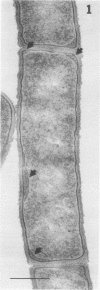
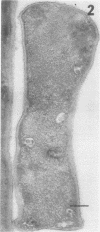
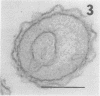
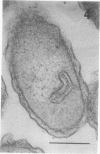
Full text
PDF




















































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER M., WILSON P. W. Intracellular distribution of tricarboxylic acid cycle enzymes in Azotobacter vinelandii. J Bacteriol. 1956 Feb;71(2):252–253. doi: 10.1128/jb.71.2.252-253.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M. Mesosomes in blue-green algae. Arch Mikrobiol. 1972;84(3):199–206. doi: 10.1007/BF00425198. [DOI] [PubMed] [Google Scholar]
- Altenburg B. C., Suit J. C., Brinkley B. R. Ultrastructure of deoxyribonucleic acid-membrane associations in Escherichia coli. J Bacteriol. 1970 Oct;104(1):549–555. doi: 10.1128/jb.104.1.549-555.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburg B. C., Suit J. C. Relation between deoxyribonucleic acid and intracytoplasmic membranes in Escherichia coli O111a. J Bacteriol. 1970 Jul;103(1):227–237. doi: 10.1128/jb.103.1.227-237.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell M., Work T. S. The biogenesis of mitochondria. Annu Rev Biochem. 1970;39:251–290. doi: 10.1146/annurev.bi.39.070170.001343. [DOI] [PubMed] [Google Scholar]
- Attardi G., Ojala D. Mitochondrial ribssome in HeLa cells. Nat New Biol. 1971 Feb 3;229(5):133–136. doi: 10.1038/newbio229133a0. [DOI] [PubMed] [Google Scholar]
- Avner P. R., Coen D., Dujon B., Slonimski P. P. Mitochondrial genetics. IV. Allelism and mapping studies of oligomycin resistant mutants in S. cerevisiae. Mol Gen Genet. 1973 Sep 5;125(1):9–52. doi: 10.1007/BF00292982. [DOI] [PubMed] [Google Scholar]
- BARRNETT R. J., PALADE G. E. Histochemical demonstration of the sites of activity of dehydrogenase systems with the electron microscope. J Biophys Biochem Cytol. 1957 Jul 25;3(4):577–588. doi: 10.1083/jcb.3.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D. Effects of magnesium ion deficiency on Escherichia coli and possible relation to the mode of action of novobiocin. J Bacteriol. 1962 Oct;84:679–682. doi: 10.1128/jb.84.4.679-682.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon K., White D. Phospholipid metabolism and membrane synthesis during sporulation in Bacillus megaterium. J Bacteriol. 1974 Apr;118(1):225–230. doi: 10.1128/jb.118.1.225-230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L. Corynebacterium diphtheriae and its relatives. Bacteriol Rev. 1970 Dec;34(4):378–422. doi: 10.1128/br.34.4.378-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard T. The ultrastructural differentiation of brown adipose tissue in the rat. J Ultrastruct Res. 1969 Nov;29(3):311–322. doi: 10.1016/s0022-5320(69)90109-9. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Beaton C. D. An electron microscope study of the mesosomes of a penicillinase-producing staphylococcus. J Gen Microbiol. 1968 Jan;50(1):37–42. doi: 10.1099/00221287-50-1-37. [DOI] [PubMed] [Google Scholar]
- Beining P. R., Huff E., Prescott B., Theodore T. S. Characterization of the lipids of mesosomal vesicles and plasma membranes from Staphylococcus aureus. J Bacteriol. 1975 Jan;121(1):137–143. doi: 10.1128/jb.121.1.137-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Brandt J. T., Martin A. P., Lucas F. V., Vorbeck M. L. The structure of rat liver mitochondria: a reevaluation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1097–1104. doi: 10.1016/s0006-291x(74)80091-4. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D. Bacterial mesosomes. Sci Prog. 1972 Winter;60(240):527–546. [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. The structure and development of mesosomes studied in Bacillus licheniformis strain 6346. J Ultrastruct Res. 1972 Jan;38(1):113–133. doi: 10.1016/s0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J Bacteriol. 1968 Oct;96(4):1366–1381. doi: 10.1128/jb.96.4.1366-1381.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Ultrastructure and cell division of a facultatively parasitic strain of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Mar;101(3):997–1004. doi: 10.1128/jb.101.3.997-1004.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERLETTI P., STROM R., GIORDANO M. G. REACTIVATION OF SUCCINIC DEHYDROGENASE BY PHOSPHOLIPIDS. Biochem Biophys Res Commun. 1965 Jan 18;18:259–263. doi: 10.1016/0006-291x(65)90750-3. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI S. F., HIRSCH P. BIOLOGY OF BUDDING BACTERIA. 3. FINE STRUCTURE OF RHODOMICROBIUM AND HYPHOMICROBIUM SPP. J Bacteriol. 1965 Feb;89:503–512. doi: 10.1128/jb.89.2.503-512.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTA-ROBLES E. H. ELECTRON MICROSCOPY OF PLASMOLYSIS IN ESCHERICHIA COLI. J Bacteriol. 1963 Mar;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Komai H., Hunter D. R. Isolation of a major hydrophobic protein of the mitochondrial inner membrane. Biochem Biophys Res Commun. 1973 Dec 10;55(3):655–659. doi: 10.1016/0006-291x(73)91194-7. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Coty W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. 3. Subunit composition. J Biol Chem. 1973 Nov 10;248(21):7427–7431. [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem. 1971 Aug 25;246(16):4987–4994. [PubMed] [Google Scholar]
- Cavari B. Z., Kalra V. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems: effect of chloramphenicol. J Bacteriol. 1971 Dec;108(3):1017–1025. doi: 10.1128/jb.108.3.1017-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N. C., Lark K. G. Segregation of deoxyribonucleic acid in bacteria: association of the segregating unit with the cell envelope. J Bacteriol. 1967 Aug;94(2):415–421. doi: 10.1128/jb.94.2.415-421.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Trager W. Nutritional significance of symbiotic bacteria in two species of hemoflagellates. Science. 1974 Feb 8;183(4124):531–532. doi: 10.1126/science.183.4124.531. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesbro W. R., Lampen J. O. Characteristics of secretion of penicillinase, alkaline phosphatase, and nuclease by Bacillus species. J Bacteriol. 1968 Aug;96(2):428–437. doi: 10.1128/jb.96.2.428-437.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choules G. L., Gray W. R. Peptidase activity in the membranes of Mycoplasma laidlawii. Biochem Biophys Res Commun. 1971 Nov;45(4):849–855. doi: 10.1016/0006-291x(71)90416-5. [DOI] [PubMed] [Google Scholar]
