Abstract
Fructose has recently been observed to affect brain metabolism and cognitive function in adults. Yet, possible late-onset effects by gestational fructose exposure have not been examined. We evaluated mitochondrial function in the brain of aging (15 months) male offspring of Fischer F344 rat dams fed a high-fructose diet (50% energy from fructose) during gestation and lactation. Maternal fructose exposure caused a significantly lower body weight of the offspring throughout life after weaning, while birth weight, litter size, and body fat percentage were unaffected. Isolated brain mitochondria displayed a significantly increased state 3 respiration of 8%, with the substrate combinations malate/pyruvate, malate/pyruvate/succinate, and malate/pyruvate/succinate/rotenone, as well as a significant decrease in the P/O2 ratio, compared with the control. Uncoupling protein 5 (UCP5) protein levels increased in the fructose group compared with the control (P=0.03) and both UCP5 mRNA and protein levels were inversely correlated with the P/O2 ratio (P=0.008 and 0.03, respectively), suggesting that UCP5 may have a role in the observed decreased phosphorylation efficiency. In conclusion, maternal high-fructose diet during gestation and lactation has long-term effects (fetal programming) on brain mitochondrial function in aging rats, which appears to be linked to an increase in UCP5 protein levels.
Keywords: aging, mitochondria, brain development, energy metabolism, gene regulation
Introduction
Fructose consumption has recently been compared with alcohol in terms of its adverse effects on health.1 Fructose consumption per capita in the western world has increased at a steady rate since the 1950s,2 mainly fueled by an almost fivefold increase in consumption of soft drinks sweetened with 10% to 15% sucrose or high-fructose corn syrup.3 This increased fructose ingestion has been linked in particular with obesity and type 2 diabetes,4 but is also considered as a risk factor in the development of dementia5 and a high-fructose intake is associated with impaired cognitive function in humans.6 Recently, a high-fructose intake in rats, coupled with a low omega-3 fatty acid intake, was observed to induce a metabolic syndrome like state in the brain as well as impaired cognitive function7 suggesting an interaction between fructose intake and brain function.
The last few decades it has become apparent that the fetal and early life nutrition and environment can permanently change the phenotype of the adult organism, a phenomenon termed as fetal programming,8 but more recently also termed as developmental programming, implying that fetal programming may in fact involve changes in normal development.9 Thus, a low birth weight has been observed with a maternal high-fructose diet10, 11 indicating possible developmental programming effects by a high maternal fructose intake. Taken together, these findings indicate that a high maternal fructose intake during gestation may well impact fetal growth in general as well as brain development and possibly brain function in adulthood of the offspring.
Thus, we hypothesized that feeding pregnant rats a diet with high-fructose content during gestation and lactation would affect brain mitochondrial function in late adult life of the offspring.
Materials and methods
Animals
All experimental procedures complied with guidelines laid down by The Danish Animal Experiments Inspectorate (permit 2013-15-2934-00904) and by the local animal facility at the University of Copenhagen, Denmark and were performed according to the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Female Fischer F344 rats (N=12) were obtained from Harlan Laboratories (Horst, The Netherlands) on day 1 of pregnancy and randomly assigned to two different dietary groups (ensuring an equal weight distribution per group) receiving either a high-fructose diet or a control diet (Research Diets Inc., New Brunswick, NJ, USA; Table 1) during gestation and the subsequent lactation period. The offspring was weighed <2 hours after birth and litter sizes normalized to N=8 per dam. After weaning (day 20 after birth), both groups of offspring were randomly reduced to 8 per group, with at least one pup taken from the litter of each dam, and all animals were put on a standard chow diet (Altromin 1319F; Altromin, Lage, Germany) for the rest of their lives. An Echo MRI 4-in-1 for Small Animals (EchoMRI, Houston, TX, USA) was used to monitor total body fat mass and lean mass of unanesthetized animals at 15 months of age. Body weights were measured once a week and snout–anus length measured post mortem at 15 months using a standard ruler. Offspring food and water intake was not monitored. One animal in the fructose group died during experimental procedures and one animal from each group died due to unknown causes between 12 and 15 months of age. The resulting animals (7 controls and 6 fructose exposed offspring) were killed at 15 months of age after an overnight fast and brain tissue removed for further analysis as described below.
Table 1. Diet composition.
| Diet component |
Control |
Fructose |
||
|---|---|---|---|---|
| Gram | kJ | Gram | kJ | |
| Casein | 200 | 3,347 | 200 | 3,347 |
| DL-Methionine | 3 | 50 | 3 | 50 |
| Corn starch | 535 | 8,954 | 50 | 837 |
| Maltodextrin | 125 | 2,092 | 125 | 2,092 |
| Fructose | 0 | 0 | 485 | 8117 |
| Cellulose | 50 | 0 | 50 | 0 |
| Corn oil | 50 | 1,883 | 50 | 1,883 |
| Mineral mix | 35 | 0 | 35 | 0 |
| Vitamin mix | 1 | 0 | 1 | 0 |
| Choline bitartrate | 2 | 0 | 2 | 0 |
| Total | 1,001 | 16,326 | 1,001 | 16,326 |
| Main diet components | Gram% | kJ% | Gram% | kJ% |
|---|---|---|---|---|
| Protein | 20.3 | 20.8 | 20.3 | 20.8 |
| Carbohydrate total | 65.9 | 67.7 | 65.9 | 67.7 |
| Carbohydrate from fructose | 0 | 0 | 48.5 | 49.7 |
| Fat | 5.0 | 11.5 | 5.0 | 11.5 |
| Fiber | 5.0 | 0 | 5.0 | 0 |
| Total | 100% | 100% |
Composition of the two diets given to pregnant and lactating Fischer F344 dams.
