Significance
Game theory is used throughout the social and biological sciences to study behavior in social interactions. Recent research suggests an important role for the dopamine neurotransmitter system in these types of decisions. This study used a competitive game to study how people varied in their decision-making processes and related these differences in the set of genes that carry out biological functions required for dopaminergic functioning. We found that genes differentially expressed in separate brain regions influenced distinct components of people’s decision-making processes and that a surprising degree of consistency exists with what is known at the brain level about how people make decisions in social interactions.
Keywords: neuroeconomics, experience-weighted attraction, eigenSNPs
Abstract
Game theory describes strategic interactions where success of players’ actions depends on those of coplayers. In humans, substantial progress has been made at the neural level in characterizing the dopaminergic and frontostriatal mechanisms mediating such behavior. Here we combined computational modeling of strategic learning with a pathway approach to characterize association of strategic behavior with variations in the dopamine pathway. Specifically, using gene-set analysis, we systematically examined contribution of different dopamine genes to variation in a multistrategy competitive game captured by (i) the degree players anticipate and respond to actions of others (belief learning) and (ii) the speed with which such adaptations take place (learning rate). We found that variation in genes that primarily regulate prefrontal dopamine clearance—catechol-O-methyl transferase (COMT) and two isoforms of monoamine oxidase—modulated degree of belief learning across individuals. In contrast, we did not find significant association for other genes in the dopamine pathway. Furthermore, variation in genes that primarily regulate striatal dopamine function—dopamine transporter and D2 receptors—was significantly associated with the learning rate. We found that this was also the case with COMT, but not for other dopaminergic genes. Together, these findings highlight dissociable roles of frontostriatal systems in strategic learning and support the notion that genetic variation, organized along specific pathways, forms an important source of variation in complex phenotypes such as strategic behavior.
Game theory describes strategic interactions where success of players’ actions depends on those of coplayers and has been instrumental in the quantitative analysis of social behavior (1, 2). In humans, there is substantial evidence from laboratory experiments that, in addition to learning about rewards and punishments available in the environment, people also anticipate and respond to competitive or cooperative actions of other participants (1, 3). Specifically, learning in strategic settings can be parsimoniously characterized using two learning rules across a wide range of strategic contexts and experimental conditions: (i) reinforcement-based learning (RL) through trial and error and (ii) belief-based learning through anticipating and responding to the actions of others (1, 4).
Only in the past decade, however, have researchers begun to characterize the biological substrates underlying decision making in game theoretic settings (3). At the neural level, applications of functional neuroimaging, combined with formal mathematical models of behavior, have elucidated key roles of the frontostriatal circuits and putative dopaminergic mechanisms in guiding social behavior (5, 6). In particular, during competitive strategic interactions activity in the prefrontal cortex (PFC) was found to be better accounted for by models that incorporate higher-order inferences about opponents’ behavior, rather than simpler forms of trial-and-error reinforcement learning (5, 7).
In comparison, despite the explosion in availability of genomic data as well as known heritability of economic behavior, we know much less about the molecular genetic underpinnings of the intermediate neural mechanisms (8, 9). Here we sought to shed light on the neurogenetic basis of strategic behavior by exploiting known variation in the set of genes within the dopamine pathway and studying their effects on behavior. A genetic pathway consists of a group of functionally related genes that mediate a particular biological process (e.g., dopamine functioning) (Fig. 1B) (10). For example, the DAT1 gene encodes the dopamine transporter (DAT), whose function is to remove dopamine from the synaptic cleft, thus terminating the signal of the neurotransmitter. Although these pathways are abstractions of complex biological processes that have no simple start or end points, they have been invaluable to researchers because they capture and organize our knowledge in a parsimonious and tractable manner (10).
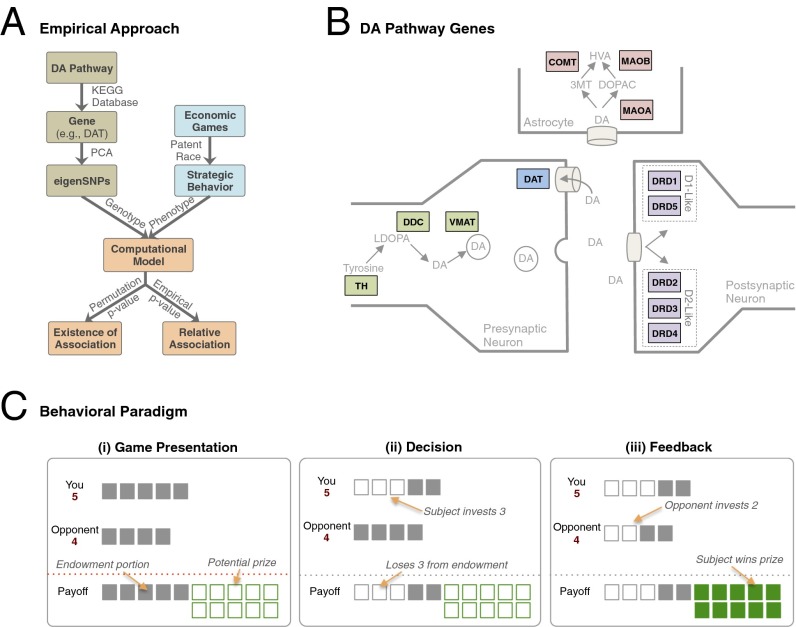
Fig. 1.
