Abstract
To address the hypothesis that elevated blood alcohol increases systemic oxidant stress, we measured urinary excretion of isoprostanes (iPs), free radical–catalyzed products of arachidonic acid. Ten healthy volunteers received acute doses of alcohol (Everclear-R) or placebo under randomized, controlled, double-blind conditions. Urinary iPF2a-III increased in a time- and dosage-dependent manner after dosing with alcohol, with the peak urinary iPF2a-III excretion correlating with the rise in blood alcohol. To determine whether oxidant stress was associated with alcohol-induced liver disease (ALD), we then studied the excretion of iP in individuals with a documented history of alcohol-induced hepatitis or alcohol-induced chronic liver disease (AC). Both urinary iPF2a-III and urinary iPF2a-VI were markedly increased in patients with acute alcoholic hepatitis. In general, urinary iPF2a-III was significantly elevated in cirrhotic patients, relative to controls, but excretion was more pronounced when cirrhosis was induced by alcohol than by hepatitis C. Excretion of iPF2a-VI, as well as 4-hydroxynonenal and the iPF2a-III metabolite, 2,3-dinor-5,6-dihydro-iPF2a-III, was also increased in AC. Vitamin C, but not aspirin, reduced urinary iPs in AC. Thus, vasoactive iPs, which serve as indices of oxidant stress, are elevated in the urine in both acute and chronic ALD. Increased generation of iPs by alcohol in healthy volunteers is consistent with the hypothesis that oxidant stress precedes and contributes to the evolution of ALD.
Introduction
Alcohol-associated cirrhosis is estimated to account for almost half of the serious liver disease in the Western world (1). Although light to moderate ingestion of alcohol may reduce the risk of coronary heart disease (2), intake of more than 30 g of alcohol per day has been associated with increased mortality due to breast cancer in women (3), and with cirrhosis in both sexes (1–5). The mechanisms involved in alcohol-induced liver disease (ALD) are poorly understood (6), although prevalence of the disease appears to follow a dose-response curve in relation to lifetime intake (4, 5).
The potential role that oxidant injury plays in the pathogenesis of ALD has received considerable scrutiny (6–9). Alcohol ingestion, through induction of the CYP4502E1 isozyme, may result in increased generation of reactive oxygen intermediates (ROIs), which have the potential to peroxidize lipids (10). The degree of lipid peroxidation may be modulated by endogenous antioxidant defenses or by xenobiotics, such as cigarette smoking or dietary fats (11). Thus, genetic and environmental factors may contribute to the variable expression of ALD as a function of alcohol intake in different populations. Evidence consistent with a causal relationship between lipid peroxidation and morphologically assessed liver damage has recently been reported in a rat model of ALD (11). Lipid peroxidation was determined indirectly by measurement of conjugated dienes and protein adduct formation (12).
Isoprostanes (iPs) are free radical–catalyzed products of arachidonic acid (13–16). Quantitation of iPs in biological fluids appears to reflect oxidant stress in vivo. We have developed specific methods for measurement of the distinct iPs iPF2α-III (formerly known as 8-iso-PGF2α; refs. 16 and 17) and iPF2α-VI (formerly known as IPF2α-1; ref. 18). Urinary excretion of both compounds is increased in syndromes of oxidant stress (19–21), and depressed by antioxidants in vitro (18) and in vivo (21, 22). Urinary iPs may be used to select effective antioxidant doses of vitamins in vivo (22). We now report that excretion of both iPs is increased in patients with acute and chronic ALD. Furthermore, the concomitant increase in excretion of an iP metabolite and an independent index of lipid peroxidation, 4-hydroxynonenal (4-HNE; ref. 23), implies that this reflects increased generation, rather than altered disposition, of the iPs in liver disease. Vitamin C, but not aspirin, suppresses iP generation in cirrhosis. Furthermore, alcohol ingestion dose dependently increases excretion of iPF2α-III in healthy volunteers. This is consistent with the possibility that alcohol-induced oxidant stress might precede liver damage and may contribute to the evolution of ALD.
Methods
Study design.
The first study was a randomized, placebo-controlled trial to determine the dose-response relationship between alcohol consumption and urinary iPF2α-III excretion. All clinical studies were approved by the Institutional Review Board and the General Clinical Research Center (GCRC) Advisory Committee, as appropriate. All patients who participated in these studies consented to do so and were compensated for incurred expenses. Ten volunteers (5 males and 5 females), between the ages of 21 and 60 years (mean: 38.0 ± 11.5 years), were studied. The sample size was based on the known coefficient of variation of the methods (maximally 4.5%) and the desire to detect a dose-dependent increase of at least 50% at the peak from baseline values, where α = 0.05 and the power (1-β) of the comparison is 0.85. A similar basis was used for sample size calculation in all subsequent studies.
Individuals with a history of liver disease, alcoholism, or intake of any medication — specifically nonsteroidal anti-inflammatory drugs (NSAIDs), anovulant contraceptives, or vitamins — in the preceding 2 months were excluded. Other exclusion criteria included pregnancy, tobacco smoking, and a history of myocardial infarction in the preceding 3 months. Volunteers desisted from all drug and alcohol intake from the time of screening (14 days before dosing) to the conclusion of the study, except as mandated by the trial design.
Volunteers were randomized under double-blind, placebo-controlled conditions for the order in which they drank 240 mL of a nonalcoholic solution of lemonade (Wyler’s Lemonade) or 0.2, 0.3, 0.4, 0.6, or 0.9 g/kg body wt of a 98% solution of alcohol (Everclear-R), made up to 240 mL with the lemonade solution. Before starting the study, a complete dietary history was obtained, using a quantitative food-frequency questionnaire. In an attempt to standardize this variable, subjects were instructed to comply with the US Dietary Goals and Guidelines for Healthy Adults. Dietary compliance was monitored by interview during each GCRC admission and by random 24-hour recalls of dietary intake, as assessed by telephone interview during the outpatient periods. During each of the 4 admissions, subjects received the same standardized diet. Meals were administered at 5 hours, 12 hours, and 24 hours after dosing. The diet provided 1.2 × basal energy expenditure (BEE) kcal per day for each study participant, which is based on the Harris-Benedict equation (24). Multiplication by a factor of 1.2 was to account for sedentary activity. Foods were prepared ahead of time and weighed to the nearest 0.1 g before serving. All foods were prepared by trained technicians in the GCRC metabolic kitchen. Participants were encouraged to consume all foods offered. However, if there were residual foods, they were weighed to the nearest 0.1 g and recorded on each subject’s daily intake chart.
