Abstract
Background
Interleukin-21 (IL-21) has been implicated in the development of Th2-mediated immune responses; however, the exact role it plays in allergic diseases is not well understood.
Objective
To elucidate the contribution of IL-21 receptor signaling to Th2-dependent immune responses in the lung.
Methods
We compared allergic airway responses in wild type BALB/c and Il21r-deficient mice exposed to local airway challenge with house dust mite.
Results
We demonstrate that IL-21R-deficiency reduces house dust mite-driven airway hyperresponsiveness (AHR) with only partial effects on airway inflammation. Concomitant with the reduction in AHR in Il21r-deficient mice, significant suppression was observed in protein levels of the Th2 cytokines IL-4, and IL-13. In contrast, IL-21R-deficiency was associated with an increase in PBS- and allergen-driven IgE levels, while IgG1 and IgG2a levels were decreased. Moreover, our results suggest that IL-21 may contribute to AHR through its ability to both directly induce Th2 cell survival and to impair regulatory T cell suppression of Th2 cytokine production. Importantly, we show that IL-21-positive cells are increased in the bronchial mucosa of asthmatics compared to non-asthmatics.
Conclusion
These results suggest that IL-21 plays an important role in the allergic diathesis by enhancing Th2 cytokine production through multiple mechanisms including the suppression of Treg inhibitory effects on Th2 cell cytokine production.
Keywords: airway hyperresponsiveness, asthma, Tregs, IL-21, IL-21R
Introduction
Allergic asthma is a chronic disease of the airways associated with airway hyperresponsiveness and airway inflammation. Although the etiology of asthma is unclear, T lymphocytes, particularly Th2 cells, are critical to the development and maintenance of asthma. However, the factors responsible for regulating the development and/or maintenance of Th2-mediated inflammation are not well understood. Recently, it was demonstrated that mice deficient in IL-21 signaling develop weaker Th2-type immune responses in several parasite infection models (1, 2), suggesting an important role for IL-21 in the maintenance or development of Th2 responses. While thought to be preferentially secreted by Th2 cells (3), IL-21 was recently shown to be produced in higher quantity by Th17 cells and T follicular helper (Tfh) cells (4–6). Interestingly, IL-21 can itself regulate Tfh generation (7). IL-21 can also inhibit Treg differentiation and activity (4, 5), and render CD4+ and CD8+ T cells resistant to Treg suppression (8, 9), suggesting that the pro-Th2 effects of IL-21 may be related to inhibition of Treg function. Another well-documented aspect of IL-21 biology is its effects on B cell function, especially as a potent suppressor of IgE production (10, 11). Importantly, IL-21 can profoundly affect the growth, survival, and function of B cells, T cells, NK cells and dendritic cells (DCs) (12–17). IL-21 signals through the common γ chain and its cognate receptor, the IL-21R, which is expressed by lymphoid (15), and by non-lymphoid tissues alike (18–20). Thus the ability of IL-21 to regulate Th2 responses may be due to effects on a number of cell types.
In light of the currently available data, we wanted to investigate whether this cytokine was involved in asthma pathogenesis and important in the development of Th2- driven allergic airway responses. In order to directly evaluate the potential role of IL-21 receptor signaling in experimental allergic asthma, we compared the responses of widltype and BALB/c Il21r-deficient mice to house dust mite (HDM) exposure in vivo and the production of IL-21 in human asthmatic airways. Our results support an important role for IL-21 in allergic airway disease by enhancing a Th2 immune response possibly through inhibition of Treg activity, thus implicating this cytokine in asthma pathogenesis.
Materials and Methods
Mice, allergen sensitization and airway measurements
Six week old male and female Il21r-deficient mice or BALB/c controls were anesthetized with ketamine/xylazine prior to intratracheal (i.t.) delivery of HDM extract (100 µg; Greer Laboratories, Lenoir, NC) or PBS on days 0, 14 and 21. 72 hours after final allergen challenge, airway responses were evaluated. To do this, mice were anaesthetized with sodium pentobarbital, intubated and respirated at a rate of 120 breaths per minute with a constant tidal volume (0.2 ml) and paralyzed with decamethonium bromide (25 mg/kg). After a stable baseline was achieved, acetylcholine (50 ug/kg) was injected into the inferior vena cava and dynamic airway pressure (cm H20 × sec) was followed for 5 minutes. Immediately after AHR measurements, blood was collected for detection of total and HDM-specific IgE, and HDM-specific IgG2a, and IgG1 by ELISA (Pharmingen, San Diego, CA). Bronchoalveolar lavage fluid (BALF), was harvested and centrifuged (300g for 8 min), the cell pellet resuspended in 1.0 ml of 10% fetal bovine serum in PBS. Slides were prepared by cytocentrifugation (Cytospin 3; Shandon Instruments, Pittsburgh, PA), and stained with Diff-Quik (Dade Behring, Düdingen, Switzerland). BALF cell differential counts were determined using morphologic criteria under a light microscope with evaluation of ≥ 500 cells/slide.
Lung cell isolation and cytokine determination
Lungs were minced and digested using Liberase CI (0.5 mg/ml) (Roche Diagnostics, Indianapolis, IN) and DNase I (0.5 mg/ml) (Sigma, St. Louis, MO) and incubated at 37°C for 45 min. To make a single cell suspension, the remaining tissue was forced through a 70 µm cell strainer. Cells were cultured at 8.3 × 105/ml in 300 µl in flat-bottom 96-well plates, and stimulated with media or HDM at 30 µg/ml. Supernatants were harvested at 72 hours and cytokine levels were measured in (IL4, IL-5: Pharmingen) or R&D Systems (IL-13: Minneapolis, MN).