The rat strain chosen for this study, the Fischer 344 strain, is the most used strain in aging research and seems to be the preferred rat model in cognitive aging research.12 We chose it primarily as its life expectancy is significantly shorter than common outbred rat strains, and as developmental programming effects, and especially effects on brain function, have a tendency to be more pronounced in late adult life.13
Preparation of Isolated Mitochondria
Mitochondria were isolated from brain (without the cerebellum) as previously described by Rosenthal et al.14 using a discontinuous Percoll gradient centrifugation.15 Overnight fasted rats were sedated with a mixture of Hypnorm (active ingredients fentanyl and fluanisone at a concentration of 0.079 and 2.5 mg/mL, respectively) and Dormicum (active ingredient midazolam at a concentration of 1.25 mg/mL) in water given subcutaneous as 0.3 mL per 100 g of body weight. After sedation, the head was quickly cutoff with a pair of scissors and the brain extracted, followed by removal of cerebellum. A small sample was frozen in liquid nitrogen and stored at −80°C for later RNA isolation. The remaining brain tissue (∼1 g) was placed in ice-cold buffer A (225 mmol/L mannitol, 75 mmol/L sucrose, 5 mmol/L Hepes, 1 mmol/L EGTA, and 1 mmol/L ATP, pH 7.4), minced with a pair of scissors, and homogenized in ∼10 volume buffer A using a Potter-Elvehjem (VWR, Radnar, PA, USA) with a loose fit Teflon pistil (10 to 15 strokes at 200 r.p.m.). Buffer A was then added to the homogenate to a total volume of 20 mL and centrifuged at 1,300 g for 3 minutes. The supernatant was transferred to a new tube and centrifuged at 20,000 g for 10 minutes. The pellet was resuspended in 2.5 mL 15% Percoll (v/v in buffer A) and transferred to a 15-mL centrifuge tube. Using a syringe, 2.5 mL 23% Percoll was added to the bottom, followed by 2.5 mL 40% Percoll, and the tube was centrifuged at 30,700 g for 10 minutes (with centrifuge brakes off). The lower fraction containing the mitochondria was carefully removed using a syringe and transferred to a new 15 mL centrifuge tube, to which buffer A was added to a total volume of 10 mL. The tube was then centrifuged at 16,600 g for 10 minutes and the supernatant gently removed. The pellet was resuspended in 10 mL buffer A and centrifuged at 6,300 g for 10 minutes. The resulting pellet was resuspended in 600 μL buffer B (225 mmol/L mannitol, 75 mmol/L sucrose, 5 mmol/L Hepes pH 7.4). A 25-μL sample for measuring citrate synthase enzymatic activity and protein determination was removed and frozen at −80°C until analysis. The rest of the mitochondrial suspension was kept on ice for high-resolution mitochondrial respirometry.
The activity of citrate synthase, which is expressed only in the mitochondrial matrix, was assayed on all mitochondrial preparations as described.16 Protein concentrations were determined as described17 using bovine serum albumin as a standard.
Mitochondrial Respiration
Mitochondrial function was tested using Oroboros Oxygraph-2K instruments (Oroboros Inc., Innsbruck, Austria) operating eight oxygraph chambers in parallel at 25°C. Briefly, 20 μL mitochondrial suspension was added to 2 mL of incubation buffer (125 mmol/L KCl, 20 mmol/L Hepes, 2 mmol/L K2HPO4, 1 mmol/L MgCl2, 0.1 mmol/L EGTA, 0.025% BSA (FFA free), pH 7.0 at room temperature) in each chamber. Stirrer speed was 600 r.p.m. Approximately 5 minutes after the mitochondria were introduced into the chambers, malate and pyruvate were added to concentrations of 1 and 0.5 mmol/L, respectively to achieve state 4 respiration. Once a steady-state 4 respiration was observed, a small known amount of ADP (0.4 μmol) was added to initiate state 3 respiration and to estimate the P/O2 ratio. Subsequently, state 3 respiration was reestablished by raising the ADP concentration to 2.0 mmol/L followed by the addition of succinate (5 mmol/L) to involve full complex II contribution to respiration. To conclude the experiment, the complex I inhibitor rotenone was added to a final concentration of 50 nmol/L.18 All measurements were performed in duplicate. Between experiments the chambers were washed twice with ethanol and water. All mitochondrial preparations had respiratory control ratios (RCR, state 3 with pyruvate/state 4) above 16, indicating consistent preservation of mitochondrial function after isolation.
RNA Isolation, Quantitative Real-Time PCR, and Affymetrix Microarrays
RNA was extracted from 20 to 30 mg brain tissue using Qiazol (Qiagen, Valencia, CA, USA) together with a Qiagen Tissuelyzer (Qiagen), as described by the manufacturer, followed by cDNA synthesis from 1.5 μg RNA in a 20-μL volume using the High Capacity cDNA Reverse Transcription Kit with RNase inhibitor and random hexamer primers (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's instructions employing an Eppendorf Thermo cycler (Applied Biosystems). A nonreverse transcription and a nontemplate control reaction were included and the cDNA product was diluted to a volume of 200 μL and stored at −20°C until further analysis.
cDNA was amplified using either SYBR Select Master mix or TaqMan Gene Expression Master Mix (Applied Biosystems) according to standard conditions ((95°C 10 minutes) × 1, (95°C 15 seconds, 60°C 1 minute, 95°C 15 seconds, 60°C 15 seconds, 95°C 15 seconds) × 50 cycles in a total volume of 10 μL with a melting curve from 60 to 100°C when using SYBR Green) in 384-well plates on an ABI PRISM 7900 sequence detector (Applied Biosystems). In addition to the samples of interest, standardized dilutions of pooled cDNA from all samples were amplified. The relative contents of the genes were quantified on the basis of the standard curve method using the standardized control dilutions for the standard curve. Samples were run in triplicate and all samples were run together allowing relative comparison between all conditions. Target gene mRNA levels were normalized to β-Actin mRNA levels.