The 6 study days were each separated by 2-week “washout” periods. The volunteers were admitted to the GCRC, and fasting and dosing occurred over a 15-minute period at 0800 hours on each study day. Blood-alcohol levels were measured at baseline, at 20, 40, and 60 minutes, and at 2, 3, 4, 6, 12, and 24 hours after dosing. Sequential urine collections were performed for iPF2α-III during the 2 hours before dosing (–2 to 0 hours), and from 0 to 6, 6 to 12, and 12 to 24 hours after dosing, in timed aliquots on each study day. Urine was aliquoted, spiked with internal standards, and stored for analysis by stable-isotope dilution gas chromatography/mass spectrometry (GC/MS).
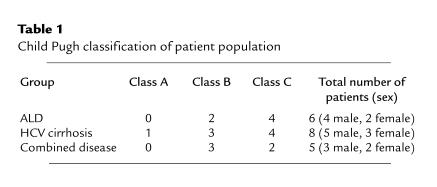
The second study addressed the hypothesis that patients with established ALD have increased ROI generation in vivo, as assessed by urinary excretion of iPF2α-III and iPF2α-VI, when compared with patients with non–alcohol-related liver disease, and with age- and sex-matched controls. Fifteen healthy age- and sex-matched controls (8 males and 7 females) were recruited through advertisements placed in local papers. Nineteen patients between the ages of 38 and 64 years (mean: 52.0 ± 6.2 years), with either biopsy-proven cirrhosis or end-stage liver failure compatible with cirrhosis, were recruited from the Hepatology and Liver Transplant Clinic at the Hospital of the University of Pennsylvania. Six of the patients had a known diagnosis of ALD, 8 had a history of serologically confirmed hepatitis C (HCV) cirrhosis, and 5 had combined ALD and HCV cirrhosis (Table 1). The severity of liver disease was comparable among the 3 patient groups, as demonstrated by Child-Pugh class (ref. 25; Table 1). Patients were excluded if they had a known diagnosis of hepatocellular carcinoma, spontaneous bacterial peritonitis, other infective processes, or a history of gastrointestinal hemorrhage within the previous month. Patients with cholestatic liver disease and hemochromatosis were excluded. Study subjects were also excluded if they had a history of established renal or pulmonary disease, or a myocardial infarct within the last 3 months. No patient gave a history of a myocardial infarct occurring in the year preceding the study. All patients and volunteers recruited were nonsmokers. Patients received a standard therapeutic regimen of a loop diuretic and a potassium-sparing diuretic. All subjects discontinued vitamin and iron supplements and abstained from taking NSAIDs and alcohol for a minimum period of 4 weeks before being studied. Eleven patients had a history of alcohol dependence according to psychiatric interview, including a history of excess alcohol intake for more than 10 years (n = 8), a history of prior rehabilitation attempts (n = 11), attendance at Alcoholics Anonymous (n = 10), and convictions for driving while impaired by alcohol (n = 7). In addition, each of these patients had a positive score on the CAGE screening questionnaire for covert alcoholism, whereas all patients with non–alcohol-related liver disease and all volunteers had negative scores (26). All 11 patients gave a history of abstinence from alcohol at the time of the study, with a range of 4 months to 4 years, and a median interval of 2 years.
Table 1.
Child Pugh classification of patient population
Subjects were admitted to the GCRC for 3 consecutive days. Each subject received a standardized diet throughout the study period. In addition, the research dietician obtained a comprehensive dietary history from each participant, and subjects were instructed to follow a standard diet for 2 weeks before the study. The subjects’ usual dietary intake was estimated by a quantitative food-frequency score, which has been previously validated (24). Baseline measurements of hepatic and renal function included aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, bilirubin, prothrombin time, creatinine, sodium, and urea. In addition, blood was drawn on admission for vitamin E and C levels. Three consecutive 24-hour urine collections were obtained for iPF2α-III. Given that little information is available on the metabolism of iPs and that the liver might be a site of metabolite formation, we developed methods to analyze a putative 2,3-dinor-5,6-dihydro-iPF2α-III metabolite of iPF2α-III (27, 28) in a subset (n = 20) of study subjects to determine if an increment in iPF2α-III might reflect reduced metabolism, rather than increased generation, of the compound. Urinary iPF2α-VI was also measured in patients with ALD, and in age- and sex-matched controls.
An additional cohort of 16 patients (13 males) with chronic ALD, aged 49.9 ± 13.0 years, were compared with a control group of 12 healthy volunteers (6 males) aged 40.3 ± 19.9 years (P = NS). Entry and exclusion criteria were as described above. A 24-hour urine sample was collected for analysis of 4-HNE and iPF2α-VI.
A third study involved assessment of iP generation in patients with acute alcoholic hepatitis. Patients were admitted directly to the hepatology service from the emergency room. Baseline measurements of hepatic and renal function included AST, ALT, albumin, bilirubin, prothrombin time, creatinine, sodium, and urea. In addition, vitamin E and C levels were obtained. Three of the 5 patients had a Maddrey score (a discriminant function) of greater than 32, indicating a poor prognosis (29). In addition to routine medical care, 3 consecutive 24-hour urine collections were obtained for iPF2α-III and iPF2α-VI in a manner identical to that in the preceding study.
