Abstract
The risk of predation can have large effects on ecological communities via changes in prey behaviour, morphology and reproduction. Although prey can use a variety of sensory signals to detect predation risk, relatively little is known regarding the effects of predator acoustic cues on prey foraging behaviour. Here we show that an ecologically important marine crab species can detect sound across a range of frequencies, probably in response to particle acceleration. Further, crabs suppress their resource consumption in the presence of experimental acoustic stimuli from multiple predatory fish species, and the sign and strength of this response is similar to that elicited by water-borne chemical cues. When acoustic and chemical cues were combined, consumption differed from expectations based on independent cue effects, suggesting redundancies among cue types. These results highlight that predator acoustic cues may influence prey behaviour across a range of vertebrate and invertebrate taxa, with the potential for cascading effects on resource abundance.
Keywords: bioacoustics, invertebrate, non-consumptive effect, predation, trophic cascade
1. Introduction
The risk of predation, even from relatively rare predators, can have large effects on ecological communities via changes in prey behaviour, morphology and reproduction that cascade to impact the abundance and biomass of basal resources [1–4]. These non-consumptive indirect effects can be large [5,6] and equivalent in magnitude to consumptive effects [2]. By matriculating through the food web, non-consumptive predator effects can even influence ecosystem functioning [3,4].
In order for non-consumptive effects to occur, prey must be able to detect their predators and assess predation risk [7]. In addition to visual or mechanical cues resulting from direct contact with predators, prey can utilize chemical/olfactory and acoustic stimuli from predators and damaged conspecifics to evaluate the risk of predation [8,9]. In marine environments, much of the research on predator recognition and avoidance has focused on water-borne chemical cues, demonstrating their importance across a wide range of taxa [6,10,11]. Because water also provides an excellent medium for the transmission of sound, acoustic signals could be a reliable cue of nearby predators, potentially increasing the strength of non-consumptive effects [7].
Despite significant research in marine bioacoustics (particularly on marine mammals and fishes), few studies have considered the potential for predator vocalizations to elicit non-consumptive effects on prey and their resources (but see [12]). Sound production is widespread in marine fishes, and serves a variety of social communication purposes, including mate attraction, territory defence, and distress and alarm calls [13,14]. Unlike in marine mammals such as dolphins, fish vocalizations have not been explicitly linked to their foraging behaviour, yet they do indicate fish presence and thus could provide reliable cues of predation risk for prey. However, their utility as cues relies also on the ability of their prey to detect these sounds.
We examined the potential for fish vocalizations to create non-consumptive effects in their crab prey, and whether these effects cascade to the crabs’ bivalve resources. Relatively little is known about marine invertebrates' response to sound [15,16], but many invertebrate species have organs known as statocysts that can detect particle motion and thus may serve as a sound receptor [17,18]. We first quantified crab hearing capability using the auditory evoked potential (AEP) method [15,19]. We then conducted two experiments to examine (1) whether crabs alter their foraging rates in response to acoustic cues from predators and non-predators, and (2) whether there are interactive effects of predator acoustic and water-borne chemical cues. We utilized a well-studied oyster reef food web consisting of vocalizing fish predators (hardhead catfish, Ariopsis felis; black drum, Pogonias cromis; oyster toadfish, Opsanus tau), mud crab (Panopeus spp.) prey, and bivalve (hard clam, Mercenaria mercenaria) resources. Previous research in this system has demonstrated strong non-consumptive predator effects resulting from the physical presence of fish and invertebrate predators [20,21], as well as from predator water-borne chemical cues [8,22].
2. Material and methods
(a). Crab hearing measurements
We quantified crab hearing capability using the AEP method, a minimally invasive method of measuring the neural response resulting from auditory stimulation [15,19,23–26]. The AEP method provides a rapid means of testing hearing capability without training of the test organisms. We tested four male and four female crabs (Panopeus spp.) of 30–50 mm carapace width collected from natural intertidal oyster reefs in the northern Gulf of Mexico within 48 h of the hearing experiments. This sample size is standard for AEP studies [15,19,23–26]. Testing for each crab took approximately 20 min, and all crabs survived the tests. We also tested one dead crab as a control.
During the AEP experiments, an individual crab was restrained on a wooden sling and suspended 10 cm underwater in a cylindrical steel tank (122 cm high, 20.3 cm diameter, 0.95 cm thick walls) containing seawater at 23°C, with the underwater speaker positioned at the bottom of the tank. Prior to submerging the crab, a stainless steel recording electrode (Rochester Electro-Medical, Tampa, FL) was inserted under the carapace near the basal joint of the antennule and statocyst [27]. A reference electrode was placed under the carapace in the body cavity. A ground electrode was placed in the water near the body of the crab. All exposed surfaces of the electrodes that were not in direct contact with the crab were coated with enamel for insulation.