RNA isolation and Purification and the Real Time PCR
Total RNA was extracted from lung tissue samples placed individually in 0.5 ml TRIzol reagent (Invitrogen). The sample was homogenized using a tissue polytron (Omni International Inc.) and total RNA was extracted according to the recommendations of the manufacturer and further purified using RNeasy Mini Kit (Qiagen). Individual sample RNA (1 µg) was reverse-transcribed using Superscript II (Invitrogen, Carlsbad, CA) and a mixture of oligo (dT) and random primers. Real- time polymerase chain reaction (RT-PCR) was performed on an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA). Relative quantities of mRNA for several genes was determined using SYBR Green PCR Master Mix (Applied Biosystems) and by the comparative threshold cycle method as described by Applied Biosystems for the ABI Prism 7700/7900 sequence detection systems. In this method, mRNA levels for each sample were normalized to hypoxanthine guanine phosphoribosyl transferase (Hprt1) mRNA levels and then expressed as a relative increase or decrease compared with levels in PBS control animals. Primers were designed using Primer Express software (Applied Biosystems). Primers for Il4, Il13 and Hprt1 were published previously (21).
Immunocytochemistry
The study was approved by the research ethics committee of McGill University (Montreal, Canada), and all subjects met ATS criteria for the diagnosis of asthma (22). Criteria included prior diagnosis and treatment for asthma, documented evidence of variable airflow obstruction greater than 15%, and bronchial hyperresponsiveness. For severe asthma, subjects were on daily inhaled steroids (2 patients were also on oral prednisone) with at least one other add-on therapy (long acting β-agonist, leukotriene receptor antagonist) (FEV1 < 80%); some moderate asthmatic patients were on daily inhaled steroids or unmedicated (FEV1 84–96%). Mild asthmatics were on either no medication or short-acting β-agonist when needed (FEV1 84–96%). Non-asthmatics were also non-atopic (FEV1 102%). Immunohistochemistry was performed on sections of major airways from 4 non-asthmatic, 3 mild, 4 moderate and 4 severe asthmatic subjects. Briefly, 10% formalin-fixed, paraffin-embedded sections (5 µm) were deparaffinized, extensively washed in PBS, blocked for 30 min in universal blocking solution, and incubated overnight at 4°C with goat anti-human IL-21 (1:1500, cat# GTX18496, GeneTex, San Antonio, TX) in a humidified chamber. The next day, slides were washed and incubated with a horseradish peroxidase-conjugated rabbit anti-goat antibody (1:500) for 45 min at RT. Immunostaining was developed with diaminobenzidine (1:100) for 20 sec at RT. All slides were counterstained with Gill’s haematoxylin, and examined. As a negative control, an isotype-matched control antibody replaced the primary antibody.
Treg inhibition assay
Lymph nodes from naïve male BALB/c mice were collected and CD4+ T cells were enriched using anti-CD4-coated magnetic beads on an autoMACS (Miltenyi Biotec, Auburn, CA). Cells were flow-sorted to separate effector T cells (CD4+CD25−) and Tregs (CD4+CD25bright). The CD4+CD25− cells were CFSE-labeled and plated at 2.5×104–5.0×104 cells per well (96–well round bottom dish) in the presence of 2.5×104–5.0×104 anti-CD3/anti-CD28-coated silicon beads (1:1 ratio) or magnetic beads (Dynabeads Mouse T-Activator, Invitrogen). Increasing amounts of CD4+CD25bright cells were incubated with effector T cells in the presence or absence of recombinant mouse IL-21 (Peprotech). Alternatively, sorted effector T cells were rested (1×106 cells/ml), and Tregs (0.5×106 cells/ml) were incubated overnight with 500 IU rhIL-2 (BD Biosciences) with or without rIL-21 (150 ng/ml). The following day, cells were washed and co-incubated as above. Day 5 supernatants were analysed for cytokines by ELISA (IL-4: BD Pharmingen, IL-2, IL-13: RnD Systems), and day 3 cells harvested for flow cytometric analysis of proliferation by CFSE dilution. The frequency of cell division was determined using FlowJo software (Tree Star, Ashland, OR).
IL-21R flow cytometric staining
Isolated lung cells from HDM-treated BALB/c mice (days 0, 14 and 21) were used. All cells were stained with a PE-conjugated anti-IL-21R mAb (eBioscience, clone eBio4A9). T cell subsets were examined using mAbs to CD4, (eBioscience, RM4–5) CD25 (eBioscience, PC61.5), and Foxp3 (eBioscience, FJK-16s). mDCs (CD11c+CD11b+Gr1−), pDCs (CD11c+CD11b−Gr1+) and PMNs (CD11c+CD11bbrightGr1bright) were quantified using antibodies to CD11c (eBioscience, N418), CD11b (eBioscience, M1/70), and Gr1 (eBioscience, RB6-8C5). Leukocytes and non-leukocytes were differentiated by CD45 (eBioscience, 30-F11) staining. Dead cells were excluded using the viability dye 7-amino-actinomycin D (eBioscience, 7-AAD). All staining reactions were performed at 4°C. Flow cytometry antibodies were purchased from eBioscience. Analysis was done using FlowJo software.
Statistical Analysis
For comparison between multiple groups, one-way analysis of variance (ANOVA) was used to determine differences between groups with post hoc comparisons using Fisher's method. Significance was assumed at p < 0.05.