A fourth study addressed the effect of the antioxidant vitamin C on iP excretion in patients with established liver disease. Given that iPF2α-III, but not iPF2α-VI, may be formed as a minor product of either of the cyclooxygenase (COX) isoforms (17, 18, 30), we also assessed the effects of aspirin. Patients were again recruited from the hepatology and liver-transplant clinics. Fifteen patients were studied: 5 with ALD, 5 with HCV cirrhosis, and 5 with combined disease. Samples for measurement of endogenous vitamin levels and urinary iPs were obtained at baseline and after taking 2.5 g of vitamin C per day for 10 days. Final samples were obtained after a 10-day washout period. The volunteers were then given 325 mg of aspirin. Urinary iP measurement was repeated in a 24-hour sample after aspirin ingestion. In this study, we also compared measurement of thiobarbituric acid–reactive substances (TBARS), an ex vivo assay used to reflect RO1 generation (23) with iP production.
Measurement of iPF2α-III.
Urinary iPF2α-III was assayed by GC/MS as described previously (17). Briefly, urine was aliquoted, spiked with [18O2]iPF2α-III, and extracted from the aqueous matrix by solid-phase extraction techniques. The samples were purified by TLC. Native and labeled iPF2α-III were derivatized as their pentafluorobenzyl ester, trimethylsilyl ethers. They were then analyzed by GC/MS in the negative-ion, chemical-ionization mode. Quantification values were expressed as the ratio of the area under the peak of the ion representing the endogenous compound to that of the internal standard. Urinary creatinine was determined by a standard automated colorimetric assay, using a Beckman Synchron CX System (Beckman Instruments Inc., Arlington Heights, Illinois, USA).
2,3-dinor-5,6-dihydro-iPF2α-III was obtained as a gift from Cayman Chemical Co. (Ann Arbor, Michigan, USA). The 18O2 compound was made using the method of Pickett and Murphy (31). Briefly, internal standard was added to urine that was acidified with formic acid. The lipid was extracted by solid-phase extraction on a RapidTrace SPE Workstation (Zymark Corp., Hopkinton, Massachusetts, USA). The sample was applied to the column and rinsed with pH 7.0 buffer followed by 1 mL of ethyl acetate, then dried under N2 and redissolved in 30 μL of ethyl acetate. The metabolite was separated from other eicosanoids by TLC (Whatman Inc., Clifton, New Jersey, USA), using a mobile phase of 20% methanol and 80% ethyl acetate. Again, the compound was derivatized as its pentaflourobenzyl ester, trimethylsilyl ether, and analyzed on an MD800 GC/MS (Finnigan Corp., San Jose, California, USA). Urinary iPF2α-VI was similarly measured, as described previously, taking advantage of the pH-dependent lactonization of this class of iPs (18).
Analysis of urinary 4-HNE.
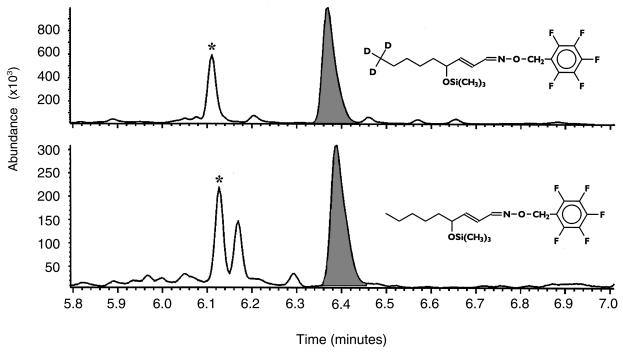
The method for measurement of 4-HNE in urine was adapted from one previously developed by van Kuijk et al. (32) to measure 4-hydroxyalkenals in oxidized LDL. Briefly, 5-mL urine samples were spiked with 5 ng of d3-HNE (kindly provided by F.J. van Kuijk, University of Texas Medical Branch, Galveston, Texas, USA), mixed well, and allowed to equilibrate for 15 minutes at room temperature. Then 2 mg of o-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine hydrochloride was added to each sample, and the samples were allowed to stand for 30 minutes at room temperature. 4-HNE was extracted using reverse-phase solid-phase extraction cartridges (C18 EC, 500 mg; International Sorbent Technology Ltd., Mid Glamorgan, United Kingdom) under the following conditions. The cartridge was conditioned with 5 mL of ethanol and washed with 1.5 mL of H2O. A sample was loaded onto the cartridge, which was washed with 3 mL of 60% ethanol. The cartridge was dried for 10 minutes, and the sample was eluted with 3 mL of ethyl acetate. The sample was then dried under a stream of N2 and dissolved in 1 mL of hexane. A second extraction used straight-phase solid-phase extraction cartridges (100 mg silica conditioned with 1 mL hexane). The sample was loaded onto the cartridge, which was washed with 1 mL of hexane. The sample was eluted with 1 mL of 30% ethyl acetate in hexane, dried, and dissolved in 15 μL of dodecane. One microliter of the sample was used for GC/MS analysis. The mass spectrometer was operated in the negative-ion, electron-capture ionization mode, using ammonia as the moderating gas. Ions monitored were of mass-to-charge (m/z) ratio 283 and 286 for 4-HNE and d3-HNE, respectively. A representative chromatogram obtained from human urine is depicted in Figure 1. Increasing amounts of d3-HNE (0.3, 0.625, 1.25, 2.5, 5, and 10 ng) were added to 5-mL control urine samples, from which 4-HNE was extracted as described above, to assess the linearity of the assay.
Figure 1.
Quantitation of 4-HNE in human urine. Five milliliters of urine was spiked with 5 ng of trideuterated 4-HNE. The upper trace (m/z 286) represents the internal standard; the lower trace (m/z 283) represents endogenous 4-HNE. Each ion trace shows 2 peaks (filled peak and asterisk-marked peak), which originate from the syn and anti isomers generated when the pentafluorobenzyloxime derivative is formed.
Other measurements.
Blood was collected from the antecubital vein, and ethanol concentration was measured using an alcohol dehydrogenase kit from Sigma Chemical Co. (St. Louis, Missouri, USA). After hexane extraction, plasma concentrations of vitamin C and serum levels of α-tocopherol were measured by HPLC on a reverse-phase C18 column (33). Serum was heated with thiobarbituric acid at a pH of less than 5.0 for measurement of TBARS. The resulting malondialdehyde adduct was measured by its absorbance at 532 nm, as described previously (27).