β-Actin, forward; 5′-ATTGGTGGCTCTATCCTGGC-3′, reverse; 5′-CAGCTCAGTAACAGTCCGC-3′. Uncoupling protein 1 (UCP1), forward; 5′-TCCCTCAGGATTGGCCTCTAC-3′, reverse; 5′-GTCATCAAGCCAGCCGAGAT-3′, probe; 5′-TAM-AACGCCTGCCTCTTTGGGAAGCAA-FAMRA-3′. UCP2, forward; 5′-GAGAGAGTCAAGGGCTAGCGC-3′, reverse; 5′-GCTTCGACAGTGCTCTGGTATCT-3′, probe; 5′-FAM-CAGGCATCGGGAGCCGCCTC-TAMRA-3′. UCP3, forward; 5′-TGGACTTCAGCCATCAGAAGTG-3′, reverse; 5′-GTGGGTTGAGCACAGGTCACT-3′, probe; 5′-FAM-TCGCCATCAGGATTCTGGCAGGC-TAMRA-3′. UCP4, forward; 5′-CGTGGCCGAGCTAGCAAC-3′, reverse; 5′-CCAGAGGGGATAATGTTCATCTTC-3′, probe; 5′-FAM-GCAAGGAGTGACGCCCGCCA-TAMRA-3′. UCP5, forward; 5′-CAGTGATTGTAAGCGGACATCAG-3′, reverse; 5′-GGTTGGCAATAGTAGATGAAATCACTC-3′, probe; 5′-FAM-AAGCCATGAGATGTCTGGTCTGAACTGGAA-TAMRA-3′.
Gene Expression Microarrays
For gene expression profiling by microarrays, RNA isolated by Qiazol from 15-month-old Fischer rats as described above was further purified using the RNeasy kit (Qiagen) and the quality of RNA assured using a Bioanalyzer (Agilent, Santa Clara, CA, USA) as described by the manufacturer (all samples had an RIN of >8.0). The samples were analyzed at the RH Microarray Center (Center of Genomic Medicine, Rigshospitalet, Copenhagen, Denmark) with cDNA prepared using the Ambion WT Expression Kit (Life Technologies, Paisley, UK) and cDNA labeling performed using the Affymetrix Whole Transcription (WT) Expression kit (Affymetrix Inc., Santa Clara, CA, USA) before being analyzed on GeneChip Rat Gene 1.0 ST Arrays (Affymetrix) according to the manufacturer's instructions. The microarrays were analyzed using R 3.0.1 (open source: http://www.r-project.org) and Bioconductor 2.12 (open source: http://www.bioconductor.org). Raw CEL files for all samples were normalized using quantile normalization and log2 probe expression values were obtained using the Robust Multi-array Average method with default parameters in R/Bioconductor. The expression level threshold (detection limit) for the arrays was set to the median of the antigenomic (negative controls) probeset expression levels+s.d. and calculated for each sample. Starting out with 29,214 probesets, control probesets, and probesets not detected on at least 50% of the arrays in each group were removed resulting in 10,288 probesets. This was further refined by removing unknown genes resulting in 8,980 known genes (Supplementary Table). We examined whether any genes were changed significantly between the control and gestational fructose group using SAM (Significance Analysis of Microarrays)19 and found no differences. To examine whether any gene sets were significantly changed we employed GSA20 and GSEA21 using gene sets from MSigDB v4.0 (Molecular Signatures Database, Broad Institute, Cambridge, MA, USA; http://www.broadinstitute.org/gsea/msigdb) and found no differences. To obtain the mitochondrial genes, the 8,980 known genes were filtered by selecting all genes containing the string ‘mitochon' in the GO cellular component annotation database resulting in 923 genes (data not shown). The raw CEL files as well as the Robust Multi-array Average normalized data have been deposited in the NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo) and are accessible through GEO series accession number GSE50151.
Western Blotting
Twenty micrograms of mitochondrial protein of each sample was subjected to SDS-PAGE (4% to 12% Bis-Tris precast gels, NuPage) followed by electroblotting onto a PVDF membrane. The membrane was blocked for 1 hour in 5% skim milk in Tris-Buffered Saline containing 0.1% Tween-20 (TBS-T) followed by incubation overnight with an affinity-purified rabbit antibody against rat UCP5 (UCP51-A; Alpha Diagnostic Intl. Inc., San Antonio, TX, USA) at a 1:250 dilution in 5% skim milk in TBS-T and incubated overnight at 4°C. The membrane was washed in TBS-T and incubated for 1 hour at room temperature with horseradish peroxidase-conjugated anti-rabbit (Dako, Glostrup, Denmark) diluted 1:10,000) in 5% skim milk in TBS-T. After washing, specific binding was detected using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Pierce Technology, Omaha, NE, USA) and visualized using a Syngene G-Box gel imaging system (Syngene, Cambridge, UK). Quantification was performed using the Image J gel analysis software (open source: http://rsbweb.nih.gov) and UCP5 protein amounts were normalized to the mean of the control samples. All buffers and gels were purchased from Invitrogen (Carlsbad, CA, USA).
Statistics
Differences between groups were tested by Student's t-test. Correlation was tested using Pearson's correlation using all data points. The mRNA data were log transformed to ensure a normal distribution. All statistical analyses were performed using SAS 9.2 (The SAS Institute, Cary, NC, USA). A P value of <0.05 was considered as significant while a P value between 0.05 and 0.1 was considered as a trend. A P value above 0.1 was considered as nonsignificant (NS). All data except mRNA levels are presented as means±standard error of the mean (s.e.m.). The mRNA levels are presented as geometric means with 95% confidence intervals.