Results
Role of IL-21 signaling in allergen-driven airway hyperresponsiveness
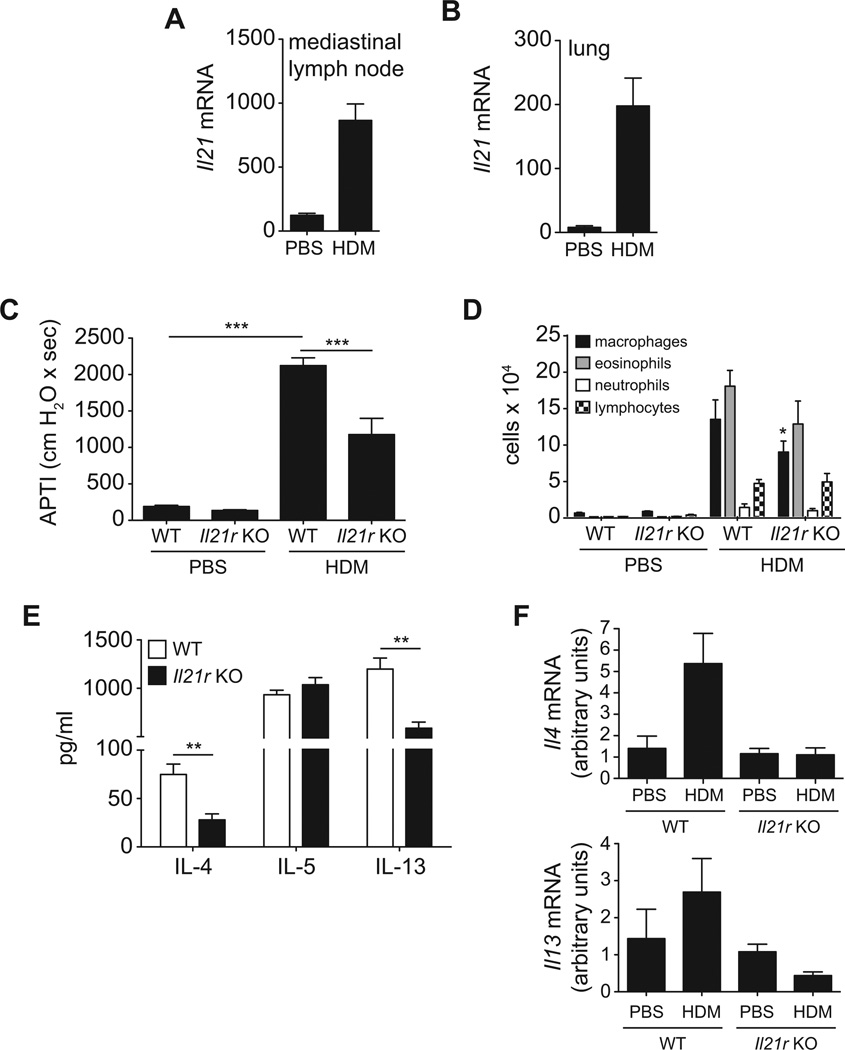
Because IL-21 is induced during Th2-mediated immune responses in vivo (2), we sought to determine if it was upregulated by pulmonary allergen exposure. After allergen treatment, Il21 transcripts are strongly upregulated in the lungs and lung-draining lymph nodes (Fig. 1A, B) compared to PBS-challenged mice, suggesting that IL-21 may be important in promoting allergen-induced Th2 responses. To delineate the role of IL-21 in regulation of Th2-driven allergic airway responses, we compared HDM-induced AHR in Il21r-deficient and wildtype BALB/c mice. Compared to PBS-treated mice, intratracheal exposure to HDM induced significant increases in AHR to cholinergic agonist stimulation (Fig. 1C). However, compared to WT controls, HDM-induced AHR in Il21r-deficient mice was significantly reduced. Concomitant with the increase in AHR, significant increases in the numbers of BAL macrophages, eosinophils, neutrophils, and lymphocytes were observed in wildtype HDM-treated mice (Fig. 1D). The number of BAL macrophages was significantly reduced in the Il21r-deficient as compared to wildtype. Similar reductions were observed in eosinophil and neutrophil numbers in Il21r-deficient relative to wildtypes, but did not reach statistical significance. Thus, the incomplete suppression of these responses suggests that IL-21 participates, but is not the only factor involved in allergic airway responses.
Figure 1. Il21r deficiency attenuates allergen-driven airway hyperresponsiveness.
A/J mice were exposed to HDM intratracheally on days 0 and 14 and sacrificed on day 17. Il21 mRNA was measured in lungs and mediastinal lymph nodes by real-time PCR (A, B). Expression of Il21 was normalized to ribosomal protein S14 mRNA. Wildtype BALB/c and Il21r-deficient mice were sensitized and challenged with PBS or HDM as described in Materials and Methods. Airway responsiveness to i.v. acetylcholine was assessed prior to sacrifice, 72 hours after final HDM challenge (C). BALF was harvested at time of sacrifice and BAL macrophages, eosinophils, and neutrophils were enumerated (D). Lung cells were cultured ex vivo and restimulated for 72 hours with 30 µg/ml HDM extract, and IL-4, IL-5, and IL-13 levels in culture supernatants were measured by ELISA (E). Levels of Il4 and Il13 mRNA were measured by real-time PCR from total lung RNA (F). Data represents mean ± SEM. n = 5–8 mice per group (*p<0.05, **p<0.01, ***p<0.001). Data is representative of 2 independent experiments.
IL-21R deficiency reduces Th2 cytokine production
To determine whether the effects of Il21r deficiency on AHR were mediated via alteration of Th2 cytokine production, IL-4, IL-5 and IL-13 were measured in supernatants from cultures of lung cells derived from HDM-exposed wildtype and Il21r-deficient mice (Fig. 1E). We found that in vitro HDM restimulation of lung cells from HDM-treated mice had significantly increased IL-4 production, however, IL-4 levels were significantly decreased in lung cells from Il21r-deficient mice compared to wildtype animals (Fig. 1E). A similar trend was observed with IL-13 (Fig. 1E). Lung cells from Il21r-deficient mice secreted less IL-13 than those from HDM-challenged wildtype mice. IL-5 was increased in cells from mice challenged with HDM, but the lack of IL-21R did not affect IL-5 levels. No appreciable IFNγ was observed in HDM-stimulated lung cells from either wildtype or Il21r-deficient mice (data not shown).