Sound stimuli were presented and AEP waveforms were collected using a Tucker-Davis Technologies physiology apparatus with SigGen and BioSig software. Sounds were computer generated using TDT software and hardware (RP2.1 Real-Time Processor) at a 24 kHz sample rate, and passed through a power amplifier (Halfer P1000) connected to the underwater speaker (University Sound UW-30). Tone bursts were 50 ms in total duration and were gated with a Hanning window (similar to the conditions of past AEP studies [24–26]). Sounds were presented 17.5 times s−1 with alternating phase on each presentation. Responses to each tone burst at each sound pressure level (SPL) were collected using the BioSig software package using a DB4 biological amplifier with gain set to 10 000×, and a 3000 Hz low-pass filter. Near threshold, 1000 responses were averaged for each stimulus frequency and level combination. In order to speed up testing, the program was advanced to the next test condition if an AEP was obvious before 1000 averages were reached. The SPLs of each presented frequency were confirmed using a calibrated underwater hydrophone (HTI 96 min; sensitivity: −164.4 dB re 1 V µPa−1). Sound pressure gradients were calculated by measuring the sound pressure at locations 5 cm in each direction (x, y and z) from where the crab was centred with the same hydrophone using the BioSig software, which creates a time-locked recording. The set-up for calibration was exactly the same, except the crab was not present. The sound pressure gradients were then converted to particle velocities for each frequency and location in the test tank as a function of √(x2 + y2 + z2).
The SPL at each frequency was initially set to a level that produced a response and then decreased in 6 dB steps until a stereotypical AEP was no longer seen. The frequencies tested were 75, 100, 200, 400, 800 and 1600 Hz. Initial levels were: 75 Hz/160 dB; 100 Hz/158 dB; 200 Hz/146 dB; 400 Hz/151 dB; 800 Hz/144 dB; 1600 Hz/139 dB. The sound was then repeated at the next lower level to ensure that threshold had been reached. Threshold was defined as the lowest level at which a response could be detected in the Fourier transform of the AEP that was 6 dB above background noise at twice the stimulus frequency.
(b). Predator acoustic cue experiment
We examined the effects of predator acoustic cues on crab foraging behaviour by quantifying consumption of crab resources (juvenile clam, Mercenaria mercenaria) in an outdoor mesocosm experiment at the Florida State University Coastal and Marine Laboratory (FSUCML) in August 2012. We utilized publicly available recorded vocalizations of three fish predators that are known to consume mud crabs on oyster reefs in our study area: the black drum (Pogonias cromis; audio file courtesy of James Locascio and David Mann, University of South Florida College of Marine Science; electronic supplementary material, audio file S1), the hardhead catfish (Ariopsis felis; from dosits.org, copyright University of Rhode Island Office of Marine Programs, 2002–2007; electronic supplementary material, audio file S2) and the oyster toadfish (Opsanus tau; from dosits.org, copyright University of Rhode Island Office of Marine Programs, 2002–2007; electronic supplementary material, audio file S3). Toadfish are among the best-studied vocal fish; they produce a range of sounds related to mate attraction, nest defence and agonism [13,28,29]. Catfish vocalizations have been documented in relation to schooling behaviour, reproduction and distress, and they may also function as a form of echolocation [30,31]. The ‘drum’ call of the black drum has been linked to territoriality and spawning; these fish also produce a staccato disturbance call [32]. None of the fish are known to vocalize specifically when foraging, yet we hypothesized that these sounds may still provide a reliable cue to crabs of predation risk.
Each experimental mesocosm (n = 20) consisted of a 78.5 l round plastic tub (diameter = 42 cm; area = 0.55 m2) with two drains: a 2.5 cm hole drilled 8.0 cm from the top of the tub prevented overflow of seawater, and a barbed reducer (1/4 inch), 8.0 cm from the bottom, allowed a gradual outflow of water as in [22]. Mesocosms were filled with 18.6 l (an approximate depth of 6 cm) of sieved sand and approximately 3 l of dead oyster shell to provide structure and habitat for the crabs. All mesocosms received flow-through, sand-filtered seawater from the Gulf of Mexico via the seawater system at FSUCML to simulate high tide during the trials. Each mesocosm was fitted with a plastic mesh cover (1/4 inch openings) lined with window screen to reduce light and prevent visual stimuli from outside the mesocosm from influencing crab behaviour.
We ran three independent trials of the mesocosm experiment, using different crabs in each trial. Within a trial, we conducted three separate treatment exposures within a 5-day period. Adult mud crabs (Panopeus spp.) between 20 and 40 mm carapace width were hand collected from natural reefs in Apalachee Bay, Florida, within 3 days of the start of each trial, held in tanks with flow-through seawater, and fed juvenile clams ad libitum. On the first exposure day of the trial, five crabs were added to each mesocosm and allowed to acclimate to their surroundings for at least 30 min. This density is within the range found on natural reefs in the region [22]. Live juvenile clams less than 10 mm in shell diameter were affixed with super glue to the inner surface of dead oyster shells (five clams per shell) the morning of each run to serve as the resource for the crabs. These shells were then marked along the shell margin with a 1–2 cm stripe of brightly coloured enamel to facilitate identification and retrieval at the end of the experiment. Five of these shells (n = 25 clams per mesocosm) were placed in each mesocosm, intermixed with the other dead oyster shell.
