Abstract
T cell development in the thymus produces multiple lineages of cells, including innate T cells. Studies in mice harboring alterations in TCR signaling proteins or transcriptional regulators have revealed an expanded population of CD4+ innate T cells in the thymus that produce IL-4 and express the transcription factor PLZF. In these mice, IL-4 produced by the CD4+ PLZF+ T cell population leads to the conversion of conventional CD8+ thymocytes into innate CD8+ T cells resembling memory T cells expressing Eomesodermin. The expression of PLZF, the signature iNKT cell transcription factor, in these innate CD4+ T cells suggests that they might be a subset of αβ or γδ TCR+ NKT cells or MAIT cells. To address these possibilities, we characterized the CD4+ PLZF+ innate T cells in itk-/- mice. We show that itk-/- innate PLZF+ CD4+ T cells are not CD1d-dependent NKT cells, MR1-dependent MAIT cells, nor γδ T cells. Further, although the itk-/- innate PLZF+ CD4+ T cells express αβ TCRs, neither β2m-dependent MHC class I nor any MHC class II molecules are required for their development. In contrast to iNKT cells and MAIT cells, this population has a highly diverse TCRα chain repertoire. Analysis of peripheral tissues indicates that itk-/- innate PLZF+ CD4+ T cells preferentially home to spleen and mesenteric lymph nodes due to increased expression of gut-homing receptors, and that their expansion is regulated by commensal gut flora. These data support the conclusion that itk-/- innate PLZF+ CD4+ T cells are a novel subset of innate T cells.
Introduction
The mature immune system is comprised of multiple lineages of T lymphocytes. Conventional CD4+ and CD8+ T cells, the majority populations, develop in the thymus as naïve cells with TCRs specific for classical MHC class II or MHC class I molecules, respectively. In addition to these conventional T cell lineages, several subsets of T cells develop in the thymus with pre-programmed effector functions, including the ability to rapidly secrete cytokines and to home to specific extrathymic lymphoid or non-lymphoid organs (1, 2). This latter group of T cells, collectively referred to as innate T cells, includes TCRαβ+ T cells such as iNKT cells, mucosal-associated invariant T (MAIT) cells, and H2-M3-specific T cells, as well as several lineages of TCRγδ+ T cells (1, 2). For several of these subsets, their specific effector functions and homing properties are correlated with the expression of an invariant, or nearly invariant TCR sequence (3, 4). An additional common element is that many of these cell types express TCRs that are specific for nonclassical MHC class Ib molecules, rather than for classical MHC class Ia or class II molecules (5-7).
Molecular analysis of several innate T cell lineages has identified key transcription factors that regulate the signature functions of each cell subset. One such transcription factor is promyelocytic leukemia zinc finger, (PLZF, also known as zbtb16). PLZF is expressed in iNKT cells, human peripheral MAIT cells, and Vγ1.1/Vδ6.3+ NKT cells (8-15), and PLZF confers dual IFNγ plus IL-4 secreting capabilities on the cells, along with a preference to emigrate from the thymus to non-lymphoid tissues. In the two subsets of NKT cells (αβ TCR+ and γδ TCR+), PLZF is first detected in immature thymic subsets expressing the invariant TCRs, and is then down-regulated as the T cells mature (8, 9, 12). A second shared feature of these cells is their dependence on the SLAM family receptor adapter protein, SAP; in SAP-deficient mice, PLZF-expressing αβ and γδ NKT cells fail to develop and/or accumulate to normal numbers (15-18).
Expanded populations of innate PLZF-expressing CD4+ T cells have also been found in several lines of genetically-modified mice. For instance, mice expressing MHC class II molecules on their thymocytes have a predominant subset of innate CD4+ T cells, known as T-CD4 cells, that co-express IFNγ and IL-4 when stimulated (19). In addition, klf2-/-, id3-/-, SLP76 (Y145F), and itk-/- mice all have increased numbers of PLZF-expressing innate CD4+ T cells that are responsible for inducing thymic CD8+ T cells to develop as innate T cells expressing the T-box transcription factor, Eomesodermin (Eomes) (20-22). In several of these cases, studies have shown that these expanded PLZF+ CD4+ thymic populations are eliminated in mice also lacking SAP (21, 23), suggesting an important role for homotypic thymocyte-thymocyte interactions.
To date, a detailed characterization of the CD4+ PLZF+ T cell populations arising in itk-/-, as well as the other lines of mice sharing this phenotype, is lacking. Here we show that these cells are not CD1d-specific iNKT cells, nor are they cells specific for the nonclassical MHC class I molecule, MR1. We also demonstrate that the itk-/- CD4+ PLZF+ cells are not dependent on classical MHC class I or MHC class II molecules, nor are they a lineage-confused population of γδ T cells. Instead, we find that these cells develop independently of β2m and MHC class II molecules, have high expression of gut-homing receptors, preferentially migrate from the thymus to the spleen and mesenteric lymph nodes (mLN), and are dependent on commensal gut flora for their expansion. These data identify a novel population of innate CD4+ T cells with striking similarities to human PLZF+ T-CD4 and MAIT cells.
Materials and Methods
Mice
Wild-type (WT) C57Bl/6 mice were purchased from either Taconic Farms, Inc. (Hudson, NY), Jackson Laboratories (Bar Harbor, ME), or Charles River Laboratories International, Inc. (Wilmington, MA). itk-/- mice were previously described (24-26) and housed at the University of Massachusetts Medical School in accordance with the institutional animal care and use committee (IACUC) and in a specific-pathogen free environment. IL-4 reporter (4get) mice (27) were a gift from Markus Mohrs (Trudeau Institute, Saranac Lake, NY) and were crossed to itk-/- at the University of Massachusetts Medical School. cd1d-/- mice were a gift from the laboratory of Raymond Welsh and were crossed to itk-/- mice at the University of Massachusetts Medical School. mr1-/- and il-15-/- mice were a gift from Joonsoo Kang and were also crossed to itk-/- mice at the University of Massachusetts Medical School. H2dlAb1-Ea mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were crossed to itk-/- at the University of Massachusetts Medical School. itk-/- β2m-/- mice were a kind gift from Pamela Schwartzberg (National Institute of Health). itk-/- β2m-/- were crossed to itk-/- H2dlAb1-Ea mice at the University of Massachusetts Medical School. β2m-/-/H2-ab1-/- were purchased from Taconic Farms, Inc. (Hudson, NY). tcrd-/- were a gift from the laboratory of Raymond Welsh and were crossed to itk-/- mice at the University of Massachusetts Medical School as previously described (11). Mice were between the ages of 3-12 weeks at the time of experiment.
Antibiotic Treatment
In order to examine the role of commensal gut flora in the expansion of itk-/- innate PLZF+ CD4+ T cells, we provided WT and Itk-deficient mice with a strong course of antibiotics via drinking water from birth until the time of the experiment. This antibiotic cocktail consisted of ampicillin (1 g/L, A9518), neomycin (1 g/L, N6386), metronidazole (1g/L, M3761) (Sigma-Aldrich, St. Louis, MO), vancomycin (0.5 g/L, V870) (PhytoTechnology Laboratories, Shawnee Mission, KS), and the sweetener Equal (5 g/L). This antibody cocktail has previously been described to abrogate commensal gut flora in mice (28, 29).
Cell Preparation
Thymus, spleen, peripheral lymph nodes (pLN: including cervical, axillary, brachial and inguinal), mesenteric lymph nodes (mLN), liver and small intestine were harvested from mice and stored in RPMI (Gibco by Invitrogen, Grand Island, New York) supplemented with fetal bovine serum (FBS), L-glutamine, penicillin, streptomycin, β-mercaptoethanol, and Hepes (RPMI-10). Thymi were processed using forceps and frosted microscope slides. Spleen, pLN, and mLN were processed using only frosted microscrope slides. Thymus, spleen, pLN, and mLN were lysed with red blood cell (RBC) lysis buffer prior to single cell suspension with RPMI-10. Before harvesting, livers were perfused with 5 mL ice-cold 1X phosphate buffered saline (PBS). Livers were processed by cutting with a razor blade prior to suspending in 0.05% collagenase. After liver tissue was dissolved, red blood cells were lysed prior to suspending the cells in a discontinuous 40/70% Percoll gradient to isolate lymphocytes. A single cell suspension was made from the lymphocytes after the Percoll gradient was performed. Intestinal intraepithelial lymphocytes (iIELs) were isolated by removing fecal matter and Peyer's patches from the small intestine prior to shaking in media. Cell suspensions were filtered prior to suspending cells in a discontinuous 40/70% Percoll gradient. Single cell suspensions of the iIELs were made after the gradient was performed.
Cell Stimulation
Thymocytes from WT or itk-/- mice were plated at 106-107 cells per well prior to stimulating with phorbol myristate acetate (PMA, 10 ng/mL) (Sigma-Aldrich, St. Louis, MO) and ionomycin (1 μg/mL) (Sigma-Aldrich, St. Louis, MO) for six hours at 37°C in the presence of brefeldin A (BD Biosciences, San Jose, CA) and monensin (BD Biosciences, San Jose, CA).
