Significance
The small G protein ADP-ribosylation factor 6 (Arf6) and the exchange factor for Arf6 (EFA6) are involved in endocytic vesicular transport, but their precise functions remain unclear. The Bin/Amphiphysin/Rvs (BAR) domain containing endophilin is known to couple fission to uncoating of the clathrin-coated vesicles. Here, we identified endophilin as a direct interactor of EFA6. We analyzed in vitro the effect of this interaction on EFA6 guanine nucleotide exchange factor activity, and on endophilin lipid binding and remodeling activities. We then studied in vivo the role of the two proteins in transferrin receptor endocytosis. Our results suggest a model in which EFA6 recruits endophilin on flat areas of endocytic zones of the plasma membrane, where endophilin cooperates with EFA6 to activate Arf6 and regulate clathrin-mediated endocytosis.
Keywords: small GTP-binding proteins, membrane curvature, vesicular trafficking
Abstract
Members of the Arf family of small G proteins are involved in membrane traffic and organelle structure. They control the recruitment of coat proteins, and modulate the structure of actin filaments and the lipid composition of membranes. The ADP-ribosylation factor 6 (Arf6) isoform and the exchange factor for Arf6 (EFA6) are known to regulate the endocytic pathway of many different receptors. To determine the molecular mechanism of the EFA6/Arf6 function in vesicular transport, we searched for new EFA6 partners. In a two-hybrid screening using the catalytic Sec7 domain as a bait, we identified endophilin as a new partner of EFA6. Endophilin contains a Bin/Amphiphysin/Rvs (BAR) domain responsible for membrane bending, and an SH3 domain responsible for the recruitment of dynamin and synaptojanin, two proteins involved, respectively, in the fission and uncoating of clathrin-coated vesicles. By using purified proteins, we confirmed the direct interaction, and identified the N-BAR domain as the binding motif to EFA6A. We showed that endophilin stimulates the catalytic activity of EFA6A on Arf6. In addition, we observed that the Sec7 domain competes with flat but not with highly curved lipid membranes to bind the N-BAR. In cells, expression of EFA6A recruits endophilin to EFA6A-positive plasma membrane ruffles, whereas expression of endophilin rescues the EFA6A-mediated inhibition of transferrin internalization. Overall, our results support a model whereby EFA6 recruits endophilin on flat areas of the plasma membrane to control Arf6 activation and clathrin-mediated endocytosis.
The ADP ribosylation factor family, which includes six members, is known to regulate different stages of vesicular trafficking (reviewed in refs. 1, 2). The most abundant isoform, Arf1, controls the membrane trafficking at the level of the Golgi apparatus by regulating in a GTP-dependent manner the recruitment of the COPI coat complex (3–5) and the two clathrin adaptors AP-1 (6, 7) and GGAs (8) onto the Golgi membranes. By activating lipid-modifying enzymes, Arf1 is able to change the lipid composition of the donor compartment membrane, thus facilitating membrane dynamic (9–12). ADP-ribosylation factor 6 (Arf6), the most distant isoform, is thought to regulate plasma membrane and endosomal trafficking. Similarly to Arf1, Arf6 activates the PLD (13, 14) and the type I PI4P5K (15, 16) to produce, respectively, phosphatidic acid, which is a fusogenic lipid, and phosphatidylinositol 4–5 bisphosphate, which is known to regulate clathrin-dependent endocytosis. Arf6 and PIP2 cooperate (at least in vitro) to recruit AP-2 onto lipid membranes, suggesting a role for Arf6 in the formation of clathrin-coated pits (17). In addition to this putative role during the initial steps of internalization, Arf6 has been shown to interact and recruit Nm23-H1, a protein believed to control the dynamin-dependent fission of endocytic vesicles (18). These different observations have clarified the molecular basis of the role of Arf6 in clathrin-dependent and -independent endocytosis. Arf6 has been shown to be involved in the internalization of different cargos, such as β1 integrin, E-cadherin, MHC class I, G protein-coupled receptors, and poly-Ig receptor (19–22). Moreover, EFA6, the Arf6 specific exchange factor, has been shown recently to activate Arf6 in response to β2AR stimulation in a β-arrestin–dependent manner (23). This ligand-mediated activation of Arf6 couples stimulation and Arf6-dependent trafficking of the G protein-coupled receptor. In addition to its role in internalization, Arf6 also seems to be required for the recycling of endosomes. Indeed, activated Arf6 controls the fast recycling of the transferrin receptor (Tfn-R) via two effectors, JIP3/4 (24) and the Sec10 subunit of the exocyst (25), and the recycling of the β2AR, probably through Rab4 activation (23). Also, Arf6 has been involved in the recycling of the IL2 receptor α-subunit, syndecan, integrin β1, and MHC class I (19, 26–28).