Mesocosms were randomly assigned to one of the acoustic cue treatments for each trial (n = 4 per treatment per trial): black drum (predator), hardhead catfish (predator), toadfish (predator), snapping shrimp (non-predator control; Alpheus heterochaelis; courtesy of Paul Perkins, NUWC Engineering, Test and Evaluation Department; from dosits.org, copyright University of Rhode Island Office of Marine Programs, 2002–2007; electronic supplementary material, audio file S4) and silent recording (control; electronic supplementary material, audio file S5). Acoustic treatments were maintained for each treatment exposure in a given trial (i.e. the same crabs experienced the same acoustic cue in each of the 3 exposure days per trial). Playback treatments consisted of a single 5 min playback of a looped-mode recording created by a brief (5–10 s) clip of the appropriate sound with 3–4 s of silence between sounds. Cues were broadcast from a portable MP3 player that was connected to a 120 V amplifier (OSC GX3) and then to a 30 W underwater speaker (Electro-Voice UW-30). The speaker was placed along one side of the mesocosm, facing the interior of the tank. We tested each of our predator cues in a replicate mesocosm set up at USF, because calibration equipment was not available at the time of the experiment. The hydrophone was placed 20 cm away from the underwater speaker, mimicking the crabs’ position during the mesocosm experiments (maximum distance was 35 cm). SPLs (RMS between 100 and 1000 Hz) were toadfish = 144 dB re 1 µPa; drum = 138 dB re 1 µPa; catfish = 137 dB re 1 µPa; snapping shrimp = 133 dB re 1 µPa; silent = 112 dB re 1 µPa). Cues were played sequentially across mesocosms; clams were added immediately prior to the acoustic cue playback in each mesocosm to prevent crab foraging prior to applying the treatment.
Within 4.0 h of the cue treatments, we retrieved the marked oyster shells and counted the number of live clams remaining to quantify clam consumption. Direct observations of crab behaviour were not possible because of low water clarity.
(c). Predator acoustic cue versus water-borne cue experiment
To test the strength of predator acoustic effects relative to a different predator cue on crab foraging behaviour, we conducted a factorial manipulation of catfish acoustic cues (present, absent) and catfish water-borne cues (present, absent) in the mesocosms described above. Treatments were randomly assigned to mesocosms (n = 5 per treatment per trial). We utilized a coupled, flow-through predator cue system similar to previous experimental manipulations testing non-consumptive predator effects (see e.g. [6,22,33,34]). We ran two independent trials using different crabs, with a single treatment exposure in each. Adult mud crabs (Panopeus spp.) between 20 and 40 mm carapace width were hand collected from natural reefs in Apalachee Bay within 3 days of the start of each trial, held in tanks with flow-through seawater and fed juvenile clams ad libitum. Five crabs were added to each mesocosm at least 30 min prior to the start of each trial, and all mesocosms received flow-through, sand-filtered seawater from the Gulf of Mexico via the seawater system at FSUCML. Five marked oyster shells with live juvenile clams affixed with super glue (five clams per shell) were added to each mesocosm just prior to the cue treatments to prevent crabs from consuming resources before the application of each treatment. After the marked oyster shells were added, the inflow tubing for mesocosms assigned to the water-borne cue treatment was switched from the regular FSUCML seawater system line to a line with seawater that first circulated through a single 100-gallon flow-through tank (area = 1.04 m2) that housed two predators (hardhead catfish, Ariopsis felis) for the duration of the trial (see [22] for additional details on mesocosm set-up; catfish were housed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the protocol was approved by the Animal Care and Use Committee at the Florida State University, Permit no. 1106). Mesocosms not receiving water-borne predator cues continued to receive seawater directly from the FSUCML seawater system at an equivalent flow rate for the duration of the trial. Immediately after the water-borne cue treatment was established, the acoustic cue treatment was applied. Each mesocosm received a single 5 min playback loop of the predator (hardhead catfish) recording or a silent recording. After 2 h, we ended the water-borne cue treatments, collected the marked oyster shells and quantified the number of clams consumed in each mesocosm as above.
(d). Statistical analysis
We used a generalized linear mixed-effects model selection approach using R software (v. 2.15.0, lme4, lattice, multcomp and glmmADMB packages) to examine the proportion of clams consumed across treatments. In all analyses, experimental trial and mesocosm were included as random effects. For the first experiment, predator cue was treated as a fixed factor, and exposure day was nested within mesocosm. We also ran a separate analysis using only the data from the first exposure day of each trial. For the second experiment, catfish acoustic cues (present/absent) and water-borne chemical cues (present/absent) were designated as fixed factors. One mesocosm was excluded from each trial in the second experiment because of failure in the delivery of water from the predator tank.
For each analysis, we fitted the data to a series of nested models using a binomial error distribution to determine which best explained the observed results (electronic supplementary material, appendix S6). These models included a null model with an intercept of 1 and only the random effects, and models that included random effects with single, additive and interactive effects between fixed factors. We then performed model selection using Akaike's information criterion corrected for small sample sizes (AICc [35]). The identification of the best candidate model was based on Akaike weight (wi), which was calculated as the model likelihood normalized by the sum of all model likelihoods. Weights close to 1 indicate greater support for the model in the data [36]. Models were considered to have substantial support if their ΔAICc score was less than 2.0. We then conducted mean comparisons using a Tukey test in the multcomp package. In the second experiment, we also used a multiplicative risk model on the proportion of clams surviving to calculate expected consumption in the combined cue treatment [37]. We then compared expected and observed values using Student's t-test.