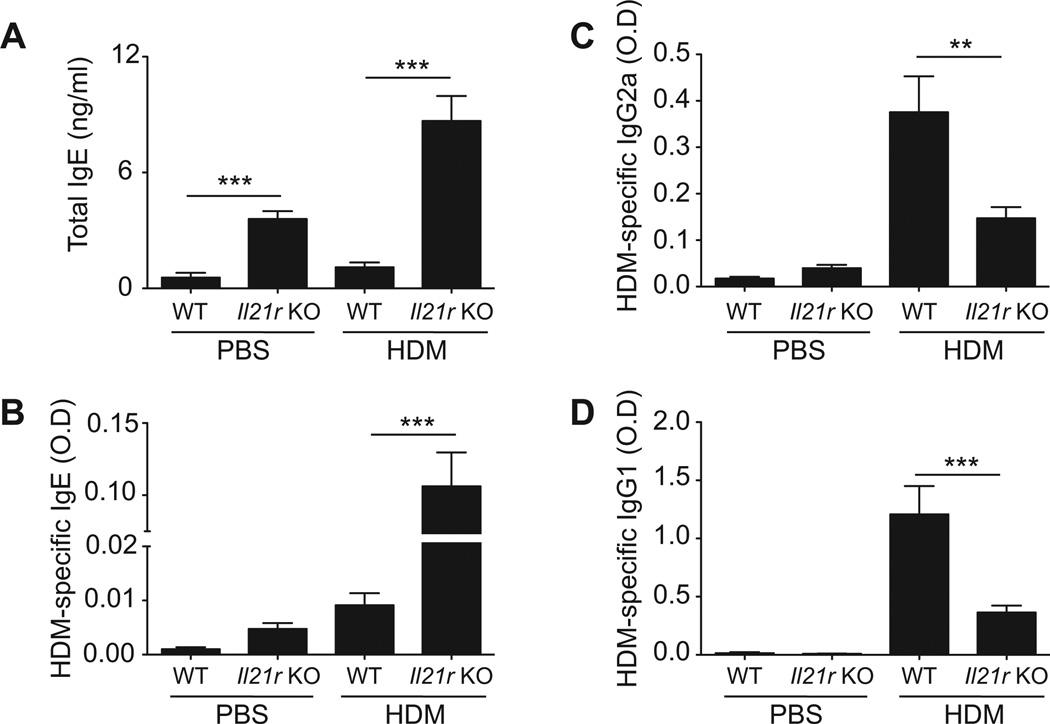
IL-21 decreases allergen-induced IgE synthesis
As IL-21 has previously been shown to regulate class switching in B cells, we examined the effect of IL-21R deficiency on serum immunoglobulin levels following HDM-immunization. Interestingly, baseline levels of IgE were significantly greater in PBS-treated Il21r-deficient mice as compared to wildtype controls (Fig. 2A, B). Likewise, HDM exposure induced significantly higher levels of serum IgE levels in Il21r-deficient mice as compared to wildtype HDM-exposed mice. In contrast, Il21r deficiency resulted in reduced serum HDM-specific IgG1 and IgG2a levels as compared to HDM-sensitized wildtype animals (Fig. 2C, D).
Figure 2. IL-21 regulates allergen-induced IgE synthesis.
Serum levels of total IgE (A), HDM-specific IgE (B), HDM-specific IgG2a (C), and HDM-specific IgG1 (D) were measured by ELISA 72 hours after the final HDM challenge. Data shown are mean ± SEM. n = 8 mice per group (*p<0.05, **p<0.01, ***p<0.001). Data is representative of 2 independent experiments.
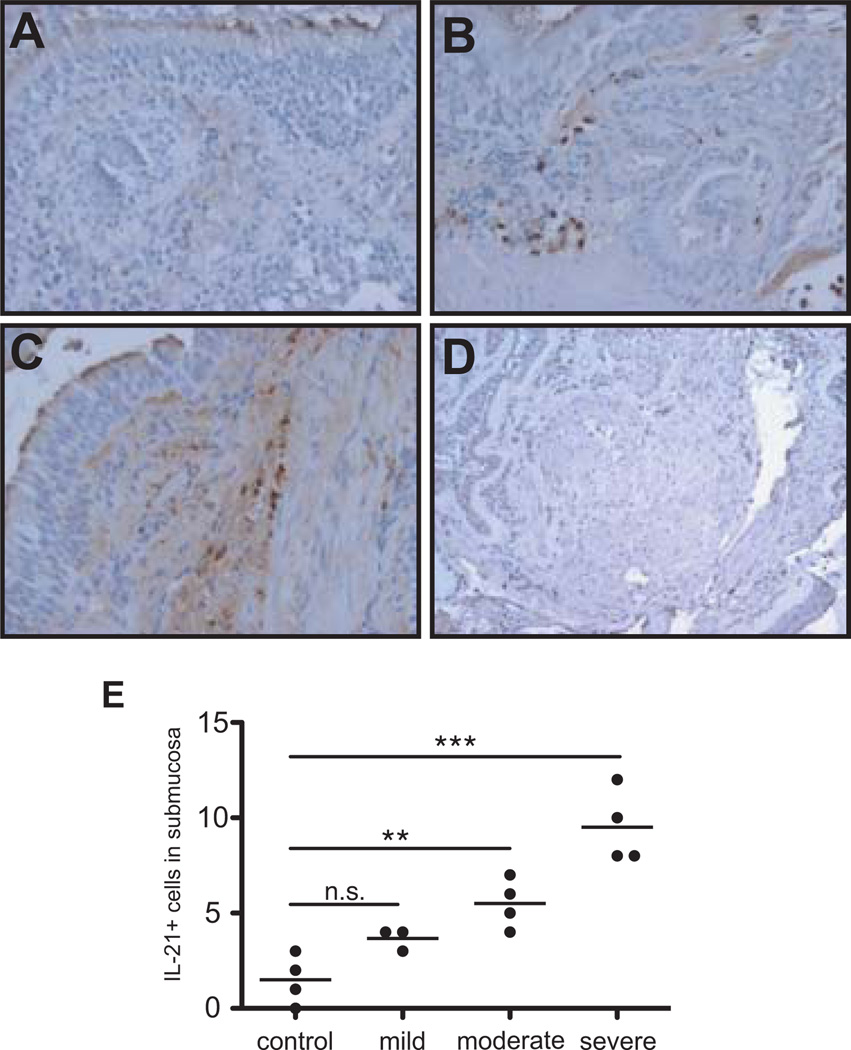
IL-21 is increased in human asthmatic airways
As IL-21 is strongly increased in experimental asthma, we wanted to examine its expression in human disease. IL-21 expression was assessed in bronchial biopsies from mild, moderate, and severe asthmatic patients and non-asthmatics by immunocytochemistry (Fig. 3). IL-21 positive cells were either absent or present in very small numbers in non-asthmatics airways (Fig.3A). Stronger staining was observed in the sections from moderate (Fig. 3B) and severe asthmatic individuals (Fig. 3C). The IL-21 staining appeared to be associated with infiltrating inflammatory cells in the submucosa. An isotype stained severe asthmatic section showed no signal (Fig. 3D). To our knowledge, this is the first report showing elevated IL-21 expression in human asthma, and that it correlates with disease severity (Fig. 3E).