- Coen D., Deutsch J., Netter P., Petrochilo E., Slonimski P. P. Mitochondrial genetics. I. Methodology and phenomenology. Symp Soc Exp Biol. 1970;24:449–496. [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Poindexter J. S. The internal membranes of Caulobacter crescentus. J Gen Microbiol. 1966 Feb;42(2):301–308. doi: 10.1099/00221287-42-2-301. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. Are/were mitochondria and chloroplasts microorganisms? Am Sci. 1970 May-Jun;58(3):281–289. [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S. F., Jacobs N. J., Gray C. T. Ultrastructure and respiratory capacity of Staphylococcus and Bacillus grown under aerobic and anaerobic conditions. J Bacteriol. 1968 Aug;96(2):554–556. doi: 10.1128/jb.96.2.554-556.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota-Robles E. H. Internal membranes in cells of Escherichia coli. J Ultrastruct Res. 1966 Dec;16(5):626–639. doi: 10.1016/s0022-5320(66)80010-2. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Higgins M. L. Morphokinetic reaction of Streptococcus faecalis (ATCC 9790) cells to the specific inhibition of macromolecular synthesis: nucleoid condensation on the inhibition of protein synthesis. J Bacteriol. 1972 Mar;109(3):1210–1220. doi: 10.1128/jb.109.3.1210-1220.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Aspects of membrane synthesis during the cell cycle of Bacillus megaterium and Escherichia coli. J Gen Microbiol. 1969 Nov;58(3):iv–iv. [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C. Origin of mitochondria. Science. 1973 Oct 5;182(4107):85–85. doi: 10.1126/science.182.4107.85. [DOI] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C. Restoration of deoxycholate-disrupted membrane oxidases of Micrococcus lysodeikticus. J Bacteriol. 1971 Dec;108(3):964–972. doi: 10.1128/jb.108.3.964-972.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Freer J. H. The isolation and characterisation of mesosome material from Micrococcus lysodeikticus. J Gen Microbiol. 1969 Nov;58(3):vii–vii. [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Thomas T. D., Posgate J. A. Properties of mesosomal membranes isolated from Micrococcus lysodeikticus and Bacillus megaterium. Biochem J. 1971 May;122(5):44P–45P. doi: 10.1042/bj1220044p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Cytological and chemical studies of the growth of protoplasts of Bacillus megaterium. J Biophys Biochem Cytol. 1958 May 25;4(3):257–266. doi: 10.1083/jcb.4.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías R. N., Londero L., Trucco R. E. Effect of membrane lipid composition on the allosteric inhibition by sodium of the Ca 2+ )-adenosine triphosphatase from Escherichia coli. J Bacteriol. 1972 Jan;109(1):471–473. doi: 10.1128/jb.109.1.471-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandes B., Chaix P., Ryter A. Localisation des cytochromes de Bacillus subtilis dans les structures mésosomiques. C R Acad Sci Hebd Seances Acad Sci D. 1966 Nov 21;263(21):1632–1635. [PubMed] [Google Scholar]
- Ferrandes B., Frehel C., Chaix P. Fractionment et purification des systèmes membranaires cytoplasmiques et mésosomiquees de lbacillus subtilis. Etude de quelques-unes de leurs propríetés oxydo-réductricwa associées à la chaine respiratoire. Biochim Biophys Acta. 1970 Dec 8;223(2):292–308. doi: 10.1016/0005-2728(70)90186-6. [DOI] [PubMed] [Google Scholar]
- Fielding P., Fox C. F. Evidence for stable attachment of DNA to membrane at the replication origin of Escherichia coli. Biochem Biophys Res Commun. 1970 Oct 9;41(1):157–162. doi: 10.1016/0006-291x(70)90482-1. [DOI] [PubMed] [Google Scholar]
- Firshein W., Gillmor R. G. DNA-membrane complex: macromolecular content and stimulation of enzymatic activity by polyadenylic acid. Science. 1970 Jul 3;169(3940):66–68. doi: 10.1126/science.169.3940.66. [DOI] [PubMed] [Google Scholar]
- Firshein W. In situ activity of enzymes on polyacrylamide gels of a deoxyribonucleic acid-membrane fraction extracted from pneumococci. J Bacteriol. 1974 Jun;118(3):1101–1110. doi: 10.1128/jb.118.3.1101-1110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. Mitochondria and chloroplasts as descendants of prokaryotes. Biochem Genet. 1972 Jun;6(4):275–291. doi: 10.1007/BF00486121. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Ward J. B. N-acetylmuramyl-L-alanine amidase of Bacillus licheniformis and its L-form. J Bacteriol. 1972 Jun;110(3):878–888. doi: 10.1128/jb.110.3.878-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J., Kim K. S., Krauss M. R., Beaman L., Barksdale L. Ultrastructural changes in bacteria isolated from cases of leprosy. J Bacteriol. 1969 Nov;100(2):1062–1075. doi: 10.1128/jb.100.2.1062-1075.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C. Activité succinodéshydrogénasique des membranes cytoplasmiques et mésosomiques au cours de la sporulation de B. subtilis. Analyse enzymatique et étude cytochimique. Biochimie. 1974;56(4):571–581. doi: 10.1016/s0300-9084(74)80076-3. [DOI] [PubMed] [Google Scholar]
- Frehel C., Dubray G., Wolff A. Activité estérasique des membranes cytoplasmiques et mésosomiques au cours de la sporulation de Bacillus subtilis. Biochimie. 1974;56(4):583–598. doi: 10.1016/s0300-9084(74)80077-5. [DOI] [PubMed] [Google Scholar]
- Frehel C., Ferrandes B., Ryter A. Réactions d'oxydo-réduction au niveau des membranes cytoplasmiaues et mésosomiques de Bacillus subtilis. Biochim Biophys Acta. 1971 May 11;234(2):226–241. doi: 10.1016/0005-2728(71)90078-8. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Hanawalt P. Isolation and characterization of the DNA replication complex from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):301–322. doi: 10.1016/0022-2836(70)90032-x. [DOI] [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V., McQUILLEN K. The chemical composition of the protoplast membrane of Micrococcus lysodeikticus. Biochim Biophys Acta. 1958 Jul;29(1):21–29. doi: 10.1016/0006-3002(58)90141-0. [DOI] [PubMed] [Google Scholar]