3. Results
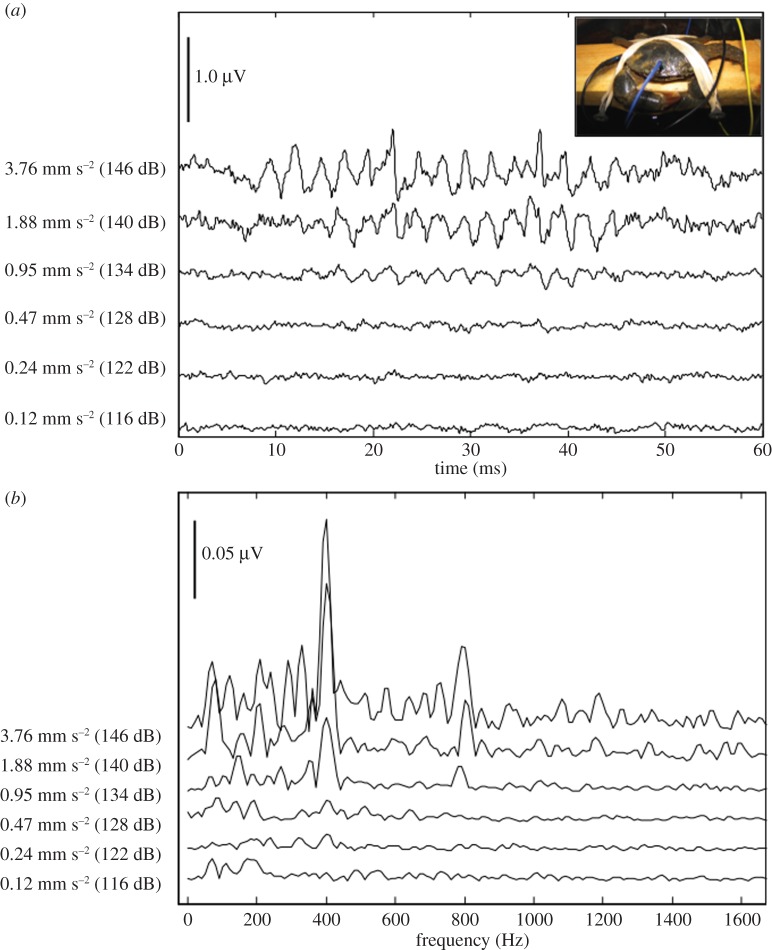
AEPs (indicating neural responses) were obtained for all live crabs tested, and showed a frequency doubling effect, where the AEP signal was twice the frequency of the stimulus frequency (figure 1). The acoustic pressure thresholds were all very high (>133 dB re 1 µPa; figure 2a), and no clear pattern of sensitivity was apparent. When the data were plotted as a function of acoustic particle acceleration, best sensitivity was at 75 Hz with decreasing sensitivity up to 1600 Hz (figure 2b).
Figure 1.
Evoked potentials for an individual crab in response to 200 Hz sound presentation of tonal bursts at several sound levels. Sound levels are shown in terms of both particle acceleration and acoustic pressure. (a) Evoked potentials as a function of time from stimulus presentation. Vertical scale bar represents 1.0 μV amplitude. Inset: experimental crab on wooden sling prior to placement in the tank for the sound presentation. (b) Evoked potentials as a function of frequency (FFT of traces in (a)). Vertical scale bar represents 0.05 μV amplitude. (Online version in colour.)
Figure 2.
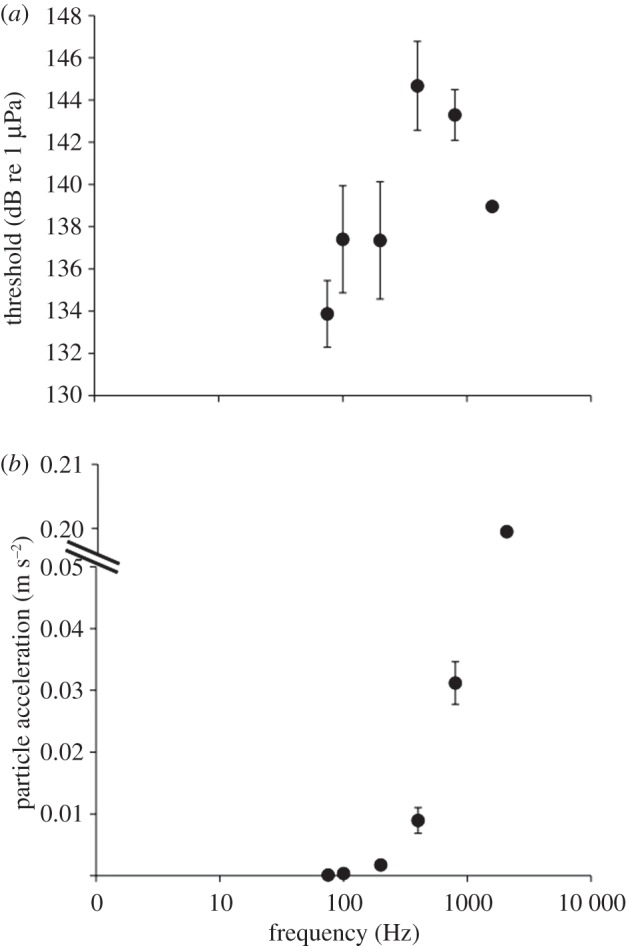
Evoked potential audiograms in terms of (a) acoustic pressure and (b) acoustic particle acceleration, showing the lowest sound level that produced a detectable evoked potential. Eight crabs were tested at all frequencies. Lower values for thresholds and particle accelerations indicate greater sensitivity. Error bars represent ±1 s.e.