EFA6 belongs to the Sec7 domain-containing protein family that acts as guanine nucleotide exchange factor (GEF) for Arf proteins (reviewed in ref. 29). In humans, the EFA6 protein family contains four isoforms, and shares a common domain organization consisting of a Sec7 domain bearing the catalytic activity, a pleckstrin homology domain responsible for the plasma membrane localization by interacting with PIP2 and F-actin, a C-terminal region containing a putative coiled-coil motif and two proline rich motifs responsible for F-actin reorganization, and an N-terminal domain of unknown function whose size and primary sequence are the least conserved across the four isoforms. EFA6 is highly selective for Arf6, and is known to coordinate plasma membrane trafficking with actin cytoskeleton remodeling (30). EFA6 interacts directly with F-actin (31) and α-actinin (32), and its overexpression leads to the formation of F-actin rich microvilli at the plasma membrane (30, 33). EFA6 is involved in the endocytic/recycling transport of several membrane proteins such as the Tfn-R, β2AR and K+ channel Twik1, and in the assembly of the tight junction in epithelial cells (23, 34–36). However, even though EFA6 functions are starting to be uncovered, little is known regarding its regulation.
Endophilins are members of the large and heterogeneous family of Bin/Amphiphysin/Rvs (BAR) domain proteins. They are encoded by endophilin 1–3 (i.e., A1-3) and B1-2 genes (reviewed in ref. 37). They function in synaptic vesicle recycling, membrane receptor trafficking, and different processes that require membrane remodeling. Endophilin BAR domain is part of the N-BAR subfamily (that contains an N-terminal amphipathic helix) that folds into a crescent-shaped dimer able to sense membrane curvature in vitro and to induce the deformation and tubulation of liposomes (reviewed in refs. 38, 39). The N-terminal BAR domain of endophilin is followed after a short linker by a C-terminal SH3 domain. This SH3 domain has been shown to bind dynamin and synaptojanin, two proteins involved in endocytic vesicle scission and uncoating, respectively (40–42). Although endophilin has been extensively investigated in vitro, its precise role in the cell has yet to be clarified.
To unravel at the molecular level the function of the EFA6/Arf6 pathway in membrane trafficking, we have looked for new partners of EFA6. Here, we identified the endophilin N-BAR domain as a direct interactor of the EFA6A Sec7 domain. We analyzed in vitro the effect of this interaction on the GEF activity of EFA6A, and on the lipid binding and remodeling activities of endophilin. We then studied in vivo the role of the two proteins in the endocytosis of the Tfn-R. Our results suggest a model in which EFA6 recruits endophilin on flat areas of endocytic zones of the plasma membrane, where endophilin cooperates with EFA6 to activate Arf6 and to regulate clathrin-mediated endocytosis.
Results
Endophilin Interacts with EFA6A.
To uncover regulators of the nucleotide exchange factor activity of EFA6A, we performed a yeast two-hybrid screen by using its catalytic Sec7 domain as a bait. We identified several clones encoding for endophilin B1. GST pull-down experiments using lysates from transfected cell confirmed the interaction and showed that EFA6A could interact with endophilin A1, A2, and B1 isoforms (Fig. S1). From these experiments, we concluded that EFA6A interacts with members of the two endophilin subfamilies A and B.