(A) Starting with the Kyoto Encyclopedia of Genes and Genomes dopamine pathway, we selected a set of genes directly related to dopamine functioning (Table 1). For each gene, we took all available SNPs in the GWA dataset and conducted PCA to account for correlation due to LD. On the phenotype side, we used a laboratory-based economic game (patent race). These were then combined in our computational model, where parameters were estimated using maximum likelihood. Hypothesis testing was done using two different methods: (i) permutation P values under the null hypothesis of no association and (ii) empirical P values by comparing to randomly matched genes in the GWA dataset. (B) Dopamine pathway genes are represented in a stylized version of the dopamine synapse and include dopamine genes directly involved in synthesis (green), uptake (blue), and metabolism (pink) and receptors (violet). Certain details, such as presynaptic autoreceptors, have been omitted for clarity. (C) In the patent race, subjects were presented with (i) the game with information regarding their endowment, the endowment of the opponent, and the potential prize. (ii) Subjects inputted the decision (self-paced) by pressing a button mapped to the desired investment amount from the initial endowment. (iii) After a brief delay, the opponent’s choice was revealed. If the subject’s investment was strictly more than those of the opponent, the subject won the prize; otherwise, the subject lost the prize. In either case, the subject kept the portion of the endowment not invested.
Studying the molecular basis of social and strategic behavior in the context of economic games and specific biological pathways offers a number of important advantages. First, the dopamine pathway seems to play a fundamental role in social behavior in all known vertebrate species by virtue of its involvement in (social) reward and decision-making processes (11, 12). Unlike economic phenotypes such as wealth that are far removed from the proximate biological mechanisms, models of strategic learning provide highly parsimonious and mathematically rigorous descriptions of behavior and have been shown to have substantial predictive validity at both behavioral and neural levels (3, 9).
Importantly, focusing on specific biological pathways allows us to exploit existing knowledge regarding the biological mechanisms underlying behavior, and in particular known relationships between gene and brain. The dopamine system is known to exhibit remarkable regional variation in expression levels of genes coding for the set of enzymes, receptors, and transporters involved in dopamine functioning (Fig. 1C) (11, 13). In the PFC, where DAT1 expression is low, genes regulating enzymatic breakdown, in particular catechol-O-methyl transferase (COMT) and to a lesser extent isoforms of the monoamine oxidase (MAO) genes, are important determinants of dopamine flux (14). In contrast, these genes have much less impact on striatal dopamine levels, where DAT1 expression is high (15). On the receptor side, regional variation results from distribution of dopamine receptor types (16). Receptors of the D1 family, D1 and D5, are expressed throughout the brain. In contrast, receptors in the D2 family exhibit more regional specificity: D2 receptors are expressed primarily in the dorsal striatum, D3 receptors in the ventral striatum, including nucleus accumbens but less so in dorsal striatum, and D4 receptors in the frontal cortex and limbic regions (16).
These differences have known important consequences for cognition and behavior (14, 15) but to our knowledge have not been explored in strategic or social behavior in humans. Here we studied the behavior of 218 participants in a multistrategy competitive game, the so-called patent race, in a stylized but well-characterized setting of a population with many anonymously interacting agents and low probability of re-encounter (Fig. 1C and SI Materials and Methods) (5).
Moreover, using this game, previous neuroimaging results have been able to disaggregate trial-by-trial variation in neural responses along frontostriatal circuits to distinct computational signatures of RL and belief learning processes (5). In particular, whereas the medial PFC was found to respond selectively to belief-based inputs and reflected individual differences in degree of engagement of belief learning, striatal activity was correlated with both reinforcement and belief-based signals, suggesting possible convergence of these signals in the striatum (5). Building upon these findings, therefore, we investigated (i) the degree to which variation in strategic learning can be captured by variation in genes in the dopamine pathway and (ii) the extent to which these variations are organized along dissociable prefrontal and striatal neural systems.
Consistent with our goal of capturing overall variation in dopamine functioning and its effects on strategic learning, we included not only exonic polymorphisms that exert direct effects on protein sequence and functions but also those in intronic and UTRs, as well as variable-number tandem repeats (VNTRs) and synonymous exonic polymorphisms (Fig. 1B and SI Materials and Methods). Although long thought to have no biological effect, intronic and synonymous mutations are now known to affect gene translational and transcriptional efficiencies, and consequently protein levels, as opposed to altering protein structure itself (17). To account for correlated regressors owing to linkage disequilibrium (LD), we took a dimension reduction approach and created a set of eigenSNPs using principal components analysis (PCA) (Fig. 1B and Materials and Methods). Compared with traditional candidate gene approaches, this multilocus approach can be used to detect association between a phenotype and groups of SNPs (genes) and is more efficient when there exist weaker but coordinated effects arising from multiple SNP markers (10).
Results
Model-Based Characterization of Behavior.