Extracellular/Intracellular Staining
Cells were plated at 106-107 cells per well prior to washing with fluorescence-activated cell sorting (FACS) buffer (1X PBS supplemented with 2% FBS). Fc receptors were blocked using supernatant from 2.4G2 hybridomas grown in the lab prior to staining with the CD1d tetramer loaded with PBS57 (a gift from the NIH). Cells were stained with various combinations of the cell surface antibodies (BD Pharmingen, San Jose, CA, and eBioscience, San Diego, CA) against CD4 (RM4-5), CD8 (53-6.7 or 5H10), TCRβ (H57-597), TCRδ (GL3), Heat-shock antigen (HSA, CD24) (30-F1 or M1/69), CD44 (IM7), CD62L (MEL-14), CD127 (IL-7Rα) (A7R34 or SB/199), CD124 (IL-4Rα) (mIL4R-M1), CD122 (IL-2Rβ) (5H4 or TM-β1), CXCR3 (CXCR3-173), α4β7 (DATK32), CD103 (2E7), CCR6 (140706), Vα2 (B20.1), Vα3.2 (RR3-16), Vα8.3 (B21.14), Vβ3 (KJ25), Vβ4 (KT4), Vβ6 (RR4-7), Vβ7 (TR310), Vβ8.3 (1B3.3), Vβ10b (B21.5), Vβ11 (RR3-15), and Vβ14 (14-2). Cells were then permeabilized using either the eBioscience Foxp3/transcription factor staining kit or BD Cytofix/Cytoperm according to the manufacturer's protocol prior to staining with antibodies against the transcriptions factors, Eomesodermin (Dan11mag, eBioscience, San Diego, CA) and PLZF (D-9, Santa Cruz Biotechnology, Inc., Dallas, TX) with an IgG1 secondary (A85-1, BD Pharmingen, San Jose, CA), and the cytokines (BD Pharmingen or eBioscience), IL-4 (BVD6-24G2) and IFNγ (XMG1.2)
SMART-RACE PCR
Thymocytes from itk-/- mice expressing the IL-4 reporter (4get) were harvested and processed as described above using DMEM supplemented with azide (0.1%), Hepes (10 mM), and FCS (2%) and ammonium-chloride-potassium lysis buffer to lyse red blood cells. CD8+ thymocytes were depleted using Dynal beads (Invitrogen, Grand Island, NY) prior to staining with CD1d tetramer, TCRβ, CD4, CD8, HSA, and CD44. CD4SP CD1d-tetramerneg TCRβ+ HSAlow CD44high GFP+ thymocytes were sorted into TRIzol (Invitrogen, Grand Island, NY) on a FACS Aria (Becton Dickinson, Dallas, TX). Afterwards, RNA was isolated according to the manufacturer's protocol prior to converting to cDNA using the SMART Race cDNA amplification kit from Clontech using a modified manufacturer's protocol in which the provided PowerScript was replaced with Supercript III (Invitrogen, Grand Island, NY). To amplify the TCRα specific genes the Advantage2 system was used according to manufacturer's protocol (Clontech, Mountain View, CA). For the 5′ forward primer, the Universal Primer Mix from the SMART Race cDNA amplification kit was used. To amplify the genes of interest a 3′ reverse primer specific to the TCRα region was used and reported previously (30). The PCR amplification program used was provided in the manufacturer's protocol. The resulting PCR product of appropriate size (∼500-700 bp) was gel purified using the NucleoSpin Extract II kit according to the manufacturer's protocol (Clontech, Mountain View, CA). The purified PCR product was ligated into pCR4 vector from the TOPO TA cloning kit (Invitrogen, Grand Island, NY) according to the manufacturer's protocol and transformed into DH5α E. coli from One Shot TOP10 chemically competent cells (Invitrogen, Grand Island, NY) using the manufacturer's procedures. Colonies were selected and amplified overnight for sequencing. Amplified colonies are preserved in 20% glycerol on dry ice and sent to Agencourt Bioscience (Beverly, MA) for sequencing. The resulting sequences were aligned using Sequencher software (Gene Codes Corporation, Ann Arbor, MI) and analyzed using IMGT/V-quest (http://www.imgt.org) (31, 32).
Results
CD4+ PLZF+ CD1d-tetramerneg αβ T cells expressing IL-4 mRNA develop in the absence of Itk
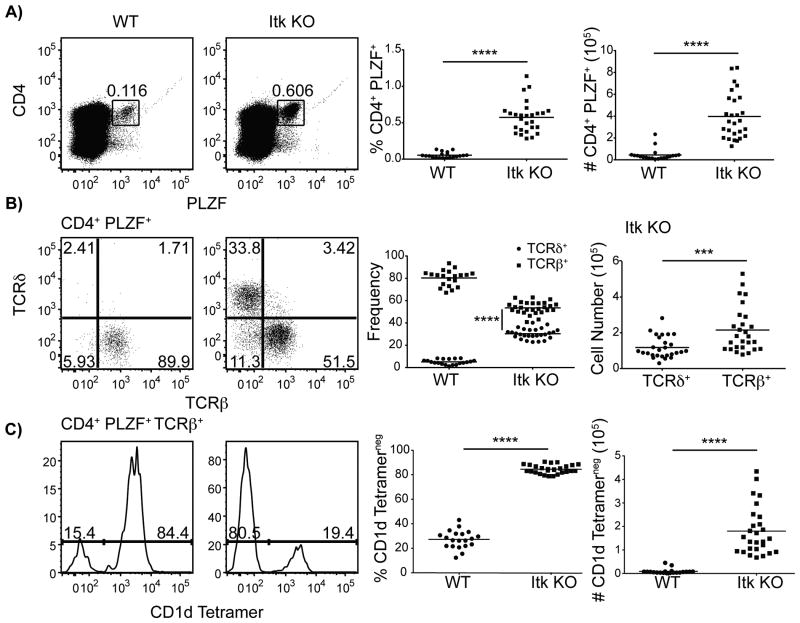
In the absence of Itk, the frequency and number of CD4+ PLZF+ thymocytes is substantially increased (Figure 1A). When further examined based on surface expression of TCRβversus TCRδ, it is apparent that in WT, the vast majority of CD4+ PLZF+ thymocytes are αβ T cells, whereas in itk-/-, there are nearly comparable proportions of αβ and γδ T cells in this population (Figure 1B). Interestingly, WT CD4+ PLZF+ TCRβ+ thymocytes are predominantly iNKT cells, as they bind to CD1d/α-galcer tetramers (hereafter referred to as ‘CD1d-tetramers’) (Figure 1C); in contrast, only a modest proportion of itk-/- CD4+ PLZF+ TCRβ+ thymocytes are iNKT cells, indicating a significant increase in a non-iNKT cell population in the absence of Itk (Figure 1C).
Figure 1. CD4+ PLZF+αβ T cells develop in the absence of Itk.
Thymocytes from WT and itk-/- mice were isolated and stained with CD1d-tetramer and antibodies to CD4, TCRδ, TCRβ, and PLZF.
(A) Dot-plots show CD4 versus PLZF staining of total thymocytes; numbers indicate the percentage of CD4+ PLZF+ cells. Graphs show a compilation of data indicating the percentages (left) and absolute numbers (right) of CD4+ PLZF+ cells.
(B) Dot-plots show TCRβ versus TCRδ staining on CD4+ PLZF+ thymocytes. Graphs show the relative proportion of CD4+ PLZF+ thymocytes expressing TCRδ or TCRβ (left), and the absolute numbers of cells in each population in itk-/- mice (right).
(C) Histograms show CD1d-tetramer staining on CD4+ PLZF+ TCRβ+ thymoyctes in WT (left) or itk-/- (right). Graphs show percentages (left) and absolute numbers (right) of CD4+ PLZF+ TCRβ+ CD1d-tetramerneg thymocytes.
n = 20-27 mice per group. Results are representative of six independent experiments. Statistical analysis was performed using a Mann Whitney t test. ***p < 0.0005 ****p < 0.0001
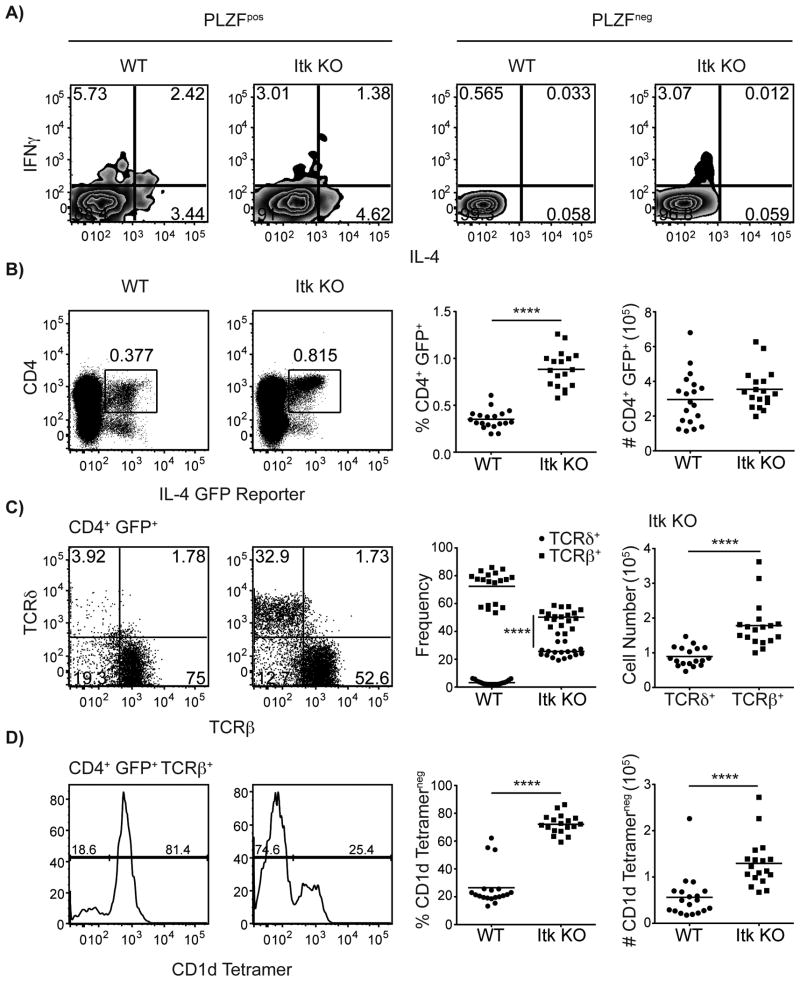
To first determine that PLZF+ thymocytes were the sole producers of IL-4 in both WT and Itk-deficient mice, we stimulated WT and itk-/- thymocytes ex vivo with PMA and ionomycin. In agreement with previous studies involving αβ iNKT cells (8, 9), we found that only PLZF+ thymocytes populations are able to produce IL-4 (Figure 2A). To further determine if the novel population of itk-/- CD4+ PLZF+ TCRβ+ thymocytes described in Figure 1 was constitutively expressing IL-4 mRNA, we crossed itk-/- mice to mice carrying a GFP reporter in the IL-4 locus (27). In these IL-4 reporter mice, known as ‘4get’ mice, GFP expression is a direct readout of IL-4 mRNA production, and previous studies with T-CD4 cells demonstrate that GFP expression correlates with expression of PLZF (33). As shown in Figure 2B-D, itk-/- thymocytes include an increased percentage of CD4+ GFP+ cells compared to WT. Further, among WT thymocytes, the vast majority of CD4+ GFP+ cells are TCRβ+; in contrast, itk-/- thymocytes have substantial populations of both αβ and γδ lineage cells expressing CD4 and GFP, with about a 2:1 ratio between the two cell types (Figure 2C). Similar to the pattern seen for PLZF expression, analysis of CD4+ GFP+ TCRβ+ thymocytes for binding to the CD1d-tetramer indicated that the majority of these cells in the WT thymus are iNKT cells, whereas in itk-/- mice iNKT cells represent only a modest percentage of this GFP+ population (Figure 2D). Thus, this novel population of itk-/- CD4+ PLZF+ TCRβ+ CD1d-tetramerneg thymocytes is also constitutively expressing IL-4 mRNA; previous studies have determined that IL-4 contributes to the conversion of conventional αβ T cells into innate-like T cells expressing the transcription factor, Eomes (20-22, 34).
Figure 2. CD4SP thymocytes in itk-/- mice constitutively express IL-4 mRNA.
(A) Thymocytes from WT and itk-/- mice were isolated and stimulated with PMA and ionomycin for six hours at 37°C in the presence of brefeldin A and monensin prior to intracellular staining with antibodies against PLZF, IFNγ, and IL-4. Dot plots show IFNγ versus IL-4 production of PLZFpos thymocytes (right) and PLZFneg thymocytes (left). Figure is representative of two independent experiments.