Statistical analysis.
Data were initially subjected to Kruskal-Wallis ANOVA, with subsequent pairwise analysis using a 2-tailed distribution-free test, as appropriate. Data are expressed as the mean ± SEM. A Bonferroni correction was used when multiple paired comparisons were performed.
Results
Alcohol increases iP excretion in healthy volunteers.
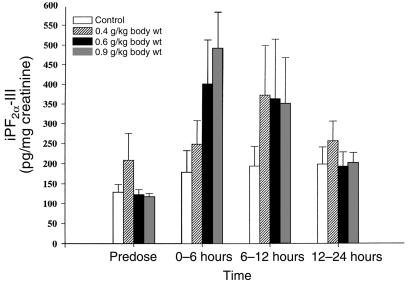
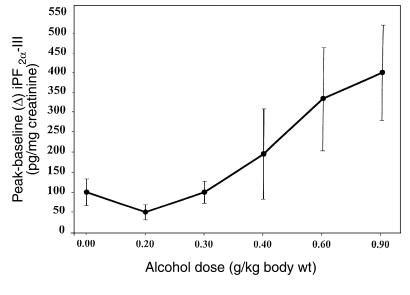
No alteration in urinary iPF2α-III was observed after administration of the control solution. However, urinary iPF2α-III excretion rose from 116 ± 10.1 pg/mg creatinine before dosing, to 491 ± 90, 349.5 ± 117.5, and 201.8 ± 25.6 pg/mg creatinine at 0–6 hours, 6–12 hours, and 12–24 hours after dosing with 0.9 gm/kg alcohol, respectively (P < 0.001; Figure 2). The time dependence of this response reflected the acute increase in blood alcohol. Alcohol also significantly (P < 0.01) increased peak urinary iPF2α-III excretion in a dose-dependent manner. For example, the increase from baseline was 50 ± 19.6, 102 ± 28, 197 ± 112, 335 ± 130, and 401 ± 120 pg/mg creatinine after dosing with 0.2, 0.3, 0.4, 0.6, and 0.9 gm/kg alcohol, respectively (Figure 3). Paired comparisons revealed significant elevations at the 2 highest doses of alcohol. The corresponding peak plasma alcohol levels were 30.2 ± 5.3, 64.6 ± 6.1, 81.2 ± 4.2, 105.1 ± 4.2, and 130.6 ± 5.6 mg/dL. While mean peak plasma alcohol levels and mean peak urinary iPF2α-III excretion were significantly correlated (P < 0.001), the relationship between individual alcohol and urinary iPF2α-III levels across the study did not attain significance. This may reflect the discordant timing of plasma versus urinary measurements, and/or differences in the kinetics of alcohol and iPs in a study of this sample size.
Figure 2.
Time- and dose-dependent increase in excretion of iPF2α-III in healthy volunteers administered alcohol. Acute oral doses (240 mL) of alcohol or a control solution were administered under randomized, double-blind, controlled conditions. Urinary iPF2α-III increased significantly (P < 0.001) as a function of dose. Alcohol also increased urinary iPF2α-III in a time-dependent manner, with the 3 highest dose groups (P < 0.01) peaking in the fraction collected 0–6 hours after dosing.
Figure 3.
The dose-related increment in iPF2α-III excretion in healthy volunteers. Alcohol significantly increased the maximal (vs. control) urinary iPF2α-III (P < 0.01) in a dose-dependent manner. Subsequent to ANOVA, paired comparisons indicated significant increases at the 2 highest doses.
Urinary iPs and 4-HNE are elevated in acute and chronic ALD.
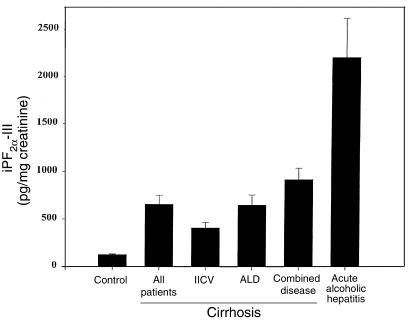
All patients with cirrhosis had elevated levels of urinary iPF2α-III when compared with noncirrhotic controls (664 ± 95 vs. 127.2 ± 7.9 pg/mg creatinine; P < 0.001). Urinary iPF2α-III excretion was significantly increased in patients with HCV cirrhosis (411.7 ± 59.8 pg/mg creatinine; P < 0.01). However, this increase was even more marked in patients with ALD (656.9 ± 105.8 pg/mg creatinine; P < 0.001) and in those with combined disease (921.9 ± 120 pg/mg creatinine; P < 0.001) (Figure 4). There was no significant variability in the 3 consecutive measurements in the patients with cirrhosis.
Figure 4.
Significant increases in urinary excretion of iPF2α-III in patients with cirrhosis due to alcoholism (ALD), viral hepatitis (HCV), or a combination of the 2 etiologies. Levels were significantly elevated in cirrhosis compared with controls. Excretion of iPF2α-III was most marked in acute alcoholic hepatitis.
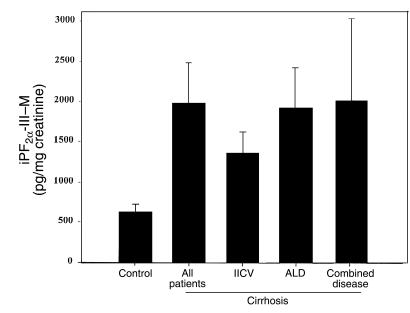
Urinary excretion of 2,3-dinor-5,6-dihydro-iPF2α-III, a putative metabolite of iPF2α-III (29, 30), was higher than that of parent iPF2α-III in cirrhosis (1,926 ± 497 vs. 664 ± 95 pg/mg creatinine; P < 0.01). Like the parent iP, excretion of the metabolite was increased in cirrhosis; this was particularly apparent in patients with a history of alcoholism (Figure 5). Thus, the increment in urinary iPF2α-III is likely to reflect increased generation of the iP, rather than its decreased metabolism in cirrhosis.