- Garland J. M. Mesosomes in Bacillus cereus 569 and the production of extra membranes by treatment with actinomycin-D. J Gen Microbiol. 1974 Feb;80(2):419–431. doi: 10.1099/00221287-80-2-419. [DOI] [PubMed] [Google Scholar]
- Garrard W. T. Synthesis, assembly, and localization of periplasmic cytochrome c. J Biol Chem. 1972 Sep 25;247(18):5935–5943. [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fractionation and characterization of the plasma and mesosome membrane of Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):426–440. doi: 10.1128/jb.97.1.426-440.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht P., Ruska H. Uber Veränderungen der Feinstrukturen von Bakterien unter der Einwirkung von Chloramphenicol. Klin Wochenschr. 1968 Jun 1;46(11):575–582. doi: 10.1007/BF01747836. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. Localization of monoamine oxidase in rat liver mitochondria. Adv Biochem Psychopharmacol. 1972;5:207–226. [PubMed] [Google Scholar]
- Greenawalt J. W., Schnaitman C. An appraisal of the use of monoamine oxidase as an enzyme marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1970 Jul;46(1):173–179. doi: 10.1083/jcb.46.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of intracellular membranes of Escherichia coli 0111a. Methods Enzymol. 1974;31:633–642. doi: 10.1016/0076-6879(74)31069-5. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of outer and inner mitochondrial membranes. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes G. W., Mahler H. R., Perlman P. S. Letter: Mitochondrial morphology. Science. 1974 Aug 16;185(4151):630–631. doi: 10.1126/science.185.4151.630. [DOI] [PubMed] [Google Scholar]
- Guarnieri M., Stechmiller B., Lehninger A. L. Use of an antibody to study the location of cardiolipin in mitochondrial membranes. J Biol Chem. 1971 Dec 25;246(24):7526–7532. [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of Streptomyces coelicolor. II. The nuclear material. J Biophys Biochem Cytol. 1960 Sep;8:267–278. doi: 10.1083/jcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. States of activity and structure in mitochondrial membranes. Ann N Y Acad Sci. 1972 Jun 20;195:492–505. [PubMed] [Google Scholar]
- Hagen P. O., Goldfine H., Williams P. J. Phospholipids of bacteria with extensive intracytoplasmic membranes. Science. 1966 Mar 25;151(3717):1543–1544. doi: 10.1126/science.151.3717.1543. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Cammack R., Rao K. K. The iron-sulphur proteins: evolution of a ubiquitous protein from model systems to higher organisms. Orig Life. 1974 Jul-Oct;5(3):363–386. [PubMed] [Google Scholar]
- Hall D. O., Greenawalt J. W. The preparation and biochemical properties of mitochondria from Neurospora crassa. J Gen Microbiol. 1967 Sep;48(3):419–430. doi: 10.1099/00221287-48-3-419. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Palmer J. M. Mitochondrial research today. Nature. 1969 Feb 22;221(5182):717–723. doi: 10.1038/221717a0. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. The iron-sulphur proteins: structure, function and evolution of a ubiquitous group of proteins. Sci Prog. 1975 Summer;62(246):285–317. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley E. S., Greenawalt J. W. Biogenesis of mitochondrial membranes in Neurospora crassa. Mitochondrial protein synthesis during conidial germination. Eur J Biochem. 1975 Jun;54(2):585–601. doi: 10.1111/j.1432-1033.1975.tb04171.x. [DOI] [PubMed] [Google Scholar]
- Higashi T., Bogin E., Brodie A. F. Separation of a factor indispensable for coupled phosphorylation from the particulate fraction of Mycobacterium phlei. J Biol Chem. 1969 Jan 25;244(2):500–502. [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L. Factors influencing the frequency of mesosomes observed in fixed and unfixed cells of Streptococcus faecalis. J Cell Biol. 1974 May;61(2):288–300. doi: 10.1083/jcb.61.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L. Morphokinetic reaction of cells of Streptococcus faecalis (ATCC 9790) to specific inhibition of macromolecular synthesis: dependence of mesosome growth on deoxyribonucleic acid synthesis. J Bacteriol. 1972 Mar;109(3):1221–1231. doi: 10.1128/jb.109.3.1221-1231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Early changes in the ultrastructure of Streptococcus faecalis after amino acid starvation. J Bacteriol. 1970 Jul;103(1):244–253. doi: 10.1128/jb.103.1.244-253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of cell growth and mesosomal structure of Bacillus licheniformis. J Ultrastruct Res. 1969 Jan;26(1):130–147. doi: 10.1016/s0022-5320(69)90040-9. [DOI] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of mesosomes in Bacillus subtilis. J Ultrastruct Res. 1970 May;31(3):260–271. doi: 10.1016/s0022-5320(70)90130-9. [DOI] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of the structure of mesosomal membranes in Bacillus licheniformis. J Ultrastruct Res. 1970 May;31(3):247–259. doi: 10.1016/s0022-5320(70)90129-2. [DOI] [PubMed] [Google Scholar]
- Highton P. J., Hobbs D. G. Penicillin and cell wall synthesis: a study of Bacillus licheniformis by electron microscopy. J Bacteriol. 1971 May;106(2):646–658. doi: 10.1128/jb.106.2.646-658.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Scheffler I., Jacob F. Genetic approach to DNA replication and its control in Escherichia coli. Fed Proc. 1972 Sep-Oct;31(5):1422–1427. [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Avers C. J. Mitochondrion of yeast: ultrastructural evidence for one giant, branched organelle per cell. Science. 1973 Aug 24;181(4101):749–751. doi: 10.1126/science.181.4101.749. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Geftic S. G., Heymann H., Adair F. W. Mesosomes in Pseudomonas aeruginosa. J Bacteriol. 1973 Apr;114(1):434–438. doi: 10.1128/jb.114.1.434-438.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak I., Coon H. G., Dawid I. B. Interspecific recombination of mitochondrial DNA molecules in hybrid somatic cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1828–1832. doi: 10.1073/pnas.71.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Trembath M. K., Linnane A. W., Lukins H. B. Biogenesis of mitochondria. 30. An analysis of polarity of mitochondrial gene recombination and transmission. Mol Gen Genet. 1973 Mar 27;122(1):37–51. doi: 10.1007/BF00337972. [DOI] [PubMed] [Google Scholar]