(B-D) Thymocytes from WT-4get and itk-/--4get mice were isolated and stained with CD1d-tetramer and antibodies to CD4, TCRδ, and TCRβ.
(B) Dot-plots show GFP fluorescence versus CD4 staining; numbers indicate the percentages of CD4+ GFP+ cells. Graphs show a compilation of data indicating the percentages and absolute numbers of CD4+ GFP+ cells.
(C) Dot-plots show TCRβ versus TCRδ staining on CD4+ GFP+ cells. Graphs show a compilation of data indicating the proportions of CD4+ GFP+ cells in the αβ versus γδ lineage (left), and the absolute numbers of CD4+ GFP+ cells in each lineage among itk-/--4get thymocytes (right).
(D) Histograms show CD1d-tetramer staining on CD4+ GFP+ TCRβ+ cells. Graphs show percentages and absolute numbers of CD1d-tetramerneg CD4+ GFP+ TCRβ+ thymocytes. n = 18-19 mice per group. Results are representative of four independent experiments. Statistical analysis was performed using a Mann Whitney t test. ****p < 0.0001
PLZF+ CD1d-tetramerneg αβ T cells home to the spleen and mesenteric lymph node
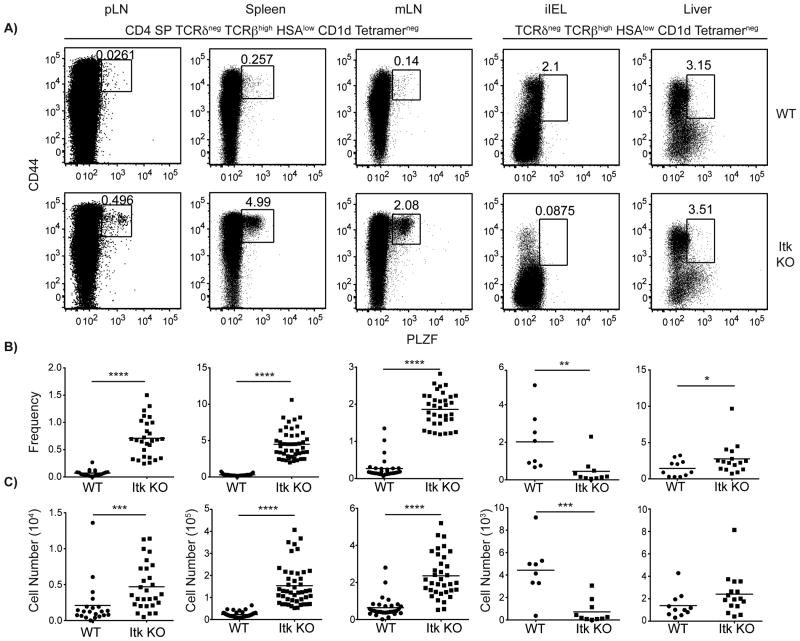
To determine the preferred site of residence of the PLZF+ CD1d-tetramerneg αβ T cells found in itk-/- mice, we examined a variety of peripheral organs for the presence of these cells. Upon examination of the peripheral lymph nodes (pLN: inguinal, auxiliary, brachial, and cervical), spleen, mesenteric lymph nodes (mLN), intestinal intraepithelial lymphocytes (iIELs), and liver, we found that PLZF+ CD1d-tetramerneg αβ T cells preferentially homed to the spleen and mLN in itk-/- mice (Figure 3). Although we did see a significant increase in the frequency and cell number of PLZF+ CD1d tetramerneg αβ T cells in the pLN of itk-/- versus WT mice, the numbers of these cells in the pLN were 10-100 fold less than the numbers found in the spleen or mLN (Figure 3C). We also saw a significant increase in the frequency and number of PLZF+ CD1d-tetramerneg αβ T cells in the liver of Itk-deficient mice (Figure 3B), but as we saw in the pLN, the numbers of these cells were 100 fold less than the numbers found in the spleen or mLN (Figure 3B, C). Interestingly, we also observed a significant decrease in the frequency and numbers of PLZF+ CD1d-tetramerneg αβ T cells in the iIEL compartment of itk-/- mice (Figure 3B-C). These data indicated that itk-/- PLZF+ CD1d-tetramerneg αβ T cells were likely to be expressing adhesion/homing molecules known to direct T cells to mucosal-associated tissues.
Figure 3. CD4+ innate PLZF+ CD4+ cells preferentially home to spleen and mesenteric lymph nodes (mLN).
Lymphocytes were isolated from peripheral lymph nodes (pLN: inguinal, auxiliary, brachial, and cervical), spleens, mLNs, iIELs, and livers and were stained with CD1d-tetramer and antibodies to CD4, CD8, TCRδ, TCRβ, HSA, CD44, and PLZF.
(A) Dot-plots show PLZF versus CD44 staining on TCRβhigh TCRδneg HSAlow CD1d-tetramerneg cells in each organ; for pLN, spleen, and mLN, cells were also gated on CD4+ CD8-. Numbers indicate the percentages of PLZF+ CD44hi cells in each sample. Graphs show compilations of data indicating percentages (B) and absolute numbers (C) of CD44hi PLZF+ in each organ. n > 20 mice per group and the results are representative of more than five independent experiments for pLN, spleen, and mLN. n = 8-10 samples and the results are representative of two independent experiments for iIELs. n = 11-16 mice per group and is representative of two independent experiments for liver. Statistical analysis was performed using a Mann-Whitney t test. *p < 0.05 **p < 0.005 ***p < 0.0005 ****p < 0.0001
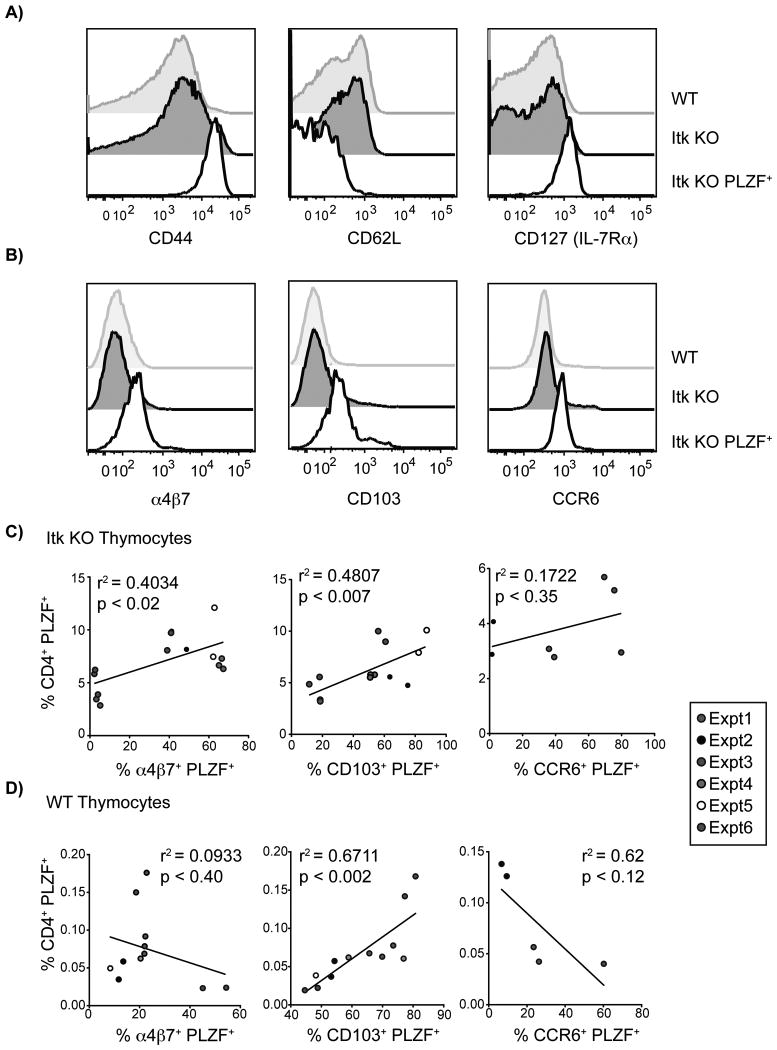
To address this issue, we examined the surface receptors expressed on itk-/- CD4+ PLZF+ CD1d-tetramerneg αβ thymocytes, and compared these to WT conventional CD4SP thymocytes and itk-/- PLZFneg CD4SP thymocytes. We first observed that the itk-/- CD4+ PLZF+ CD1d-tetramerneg αβ thymocytes had an activated phenotype, as they expressed high levels of CD44 and low levels of CD62L (Figure 4A). We also found increased IL-7Rα (CD127) expression on CD4+ PLZF+ CD1d-tetramerneg αβ thymocytes relative to WT or itk-/- PLZFneg CD4SP thymocytes (Figure 4A). This expression level of CD127 is similar to that seen on iNKT cells (data not shown), suggesting that itk-/- CD4+ PLZF+ CD1d-tetramerneg αβ T cells may be a type of non-canonical NKT cell.
Figure 4. Innate PLZF+ CD4+ αβ T cells in itk-/- mice have an activated phenotype and express gut-homing receptors.
Thymocytes from WT and itk-/- mice were isolated and stained with CD1d-tetramer and antibodies to CD4, CD8, TCRδ, TCRβ, HSA, CD44, CD62L, CD127, α4β7, CD103, CCR6, and PLZF.
(A) Histograms show CD44, CD62L, and CD127 expression on WT conventional CD4SP thymocytes (CD4SP TCRδneg TCRβhigh CD1d-tetramerneg HSAlow; gray shaded histograms), itk-/- conventional thymocytes (CD4SP TCRδneg TCRβhigh CD1d-tetramerneg HSAlow; black shaded histograms), and itk-/- innate PLZF+ CD4+ cells (CD4+ PLZF+ TCRδneg TCRβhigh CD1d-tetramerneg; black open histograms). Results are representative of five or more independent experiments.
(B) Histograms show gut homing receptor (α4β7, CD103, and CCR6) expression on WT conventional CD4SP thymocytes (CD4SP TCRδneg TCRβhigh CD1d-tetramerneg HSAlow; gray shaded histograms), itk-/- conventional thymocytes (CD4SP TCRδneg TCRβhigh CD1d-tetramerneg HSAlow; black shaded histograms), and itk-/- innate PLZF+ CD4+ cells (CD4+ PLZF+ TCRδneg TCRβhigh CD1d-tetramerneg; black open histograms). Results are representative of 4-6 independent experiments.
(C,D) Gut-homing receptor expression correlates with the frequency of CD4+ PLZF+ thymocytes in itk-/- mice. Graphs display the percentages of either (C) itk-/- CD4 SP TCRδneg TCRβhigh CD1d tetramerneg HSAlow or (D) WT CD4+ PLZF+ thymocytes and the proportion of these cells expressing α4β7, CD103, or CCR6 in each individual mouse. Legend on the right indicates percentages from individual experiments. n = 5-14 mice per group. Results are representative of 3-6 independent experiments.