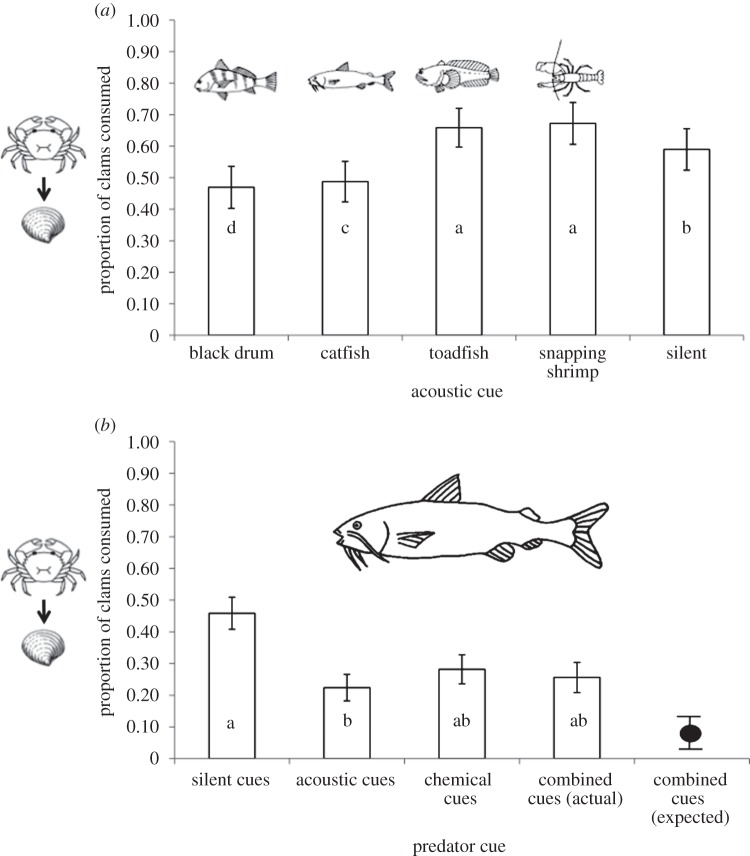
In our first mesocosm experiment, acoustic cue treatment explained a large percentage of the variation in crab foraging behaviour (wi = 1.0, table 1). Acoustic cues differentially affected consumption of bivalve prey (figure 3a): foraging rates were lowest when crabs were exposed to the black drum (predator) cue, intermediate in response to the catfish (predator) cue, and equivalently high in the toadfish (predator) and snapping shrimp (non-predator control) treatments. The results were consistent when we restricted our analysis to the first day of exposure in each trial (table 1).
Table 1.
Results of model selection analyses for the mesocosm experiments. Italic indicates best model; parentheses denote random effects and solid denotes nested effects. ΔAICc is the difference between the AICc of a particular model compared with the lowest AICc observed. Models with ΔAICc scores of less than 2.0 have significant explanatory power. The Akaike weight is calculated as the model likelihood normalized by the sum of all model likelihoods; values close to 1.0 indicate greater confidence in the selection of a model.
| model | df | ΔAICc | weight |
|---|---|---|---|
| predator acoustic cue experiment | |||
| all data | |||
| proportion clams consumed ∼ 1 + (trial) + (tank/exposure day) | 4 | 62.8 | <0.001 |
| proportion clams consumed ∼ acoustic treatment + (trial) + (tank/exposure day) | 8 | 0 | 1.0 |
| first exposure day per trial only | |||
| proportion clams consumed ∼ 1 + (trial) + (tank) | 3 | 46.7 | <0.001 |
| proportion clams consumed ∼ acoustic treatment + (trial) + (tank) | 7 | 0 | 1.0 |
| predator acoustic cue versus chemical cue experiment | |||
| proportion clams consumed ∼ 1 + (trial) + (tank) | 3 | 1.9 | 0.153 |
| proportion clams consumed ∼ acoustic cue + (trial) + (tank) | 4 | 0 | 0.403 |
| proportion clams consumed ∼ chemical cue + (trial) + (tank) | 4 | 3.3 | 0.076 |
| proportion clams consumed ∼ acoustic cue + chemical cue + (trial) + (tank) | 5 | 1.5 | 0.190 |
| proportion clams consumed ∼ acoustic cue × chemical cue + (trial) + (tank) | 6 | 1.6 | 0.178 |
Figure 3.
Crab foraging responses to predatory cues. (a) Effects of predator and non-predator acoustic cues. Crab foraging activity (proportion of clams consumed) varied in response to both predator and non-predator cues compared with the silent cue control. (b) Effects of predator acoustic cues and predator chemical cues. Crab foraging activity decreased in response to short-term catfish acoustic cues, persistent water-borne catfish cues and their combination. Consumption rates in the combined cue treatment were greater than the expectation calculated with the multiplicative risk model (black circle). Letters indicate significant differences based on Tukey post hoc tests. Error bars represent ±1 s.e. Line drawings by T. Rogers.
