Abstract
The histological identification of increased eosinophils in the gastrointestinal tract occurs in numerous clinical disorders; however, there is a limited understanding of the mechanisms regulating eosinophil trafficking into this mucosal surface. The results presented in this study characterize the processes regulating eosinophil homing into the gastrointestinal tract at baseline. Eosinophils were found to be present in the lamina propria of 19-day-old embryos and germ-free adult mice at concentrations comparable to those present in non–germ-free adult mice. Furthermore, eosinophil gastrointestinal levels were not altered by increasing circulating eosinophils after pulmonary allergen challenge. Gastrointestinal eosinophil levels were partially reduced in mice deficient in recombinase activating gene-1 (RAG-1), IL-5, or the β common chain (βc), but these reductions paralleled reductions in circulating eosinophils. In contrast, mice deficient in eotaxin had a marked reduction in gastrointestinal eosinophils but normal levels of eosinophils in the hematopoietic compartments. Furthermore, eotaxin was important for regulating eosinophil levels, even in the presence of high levels of IL-5. These investigations demonstrate eosinophil homing into the gastrointestinal tract during embryonic development occurring independently of viable intestinal flora. Furthermore, eotaxin is identified as the primary regulator of eosinophil gastrointestinal homing under homeostatic states, and may therefore have a fundamental role in innate immune responses.
J. Clin. Invest. 103:1719–1727 (1999).
Introduction
Eosinophils are proinflammatory cells implicated in the pathogenesis of numerous inflammatory processes, especially allergic disorders (1, 2). They are known to be recruited from the circulation into inflammatory foci, where they can modulate responses by releasing an array of molecules, including cytotoxic proteins, cytokines, and lipid mediators (3). Increased levels of eosinophils are frequently seen in gastrointestinal tissue obtained from patients with a variety of disorders, e.g., food allergy. In some of these diseases, such as eosinophilic gastroenteritis, eosinophils are believed to be the principal effector cell, whereas in other diseases (e.g., inflammatory bowel disease and gastroesophageal reflux), the finding of eosinophils in tissues is an enigma, since eosinophils may have a regulatory function (1–4). Eosinophil numbers in the gastrointestinal tract are substantially higher than in hematopoietic sites. Nevertheless, most investigations of eosinophils have focused on evaluating their functions in hematopoietic and pulmonary tissues. Understanding the processes that regulate eosinophil trafficking in the gastrointestinal tract is not only important in treating clinical diseases, but may also have important implications in further understanding the role of eosinophils in innate immune responses and in immune surveillance of healthy tissues.
Eosinophils are generated in the bone marrow from pluripotential stem cells. IL-3, IL-5, and GM-CSF regulate eosinophil development by binding to receptors that share a common β chain (βc) and have unique α chains. In addition to βc, mouse IL-3 also binds to a mouse receptor that contains a separate βIL-3 chain. Of these 3 cytokines, IL-5 (also known as eosinophil differentiation factor) is the most specific to the eosinophil lineage (5). IL-5 also stimulates the release of eosinophils from the bone marrow into the peripheral circulation (6). The critical role of IL-5 in regulating the production of eosinophils is most clearly demonstrated by genetic manipulation of mice. Overproduction of IL-5 in transgenic mice results in marked peripheral blood eosinophilia; deletion of the IL-5 gene inhibits allergen-induced peripheral blood and pulmonary eosinophilia (7–9). Interestingly, the baseline levels of eosinophils in the bone marrow or blood of mice deficient in IL-5, βc, GM-CSF, or both βc and βIL-3, are similar or only modestly reduced when compared with wild-type mice (8, 10–12). This indicates the importance of other factors and/or receptors in maintaining the steady-state baseline level of eosinophils.
The accumulation of eosinophils in tissues is regulated by locally generated chemoattractants, especially chemokines, a large family of chemotactic cytokines that have pleiotropic effects (13–15). Chemokines are divided into 4 families, termed CXC, CC, C, and CX3C, depending upon the spacing of conserved cysteines. Eosinophil-active chemokines are mainly restricted to members of the CC group, and include monocyte chemoattractant protein-2 (MCP-2), MCP-3, MCP-4, RANTES, macrophage inflammatory protein-1α (MIP-1α), eotaxin, and eotaxin-2 (16). Eotaxin is a biologically significant eosinophil chemoattractant because it is the most selective eosinophil chemoattractant in vivo identified to date (17–20), and neutralization of its activity has profound effects on eosinophil function (21–25). In addition, its receptor, CC chemokine receptor-3, is the main chemokine receptor expressed on eosinophils (26–28). Eotaxin production is increased in diseased tissues that are characterized by eosinophilic inflammation, including lesions in patients with inflammatory bowel disease (29), asthma (24, 30), allergic rhinitis, and nasal polyps (31, 32); it is also increased in response to respiratory allergen challenge in animals (16, 17) and humans (31, 33). In addition, its biological activity is enhanced by IL-5, which increases the circulating pool of eosinophils and primes eosinophils to have enhanced responsiveness to eotaxin (6, 34, 35).
We demonstrated recently that eotaxin is constitutively produced by mononuclear cells in the lamina propria of the mouse lower small intestine (25). We also showed that eotaxin is critical for the maintenance of eosinophils in this tissue. We now further define the cells, cytokines, and processes that regulate eosinophils throughout the gastrointestinal tract at baseline.
Methods
Mice.
