Abstract
CD19 receptor is expressed at high levels on human B-lineage lymphoid cells and is physically associated with the Src protooncogene family protein-tyrosine kinase Lyn. Recent studies indicate that the membrane-associated CD19-Lyn receptor-enzyme complex plays a pivotal role for survival and clonogenicity of immature B-cell precursors from acute lymphoblastic leukemia patients, but its significance for mature B-lineage lymphoid cells (e.g., B-lineage lymphoma cells) is unknown. CD19-associated Lyn kinase can be selectively targeted and inhibited with B43-Gen, a CD19 receptor-specific immunoconjugate containing the naturally occurring protein-tyrosine kinase inhibitor genistein (Gen). We now present experimental evidence that targeting the membrane-associated CD19-Lyn complex in vitro with B43-Gen triggers rapid apoptotic cell death in highly radiation-resistant p53-Bax- Ramos-BT B-lineage lymphoma cells expressing high levels of Bcl-2 protein without affecting the Bcl-2 expression level. The therapeutic potential of this membrane-directed apoptosis induction strategy was examined in a scid mouse xenograft model of radiation-resistant high-grade human B-lineage lymphoma. Remarkably, in vivo treatment of scid mice challenged with an invariably fatal number of Ramos-BT cells with B43-Gen at a dose level < 1/10 the maximum tolerated dose resulted in 70% long-term event-free survival. Taken together, these results provide unprecedented evidence that the membrane-associated anti-apoptotic CD19-Lyn complex may be at least as important as Bcl-2/Bax ratio for survival of lymphoma cells.
Full text
PDF

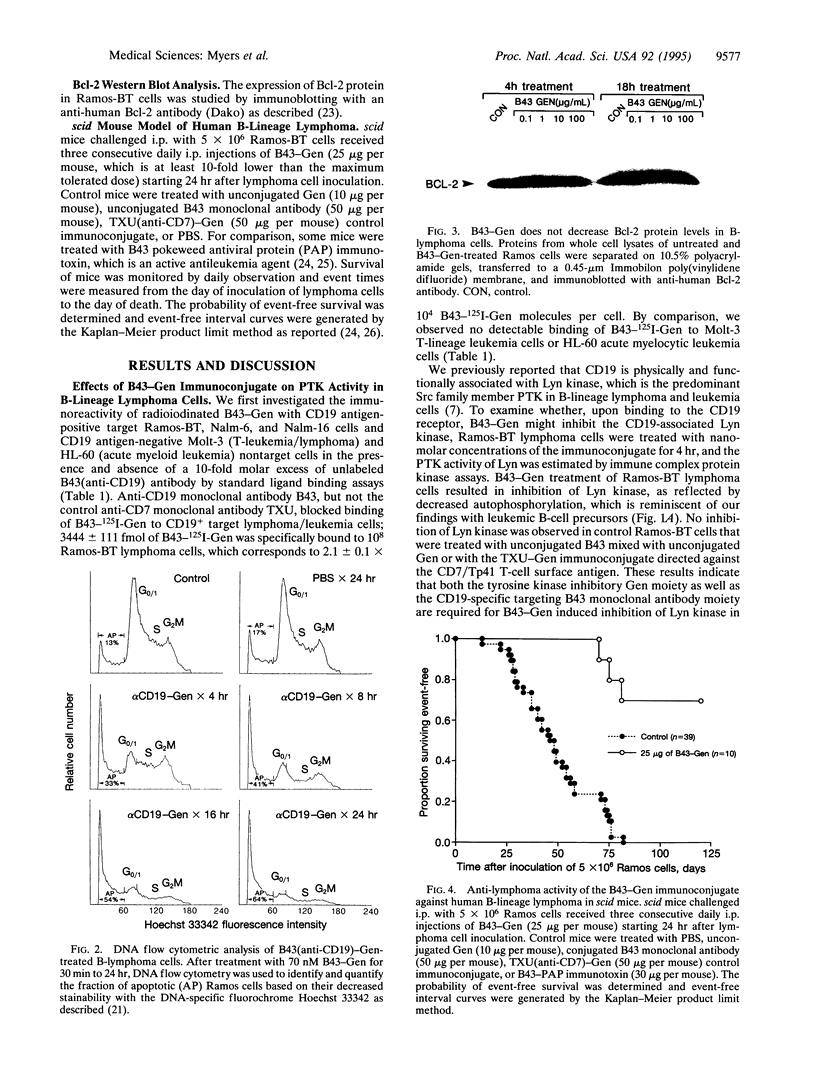
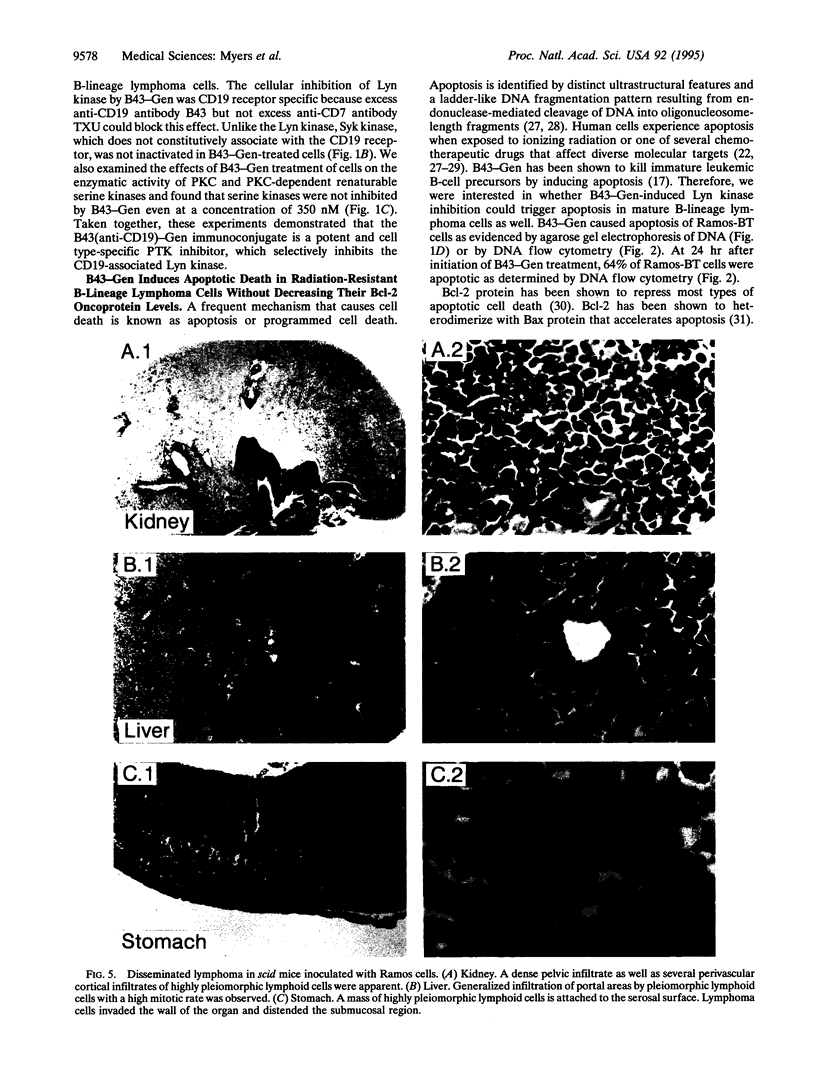

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bolen J. B., Rowley R. B., Spana C., Tsygankov A. Y. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992 Dec;6(15):3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- Bradbury L. E., Goldmacher V. S., Tedder T. F. The CD19 signal transduction complex of B lymphocytes. Deletion of the CD19 cytoplasmic domain alters signal transduction but not complex formation with TAPA-1 and Leu 13. J Immunol. 1993 Sep 15;151(6):2915–2927. [PubMed] [Google Scholar]
- Callard R. E., Rigley K. P., Smith S. H., Thurstan S., Shields J. G. CD19 regulation of human B cell responses. B cell proliferation and antibody secretion are inhibited or enhanced by ligation of the CD19 surface glycoprotein depending on the stimulating signal used. J Immunol. 1992 May 15;148(10):2983–2987. [PubMed] [Google Scholar]