Figure 5.
Significant increases in urinary excretion of 2,3-dinor-5,6-dihydro-iPF2α-III (iPF2α-III–M), the putative dinor-dihydro metabolite of iPF2α-III, in patients with cirrhosis induced by alcohol (ALD), hepatitis C (HCV), or a combination of etiologies. Like the parent iPF2α-III, excretion of the metabolite was increased in all forms of cirrhosis, but particularly in ALD.
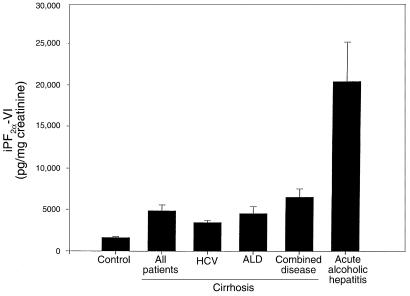
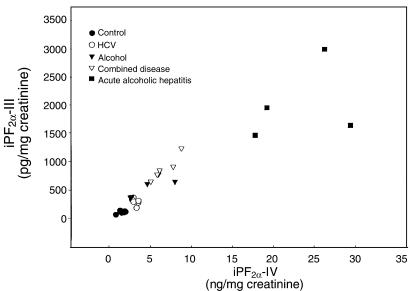
Evidence for increased generation of iPs in cirrhosis was also supported by measurement of an iP from a second class, iPF2α-VI, in a subset of patients and controls. Urinary iPF2α-VI was also significantly elevated over control in cirrhosis (4,841 ± 720 vs. 1,642 ± 110 pg/mg creatinine; P < 0.001) (Figure 6). Excretion of iPF2α-III and iPF2α-VI was highly correlated in patients with cirrhosis (r = 0.90, P < 0.001; Figure 7). Excretion of both iPF2α-III and iPF2α-VI was even more markedly elevated (P < 0.001) in patients with acute alcoholic hepatitis (2,205 ± 4,008 and 20,405 ± 4,772 pg/mg creatinine, respectively) than in those with chronic ALD. Finally, confirmation of increased oxidant stress in patients with chronic ALD was afforded by measurement of 4-HNE, using a novel assay for this compound in urine. The relationship of measured 4-HNE with added authentic spike was linear (r2 = 0.96, P < 0.0001) in human urine. The assay was also highly reproducible. Analysis of 5 samples, frozen at –70°C and thawed for analysis on 4 successive days, gave m/z ratios for ions 283/286 of 187.3 ± 13.6, 175.6 ± 6.4, 181.6 ± 11.8, and 179.25 ± 4.8, none of which differed significantly from each other (P > 0.05). Excretion of this independent marker of lipid peroxidation (23, 32) was significantly (P < 0.01) elevated in patients with chronic ALD (1.19 ± 0.3 ng/mg creatinine) compared with controls (0.49 ± 0.07 ng/mg creatinine). We failed to observe sex-based differences in urinary iPs or 4-HNE in our patients with ALD.
Figure 6.
Significant increases in iPF2α-VI excretion in patients with cirrhosis due to alcoholism, hepatitis C virus (HVC), or a combination of the 2 etiologies. Levels were significantly elevated in cirrhosis compared with controls. iP excretion was most marked in acute alcoholic hepatitis.
Figure 7.
Correlation excretion of urinary iPF2α-III and urinary iPF2α-VI in a subset of patients with cirrhosis of various etiologies (HCV, alcohol, and combined disease) and acute alcoholic hepatitis.
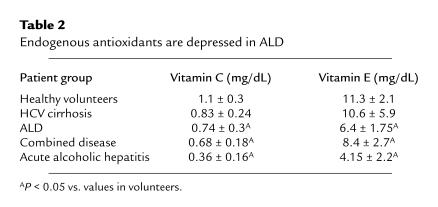
Both plasma vitamin C and serum α-tocopherol were significantly (P < 0.05) lower in all patient groups with ALD when compared with controls. Levels of the endogenous vitamins were not significantly depressed in patients with HCV cirrhosis (Table 2); however, this may reflect the comparative severity, rather than the origin, of the disease in the 2 groups and/or the constraints of a small sample size. The differences were most marked in patients with acute alcoholic hepatitis. However, levels of both vitamins fell within their normal ranges for this laboratory in all patient groups (0.2–1.9 and 4.6–14.5 mg/dL plasma, respectively).
Table 2.
Endogenous antioxidants are depressed in ALD
Vitamin C suppresses iP excretion in chronic ALD.
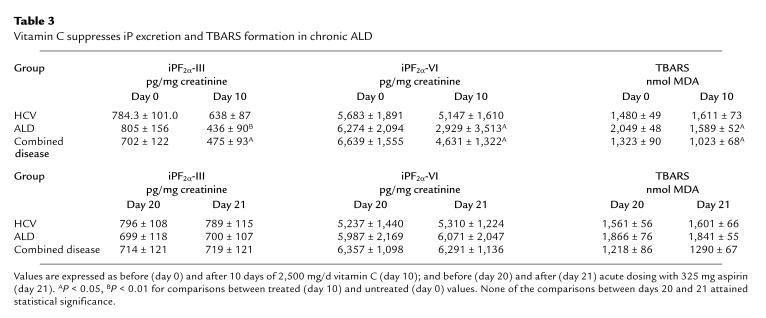
In this study, both urinary iPF2α-III (764 ± 139 vs. 53 ± 92 pg/mg creatinine; P < 0.01) and iPF2α-VI (6,198 ± 1,843 vs. 423 ± 109 pg/mg creatinine; P < 0.01) were again elevated in all patients with established cirrhosis compared with controls. Administration of vitamin C suppressed TBARS (P < 0.05), urinary iPF2α-III, and urinary iPF2α-VI in patients with ALD (P < 0.05) and with combined disease (P < 0.05). Vitamin C had no significant effect in patients with HCV cirrhosis, in whom endogenous antioxidants were not significantly depleted (Table 3). After a 10-day washout period, administration of aspirin failed to suppress urinary excretion of either iP or TBARS (Table 3). Again, endogenous levels of vitamin C (Table 3) and α-tocopherol (data not shown) were lower than in controls, but fell within the normal range.