Results
Dam and Offspring Physiologic Data
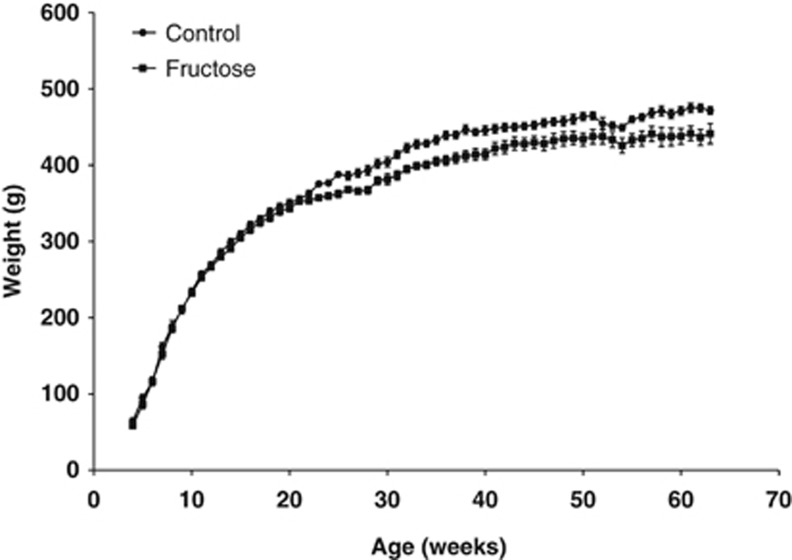
Pregnant rat dams were subjected to two different but isocaloric diet regimes from day 1 of pregnancy until the end of lactation (day 20 after birth): (1) a high-fructose diet (50% energy from fructose) and (2) a control diet (fructose exchanged with corn starch) (Table 1). The fructose group tended to have an increased food intake during pregnancy compared with control (P=0.09) and had a significantly higher weight gain during gestation (E0-E20 body weight gain, P=0.005) (Table 2). Nevertheless, there was no difference in birth weight or litter size between groups. Interestingly, the fructose exposed offspring displayed a lower body weight throughout life, except between 1 month (4 weeks) and 5 months (22 weeks) of age (Figure 1; Table 2), and had a significantly shorter snout–anus span at 15 months of age (P=0.035), while body fat and lean mass at the same time point were unaffected by gestational fructose exposure (Table 2).
Table 2. Dam and offspring data.
| Dam and birth data | Control, N=6 | Fructose, N=6 | P value |
|---|---|---|---|
| Dam body weight E0 (g) | 179±3.0 | 175±3.0 | NS |
| Dam body weight E20 (g) | 244±3.5 | 248±3.0* | 0.085 |
| Dam body weight increase E0-E20 (g) | 66±0.9 | 73±1.8* | 0.005 |
| Dam food intake E0-E20 (kJ) | 3,896±132 | 4,210±103 | 0.09 |
| Litter size | 8.5±0.6 | 9.5±0.5 | NS |
| Birth weight (mean of means) (g) | 4.64±0.34 | 4.68±0.35 | NS |
| Male offspring data | Control, N=7–8 | Fructose, N=6–8 | P value |
|---|---|---|---|
| Body weight at weaning (g) | 30.4±0.7 | 28.2±0.5* | 0.014 |
| Body weight at 3 months (g) | 269±4.0 | 266±3.9 | NS |
| Body weight at 6 months (g) | 388±2.9 | 362±4.8* | 0.001 |
| Body weight at 9 months (g) | 447±6.0 | 412±7.1* | 0.002 |
| Body weight at 12 months (g) | 465±4.9 | 437±9.5* | 0.025 |
| Body weight at 15 months (g) | 473±5.2 | 437±12.6* | 0.046 |
| Fasted body weight at 15 months (g) | 438±5.7 | 407±10.0* | 0.016 |
| Fat percentage at 15 months (%) | 14.2±1.1 | 14.1±2.2 | NS |
| Lean percentage at 15 months (%) | 68.8±0.7 | 69.5±1.9 | NS |
| Snout–anus length at 15 months (cm) | 25.4±0.2 | 24.7±0.3* | 0.035 |
NS, nonsignificant.
Pregnant Fischer F344 dams were subjected to either a control or a high-fructose diet during gestation and lactation and their male offspring was followed until 15 months of age where brain mitochondria were isolated. Figures are means±s.e.m.
* Indicates P<0.05 compared with control rats by Student's t-test. For male offspring data, N=8 in both the control and fructose groups from 0 to 12 months. At 15 months, N=7 for the control group and N=6 for the fructose group.
Figure 1.
Growth curves. Growth curves of Fischer F344 rat offspring subjected to either a control diet or a fructose diet during gestation and lactation. N=7–8 and 6–8 for controls and fructose-fed animals, respectively.
Brain Mitochondria
The mitochondrial function was examined in isolated mitochondria (Table 3). With malate and pyruvate as substrates, the fructose exposed offspring showed unchanged state 4, but increased state 3 respiration from 197±5.3 to 213±4.4 nmol/min per mg mitochondrial protein (P=0.040) and similarly an increase from 283±7.4 to 307±6.9 nmol/min·per mg mitochondrial protein (P=0.037) with malate, pyruvate, and succinate as substrates. When inhibiting complex I with rotenone, respiration was still higher in the fructose exposed offspring compared with controls (182±4.1 and 168±4.5, respectively, P=0.048). Furthermore, the fructose exposed offspring exhibited a decrease in the P/O2 ratio (P=0.029) with malate and pyruvate as substrates.