In our second experiment, catfish acoustic cues best explained crab foraging behaviour (figure 3b; wi = 0.40, table 1), along with additive (ΔAICc = 1.5, wi = 0.19, table 1) and interactive (ΔAICc = 1.6, wi = 0.18, table 1) effects of catfish chemical cues. Consumption of clams was lower in the presence of predator cues (acoustic only, chemical only or combined acoustic and chemical) than in the absence of cues, yet only the acoustic cue treatment was significantly different than the control treatment in post hoc tests. In addition, the combined effects of these two different cue types differed from expectations based on the single cue treatments: the observed consumption rate in the multiple cue treatment was greater than the expected consumption rate based on the multiplicative risk model (figure 3b; t-test, p = 0.01), even if one combined cue replicate was excluded because of unusually high consumption (t-test, p = 0.05).
4. Discussion
Crab neural responses indicated that they could detect a range of frequencies that coincide with the low-frequency vocalizations of many sonic marine fishes [14]. In addition, crabs reduced their foraging activity in the presence of experimental acoustic stimuli from two predatory fish species. The doubling effect that we observed in the auditory AEPs has been seen previously in squid and fish [15,19], and is indicative of a statocyst system with directionally sensitive sensory cells. Crabs' lack of sensitivity to sound pressure is consistent with the fact that they do not contain air bubbles and suggests that they are most likely to detect fish sounds when the fish is nearby.
Variable crab responses to individual predator species may reflect differences in risk that each of these predators poses to crabs. Although we do not have information on the relative risk posed by each predator, stomach contents indicate that all predator species tested in our experiment consume mud crabs (toad fish [20]; black drum [38]; catfish [39]). The reliability of the vocalizations as a cue of predation risk may also vary by predator species due to the nature of the call itself. For instance, the well-studied toadfish ‘boatwhistle’ call used in our playback experiment is generated by males to attract females to their nest site [13]. Because the male toadfish then guards the eggs and embryos at the nest site until they are free-swimming, this call is probably not associated with high predation risk for mud crabs. In addition, toadfish are resident on reefs, whereas catfish and black drum move on and off the reef with the tides (D. Kimbro 2010, unpublished data). Thus, catfish and black drum vocalizations may be a strong indicator of a sudden increase in predation risk for mud crabs. Furthermore, black drum calls have relatively high source levels (165 dB re 1 µPa [32]), which will increase their detectable range in comparison with fish producing lower sound levels (e.g. toadfish: 123–126 dB re 1 µPa [32]; catfish: 97 dB re 1 µPa [31]).
Although it is difficult to compare acoustic and chemical cue manipulations directly (i.e. are five minutes of acoustic cues equivalent to five minutes of chemical cues?), it is clear that catfish acoustic cues elicit non-consumptive effects of approximately the same magnitude and direction as their water-borne chemical cues. Water-borne chemical cues from predators are highly influenced by hydrodynamic conditions such as velocity and turbulence, particularly in structured habitats such as oyster reefs [8,11,40]. Acoustic cues may be less readily attenuated at these small spatial scales, and thus may provide a more reliable cue of predator presence and proximity (but see [41]). Further, our acoustic cue treatment consisted of a single 5 min playback whereas the chemical cues were continuous for the duration of the experiment, demonstrating that even short-duration acoustic cues can have lingering ecological effects. The difference between observed and expected consumption with combined acoustic and chemical cues suggests that there may also be redundancies among cue types.
Acoustically distinctive habitat cues (marine ‘soundscapes’) are increasingly recognized as an important sensory cue for fish, as well as for invertebrate larvae [42–44]. Biological sources that characterize distinct habitats, particularly at dawn and dusk (when many marine organisms forage), include soniferous animals such as snapping shrimp, fish and sea urchins [45]. However, anthropogenic noise (e.g. boats) can dominate soundscapes at other times of day, with subsequent effects on animal behaviour and physiology [46]. Although few studies have examined the effects of anthropogenic noise on invertebrates [46], recent work demonstrated that another crab species (Carcinus maenas) exhibits disrupted feeding, decreased ability to find refuge habitat and increased oxygen consumption when exposed to playbacks of ship noise [47,48]. In addition to illustrating the potential for predator acoustic cues to be masked or disrupted by human activities, this work reinforces the importance of acoustic cues of a variety of sources for marine invertebrate foraging behaviour and physiology.
Many marine invertebrates have statocysts that sense movement and vibrations [17,18], and acoustic cues have been documented as a form of conspecific communication in several crustacean species, such as fiddler crabs and lobsters [16]. Conspecific acoustic cues are similarly important in terrestrial insects such as crickets for mating and social interactions [49]. Given the ability of invertebrates to detect and respond to sound, and the prevalence of soniferous predators on land and in the sea, we expect that non-consumptive effects of predator acoustic cues on invertebrates may be more common than currently appreciated.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank R. Diaz de Villegas for his curiosity, and T. Rogers and H. Tahboub for field and laboratory assistance. C. Peterson provided the catfish used in our experiment. T. Rogers created the line drawings of the experimental organisms in figure 3. T. Gouhier, J. Grabowski, C. Stallings and two anonymous reviewers provided helpful comments on the manuscript.
Data accessibility
Data are available at Dryad doi:10.5061/dryad.v7g26.
Funding statement
This research was supported by National Science Foundation (NSF) grant OCE-0961633 to D.L.K. and A.R.H. This is contribution no. 315 from the Northeastern University Marine Science Center.