- Huff E., Cole R. M., Theodore T. S. Lipoteichoic acid localization in mesosomal vesicles of Staphylococcus aureus. J Bacteriol. 1974 Oct;120(1):273–281. doi: 10.1128/jb.120.1.273-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Janki R. M., Aithal H. N., McMurray W. C., Tustanoff E. R. The effect of altered membrane-lipid composition on enzyme activities of outer and inner mitochondrial membranes of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1078–1085. doi: 10.1016/s0006-291x(74)80298-6. [DOI] [PubMed] [Google Scholar]
- John Philip, Hamilton W. A. Respiratory control in membrane particles from Micrococcus denitrificans. FEBS Lett. 1970 Oct 16;10(4):246–248. doi: 10.1016/0014-5793(70)80639-1. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kalf G. F., Faust A. S. The inner membrane of the rat liver mitochondrion as the site of incorporation of radioactively labeled precursors into nucleic acid and protein in vitro. Arch Biochem Biophys. 1969 Oct;134(1):103–112. doi: 10.1016/0003-9861(69)90256-2. [DOI] [PubMed] [Google Scholar]
- Kats L. N., Tordzhian I. Zh. Izmenenie membrannykh struktur stafilokokkav razlichnykh usloviiakh kul'tivirovaniia. Mikrobiologiia. 1968 Sep-Oct;37(5):890–896. [PubMed] [Google Scholar]
- Kawakami M., Landman O. E. Retention of episomes during protoplasting and during propagation in the L state. J Bacteriol. 1966 Aug;92(2):398–404. doi: 10.1128/jb.92.2.398-404.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Allison V. F., Butow R. A. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. II. Evidence for the association of cytoplasmic ribosomes with the outer mitochondrial membrane in situ. J Biol Chem. 1974 May 25;249(10):3297–3303. [PubMed] [Google Scholar]
- Kellems R. E., Butow R. A. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. 3. Changes in the amount of bound ribosomes in response to changes in metabolic state. J Biol Chem. 1974 May 25;249(10):3304–3310. [PubMed] [Google Scholar]
- Kellems R. E., Butow R. A. Cytoplasmic-type 80 S ribosomes associated with yeast mitochondria. I. Evidence for ribosome binding sites on yeast mitochondria. J Biol Chem. 1972 Dec 25;247(24):8043–8050. [PubMed] [Google Scholar]
- Koike K., Wolstenholme D. R. Evidence for discontinuous replication of circular mitochondrial DNA molecules from Novikoff rat ascites hepatoma cells. J Cell Biol. 1974 Apr;61(1):14–25. doi: 10.1083/jcb.61.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koke J. R., Gupta P. D., Malhotra S. K. A succinic dehydrogenase activity in "mesosomes" of Neurospora crassa. Biochem Biophys Res Commun. 1971 Feb 5;42(3):576–582. doi: 10.1016/0006-291x(71)90410-4. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Kuriyama Y., Luck D. J. Membrane-associated ribosomes in mitochondria of Neurospora crassa. J Cell Biol. 1973 Dec;59(3):776–784. doi: 10.1083/jcb.59.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O. Release of penicillinase by Bacillus licheniformis. J Gen Microbiol. 1967 Aug;48(2):261–268. doi: 10.1099/00221287-48-2-261. [DOI] [PubMed] [Google Scholar]
- Landman O. E., Ryter A., Fréhel C. Gelatin-induced reversion of protoplasts of Bacillus subtilis to the bacillary form: electron-microscopic and physical study. J Bacteriol. 1968 Dec;96(6):2154–2170. doi: 10.1128/jb.96.6.2154-2170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastras M., Munõz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus: effect of millimolar Mg2+ in modulating the properties of the membrane-bound enzyme. J Bacteriol. 1974 Aug;119(2):593–601. doi: 10.1128/jb.119.2.593-601.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leene W., van Iterson W. Tetranitro--blue tetrazolium reduction in Bacillus subtilis. J Cell Biol. 1965 Oct;27(1):237–241. doi: 10.1083/jcb.27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Levy M., Sauner M. T. Composition en phospholipides des membranes interne et externe des mitochondries. C R Seances Soc Biol Fil. 1967 Jul 20;161(2):277–279. [PubMed] [Google Scholar]
- Lin E. C., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. VI. Use of a methocel-autoradiographic method for the study of cellular division in Escherichia coli. J Bacteriol. 1971 Oct;108(1):375–385. doi: 10.1128/jb.108.1.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Luria S. E. Phage, colicins, and macroregulatory phenomena. Science. 1970 Jun 5;168(3936):1166–1170. doi: 10.1126/science.168.3936.1166. [DOI] [PubMed] [Google Scholar]
- MARR A. G. Enzyme localization in bacteria. Annu Rev Microbiol. 1960;14:241–260. doi: 10.1146/annurev.mi.14.100160.001325. [DOI] [PubMed] [Google Scholar]
- MUDD S. Cytology of bacteria. I. The bacterial cell. Annu Rev Microbiol. 1954;8:1–22. doi: 10.1146/annurev.mi.08.100154.000245. [DOI] [PubMed] [Google Scholar]
- MUDD S., KAWATA T., PAYNE J. I., SALL T., TAKAGI A. Plasma membranes and mitochondrial equivalents as functionally coordinated structures. Nature. 1961 Jan 7;189:79–80. doi: 10.1038/189079a0. [DOI] [PubMed] [Google Scholar]
- MacLeod R. A., Thurman P., Rogers H. J. Comparative transport activity of intact cells, membrane vesicles, and mesosomes of Bacillus licheniformis. J Bacteriol. 1973 Jan;113(1):329–340. doi: 10.1128/jb.113.1.329-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S. K. Mesosome-like structures in mitochondria of poky Neurospora. Nature. 1968 Sep 21;219(5160):1267–1268. doi: 10.1038/2191267a0. [DOI] [PubMed] [Google Scholar]