Endophilin Directly Interacts with EFA6A and Stimulates Its Nucleotide Exchange Activity for Arf6.
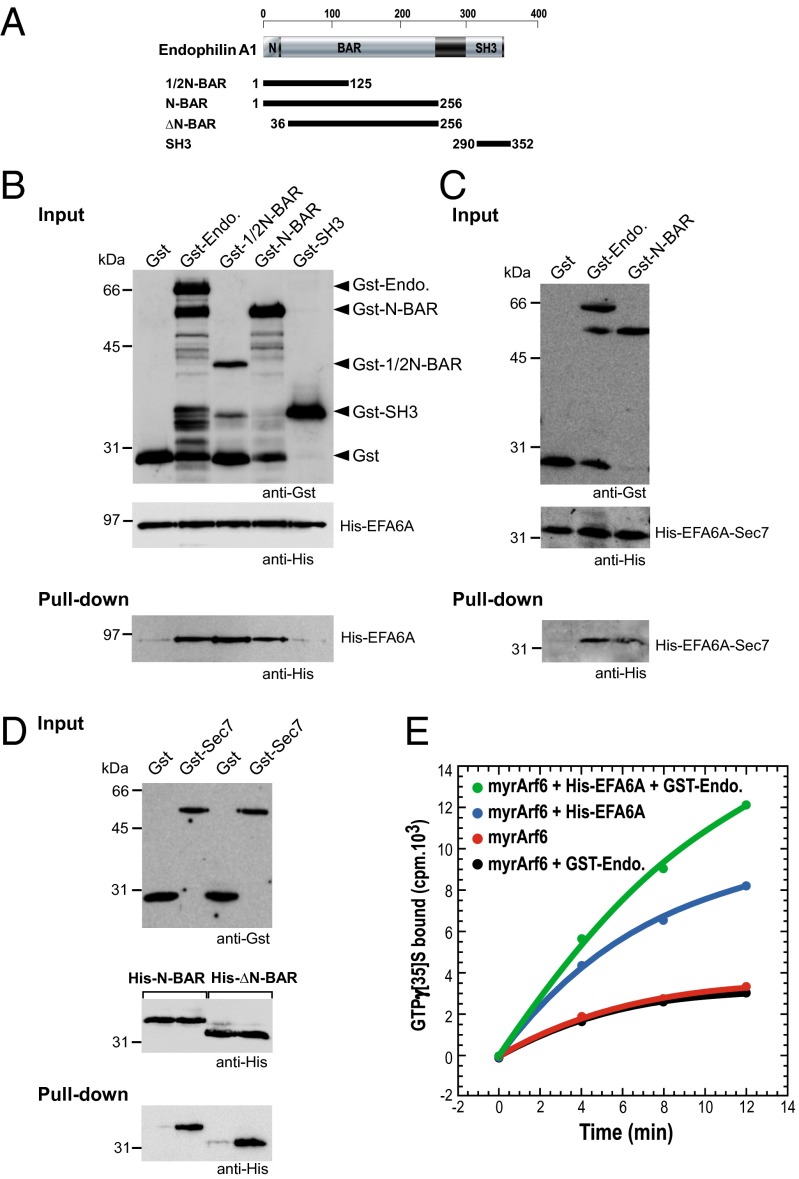
Next, we used purified recombinant proteins and different constructs of endophilin A1 (Fig. 1A) to show that His-EFA6A interacts directly with the first 125 aa of the N-BAR domain (1/2-N-BAR; Fig. 1B), known to retain the activity of lipid binding and tubule formation (43). As the Sec7 domain of EFA6A was used as bait in the two-hybrid screen, we confirmed that the purified recombinant Sec7 domain interacted with the GST-N-BAR (Fig. 1C). Moreover, we observed that the deletion of the N-terminal helix (residues 1–36) of the N-BAR did not abolish the interaction with the Sec7 domain (Fig. 1D). Considered together, these data demonstrated a direct and specific binding of the Sec7 domain of EFA6A to a subdomain (residues 36–125) of the N-BAR domain of endophilin. We then studied the specificity of the interaction by using two other purified BAR domain-containing proteins. In contrast to GST-endophilin, neither GST-arfaptin nor GST-amphiphysin were able to interact directly with His-EFA6ASec7 (Fig. S2). It suggests that the primary sequence, together with the 3D structure, is responsible for the interaction with EFA6A-Sec7 domain.