Next, we examined the expression of the gut-homing receptors α4β7, CD103, and CCR6 on itk-/- CD4+ PLZF+ CD1d-tetramerneg αβ T cells, and found that all three receptors were elevated on these cells relative to WT or itk-/- PLZFneg CD4SP thymocytes (Figure 4B). However, we did observe substantial variability in the expression of these gut-homing receptors between independent experiments. Specifically, we found that itk-/- CD4+ PLZF+ CD1d-tetramernegαβ thymocytes expressed α4β7 and CD103 in 67% of experiments (4 out of 6), and CCR6 expression in 67% of experiments (2 out of 3). One possible explanation for this variability is that, like MAIT cells (7), these itk-/- CD4+ PLZF+ thymocytes might be dependent on the commensal gut flora for their maintenance, and thus be subject to variability of the microbial environment in different mice.
The possibility that itk-/- CD4+ PLZF+ T cells might be influenced by environmental factors led us to consider whether the size of this population might be correlated with the expression of gut-homing receptors on these cells. To assess this possibility, we compared the total percentage of itk-/- CD4+ PLZF+ CD1d-tetramerneg αβ thymocytes with the percentage of these cells expressing α4β7, CD103, or CCR6. As shown in Figure 4C, we found a significant, positive correlation between the size of the itk-/- CD4+ PLZF+ population and the proportion of these cells expressing α4β7 or CD103. No such correlation was observed for CCR6. When we examined CD4+ PLZF+ thymocytes from WT mice, we found that only CD103 expression correlated positively with an increase in the percentage of these cells (Figure 4D). We did see an inverse correlation between the expression of CCR6 and the abundance of CD4+ PLZF+ WT thymocytes, but this correlation was not significant (Figure 4D). This was of interest since αβ iNKT cells have recently been shown to increase in number in the mesenteric lymph nodes of germ-free mice. This accumulation is thought to occur to due to the regulation of CXCL16 by microbiota (35). Despite this increase in receptors associated with gut-homing on itk-/- CD4+ PLZF+ αβ T cells, we did not see an overall increase of these cells in the iIEL (Figure 3). This may be due to a slight defect in actin polymerization that has been previously described in Itk-deficient T cells that may impede cell migration (36, 37). Additionally, Itk/CTLA4 DKO mice are defective in tissue-homing, which prevents organ infiltration of autoreactive T cells (38). To determine if the expression of the gut homing receptors α4β7, CD103, and CCR6 was specific to innate PLZF+ CD4+ T cells, we examined the expression of these receptors on mature, conventional WT and Itk-deficient thymocytes. We found that the presence of WT conventional CD4SP thymocytes did not correlate with the expression of α4β7, CD103, and CCR6. In itk-/- mice, the presence of conventional CD4SP thymocytes had only a slight inverse, but insignificant, correlation (r2: 0.06-0.17) with the expression of α4β7 and CCR6 (data not shown). However, the expression of CD103 did inversely and significantly correlates with the presence of itk-/- conventional CD4SP thymocytes (r2 = 0.4478, p = 0.0089, data not shown). This suggests that Itk may be involved either directly or indirectly with the regulation of CD103 on conventional T cells. Overall, these data support the notion that itk-/- CD4+ PLZF+ thymocytes are influenced by environmental factors, most likely the microbial flora in the gut, reinforcing the similarity of these cells to previously-described subsets of innate cells, such as MAIT cells.
itk-/- innate PLZF+ CD4+ T cells have a diverse TCR repertoire
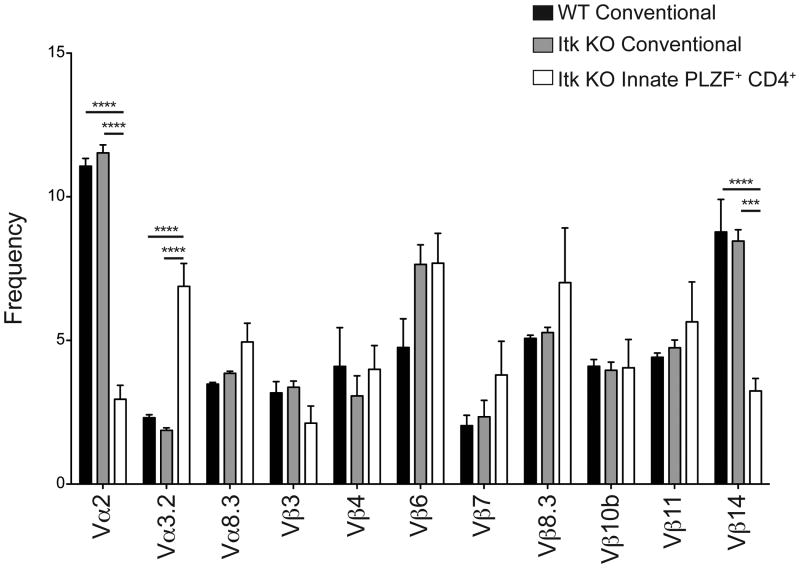
To date, the majority PLZF+ innate T cell subsets each utilize a highly restricted TCR repertoire. γδ NKT cells express a TCR composed of Vγ1.1 paired with Vδ6.3, iNKT cells express Vα14/Jα18 paired with TCRβ chain specificities, and MAIT cells express Vα19/Jα33 (3, 4, 7, 11, 12, 39-41). Only T-CD4 cells have been described to have a diverse TCR repertoire and express PLZF (42) To address whether itk-/- CD4+ PLZF+ T cells also expressed a restricted TCR repertoire, we first stained thymocytes from WT and itk-/- mice with available Vβ– and Vα–specific antibodies. As shown, we found significant decreases in Vβ14 and Vα2 expression on itk-/- CD4+ PLZF+ thymocytes relative to WT or itk-/- conventional PLZFneg CD4SP thymocytes (Figure 5). In contrast, the proportion of itk-/- CD4+ PLZF+ thymocytes expressing Vα3.2 was significantly increased compared to the expression of this Vα segment among conventional CD4SP thymocytes. This slightly skewed TCR repertoire did not appear to be a function of altered thymic selection due to the ITK-deficiency per se, as the Vα and Vβ usage of itk-/- PLZFneg CD4SP thymocytes mirrored that of WT cells in all cases. However, we were unable to definitively rule out the possibility of alterations in thymic selection in the absence of Itk since innate PLZF+ CD4+αβ T cells expand; thus, this phenotype may be due to altered negative selection, since PLZF+ thymocytes require strong TCR signaling for expansion (43). To further determine if the itk-/- PLZF+ CD4SP cells expressed an invariant TCR, we performed SMART-RACE PCR followed by sequencing of mature (TCRβ+ HSAlow) CD1d-tetramerneg GFP+ CD4SP thymocytes from itk-/- mice expressing the IL-4 reporter (GFP+). Despite seeing minor skewing in our Vα and Vβ staining, direct sequence analysis indicated that the repertoire of itk-/- CD4SP 4get+ thymocytes was highly diverse (Table I). No individual Vα-Jα combination was present at a frequency greater than 5/48, and of these five that shared the TRAV12D-1*01 F rearrangement, three different amino acid sequences were present at the V-J junctions. Thus, we conclude that although itk-/- CD4+ PLZF+ cells share features with iNKT, γδ NKT, and MAIT cells, this subset of innate T cells has a diverse TCR repertoire, similar to T-CD4 T cells (42, 44).
Figure 5. itk-/- innate PLZF+ CD4+ cells have a diverse TCR repertoire.
Thymocytes from WT and itk-/- mice were isolated and stained with CD1d-tetramer and antibodies to CD4, CD8, TCRδ, TCRβ, HSA, and PLZF, in addition to antibodies to Vα2, Vα3.2, Vα8.3, Vβ Vβ4, Vβ6, Vβ7, Vβ8.3, Vβ10b, Vβ11, or Vβ14. For Vβanalysis, thymocytes were gated on CD4SP TCRβhigh HSAlow cells; for Vαanalysis, thymocytes were gated on CD4SP TCRδneg TCRβhigh CD1d-tetramerneg HSAlow cells.
The graph shows the percentages of cells in each population expressing the indicated Vα or Vβ. Black bars indicate WT conventional CD4 αβ T cells; gray bars indicate conventional itk-/- CD4 αβ T cells; white bars indicate itk-/- innate PLZF+ CD4+ αβ T cells. n = 6-7 mice per group. Results are representative of two independent experiments. Statistical analysis was performed using a one-way ANOVA. ***p < 0.0005 ****p < 0.0001
Table I. itk-/- innate PLZF+ CD4+ T cells have a diverse TCR repertoire.
RNA from mature (TCRβhi HSAlo) CD4SP CD1d-tetramerneg thymocytes from itk-/- mice expressing the IL-4 reporter (GFP+) was isolated and converted into cDNA before generating amplicons specific for the TCRα chain. These amplicons were sequenced to indicate that itk-/- MAIT-like cells have diverse TCRα repertoire.