Eotaxin-deficient inbred mice of the 129 SvEv strain were maintained with age- and sex-matched controls from Taconic Farms (Germantown, New York, USA) as described (21). Germ-free Black Swiss mice with matched controls were also obtained from Taconic Farms. These mice were verified to be germ free by Taconic Farms by negative growth of fecal samples cultured under both aerobic and anaerobic conditions. Stool was also examined under phase microscopy to verify the axenic status of the animals before shipping the mice in sterile germ-free containers. IL-5 gene-deficient mice (36) were kindly provided along with their F10 matched control littermates by Klaus Matthaei (Australian National University , Canberra,Australia)(8). These mice were backcrossed into the BALB/c background for 10 generations. Mice transgenic for IL-5 (on a CBA background) were originally obtained from Colin Sanderson (TVW Telethon Institute for Child Health and Research, West Perth, Australia), and were backcrossed into the BALB/c background and analyzed after 4–6 backcrosses (7). IL-5 transgenic mice carrying the eotaxin wild-type or gene-targeted allele were generated by breeding F1 littermates (from eotaxin gene-targeted mice crossed with IL-5 transgenic mice) with eotaxin gene-targeted mice, and screening tail DNA by Southern analysis for the eotaxin gene-targeted allele and the IL-5 transgene. GM-CSF and βc receptor gene-targeted mice (C57BL/6) were kindly provided by Jacquelyn Reed and Jeffrey Whitsett (Children’s Hospital Medical Center, Cincinnati, Ohio, USA). Strain-matched wild-type mice and mice containing recombinase activating gene-1 (RAG-1) (BALB/c; ref. 37) or MIP-1α deficiency (C57BL/6; ref. 38), and their littermate controls, were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). All mice were housed under pathogen-free conditions and were examined between 10 and 24 weeks of age.
Analysis of gastrointestinal eosinophils.
The small intestines of adult mice were divided into the duodenum (2 cm distal to the pylorus), jejunum, and ileum by collecting tissue 7 cm and 2 cm proximal to the cecum, respectively. In perinatal mice, the middle 3–4 cm of the small intestine was harvested. Segments of the gastrointestinal tract were fixed with 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and immunostained with antiserum against mouse eosinophil major basic protein (anti-MBP) as described (25). Quantification of immunoreactive cells was carried out by 2 different methods. In the duodenum, jejunum, and ileum, eosinophils were counted randomly from the base to the tip of ∼20 villi cut in longitudinal sections and expressed as eosinophils per villus/crypt unit. Alternatively, gastrointestinal sections were counted using an ocular micrometer (10 × 10 μm; B & B Microscopes Ltd., Warrendale, Pennsylvania, USA), and eosinophil levels are expressed as the number of eosinophils per squared millimeter.
Blood and bone marrow eosinophil analysis.
Peripheral blood parameters were analyzed by automated processing (Cell Dyne model 3500; Abbott Laboratories, Abbott Park, Illinois, USA). The percentage of eosinophils in blood smears was recorded manually by counting 200–300 leukocytes on May-Grünwald–stained slides. Bone marrow analysis was performed by irrigating each femur with 5 mL PBS (pH 7.4) to isolate femoral marrow leukocytes. Displaced cells were gently resuspended using a 23-gauge needle. Cells were then centrifuged at 400 g at 20°C, and erythrocytes were removed using hypotonic lysis. The cells were recentrifuged, resuspended in PBS, and counted with a Neubauer hemacytometer (Hausser Scientific, Morsham, Pennsylvania, USA) for total cell numbers. Differential cell counts were obtained from cytocentrifuge preparations stained with Diff-Quick (Dade Diagnostics of Puerto Rico Inc., Aguada, Puerto Rico).
Northern analysis.
RNA was extracted from gastrointestinal segments using Trizol reagent (GIBCO BRL, Grand Island, New York, USA) following the manufacturer’s protocol. Twenty micrograms of total RNA from each sample was processed as described previously (16, 25). Total lung RNA from IL-4 transgenic Clara cell (CC-10) lung transgenic mice was used as a positive control (39). Two different sets of animals were used for each organ, except for tongue and esophagus RNA, which was pooled from multiple animals before isolating the total RNA. Film was exposed for 1–2 days.
Allergen treatment of mice.
A mouse model of allergic lung disease was established using methods described previously (40, 41). Bronchoalveolar lavage fluid (BALF) was obtained with 2 replicate volumes (0.8 mL) of normal saline containing 0.5 mM EDTA and was analyzed as reported previously (21, 34).
Statistical analysis.
Data are expressed as mean ± SEM. Statistical significance comparing different sets of mice was determined by Student’s t test.
Results
Eosinophils are normal constituents of the gastrointestinal tract.