Table 3. Respiration rate and phosphorylation efficiency (P/O2 ratio) of isolated brain mitochondria.
| Respiratory state and substrates | Control, N=7 | Fructose, N=6 | P |
|---|---|---|---|
| State 4: 0.5 mmol/L pyruvate | 10.4±0.4 | 11.1±0.2 | NS |
| State 3: 0.5 mmol/L pyruvate+2 mmol/L ADP | 197±5.3 | 213±4.4* | 0.040 |
| State 3+5 mmol/L succinate | 283±7.4 | 307±6.9* | 0.037 |
| State 3+5 mmol/L succinate+50 μmol/L Rotenone | 168±4.5 | 182±4.1* | 0.048 |
| P/O2, pyruvate (mole ATP per mole oxygen) | 4.86±0.05 | 4.69±0.04* | 0.029 |
NS, nonsignificant.
Mitochondria isolated from whole brain (without cerebellum) of 15-month-old Fischer F344 rats whose mothers were subjected to either a control diet or a high-fructose diet during gestation and lactation. Mitochondria were examined by high-resolution respirometry. The oxygen consumptions are given as nmol O2/min·(per mg mitochondrial protein). Malate (1 mmol/L) was present in all incubations in addition to the substrate additions indicated. Figures are means±s.e.m.
* Indicates P<0.05 compared with control rats by Student's t-test.
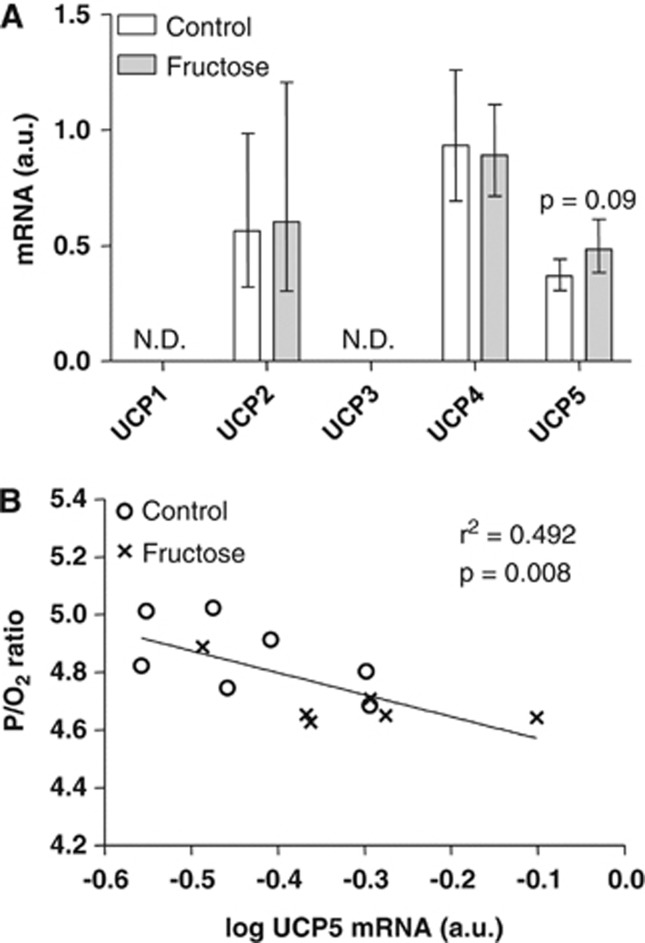
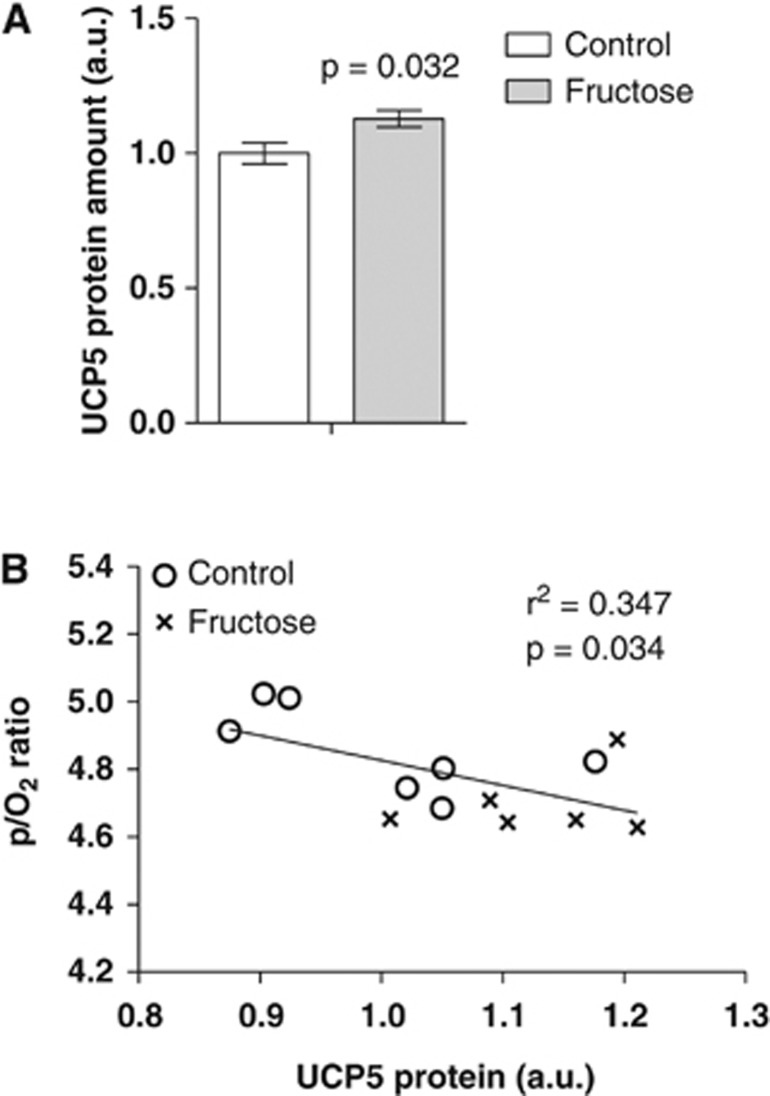
Taken together, these results prompted us to examine the mRNA levels of the brain UCPs 1 to 5 by quantitative real-time PCR (Figure 2A). The mRNA levels of UCP1 and UCP3 were below the detection limit and there was no change in the mRNA levels of UCP2 and UCP4. However, we observed a tendency (P=0.09) toward an increase in UCP5 mRNA levels in the fructose offspring compared with control, and interestingly, we found a highly significant inverse correlation between UCP5 mRNA levels and the P/O2 ratio (P=0.008) (Figure 2B). There was, however, no correlation between P/O2 ratio and UCP2 or UCP4 mRNA levels (data not shown). The protein levels of UCP5 were similarly increased in the fructose offspring compared with control (P=0.032) (Figure 3A), and inversely correlated with the P/O2 ratio (P=0.034) (Figure 3B). This suggests that increased levels of UCP5 might be causally linked with the observed P/O2 ratio decrease. There was no difference in citrate synthase activity of the isolated brain mitochondria between the control and fructose exposed offspring (3.55±0.09 and 3.71±0.05 U·(per mg mitochondrial protein), respectively, P=0.16).