- Carter R. H., Fearon D. T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992 Apr 3;256(5053):105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- Chalupny N. J., Kanner S. B., Schieven G. L., Wee S. F., Gilliland L. K., Aruffo A., Ledbetter J. A. Tyrosine phosphorylation of CD19 in pre-B and mature B cells. EMBO J. 1993 Jul;12(7):2691–2696. doi: 10.1002/j.1460-2075.1993.tb05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kiener P. A., Rankin B. M., Burkhardt A. L., Schieven G. L., Gilliland L. K., Rowley R. B., Bolen J. B., Ledbetter J. A. Cross-linking of Fc gamma receptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993 Nov 15;268(32):24442–24448. [PubMed] [Google Scholar]
- Ledbetter J. A., Rabinovitch P. S., June C. H., Song C. W., Clark E. A., Uckun F. M. Antigen-independent regulation of cytoplasmic calcium in B cells with a 12-kDa B-cell growth factor and anti-CD19. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1897–1901. doi: 10.1073/pnas.85.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler L. M., Anderson K. C., Marti G., Bates M., Park E., Daley J. F., Schlossman S. F. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983 Jul;131(1):244–250. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Otani H., Erdos M., Leonard W. J. Tyrosine kinase(s) regulate apoptosis and bcl-2 expression in a growth factor-dependent cell line. J Biol Chem. 1993 Oct 25;268(30):22733–22736. [PubMed] [Google Scholar]
- Stamenkovic I., Seed B. CD19, the earliest differentiation antigen of the B cell lineage, bears three extracellular immunoglobulin-like domains and an Epstein-Barr virus-related cytoplasmic tail. J Exp Med. 1988 Sep 1;168(3):1205–1210. doi: 10.1084/jem.168.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Isaacs C. M. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol. 1989 Jul 15;143(2):712–717. [PubMed] [Google Scholar]
- Tuveson D. A., Carter R. H., Soltoff S. P., Fearon D. T. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993 May 14;260(5110):986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Burkhardt A. L., Jarvis L., Jun X., Stealey B., Dibirdik I., Myers D. E., Tuel-Ahlgren L., Bolen J. B. Signal transduction through the CD19 receptor during discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1993 Oct 5;268(28):21172–21184. [PubMed] [Google Scholar]
- Uckun F. M., Chelstrom L. M., Finnegan D., Tuel-Ahlgren L., Manivel C., Irvin J. D., Myers D. E., Gunther R. Effective immunochemotherapy of CALLA+C mu+ human pre-B acute lymphoblastic leukemia in mice with severe combined immunodeficiency using B43 (anti-CD19) pokeweed antiviral protein immunotoxin plus cyclophosphamide. Blood. 1992 Jun 15;79(12):3116–3129. [PubMed] [Google Scholar]