Fig. 1.
Endophilin directly interacts with EFA6 and stimulates its GEF activity on Arf6. (A) Schematic representation of endophilin A1 and the different GST constructs used in this study. (B and C) GST pull-down of purified His-tagged EFA6A (B) or EFA6A-Sec7 domain (C) by different constructs of endophilin A1 fused to GST. (D) GST pull-down of purified His-tagged N-BAR or ΔN-BAR domain by EFA6-Sec7 domain fused to GST. (E) Kinetics of [35S]GTPγS binding to purified and myristoylated Arf6 (2 μM) were measured (Methods) in the presence of phospholipid vesicles and in the presence or the absence of purified His-tagged EFA6A (∼200 nM) and endophilin A1 fused to GST (2 μM). The results are representative of at least three independent experiments.
As endophilin interacted with the catalytic Sec7 domain, we investigated whether it could modulate the GEF activity of EFA6A on Arf6. We examined in vitro the kinetics of the spontaneous and His-EFA6A catalyzed Arf6 activation in the presence or absence of GST-endophilin (Fig. 1E). We carried out our assay in conditions closest to normal physiology; that is, at the surface of large liposomes and by using myristoylated Arf6. The presence of endophilin did not affect the spontaneous activation of Arf6, whereas it strongly stimulated that catalyzed by EFA6A. These results suggested first that EFA6A binds simultaneously Arf6 and endophilin, and second that, by interacting directly with the Sec7 domain, the endophilin acts as a positive regulator of the GEF activity.
The EFA6A-Sec7 Domain Competes with Lipids to Bind the N-BAR Domain.
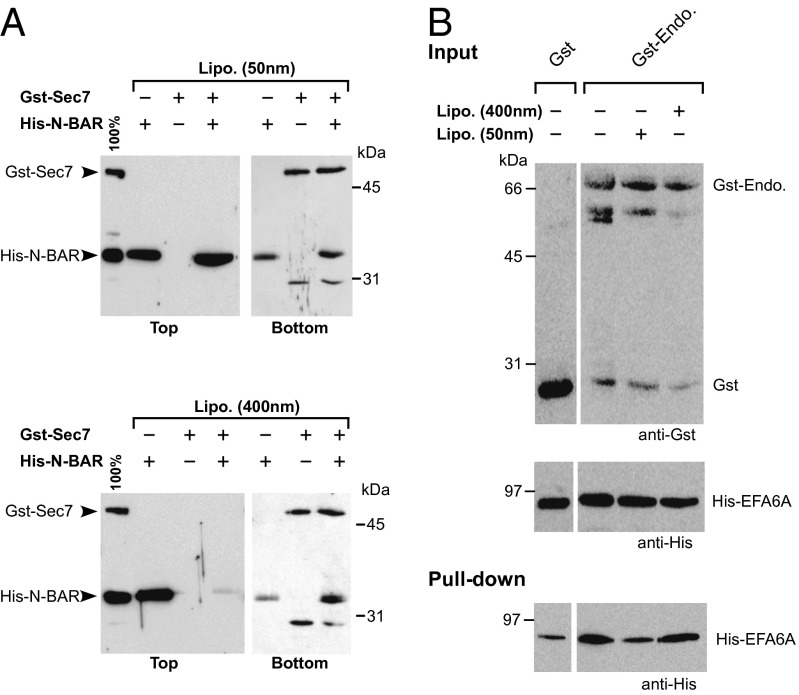
As N-BAR domains are essentially known to interact with curved lipid membranes, we examined whether the Sec7 domain could affect the binding of endophilin N-BAR domain to lipid vesicles of different sizes. To evaluate in a direct manner the binding of endophilin N-BAR and EFA6A-Sec7 to liposomes, we used a flotation assay in which the lipids and the associated proteins are recovered by centrifugation at the top of a sucrose gradient. We tested two sizes of liposomes: large liposomes with a diameter of ∼0.4 μm mimicking flat membranes, and small liposomes (∼0.05 μm in diameter) simulating endocytic vesicles. Although the affinity