Figure 3. IL-21 expression is increased in human asthmatic airways.
Immunohistochemical analysis for IL-21 was conducted on sections from bronchial biopsies obtained from non-asthmatic (A), moderate asthmatic (B), and severe asthmatic (C) individuals (20× magnification). A section from a severe patient was also stained with an isotype control (D). Quantification of the IL-21+ cells/field in the subepithelium is presented in panel E (*p < 0.05, **p < 0.01).
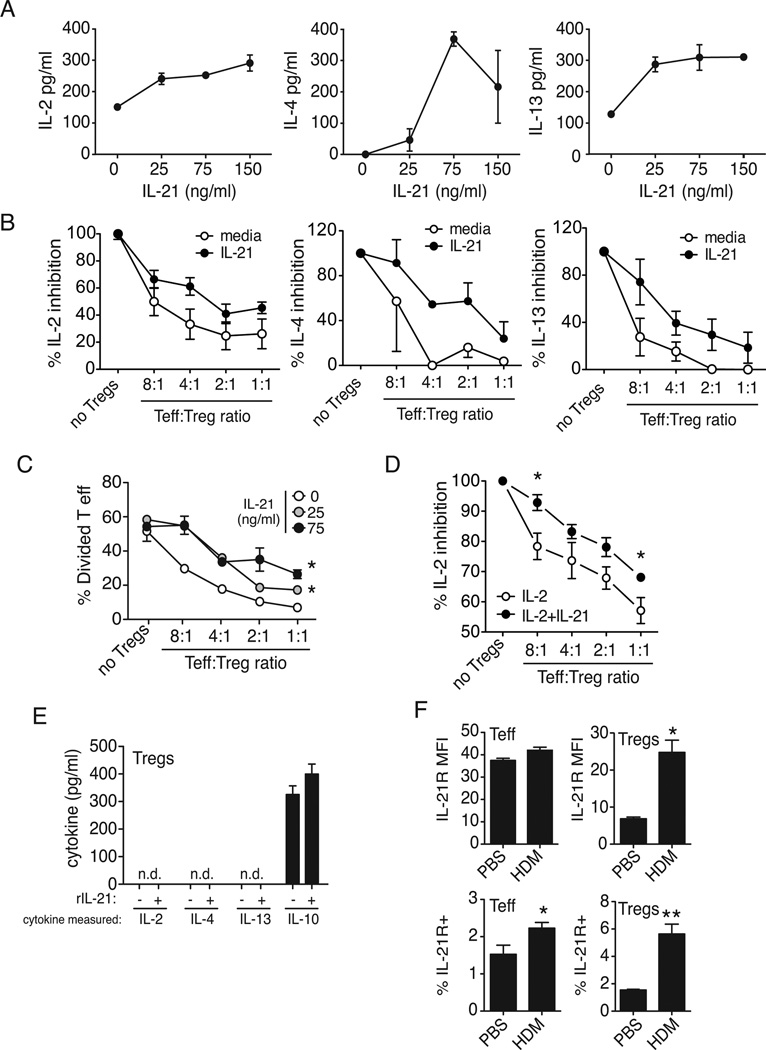
IL-21 impairs Treg function
To determine whether IL-21 enhances Th2 cytokine production directly, we treated flow-sorted lymph node effector T cells (CD4+CD25−) from naïve BALB/c mice with rIL-21 and examined cytokine production. We show that secretion of IL-2, IL-4 and IL-13 was increased (Fig. 4A) suggesting that IL-21 can directly enhance T cell cytokine production. As it has previously been shown that IL-21 may regulate Tregulatory cell function and we have previously shown that Tregs protect against the development of experimental allergic asthma(23), we next sought to determine whether IL-21 could enhance effector T cell function by impairing Tregs. To this end, we performed coculture experiments using flow-sorted lymph node effector T cells (CD4+CD25−) and Tregs (CD4+CD25bright) from naïve BALB/c mice stimulated with anti-CD3/anti-CD28− coated beads. As expected, cytokine production (IL-2, IL-4, IL-13) was decreased as the ratio of Tregs to effector T cells increased (Fig. 4B). However, addition of IL-21 to the co-cultures partially alleviated the suppressive effect of Tregs on cytokine secretion, thus requiring more Tregs to achieve total suppression than without IL-21 (Fig. 4B). Consistent with Treg-mediated suppression of cytokine production, increasing numbers of Tregs decreased the number of dividing CFSE-labeled effector T cells (Fig. 4C). While we observed that IL-21 failed to induce effector T cell division, it was able to partially prevent Treg-mediated inhibition of T cell proliferation (Fig. 4C). To directly examine the effect of IL-21 on Treg function, Tregs were pre-treated with IL-21 prior to incubation with effector T cells, and found that IL-21 directly impedes the suppressive ability of Tregs (Fig. 4D), As IL-10 is thought to be important in the suppression ability of Tregs, we treated Tregs with IL-21 and measured IL-10 production. We found that IL-21 had no impact on IL-10 secretion in sort-purified Tregs (Fig. 4E). Moreover, in the Treg inhibition assay, TGFb1 was not inhibited by IL-21 (Supplementary Fig. 2). This suggests that if IL-21 directly impedes Treg function, it is through a mechanism independent of IL-10 or TGFb1 production, consistent with previously published data that Tregs may suppress independently of TGFb or IL-10 (24, 25).
