts:GFP and E-cad at adherens junctions in discs of en-Gal4 UAS-dcr2 UAS-RFP plus, where indicated, UAS-rok-RNAi or UAS-sqh.EE, represented as the mean intensity of junctional fluorescence in P compartment regions divided by the mean intensity in A compartment regions (N= 5 discs per genotype, with 5 measurements per disc). H) Wing disc expressing GFP:Wts (green), and stained for Ex (magenta) and Sav (red), as indicated. Panels marked –z show vertical sections, white arrow points to location of a GFP:Wts puncta. I) Model for regulation of Hippo signaling by cytoskeletal tension through Jub, see text for details. Error bars show sem. See also Fig. S6.
Recently, it has been reported that Wts or LATS proteins expressed in cultured cells could localize to the plasma membrane in a Merlin-dependent manner, and that forced membrane localization via myristylation could activate Wts (Yin et al., 2013). These observations, together with prior studies on the localization of Wts regulatory proteins, implicate the apical membrane as a site of Wts activation. To begin to explore the relationship between localization of endogenous Wts and Wts activators, we compared Wts:GFP to two key Wts activators in the wing, Ex and Salvador (Sav). Ex is largely apical to Wts (Fig. 7H, S6F), and moreover when their distributions are overlayed by projecting through several confocal sections, Ex immunofluorescence exhibits an irregular distribution with no evident correlation to that of Wts (Fig. 7H). The lack of co-localization was confirmed by calculation of Pearson’s Correlation Coefficient, which was negative (Fig. S6G). Sav exhibits a more continuous distribution, but predominantly overlaps with Ex rather than Wts (Fig. 7H). Thus, in vivo Wts exhibits little or no detectable overlap with these Wts-activators.
DISCUSSION
Mechanical forces have long been known to modulate the proliferation of cultured cells in vitro (Curtis and Seehar, 1978; Huang and Ingber, 1999), and have received attention as an attractive mechanism for modulating organ growth in vivo (Aegerter-Wilmsen et al., 2007; 2012; Hufnagel et al., 2007; Shraiman, 2005). Moreover, increased stiffness is well known to correlate with tumor progression (Butcher et al., 2009). While progress has been reported in identifying components of growth regulatory pathways that could respond to mechanical force (Halder et al., 2012; Samuel et al., 2011), our understanding of how mechanical signals are integrated into growth regulatory pathways has remained poor. Here, we have documented an influence of cytoskeletal tension on wing growth in Drosophila, and delineated a molecular pathway linking this cytoskeletal tension to the regulation of growth through inhibition of the Hippo pathway.
Our observations identify Jub as a protein regulated by mechanical tension, as its localization to foci at adherens junctions is elevated during normal development along compartment boundaries, which are sites of increased tension, and its localization to junctions can be increased or decreased by increasing or decreasing, respectively, Myo II activity. Since this localization requires α-catenin, which associates with Jub, and α-catenin has been identified as a mechanotransducer (Yonemura et al., 2010), we propose that this modulation of Jub localization occurs as a consequence of a tension-induced conformational change of α-catenin that increases its binding to Jub (Fig. 7I). α-catenin is well positioned to act as a mechanotransducer (Huveneers and de Rooij, 2013): it interacts with the ß-catenin:E-cad complex, which is effectively anchored through binding E-cad in neighboring cells, and it also associates with the F-actin cytoskeleton, which could pull on α-catenin through myosin-mediated contraction (Yonemura et al., 2010)(Fig. 7I). We further propose that association with α-catenin increases the binding of Jub to Wts, leading to an inhibitory recruitment of Wts to apical junctions, and consequently increased Yki activity (Fig. 7I). This proposal is supported by the observations that Wts co-localizes with and physically associates with Jub in vivo, the promotion of this physical association by Myo II activity, and genetic and biochemical evidence that wts and jub are required for tension-dependent modulation of Yki activity.