Figure 2.
Levels of brain uncoupling protein (UCP) mRNA. (A) Brain mRNA levels of 15-month-old Fischer F344 rats subjected to either a control diet or a fructose diet during gestation and lactation. The mRNA levels were normalized to β-actin and are given as geometric means with 95% confidence intervals. ND, not detected. (B) Pearson's correlation between log-transformed UCP5 mRNA levels and P/O2 ratios (see Table 3).
Figure 3.
Brain uncoupling protein 5 (UCP5) levels. (A) Brain UCP5 protein levels (mean±s.e.m.) of 15-month-old Fischer F344 rats subjected to either a control diet or a fructose diet during gestation and lactation. (B) Pearson's correlation between UCP5 protein levels and P/O2 ratios (see Table 3).
Brain Gene Expression Profiling
In an attempt to examine whether other genes might be able to explain the observed fetal programming effects of fructose exposure in early life, we also examined the brain gene expression profile of the two groups of animals at age 15 months using Affymetrix microarrays. No changes in mRNA expression levels were observed (Supplementary Table) and perhaps more importantly, no genes were changed when examining mitochondrial genes separately (data not shown). Furthermore, there were neither changed gene sets when examining the data using either GSEA (26) or GSA (25) nor were any other mitochondrial genes significantly correlated with the P/O2 ratio (data not shown). Uncoupling protein 5 mRNA levels were below the detection limit of the microarrays, despite being detected by quantitative real-time PCR, most likely due to the lower sensitivity of microarrays compared with quantitative real-time PCR.
Discussion
In the present study, we examined the possible developmental programming effects of high-fructose exposure in early life upon mitochondrial function in the aging brain of 15-month-old offspring. We found that the high-fructose exposure resulted in significant differences in the function of brain mitochondria isolated from the aged offspring. Thus, mitochondria from the fructose group displayed an increased state 3 respiration rate, a decreased P/O2 ratio, and a strong inverse correlation between the P/O2 ratio and the UCP5 mRNA as well as UCP5 protein levels. In other words, we show for the first time that gestational fructose exposure has a pronounced developmental programming effect on brain mitochondrial function at old age in the offspring.
Developmental Programming of Mitochondrial Function by Maternal Fructose Exposure
The brain mitochondria in the fructose group presented with an increased respiration and a lower energy conservation efficiency as reflected by a decreased P/O2 ratio as examined with malate and pyruvate as substrates. Thus, it would seem that respiration was increased to compensate for a lower ATP production caused by an enhanced UCP5 generated uncoupling. Accordingly, the actual state 3 ATP production was similar in the control and the fructose groups (1002±17.9 and 954±25.4 nmol/min per mg protein, respectively; calculated as the P/O2 ratio multiplied by the state 3 oxygen consumption; P=0.16). Recently, a decrease in overall P/O2 ratios in brain mitochondria was suggested to be related to a decrease in flux through Complex I of the electron transport chain.22 This seems at variance with our results, at least in the sense that gestational fructose exposure increased respiration also in the presence of rotenone (168±4.5 and 182±4.1 nmol/min per·mg mitochondrial protein, respectively, Table 3).
While the observed changes in mitochondrial phosphorylation efficiency after fructose exposure are statistically significant, they are nominally small (4% to 9%). It may be argued that such a small change, in spite of being statistically significant, may not be significant in terms of brain function, and this aspect needs to be examined further. However, it should be noted that a recent study found a similar small change in the P/O2 ratio in aging rat brain compared with younger animals,23 suggesting that even nominally small changes in mitochondrial efficiency may actually have a functional role in or be a marker of aging. Seen in this perspective we speculate that gestational fructose exposure may provoke/accelerate aging-related metabolic changes in the brain.
In rats, there seems to be a direct link between increased fructose intake in adult rats and impaired whole body metabolic function (hyperlipidemia and insulin resistance) as well as a cognitive function, for example, memory deficit.7 It remains however, to be examined whether a lower efficiency of mitochondrial ATP production in maternal fructose exposed offspring, as observed in the present study, can be similarly linked with a change in cognitive function.