Figure 4. IL-21 overrides Treg-mediated suppression of T effector cytokine production and proliferation.
CFSE-labeled effector T cells (CD4+CD25−) derived from lymph nodes were stimulated with anti-CD3 and anti-CD28 and treated with IL-21 in the presence of increasing numbers of Tregs (CD4+CD25bright). After 5 days, (A, B) supernatants were collected for cytokine analysis, and (C) cells harvested for detection of proliferation by CFSE dilution. Proliferation is expressed as % divided T cells and expressed as the mean ± SEM of three wells. Effector T cells (CD4+CD25−) derived from lymph nodes were in were incubated with increasing numbers of IL-2 or IL-2+IL-21-pretreated Tregs (CD4+CD25bright), after 5 days supernatants were collected for IL-2 analysis (D). Supernatants from CD4+CD25bright cells (5.0×104/well) were collected for cytokine analysis, from media or IL-21-treated Tregs, data is expressed as the mean ± SEM of three wells (C). The influx of IL-21R+ Tregs (CD4+CD25+Foxp3+) in the lungs is greater than IL-21R+ effector T cells after HDM challenge. Tregs also strongly upregulate the IL-21R on their surface (MFI) compared to CD4+ effector T cells following allergen exposure (F), data is expressed as the mean ± SEM of 4–8 mice (*p < 0.05, **p < 0.01). Data is representative of 2–5 independent experiments.
To determine whether effector T cells and Tregs could have differential sensitivity to IL-21, we examined IL-21R expression on lung effector and regulatory T cells in PBS- or HDM- treated wildtype BALB/c mice. We show that while effector T cells have a higher baseline of surface IL-21R compared to Tregs, exposure to allergen had little effect on IL-21R expression in effector T cells, but strongly upregulated its expression in Tregs (~4 fold) (Fig. 4F). We found that of all lung cell types we analyzed (Supplementary Fig.1), Tregs showed the greatest fold increase in surface expression of IL-21R after in vivo HDM treatment, suggesting their heightened sensitivity to IL-21 during an allergic response. Taken together these results suggest that IL-21 may enhance Th2 cytokine production and the development of the allergic diathesis via suppression of Treg inhibitory effects on effector T cell proliferation or survival as well as cytokine production.
Discussion
Herein, we demonstrate increased IL-21 levels in the lungs of allergen challenged mice and in the airways of asthmatic individuals. We also show that allergen-induced AHR is significantly reduced in Il21r-deficient mice compared to wildtype mice, and this is accompanied by decreased production of Th2 cytokines. Interestingly, total and HDM-specific IgE levels were significantly elevated in Il21r-deficient mice compared to wildtype while HDM-specific IgG1 and IgG2a were reduced. Our results suggest that the mechanism by which IL-21 increases the AHR and Th2 cytokine production is by enhancing T cell survival and/or by decreasing Treg function. We show that IL-21 promotes cytokine production in effector T cells, and could partially reverse the inhibitory effect of Tregs on T cell proliferation and cytokine production. Taken together these results suggest that IL-21 plays an important role in the asthmatic phenotype.
Despite enhancing aspects of Th2 immune response in vivo, IL-21 is a potent inhibitor of IgE class switching in IL-4-stimulated murine B cells (26), and administration of IL-21 to mice decreases antigen-specific IgE (26, 27). We observed dramatically higher IgE levels in the naive and sensitized Il21r-deficient mice compared to controls. Although Il21r-deficient mice have reduced IL-4 and IL-13 levels, the remaining levels are likely sufficient for effective class switch to IgE. Our data recapitulates that generated by other groups showing increased IgE in the absence of IL-21 signaling (2, 11). Interestingly, we show that even in the presence of excessive amounts of IgE, the Il21r-deficient mice still exhibit less AHR; whether this is because IgE is not required for airway responses in mice, or that the lack of IL-21 signaling supersedes any pro-asthmatic effects due to elevated IgE remains unknown. Nonetheless, this highlights the lack of absolute requirement of IgE for AHR. While antigen-specific IgE has been shown to be required for the induction of AHR in some models (28, 29), it is not necessary for the development of airway reactivity in IgE-deficient animals (28, 30). Interestingly, we and others (1, 2) show that IL-21R deficiency results in a decrease in allergen-specific IgG1 and IgG2a levels, suggesting that in the absence of IL-21 signaling, B cells have unrestricted class switch to IgE at the expense of the IgG subclasses.
In contrast to the stimulatory effects of Il21r deficiency on IgE levels, Il21r-deficient mice had significantly reduced HDM-induced AHR. Our findings are consistent with studies in an OVA model of experimental asthma in which Il21r-deficient mice developed less AHR (1). The partial protection against allergen-induced AHR we observed in Il21r-deficient mice is likely mediated via IL-21’s ability to directly regulate IL-4 and IL-13 production, as we observe that IL-4 and IL-13 levels in lung cells from Il21r-deficient mice were significantly reduced. Similar reductions in Th2 cytokines have been observed in Il21r-deficient mice following parasite infection or OVA challenge (1, 2). Of note in our model, the decrease in AHR is accompanied by only a very minor reduction in BAL eosinophil numbers in the Il21r-deficient mice compared to their wild type controls. The dissociation between AHR and BAL eosinophilia in Il21r-deficient mice is likely explained by the differential effect of IL-21R signaling on IL-4/IL-13 and IL-5 production, as IL-5 levels did not differ between Il21r-deficient and widltype mice. Similarly, IL-4/IL-13-dependent granuloma formation in Schistosoma mansoni-infected Il21r-deficient mice was impaired relative to wildtype controls, while no changes were observed in tissue eosinophil numbers (2).