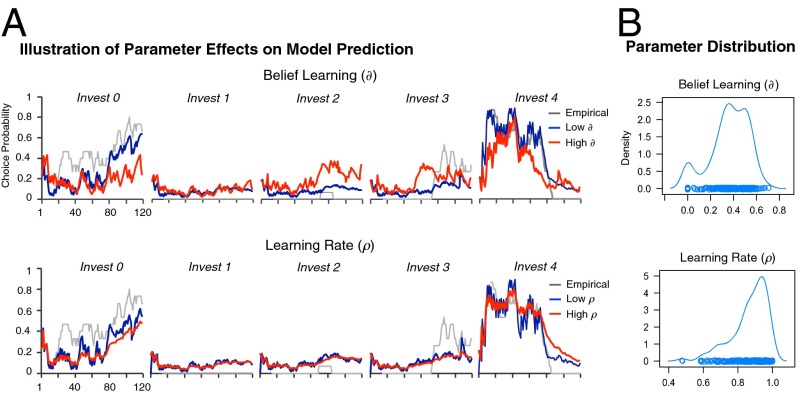
To characterize individual variation in choice behavior, we adopted a hybrid model—experience weighted attraction (EWA)—that combines and nests both reinforcement and belief learning (1, 4). Specifically, choice behavior in EWA is governed by two key parameters capturing distinct computational components involved in updating players’ action values and has been highly successful in explaining observations across a wide range of games at both behavioral and neural levels (Materials and Methods, Fig. S1, and Table S1). First, the belief learning parameter δ captures a player’s sensitivity toward actions of opponents as opposed to received payoffs. An individual responding only to received payoffs is captured by δ = 0, corresponding to a pure RL player, whereas a player driven entirely by belief learning is captured by δ = 1. Using choice behavior and simulations for a single subject as illustration (Fig. 2A, Upper), a larger belief learning parameter is most saliently reflected in an increased probability of investing 2 and 3 in rounds 70–100, corresponding to periods when strong players invested 1–2 units with increased likelihood. Second, learning rate ρ governs how action values depreciate over time, capturing the degree to which players are sensitive to more recent observations relative to past ones. A player highly sensitive to recent observations, captured by a low ρ, will therefore adapt faster, for example during rounds 80–100 in Fig. 2A, Lower, whereas a player with a large ρ is similarly sensitive to recent and past observations and adapts more slowly.
Fig. 2.
(A) Illustration of parameter effects on model predictions using a single subject in the weak role. Actual subject choice behavior is presented as a time series smoothed using a 15-round bin average. Simulated choice probabilities were calculated using different belief learning and learning rate parameters. Each panel refers to a separate investment level. Note that a change in belief learning primarily affects the distribution of investment choices; in contrast, a change in the learning rate primarily affects the smoothness of adaption across rounds. (B) Density plot of individual-level belief learning (δ) and learning rate (ρ) parameters.
Consistent with previous studies of strategic learning (4, 5), we found that the hybrid model significantly outperformed both reinforcement and belief-based learning models alone as measured using the likelihood ratio test (P < 0.001 for each) as well as the Akaike information criterion penalizing for number of parameters (P < 0.001 for each). To capture individual variation in behavior, we estimated a saturated (fixed effects) model where each participant was coded with individual belief learning and learning rate parameters, (mean = 0.36, SD = 0.17) and (mean = 0.86, SD = 0.10), respectively (Fig. 2B). This generated a set of individual-level belief learning parameters that we use in subsequent genetic analyses. Furthermore, we found that the individual estimates of the two parameters were largely uncorrelated (Spearman ρ = 0.13), which allowed us to characterize potential separable genetic contributions to behavior.
Characterization of Genetic Variation in Dopamine Pathway.
We next sought to summarize variation of genes along the dopamine pathway. Using PCA and a 90% cutoff rule (Materials and Methods), we found SNPs within gene were highly correlated, consistent with nearby markers being in strong LD (Table 1). For example, four eigenSNPs contained 91% of the variation in the COMT gene, for which our genome-wide association (GWA) data contained 17 SNPs that exceeded a minor allele frequency (MAF) threshold of 0.1 (Table 1). Critically for our goal of identifying contribution of individual dopamine genes to behavior, we found using canonical correlation analysis that variation across genes are essentially uncorrelated (mean = 1.9 ± 1.4%; Table S2), consistent with distant marker being in weak LD.
Table 1.
