Full text
PDF

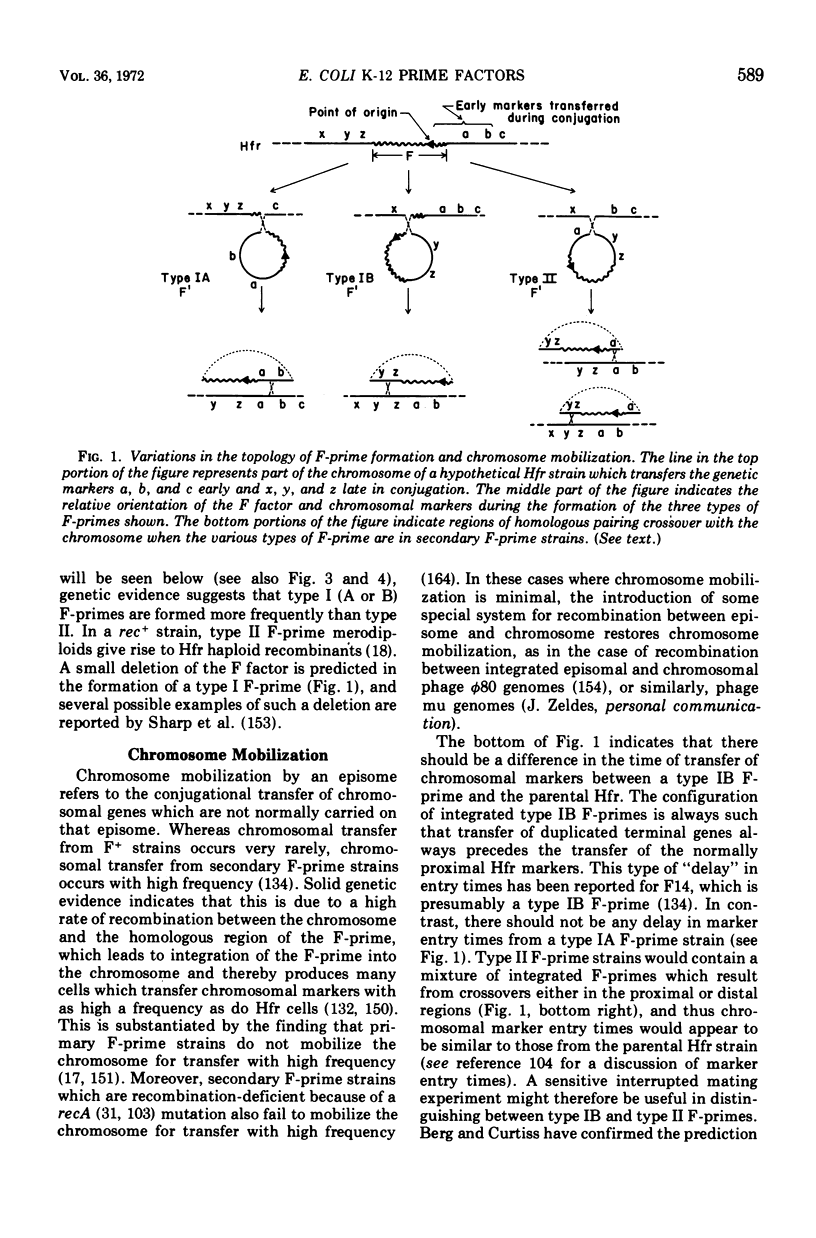




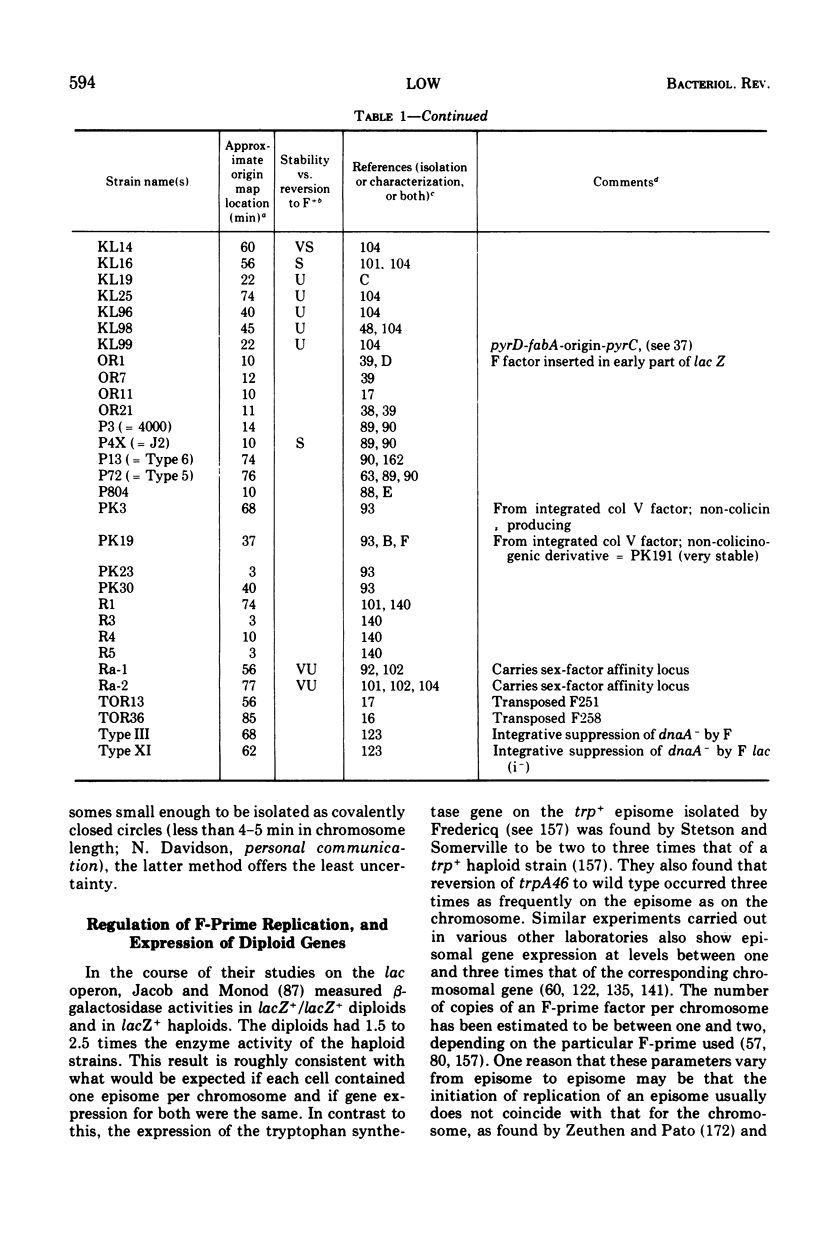
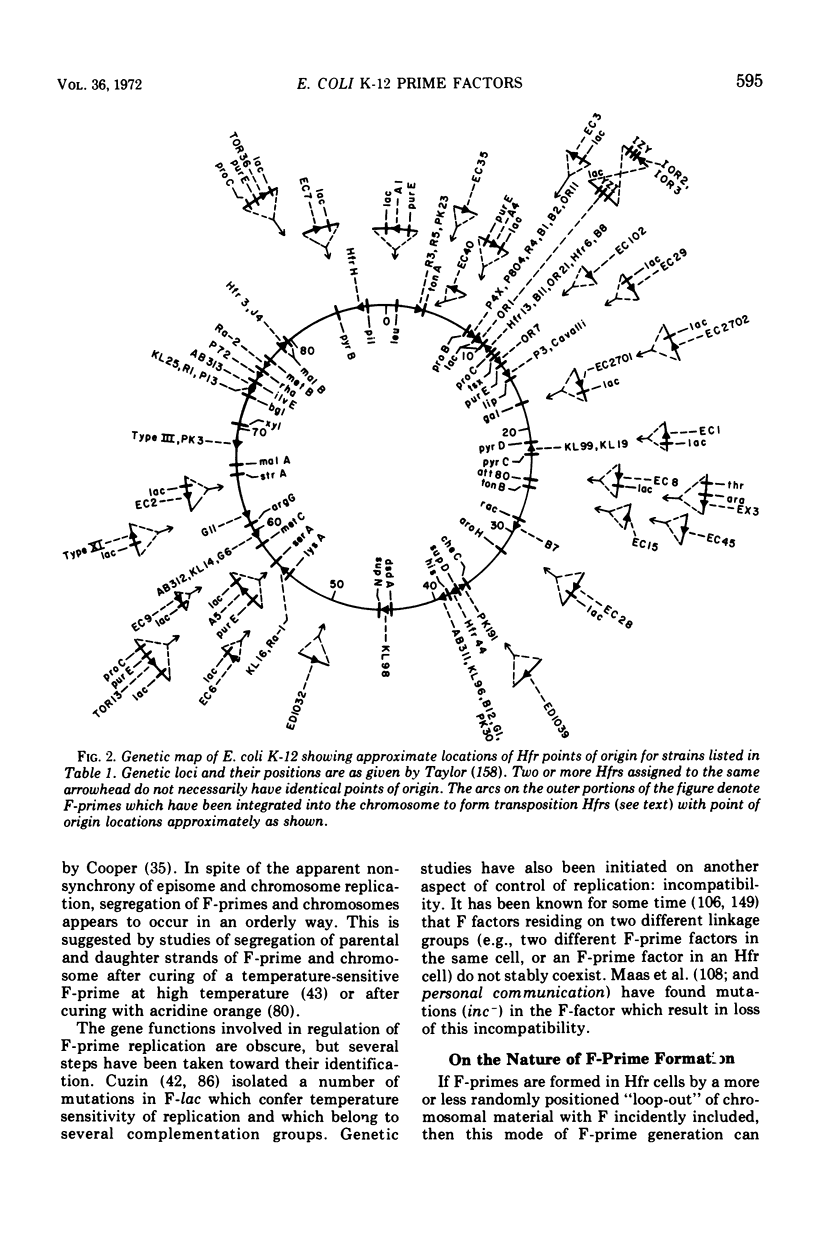

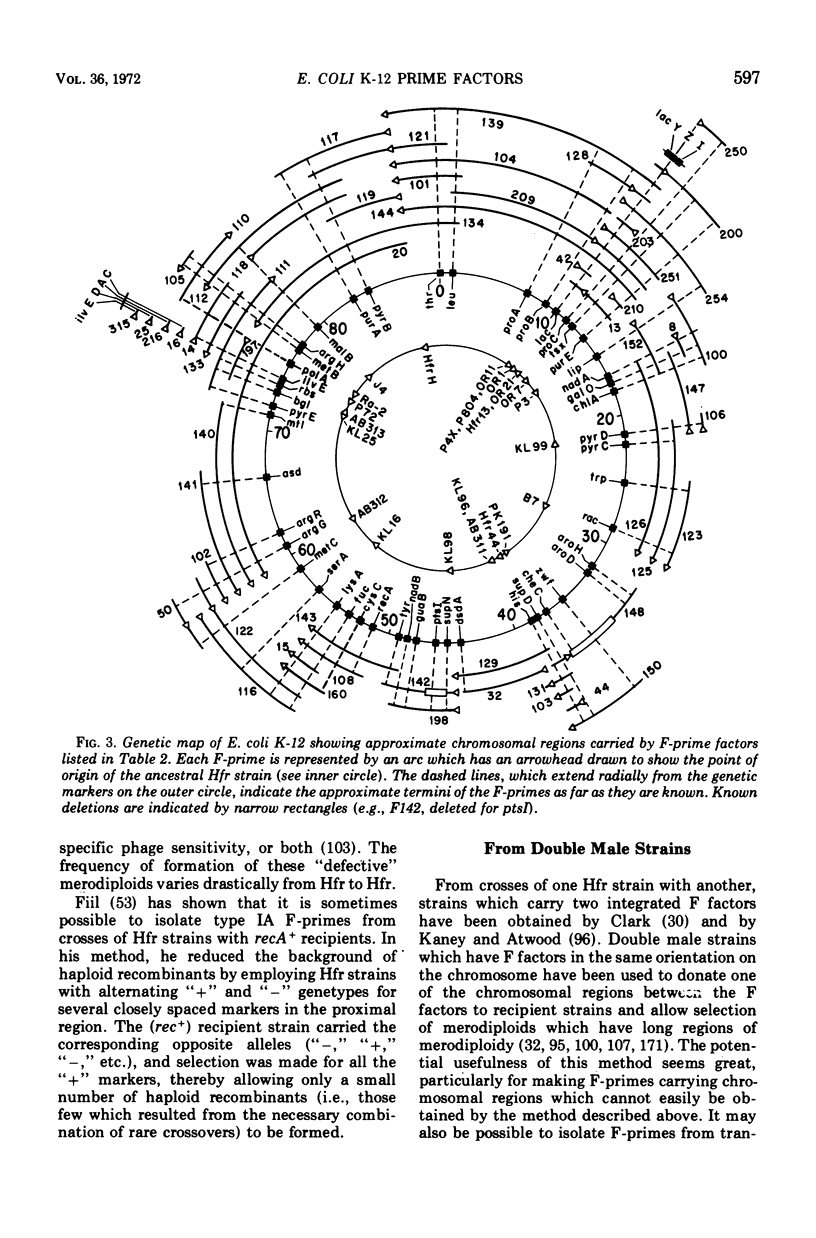









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi H., Nakano M., Inuzuka M., Tomoeda M. Specific role of sex pili in the effective eliminatory action of sodium dodecyl sulfate on sex and drug resistance factors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1114–1124. doi: 10.1128/jb.109.3.1114-1124.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelberg E. A., Bergquist P. The stabilization of episomal integration by genetic inversion: a general hypothesis. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2061–2065. doi: 10.1073/pnas.69.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Loper J. C. Transcription initiation in the histidine operon of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1970 Apr;65(4):925–932. doi: 10.1073/pnas.65.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S. J., Tittawella I. P., Hayward R. S., Scaife J. G. Amber mutations of Escherichia coli RNA polymerase. Nat New Biol. 1971 Aug 4;232(31):133–136. doi: 10.1038/newbio232133a0. [DOI] [PubMed] [Google Scholar]
- Austin S., Scaife J. A new method for selecting RNA polymerase mutants. J Mol Biol. 1970 Apr 14;49(1):263–267. doi: 10.1016/0022-2836(70)90394-3. [DOI] [PubMed] [Google Scholar]
- Avitabile A., Carlomagno-Cerillo S., Favvre R., Blasi F. Isolation of transducing bacteriophages for the histidine and isoleucine-valine operons in Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):40–47. doi: 10.1128/jb.112.1.40-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastarrachea F., Willetts N. S. The elimination by acridine orange of F30 from recombination-deficient strains of Escherichia coli K12. Genetics. 1968 Jun;59(2):153–166. doi: 10.1093/genetics/59.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzicalupo P., Tocchini-Valentini G. P. Curing of an Escherichia coli episome by rifampicin (acridine orange-F + -F - -Hfr-lac). Proc Natl Acad Sci U S A. 1972 Feb;69(2):298–300. doi: 10.1073/pnas.69.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R. Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J Mol Biol. 1966 Aug;19(2):254–265. doi: 10.1016/s0022-2836(66)80003-7. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Gallant J. A. Tests of reciprocality in crossingover in partially diploid F strains of Escherichia coli. Genetics. 1971 Aug;68(4):457–472. doi: 10.1093/genetics/68.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist P. L., Adelberg E. A. Abnormal excision and transfer of chromosomal segments by a strain of Escherichia coli K-12. J Bacteriol. 