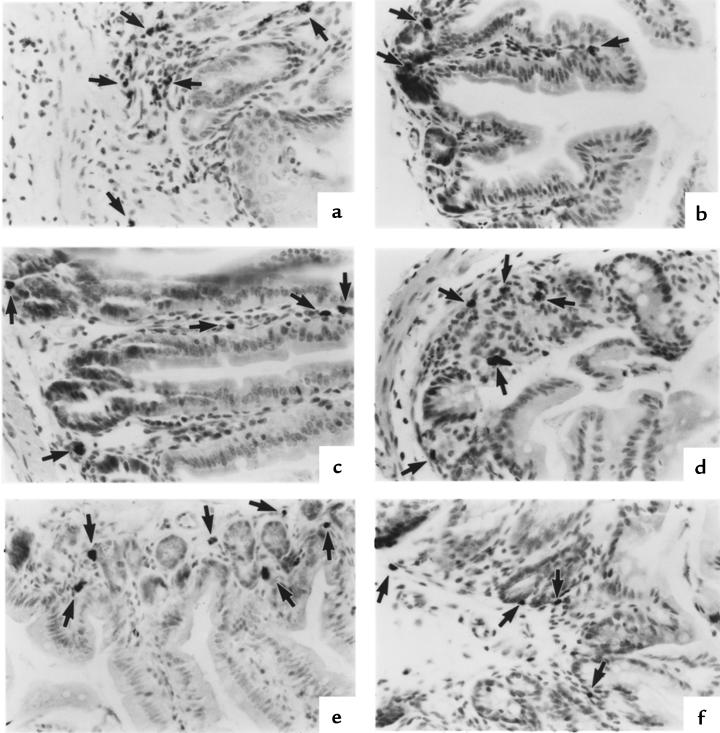
Eosinophils have been noted to be present at low levels in numerous tissues, but their distribution patterns have not been extensively analyzed. We were therefore interested in examining these cells in mice, because this species would allow unique experimental manipulation. Eosinophils throughout the gastrointestinal tract of conventional healthy mice (untreated mice maintained under pathogen-free conditions) were immunohistochemically identified (25). Eosinophils were found to be present in the lamina propria of the stomach, small intestine, cecum, and colon (Figure 1). No eosinophils were detected in the esophagus or tongue. This distribution pattern is similar to that observed in humans (4). Eosinophils were found at similar levels in all mouse strains analyzed (BALB/c, C57BL/6, 129 SvEv), predominantly located in the submucosa and not in the mucosal or serosal layers of the gastrointestinal tract. The distribution pattern varied in different regions of the small intestine. In the duodenum, eosinophils were primarily detected through the entire length of villi (Figure 1b). In the jejunum (Figure 1c) and ileum (Figure 1d), most eosinophils were at the base of villi in the region of the crypt of Lieberkühn.
Figure 1.
Immunohistochemical identification of eosinophils in different segments of the gastrointestinal tract of mice. Eosinophils are found in the lamina propria of the stomach (a), duodenum (b), jejunum (c), ileum (d), colon (e), and cecum (f). In the small intestine (b–d), eosinophils are predominantly located in the crypt region. In the duodenum (b), eosinophils are also seen throughout the length of the villus. Eosinophils were not routinely seen in the mucosal or serosal layers. These representative photomicrographs were taken from 129 SvEv mice. Each section is immunostained with anti-MBP antiserum and processed by nickel/cobalt enhancement of peroxidase activity. Eosinophils are indicated by arrows. ×125.
Eosinophils become localized in the gastrointestinal tract during the perinatal period.
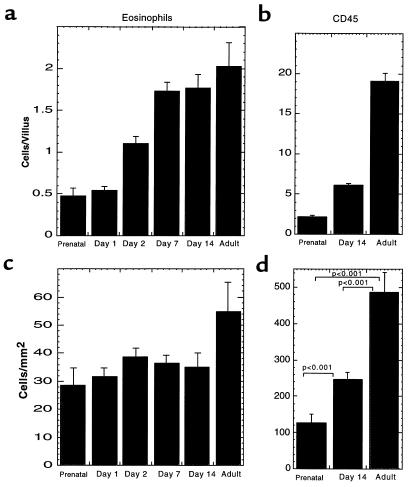
We were interested in determining whether eosinophils migrate into the gastrointestinal tract as a normal developmental process during gestation, or migrate during the postnatal period in response to an extrinsic stimulus. Migration during the postnatal period would suggest that factors such as gastrointestinal colonization with bacteria are involved in initiating eosinophil homing. We therefore analyzed the number of intestinal eosinophils in the mice 1 day before birth (embryonic day 19) and during the first 2 weeks postpartum. Prenatal mice had readily detectable eosinophils located in regions similar to those observed in adults (data not shown). Because of the significant morphological differences between the gastrointestinal tracts of perinatal, postnatal, and adult mice, it was necessary to standardize the eosinophil numbers per unit area in order to make comparisons between the experimental groups. First, eosinophil levels were normalized to the villus/crypt unit, a region that is increasing in size during development. Using this normalization, eosinophil levels increased with age (Figure 2a). However, when eosinophil levels were normalized to the area of the lamina propria, their concentrations were found to be similar at all ages (Figure 2c). As a control, we also examined total leukocytes in the gastrointestinal tract by staining for CD45, a pan-leukocyte marker. CD45+ cells were substantially reduced in the perinatal mice compared with adult mice (Figure 2, b and d). These data indicate the occurrence of eosinophil homing early in perinatal development.
Figure 2.
Quantitation of gastrointestinal eosinophil numbers in embryonic and perinatal mice. The number of eosinophils (a and c) and CD45+ cells (b and d) residing in the small intestine of wild-type mice was determined in day- 19 embryos and in postnatal mice during the first 2 weeks of life. In a and b, cell levels were normalized to the villus/crypt unit. In c and d, cell numbers were normalized to the unit area (mm2) of the lamina propria. The mean ± SEM for littermate 129 SvEv mice (n = 5–12 mice in each group) is shown. In b, there was no significant difference between any of the groups (P > 0.05).