- Marchant R., Smith D. G. Membranous structures in yeasts. Biol Rev Camb Philos Soc. 1968 Nov;43(4):459–480. doi: 10.1111/j.1469-185x.1968.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Matheson A., Kwong M. C. Electron-dense particles resembling ribosomes in mesosomes of Bacillus subtilis. J Bacteriol. 1972 May;110(2):747–750. doi: 10.1128/jb.110.2.747-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson A., Yang M. K., Smith R. P. Demonstration of ribosomes in mesosomes associated with Bacillus subtilis protoplasts. J Bacteriol. 1973 Jul;115(1):349–357. doi: 10.1128/jb.115.1.349-357.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Girard A. E., Kornfeld J. M. Fine structure of resting and germinating Penicillium chrysogenum conidiospores. Protoplasma. 1971;73(3):443–456. doi: 10.1007/BF01273945. [DOI] [PubMed] [Google Scholar]
- McGill M., Hsu T. C., Brinkley B. R. Electron-dense structures in mitochondria induced by short-term ethidium bromide treatment. J Cell Biol. 1973 Oct;59(1):260–265. doi: 10.1083/jcb.59.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. A., Gilula N. B., Karnovsky M. J. Cryoprotectant-induced redistribution of intramembranous particles in mouse lymphocytes. J Cell Biol. 1974 Jan;60(1):192–203. doi: 10.1083/jcb.60.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Dales S. Membrane synthesis in Bacillus subtilis. 3. The morphological localization of the sites of membrane synthesis. J Cell Biol. 1972 Oct;55(1):32–41. doi: 10.1083/jcb.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Molecular weight, amino acid composition and other properties of membrane-bound ATPase from Bacillus megaterium KM. Biochim Biophys Acta. 1973 Jan 26;291(2):480–488. doi: 10.1016/0005-2736(73)90499-9. [DOI] [PubMed] [Google Scholar]
- Mishra N. C., Tatum E. L. Non-Mendelian inheritance of DNA-induced inositol independence in Neurospora. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3875–3879. doi: 10.1073/pnas.70.12.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Chan B., Rose H. M. Electron microscopy of magnesium-depleted bacteria. J Bacteriol. 1966 Feb;91(2):891–895. doi: 10.1128/jb.91.2.891-895.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Stocker B. A. How genes determine the structure of the Salmonella lipopolysaccharide. J Gen Microbiol. 1969 Aug;57(3):vi–vi. [PubMed] [Google Scholar]
- Nachbar M. S., Salton M. R. Characteristics of a lipid-rich NADH dehydrogenase-containing particulate fraction obtained from Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):309–320. doi: 10.1016/0005-2728(70)90187-8. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Preservation of the ultrastructure of Bacillus subtilis by chemical fixation as verified by freeze-etching. J Cell Biol. 1969 Sep;42(3):733–744. doi: 10.1083/jcb.42.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Structural features of mesosomes (chondrioids) of Bacillu subtilis after freeze-etching. J Cell Biol. 1968 Nov;39(2):251–263. doi: 10.1083/jcb.39.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. The mesosome of Bacillus subtilis as affected by chemical and physical fixation. J Cell Biol. 1971 Jan;48(1):219–224. doi: 10.1083/jcb.48.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA: Advances, Problems, and Goals. Science. 1969 Jul 4;165(3888):25–35. doi: 10.1126/science.165.3888.25. [DOI] [PubMed] [Google Scholar]
- Nass M. M. The circularity of mitochondrial DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1215–1222. doi: 10.1073/pnas.56.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth. Science. 1974 Jun 7;184(4141):1043–1050. doi: 10.1126/science.184.4141.1043. [DOI] [PubMed] [Google Scholar]
- Olson J. M. The evolution of photosynthesis. Science. 1970 Apr 24;168(3930):438–446. doi: 10.1126/science.168.3930.438. [DOI] [PubMed] [Google Scholar]
- Onishi Y. "Phospholipids of virus-induced membranes in cytoplasm of Escherichia coli. J Bacteriol. 1971 Sep;107(3):918–925. doi: 10.1128/jb.107.3.918-925.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y., Kuwano M. Growth inhibition and appearance of the membranous structure in Escherichia coli infected with bacteriophage fd. J Virol. 1971 May;7(5):673–678. doi: 10.1128/jvi.7.5.673-678.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D activity in Haemophilus parainfluenzae. J Bacteriol. 1970 Jul;103(1):111–115. doi: 10.1128/jb.103.1.111-115.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., White D. C. Consequences of the inhibition of cardiolipin metabolism in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1065–1071. doi: 10.1128/jb.108.3.1065-1071.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. D., Salton M. R. Localization and distribution of Micrococcus lysodeikticus membrane ATPase determined by ferritin labeling. Biochim Biophys Acta. 1973 Mar 16;298(2):297–322. doi: 10.1016/0005-2736(73)90360-x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Owen P., Freer J. H. Isolation and properties of mesosomal membrane fractions from Micrococcus lysodeikticus. Biochem J. 1972 Oct;129(4):907–917. doi: 10.1042/bj1290907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L. Functional organization of intramembrane particles of mitochondrial inner membranes. J Bioenerg. 1972 May;3(1):115–127. doi: 10.1007/BF01516002. [DOI] [PubMed] [Google Scholar]
- Packer L. Membrane structure in relation to function of energy-transducing organelles. Ann N Y Acad Sci. 1974 Feb 18;227:166–174. doi: 10.1111/j.1749-6632.1974.tb14380.x. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Hall D. O. The mitochondrial membrane system. Prog Biophys Mol Biol. 1972;24:125–176. doi: 10.1016/0079-6107(72)90006-5. [DOI] [PubMed] [Google Scholar]
- Pangborn J., Marr A. G., Robrish S. A. LOCALIZATION OF RESPIRATORY ENZYMES IN INTRACYTOPLASMIC MEMBRANES OF AZOTOBACTER AGILIS. J Bacteriol. 1962 Oct;84(4):669–678. doi: 10.1128/jb.84.4.669-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E. Expansive growth of the nuclear envelope and formation of mitochondria in ganglionic neuroblasts. Z Zellforsch Mikrosk Anat. 1966;72(3):295–324. doi: 10.1007/BF00341538. [DOI] [PubMed] [Google Scholar]
- Pannese E. Structures possibly related to the formation of new mitochondria in spinal ganglion neuroblasts. J Ultrastruct Res. 1966 Apr;15(1):57–65. doi: 10.1016/s0022-5320(66)80093-x. [DOI] [PubMed] [Google Scholar]