| Frequency | V-GENE and allele | AA JUNCTION |
|---|---|---|
| 3 | TRAV15D-2/DV6D-2*03 F or TRAV15N-2*01 F | CALSELISGGYKVVF |
| 2 | TRAV13N-4*01 F | CAMERTNYGSSGNKLIF |
| 2 | TRAV12D-1*01 F | CALSEGPGYQNFYF |
| 2 | TRAV8D-2*02 (F) or TRAV8N-2*01 F | CATYRGSALGRLHF |
| 2 | TRAV9N-3*01 F | CAVRGFASALTF |
| 2 | TRAV7-4*02 F or TRAV7D-4*01 F | CAASENQGGSAKLIF |
| 1 | TRAV14-1*01 F or TRAV14N-1*01 F | CADMNTGGLSGKLTF |
| 1 | TRAV10*02 (F) | CAASMSNYNVLYF |
| 1 | TRAV15D-2/DV6D-2*03 F or TRAV15N-2*01 F | CALSELGTNYNVLYF |
| 1 | TRAV4D-4*03 (F) or TRAV4N-4*01 F | CAAERVNSGTYQRF |
| 1 | TRAV8D-2*03 F | CATHRGSALGRLHF |
| 1 | TRAV6D-7*04 F or TRAV6N-7*01 F | CALGRGSALGRLHF |
| 1 | TRAV7-4*02 F or TRAV7D-4*01 F | CAASDPMNYNQGKLIF |
| 1 | TRAV16N*01 F | CAMREPFPNTNKVVF |
| 1 | TRAV7-5*01 F or TRAV7D-5*01 F | CAASSGSWQLIF |
| 1 | TRAV12D-1*01 F | CALSAWSNYNVLYF |
| 1 | TRAV6D-6*02 F | CALGAWTNAYKVIF |
| 1 | TRAV13N-1*01 F | CAMEPPGYQNFYF |
| 1 | TRAV12D-2*01 F | CALSAHDTNAYKVIF |
| 1 | TRAV12-3*03 (F) | CALRYNYAQGLTF |
| 1 | TRAV13N-2*01 F | CAIACNYAQGLTF |
| 1 | TRAV7-4*02 F or TRAV7D-4*01 F | CAARSSNTNKVVF |
| 1 | TRAV14-2*02 F | CAARYNQGKLIF |
| 1 | TRAV8D-2*01 F, TRAV8D-2*02 (F), or TRAV8N-2*01 F | CATISGSFNKLTF |
| 1 | TRAV14D-3/DV8*01 F or TRAV14N-3*01 F | CATPGTNSAGNKLTF |
| 1 | TRAV13-2*02 (F) | CAARTGGYKVVF |
| 1 | TRAV14-2*01 F, TRAV14D-2*03 F, or TRAV14N-2*01 F | CAASGNYSNNRLTL |
| 1 | TRAV13-4/DV7*02 (F) | CAMERPSGSWQLIF |
| 1 | TRAV9N-3*01 F | CAGRSNYNVLYF |
| 1 | TRAV3-3*01 F | CAVGVTGSGGKLTL |
| 1 | TRAV6D-7*04 F or TRAV6N-7*01 F | CALGVNSNNRIFF |
| 1 | TRAV12D-1*01 F | CASNTGNYKYVF |
| 1 | TRAV13N-4*01 F | CAIASSGSWQLIF |
| 1 | TRAV9N-3*01 F | CAVSMSMGYKLTF |
| 1 | TRAV5D-4*02 (F) | CAAMDNNAPRF |
| 1 | TRAV7-4*02 F or TRAV7D-4*01 F | CAASEWRGNTGKLIF |
| 1 | TRAV4D-4*02 (F) | CAAGTSNTNKVVF |
| 1 | TRAV13D-1*02 (F) | CALERNSNNRIFF |
| 1 | TRAV13N-4*01 F | CAMFSNYNVLYF |
| 1 | TRAV12D-2*01 F | CALRNNYAQGLTF |
| 1 | TRAV7-3*04 F | CAVSRGSALGRLHF |
itk-/- innate PLZF+ CD4+ T cells develop independently of MR1 and CD1d
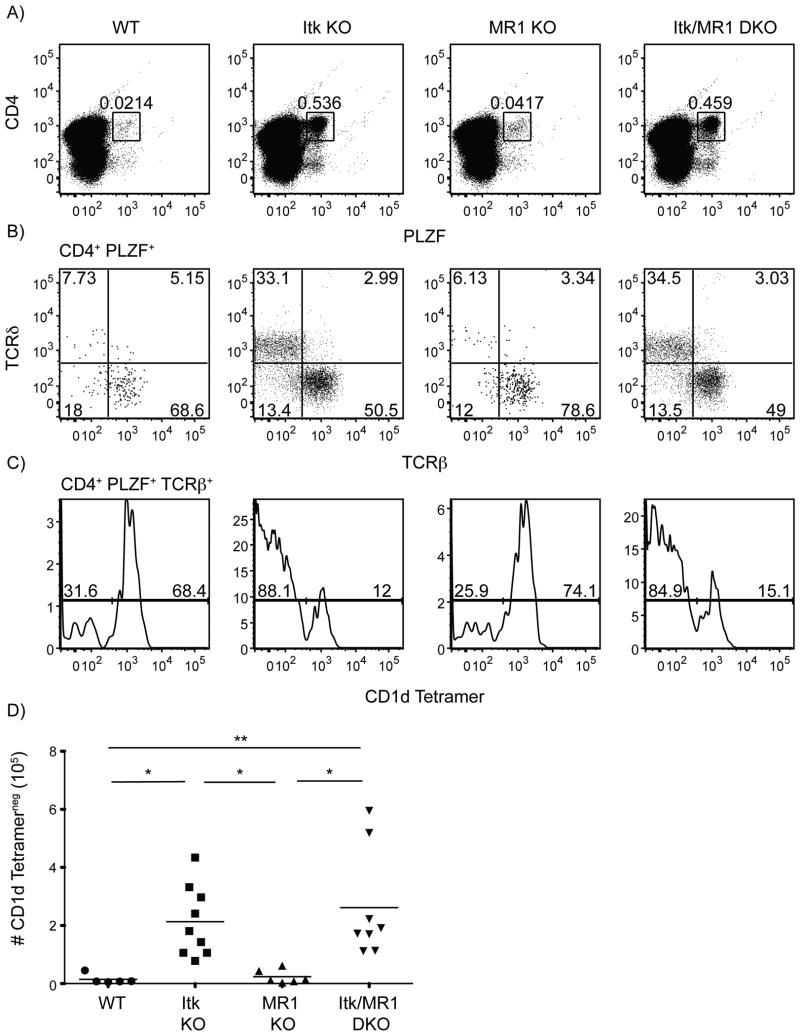
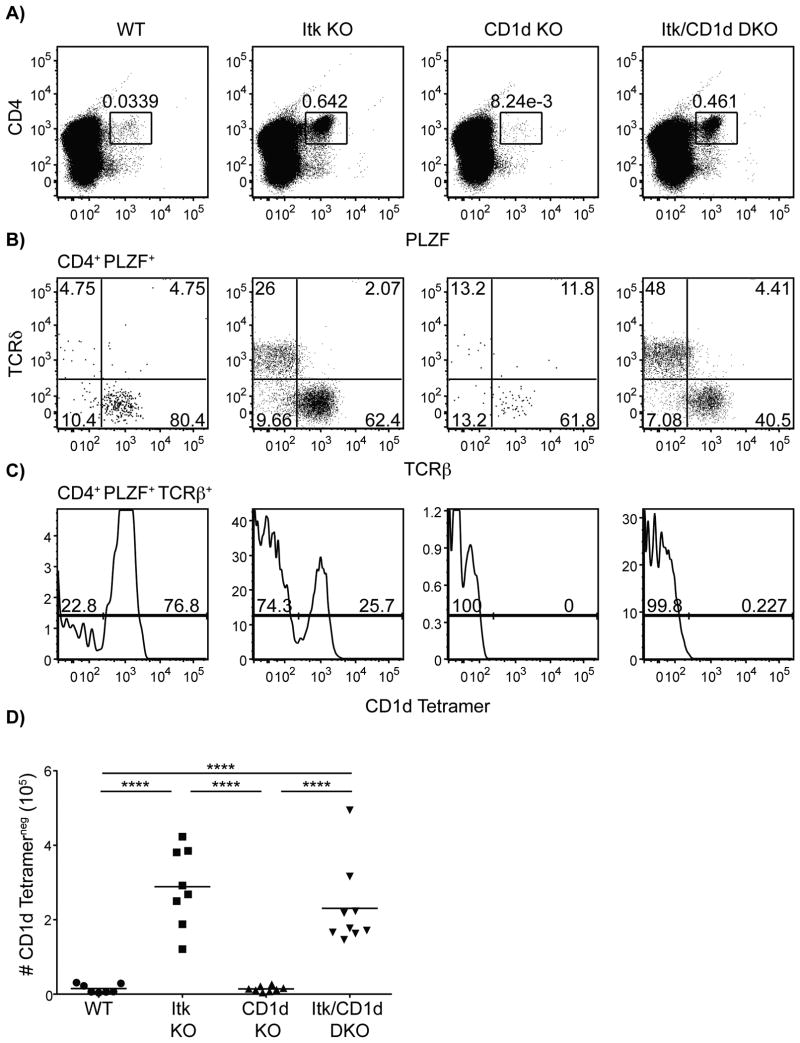
As described, itk-/- innate PLZF+ CD4+ T cells share a number of characteristics with iNKT cells and MAIT cells. To determine whether these itk-/- CD4+ PLZF+ cells were, in fact, a subset of MAIT cells or non-canonical NKT cells, we examined the impact of genetic deficiencies in MR1 or CD1d, respectively, on the development of these cells. Although mouse MAIT cells have not been found to express PLZF (10), these experiments were performed in peripheral CD8+ and DN Vα19-Jα33 TCR transgenic cells. It's plausible that Itk may be required for a maturation step in the development of mouse MAIT cells; therefore, itk-/- CD4+ PLZF+ cells may be developmentally arrested in a phase that is normally rapid and transient in WT mice. However, in the absence of MR1, we observed no change in the frequency or absolute numbers of itk-/- CD4+ PLZF+ thymocytes relative to that seen in mice lacking only Itk (Figure 6). Similarly, the absence of CD1d also had no effect on the development of itk-/- CD4+ PLZF+ T cells (Figure 7).
Figure 6. The development of itk-/- CD4+ PLZF+ αβ T cells is independent of MR1.
Thymocytes from WT, itk-/-, mr1-/- and itk/mr1-/- mice were isolated and stained with CD1d-tetramer and antibodies to CD4, TCRδ, TCRβ, and PLZF.
(A) Dot-plots show CD4 versus PLZF staining of total thymocytes; numbers indicate the percentages of CD4+ PLZF+ cells.
(B) Dot-plots show TCRβ versus TCRδ staining on CD4+ PLZF+ thymocytes; numbers indicate the percentages of cells in each quadrant.
(C) Histograms show CD1d-tetramer staining on CD4+ PLZF+ TCRβ+ thymocytes.
(D) Graph shows a compilation of data indicating absolute numbers of CD4+ PLZF+ TCRβ+ CD1d-tetramerneg thymocytes.
n = 5-8 mice per group. Results are representative of three independent experiments. Statistical analysis performed using a one-way ANOVA. *p <0.05 **p < 0.005 ****p < 0.0001
Figure 7. The development of itk-/- CD4+ PLZF+ αβ T cells is independent of CD1d.
Thymocytes from WT, itk-/-, cd1d-/- and itk/cd1d-/- mice were isolated and stained with CD1d-tetramer and antibodies to CD4, TCRδ, TCRβ, and PLZF.
(A) Dot-plots show CD4 versus PLZF staining on total thymocytes; numbers indicate the percentages of CD4+ PLZF+ cells.
(B) Dot-plots show TCRβ versus TCRδ staining on CD4+ PLZF+ thymocytes. Numbers indicate the percentages of cells in each quadrant.
(C) Histograms show CD1d-tetramer staining on CD4+ PLZF+ TCRβ+ thymocytes.