Summary of dopamine pathway genes and parameter estimates
| Belief learning (δ) |
Learning rate (ρ) |
||||||||||||
| Function | Gene | SNPs | PCs | % Var | k* | LLR | punc | pperm | pemp | LLR | punc | pperm | pemp |
| Synthesis | TH | 2 | 2 | 100 | 1,089 | 0.98 | 0.374 | 0.898 | 0.888 | 0.9 | 0.400 | 0.913 | 0.932 |
| DDC | 20 | 4 | 90 | 162 | 3.45 | 0.141 | 0.943 | 0.963 | 29.3 | 0.000 | 0.257 | 0.278 | |
| VMAT2 | 16 | 8 | 92 | 22 | 31.1 | 0.000 | 0.420 | 0.410 | 12.3 | 0.002 | 0.969 | 1.000 | |
| Transport/clearance | DAT1 | 9 | 5 | 93 | 73 | 9.35 | 0.002 | 0.821 | 0.808 | 68.6 | 0.000 | 0.024 | 0.027 |
| VNTR | 0.22 | 0.510 | 0.796 | 34.5 | 0.000 | 0.008 | |||||||
| Joint | 9.7 | 0.007 | 0.877 | 86.9 | 0.000 | 0.014 | |||||||
| COMT | 17 | 4 | 91 | 191 | 57.3 | 0.000 | 0.005 | 0.005 | 49.8 | 0.000 | 0.038 | 0.031 | |
| MAOA | 22 | 1 | 94 | 4 | 12.3 | 0.000 | 0.082 | 0.25 | 0.2 | 0.495 | 0.834 | 1.000 | |
| VNTR | 3.5 | 0.136 | 0.687 | 17.4 | 0.000 | 0.498 | |||||||
| Joint | 32.1 | 0.000 | 0.029 | 32.7 | 0.000 | 0.691 | |||||||
| MAOB | 28 | 3 | 95 | 70 | 32.7 | 0.000 | 0.035 | 0.029 | 1.2 | 0.000 | 0.585 | 0.586 | |
| Receptor | DRD1 | 5 | 3 | 99 | 275 | 9.22 | 0.000 | 0.522 | 0.510 | 9.76 | 0.000 | 0.639 | 0.647 |
| DRD2 | 17 | 5 | 94 | 159 | 24.8 | 0.000 | 0.295 | 0.296 | 67.5 | 0.000 | 0.036 | 0.025 | |
| DRD3 | 6 | 3 | 97 | 289 | 2.49 | 0.174 | 0.881 | 0.917 | 23.9 | 0.000 | 0.219 | 0.201 | |
| DRD4 | 1 | 1 | 100 | 975 | 3.40 | 0.009 | 0.335 | 0.396 | 9.46 | 0.000 | 0.193 | 0.183 | |
| VNTR | 11.9 | 0.000 | 0.247 | 12.5 | 0.000 | 0.314 | |||||||
| Joint | 12.0 | 0.000 | 0.398 | 25.8 | 0.000 | 0.207 | |||||||
PCs, principal components; % Var, percent of total variance captured by included PCs; punc, P value using likelihood ratio test; pperm, permutation P value (see SI Materials and Methods); pemp, empirical P value (see SI Materials and Methods); TH, tyrosine hydroxylase; VMAT2, vesicular monoamine transporter 2.
Number of matched comparison genes chosen from the GWA dataset.
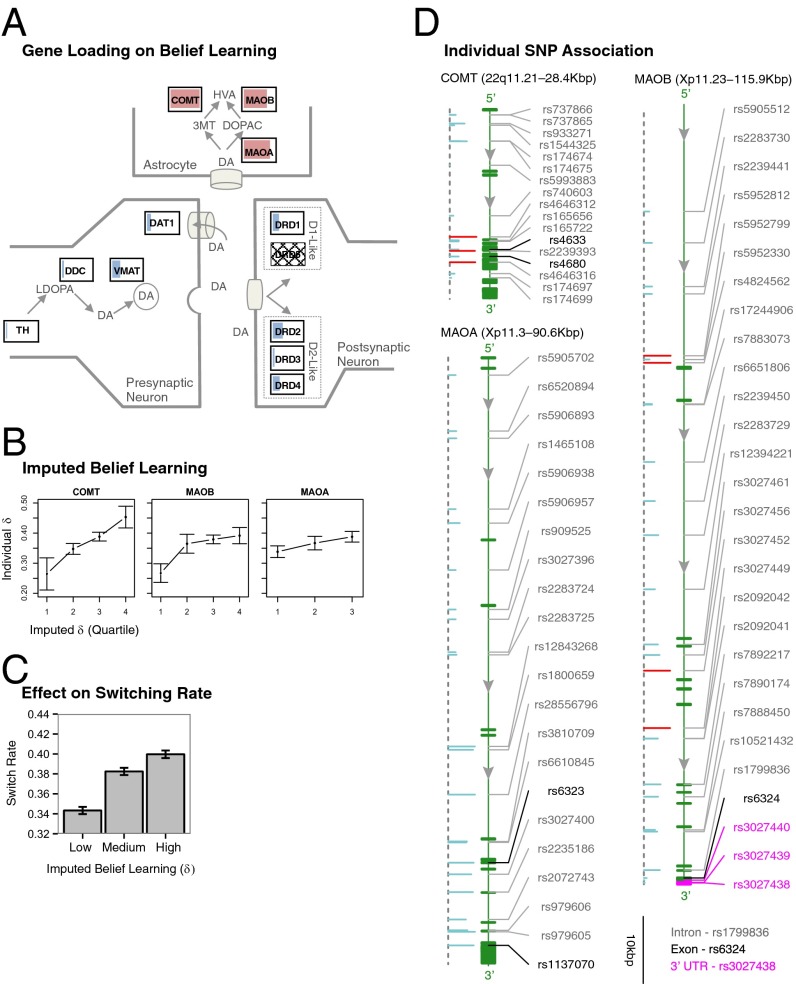
Prefrontal Dopamine Genes Selectively Contribute to Variation in Belief Learning.