1972 Jul;111(1):119–128. doi: 10.1128/jb.111.1.119-128.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T. P1 transduction: formation of heterogenotes upon cotransduction of bacterial genes with a P2 prophage. Virology. 1972 Jan;47(1):76–93. doi: 10.1016/0042-6822(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Brandriss M. C., Calvo J. M. Recognition of an Escherichia operator by a Salmonella repressor. J Bacteriol. 1971 Dec;108(3):1431–1433. doi: 10.1128/jb.108.3.1431-1433.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda P., Meacock P., Achtman M. Early transfer of genes determining transfer functions by some Hfr strains in Escherichia coli K12. Mol Gen Genet. 1972;116(4):336–347. doi: 10.1007/BF00270090. [DOI] [PubMed] [Google Scholar]
- Broda P., Meacock P. Isolation and characterisation of Hfr strains from a recombination-deficient strain of Escherichia coli. Mol Gen Genet. 1971;113(2):166–173. doi: 10.1007/BF00333190. [DOI] [PubMed] [Google Scholar]
- CAVALLI-SFORZA L. L. La sessualità nei batteri. Boll Ist Sieroter Milan. 1950 Sep-Oct;29(9-10):281–289. [PubMed] [Google Scholar]
- CLARK A. J. Genetic analysis of a "double male" strain of Escherichia coli K-12. Genetics. 1963 Jan;48:105–120. doi: 10.1093/genetics/48.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLWES R. C., MOODY E. E., PRITCHARD R. H. THE ELIMINATION OF EXTRACHROMOSOMAL ELEMENTS IN THYMINELESS STRAINS OF ESCHERICHIA COLI K12. Genet Res. 1965 Feb;6:147–152. doi: 10.1017/s0016672300004018. [DOI] [PubMed] [Google Scholar]
- Chater K. F. Dominance of the wild-type alleles of methionine regulatory genes in Salmonella typhimurium. J Gen Microbiol. 1970 Sep;63(1):95–109. doi: 10.1099/00221287-63-1-95. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Maas W. K., Low B. Production of a merodiploid strain from a double male strain of E. coli K12. Mol Gen Genet. 1969;105(1):1–15. doi: 10.1007/BF00750309. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Clowes R. C., Moody E. E. Chromosomal transfer from "recombination-deficient" strains of Escherichia coli K-12. Genetics. 1966 Apr;53(4):717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Relationship of Flac replication and chromosome replication. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2706–2710. doi: 10.1073/pnas.69.9.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Silbert D. F., Wulff D. L. Mapping of the fabA locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1972 Oct;112(1):206–211. doi: 10.1128/jb.112.1.206-211.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Stallions D. R. Probability of F integration and frequency of stable Hfr donors in F+ populations of Escherichia coli K-12. Genetics. 