Although the mechanism(s) by which IL-21 regulates IL-4 and IL-13 production are unclear, our studies suggest that the effects of IL-21 might be direct as IL-21 treatment of sort-purified effector T cells in vitro enhanced their production of IL-2, IL-4, and IL-13. As previous studies demonstrate that IL-21 does not directly regulate Th2 polarization(11), and there is no evidence that IL-21 promotes transcription of genes coding for Th2 cytokine, the effect of IL-21 might be to expand already polarized effector T cells. However, our data and that of others indicate that IL-21 does not have a strong mitogenic effect on effector cells (13, 14, 31). Alternatively since IL-21 is an important survival factor for cells that are known to produce IL-4 and IL-13, such as NK, NKT and T cells (13, 14, 17, 32), IL-21 signaling might enhance the survival of effector cells, resulting in increased cytokine output.
In addition to its direct effects on effector T cells, our studies suggest that IL-21 may enhance Th2 cytokine production by inhibiting the suppressive effects of Tregs. Specifically, we show that IL-21 treatment of Teffector/Treg cocultures increases the numbers of Tregs required to achieve the same level of inhibition of cytokine production and proliferation as in cultures without IL-21. We also show that IL-21-treated Tregs are less suppressive than control Tregs in cocultures with effector T cells, showing that IL-21 directly hampers Treg function. Our findings are consistent with recent studies demonstrating that IL-21 can inhibit Treg development, and function (4, 5, 8, 9). The mechanism by which IL-21 impairs Treg function is unclear. However, there is evidence that IL-21 may inhibit Treg function through actions directly on Tregs and on Teffector themselves. In this regard, IL-21 decreases the number of Foxp3+ cells from human PBMC cultures (8), and a greater number of Tregs can be generated from Il21-deficient naïve T cells than from wildtype (5), suggesting that IL-21 may prevent optimal Treg function and development by inhibiting Foxp3. Additionally, IL-21 can prevent Treg-induced apoptosis of effector T cells (33) by maintaining or increasing anti-apoptotic molecules such as Bcl-2 in effector T cells (17, 33). IL-21 can also activate the PI3K pathway (17, 34) which renders effector T cells resistant to Treg suppression (35). Although IL-21 signaling may contribute to both decreased Treg function and enhanced resistance of Teffectors to Treg-mediated suppression, we show that the predominant effect of IL-21 in our model is likely due to impaired Treg function as HDM exposure leads to a robust upregulation of IL-21R expression on lung Tregs compared to effector T cells, rendering them more responsive to IL-21 during allergic inflammation. Hence, the upregulation of IL-21 during allergic responses may lead to enhanced Th2-mediated immune responses via multiple mechanisms including: enhanced Teffector survival, direct suppression of inhibitory Treg signals, and enhanced Teffector resistance to Treg-mediated suppression. Interestingly, we saw IL-21R expression on lung mDCs and pDCs (supplementary Fig. 1), while little is known about the function of IL-21 on DCs, an early report showed that it decreased antigen presentation (16), whether this is also true in the context of a complex allergenic source like HDM remains to be determined. While this may suggest a protective effect for IL-21, overall, a deficiency in IL-21 signaling protects against HDM-induced allergic asthma.
In summary, we demonstrate that IL-21 is elevated in the lungs of both human asthmatics and in allergen-exposed mice and that IL-21R signaling partially modulates several manifestations of allergic asthma including AHR, and Th2 cytokine production. Moreover, our data suggest that IL-21 may enhance Th2-mediated immune responses through the suppression of the anti-inflammatory arm of the immune response (Tregs) and/or enhanced Th2 effector cell survival, ultimately leading to greater Th2 cytokine production. Taken together these results suggest that dysregulated IL-21 production may play an important role in the pathogenesis of allergic airway disease.
Supplementary Material
After HDM, the surface level (MFI) of the IL-21R and the frequency (%IL-21R+) of IL-21R+ leukocytes (CD45+) and non-leukocyte (CD45−) populations are increased in the lungs (A). The frequency of IL-21R+ myeloid DCs (mDC - CD11c+CD11b+, Gr1+) and surface expression are also increased following allergen. However, frequency (%) and surface expression (MFI) of IL-21R is unchanged after treatment in B cells, polymorphonuclear leukocytes (PMNs - CD11c+, CD11b+. Gr1+) and plasmacytoid DCs (pDC – CD11clo, CD11b-, Gr1+) (B).
IL-21 pre-treated Tregs (CD4+CD25bright) were co-incubated with effector T cells (CD4+CD25−) derived from lymph nodes, and were stimulated with anti-CD3 and anti-CD28 After 5 days, supernatants were collected for TGFb1 analysis. Data is expressed as pg/ml (A) or as % of the “no Tregs” condition (B) (data expressed as mean ± SEM of four wells). Representative of 2 independent experiments.
Acknowledgements
This work was supported by NHLBI grants HL67736 and HL076383 to MWK.
Abbreviations
- AHR
airway hyperresponsiveness
- HDM
house dust mite
- BALF
bronchoalveolar lavage fluid
Footnotes
The authors have no conflicting financial interests.