Table 3.
Vitamin C suppresses iP excretion and TBARS formation in chronic ALD
Discussion
Although ALD is a significant cause of chronic liver disease in the developed world, it remains an enigma. It is unclear which mechanisms underlie the evolution of cirrhosis in chronic alcoholics. Putative factors include alcohol dose, sex, and comorbid conditions. Increasing lifetime alcohol dose is associated with greater prevalence of alcoholic liver disease above a threshold of 30 g of ethanol per day (5, 34). The threshold to initiate ALD may be lower, and the dose-response curve may be altered in women (4, 5). Comorbidity with viral infection, particularly chronic HCV infection, is associated with more severe liver injury in alcoholics, especially in those with a high alcohol intake (35). Consumption of diverse alcoholic beverages may be more injurious, while alcohol intake at mealtime may be less hazardous than in the fasting state (5).
Recently, there has been increasing interest in the possibility that ROIs might contribute to the evolution of ALD (7–9, 36–38). The development of hepatic disease in response to alcohol feeding in the rat is associated with an increase in lipid peroxides and in conjugated dienes in plasma (39, 40). Alcohol administration depletes the liver of the antioxidant glutathione. Administration of methionine, a precursor of glutathione, protects against alcohol-induced hepatic injury in rodents (39). Similarly, administration of polyenylphosphatidylcholine, which suppresses lipid peroxidation, attenuates alcohol-induced liver injury in baboons (41). Conversely, dietary supplementation with unsaturated fatty acids, a target for lipid peroxidation, increases susceptibility to ALD (39, 40).
Chronic alcoholics frequently consume inadequate diets, which may be deficient in antioxidant vitamins (42–44). It is possible that genetic variability in enzymes intrinsic to antioxidant defense or alcohol metabolism (11, 45, 46), or variability in consumption of antioxidants in the diet, may contribute to ethnic- and sex-based variability in the expression of ALD. Indeed, the antioxidant content of red wine has been suggested to contribute to the cardiovascular benefit associated with this form of alcohol consumption (36, 37, 47). While alcohol enhances cell-mediated lipoprotein oxidation in vitro, the same concentration of red wine suppresses this effect (47, 48). Red wine and its antioxidant polyphenols have been shown to reduce the progression of atherosclerosis in mice (49).
This study uses a noninvasive approach, based on highly specific GC/MS assays, to investigate the relationship between ROI generation in vivo and ALD. Isoeicosanoids are a family of free radical–catalyzed products of arachidonic acid that are isomers of the enzymatically formed eicosanoids. These compounds, in contrast with conventional indices of oxidant stress, are chemically stable and may be measured specifically with GC/MS (16). Attention has focused upon the F2-iPs, which circulate in plasma and are excreted in urine. Up to 64 of these compounds exist, grouped in 4 structural classes (16, 50, 51). Nanji et al. (52) have reported elevated levels of circulating immunoreactive iPF2α-III in a rodent model of ALD. However, these investigators used a commercial immunoassay that was subsequently withdrawn because of problems with precision and quantitation. No available immunoassays have been checked for cross-reactivity with the parent F2-iPs or their potential metabolites. GC/MS–based approaches using a heterologous prostaglandin internal standard to estimate circulating total iPs have suggested an increase in lipid peroxidation in patients with chronic ALD (53). However, the complexity of these species and the potential for differences in their recovery and detection represent a limitation of this approach (16). We have favored specific analysis by GC/MS of individual iPs using homologous internal standards (16).
We have now developed assays for members of 2 iP classes, iPF2α-III and iPF2α-VI, using GC/MS. Urinary excretion of these iPs is increased in a wide variety of syndromes of oxidant stress, including cigarette smoking (19) and during reperfusion after coronary ischemia (20, 21). Excretion is also increased in patients with hypercholesterolemia (54, 55). We have immunolocalized iPF2α-III to monocytes and smooth muscle cells in human atherosclerotic plaque (56). The virtue of specific iP analysis has recently been established in a mouse model of atherosclerosis in which both serum cholesterol and urinary iPs are increased. Suppression of urinary iPF2α-VI was used to identify a dosing regimen of vitamin E that suppressed lipid peroxidation while leaving serum cholesterol unchanged (22). Because atherogenesis was reduced by 40%, this approach established the functional importance of lipid peroxidation in this disease.
We previously reported that urinary iPF2α-III is elevated in patients with cirrhosis (57). However, the majority of these patients had HCV disease, and in those with a history of alcohol intake, it was poorly documented. This study extends those findings to elucidate the role of alcohol-induced oxidant stress in both healthy volunteers and in patients with ALD. Excretion of iPF2α-III was increased in patients with established cirrhosis and a history of alcohol abuse. Indeed, the increment in iP excretion was more marked than that observed in cirrhotic patients with a history of HCV. The larger increment that was noted in patients with combined etiology is consistent with the reported additive effect of alcohol and HCV infection to produce a more severe degree of injury (34). Furthermore, excretion of iPs was increased in patients with acute alcoholic liver disease and, in a dose-dependent manner, in healthy volunteers who were administered alcohol (33). Thus, oxidant stress in vivo was increased in patients with acute alcoholic hepatitis and in patients with cirrhosis and a history of alcohol abuse, and was provoked by alcohol administration to healthy volunteers. In addition to the increment in urinary iPF2α-III, we report that excretion of 2,3-dinor-5,6-dihydro-iPF2α-III, which may be formed as a metabolite of iPF2α-III (27), is also increased in chronic ALD. This suggests that the increment in urinary iPF2α-III reflects an increase in its generation in vivo, rather than reduced oxidative metabolism due to impairment of hepatic function. Finally, we have developed a method for analysis of 4-HNE in urine, using GC/MS. Pawlosky et al. (57) have reported that rhesus monkeys fed a diet for 3 years comprising 24% of total daily calories and low in vitamins C and E develop hepatic fibrosis and have elevated levels of iPF2α-III and 4-HNE in plasma. In accordance with these observations, both 4-HNE and iPF2α-VI are significantly elevated in the urine of patients with ALD.