1969 Sep;63(1):27–38. doi: 10.1093/genetics/63.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Existence chez Escherichia coli K12 d'une unité génétique de transmission formée de différents réplicons. Ann Inst Pasteur (Paris) 1967 May;112(5):529–545. [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- DEWITT S. K., ADELBERG E. A. Transduction of the attached sex factor of Escherichia coli. J Bacteriol. 1962 Mar;83:673–678. doi: 10.1128/jb.83.3.673-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries J. K., Maas W. K. Chromosomal integration of F' factors in recombination-deficient Hfr strains of Escherichia coli. J Bacteriol. 1971 Apr;106(1):150–156. doi: 10.1128/jb.106.1.150-156.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Jewett S., Fox C. F. Isolation and mapping of phosphotransferase mutants in Escherichia coli. J Bacteriol. 1970 Nov;104(2):793–797. doi: 10.1128/jb.104.2.793-797.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenchik Z., Stacey K. A., Hayes W. Ultraviolet induction of chromosome transfer by autonomous sex factors in Escherichia coli. J Gen Microbiol. 1969 Apr;56(1):1–14. doi: 10.1099/00221287-56-1-1. [DOI] [PubMed] [Google Scholar]
- FALKOW S., CITARELLA R. V. MOLECULAR HOMOLOGY OF F-MEROGENOTE DNA. J Mol Biol. 1965 May;12:138–151. doi: 10.1016/s0022-2836(65)80288-1. [DOI] [PubMed] [Google Scholar]
- Fan D. P. Deletions in limited homology recombination in Escherichia coli. Genetics. 1969 Feb;61(2):351–361. doi: 10.1093/genetics/61.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill N. A functional analysis of the rel gene in Escherichia coli. J Mol Biol. 1969 Oct 28;45(2):195–203. doi: 10.1016/0022-2836(69)90099-0. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Roth J. R. Histidine regulatory mutants in Salmonella typhiumium. VI. Dominance studies. J Mol Biol. 1968 May 14;33(3):547–557. doi: 10.1016/0022-2836(68)90305-7. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G., Banerjee S. A mutation increasing the amount of a constitutive enzyme in Escherichia coli, glucose 6-phosphate dehydrogenase. J Mol Biol. 1971 Feb 28;56(1):183–194. doi: 10.1016/0022-2836(71)90093-3. [DOI] [PubMed] [Google Scholar]
- Frame R., Bishop J. O. The number of sex-factors per chromosome in Escherichia coli. Biochem J. 1971 Jan;121(1):93–103. doi: 10.1042/bj1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Studies on Escherichia coli sex factors. IV. Molecular weights of the DNA of several F' elements. J Mol Biol. 1968 Jul 14;35(1):95–102. doi: 10.1016/s0022-2836(68)80039-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Complementation in vivo between structural mutants of alkaline phosphatase from E. coli. J Mol Biol. 1963 Jul;7:13–22. doi: 10.1016/s0022-2836(63)80015-7. [DOI] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N. Pseudoinversions in the chromosome of Escherichia coli K-12. Genetics. 