In an attempt to identify the stimulus for eosinophil homing, we assessed the role of endogenous intestinal bacterial flora on eosinophil homing into the gastrointestinal tract by examining eosinophil levels in germ-free mice. Germ-free mice have never come in contact with viable bacteria and have been shown to have decreased levels of lymphocytes in the lamina propria (42). These mice were received from a commercial source and were immediately analyzed for the level of gastrointestinal eosinophils present before they became colonized. Germ-free mice were found to have eosinophil levels that were similar to those in control colonized mice (29 ± 14 vs. 25 ± 6.3 eosinophils/mm2 [mean ± SEM, n = 5] in germ-free vs. control mice, respectively). Taken together, these results suggest that eosinophil homing into the gastrointestinal tract occurs prenatally and is independent of the presence of viable bacterial flora. Furthermore, eosinophil homing into the gastrointestinal tract appears to be regulated by mechanisms distinct from those regulating other gastrointestinal leukocytes (e.g., mast cells and lymphocytes) that have previously been shown to take at least 1 month to reach adult levels (43–46).
Gastrointestinal eosinophils are not affected by pulmonary allergen challenge.
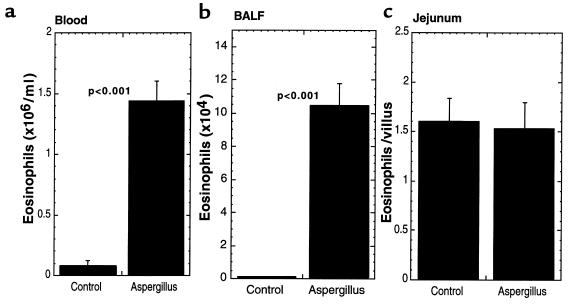
We were interested in determining whether the level of gastrointestinal eosinophils was increased in parallel with increases in peripheral blood eosinophils. This would establish whether gastrointestinal levels were directly related to circulating levels of eosinophils, or whether local and specific mechanisms in the gastrointestinal tract were operational. To induce peripheral blood eosinophilia, mice were challenged with repeated intranasal inoculations of Aspergillus fumigatus antigens using a well-established protocol (40, 41). Aspergillus challenge induced a significant increase in the level of eosinophils in the peripheral blood and BALF; however, no change in the level of gastrointestinal eosinophils was observed (Figure 3). These results imply the specific involvement of local processes within the gastrointestinal tract in regulating gastrointestinal eosinophil numbers.
Figure 3.
Eosinophil levels after pulmonary allergen challenge. Mice (129 SvEv) were challenged with Aspergillus fumigatus allergen intranasally 3 days per week for 3 weeks. Eighteen hours after the last allergen exposure, the mice were sacrificed and the number of eosinophils in the blood (a), BALF (b), and jejunum (c) was analyzed. The data are expressed as mean ± SEM (n = 15–18 mice.
Eotaxin is constitutively expressed in the gastrointestinal tract.
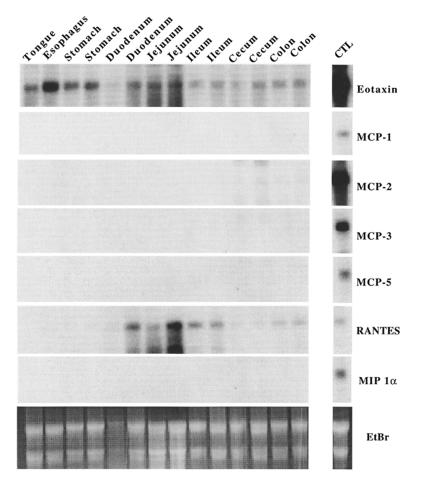
When the eotaxin cDNA from guinea pigs, mice, and humans was identified, the mRNA for this chemokine was found to be constitutively expressed in a variety of tissues (19, 29, 47). A finding in all species was that the intestine expressed relatively high levels of eotaxin mRNA; however, the segmental distribution of eotaxin mRNA within the intestine was not examined. Examination of multiple segments of the mouse gastrointestinal tract for expression of eotaxin mRNA indicated that this chemokine was ubiquitously expressed, at variable levels, in all segments (Figure 4). Notably, the constitutive expression of eotaxin was distinct from the expression of related eosinophil-active chemokines, which were not readily detectable. Only RANTES was detectable, but it was not ubiquitously expressed (Figure 4).
Figure 4.
Northern analysis of chemokine expression in the gastrointestinal tract. Total RNA (20 μg) from various gastrointestinal segments of wild-type 129 SvEv mice was electrophoresed and transferred to a membrane that was hybridized under conditions of high stringency with cDNA probes for murine eotaxin, MCP-1, MCP-2, MCP-3, MCP-5, RANTES, and MIP-1α, using methods described previously (17). Control RNA is derived from the lungs of IL-4 transgenic Clara cell lung in transgenic mice (39). Each lane represents RNA from a different animal, except the lanes for the tongue and esophagus, which were pooled from multiple animals. Autoradiographs were exposed for 1–2 days. The chemokine cDNA probes were described previously (16), except for MCP-2, which has recently been cloned (M.N. Sarafi and A.D. Luster, unpublished data). The ethidium bromide–stained gel (EtBr) is also shown.
Eotaxin is required for baseline eosinophil accumulation throughout the gastrointestinal tract.