- Parsons D. F. Recent advances correlating structure and function in mitochondria. Int Rev Exp Pathol. 1965;4:1–54. [PubMed] [Google Scholar]
- Parsons D. F., Yano Y. The cholesterol content of the outer and inner membranes of guinea-pig liver mitochondria. Biochim Biophys Acta. 1967 May 2;135(2):362–364. doi: 10.1016/0005-2736(67)90132-0. [DOI] [PubMed] [Google Scholar]
- Patch C. T., Landman O. E. Comparison of the biochemistry and rates of synthesis of mesosomal and peripheral membranes in Bacillus subtilis. J Bacteriol. 1971 Jul;107(1):345–357. doi: 10.1128/jb.107.1.345-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Johnson J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. II. Structure and formation of rhapidosomes. J Cell Biol. 1967 Oct;35(1):15–35. doi: 10.1083/jcb.35.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. I. Structure and formation of mesosomes. J Cell Biol. 1967 Oct;35(1):1–13. doi: 10.1083/jcb.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontefract R. D., Bergeron G., Thatcher F. S. Mesosomes in Escherichia coli. J Bacteriol. 1969 Jan;97(1):367–375. doi: 10.1128/jb.97.1.367-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontefract R. D., Thatcher F. S. An electron microscopy study of mesosomes in irradiation-resistant mutants of Escherichia coli. J Ultrastruct Res. 1970 Jan;30(1):78–86. doi: 10.1016/s0022-5320(70)90065-1. [DOI] [PubMed] [Google Scholar]
- Popkin T. J., Theodore T. S., Cole R. M. Electron microscopy during release and purification of mesosomal vesicles and protoplast membranes from Staphylococcus aureus. J Bacteriol. 1971 Sep;107(3):907–917. doi: 10.1128/jb.107.3.907-917.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz M., Swift H. Mitochondrial nucleic acids and their relation to the biogenesis of mitochondria. Physiol Rev. 1970 Jul;50(3):376–427. doi: 10.1152/physrev.1970.50.3.376. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Mahler H. R. The non symbiotic origin of mitochondria. Science. 1972 Aug 18;177(4049):575–582. doi: 10.1126/science.177.4049.575. [DOI] [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H. Cell morphology of Bacillus subtilis: the effect of genetic background on the expression of a rod - gene. Mol Gen Genet. 1972;119(1):11–26. doi: 10.1007/BF00270440. [DOI] [PubMed] [Google Scholar]
- Reichenbach H., Voelz H., Dworkin M. Structural changes in Stigmatella aurantiaca during myxospore induction. J Bacteriol. 1969 Feb;97(2):905–911. doi: 10.1128/jb.97.2.905-911.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Valois F. W., Watson S. W. Fine structure of the cytomembranes of Nitrosocystis oceanus. J Bacteriol. 1967 Aug;94(2):422–433. doi: 10.1128/jb.94.2.422-433.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Burger M. M. Distribution of marker enzymes between mesosomal and protoplast membranes. J Biol Chem. 1974 Aug 25;249(16):5337–5345. [PubMed] [Google Scholar]
- Reusch V. M., Jr, Burger M. M. The bacterial mesosome. Biochim Biophys Acta. 1973 Apr 3;300(1):79–104. doi: 10.1016/0304-4157(73)90012-9. [DOI] [PubMed] [Google Scholar]
- Robinson J. P., Robinson R. D., Hash J. H. Electron microscopy of Staphylococcus aureus cells and cell walls after treatment with lysozyme Chalaropsis. J Bacteriol. 1974 Feb;117(2):900–903. doi: 10.1128/jb.117.2.900-903.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucinsky T. E., Cota-Robles E. H. Mesosome structure in Chromobacterium violaceum. J Bacteriol. 1974 May;118(2):717–724. doi: 10.1128/jb.118.2.717-724.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Jacob F. DNA-membrane complex and nuclear segregation in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:669–676. doi: 10.1101/sqb.1968.033.01.076. [DOI] [PubMed] [Google Scholar]
- Ryter A., Jacob F. Etude morphologique de la liaison du noyau à la membrane chez E. coli et chez les protoplastes de B. subtilis. Ann Inst Pasteur (Paris) 1966 Jun;110(6):801–812. [PubMed] [Google Scholar]
- Ryter A. Structure and functions of mesosomes of gram positive bacteria. Curr Top Microbiol Immunol. 1969;49:151–177. doi: 10.1007/978-3-642-46166-8_4. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., CHAPMAN J. A. Isolation of the membranemesosome structures from Micrococcus lysodeikticus. J Ultrastruct Res. 1962 Jun;6:489–498. doi: 10.1016/s0022-5320(62)80004-5. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. V. The action of lysozyme on cell walls of some lysozyme-sensitive bacteria. Biochim Biophys Acta. 1956 Dec;22(3):495–506. doi: 10.1016/0006-3002(56)90060-9. [DOI] [PubMed] [Google Scholar]
- SHINOHARA C., FUKUSHI K., SUZUKI J. Mitochondria-like structures in ultrathin sections of Mycobacterium avium. J Bacteriol. 1957 Sep;74(3):413–415. doi: 10.1128/jb.74.3.413-415.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Bacterial membranes. CRC Crit Rev Microbiol. 1971 May;1(1):161–197. doi: 10.3109/10408417109104480. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H., Ellar D. J. Electron transport components localized in a lipid-depleted sheet isolated from Micrococcus lysodeikticus membranes by deoxycholate extraction. Biochem Biophys Res Commun. 1968 Dec 30;33(6):909–915. doi: 10.1016/0006-291x(68)90398-7. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Subunit structure and properties of two forms of adenosine triphosphatase released from Micrococcus lysodeikticus membranes. Biochem Biophys Res Commun. 1972 Oct 17;49(2):350–357. doi: 10.1016/0006-291x(72)90417-2. [DOI] [PubMed] [Google Scholar]