(D) Graph shows a compilation of data indicating absolute numbers of CD4+ PLZF+ TCRβ+ CD1d-tetramerneg thymocytes.
n = 7-9 mice per group. Results are from four independent experiments. Statistical analysis performed using a one-way ANOVA. ****p < 0.0001****p < 0.0001
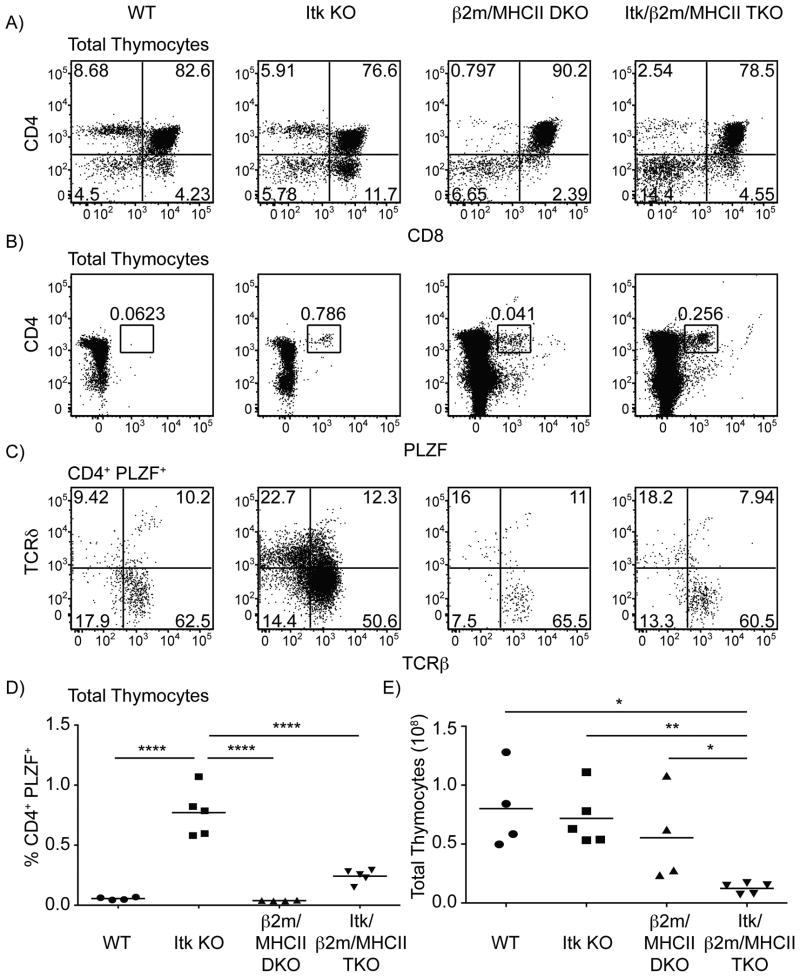
To determine whether itk-/- CD4+ PLZF+ T cells might be selected on another nonclassical MHC class Ib molecule, or on classical MHC class I, we crossed itk-/- mice to mice lacking all β2m expression. As can be seen in Supplemental Figure 1, itk-/- β2m-/- mice had comparable numbers of CD4+ PLZF+ T cells in their thymus to the numbers in the itk-/- mice. These data indicate one of several possible conclusions. itk-/- innate PLZF+ CD4+ T cells may be dependent on a nonclassical MHC class I molecule that does not pair with β2m, they may be dependent on MHC class II, or finally, these cells may not require any MHC ligand for their maturation, similar to γδ T cells.
Class II MHC restricted cells regulate the expansion of itk-/- innate PLZF+ CD4+ T cells
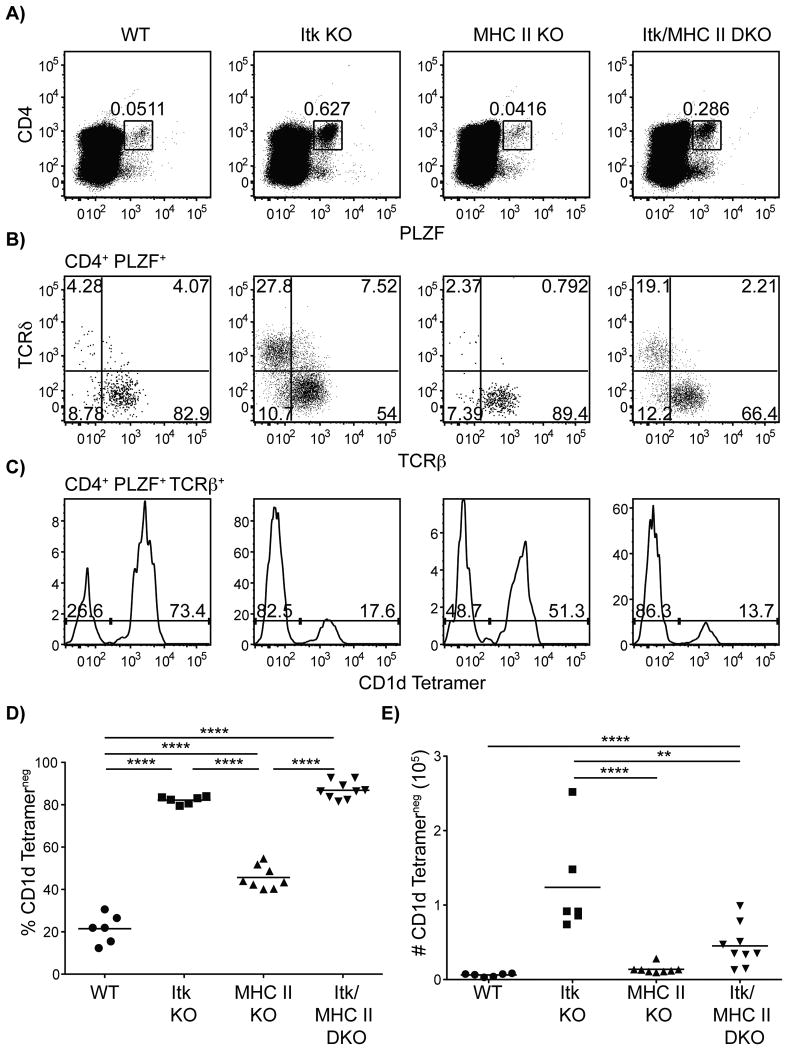
To address the possibility that itk-/- innate PLZF+ CD4+ T cells might require MHC class II molecules for their development and/or selection, we crossed itk-/- mice to MHC class II-deficient mice. When the Itk/MHC class II double-deficient mice were analyzed, we found that the numbers of CD4+ PLZF+ CD1d-tetramerneg αβ T cells were reduced compared to the numbers seen in the single Itk-deficient mice (Figure 8). However, in spite of the reduced numbers, a clear population of CD4+ PLZF+ CD1d-tetramerneg αβ thymocytes was observed in itk-/-/H2dlAb1-Ea mice. These data indicate that MHC class II molecules are not required for the development of itk-/- CD4+ PLZF+ T cells, but may be necessary for their expansion.
Figure 8. itk-/- innate PLZF+ CD4+ cells are dependent on conventional CD4+ T cells for expansion.
Thymocytes from WT, itk-/-, H2dlAb1-Ea and itk/H2dlAb1-Ea mice were isolated and stained with CD1d-tetramer and antibodies to CD4, TCRδ, TCRβ, and PLZF.
(A) Dot-plots show CD4 versus PLZF staining of total thymocytes; numbers indicate the percentages of CD4+ PLZF+ cells.
(B) Dot-plots show TCRδ versus TCRβ staining on CD4+ PLZF+ thymocytes; numbers indicate the percentages of cells in each quadrant.
(C) Histograms show CD1d-tetramer staining on CD4+ PLZF+ TCRβ+ thymocytes.
(D) Graph shows a compilation of data indicating percentage of CD4+ PLZF+ TCRβ+ CD1d-tetramerneg thymocytes.
(E) Graph shows a compilation of data indicating absolute numbers of CD4+ PLZF+ TCRβ+ CD1d-tetramerneg thymocytes.
n = 6-8 mice per group. Data is representative from three independent experiments. Statistical analysis was performed using a one-way ANOVA. **p < 0.005 ****p < 0.00001
MHC molecules regulate the development of CD4+ PLZF+ thymocytes
The absence of β2m or class II MHC did not prevent the development of itk-/- innate PLZF+ CD4+ T cells, a feature reminiscent of γδ T cells. Thus, we reasoned that itk-/- innate PLZF+ CD4+ T cells were either promiscuous in their recognition of MHC class I and II molecules or did not require MHC molecules for their development. Therefore, we crossed Itk/β2m DKO mice to Itk/MHCII DKO mice to obtain mice deficient in Itk, β2m, and MHCII (TKO). In these mice, we still detected the development of itk-/- innate PLZF+ CD4+ cells, although at a reduced frequency (Figure 9A-D). Interestingly, the total thymic cellularity of TKO mice was significantly reduced when compared to WT, Itk KO, or β2m/MHCII DKO mice (Figure 9E). This result suggests that SP thymocytes promote the expansion of developing T cells in the absence of Itk and is consistent with our data demonstrating that the conventional CD4+ T cell subset promotes the expansion of itk-/- innate PLZF+ CD4+ T cells.
Figure 9. Development of itk-/- innate PLZF+ CD4+ cells develop is regulated by MHC molecules.
Thymocytes from WT, itk-/-, β2m-/-/H2-ab1-/- and itk-/-/β2m-/-/H2dlAb1-Ea mice were isolated and stained with CD1d-tetramer and antibodies to CD8, CD4, TCRδ, TCRβ, and PLZF.
(A) Dot-plots show CD4 versus CD8 staining of total thymocytes; numbers indicate the percentages of CD4+ CD8+ DP cells.
(B) Dot-plots show CD4 versus PLZF staining of total thymocytes; numbers indicate the percentages of CD4+ PLZF+ cells.
(C) Dot-plots show TCRδ versus TCRβ staining on CD4+ PLZF+ thymocytes; numbers indicate the percentages of cells in each quadrant.
(D) Graph shows a compilation of data indicating percentage of CD4+ PLZF+ thymocytes.
(E) Graph shows a compilation of data indicating the absolute number of total thymocytes.
n = 4-5 mice per group. Data is representative from two independent experiments. Statistical analysis was performed using a one-way ANOVA. *p < 0.05, **p < 0.005 ****p < 0.00001
Since itk-/- innate PLZF+ CD4+ T cells developed in the absence of β2m and MHCII, we considered whether these cells might be‘confused’ dual-lineage αβ/γδ T cells that would require the γδ TCR for their development. However, examination of itk-/- tcrd-/- mice for the presence of mature (TCRβhigh HSAlow) CD4SP CD1d-tetramerneg thymocytes demonstrated no reduction in the frequency or number of itk-/- innate PLZF+ CD4+ cells between itk-/- and itk-/- tcrd-/- mice (Supplemental Figure 2).
IL-15 regulates the expansion of itk-/- innate PLZF+ CD4+ cells in the periphery
IL-15 is an important cytokine required for the terminal maturation and maintenance of iNKT cells, as well as the persistence of memory-phenotype conventional and innate CD8+ T cells. In addition, IL-15 is an important regulator of αβ and γδ iIELs (45, 46). As itk-/- innate PLZF+ CD4+ cells home to the mLN, we considered whether these cells might also require IL-15 for their maintenance. Whereas the absence of IL-15 had no effect on the development of itk-/- innate PLZF+ CD4+ cells in the thymus, we observed a significant increase in the numbers of itk-/- innate PLZF+ CD4+ cells in the spleen and mLN of mice lacking IL-15 (Supplemental Figure 3). Thus, in the absence of IL-15, and all IL-15-dependent lymphocyte subsets, itk-/- innate PLZF+ CD4+ cells undergo enhanced expansion in the periphery.