Having summarized overall variation at both behavioral and genetic levels, we sought to identify genetic contributions to individual variation in the degree of belief learning, captured by the parameter δ. Specifically, for each gene in the dopamine pathway, we allowed the δ parameter in our computational model to vary according to the set of associated eigenSNPs, which can be interpreted as genetic variation that affects neural sensitivity to specific reward-related inputs (Materials and Methods). For example, in the case of the MAOB gene, in addition to the population parameter δ we included three additional parameters, {δE1,δE2,δE3}, corresponding to the three eigenSNPs of the MAOB gene (Table 1 and SI Materials and Methods). Motivated by our previous neuroimaging findings suggesting PFC involvement in belief learning (5), we first examined genes known to regulate prefrontal dopamine levels. Specifically, we included the COMT gene and the two monoamine oxidase genes (MAO A and B) that code for isoforms of enzymes that break down extracellular dopamine. Functionally, MAOB is known to preferentially metabolize dopamine, whereas MAOA is more selective toward serotonin (18). Animal experiments using COMT knock-out mice suggest that MAO contributed to ∼20% of dopamine degradation, approximately half that of COMT (18).
Using permutation tests to assess the null hypothesis of no association, we found that allowing belief learning to vary according to COMT genotype significantly improved model fit (permutation P < 0.005, Table 1). In addition, and consistent with animal data on relative efficiency of the different enzymes in dopamine breakdown, we found that MAOB exerted a significant (permutation P < 0.05) albeit weaker influence on belief learning in terms of both significance as well as improvement in log likelihood (Table 1 and Materials and Methods). For MAOA, which has greater affinity to serotonin compared with dopamine (18), we found an even weaker association (permutation P < 0.1; Table 1). Interestingly, incorporating the 30-base repeat sequence VNTR, a highly studied polymorphism in the promoter region that has been implicated in behavioral traits such as aggression (19), together with SNP data significantly improved the model (permutation P < 0.05; Table 1). We then characterized genes that primarily modulated striatal genes, as well as other genes in our dopamine pathway, including receptors and those involved in dopamine synthesis. In contrast, we did not find any of these to significantly improve model fit (minimum permutation P < 0.30; Table 1).
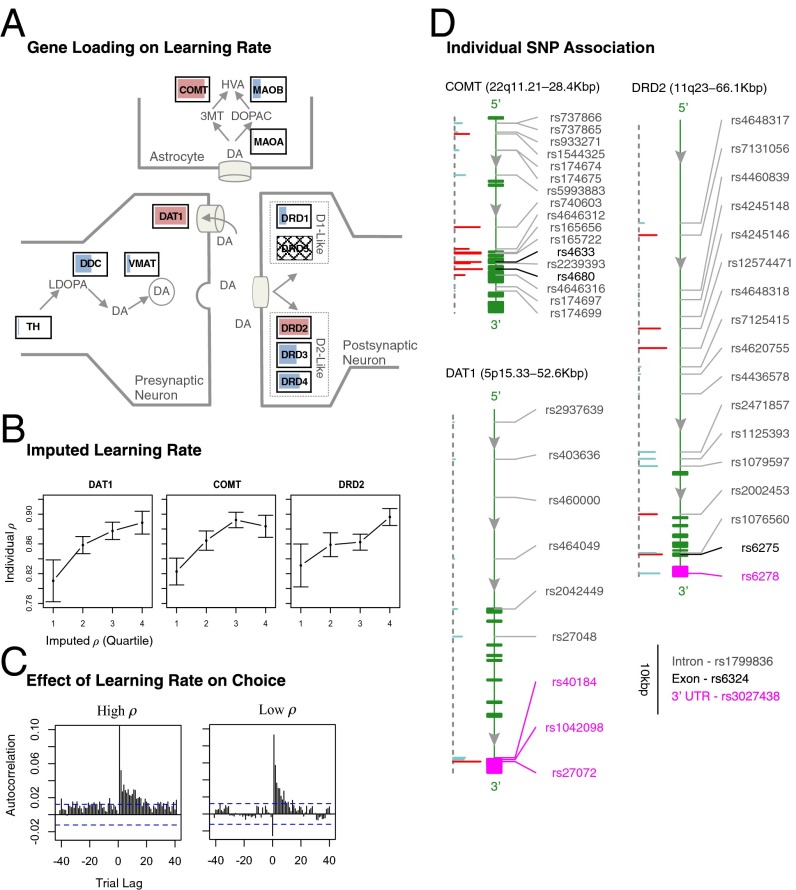
Multiplexed Contribution of Dopamine Genes to Variation in Learning Rate.