References
- 1.Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109(5):2023–2031. doi: 10.1182/blood-2006-05-021600. Epub 2006/11/02. [DOI] [PubMed] [Google Scholar]
- 2.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116(7):2044–2055. doi: 10.1172/JCI27727. Epub 2006/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196(7):969–977. doi: 10.1084/jem.20020620. Epub 2002/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. Epub 2007/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. Epub 2007/06/22. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. Epub 2007/06/22. [DOI] [PubMed] [Google Scholar]
- 7.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–137. doi: 10.1016/j.immuni.2008.06.001. Epub 2008/07/08. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111(1):229–235. doi: 10.1182/blood-2007-05-089375. Epub 2007/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178(2):732–739. doi: 10.4049/jimmunol.178.2.732. Epub 2007/01/05. [DOI] [PubMed] [Google Scholar]
- 10.Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172(9):5154–5157. doi: 10.4049/jimmunol.172.9.5154. Epub 2004/04/22. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630–1634. doi: 10.1126/science.1077002. Epub 2002/11/26. [DOI] [PubMed] [Google Scholar]
- 12.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16(4):559–569. doi: 10.1016/s1074-7613(02)00295-9. Epub 2002/04/24. [DOI] [PubMed] [Google Scholar]
- 13.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. doi: 10.1038/35040504. Epub 2000/11/18. [DOI] [PubMed] [Google Scholar]
- 14.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178(5):2827–2834. doi: 10.4049/jimmunol.178.5.2827. Epub 2007/02/22. [DOI] [PubMed] [Google Scholar]
- 15.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5(9):688–698. doi: 10.1038/nri1688. Epub 2005/09/03. [DOI] [PubMed] [Google Scholar]
- 16.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102(12):4090–4098. doi: 10.1182/blood-2003-03-0669. Epub 2003/08/02. [DOI] [PubMed] [Google Scholar]
- 17.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukoc Biol. 2007;82(3):645–656. doi: 10.1189/jlb.0806494. Epub 2007/06/08. [DOI] [PubMed] [Google Scholar]
- 18.Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, et al. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55(12):1774–1780. doi: 10.1136/gut.2006.093187. Epub 2006/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungel A, Distler JH, Kurowska-Stolarska M, Seemayer CA, Seibl R, Forster A, et al. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50(5):1468–1476. doi: 10.1002/art.20218. Epub 2004/05/18. [DOI] [PubMed] [Google Scholar]
- 20.Distler JH, Jungel A, Kowal-Bielecka O, Michel BA, Gay RE, Sprott H, et al. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum. 2005;52(3):856–864. doi: 10.1002/art.20883. Epub 2005/03/08. [DOI] [PubMed] [Google Scholar]
- 21.Miller SD, Shevach EM. Immunoregulation of experimental autoimmune encephalomyelitis: editorial overview. Res Immunol. 1998;149(9):753–759. doi: 10.1016/s0923-2494(99)80002-9. Epub 1999/01/29. [DOI] [PubMed] [Google Scholar]
- 22.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162(6):2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. Epub 2000/12/09. [DOI] [PubMed] [Google Scholar]
- 23.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202(11):1549–1561. doi: 10.1084/jem.20051506. Epub 2005/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10(12):1969–1980. doi: 10.1093/intimm/10.12.1969. Epub 1999/01/14. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. Epub 1998/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100(13):4565–4573. doi: 10.1182/blood-2002-04-1115. Epub 2002/10/24. [DOI] [PubMed] [Google Scholar]
- 27.Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, et al. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. 2007;179(10):7157–7165. doi: 10.4049/jimmunol.179.10.7157. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
- 28.Hamelmann E, Oshiba A, Schwarze J, Bradley K, Loader J, Larsen GL, et al. Allergen-specific IgE and IL-5 are essential for the development of airway hyperresponsiveness. Am J Respir Cell Mol Biol. 1997;16(6):674–682. doi: 10.1165/ajrcmb.16.6.9191469. Epub 1997/06/01. [DOI] [PubMed] [Google Scholar]
- 29.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, et al. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest. 1996;97(6):1398–1408. doi: 10.1172/JCI118560. Epub 1996/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, et al. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci U S A. 1997;94(4):1344–1349. doi: 10.1073/pnas.94.4.1344. Epub 1997/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. Epub 2005/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onoda T, Rahman M, Nara H, Araki A, Makabe K, Tsumoto K, et al. Human CD4+ central and effector memory T cells produce IL-21: effect on cytokine-driven proliferation of CD4+ T cell subsets. Int Immunol. 2007;19(10):1191–1199. doi: 10.1093/intimm/dxm090. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 33.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
- 34.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109(10):4135–4142. doi: 10.1182/blood-2006-10-054973. Epub 2007/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wohlfert EA, Clark RB. 'Vive la Resistance!'--the PI3K-Akt pathway can determine target sensitivity to regulatory T cell suppression. Trends Immunol. 2007;28(4):154–160. doi: 10.1016/j.it.2007.02.003. Epub 2007/03/03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After HDM, the surface level (MFI) of the IL-21R and the frequency (%IL-21R+) of IL-21R+ leukocytes (CD45+) and non-leukocyte (CD45−) populations are increased in the lungs (A). The frequency of IL-21R+ myeloid DCs (mDC - CD11c+CD11b+, Gr1+) and surface expression are also increased following allergen. However, frequency (%) and surface expression (MFI) of IL-21R is unchanged after treatment in B cells, polymorphonuclear leukocytes (PMNs - CD11c+, CD11b+. Gr1+) and plasmacytoid DCs (pDC – CD11clo, CD11b-, Gr1+) (B).
IL-21 pre-treated Tregs (CD4+CD25bright) were co-incubated with effector T cells (CD4+CD25−) derived from lymph nodes, and were stimulated with anti-CD3 and anti-CD28 After 5 days, supernatants were collected for TGFb1 analysis. Data is expressed as pg/ml (A) or as % of the “no Tregs” condition (B) (data expressed as mean ± SEM of four wells). Representative of 2 independent experiments.