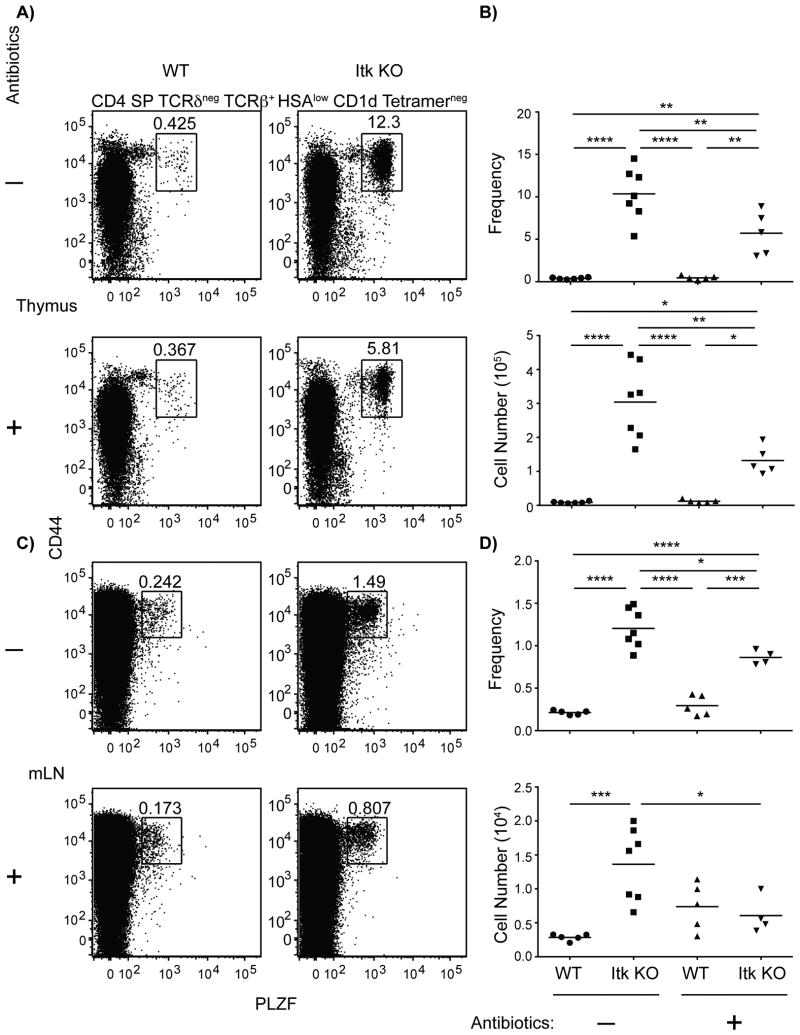
Commensal flora regulate the expansion of itk-/- innate PLZF+ CD4+ cells
Although we found that itk-/- innate PLZF+ CD4+ cells were not dependent on MR1 for their development, the variability in gut-homing receptor expression we observed (Figure 4) suggested that these cells might require commensal gut flora for their peripheral maintenance, a feature reminiscent of MAIT cells (7). Hence, we examined the influence of antibiotics on the presence of itk-/- innate PLZF+ CD4+ cells. The course of antibiotics utilized in this study has previously been described to abrogate commensal gut flora in mice (28, 29), and we began treating mice with the antibiotic cocktail from birth. Strikingly, we found that Itk-deficient mice receiving antibiotic treatment had a significantly decreased frequency and number of innate PLZF+ CD4+ cells in the thymus and mLN (Figure 10). However, we did not find any significant differences in the spleen (data not shown). Thus, commensal gut flora appear to promote the expansion of itk-/- innate PLZF+ CD4+ cells in the both the thymus and mLN.
Figure 10. Commensal gut flora promote the expansion of itk-/- innate PLZF+ CD4+ cells.
Thymocytes and mLN from untreated or treated WT and itk-/- mice were isolated and stained with CD1d-tetramer and antibodies to CD4, CD8, TCRδ, TCRβ, HSA, CD44, and PLZF.
(A) Dot-plots show CD44 versus PLZF staining of CD4SP TCRδneg TCRβ+ CD1d tetramerneg HSAlow thymocytes from untreated (-) or antibiotic treated (+) mice.
(B) Graphs depict the frequency (top) and number (bottom) of CD44hi PLZF+ CD4SP TCRδneg TCRβ+ CD1d tetramerneg HSAlow thymocytes from untreated (-) or antibiotic treated (+) mice.
(C) Dot-plots show CD44 versus PLZF staining of CD4SP TCRδneg TCRβhi CD1d tetramerneg HSAlo of lymphocytes from the mLN of untreated (-) or antibiotic treated (+) mice.
(B) Graphs depict the frequency and number of CD44hi PLZF+ CD4SP TCRδneg TCRβ+ CD1d tetramerneg HSAlow lymphocytes from the mLN of untreated (-) or antibiotic treated (+) mice.
n = 4-7 mice per group. Data are representative of two independent experiments. Statistical analysis was performed using a one-way ANOVA. *p < 0.05 **p< 0.005 ***p < 0.0005 ****p < 0.0001
To further determine the role of itk-/- innate PLZF+ CD4+ in the development of Eomes+ innate-like CD4+ and CD8+ T cells, we analyzed the presence of these subsets in WT or itk-/- mice treated with antibiotics. We found that overall thymic cellularity was similar between mice untreated or treated with antibiotics (Supplemental Figure 4A); however, there were diminished numbers of Eomes+ innate-like CD4+ and CD8+ T cells in antibiotic treated itk-/- mice compared to untreated controls (Supplemental Figure 4C, E). While antibiotic treatment influenced the expansion of itk-/- innate PLZF+ CD4+ thymocytes, this treatment did not affect CD4+ TCRδ+ PLZF+ thymocytes in itk-/- mice (Supplemental Figure 4G). Intriguingly, antibiotic treatment did promote the expansion of itk-/- iNKT cells (Supplemental Figure 4I), which suggests that these two subsets may compete for a thymic niche during development in Itk-deficient mice. Alternatively, and most likely, microbiota have recently been shown to regulate the expression of CXCL16; thus, in germ-free mice upregulation of CXC16 promotes iNKT cell accumulation (35), a phenomenon that may also be occurring upon antibiotic treatment of Itk-deficient mice. In the periphery, we did not see statistically significant differences in Eomes+ CD4+ or CD8+ T cells, γδ NKT cells, or iNKT cells between itk-/- mice untreated or treated with antibiotics (Supplemental Figure 4D, F, H, J); however, we did find a significant decrease in the overall cellularity of the mesenteric lymph node (Supplemental Figure 4B). Thus, itk-/- innate PLZF+ CD4+ T cells may promote the overall expansion of additional innate cell subsets; however, the reduction in numbers of itk-/- innate PLZF+ CD4+ T cells upon treatment with antibiotics may not fully abrogate the expansion of these other innate cell subsets due to the continued presence of itk-/- γδ NKT cells.
Discussion
Recent studies in a variety of genetically-modified mouse strains have revealed an expanded population of IL-4-producing CD4+ T cells. These cells express the transcription factor PLZF, normally found in αβ and γδ NKT cells, as well as in human MAIT cells (8, 9, 11, 12, 14, 20, 21). Mouse lines exhibiting this large number of PLZF+ innate T cells include those lacking Itk, Id3, KLF2, mice expressing a mutant form of SLP-76 that no longer interacts with Itk (SLP-76(Y145F)), mice expressing a stabilized form of β-catenin, and finally, mice expressing MHC class II molecules on their thymocytes (20-22, 42, 47, 48). One common feature shared by all of these cell types, those present in WT mice as well as those found in the mutant lines described above, is their dependence on the SLAM-family receptor signaling protein, SAP (16-18, 21). Additionally, we have found that itk-/- innate PLZF+ CD4+ cells require SAP for their development (Prince et al, accompanying manuscript), and when we further analyze this population between WT and SAP-deficient mice, we see a significant decrease in CD4+ PLZF+ thymocytes (p < 0.0002 using a Mann-Whitney t test). Thus, this CD4+ PLZF+ population described in Itk-deficient mice could be a minute, but present, population in WT mice. Interestingly, SAP is not required for the development of conventional naïve CD4+ or CD8+ T cells (49, 50), indicating the itk-/- PLZF+ T cells, along with all the others, are selected via a distinct pathway from conventional αβ T cells. Furthermore, the requirement for SAP suggests a role for homotypic interactions between thymocytes as a necessary component of this developmental pathway. In the thymus, SAP is most highly expressed in CD4+ CD8+ thymocytes, as well as in γδ T cells (www.immgen.org). Our data rule out an important role for γδ T cells in the development and/or expansion of PLZF+ T cells, at least in itk-/- mice, pointing instead to a likely role for SAP in αβthymocytes that are the likely precursors to the CD4+ PLZF+ T cells in these mice.
A second feature shared by iNKT, γδ NKT, and MAIT PLZF+ T cell subsets is their expression of a highly restricted, if not invariant, TCR repertoire. Additionally, while T-CD4 cells have been shown to develop utilizing a Vα2+ Vβ8.2+ transgenic TCR (T3 TCR), these cells maintain a diverse TCR repertoire (33, 42). Similarly, we determined that itk-/- innate PLZF+ CD4+ T cells also had a diverse TCR repertoire. Since itk-/- innate PLZF+ CD4+ T cells develop independently of MHC class Ia, class Ib, and MHC class II protein expression, it is unlikely that they require a TCR ligand-dependent selection process. Instead, their maturation may be more similar to that of adult γδT cells, which have been proposed to require a functional TCR complex, but not necessarily a particular ligand specificity, for their maturation and survival (51). Although itk-/- innate PLZF+ CD4+ T cells did not require a known MHC molecule for selection, we found that this cell type was highly dependent on the presence of SP thymocytes for their expansion. In the T3 model system for T-CD4 cells, this transgenic TCR is only efficiently selected upon thymocyte-thymocyte interactions where the CIITA transgene is expressed (33). Our data further support the crucial role of thymocyte-thymocyte interactions for the development of PLZF+ T cells and also suggest that interactions with SP thymocytes are critical for the expansion of PLZF-expressing innate-like lymphocytes.