Next, we characterized genes that explained the other key parameter of our computational model—the learning rate ρ. As with the belief learning parameter, we allowed ρ to vary according the set of eigenSNPs in each dopamine gene. Motivated by our hypothesis that learning rate is primarily regulated by striatal functioning, we first characterized dopamine genes that disproportionately affected striatal dopamine functioning, in particular DAT1, and also dopamine receptor (DR) D2 and DRD3 (20, 21). We found that the DAT1 gene was significantly associated with variation in individual learning rates (permutation P < 0.05; Table 1). The existence of association is further enhanced by the fact that the DAT1 VNTR was also significantly associated with ρ (permutation P < 0.01; Table 1), as well as being jointly significant (permutation P < 0.02; Table 1).
Next we characterized dopamine receptor genes DRD2 and DRD3, which primarily affect dorsal and ventral striatal dopamine functioning, respectively. In previous neuroimaging results, activity in the dorsal striatum, in particular the putamen, but not the ventral striatum, was correlated with both reinforcement and belief prediction errors. However, there are reasons to suspect that the ventral striatum may also be involved, because it is widely implicated in neuroimaging studies on reward and decision making (22). We found that DRD2 was significantly associated with the learning rate (permutation P < 0.05), but not DRD3 (permutation P > 0.2; Table 1).
We then characterized genes that primarily affect prefrontal dopamine functioning. However, there are studies that suggest COMT exerts an indirect effect on striatal dopamine (23, 24). In contrast, we are not aware of human or animal studies demonstrating such indirect effects for MAO. Intriguingly, we found that COMT variation was significantly associated with the learning rate (P < 0.05), but not for either MAOA or MAOB (P > 0.5 for each; Table 1). Finally, we characterized dopamine synthesis genes as well as receptor genes that do not exhibit regional specificity and did not find that these genes are significantly associated with behavior (minimum P = 0.19; Table 1).
Distribution of Association Across the Genome.
In the above results we have focused on permutation tests to guard against spurious associations compared with a random genotype. It is possible, however, that our evidence of association does not rise above the background association compared with the genome at large. To investigate this possibility, we compared the fit of models using dopamine genes relative to matching non-dopamine genes in the GWA dataset to generate an “empirical” null distribution (SI Materials and Methods). Strikingly, despite varying sizes of the comparison gene sets (Table S3), we found that the empirical null distributions, and consequently P values, tracked the permutation null closely in all dopamine genes tested (Table 1).
In addition, to formally compare effect size of prefrontal and striatal dopamine genes on choice behavior, we contrasted, using a bootstrap procedure, the mean eigenSNPs coefficient for COMT and MAOB against those for DAT1 and DRD2 (SI Materials and Methods). We found a strong dissociation between the two gene sets in the belief learning parameter δ, such that prefrontal genes exerted a significantly greater effect than striatal dopamine genes (bootstrap P = .004). In contrast, likely owing to the significant contribution of COMT to learning rate, we only found a weak dissociation in favor of striatal genes for the ρ parameter (bootstrap P = .097).
Mapping Genetic Variation to Behavioral Variation.
Here we performed two types of model checks to illustrate how estimated genetic effects captured variation at the level of model parameters and choice behavior. First, to illustrate estimated genetic contribution to variation in the belief learning parameter δ, we imputed, for each individual, a gene-weighted parameter estimates by multiplying δEj estimates with individual eigenSNP scores (SI Materials and Methods). Using these imputed parameters, we found that the variation in consistently reflected individual differences summarized by from the saturated model using fixed effects (Fig. 3B). For example, for the COMT gene, we found that the lowest quartile of estimates corresponded to a mean value of 0.26, compared with 0.45 for the highest quartile. Using the same procedure for the learning rate parameter ρ, we found a similar relationship with the gene-weighted parameter estimates . For the DAT1 gene, for example, the lowest quartile of estimates corresponded to a mean value of 0.81, compared with 0.89 for the highest quartile (Fig. 4B).
Fig. 3.
(A) Permutation tests showed significant association of COMT and MAOB with individual variation in belief learning (P < 0.005 and P < 0.05, respectively), whereas MAOA was marginally significant (P < 0.1). No other genes were found to be significantly associated. Lengths of bars indicate likelihood improvement per principal component. Colors represent permutation P values. (B) To capture how genetic variation affects the degree of belief learning, we split, for each gene, the gene-weighted values into quartiles (terciles in the case of MAOA owing to limited genetic variation) and calculated the mean individual-level values (error bars are SEM). The former was calculated using the calibrated eigenSNP model, whereas the latter was estimated using fixed effects in a saturated model. (C) Effects of gene variation on switching rate were calculated as the probability that participants switched investment amounts between trials t and t+1, separated by the imputed gene-weighted learning parameter .Consistent with model predictions, individuals with higher values switched at a higher rate than those with lower values. (D) Individual SNP associations for belief learning parameter δ are shown on DNA strand. Thick green bands indicate exonic regions, purple bands UTRs, and otherwise intronic regions. Bar lengths indicate log-likelihood ratio (LLR) improvement, where red indicates significance at P < 0.05 and blue indicates nonsignificance. Scale is given at bottom right.
Fig. 4.