We have previously shown that iPF2α-III may be formed as a minor product of both COX isoforms (17, 32). However, evidence in healthy volunteers and in syndromes of COX activation suggests that these pathways contribute trivially, if at all, to urinary concentrations of this iP (18, 19). Consistent with these observations, vitamin C, but not aspirin, suppressed urinary iPF2α-III, urinary iPF2α-VI, and circulating TBARS in patients with alcoholic cirrhosis.
The mechanisms that underlie the increase in oxidant stress in ALD are unclear. Although alcoholics often consume a diet deficient in antioxidant vitamins, the patients in this study that had chronic ALD were stabilized in the GCRC on a standardized diet for 2 weeks before participation in the study. They also had abstained from alcohol for a minimum of 4 months before being studied. Patients with acute alcoholic hepatitis gave a history of drinking within 24 hours of admission, but were studied 1–3 days after admission. Patients with ALD had higher urinary iPs, lower levels of endogenous vitamins E and C, and a significant fall in urinary iPs on vitamin C in contrast to those with HCV cirrhosis. An attempt was made to standardize the clinical severity of the groups of patients with chronic liver disease in this study. However, in patients with more severe HCV cirrhosis, in whom endogenous antioxidant defenses were depleted, supplementation with exogenous vitamin E significantly reduced their elevated urinary iPF2α-III (57).
Measured with precision and sensitivity in urine using GC/MS, iPs are chemically stable indices of oxidant stress. This noninvasive approach is readily applicable to clinical trials. The effect of relatively modest alcohol ingestion on urinary iPs in volunteers raises the possibility that during chronic alcohol intake, the vasoconstrictor and proliferative effects of iPs (59, 60) may contribute to the evolution of ALD. Irrespective of this possibility, these noninvasive markers of lipid peroxidation, augmented by the measurement of 4-HNE, may be used to discriminate between the oxidant effects of various forms of alcohol in humans, and to titrate the dosage of antioxidant strategies designed to modify the evolution of cirrhosis in animal models of ALD.
Acknowledgments
This work was supported by grants MO1 RR-00040, HL-4500, T32 DK-07066, and DK-44730 from the National Institutes of Health (NIH), and by AMX-360 NMR Instrument Grant CHE-9013145 from the National Science Foundation. E.A. Meagher holds a Clinical Associate Physician Award from the NIH. G.A. FitzGerald is the Robinette Foundation Professor of Cardiovascular Medicine.
References
- 1.Doll R, Peto R, Hall E, Wheatley K, Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. Br Med J. 1994;309:911–918. doi: 10.1136/bmj.309.6959.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs CS, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 3.Garfinkel L, Boffetta P, Stellman SD. Alcohol and breast cancer: a cohort study. Prev Med. 1988;17:686–693. doi: 10.1016/0091-7435(88)90086-2. [DOI] [PubMed] [Google Scholar]
- 4.Becker U, et al. Prediction of risk of liver disease by alcohol intake, sex and age: a prospective study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 5.Bellentani S, et al. Drinking habits as a cofactor of risk for alcohol induced liver damage. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 7.Britton RS, Bacon BR. Role of free radicals in liver diseases and hepatic fibrosis. Hepatogastroenterology. 1994;41:343–348. [PubMed] [Google Scholar]
- 8.Lieber CS, DeCarli LM. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970;245:2505–2512. [PubMed] [Google Scholar]
- 9.Tsukamoto H. Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol. 1993;10:465–467. doi: 10.1016/0741-8329(93)90066-w. [DOI] [PubMed] [Google Scholar]
- 10.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 11.Nanji AA, French SW. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989;44:223–227. doi: 10.1016/0024-3205(89)90599-7. [DOI] [PubMed] [Google Scholar]
- 12.Smith CV, Anderson RE. Methods for determination of lipid peroxidation in biological samples. Free Radic Biol Med. 1987;3:341–344. doi: 10.1016/s0891-5849(87)80044-8. [DOI] [PubMed] [Google Scholar]
- 13.Morrow JD, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad JA, Morrow JD, Takahashi K, Roberts LJ. Identification of non-cyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J Biol Chem. 1993;268:4161–4169. [PubMed] [Google Scholar]
- 15.Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease [review] Arterioscler Thromb Vasc Biol. 1997;7:2309–2315. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- 16.Lawson, J.A., Rokach, J., and FitzGerald, G.A. 1999. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo [review]. J. Biol. Chem. In press. [DOI] [PubMed]
- 17.Praticò D, Lawson JA, FitzGerald GA. Cyclooxygenase-dependent formation of 8-epi prostaglandin F2α in human platelets. J Biol Chem. 1995;270:9800–9808. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- 18.Praticò D, et al. IPF2α-I: an index of lipid peroxidation in humans. Proc Natl Acad Sci USA. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Delanty N, et al. 8-Epi PGF2α generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 21.Reilly M, et al. Increased generation of the F2 isoprostanes, IPF2α-I and 8-epi PGF2α in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–3320. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- 22.Praticò D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 23.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 24.Smucker R, Block G, Coyle L, Harvin A, Kessler L. A dietary and risk factor questionnaire and analysis system for personal computers. Am J Epidemiol. 1989;129:445–449. doi: 10.1093/oxfordjournals.aje.a115149. [DOI] [PubMed] [Google Scholar]
- 25.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 26.Beresford T, Blow FC, Hiu EM, Singer K, Lucey MR. Comparison of CAGE questionnaire and computer-assisted laboratory profiles in screening for covert alcoholism. Lancet. 1990;336:462–485. doi: 10.1016/0140-6736(90)92022-a. [DOI] [PubMed] [Google Scholar]