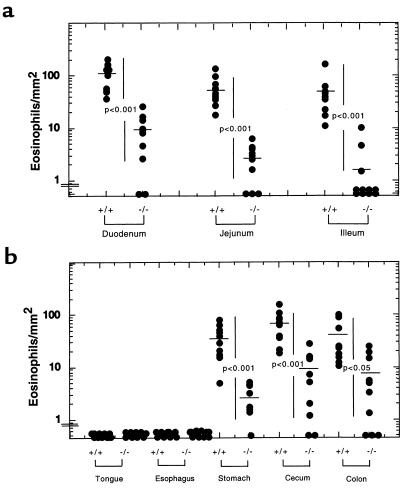
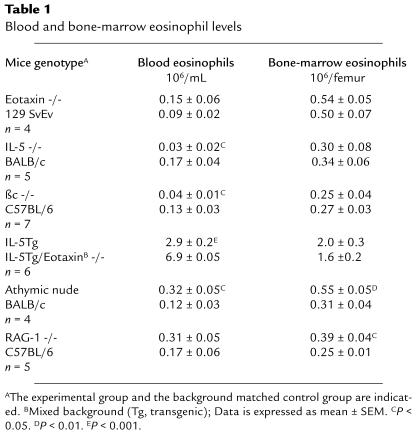
Data on the expression patterns of eosinophil-active chemokines at baseline indicated that eotaxin might be involved in the selective regulation of eosinophil homing in the gastrointestinal tract. To test this hypothesis, the number of eosinophils in the jejunum of mice deficient in eotaxin was initially examined (21) and shown to be significantly lower than in wild-type mice (25). We next examined the entire length of the small intestine for the level of tissue eosinophils, and found a marked reduction in the eotaxin gene-targeted mice when the data were normalized per villus and per unit area (Figure 5a). The duodenum, jejunum, and ileum of wild-type mice had 6.2 ± 1.0, 2 ± 0.4, and 1.7 ± 0.5 eosinophils per villus in comparison with eotaxin-deficient mice, which had 0.52 ± 0.14, 0.09 ± 0.02, and 0.05 ± 0.03 eosinophils per villus, respectively (data not shown). Next we examined other gastrointestinal segments in order to determine if eotaxin had a similar role in other regions of the gastrointestinal tract. Interestingly, eosinophils were rarely encountered in any segments of the gastrointestinal tract of eotaxin-deficient mice (Figure 5b). The eosinophil numbers (mean ± SEM) in the stomach, cecum, and colon of wild-type mice were 36.7 ± 7.5, 69.3 ± 14, and 39 ± 10 cells/mm2, respectively. Eotaxin gene-targeted mice had 2.6 ± 0.6, 8.9 ± 2.9, and 7.4 ± 2.8 cells/mm2, respectively (Figure 5b). No significant differences were observed in the levels of bone marrow and peripheral blood eosinophils in these 2 groups of mice (Table 1). As a control, we also examined the level of gastrointestinal eosinophils in mice that were deficient in another CC chemokine gene, MIP-1α. These gene-targeted mice did not have an alteration in the level of gastrointestinal eosinophils when compared with wild-type mice (2.2 ± 0.3 vs. 2.4 ± 0.5 eosinophils per villus in MIP-1α–deficient and wild-type mice, respectively).
Figure 5.
Quantitation of eosinophils in different gastrointestinal segments of wild-type and eotaxin-deficient mice. Eosinophils were counted from tissue sections of wild-type (+/+) and eotaxin-deficient (–/–) mice immunostained with anti-MBP antiserum. Eosinophils are normalized per squared millimeter. Each data point represents an individual mouse. The horizontal line is the mean value. Levels of eosinophils indicated near the ordinate were undetectable.
Table 1.
Blood and bone-marrow eosinophil levels
Eotaxin is required for IL-5–mediated increases in gastrointestinal eosinophils.
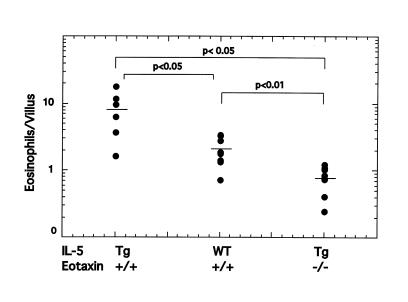
In light of known synergistic effects between eotaxin and IL-5 (6, 34, 35), we examined the relationship between eotaxin and IL-5 in regulating gastrointestinal eosinophil levels. Transgenic mice that overexpress IL-5 under control of the T-cell promoter CD2 have an increase of approximately 10- to 20-fold in the number of eosinophils in the hematopoietic organs (Table 1) (7). Because T cells also reside in the gastrointestinal tract, and local overexpression of IL-5 can promote eosinophil accumulation (48), we hypothesized that these transgenic mice would have increased levels of gastrointestinal eosinophils. IL-5 transgenic mice were demonstrated to have a 4-fold increase in the level of eosinophils in the lamina propria of the small intestine compared with wild-type mice (Figure 6). Interestingly, IL-5 transgenic mice also had increased eosinophils in the esophagus (wild-type and IL-5 transgenic mice had 0.44 ± 0.23 and 21.6 ± 6.9 eosinophils/mm2, respectively [mean ± SEM, n = 3–4]) (data not shown). To test the dependency of gastrointestinal eosinophil levels on eotaxin in the presence of elevated levels of IL-5, we generated mice that were transgenic for IL-5 and deficient in eotaxin. These mice had a significantly reduced level (∼10%) of eosinophils within the jejunum compared with IL-5 transgenic mice that contained wild-type eotaxin (Figure 6). Furthermore, the IL-5 transgenic mice that were deficient in eotaxin had levels of circulating eosinophils that were more than 2-fold higher than in IL-5 transgenic mice that had wild-type eotaxin (Table 1). Collectively, these data suggest that the elevation in circulating eosinophils was a consequence of inhibition of eosinophil recruitment into the gastrointestinal tract in the absence of eotaxin. Furthermore, the data indicate that eotaxin, even in the presence of high levels of IL-5 and circulating eosinophils, is critical for maintaining gastrointestinal eosinophil levels.