(A) Permutation tests showed significant association of DAT1, DRD2, and COMT with individual variation in learning rate (all P < 0.05). Color coding and interpretation are identical to Fig. 3A. (B) To capture how genetic variation affects the learning rate, we split gene-weighted values into quartiles and calculated the mean individual-level values (error bars are SEM). (C) Effect of genetic variation on choice behavior is illustrated using correlogram of investment level at time t with payoff deviation at time t± lag. The x axis represents different lags in number of rounds. Blue dashed lines indicate theoretical 95% confidence interval. Note the higher correlation values between periods 15–30 in high-ρ relative to low-ρ individuals. (D) Individual SNP associations for learning rate parameter ρ are shown on DNA strand. Color coding and interpretation are identical to Fig. 3D.
Next, we sought to quantify and visualize estimated genetic effects at the choice behavior level. First, as shown in previous theoretical and behavioral studies (4, 5), a key feature distinguishing belief and reinforcement learning is an increased rate of switching strategies across rounds by belief learners, owing to sensitivity of belief learners to the action of opponents (5). Consistent with this model prediction, we found that individuals with higher values indeed exhibited higher switching rates compared with those with lower values (Fig. 3C). Second, we sought to capture the influence of learning rate on choice behavior. Under EWA, individuals with lower values should be influenced by more recent outcomes compared with those with higher values (4, 5). We therefore calculated a correlogram to measure how investment levels were influenced by payoff information at different lags (Fig. 4C). Consistent with model predictions, we found that for high individuals past experiences continue to exert an effect well into 20 rounds in to the future, whereas for low individuals this effect drops sharply after 10 rounds (Fig. 4C).
Distribution of Association Across SNPs.
Next we sought to understand how identified behavioral effects are distributed across SNPs. Of SNPs in dopamine genes with a significant association with behavioral parameters, only one is associated with changes in protein sequence: rs4680 (Val158Met) in COMT, a polymorphism that causes changes in the catalytic activity of COMT and has been widely studied in the literature (Figs. 2D and 3D) (25). Seven other SNPs produce synonymous mutations that do not cause a change in the protein sequence. The vast majority of SNPs are located in intron sequences (60/79) or in the 3′ or 5′ UTRs of the target genes (11/79) (Figs. 3D and 4D). Furthermore, we computed the fit improvement for each SNP in the implicated genes (Figs. 3D and 4D and SI Materials and Methods). Consistent with the idea of multiple SNPs each exerting a weak influence on behavior, we observed that most SNPs exerted a small effect on our two learning parameters (Figs. 3D and 4D). We also explored the complementary notion that interactions between SNPs account for variation, finding qualitatively similar results (Table S4 and SI Results).
Discussion
There is now increasingly detailed knowledge of two physical substrates responsible for behavior: the brain and the genome (9). Here, we build upon these insights to shed light on the complex process by which genomic variation influences behavior through its impact on neural circuitry. Importantly, and similar to previous discussions in the computational neuroimaging literature, results from our computational approach should not be interpreted as an exercise to “localize Greek letters” in the genome (26). Just as it would be erroneous for neuroimaging researchers to interpret a particular brain region as a “prediction error module” or “a region encoding δ,” in the same way it would be mistaken to interpret our results as suggesting that dopamine genes function as “belief learning genes” or “genes encoding δ.” Rather, our goal is to test hypotheses regarding how variations in dopamine genes serve to constrain and regulate the computational properties of neural circuits subserved by dopamine.
More specifically, our results add to growing evidence that dopamine mechanisms critically underlie a wide class of value-based decision making across both social and nonsocial settings (11). They are consistent with a mechanism whereby neural computations related to the anticipation and response of actions of others are governed by dopamine genes involved in signal termination in PFC—primarily COMT and MAOA/B. Because prefrontal dopamine clearance mechanisms are slower than striatal, the PFC is thought to be sensitive to tonic, but not phasic, dopamine (27). This has led to the hypothesis that tonic prefrontal dopamine levels are important in maintaining active representations of relevant information and mediate learning after negative consequences (28). In our case, these functions might be relevant for maintaining a model of the partner’s behavior and learning through belief-based prediction errors (5).
Furthermore, and in common with basic RL mechanisms, our results suggest that variation in proteins that affect dopamine signaling and clearance mechanisms in the striatum influence valuation of past experience in action selection. A possible mechanism proposed in the literature suggests that these variants modulate dopamine concentrations in meso-temporal scale (tens of milliseconds) by regulating phasic dopaminergic signaling in the striatum. In contrast, the effects of COMT on the learning rate is likely indirect and primarily operates through its effects on the balance of dopamine levels in frontostriatal circuits (23). In humans, these hypotheses can be tested indirectly by pharmacological manipulations on protein function (29).
At the molecular level, the explanatory power of polymorphisms not affecting protein structure raises the intriguing possibility that biochemical differences are caused by polymorphisms that do not directly affect protein sequence and function. For example, synonymous mutations in COMT have been shown to affect catalytic efficiency through regulation of translational efficiency (30). More generally, synonymous mutations are known to affect mRNA stability, transcriptional machinery binding affinity, and splicing, which can have significant consequences, as evidenced by the fact that they are often under selective pressure. UTR and intronic mutations, however, are likely to fall in upstream/downstream regulatory sequences where they could affect translation efficiency and protein levels (31).