Cytoskeletal tension has previously been reported to increase YAP activity in cultured mammalian cells (Aragona et al., 2013; Dupont et al., 2011). The molecular mechanism that mediates this mechano-regulation of YAP is unknown, but was determined to be independent of Hippo signaling, because it did not involve LATS (the mammalian homologue of Wts). This clearly distinguishes it from the Hippo-pathway dependent mechano-regulation of Yki in wing discs that we describe here. Among many differences in experimental conditions, we note that experiments on cultured cells frequently involve manipulating cytoskeletal tension through cell – extracellular matrix attachment, whereas cytoskeletal tension at cell-cell attachments of epithelial cells was manipulated in our experiments. Whether this accounts for the distinct mechanisms involved remains to be determined, but just as there are multiple ligand-regulated biochemical signal transduction pathways that influence organ growth, there are likely also multiple mechanically-regulated pathways that influence organ growth. Indeed, other experimental regimes of altered attachment of cultured cells to matrix in vitro have been associated with LATS-dependent effects on YAP activity (Wada et al., 2011; Zhao et al., 2012). Moreover, in Drosophila, increased accumulation of F-actin elevates Yki activity (Fernández et al., 2011; Sansores-Garcia et al., 2011), also potentially consistent with mechanical regulation of Hippo signaling. However, it has recently been reported that F-actin levels modulate interaction between Wts and the Wts activator Merlin (Yin et al., 2013), and further studies are needed to address the mechanisms by which changes in F-actin levels associated with genetic or pharmacological manipulations impinge on Hippo signaling.
Despite the crucial role of Wts as the key direct regulator of Yki within the Hippo pathway, the endogenous localization of Wts protein in vivo had not previously been characterized. Our observations that Wts associates with Jub at cell junctions, and that this association is increased under conditions leading to elevated Yki activity, indicate that recruitment of Wts to junctions is associated with inhibition of Wts. Several positive upstream regulators of Wts also localize near cell junctions (Enderle and Mcneill, 2013; Staley and Irvine, 2012; Yu and Guan, 2013), and it was recently proposed that recruitment of Wts to the membrane is associated with Wts activation (Yin et al., 2013). However, as we show here, at least some membrane-associated activators exhibit distinct localization profiles from Wts. These observations suggest that there could be distinct sites for Wts activation and Wts inhibition at apical junctions, with most junctional Wts localized to an inhibitory complex. It could be that only a small fraction of endogenous Wts is normally active, or that Wts activation involves transient association of Wts with activators, as opposed to the stable association of Wts with Jub revealed by our studies.
While the results described here identify Jub as a key player in mechano-regulation of Hippo signaling, other recent studies have identified Jub, and two its mammalian homologues, LIMD1 and WTIP, as targets for cross-regulation of Hippo signaling by the EGFR and JNK signaling pathways (Reddy and Irvine, 2013; Sun and Irvine, 2013). These observations emphasize the importance of Ajuba family proteins as a key regulatory node within the Hippo signaling pathway, and a point of convergence between mechanical and biochemical signaling pathways. Indeed, organ growth in vivo must integrate multiple inputs. For example, the dorsal-ventral compartment boundary is not only a region of elevated cytoskeletal tension, but also, in late third instar discs, a region of low cell proliferation (O’Brochta and Bryant, 1987), due to repression of cell proliferation downstream of Notch signaling (Herranz et al., 2012). Thus, at late third instar Notch activation might override or bypass the influence of tension on growth along the compartment boundary.
Our observation of Wts complexing with Jub at apical junctions has implications for how growth is controlled in developing tissues. These complexes are promoted by cytoskeletal tension, which provides a mechanism for tension-dependent regulation of growth. However, even under conditions of constant tension, an increase in the apical perimeter of cells at junctions could result in more of these Wts-inhibitory Jub complexes, whereas a decrease in apical perimeter could lead to fewer of these Wts-inhibitory complexes. This suggests the potential of shape-dependent regulation of Hippo-signaling, with cells of small apical perimeter having higher Hippo signaling, and consequently reduced growth. Reduced apical area (and consequently perimeter) correlates with reduced cell proliferation both in vivo and in cell culture models (Aegerter-Wilmsen et al., 2012; Aragona et al., 2013; Puliafito et al., 2012). Apical area is also expected to be affected by relative mechanical forces (e.g. how much is a cell stretched by its neighbors), and thus we speculate that it could be used by cells to assess not only their own tension, but the mechanical environment in which they reside. Effects of tension on shape and the formation of junctional Wts-inhibitory complexes could thus provide a mechanism for cell density-dependent regulation of organ size.
EXPERIMENTAL PROCEDURES
Histology and imaging
Discs were fixed in 4% paraformaldehyde and stained using as primary antibodies rabbit anti-Yki (Oh and Irvine, 2008), mouse anti-ß-galacotsidase (DSHB), mouse anti-Wg (DSHB), mouse anti-Diap1 (B. Hay), rat anti-E-cad (DCAD2; DSHB), rabbit anti-Dcr2 (Abcam), guinea pig anti-α-catenin (U. Tepass), mouse anti-Dlg (DSHB), rabbit anti-cleaved caspase (Dcp-1; Cell Signaling Techonology), mouse anti-V5 (Invitrogen, R960-25), guinea pig anti-Ex (R. Fehon), rabbit anti-Sav (J. Jiang). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories and Invitrogen. F-actin was stained using Alexa Fluor 546-phalloidin (Invitrogen), and DNA was stained using Hoechst (Invitrogen). Confocal images were captured on a Leica SP5 or a Perkin Elmer Ultraview.