Figure 6.
Gastrointestinal eosinophils in mice carrying a combination of the CD2 IL-5 transgene and the wild-type or eotaxin gene-targeted allele. The level of eosinophils in the jejunum of littermate mice that are transgenic (Tg) or wild-type (WT) for IL-5, and carrying the wild-type (+/+) or homozygous deletion (–/–) of eotaxin, is shown. Each data point represents an individual mouse. The horizontal line is the mean value.
IL-5 and GM-CSF partially regulate gastrointestinal eosinophils.
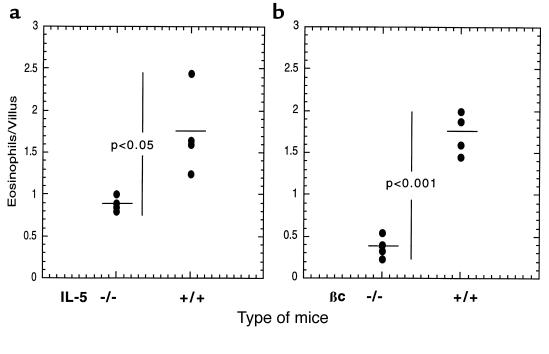
We have shown that overexpression of IL-5 in T cells is associated with an increase in the level of gastrointestinal eosinophils. We were also interested in determining if physiological levels of IL-5 and the related cytokine GM-CSF were involved in the regulation of baseline levels of gastrointestinal eosinophils. These cytokines are not only involved in the production of eosinophils from progenitor cells, but they can also regulate eosinophil survival in tissues (49, 50). Additionally, activated eosinophils and eosinophils exposed to tissue components such as fibronectin have been shown to produce these cytokines — GM-CSF in particular (51). It has been hypothesized that an autocrine process may be responsible, at least in part, for eosinophil tissue survival. We therefore examined eosinophil levels in IL-5– and GM-CSF–deficient mice. The IL-5–deficient mice, but not the GM-CSF–deficient mice (data not shown), had ∼50% fewer gastrointestinal eosinophils compared with age-, sex-, and background-controlled mice (Figure 7a). We next examined mice that were deficient in the functional receptor for IL-5 and GM-CSF (βc gene-targeted mice). The βc-deficient mice (C57BL/6) had ∼80% fewer gastrointestinal eosinophils compared with control mice (Figure 7b). We know that the effect of IL-5 and βc was not restricted to the gastrointestinal tract because mice deficient in these molecules had a reduction of ∼75% in levels of circulating eosinophils (Table 1). These data suggest that GM-CSF and IL-5 have a combined effect in regulating eosinophil levels in the gastrointestinal tract; however, this effect appears to be secondary to their effect on regulating the pool of circulating eosinophils.
Figure 7.
Quantitation of gastrointestinal eosinophils in mice deficient in IL-5 or βc. The number of eosinophils residing in the small intestine (jejunum) of IL-5 (a) and βc (b) gene-targeted mice was compared with that in control wild-type mice. Each data point represents the number of eosinophils in 1 mouse. The horizontal line is the mean value.
Gastrointestinal eosinophils are partially reduced in lymphocyte-deficient mice.
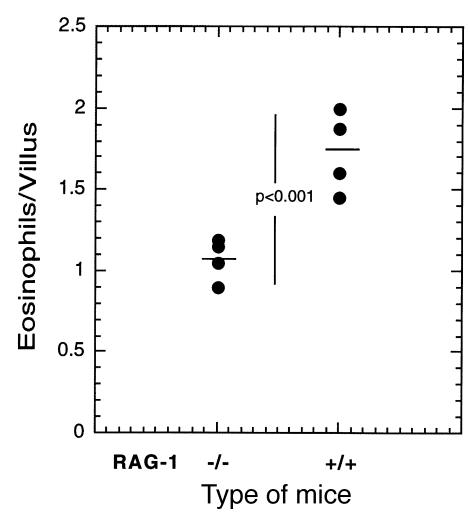
T cells and their products (e.g., IL-5) are known to regulate the development of peripheral blood eosinophilia and eosinophil infiltrates into the lung after allergen challenge (52). We therefore wanted to analyze the role of T cells in the maintenance of gastrointestinal eosinophil levels at baseline. Athymic nude mice are deficient in the majority of T cells and have been shown to have impaired eosinophil responses to allergens and parasites (53). However, these mice had no difference in the level of eosinophils in the jejunum (1.7 ± 0.3 vs. 1.07 ± 0.15 [mean ± SEM, n = 8] for control and athymic mice, respectively). To evaluate further the role of residual T cells (e.g., intraepithelial γδ T cells) and B cells in the athymic nude mice, mice deficient in RAG-1 were examined (Figure 8) (37). These mice were found to have a reduction of ∼50% in jejunum eosinophils compared with strain-matched controls. Analysis of blood and bone marrow levels of eosinophils in the RAG-1–deficient and athymic nude mice revealed significant increases in eosinophils (Table 1). These data indicate that in contrast to their critical role in regulating eosinophils during inflammatory conditions, lymphocytes do not have a major role in maintaining homeostatic levels of eosinophils in the gastrointestinal tract.