References
- 1.Lima SL. 1998. Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 2.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 3.Schmitz OJ. 2008. Effects of predator hunting mode on grassland ecosystem function. Science 319, 952–954. ( 10.1126/Science.1152355) [DOI] [PubMed] [Google Scholar]
- 4.Schmitz OJ, Hawlena D, Trussell GC. 2010. Predator control of ecosystem nutrient dynamics. Ecol Lett 13, 1199–1209. ( 10.1111/J.1461-0248.2010.01511.X) [DOI] [PubMed] [Google Scholar]
- 5.Schmitz OJ, Beckerman AP, O'Brien DL. 1997. Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology 78, 1388–1399. ( 10.1890/0012-9658(1997)078[1388:BMTCEO]2.0.CO;2) [DOI] [Google Scholar]
- 6.Trussell GC, Ewanchuk PJ, Matassa CM. 2006. Habitat effects on the relative importance of trait- and density-mediated indirect interactions. Ecol. Lett. 9, 1245–1252. ( 10.1111/J.1461-0248.2006.00981.X) [DOI] [PubMed] [Google Scholar]
- 7.Luttbeg B, Trussell GC. 2013. How the informational environment shapes how prey estimate predation risk and the resulting indirect effects of predators. Am. Nat. 181, 182–194. ( 10.1086/668823) [DOI] [PubMed] [Google Scholar]
- 8.Smee DL, Weissburg MJ. 2006. Clamming up: environmental forces diminish the perceptive ability of bivalve prey. Ecology 87, 1587–1598. ( 10.1890/0012-9658(2006)87[1587:CUEFDT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 9.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334, 1398–1401. ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 10.Kelley JL, Magurran AE. 2003. Learned predator recognition and antipredator responses in fishes. Fish. Fish. 4, 216–226. ( 10.1046/j.1467-2979.2003.00126.x) [DOI] [Google Scholar]
- 11.Weissburg MJ, Zimmer-Faust RK. 1993. Life and death in moving fluids: hydrodynamic effects on chemosensory-mediated predation. Ecology 74, 1428–1443. ( 10.2307/1940072) [DOI] [Google Scholar]
- 12.Remage-Healey L, Nowacek DP, Bass AH. 2006. Dolphin foraging sounds suppress calling and elevate stress hormone levels in a prey species, the Gulf toadfish. J. Exp. Biol. 209, 4444–4451. ( 10.1242/jeb.02525) [DOI] [PubMed] [Google Scholar]
- 13.Maruska KP, Mensinger AF. 2009. Acoustic characteristics and variations in grunt vocalizations in the oyster toadfish Opsanus tau. Environ Biol Fish 84, 325–337. ( 10.1007/s10641-009-9446-y) [DOI] [Google Scholar]
- 14.Mann DA, Hawkins AD, Jech JM. 2008. Active and passive acoustics to locate and study fish. In Fish bioacoustics (eds Webb JF, Fay RR, Popper AN.), pp. 279–310. New York: Springer. [Google Scholar]
- 15.Mooney TA, Hanlon RT, Christensen-Dalsgaard J, Madsen PT, Ketten DR, Nachtigall PE. 2010. Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J. Exp. Biol. 213, 3748–3759. ( 10.1242/jeb.048348) [DOI] [PubMed] [Google Scholar]
- 16.Popper AN, Salmon M, Horch KW. 2001. Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. A 187, 83–89. ( 10.1007/s003590100184) [DOI] [PubMed] [Google Scholar]
- 17.Budelmann BU. 1992. Hearing in nonarthropod invertebrates. In The evolutionary biology of hearing (eds Webster D, Fay R, Popper A.), pp. 141–155. New York: Springer. [Google Scholar]
- 18.Cohen MJ. 1960. The response patterns of single receptors in the crustacean statocyst. Proc. R. Soc. Lond. B 152, 30–49. ( 10.1098/rspb.1960.0020) [DOI] [PubMed] [Google Scholar]
- 19.Egner SA, Mann DA. 2005. Auditory sensitivity of sergeant major damselfish (Abudefduf saxatillis) from post-settlement juvenile to adult. Mar. Ecol.-Prog. Ser. 285, 213–222. ( 10.3354/meps285213) [DOI] [Google Scholar]
- 20.Grabowski JH. 2004. Habitat complexity disrupts predator-prey interactions but not the trophic cascade on oyster reefs. Ecology 85, 995–1004. ( 10.1890/03-0067) [DOI] [Google Scholar]
- 21.Grabowski JH, Hughes AR, Kimbro DL. 2008. Habitat complexity influences cascading effects of multiple predators. Ecology 89, 3413–3422. ( 10.1890/07-1057.1) [DOI] [PubMed] [Google Scholar]
- 22.Hughes AR, Rooker K, Murdock M, Kimbro DL. 2012. Predator cue and prey density interactively influence indirect effects on basal resources in intertidal oyster reefs. PLoS ONE 7, e44839 ( 10.1371/journal.pone.0044839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corwin JT, Bullock TH, Schweitzer J. 1982. The auditory brainstem response in five vertebrate classes. Eclectroencephalagr Clin. Neurophysiol 54, 629–641. ( 10.1016/0013-4694(82)90117-1) [DOI] [PubMed] [Google Scholar]
- 24.Mann DA, Cott PA, Hanna BW, Popper AN. 2007. Hearing in eight species of northern Canadian freshwater fishes. J Fish Biol 70, 109–120. ( 10.1111/j.1095-8649.2006.01279.x) [DOI] [Google Scholar]