Immunoblotting and immunoprecipitation
To examine effects of tension on Jub:GFP - Wts or α-catenin binding, UAS-sqh.E20E21 or w- (control) females were crossed to nub-Gal4 UASdcr2; Jub:GFP or w- (control) males and cultured at 29°C. Lysates from approximately 200 wing discs of third instar larvae were used for each genotype per co-immunoprecipitation, and at least three repetitions were performed. For Yki blots, lysates from 20 discs were used for each blot, and four repetitions were performed. Discs were lysed in 50mM Tris·HCl pH7.4, 150mM NaCl, 1% Triton X-100, 0.1% CHAPS, 0.1% NP-40, 1mM EDTA, 5% glycerol, supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Calbiochem). For co-immunoprecipitation, protein samples were incubated with GFP-Trap_A agarose beads (Chemotek) for 3h at 4°C, then washed. Protein samples were applied to 4-15% gradient gels (Bio-rad). Antibodies used for immunoblotting include rabbit anti-Wts (1:10000) (Cho et al., 2006), rabbit anti-Yki (1:2000) (Oh and Irvine, 2008), rabbit anti-phospho-Yki (1:1000, D. Pan), rabbit anti-GFP (1:2000, Life Technologies), mouse anti-tubulin (1:8000, Sigma). Blots were visualized and quantified using fluorescent-conjugated secondary antibodies and an Odyssey Imaging System (Li-Cor Biosciences).
Supplementary Material
Cytoskeletal tension regulates Drosophila wing growth through the Hippo pathway
The Ajuba protein Jub is regulated by tension and links tension to Hippo signaling
Jub inhibits Warts and recruits Warts to junctions in a tension-dependent manner
Our observations delineate a molecular mechanism that links tension to growth control
ACKNOWLEDGEMENTS
We thank Yongqiang Feng for Wts:V5-expressing flies, Spencer Irvine for initial characterization of rok RNAi phenotypes, and Martha Soto for helpful discussion in suggesting α-catenin experiments. We thank R. Basto, C. Bökel, R. Fehon, B. Hay, J. Jiang, D. Kiehart, L. Luo, G. Longmore, T. Lecuit, D. Pan, U. Tepass, J. Zallen, the Developmental Studies Hybridoma Bank, the Gene Disruption Project, and the Bloomington stock center for antibodies and Drosophila stocks, and the Drosophila Genomics Resource Center for plasmids. This research was supported by the Howard Hughes Medical Institute and NIH grant R01 GM078620.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
YP: tension measurements by laser cutting, GS: co-immunoprecipitation and western blotting, SS: all analysis of Wts localization, CR: all other experiments and manuscript preparation, KI: data analysis and manuscript preparation.
Additional experimental procedures are in the Supplemental Material.
REFERENCES
- Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124:318–326. doi: 10.1016/j.mod.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Aegerter-Wilmsen T, Heimlicher MB, Smith AC, de Reuille PB, Smith RS, Aegerter CM, Basler K. Integrating force-sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development. 2012;139:3221–3231. doi: 10.1242/dev.082800. [DOI] [PubMed] [Google Scholar]
- Aliee M, Röper J-C, Landsberg KP, Pentzold C, Widmann TJ, Jülicher F, Dahmann C. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr Biol. 2012;22:967–976. doi: 10.1016/j.cub.2012.03.070. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Bertet C, Rauzi M, Lecuit T. Repression of Wasp by JAK/STAT signalling inhibits medial actomyosin network assembly and apical cell constriction in intercalating epithelial cells. Development. 2009;136:4199–4212. doi: 10.1242/dev.040402. [DOI] [PubMed] [Google Scholar]
- Bryant PJ, Levinson P. Intrinsic growth control in the imaginal primordia of Drosophila, and the autonomous action of a lethal mutation causing overgrowth. Dev Biol. 1985;107:355–363. doi: 10.1016/0012-1606(85)90317-3. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Cho E, Feng Y, Feng Y, Rauskolb C, Rauskolb C, Maitra S, Maitra S, Fehon R, Fehon R, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Curtis ASG, Seehar GM. The control of cell division by tension or diffusion. Nature. 1978;274:52–53. doi: 10.1038/274052a0. [DOI] [PubMed] [Google Scholar]
- Degoutin JL, Milton CC, Yu E, Tipping M, Bosveld F, Yang L, Bellaïche Y, Veraksa A, Harvey KF. Riquiqui and minibrain are regulators of the hippo pathway downstream of Dachsous. Nat Cell Biol. 2013;15:1176–1185. doi: 10.1038/ncb2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Enderle L, Mcneill H. Hippo gains weight: added insights and complexity to pathway control. Sci Signal. 2013;6:re7. doi: 10.1126/scisignal.2004208. [DOI] [PubMed] [Google Scholar]
- Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17:2095–2104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes S, de M, Röper J-C, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 2011;350:255–266. doi: 10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Herranz H, Pérez L, Martín FA, Milán M. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. Embo J. 2012;27:1633–1645. doi: 10.1038/emboj.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun Integr Biol. 2008;1:192–195. doi: 10.4161/cib.1.2.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci USA. 2007;104:3835–3840. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci. 2013 doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Jülicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Legoff L, Rouault H, Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Hoppe A, Kester L, Thompson BJ, Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. Embo J. 2013 doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, Moss SJ, Troyanovsky S, Attwell D, Longmore GD, et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J Biol Chem. 2003;278:1220–1228. doi: 10.1074/jbc.M205391200. [DOI] [PubMed] [Google Scholar]
- Martín FA, Herrera SC, Morata G. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development. 2009;136:3747–3756. doi: 10.1242/dev.038406. [DOI] [PubMed] [Google Scholar]
- Nienhaus U, Aegerter-Wilmsen T, Aegerter CM. Determination of mechanical stress distribution in Drosophila wing discs using photoelasticity. Mech Dev. 2009;126:942–949. doi: 10.1016/j.mod.2009.09.002. [DOI] [PubMed] [Google Scholar]
- O’Brochta DA, Bryant PJ. Distribution of S-phase cells during the regeneration of Drosophila imaginal wing discs. Dev Biol. 1987;119:137–142. doi: 10.1016/0012-1606(87)90215-6. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 2010;20:410–417. doi: 10.1016/j.tcb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliafito A, Hufnagel L, Neveu P, Streichan S, Sigal A, Fygenson DK, Shraiman BI. Collective and single cell behavior in epithelial contact inhibition. Proc Natl Acad Sci USA. 2012;109:739–744. doi: 10.1073/pnas.1007809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Pan G, Reddy BVVG, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BVVG, Irvine KD. Regulation of Hippo Signaling by EGFR-MAPK Signaling through Ajuba Family Proteins. Dev Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino D, Brown NH, Basto R. Drosophila Ajuba is not an Aurora-A activator but is required to maintain Aurora-A at the centrosome. J Cell Sci. 2011;124:1156–1166. doi: 10.1242/jcs.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schröder E, Zhou J, Brunton VG, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K-I, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. Embo J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal R, Pellikka M, Patel RR, Hui FYW, Godt D, Tepass U. Mutational analysis supports a core role for Drosophila α-catenin in adherens junction function. J Cell Sci. 2012;125:233–245. doi: 10.1242/jcs.096644. [DOI] [PubMed] [Google Scholar]
- Schluck T, Nienhaus U, Aegerter-Wilmsen T, Aegerter CM. Mechanical control of organ size in the development of the Drosophila wing disc. PLoS ONE. 2013;8:e76171. doi: 10.1371/journal.pone.0076171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraiman BI. Mechanical feedback as a possible regulator of tissue growth. Proc Natl Acad Sci USA. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Hippo signaling in Drosophila: Recent advances and insights. Dev Dyn. 2012;241:3–15. doi: 10.1002/dvdy.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD. Ajuba Family Proteins Link JNK to Hippo Signaling. Sci Signal. 2013;6:ra81. doi: 10.1126/scisignal.2004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur, Das M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJT, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, Carlson JW, Levis RW, Spradling AC, Hoskins RA, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Meth. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Warner SJ, Yashiro H, Longmore GD. The Cdc42/Par6/aPKC Polarity Complex Regulates Apoptosis-Induced Compensatory Proliferation in Epithelia. Curr Biol. 2010 doi: 10.1016/j.cub.2010.03.025. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Jülicher F, ález-GaitánGonz M. Dynamics of Dpp signaling and proliferation control. Science. 2011;331:1154–1159. doi: 10.1126/science.1200037. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- Yu F-X, Guan K-L. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Deng W-M, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang C-Y, Yu J, Guan K-L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.