In our studies, we addressed whether the population of CD4+ PLZF+ T cells that expanded in itk-/- mice were one of the known subsets of PLZF+ T cells found in WT mice. However, elimination of all γδ T cells, all β2m-dependent MHC class I molecules, as well as the specific selecting MHC ligands for iNKT cells and MAIT cells, had no impact on the numbers of CD4+ PLZF+ T cells in itk-/- mice. In addition, these cells were not dependent on MHC class II expression, ruling out their identity as conventional CD4+ T cells that were converted into innate cells. Together, these data indicate that itk-/- CD4+ PLZF+ T cells are a novel subset of innate T cells, and not one of the previously identified populations known to express PLZF. While it is plausible that this is an artifact of the absence of Itk, it is striking that a variety of genetically-modified mice have an increase in CD4+ PLZF+ T cells, including klf2-/-, id3-/-, SLP76(Y145F), and CIITA transgenic mice (20-22, 42, 48). Thus, it seems unlikely that this is solely an artifact of the absence of Itk and may instead offer an alternative model for examining the development of innate-like lymphocytes.
Although itk-/- CD4+ PLZF+ T cells are not αβ or γδ NKT cells, nor are they MR1-dependent MAIT cells, this population shares individual features with each of these subsets. Like all PLZF+ cells, those expanded in the itk-/- mice are constitutively-expressing the IL-4 gene. Similar to γδ T cells, the itk-/- CD4+ PLZF+ T cells do not require either MHC class I or MHC class II molecules for their development and functional maturation. Further, like the human MAIT cell population, itk-/- CD4+ PLZF+ T cells generally express gut-homing receptors, such as α4β7 and CD103, and preferentially home to mesenteric lymph nodes, rather than to other peripheral lymph nodes or to liver, an organ favored by αβ and γδ NKT cells. Interestingly, in spite of their increased numbers in the mesenteric lymph node, itk-/- CD4+ PLZF+ T cells are not found in the iIEL compartment. This latter feature may reflect an inability of itk-/- T cells to migrate in response to the appropriate chemokine signals that direct WT cells into the iIEL tissue, a possibility that is consistent with recent studies demonstrating that CTLA4-deficient mice are rescued from fatal lymphocyte infiltration into tissues in the absence of Itk (38).
One final feature of itk-/- CD4+ PLZF+ is the regulation of their expansion by commensal gut flora. This data demonstrated that the numbers and phenotype of itk-/- CD4+ PLZF+ T cells are regulated in part by the gut microbial environment in each mouse. This feature, shared with MAIT cells in WT mice (7), accounts for the wide variability seen in itk-/- CD4+ PLZF+ T cells. Not only do the numbers of CD4+ PLZF+ T cells in itk-/- mice range over 0.28-1.10 as a percentage of total thymocytes, but the expression of gut-homing receptors on these cells also varies substantially in a manner that correlates with the cell numbers. In addition, analysis of itk-/- il-15-/- mice indicates enhanced peripheral expansion of this population in the absence of IL-15. As previous studies have documented competition among other innate T cell subsets, in some cases due to limiting amounts of IL-15 (52, 53), we suggest that the increased numbers of itk-/- CD4+ PLZF+ T cells found in the absence of IL-15 may reflect the absence of other competing populations in these compartments. As itk-/- CD4+ PLZF+ T cells express high levels of CD127, the IL-7 receptor αchain, peripheral itk-/- CD4+ PLZF+ T cells may have enhanced exposure to IL-7 in the mesenteric lymph nodes and spleen, in the absence of the IL-15-dependent subsets. Alternatively, alterations in the numbers of peripheral itk-/- CD4+ PLZF+ T cells may also arise due to indirect effects on the microbial environment found in these mice due to changes in their immune homeostasis.
Interestingly, this itk-/- CD4+ PLZF+ T cell subset may help regulate the expansion of other innate T cell subsets. We found that upon treatment of Itk-deficient mice with antibiotics, there were diminished numbers of Eomes+ innate-like thymocytes, and further, we saw an overall decrease in the cellularity of the mesenteric lymph nodes in these mice (Supplemental Figure 4). These data suggest that while itk-/- γδ NKT cells may be sufficient to induce Eomes expression in conventional T cells, innate CD4+ PLZF+ T cells promote the expansion and/or maintenance of these cells in the thymus (Figure 10 and Supplemental Figure 4). Further, it's plausible that itk-/- innate CD4+ PLZF+ T cells do not regulate the expansion and/or maintenance of other innate lymphocytes directly. Instead, itk-/- innate CD4+ PLZF+ T cells may regulate the microbiota that promote the expansion of other innate lymphocyte subsets.
Strikingly, T-CD4 cells that are PLZF+ and have a diverse TCR repertoire have been described in mice expressing a transgene for human MHC class II (19, 42, 44, 48, 54). This population was recently described to regulate adaptive immune responses during infection with Listeria monocytogenes or Helicobacter pylori (55). Interestingly, neonatal mice have been described to be immuno compromised in order to promote microbiome development (56). Thus, it is possible that innate CD4+ PLZF+ T cells play a role in this immunosuppression. In fact, the presence of T-CD4 cells have been described in human fetal thymus and spleen (42). Therefore, our data described here may reflect a novel model system for studying innate lymphocyte development that may be similar to the development of these subsets in humans. Further studies are needed to continue the characterization of innate lymphocytes in order to truly understand the role of these subsets and the interactions with the gut microbiota in order to prevent microbiome-associated diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Sharlene Hubbard, Regina Whitehead, and Dr. Dipti Karmarkar for technical assistance, the UMass flow cytometry core for cell sorting, and the NIH for the use of CD1d tetramer.
Footnotes
Funding for this work was provided by the NIH grant AI084987 to LJB
References
- 1.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8 (+) T-cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 2.Veillette A, Dong Z, Latour S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity. 2007;27:698–710. doi: 10.1016/j.immuni.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 5.Chiu NM, Wang B, Kerksiek KM, Kurlander R, Pamer EG, Wang CR. The selection of M3-restricted T cells is dependent on M3 expression and presentation of N-formylated peptides in the thymus. J Exp Med. 1999;190:1869–1878. doi: 10.1084/jem.190.12.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 7.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 8.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant'Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadelta T cells is pivotal for controlling IgE production in vivo. Proceedings of the National Academy of Sciences. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin CC, Cho OH, Sylvia KE, Narayan K, Prince AL, Evans JW, Kang J, Berg LJ. The Tec kinase ITK regulates thymic expansion, emigration, and maturation of γδ NKT cells. The Journal of Immunology. 2013;190:2659–2669. doi: 10.4049/jimmunol.1202531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odumade OA, Weinreich MA, Jameson SC, Hogquist KA. Krüppel-like factor 2 regulates trafficking and homeostasis of gammadelta T cells. The Journal of Immunology. 2010;184:6060–6066. doi: 10.4049/jimmunol.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira P, Boucontet L. Innate NKTγδ and NKTαβ cells exert similar functions and compete for a thymic niche. Eur J Immunol. 2012;42:1272–1281. doi: 10.1002/eji.201142109. [DOI] [PubMed] [Google Scholar]
- 15.Verykokakis M, Boos MD, Bendelac A, Adams EJ, Pereira P, Kee BL. Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent “innate” gammadelta T cells. PLoS ONE. 2010;5:e9303. doi: 10.1371/journal.pone.0009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 17.Pasquier B, Yin L, Fondanèche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8 (+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin J, Alonzo ES, Haimm E, Sant'Angelo DB, Koretzky GA, Reiner SL, Jordan MS. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. The Journal of Immunology. 2011;186:4573–4578. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherly LO, Brehm MA, Welsh RM, Berg LJ. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol. 2006;176:1571–1581. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- 25.Lucas JA, Atherly LO, Berg LJ. The absence of Itk inhibits positive selection without changing lineage commitment. J Immunol. 2002;168:6142–6151. doi: 10.4049/jimmunol.168.12.6142. [DOI] [PubMed] [Google Scholar]
- 26.Schaeffer EM, Yap GS, Lewis CM, Czar MJ, McVicar DW, Cheever AW, Sher A, Schwartzberg PL. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol. 2001;2:1183–1188. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 27.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Karmarkar D, Rock KL. Microbiota signaling through MyD88 is necessary for a systemic neutrophilic inflammatory response. Immunology. 2013 doi: 10.1111/imm.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM, Selin LK. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006;116:1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–8. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–12. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Qiao Y, Choi ES, Das J, Sant'Angelo DB, Chang CH. A transgenic TCR directs the development of IL-4+ and PLZF+ innate CD4 T cells. The Journal of Immunology. 2013;191:737–744. doi: 10.4049/jimmunol.1300862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labno CM, Lewis CM, You D, Leung DW, Takesono A, Kamberos N, Seth A, Finkelstein LD, Rosen MK, Schwartzberg PL, Burkhardt JK. Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP. Curr Biol. 2003;13:1619–1624. doi: 10.1016/j.cub.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grasis JA, Browne CD, Tsoukas CD. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J Immunol. 2003;170:3971–3976. doi: 10.4049/jimmunol.170.8.3971. [DOI] [PubMed] [Google Scholar]
- 38.Jain N, Miu B, Jiang JK, McKinstry KK, Prince A, Swain SL, Greiner DL, Thomas CJ, Sanderson MJ, Berg LJ, Kang J. CD28 and ITK signals regulate autoreactive T cell trafficking. Nat Med. 2013;19:1632–1637. doi: 10.1038/nm.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lees RK, Ferrero I, MacDonald HR. Tissue-specific segregation of TCRgamma delta+ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR alphabeta+NKT cells. Eur J Immunol. 2001;31:2901–2909. doi: 10.1002/1521-4141(2001010)31:10<2901::aid-immu2901>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 42.Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. Journal of Experimental Medicine. 2010;207:237–246. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao Y, Zhu L, Sofi H, Lapinski PE, Horai R, Mueller K, Stritesky GL, He X, Teh HS, Wiest DL, Kappes DJ, King PD, Hogquist KA, Schwartzberg PL, Sant'Angelo DB, Chang CH. Development of promyelocytic leukemia zinc finger-expressing innate CD4 T cells requires stronger T-cell receptor signals than conventional CD4 T cells. Proceedings of the National Academy of Sciences. 2012;109:16264–16269. doi: 10.1073/pnas.1207528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD, Simpson E, Park SH. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005;23:387–396. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alonzo ES, Sant'Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011;23:220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. The Journal of Immunology. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, Sharpe AH, Biron CA, Terhorst C. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 50.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, Berg LJ, Kang J, Immunological Genome Project Consortium Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol. 2012;13:511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Dubois S, Waldmann TA, Müller JR. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci U S A. 2006;103:12075–12080. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, Kim MG, Gourley S, McCarthy BP, Sant'Angelo DB, Chang CH. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23:375–386. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Qiao Y, Gray BM, Sofi MH, Bauler LD, Eaton KA, O'Riordan MXD, Chang CH. Innate-like CD4 T cells selected by thymocytes suppress adaptive immune responses against bacterial infections. Open J Immunol. 2012;2:25–39. doi: 10.4236/oji.2012.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71 (+) erythroid cells compromise neonatal host defence against infection. Nature. 2013 doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.