- Santo L. Y., Doi R. H. Ultrastructural analysis during germination and outgrowth of Bacillus subtilis spores. J Bacteriol. 1974 Oct;120(1):475–481. doi: 10.1128/jb.120.1.475-481.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Localization of cell-bound penicillinase in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1329–1338. doi: 10.1128/jb.96.4.1329-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Membrane synthesis in synchronous cultures of Bacillus subtilis 168. J Bacteriol. 1973 Oct;116(1):397–409. doi: 10.1128/jb.116.1.397-409.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H., Grossfeld H., Littauer U. Z. Mitochondrial biogenesis during differentiation of Artemia salina cysts. J Cell Biol. 1973 Sep;58(3):643–649. doi: 10.1083/jcb.58.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Intracytoplasmic membranes in Escherichia coli. J Bacteriol. 1966 Sep;92(3):780–783. doi: 10.1128/jb.92.3.780-783.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebli H. P., Vatter A. E., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Molecular weight, subunit structure, and amino acid composition. J Biol Chem. 1970 Mar 10;245(5):1122–1127. [PubMed] [Google Scholar]
- Sedar A. W., Burde R. M. The demonstration of the succinic dehydrogenase system in Bacillus subtilis using tetranitro--blue tetrazolium combined with techniques of electron microscopy. J Cell Biol. 1965 Oct;27(1):53–66. doi: 10.1083/jcb.27.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. B., King H. K. The action of lysozyme on bacterial electron transport systems. J Gen Microbiol. 1966 Jul;44(1):1–13. doi: 10.1099/00221287-44-1-1. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Siccardi A. G., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. Membrane prtein alterations associated with mutations affecting the initiation of DNA synthesis. J Mol Biol. 1970 Aug 28;52(1):75–89. doi: 10.1016/0022-2836(70)90178-6. [DOI] [PubMed] [Google Scholar]
- Shively J. M., Decker G. L., Greenawalt J. W. Comparative ultrastructure of the thiobacilli. J Bacteriol. 1970 Feb;101(2):618–627. doi: 10.1128/jb.101.2.618-627.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Kaback H. R. Active transport in isolated bacterial membrane vesicles. V. The transport of amino acids by membrane vesicles prepared from Staphylococcus aureus. J Biol Chem. 1972 Jan 10;247(1):298–304. [PubMed] [Google Scholar]
- Silva M. T. Changes induced in the ultrastructure of the cytoplasmic and intracytoplasmic membranes of several Gram-positive bacteria by variations in OsO 4 fixation. J Microsc. 1971 Jun;93(3):227–232. doi: 10.1111/j.1365-2818.1971.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C. Ultrastructure of the cell wall and cytoplasmic membrane of gram-negative bacteria with different fixation techniques. J Bacteriol. 1973 Feb;113(2):953–962. doi: 10.1128/jb.113.2.953-962.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Smith D. G., Marchant R., Maroudas N. G., Wilkie D. A comparative study of the mitochondrial structure of petite strains of Saccharomyces cerevisiae. J Gen Microbiol. 1969 Apr;56(1):47–54. doi: 10.1099/00221287-56-1-47. [DOI] [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Sequence homologies among bacterial and mitochondrial superoxide dismutases. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3725–3729. doi: 10.1073/pnas.70.12.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Blumberg P. M., Strominger J. L. Inhibition of the Bacillus subtilis membrane-bound D-alanine carboxypeptidase by 6-aminopenicillanic acid covalently coupled to sepharose. J Bacteriol. 1974 Feb;117(2):783–785. doi: 10.1128/jb.117.2.783-785.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Attardi G. Expression of the mitochondrial genome in HeLa cells. XVII. Heterogeneity of isolated HeLa cell mitochondria as assayed for their enzymatic and in vivo biosynthetic activities. J Biol Chem. 1973 Aug 25;248(16):5826–5834. [PubMed] [Google Scholar]
- Strätling W., Knippers R. Deoxyribonucleic acid synthesis in isolated DNA-membrane complexes. J Mol Biol. 1971 Nov 14;61(3):471–487. doi: 10.1016/0022-2836(71)90060-x. [DOI] [PubMed] [Google Scholar]
- Takazoe I., Ennever J. Ultrastructure of Bacterionema matruchotii. Bull Tokyo Dent Coll. 1969 May;10(2):45–60. [PubMed] [Google Scholar]
- Theodore T. S., Panos C. Protein and fatty acid composition of mesosomal vesicles and plasma membranes of Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):571–576. doi: 10.1128/jb.116.2.571-576.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore T. S., Popkin T. J., Cole R. M. The separation and isolation of plasma membranes and mesosomal vesicles from Staphylococcus aureus. Prep Biochem. 1971;1(3):233–248. doi: 10.1080/00327487108081942. [DOI] [PubMed] [Google Scholar]
- Theodore T. S., Weinbach E. C. Respiratory activities associated with mesosomal vesicles and protoplast membranes of Staphylococcus aureus. J Bacteriol. 1974 Oct;120(1):562–564. doi: 10.1128/jb.120.1.562-564.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. Y., Wilkie D. Recombination of mitochondrial drug-resistance factors in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1968 Feb 26;30(4):368–372. doi: 10.1016/0006-291x(68)90753-5. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. Bactoprenol, ATPase and acetate activating enzymes of a vesicular fraction from Lactobacillus casei. Eur J Biochem. 1969 Dec;11(3):582–591. doi: 10.1111/j.1432-1033.1969.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. The bactoprenol content of plasma and mesosome membranes from Lactobacillus casei. Biochem J. 1971 May;122(5):45P–46P. doi: 10.1042/bj1220045p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. The occurrence of bactoprenol in the mesosome and plasma membranes of Lactobacillus casei and Lactobacillus plantarum. J Gen Microbiol. 1972 Apr;70(1):87–98. doi: 10.1099/00221287-70-1-87. [DOI] [PubMed] [Google Scholar]
- Tichy P., Landman O. E. Transformation in quasi spheroplasts of Bacillus subtilis. J Bacteriol. 1969 Jan;97(1):42–51. doi: 10.1128/jb.97.1.42-51.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte M. E., Zupnik J. S. Freeze-fractured Acholeplasma laidlawii membranes: nature of particles observed. Science. 1973 Jan 5;179(4068):84–86. doi: 10.1126/science.179.4068.84. [DOI] [PubMed] [Google Scholar]