1967 Jan;55(1):49–61. doi: 10.1093/genetics/55.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E. P., Cater M. S., Matney T. S., Butler M. A., Greene A. Genetic analysis of the histidine operon in Escherichia coli K12. Genetics. 1970 Oct;66(2):219–229. doi: 10.1093/genetics/66.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Beckwith J. R. Directed transposition of the arabinose operon: a technique for the isolation of specialized transducing bacteriophages for any Escherichia coli gene. J Mol Biol. 1969 Aug 28;44(1):117–127. doi: 10.1016/0022-2836(69)90408-2. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- HAYES W. The mechanism of genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1953;18:75–93. doi: 10.1101/sqb.1953.018.01.016. [DOI] [PubMed] [Google Scholar]
- HAYES W. [Observations on a transmissible agent determining sexual differentiation in Bacterium coli]. J Gen Microbiol. 1953 Feb;8(1):72–88. doi: 10.1099/00221287-8-1-72. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Howard-Flanders P. Recombinant F' factors from Escherichia coli K-12 strains carrying recB or recC. J Bacteriol. 1972 May;110(2):578–584. doi: 10.1128/jb.110.2.578-584.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Herman R. K. Identification of recombinant chromosomes and F-merogenotes in merodiploids of Escherichia coli. J Bacteriol. 1968 Jul;96(1):173–179. doi: 10.1128/jb.96.1.173-179.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K. Reciprocal recombination of chromosome and F. merogenote in Escherichia coli. J Bacteriol. 1965 Dec;90(6):1664–1668. doi: 10.1128/jb.90.6.1664-1668.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Schiffer D., Berg P. Transduction of merodiploidy: induced duplication of recipient genes. J Bacteriol. 1969 Jul;99(1):274–278. doi: 10.1128/jb.99.1.274-278.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Wilhelm R. C. Genetic mapping and dominance of the amber suppressor, Su1 (supD), in Escherichia coli K-12. J Bacteriol. 1970 Jul;103(1):32–36. doi: 10.1128/jb.103.1.32-36.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M., Hatfield D. Complementation studies in the maltose-A region of the Escherichia coli K12 genetic map. J Mol Biol. 1971 Nov 14;61(3):681–694. doi: 10.1016/0022-2836(71)90072-6. [DOI] [PubMed] [Google Scholar]