- Uckun F. M., Evans W. E., Forsyth C. J., Waddick K. G., Ahlgren L. T., Chelstrom L. M., Burkhardt A., Bolen J., Myers D. E. Biotherapy of B-cell precursor leukemia by targeting genistein to CD19-associated tyrosine kinases. Science. 1995 Feb 10;267(5199):886–891. doi: 10.1126/science.7531365. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Fauci A. S., Heerema N. A., Song C. W., Mehta S. R., Gajl-Peczalska K., Chandan M., Ambrus J. L. B-cell growth factor receptor expression and B-cell growth factor response of leukemic B cell precursors and B lineage lymphoid progenitor cells. Blood. 1987 Oct;70(4):1020–1034. [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K. J., Kersey J. H., Houston L. L., Vallera D. A. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986 Feb 1;163(2):347–368. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Gesner T. G., Song C. W., Myers D. E., Mufson A. Leukemic B-cell precursors express functional receptors for human interleukin-3. Blood. 1989 Feb;73(2):533–542. [PubMed] [Google Scholar]
- Uckun F. M. Immunotoxins for the treatment of leukaemia. Br J Haematol. 1993 Nov;85(3):435–438. doi: 10.1111/j.1365-2141.1993.tb03329.x. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Jaszcz W., Ambrus J. L., Fauci A. S., Gajl-Peczalska K., Song C. W., Wick M. R., Myers D. E., Waddick K., Ledbetter J. A. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988 Jan;71(1):13–29. [PubMed] [Google Scholar]
- Uckun F. M., Kersey J. H., Haake R., Weisdorf D., Nesbit M. E., Ramsay N. K. Pretransplantation burden of leukemic progenitor cells as a predictor of relapse after bone marrow transplantation for acute lymphoblastic leukemia. N Engl J Med. 1993 Oct 28;329(18):1296–1301. doi: 10.1056/NEJM199310283291802. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Ledbetter J. A. Immunobiologic differences between normal and leukemic human B-cell precursors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8603–8607. doi: 10.1073/pnas.85.22.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M. Regulation of human B-cell ontogeny. Blood. 1990 Nov 15;76(10):1908–1923. [PubMed] [Google Scholar]
- Uckun F. M., Stewart C. F., Reaman G., Chelstrom L. M., Jin J., Chandan-Langlie M., Waddick K. G., White J., Evans W. E. In vitro and in vivo activity of topotecan against human B-lineage acute lymphoblastic leukemia cells. Blood. 1995 May 15;85(10):2817–2828. [PubMed] [Google Scholar]
- Uckun F. M., Tuel-Ahlgren L., Song C. W., Waddick K., Myers D. E., Kirihara J., Ledbetter J. A., Schieven G. L. Ionizing radiation stimulates unidentified tyrosine-specific protein kinases in human B-lymphocyte precursors, triggering apoptosis and clonogenic cell death. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9005–9009. doi: 10.1073/pnas.89.19.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]