of the N-BAR is known to be higher for curved membranes, in our conditions, we found the N-BAR to be associated with both kinds of vesicles, whereas, as expected, the Sec7 domain did not bind to either liposomes (Fig. 2A). Surprisingly, in the presence of the Sec7 domain, the N-BAR was found to be associated only with small liposomes, indicating that the Sec7 domain inhibited the binding of the N-BAR to the large liposomes. These data demonstrated that the endophilin N-BAR could not interact simultaneously with lipid membranes and EFA6A-Sec7. These results were strengthened by a GST pull-down experiment using purified recombinant proteins in the presence of liposomes. Fig. 2B shows that GST-endophilin recovered EFA6A even in the presence of large liposomes (∼0.4 μm). However, in the presence of small vesicles (∼0.05 μm), GST-endophilin was not able to retain EFA6A (Fig. 2B).
Fig. 2.
EFA6-Sec7 domain and highly curved lipid membranes compete to bind endophilin N-BAR domain. (A) Flotation assay. EFA6A-Sec7 fused to GST (1.5 μM) or His-tagged endophilin N-BAR (4 μM) or a mixture of the two proteins were incubated with small (50 nm) or large (400 nm) phospholipid liposomes (1 mM). The sample suspension was adjusted to 30% sucrose and then overlaid with two cushions of decreasing sucrose density (Methods). After centrifugation, the top (lipids and associated proteins) and bottom (unbound material) fractions were analyzed by SDS/PAGE. Proteins were stained with SYPRO Orange. (B) GST pull-down of purified His-tagged EFA6A by endophilin A1 fused to GST in the absence or the presence of small (50 nm) or large (400 nm) liposomes. The results shown are representative of at least three independent experiments.
Overall, these data demonstrated that the EFA6A-Sec7 domain competes with lipids for binding to the N-BAR. Furthermore, they demonstrated that, under our experimental conditions, highly curved lipid membranes dissociate the EFA6A-Sec7/N-BAR complex, whereas the Sec7 domain disrupts the interaction of N-BAR with flat membranes. In other words, the interaction between EFA6 and endophilin is regulated by the membrane curvature.
The Endophilin N-BAR–Induced Deformation and Tubulation of Large Liposomes Is Inhibited by the EFA6A-Sec7 Domain.