- Hohn B., Korn D. Cosegregation of a sex factor with the Escherichia coli chromosome during curing by acridine orange. J Mol Biol. 1969 Oct 28;45(2):385–395. doi: 10.1016/0022-2836(69)90113-2. [DOI] [PubMed] [Google Scholar]
- ISHIBASHI M., SUGINO Y., HIROTA Y. CHROMOSOMAL LOCATION OF THYMINE AND ARGININE GENES IN ESCHERICHIA COLI AND AN F' INCORPORATING THEM. J Bacteriol. 1964 Mar;87:554–561. doi: 10.1128/jb.87.3.554-561.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen K., Shapiro J. A., Beckwith J. R. Transposition of the lac region to the gal region of the Escherichia coli chromosome: isolation of lambda-lac transducing bacteriophages. J Bacteriol. 1971 Oct;108(1):5–9. doi: 10.1128/jb.108.1.5-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Inactivation of chromosomal fragments transferred from hfr strains. Genetics. 1971 May;68(1):1–11. doi: 10.1093/genetics/68.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., ADELBERG E. A. Transfert de caractéres génétiques par incorporation au facteur sexuel d'Escherichia coli. C R Hebd Seances Acad Sci. 1959 Jul 6;249(1):189–191. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Analyse des groupes de liaison génétique de différentes souches donatrices d'Escherichia coli K 12. C R Hebd Seances Acad Sci. 1957 Nov 18;245(21):1840–1843. [PubMed] [Google Scholar]
- Johnson E. M., Easterling S. B., Baron L. S. Conservation and transfer of Escherichia coli genetic segments by partial diploid Hfr strains of Salmonella typhosa. J Bacteriol. 1970 Nov;104(2):668–673. doi: 10.1128/jb.104.2.668-673.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P. L. Evolution of a site of specific genetic homology on the chromosome of Escherichia coli. J Bacteriol. 1969 Oct;100(1):269–275. doi: 10.1128/jb.100.1.269-275.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P. L. Isolation of high-frequency recombining strains from Escherichia coli containing the V colicinogenic factor. J Bacteriol. 1968 Jul;96(1):205–214. doi: 10.1128/jb.96.1.205-214.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaney A. R., Atwood K. C. Incompatibility of integrated sex factors in double male strains of Escherichia coli. Genetics. 1972 Jan;70(1):31–39. doi: 10.1093/genetics/70.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetkas M. J., Krisch R. E., Zelle M. R. Genetic analysis of a large-cell, radiation-resistant strain of Escherichia coli. J Bacteriol. 1970 Aug;103(2):393–399. doi: 10.1128/jb.103.2.393-399.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew K. K., Roth J. R. Genetic approaches to determination of enzyme quaternary structure. Biochemistry. 1971 Jan 19;10(2):204–207. doi: 10.1021/bi00778a002. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. The catabolite repression gene of the lac operon in Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):487–494. doi: 10.1016/s0022-2836(67)80120-7. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Inversion of transfer modes and sex factor-chromosome interactions in conjugation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):98–106. doi: 10.1128/jb.93.1.98-106.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- MAAS R. EXCLUSION OF A FLAC EPISOME BY AN HFR GENE. Proc Natl Acad Sci U S A. 1963 Dec;50:1051–1055. doi: 10.1073/pnas.50.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- Maas W. K., Goldschmidt A. D. A mutant of Escherichia coli permitting replication of two F factors. Proc Natl Acad Sci U S A. 1969 Mar;62(3):873–880. doi: 10.1073/pnas.62.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N. J., Duggan D. E. Ordering of mutant sites in the isoleucine-valine genes of Escherichia coli by use of merogenotes derived from F 14 : a new procedure for fine-structure mapping. J Bacteriol. 1972 Feb;109(2):730–740. doi: 10.1128/jb.109.2.730-740.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Matsubara K. Properties of sex factor and related episomes isolated from purified Escherichia coli zygote cells. J Mol Biol. 1968 Nov 28;38(1):89–108. doi: 10.1016/0022-2836(68)90130-7. [DOI] [PubMed] [Google Scholar]
- McFall E. Dominance studies with stable merodiploids in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1967 Dec;94(6):1982–1988. doi: 10.1128/jb.94.6.1982-1988.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. Reciprocal recombination in prophage lambda. J Cell Physiol. 1967 Oct;70(2 Suppl):113–118. doi: 10.1002/jcp.1040700409. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Roth J. R. Recessive-lethal nonsense suppressors in Salmonella typhimurium. J Mol Biol. 1971 Jul 14;59(1):63–75. doi: 10.1016/0022-2836(71)90413-x. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Reznikoff W. S., Silverstone A. E., Ippen K., Signer E. R., Beckwith J. R. Fusions of the lac and trp Regions of the Escherichia coli Chromosome. J Bacteriol. 1970 Dec;104(3):1273–1279. doi: 10.1128/jb.104.3.1273-1279.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody E. E., Hayes W. Chromosome transfer by autonomous transmissible plasmids: the role of the bacterial recombination (rec) system. J Bacteriol. 1972 Jul;111(1):80–85. doi: 10.1128/jb.111.1.80-85.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody E. E., Runge R. The integration of autonomous transmissible plasmids into the chromosome of Escherichia coli K12. Genet Res. 1972 Apr;19(2):181–186. doi: 10.1017/s0016672300014427. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. L., Klopotowski T. Genetic map position of the gluconate-6-phosphate dehydrogenase gene in Salmonella typhimurium. J Bacteriol. 1968 Apr;95(4):1279–1282. doi: 10.1128/jb.95.4.1279-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Nomura M., Engbaek F. Expression of ribosomal protein genes as analyzed by bacteriophage Mu-induced mutations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1526–1530. doi: 10.1073/pnas.69.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Nishimura Y., Hirota Y. Transfer-defective mutants of sex factors in Escherichia coli. I. Defective mutants and complementation analysis. Genetics. 1970 Feb;64(2):173–188. doi: 10.1093/genetics/64.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E. Transfer-defective mutants of sex factors in Escherichia coli. II. Deletion mutants of an F-prime and deletion mapping of cistrons involved in genetic transfer. Genetics. 1970 Feb;64(2):189–197. doi: 10.1093/genetics/64.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. II. INTERACTION BETWEEN F-MEROGENOTE AND CHROMOSOME DURING TRANSFER. J Bacteriol. 1963 Jun;85:1402–1408. doi: 10.1128/jb.85.6.1402-1408.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. III. AN ANALYSIS OF THE RECOMBINATION EVENTS OCCURRING IN THE F' MALE AND IN THE ZYGOTES. Genetics. 1964 Jun;49:995–1007. doi: 10.1093/genetics/49.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., RAMAKRISHNAN T. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI. IV. EFFECT OF A CHROMOSOMAL DELETION ON CHROMOSOME TRANSFER. J Bacteriol. 1964 Aug;88:367–373. doi: 10.1128/jb.88.2.367-373.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S. R., Mazaitis A. J., Maas W. K., Kleinschmidt A. K. Characterization by electron microscopy of fused F-prime factors in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1873–1876. doi: 10.1073/pnas.69.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Non-essentiality of the recA- mutation in the phenomenon of bacteriophage M13-induced elimination of F' factors. J Bacteriol. 1971 Jun;106(3):1040–1042. doi: 10.1128/jb.106.3.1040-1042.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajchert-Trzpil M., Dobrzański W. T. Influence of mutagenic agents on the integration of the F episome into the chromosome of Escherichia coli K12F+. J Gen Microbiol. 1968 Nov;54(1):47–57. doi: 10.1099/00221287-54-1-47. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Roth J. R. Frameshift suppressors. II. Genetic mapping and dominance studies. J Mol Biol. 1972 May 28;66(3):483–493. doi: 10.1016/0022-2836(72)90428-7. [DOI] [PubMed] [Google Scholar]