Figure 8.
Quantitation of gastrointestinal eosinophil numbers in RAG-1–deficient mice. The number of eosinophils residing in the jejunum of RAG-1 gene-targeted mice was compared with that in control wild-type mice. Each data point represents the number of eosinophils in 1 mouse, and the horizontal line is the mean value.
Discussion
We have characterized the processes involved in regulating eosinophil levels in the gastrointestinal tract, the location where most eosinophils reside at baseline. Several novel processes involved in regulating eosinophil levels in the gastrointestinal tract have been elucidated. First, we demonstrate that eosinophil accumulation in the gastrointestinal tract commences during embryonic development. By late gestation and in the perinatal period, eosinophil concentrations in the intestine are comparable to those in the adult. This is in marked contrast to other resident intestinal leukocytes. For example, mast cells home into the intestine prenatally, but their levels are low and do not reach adult levels until 1–2 months of age (43, 44). Similarly, most lymphocytes home into the gastrointestinal tract postnatally and approach adult levels only by 2 months of age (45, 46). Second, we show that eosinophil levels in the intestine are not dependent upon the presence of viable intestinal flora, demonstrating that eosinophil homing into the intestine is not triggered by low-grade chronic inflammation initiated by bacterial colonization. It is worth noting that we have not ruled out the role of enteric antigens in this process, since the germ-free mice still have exposure to bacterial antigens. Third, we demonstrate that eotaxin is ubiquitously expressed in the gastrointestinal tract and has a central role in maintaining the level of eosinophils in all gastrointestinal segments that normally have resident eosinophils. The critical role of eotaxin is further revealed in the eotaxin-deficient and IL-5 transgenic mice. IL-5 transgenic mice that are genetically engineered to be deficient in eotaxin have increased circulating eosinophils; however, they have a significant reduction in the level of their gastrointestinal eosinophils relative to IL-5 transgenic mice that have the wild-type eotaxin gene. This highlights the unique role of eotaxin in regulating eosinophil accumulation in the gastrointestinal tract at baseline, even in the presence of elevated levels of IL-5. Fourth, we demonstrate that although eotaxin is required for the maintenance of gastrointestinal eosinophil levels, eotaxin alone is unlikely to be sufficient, because the upper gastrointestinal tract (tongue and esophagus) expresses abundant eotaxin mRNA but has very low levels of eosinophils. Finally, we demonstrate that in contrast to eotaxin, other cytokines such as GM-CSF and IL-5 are only partially involved in regulating baseline levels of gastrointestinal eosinophils. In fact, mice deficient in the function of these cytokines have significantly reduced levels of circulating eosinophils, suggesting that their effect in the gastrointestinal tract is not tissue specific.
The processes involved in regulating baseline eosinophilopoiesis are less well understood than the processes regulating eosinophilia during inflammatory conditions. For example, IL-5 deficiency markedly attenuates peripheral blood and pulmonary eosinophilia after allergen challenge in the lung (8), yet mice deficient in this cytokine do not have appreciable reductions in eosinophilopoiesis in the bone marrow (Table 1). However, these mice (along with βc-deficient mice) have reduced eosinophil levels in the peripheral blood, indicating that IL-5 and/or GM-CSF are more critical in regulating bone marrow release than eosinophil development. Furthermore, mice deficient in lymphocytes (athymic nude or RAG-1–deficient mice) have modest increases in blood and bone marrow eosinophils (Table 1).
The gastrointestinal tract is known to harbor the largest reservoir of lymphoid tissue in the body. As a result, understanding the molecular and cellular processes regulating lymphocyte homing into the gastrointestinal tract has been an active area of investigation. Elegant studies have shown that gastrointestinal lymphocytes are unique when compared with peripheral lymphocytes, based on the expression pattern of specific receptors and cytokines and the use of unique homing molecules. As with gastrointestinal lymphocytes, the gastrointestinal tract also harbors a large proportion of eosinophils. The α4β7 integrin is coexpressed by eosinophils and lymphocytes (54–56), and the latter cells are known to use this pathway for homing into the same regions in which gastrointestinal eosinophils reside. We propose that the adhesion molecules required for eosinophil baseline homing into the gastrointestinal tract are likely to involve α4β7 interaction with mucosal addressin cell adhesion molecule-1 (MAdCAM-1). In support of this, mice deficient in α4β7 have elevated levels of peripheral blood eosinophils at baseline (57). Conservative estimates indicate that most eosinophils actually reside in the gastrointestinal tract rather than in hematopoietic organs. Possible functions of gastrointestinal eosinophils include having a role in innate immunity to infectious agents such as parasites. Eosinophils express complement receptors that can be used to mediate cellular cytotoxicity against helminths. Additionally, through their production of pleiotropic cytokines, eosinophils may have an immunomodulatory role (e.g., regulation of IgA production) or a role in maintaining tissue integrity. Further studies are needed to define the biological function and phenotypic characterization of gastrointestinal eosinophils.