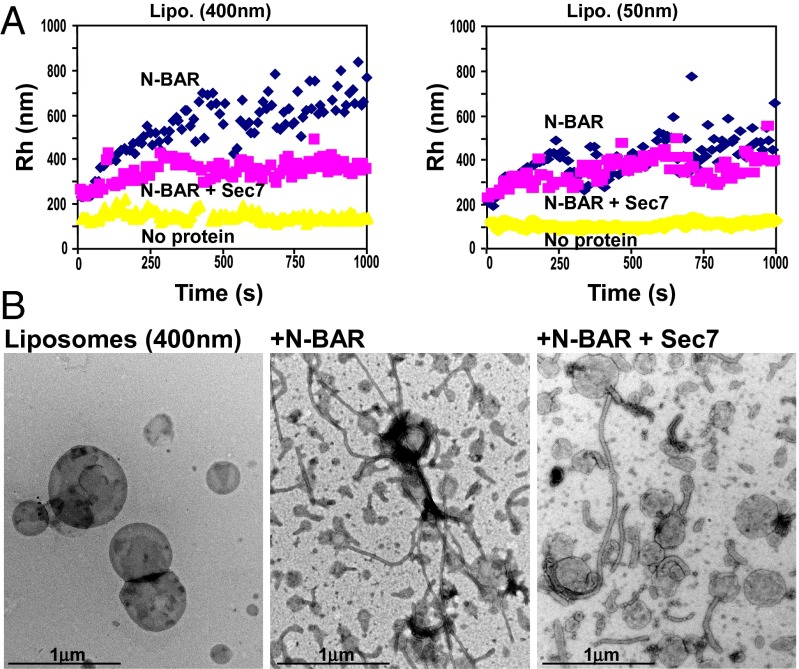
We then asked whether EFA6A-Sec7 could affect the function of the endophilin N-BAR by looking at its ability to induce the deformation and tubulation of liposomes. By dynamic light scattering, it is possible to determine the radius [i.e., hydrodynamic radius (Rh)] of the liposomes in solution. Fig. 3A shows that the presence of the N-BAR (Fig. 3A, blue diamonds) induced an increase over time of the Rh of large (Fig. 3A, Left) and small (Fig. 3A, Right) lipid vesicles in comparison with vesicles alone (Fig. 3A, yellow triangles), verifying that the N-BAR affects the form and the size of the liposomes. We used negative stain EM to visualize the change of size and to determine the nature of the deformation. As shown in Fig. 3B, a 20-min incubation of purified N-BAR domain with large liposomes led to a strong tubulation of nearly all liposomes, which then disappeared or became much smaller (Fig. 3B, Center). Interestingly, we observed that the addition of the Sec7 domain (Fig. 3B, pink squares) inhibited the N-BAR–dependent increase of the Rh of only the large liposomes (Fig. 3A). This result was confirmed by EM analysis. When the Sec7 and the N-BAR domains were added to the large lipid vesicles, only few tubules were formed, and numerous liposomes looked similar to the control ones in terms of size and shape (Fig. 3B, Right).
Fig. 3.
EFA6-Sec7 inhibits the endophilin N-BAR–induced aggregation and tubulation of large liposomes. (A) Effect of His N-BAR and GST-EFA6A-Sec7 on the aggregation and tubulation of liposomes. Large (400 nM, Left) or small (50 nM, Right) liposomes incubated alone (yellow triangles) or in the presence of His-N-BAR (blue diamonds) or His-N-BAR and GST-EFA6A-Sec7 (pink squares) were analyzed in real time by dynamic light scattering (Methods). (B) EM image of large liposomes (400 nm) incubated alone (Left) or in the presence of His-N-BAR (Center) or His-N-BAR and GST-EFA6A-Sec7 (Right). (Scale bar, 1 μM.) The results shown are representative of three separate experiments.
Thus, collectively, these results indicated that EFA6A-Sec7, by interacting with the endophilin N-BAR, inhibits its ability to generate membrane curvature. However, when membrane curvature is high, the Sec7 does not interfere with N-BAR–dependent tubulation. These observations confirmed that the Sec7 domain could not displace the complex of the N-BAR domain with highly curved membrane.
EFA6A Induces the Recruitment of Endophilin to the Plasma Membrane.
We then investigated the biological function of the EFA6/endophilin interaction. First, we examined the localization of both proteins in HeLa cells by immunofluorescence. As previously published (30, 31), EGFP-EFA6A localized essentially to the plasma membrane, and particularly in membrane ruffles and F-actin–rich microvilli-like structures induced upon its expression (Fig. 4A). In contrast, myc-tagged endophilin A2 was found mostly in the cytoplasm (Fig. 4B). When the proteins were coexpressed (Fig. 4 C and D), we noticed that a large portion of endophilin was found in EFA6A-positive structures of the plasma membrane, suggesting that EFA6A recruits endophilin to the plasma membrane. It should be noted that the EFA6A-induced structures were not affected by the presence of endophilin. Thus, the direct interaction described in vitro between the Sec7 domain of EFA6A and the N-BAR domain of endophilin could account for the EFA6A-mediated recruitment of endophilin to the plasma membrane. Furthermore, in light of our in vitro results, the interaction should preferentially occur in flat zones of the plasma membrane. Regardless, these data identified endophilin as a novel EFA6-interacting protein at the plasma membrane.