- Riva S., Fietta A. M., Silvestri L. G., Romero E. Effect of rifampicin on expression of some episomal genes in E. coli. Nat New Biol. 1972 Jan 19;235(55):78–80. doi: 10.1038/newbio235078a0. [DOI] [PubMed] [Google Scholar]
- SCAIFE J., GROSS J. D. Inhibition of multiplication of an Flac factor in Hfr cells of Escherichia coli K-12. Biochem Biophys Res Commun. 1962 May 11;7:403–407. doi: 10.1016/0006-291x(62)90324-8. [DOI] [PubMed] [Google Scholar]
- Scaife J. Episomes. Annu Rev Microbiol. 1967;21:601–638. doi: 10.1146/annurev.mi.21.100167.003125. [DOI] [PubMed] [Google Scholar]
- Scaife J. F-prime factor formation in E. coli K12. Genet Res. 1966 Oct;8(2):189–196. doi: 10.1017/s0016672300010041. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethals: a new class of nonsense suppressors in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jun;63(2):392–399. doi: 10.1073/pnas.63.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson H., Somerville R. L. Expression of the tryptophan operon in merodiploids of Escherichia coli. I. Gene dosage, gene position and marker effects. Mol Gen Genet. 1971;111(4):342–351. doi: 10.1007/BF00569786. [DOI] [PubMed] [Google Scholar]
- Taylor A L, Adelberg E A. Linkage Analysis with Very High Frequency Males of Escherichia Coli. Genetics. 1960 Sep;45(9):1233–1243. doi: 10.1093/genetics/45.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wilkins A., Gallant J., Harada B. Genetic determinant in Escherichia coli affecting thymineless death and ultraviolet sensitivity. J Bacteriol. 1971 Dec;108(3):1424–1426. doi: 10.1128/jb.108.3.1424-1426.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M. Chromosome transfer from F-lac+ strains of Escherichia coli K-12 mutant at recA, recB, or recC. J Bacteriol. 1969 May;98(2):599–604. doi: 10.1128/jb.98.2.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Bastarrachea F. Genetic and physicochemical characterization of Escherichia coli strains carrying fused F' elements derived from KLF1 and F57. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1481–1485. doi: 10.1073/pnas.69.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L., Meynell G. G. Female-specific phages and F-minus strains of Escherichia coli K12. Mol Gen Genet. 1971;113(3):222–227. doi: 10.1007/BF00339542. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yu M. T., Vermeulen C. W., Atwood K. C. Location of the genes for 16S and 23S ribosomal RNA in the genetic map of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Sep;67(1):26–31. doi: 10.1073/pnas.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen J., Pato M. L. Replication of the F'lac sex factor in the cell cycle of Escherichia coli. Mol Gen Genet. 1971;111(3):242–255. doi: 10.1007/BF00433109. [DOI] [PubMed] [Google Scholar]