In summary, these investigations dissect the cellular and molecular mechanisms involved in eosinophil homing into the gastrointestinal tract. We demonstrate that eosinophil localization within the gastrointestinal tract occurs early in development and is independent of the presence of viable intestinal flora. Furthermore, we demonstrate that eosinophil levels in the gastrointestinal tract are predominantly modulated by eotaxin and less significantly by IL-5, GM-CSF, and lymphocytes. The demonstration that eosinophils normally reside in the gastrointestinal tract and are markedly increased in a variety of diseases underscores the importance of delineating these processes and their implications in diseased states of the gastrointestinal tract.
Acknowledgments
We wish to thank Mitchell Cohen, Susan Wert, and Nives Zimmermann for their helpful discussions and review of this manuscript. We also thank K. Matthaei, G.K. Hershey, and A. Srikiatkhachorn for review of the manuscript. The study was supported in part by National Institutes of Health grant R01 AI-42422-02 (to M.E. Rothenberg) and the National Health Medical Research Council (Australia) C.J. Martin Post-doctorate Fellowship Award (to S. Hogan).
References
- 1.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Weller PF. The immunobiology of eosinophils. N Engl J Med. 1991;324:1110–1118. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 4.Furuta GT, Ackerman SJ, Wershil BK. The role of the eosinophil in gastrointestinal diseases. Curr Opin Gastroenterol. 1995;1995:541–547. [Google Scholar]
- 5.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 6.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee NA, et al. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 10.Dranoff G, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 11.Nishinakamura R, et al. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995;2:211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Nishinakamura R, Miyajima A, Mee PJ, Tybulewicz VLJ, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458–2464. [PubMed] [Google Scholar]
- 13.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 14.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 15.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 16.Luster AD, Rothenberg ME. Role of monocyte chemoattractant protein and eotaxin subfamily of chemokines in allergic inflammation. J Leukoc Biol. 1997;62:620–633. doi: 10.1002/jlb.62.5.620. [DOI] [PubMed] [Google Scholar]
- 17.Jose PJ, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponath PD, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenberg ME, Luster AD, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalo J-A, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation: the role of T cells, chemokines, and adhesion receptors. J Clin Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen- induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humbles AA, et al. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo. J Exp Med. 1997;186:601–612. doi: 10.1084/jem.186.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira MM, et al. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J Clin Invest. 1997;100:1657–1666. doi: 10.1172/JCI119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkhioued B, et al. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- 25.Matthews AN, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combadiere C, Ahuja SK, Murphy PM. Cloning and functional expression of a human eosinophil CC chemokine receptor. J Biol Chem. 1995;270:16491–16494. doi: 10.1074/jbc.270.28.16491. [DOI] [PubMed] [Google Scholar]
- 27.Daugherty BL, et al. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponath PD, et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Zepeda EA, et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 30.Ying S, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 31.Minshall E, et al. Eotaxin mRNA expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Am J Respir Cell Mol Biol. 1997;17:683–690. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- 32.Bartels J, et al. Increased eotaxin-mRNA expression in non-atopic and atopic nasal polyps: comparison to RANTES and MCP-3 expression. Rhinology. 1997;35:171–174. [PubMed] [Google Scholar]
- 33.Brown JR, et al. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998;114:137–146. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothenberg ME, et al. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 35.Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopf M, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 37.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 38.Cook DN, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 39.Rankin JA, et al. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA. 1996;93:7821–7825. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehlhop PD, et al. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci USA. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang WW, et al. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med. 1998;188:1063–1074. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson A. Models of intestinal hypersensitivity. Clin Gastroenterol. 1976;5:271–288. [PubMed] [Google Scholar]
- 43.Watkins SG, Dearin JL, Young LC, Wilhelm DI. Association of mastopoiesis with haemopoietic tissues in the neonatal rat. Experientia. 1976;32:1339–1340. doi: 10.1007/BF01953127. [DOI] [PubMed] [Google Scholar]
- 44.Woodbury RG, Neurath H. Purification of an atypical mast cell protease and its levels in developing rats. Biochemistry. 1978;17:4298–4304. doi: 10.1021/bi00613a029. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson A, Parrott DM. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972;12:477–488. [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson A, Parrott DM. Growth and development of “antigen-free” grafts of foetal mouse intestine. J Pathol. 1972;106:95–101. doi: 10.1002/path.1711060205. [DOI] [PubMed] [Google Scholar]
- 47.Rothenberg ME, Luster AD, Lilly CM, Drazen JM, Leder P. Constitutive and allergen-induced expression of eotaxin mRNA in the guinea pig lung. J Exp Med. 1995;181:1211–1216. doi: 10.1084/jem.181.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JJ, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owen WF, et al. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987;166:129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothenberg ME, et al. IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989;143:2311–2316. [PubMed] [Google Scholar]
- 51.Anwar AR, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 53.Hamelmann E, et al. Allergen-specific IgE and IL-5 are essential for the development of airway hyperresponsiveness. Am J Respir Cell Mol Biol. 1997;16:674–682. doi: 10.1165/ajrcmb.16.6.9191469. [DOI] [PubMed] [Google Scholar]
- 54.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 55.Berlin C, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 56.Bochner BS, Schleimer RP. The role of adhesion molecules in human eosinophil and basophil recruitment. J Allergy Clin Immunol. 1994;94:427–438. doi: 10.1016/0091-6749(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 57.Steeber DA, et al. Efficient lymphocyte migration across high endothelial venules of mouse Peyer’s patches requires overlapping expression of L-selectin and beta7 integrin. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]