Uncoupling Proteins and Mitochondrial Function
A decrease in P/O2 ratio is usually the effect of an increased proton leak through the inner mitochondrial membrane, for example, via an enhanced UCP activity.24 To examine whether a change in the expression level of UCPs could be responsible for the decrease in P/O2 ratio in the fructose group, we measured mRNA levels of UCP1-5 and found a trend toward an increase in UCP5 mRNA, but not in the other UCP mRNA levels. Noteworthy, we found a strong inverse correlation between the P/O2 ratio and the UCP5 mRNA as well as protein levels.
A change in UCP5 expression in relation to brain mitochondrial respiration has been observed previously. Thus, a ketogenic diet induces an increase in brain mitochondrial respiration as well as an increase in UCP5.25 Also, hypoxic conditions inducing a decrease in brain mitochondrial respiration were found to increase UCP5.26 In the present study, we did not observe an increase in basal, state 4, respiration, which has been reported by others when overexpressing UCP5 in neuroblastoma cells.27, 28 As UCP5 has also been suggested to have a role in protection against oxidative stress and mitochondrial dysfunction29 the increase in UCP5 may also be a compensatory mechanism caused by the maternal fructose-induced changes in mitochondrial function.
Coincidentally, another UCP, UCP1, has been shown to be regulated by epigenetic modification of its promoter,30 and we speculate that the increased UCP5 mRNA levels observed in this study could be due to similar modifications of the UCP5 promoter caused by maternal fructose exposure. Currently, there are however no data regarding epigenetic regulation of UCP5 expression.
Developmental Programming by Fructose
The results of the present study together with literature data support the hypothesis tested in the present study that a high-fructose intake during gestation can cause adverse developmental programming. Thus, fructose intake during gestation has been shown to cause changes in glucose metabolism,10, 11, 31 and lipid metabolism,10, 32, 33 as well as decreased fetal leptin signaling34 in the offspring. Several of the studies mentioned above also reported a fructose-induced decrease in birth weight,10, 11 whereas others including the present study (Table 2) did not see this effect.31, 33, 34 The decrease in offspring body weight in adulthood seen in the present study (Figure 1) due to a high maternal fructose intake has been reported by others as well.11, 31
In the present study, we found no difference in body fat percentage at 15 months but a lower snout-to-anus length suggesting that maternal fructose impairs development and/or the growth program of the offspring, perhaps involving changes in growth hormones during fetal life or early adulthood. One might also speculate that the observed effect of maternal fructose upon energy metabolism in offspring brain could be due to a disturbance of the hypothalamic pituitary adrenal axis, which is commonly associated with developmental programming.35
The pathophysiology of Fructose in a Metabolic Context
Fructose is thought to exhibit its long-term negative effects on metabolism by an increase in hepatic lipogenesis, primarily saturated fatty acids, causing dyslipidemia,36 an increase in low-grade inflammation,37 as well as increased oxidative stress.38 Pregnancy may exacerbate the adverse effects of fructose as several studies in rats have shown a faster and larger fructose induced increase in dam liver size during gestation compared with nonpregnant controls.31
Sufficient supply of essential fatty acids during gestation is important for normal fetal brain development39 and consuming an n-3 fatty acid deficient diet during gestation results in lasting changes in brain development in the offspring.40 One might speculate that the increase in nonessential saturated fatty acids in plasma caused by a maternal fructose diet might compete with transport of essential fatty acids across the placenta resulting in a fetal deficiency in n-3 fatty acids, and hence be one mechanism by which fructose affects the brain during gestation. Furthermore, maternal inflammation has been shown to cause adverse developmental programming of the offspring brain.41
Conclusions
The present study shows that a high-fructose intake during gestation and lactation results in developmental programming in the form of significant changes in the mitochondrial phenotype in the brain of aging offspring. The observed change in mitochondrial coupling between respiration and ATP formation most likely involves an increase in brain UCP5 protein levels.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported by The Danish Strategic Research Council grant #09-067124 and #09-059921, Danish Medical Research Council grant #271-07-0732 and #09-073413, by Købmand i Odense Johann og Hanne Weimann f. Seedorffs Legat, Gangstedfonden, Ernst Fischers mindelegat, Eva og Hans Carl Adolfs Mindelegat, and Direktør Emil Hertz og Hustru Inger Hertz Fond.