Fig. 4.
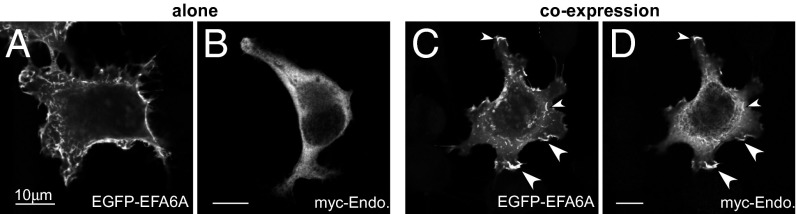
Coexpression with EFA6 induces the redistribution of endophilin to the plasma membrane. BHK-21 cells were transfected with EGFP-EFA6A (A) or with myc-endophilinA2 (B) or both (C and D). After fixation, the cells were processed for immunofluorescence (Methods). The images shown are representative of at least five separate experiments.
Endophilin Rescues the EFA6A-Induced Inhibition of Transferrin Internalization.
We have previously shown that the overexpression of EFA6A inhibited Tfn uptake and caused redistribution of the Tfn-R to the cell surface by an unknown mechanism (30). In light of our new results described here, and because endophilin is known to be a key regulator of clathrin-dependent endocytosis, one explanation could be that the overexpressed EFA6A inhibited the formation of endocytic vesicles, and thereby Tfn internalization, by sequestering the endogenous endophilin on flat regions of the plasma membrane. To test this hypothesis, we examined the internalization of fluoresceinated human Tfn in TRV-b1 cells [a CHO-derived cell overexpressing the human Tfn-R (44)] expressing EFA6A, endophilin A2, or both (Fig. 5). In untransfected cells, Tfn internalized for 3 min was found throughout the cells in small vesicular structures known to be early endosomes (Fig. 5A, Left), whereas, after 30 min, it was mostly accumulated in a single large patch in the pericentriolar area known to be the endocytic recycling compartment (Fig. 5A, Right). As previously published, we observed that overexpression of endophilin A2 had no effect on the rate of Tfn internalization. In contrast, overexpression of EFA6A strongly inhibited Tfn uptake as strongly at 3 min as at 30 min, with a ∼50% inhibition being measured (Fig. 5). However, when cells were cotransfected with EFA6A and endophilin, they internalized Tfn similarly to untransfected or endophilin-expressing cells (Fig. 5). Thus, overexpression of endophilin rescued the inhibition of the Tfn uptake mediated by EFA6A.
Fig. 5.
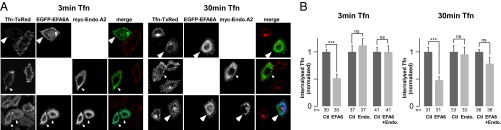
Expression of endophilin rescues the EFA6-induced inhibition of transferrin uptake. (A and B) TRVb-1 cells expressing EGFP-EFA6, myc-endophilin A2, or both were incubated at 37 °C for 3 min (Left) or 30 min (Right) with iron-saturated Texas red-conjugated human transferrin, fixed, and processed for immunofluorescence (A). Arrowheads indicate the transfected cells. Internalized transferrin was quantified (B) as described in Methods and normalized to the amount of internalized transferrin in untransfected cells in each condition (error bars represent SEM, n indicates the number of analyzed cells). Statistical analysis was performed by Student t test. ns, not significant, i.e., P > 0.1 (***P < 0.001).
Altogether, these results showed that EFA6A and endophilin are part of the same pathway that controls Tfn-R internalization.