- 25.Mann DA, Higgs DM, Tavolga WN, Souza MJ, Popper AN. 2001. Ultrasound detection by clupeiform fishes. J. Acoust. Soc. Am. 109, 3048–3054. ( 10.1121/1.1368406) [DOI] [PubMed] [Google Scholar]
- 26.Popper AN, Smith M, Cott PA, Hanna BW, MacGillivary A, Austin M, Mann DA. 2005. Effects of exposure to seismic airgun use on hearing of three fish species. J. Acoust. Soc. Am. 117, 3958–3971. ( 10.1121/1.1904386) [DOI] [PubMed] [Google Scholar]
- 27.Sandeman DC, Okajima A. 1972. Statocyst-induced eye movements in the crab Scylla serrata. J. Exp. Biol. 57, 187–204. [DOI] [PubMed] [Google Scholar]
- 28.Bass AH, McKibben JR. 2003. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 69, 1–26. ( 10.1016/S0301-0082(03)00004-2) [DOI] [PubMed] [Google Scholar]
- 29.Fine ML. 1978. Seasonal and geographical variation of the mating call of the oyster toadfish Opsanus tau L. Oecologia 36, 45–57. ( 10.1007/BF00344570) [DOI] [PubMed] [Google Scholar]
- 30.Schmidtke D, Schulz J, Hartung J, Esser K-H. 2013. Structure and possible functions of constant-frequency calls in Ariopsis seemanni (Osteichthyes, Ariidae). PLoS ONE 8, e64864 ( 10.1371/journal.pone.0064864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavolga WN. 1977. Mechanisms for directional hearing in the sea catfish (Arius felis). J. Exp. Biol. 67, 97–115. [DOI] [PubMed] [Google Scholar]
- 32.Locascio JV, Mann DA. 2011. Localization and source level estimates of black drum (Pogonias cromis) calls. J. Acoust. Soc. Am. 130, 1868–1879. ( 10.1121/1.3621514) [DOI] [PubMed] [Google Scholar]
- 33.Freeman AS, Byers JE. 2006. Divergent induced responses to an invasive predator in marine mussel populations. Science (Washington DC) 313, 831–833. ( 10.1126/science.1125485) [DOI] [PubMed] [Google Scholar]
- 34.Large SI, Smee DL, Trussell GC. 2011. Environmental conditions influence the frequency of prey responses to predation risk. Mar. Ecol. Prog. Ser. 422, 41–49. ( 10.3354/meps08930) [DOI] [Google Scholar]
- 35.Burnham KP, Anderson DR. 2002. Model selection and multimodal inference: a practical information-theoretic approach, 2nd edn, p. 488 New York: Springer. [Google Scholar]
- 36.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108. ( 10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 37.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 38.Robins CR, Ray GC. 1986. A field guide to Atlantic coast fishes of North America. Boston, MA: Houghton Mifflin Company. [Google Scholar]
- 39.Motta PJ, Clifton KB, Hernandez P, Eggold BT, Giordano SD, Wilcox R. 1995. Feeding relationships among nine species of seagrass fishes of Tampa Bay, Florida. Bull. Mar. Sci. 56, 185–200. [Google Scholar]
- 40.Weissburg MJ. 2000. The fluid dynamical context of chemosensory behavior. Biol. Bull. 198, 188–202. ( 10.2307/1542523) [DOI] [PubMed] [Google Scholar]
- 41.Wilson CJ, Wilson PS, Greene CA, Dunton KH. 2013. Seagrass meadows provide an acoustic refuge for estuarine fish. Mar. Ecol. Progr. Ser. 472, 117–127. ( 10.3354/meps10045) [DOI] [Google Scholar]
- 42.Lillis A, Eggleston DB, Bohnenstiehl DR. 2013. Oyster larvae settle in response to habitat-associated underwater sounds. PLoS ONE 8, e79337 ( 10.1371/journal.pone.0079337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C. 2006. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 51, 143–196. [DOI] [PubMed] [Google Scholar]
- 44.Stanley JA, Radford CA, Jeffs AG. 2012. Location, location, location: finding a suitable home among the noise. Proc. R. Soc. B 279, 3622–3631. ( 10.1098/rspb.2012.0697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radford CA, Stanley JA, Tindle CT, Montgomery JC, Jeffs AG. 2010. Localised coastal habitats have distinct underwater sound signatures. Mar. Ecol. Progr. Ser. 401, 21–29. ( 10.3354/meps08451) [DOI] [Google Scholar]
- 46.Morley EL, Jones G, Radford AN. 2014. The importance of invertebrates when considering impacts of anthropogenic noise. Proc. R. Soc. B 281, 20132683 ( 10.1098/rspb.2013.2683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wale MA, Simpson SD, Radford AN. 2013. Noise negatively affects foraging and antipredator behavior in shore crabs. Anim. Behav. 86, 111–118. ( 10.1016/j.anbehav.2013.05.001) [DOI] [Google Scholar]
- 48.Wale MA, Simpson SD, Radford AN. 2013. Size-dependent physiological responses of shore crabs to single and repeated playback of ship noise. Biol. Lett. 9, 20121103 ( 10.1098/rsbl.2012.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas ML, Gray B, Simmons LW. 2011. Male crickets alter the relative expression of cuticular hydrocarbons when exposed to different acoustic environments. Anim. Behav. 82, 49–53. ( 10.1016/j.anbehav.2011.03.023) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at Dryad doi:10.5061/dryad.v7g26.