Supplementary Material
References
- Lustig RH, Schmidt LA, Brindis CD. Public health: The toxic truth about sugar. Nature. 2012;482:27–29. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv Nutr. 2013;4:220–225. doi: 10.3945/an.112.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev. 2007;65:S13–S23. doi: 10.1111/j.1753-4887.2007.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Stephan BCM, Wells JCK, Brayne C, Albanese E, Siervo M. Increased fructose intake as a risk factor for dementia. J Gerontol A Biol Sci Med Sci. 2010;65:809–814. doi: 10.1093/gerona/glq079. [DOI] [PubMed] [Google Scholar]
- Ye X, Gao X, Scott T, Tucker KL. Habitual sugar intake and cognitive function among middle-aged and older Puerto Ricans without diabetes. Br J Nutr. 2011;106:1423–1432. doi: 10.1017/S0007114511001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome' in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol (Lond) 2012;590:2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen KL, Rochon C, Zhong SB, Whitcomb L. Fructose and sucrose feeding during pregnancy and lactation in rats changes maternal and pup fuel metabolism. J Nutr. 1991;121:1999–2005. doi: 10.1093/jn/121.12.1999. [DOI] [PubMed] [Google Scholar]
- Rawana S, Clark K, Zhong S, Buison A, Chackunkal S, Jen KL. Low dose fructose ingestion during gestation and lactation affects carbohydrate metabolism in rat dams and their offspring. J Nutr. 1993;123:2158–2165. doi: 10.1093/jn/123.12.2158. [DOI] [PubMed] [Google Scholar]
- LaSarge C, Nicolle M.Comparison of different cognitive rat models of human agingIn: Bizon JL, Woods A (eds). Animal Models of Human Cognitive Aging Humana Press; 200973–102. [Google Scholar]
- Kajantie E. Early-life events. Effects on aging. Hormones (Athens) 2008;7:101–113. doi: 10.1007/BF03401501. [DOI] [PubMed] [Google Scholar]
- Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7:752–758. doi: 10.1038/jcbfm.1987.130. [DOI] [PubMed] [Google Scholar]
- Tretter L, Takacs K, Hegedus V, Adam-Vizi V. Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria. J Neurochem. 2007;100:650–663. doi: 10.1111/j.1471-4159.2006.04223.x. [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide. Humana Press; 1993. [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Fritzen AJ, Grunnet N, Quistorff B. Flux control analysis of mitochondrial oxidative phosphorylation in rat skeletal muscle: pyruvate and palmitoyl-carnitine as substrates give different control patterns. Eur J Appl Physiol. 2007;101:679–689. doi: 10.1007/s00421-007-0544-2. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. On testing the significance of sets of genes. Ann Appl Stat. 2007;1:107–129. [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Cocco T, Pacelli C, Sgobbo P, Villani G. Control of OXPHOS efficiency by complex I in brain mitochondria. Neurobiol Aging. 2009;30:622–629. doi: 10.1016/j.neurobiolaging.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Vančová O, Bačiak L, Kašparová S, Kucharská J, Palacios HH, Horecký J, et al. In vivo and in vitro assessment of brain bioenergetics in aging rats. J Cell Mol Med. 2010;14:2667–2674. doi: 10.1111/j.1582-4934.2009.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluse FE. Uncoupling proteins: molecular, functional, regulatory, physiological and pathological aspects. Adv Exp Med Biol. 2012;942:137–156. doi: 10.1007/978-94-007-2869-1_6. [DOI] [PubMed] [Google Scholar]
- Kim-Han JS, Reichert SA, Quick KL, Dugan LL. BMCP1: a mitochondrial uncoupling protein in neurons which regulates mitochondrial function and oxidant production. J Neurochem. 2001;79:658–668. doi: 10.1046/j.1471-4159.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- Kwok KH-H, Ho PW-L, Chu AC-Y, Ho JW-M, Liu H-F, Yiu DC-W, et al. Mitochondrial UCP5 is neuroprotective by preserving mitochondrial membrane potential, ATP levels, and reducing oxidative stress in MPP+ and dopamine toxicity. Free Radic Biol Med. 2010;49:1023–1035. doi: 10.1016/j.freeradbiomed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu Y, Xia C, Gao P, Liu J-Z. Evidence for involvement of uncoupling proteins in cerebral mitochondrial oxidative phosphorylation deficiency of rats exposed to 5,000 m high altitude. Neurochem Res. 2013;38:282–289. doi: 10.1007/s11064-012-0917-8. [DOI] [PubMed] [Google Scholar]
- Ramsden DB, Ho PW-L, Ho JW-M, Liu H-F, So DH-F, Tse H-M, et al. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012;2:468–478. doi: 10.1002/brb3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A, Karamitri A, Kemp P, Speakman JR, Lomax MA. Role of Ucp1 enhancer methylation and chromatin remodelling in the control of Ucp1 expression in murine adipose tissue. Diabetologia. 2010;53:1164–1173. doi: 10.1007/s00125-010-1701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Arentson EJ, Teegarden D, Koser SL, Onyskow L, Donkin SS. Fructose consumption during pregnancy and lactation induces fatty liver and glucose intolerance in rats. Nutr Res. 2012;32:588–598. doi: 10.1016/j.nutres.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching RHH, Yeung LOY, Tse IMY, Sit W-H, Li ETS. Supplementation of bitter melon to rats fed a high-fructose diet during gestation and lactation ameliorates fructose-induced dyslipidemia and hepatic oxidative stress in male offspring. J Nutr. 2011;141:1664–1672. doi: 10.3945/jn.111.142299. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Kumazawa M, Sato S. Fructose intake during pregnancy up-regulates the expression of maternal and fetal hepatic sterol regulatory element-binding protein-1c in rats. Endocrine. 2013;44:79–86. doi: 10.1007/s12020-012-9815-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez L, Panadero MI, Roglans N, Otero P, Alvarez-Millán JJ, Laguna JC, et al. Fructose during pregnancy affects maternal and fetal leptin signaling. J Nutr Biochem. 2013;24:1709–1716. doi: 10.1016/j.jnutbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2013;34:27–46. doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L, Lê K-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- Liu T-T, Shih K-C, Kao C-C, Cheng W-T, Hsieh P-S. Importance of cyclooxygenase 2-mediated low-grade inflammation in the development of fructose-induced insulin resistance in rats. Chin J Physiol. 2009;52:65–71. [PubMed] [Google Scholar]
- Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y. High fructose feeding of magnesium deficient rats is associated with increased plasma triglyceride concentration and increased oxidative stress. Magnes Res. 2003;16:7–12. [PubMed] [Google Scholar]
- Rombaldi Bernardi J, de Souza Escobar R, Ferreira CF, Pelufo Silveira P. Fetal and neonatal levels of omega-3: effects on neurodevelopment, nutrition, and growth. ScientificWorldJournal. 2012;2012:202473. doi: 10.1100/2012/202473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-F, Su H-M. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J Nutr Biochem. 2013;24:70–80. doi: 10.1016/j.jnutbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–294. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.