- 27.Roberts LJ, II, Moore KP, Zackert WE, Oates JA, Morrow JD. Identification of the major urinary metabolite of the F2-isoprostane, 8-iso-prostaglandin F2α in humans. J Biol Chem. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 28.Ciabrando C, et al. Identification and measurement of endogenous beta-oxidation metabolites of 8-epi-prostaglandin F2α. J Biol Chem. 1999;15:1313–1319. doi: 10.1074/jbc.274.3.1313. [DOI] [PubMed] [Google Scholar]
- 29.Carithers RL, Jr, et al. Methylprednisone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 30.Praticò D, FitzGerald GA. Generation of 8-epiprostaglandin F2α by human monocytes: discriminate production by reactive oxygen species and prostaglandin endoperoxide synthase-2. J Biol Chem. 1996;271:8919–8924. doi: 10.1074/jbc.271.15.8919. [DOI] [PubMed] [Google Scholar]
- 31.Pickett WC, Murphy RC. Enzymatic preparation of carboxyl oxygen-18 labeled prostaglandin F2α and utility for quantitative mass spectrometry. Anal Biochem. 1981;111:115–121. doi: 10.1016/0003-2697(81)90237-2. [DOI] [PubMed] [Google Scholar]
- 32.van Kuijk FJ, Siakotos AN, Fong LG, Stephens RJ, Thomas DW. Quantitative measurement of 4-hydroxyalkenals in oxidized low-density lipoprotein by gas chromatography-mass spectrometry. Anal Biochem. 1995;224:420–424. doi: 10.1006/abio.1995.1060. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan LA, Miler JA, Stein EA. Simultaneous measurement of serum retinol, tocopherols, carotenes, and carotenoids by high performance liquid chromatography. J Clin Lab Anal. 1987;1:147–152. [Google Scholar]
- 34.Baldwin, J.N., and Tong, T.G. 1996. Alcoholism. In Textbook of therapeutics: drug and disease management. 6th edition. E.T. Herfindal and D.R. Gourley, editors. Williams & Wilkins. Baltimore, MD.
- 35.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27:915–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- 36.Marmot MG, Shipley MJ, Rose G, Thomas BJ. Alcohol and mortality: a U-shaped curve. Lancet. 1981;1:580–583. doi: 10.1016/s0140-6736(81)92032-8. [DOI] [PubMed] [Google Scholar]
- 37.Renaud S, Orgeril M. Wine alcohol, platelets and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 38.Tsukamoto H, et al. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouach H, et al. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 40.Tsukamoto H, Towner SJ, Ciofalo LM, French SW. Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology. 1986;6:814–822. doi: 10.1002/hep.1840060503. [DOI] [PubMed] [Google Scholar]
- 41.Aleynik SI, Leo MA, Ma X, Aleynik MK, Lieber CS. Polyenylphosphatidyl-choline (PPC) prevents hepatic lipid peroxidation and attenuates associated injury produced by CCl4 in rats and by alcohol in baboons. Gastroenterology. 1995;108(Suppl.):A1025. (Abstr.) [Google Scholar]
- 42.Lieber CS. Prevention and treatment of liver fibrosis based on pathogenesis. Alcohol Clin Exp Res. 1999;23:944–949. [PubMed] [Google Scholar]
- 43.Leo MA, Rosman AS, Lieber CS. Differential depletion of carotenoids and tocopherol in liver disease. Hepatology. 1993;17:977–986. [PubMed] [Google Scholar]
- 44.Sadrzadeh SM, Nanji AA, Meydani M. Effect of chronic ethanol feeding on plasma and liver alpha- and gamma-tocopherol levels in normal and vitamin E-deficient rats. Relationship to lipid peroxidation. Biochem Pharmacol. 1994;47:2005–2010. doi: 10.1016/0006-2952(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 45.Frezza M, et al. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 46.Loft S, Olesen KL, Dossing M. Increased susceptibility to liver disease in relation to alcohol consumption in women. Scand J Gastroenterol. 1987;22:1251–1256. doi: 10.3109/00365528708996472. [DOI] [PubMed] [Google Scholar]
- 47.Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 48.Rifici VA, Khachadurian AK. Polyphenols found in red wine inhibit cell mediated lipoprotein oxidation. J Invest Med. 1998;46(Suppl.):2907A. (Abstr.) [Google Scholar]
- 49.Hayek T, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 50.Rokach J, et al. The isoprostanes, a new class of natural products: synthesis and biosynthesis. Synthesis. 1998;1:569–580. [Google Scholar]
- 51.Waugh RJ, Murphy RC. Mass spectrometric analysis of four regioisomers of F2-isoprostanes formed by free radical oxidation of arachidonic acid. J Am Soc Mass Spectrom. 1996;7:490–499. doi: 10.1016/1044-0305(95)00709-1. [DOI] [PubMed] [Google Scholar]
- 52.Nanji AA, Khwaja S, Tahan SR, Sadrzadeh SMH. Plasma levels of a novel noncyclooxygenase-derived prostanoid (8-isoprostane) correlate with severity of liver injury in experimental alcoholic liver disease. J Pharmacol Exp Ther. 1994;269:1280–1285. [PubMed] [Google Scholar]
- 53.Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:192–196. [PubMed] [Google Scholar]
- 54.Reilly M, et al. Increased generation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–2828. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- 55.Davi G, et al. In vivo formation of 8-epi-prostaglandin F2α is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–3235. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 56.Praticò D, et al. Localization of distinct F2 isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iuliano L, et al. Enhanced lipid peroxidation in patients with hepatic cirrhosis. J Invest Med. 1998;46:51–57. [PubMed] [Google Scholar]
- 58.Pawlosky RJ, Flynn BM, Salem N., Jr The effects of low dietary levels of polyunsaturates on alcohol-induced liver disease in rhesus monkeys. Hepatology. 1997;26:1386–1392. doi: 10.1002/hep.510260602. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi K, et al. Glomerular action of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2α in the rat: evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992;90:136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukunaga M, et al. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am J Physiol. 1993;264:C1619–C1624. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]