Methodologically, we provide a tractable approach to connect gene and behavior by leveraging our knowledge of the intermediate neural mechanisms. Unlike unconstrained hypothesis-free tests on individual polymorphisms, focusing on biological pathways allows us to relate systems of functionally related genes to putative mechanistic models of behavior. That is, we explicitly acknowledge the inherent tension regarding our current state of knowledge (8, 10). On the one hand, we now have an immense and growing base of knowledge regarding the biological basis of economic behavior, which can explain observation across multiple biological levels and, in some cases, across multiple species (3, 8). On the other hand, our knowledge is highly incomplete. Pathway analysis based on GWA data thus can complement these studies by testing multiple dopaminergic pathway genes for association with decision-making tasks rather than solely relying on an individual SNP approach (8, 10).
Taken together, these findings highlight the dissociable roles of dopamine genes in strategic learning and support the notion that variations in molecular mechanisms, organized along specific genetic pathways and brain circuits, form an important source of variation in complex phenotypes such as strategic behavior. More generally, these data suggest the intriguing possibility that, although complex phenotypes such as economic behavior are highly polygenic, the information is sparsely distributed across the genetic code and concentrated within specific functionally defined biological pathways.
Materials and Methods
Participants.
A total of 218 (103 female) undergraduates were recruited from the Behavioral × Biological Economics and Social Sciences (B2ESS) Laboratory at the National University of Singapore. A total of 217 (103 female) were included in the final analysis after one subject was excluded owing to genotype unavailability (SI Materials and Methods).
Procedure.
Participants completed 240 rounds of the patent race game in sessions of 18–24 participants, alternating between strong and weak roles over 120 rounds, counterbalanced. Informed consent was obtained as approved by the Internal Review Board at the National University of Singapore (SI Materials and Methods).
Genotype Selection and Preprocessing.
For each dopamine gene (Fig. 1B), SNPs were included according to hg18 coordinates, and with MAF exceeding 0.1. DRD5 was excluded from the final analysis owing to lack of SNP variation in our sample. For details, including coding of VNTRs and X-chromosome genes, see SI Materials and Methods.
Computational Modeling.
Denote as strategy k (investment level) for player i, si (t) the chosen strategy by player i at period t, and s−i(t) the chosen strategy of the opponent at period t For each round, player i receives possible payoff for playing strategy in period t, and the subjective value for playing strategy k is governed by two parameters and updates according to the following:
| [1] |
where N(t) = ρi ⋅ N(t−1)+1 captures how depreciates over time (for details, see SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank M. Monakhov, T. Rong, and X. Zhang for assistance in data collection. This research was supported by National Institute of Mental Health Grant R01 MH098023 (to M.H.), the Ministry of Education, Singapore (S.Z., R.P.E., and S.H.C.), and the AXA Research Fund (R.P.E. and S.H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316259111/-/DCSupplemental.
References
- 1.Camerer C. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 2.Hofbauer J, Sigmund K. Evolutionary Games and Population Dynamics. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 3.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9(7):545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camerer CF, Ho T. Experience-weighted attraction learning in games: A unifying approach. Econometrica. 1999;67(4):827–874. [Google Scholar]
- 5.Zhu L, Mathewson KE, Hsu M. Dissociable neural representations of reinforcement and belief prediction errors underlie strategic learning. Proc Natl Acad Sci USA. 2012;109(5):1419–1424. doi: 10.1073/pnas.1116783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456(7219):245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampton AN, Bossaerts P, O’Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci USA. 2008;105(18):6741–6746. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65(6):831–844. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: Social life in molecular terms. Nat Rev Genet. 2005;6(4):257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nat Rev Genet. 2010;11(12):843–854. doi: 10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- 11.O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336(6085):1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- 12.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 13.Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30(2):215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Nemoda Z, Szekely A, Sasvari-Szekely M. Psychopathological aspects of dopaminergic gene polymorphisms in adolescence and young adulthood. Neurosci Biobehav Rev. 2011;35(8):1665–1686. doi: 10.1016/j.neubiorev.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci. 2009;12(8):1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortés R, Camps M, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: Autoradiographic distribution of D1 and D2 sites in Parkinson syndrome of different etiology. Brain Res. 1989;483(1):30–38. doi: 10.1016/0006-8993(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 17.Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12(10):683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- 18.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: From genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31(3):120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Gainetdinov RR, Caron MG. Monoamine transporters: From genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 21.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 22.Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Annu Rev Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- 23.Dreher J-C, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci USA. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yacubian J, et al. Gene–gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci USA. 2007;104(19):8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Lindenberg A, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11(9):867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly JX, Mars RB. Computational neuroimaging: Localising Greek letters? Comment on Forstmann et al. Trends Cogn Sci. 2011;15(10):450. doi: 10.1016/j.tics.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: An in vivo voltammetric study. J Neurosci. 1994;14(1):442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci USA. 2007;104(41):16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nackley AG, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314(5807):1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee S, Pal JK. Role of 5′- and 3′-untranslated regions of mRNAs in human diseases. Biol Cell. 2009;101(5):251–262. doi: 10.1042/BC20080104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.