- Towers N. R., Dixon H., Kellerman G. M., Linnane A. W. Biogenesis of mitochondria. 22. The sensitivity of rat liver mitochondria to antibiotics; a phylogenetic difference between a mammalian system and yeast. Arch Biochem Biophys. 1972 Aug;151(2):361–369. doi: 10.1016/0003-9861(72)90510-3. [DOI] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Tsien H. C., Higgins M. L. Effect of temperature on the distribution of membrane particles in Streptococcus faecalis as seen by the freeze-fracture technique. J Bacteriol. 1974 May;118(2):725–734. doi: 10.1128/jb.118.2.725-734.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Detection of a rapidly metabolizing portion of the membrane cardiolipin in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1058–1064. doi: 10.1128/jb.108.3.1058-1064.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Uzzell T., Spolsky C. Origin of mitochondria. Science. 1973 May 4;180(4085):516–517. doi: 10.1126/science.180.4085.516. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-NEGATIVE BACTERIUM. TELLURITE REDUCTION IN PROTEUS VULGARIS. J Cell Biol. 1964 Mar;20:377–387. doi: 10.1083/jcb.20.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- VOELZ H. FORMATION AND STRUCTURE OF MESOSOMES IN MYXOCOCCUS XANTHUS. Arch Mikrobiol. 1965 May 28;51:60–70. doi: 10.1007/BF00406850. [DOI] [PubMed] [Google Scholar]
- Vermeulen C. A., Venema G. Electron microscope and autoradiographic study of ultrastructural aspects of competence and deoxyribonucleic acid absorption in Bacillus subtilis: localization of uptake and of transport of transforming deoxyribonucleic acid in competent cells. J Bacteriol. 1974 May;118(2):342–350. doi: 10.1128/jb.118.2.342-350.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen C. A., Venema G. Electron microscope and autoradiographic study of ultrastructural aspects of competence and deoxyribonucleic acid absorption in Bacillus subtilis: ultrastructure of competent and noncompetent cells and cellular changes during development of competence. J Bacteriol. 1974 May;118(2):334–341. doi: 10.1128/jb.118.2.334-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. V., Stevens B. J., Huet J., André J. Mitoribosomes from Candida utilis. Morphological, physical, and chemical characterization of the monomer form and of its subunits. J Cell Biol. 1972 Sep;54(3):468–492. doi: 10.1083/jcb.54.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. Characterization of the protoplasmic constituents of bacillus megaterium. J Bacteriol. 1953 Dec;66(6):696–702. doi: 10.1128/jb.66.6.696-702.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., GYLLANG H. METABOLIC PROPERTIES OF SOME L FORMS DERIVED FROM GRAM-POSITIVE AND GRAM-NEGATIVE BACTERIA. J Bacteriol. 1965 Jun;89:1443–1447. doi: 10.1128/jb.89.6.1443-1447.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. PLASMOLYSIS IN BACILLUS MEGATERIUM. J Bacteriol. 1965 Apr;89:1151–1154. doi: 10.1128/jb.89.4.1151-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker W. E. The biochemistry of magnesium. Ann N Y Acad Sci. 1969 Aug 15;162(2):717–726. doi: 10.1111/j.1749-6632.1969.tb13003.x. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Holt S. C., Shively J. M., Decker G. L., Greenawalt J. W. Ultrastructural properties of the extra membranes of Escherichia coli O111a as revealed by freeze-fracturing and negative-staining techniques. J Bacteriol. 1973 Jan;113(1):433–444. doi: 10.1128/jb.113.1.433-444.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Shively J. M., Greenawalt J. W. Formation and ultrastructure of extra membranes in Escherichia coli. J Bacteriol. 1970 Apr;102(1):240–249. doi: 10.1128/jb.102.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeldon L. W. Products of mitochondrial protein synthesis. Biochimie. 1973;55(6):805–814. doi: 10.1016/s0300-9084(73)80033-1. [DOI] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Salton M. R. Antibody to adenosine triphosphatase from membranes of Micrococcus lysodeikticus. Biochemistry. 1970 Jul 21;9(15):3034–3040. doi: 10.1021/bi00817a015. [DOI] [PubMed] [Google Scholar]
- Wiebe W. J., Chapman G. B. Variation in the fine structure of a marine achromobacter and a marine pseudomonad grown under selected nutritional and temperature regimes. J Bacteriol. 1968 May;95(5):1874–1886. doi: 10.1128/jb.95.5.1874-1886.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., van Iterson W. Effects of treatment with sodium dodecyl sulfate on the ultrastructure of Escherichia coli. J Bacteriol. 1972 Sep;111(3):801–813. doi: 10.1128/jb.111.3.801-813.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Vermeulen C. A., Venema G. Evidence for the involvement of membranous bodies in the processes leading to genetic transformation in Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):1111–1121. doi: 10.1128/jb.92.4.1111-1121.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuyanagi Y. Un mode de différenciation de la membrane mitochondriale, évoquant le mésosome bactérien. C R Acad Sci Hebd Seances Acad Sci D. 1966 Mar 21;262(12):1348–1351. [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Chemical and physical characteristics of the deoxycholate-soluble and magnesium-reaggregated membrane nicotinamide adenine dinucleotide (reduced form) oxidase of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):51–58. doi: 10.1128/jb.109.1.51-58.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Separation of the primary dehydrogenase from the cytochromes of the nicotinamide adenine dinucleotide (reduced form) oxidase of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):59–68. doi: 10.1128/jb.109.1.59-68.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZWILLENBERG L. O. ELECTRON MICROSCOPIC FEATURES OF GRAM-NEGATIVE AND GRAM-POSITIVE BACTERIA EMBEDDED IN PHOSPHOTUNGSTATE. Antonie Van Leeuwenhoek. 1964;30:154–162. doi: 10.1007/BF02046721. [DOI] [PubMed] [Google Scholar]
- den Kamp JA O. P., van Iterson W., van Deenen L. L. Studies of the phospholipids and morphology of protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1967;135(5):862–884. doi: 10.1016/0005-2736(67)90056-9. [DOI] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W., den Kamp J. A. Bacteria-shaped gymnoplasts (protoplasts) of Bacillus subtilis. J Bacteriol. 1969 Jul;99(1):304–315. doi: 10.1128/jb.99.1.304-315.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]