Discussion
In this study, we identified and characterized a direct interaction of EFA6, the Arf6-specific exchange factor, with endophilin, a protein involved in clathrin-coated vesicle formation. This interaction, which is, to our knowledge, the first described between an ArfGEF and an N-BAR–containing protein, clearly reinforces the link between the EFA6/Arf6 pathway and endocytic vesicular transport.
Until now, the factors that trigger endophilin recruitment to the membrane have been unknown. Here, we observed that endophilin A2 and B1, representative members of the two endophilin subfamilies that are normally found diffused throughout the cytosol, are strongly recruited to the specific EFA6-enriched domains of the plasma membrane when coexpressed with EFA6. This observation indicates that EFA6 contributes to the plasma membrane localization of endophilin. Thus, our results identified and characterized EFA6 as one of the factors that recruit endophilin to the plasma membrane.
Nevertheless, the EFA6/endophilin interaction is probably not constitutive. Besides the membrane curvature that induces the dissociation of the Sec7/N-BAR complex, other regulatory signals must exist to control this interaction. A better understanding of the EFA6/endophilin pathway will require the identification of the signals and the molecular mechanisms that regulate in time and space the formation of the complex. It has been proposed that phosphorylation of a couple of sites in the N-BAR domain could modulate the endocytic function and membrane binding properties of endophilin (ref. 45; reviewed in ref. 37). It will be of interest to study if endophilin phosphorylation can also modulate the interaction of the N-BAR domain with EFA6.
Numerous proteins involved in intracellular vesicular transport have been shown to interact with phospholipid membranes in a membrane curvature-dependent manner. This dependency is given by sensor domains such as amphipathic helix, Alps motif, BAR domain. However, to our knowledge, we have now characterized for the first time a protein–protein interaction that is controlled by membrane curvature. According to our in vitro results, the recruitment of endophilin by EFA6 will occur only on flat areas of the plasma membrane. Indeed, the interaction between the catalytic Sec7 domain of EFA6 and the lipid-binding N-BAR domain of endophilin was down-regulated by the membrane curvature. Also, it implies that the Sec7 domain binding site is located in the concave face of the endophilin N-BAR that is known to interact with the lipid membranes (46).
What could be the function of this EFA6-induced plasma membrane recruited endophilin? Here we demonstrated that endophilin binds to the catalytic Sec7 domain to simulate Arf6 activation. Endophilin constitutes the first activator of the catalytic activity of EFA6 by directly interacting with its Sec7 domain. We have previously reported that in response to isoproterenol, a β2 adrenergic receptor agonist, β-arrestin was able to stimulate the EFA6-mediated activation of Arf6. However, this stimulation occurs probably by a different mechanism from that used by endophilin. Indeed, β-arrestin interacts simultaneously with EFA6 and Arf6GDP to corecruit the GEF and its substrate. Thus, β-arrestin stimulates Arf6 activation by reducing the dimensionality. An interaction with the N-BAR domain of arfaptin has been reported with Arf and Arl proteins (47, 48). However, we did not observe a direct interaction of Arf6 with endophilin. Thus, we hypothesize that endophilin modulates the GEF activity of EFA6 by affecting the 3D structure of the Sec7 domain, or by affecting the overall structural organization of the protein, leading to an increase in its affinity for Arf6. Structural analysis would be required to identify precisely the binding site and the mechanism by which endophilin enhances the GEF activity.
Regardless of the mechanism, the endophilin recruited to the plasma membrane by EFA6 would increase the pool of activated Arf6GTP. However, this stimulatory effect would occur only in the absence of a highly curved membrane. Indeed, we observed dissociation of the Sec7/N-BAR complex in the presence of small lipid vesicles, suggesting a chronological sequence of events. First, EFA6 would recruit endophilin to a flat zone of the plasma membrane, where both proteins would cooperate to activate Arf6. A high concentration of Arf6GTP will then activate the type I PIP5Kinase, recruit AP-2 and the clathrin molecules to shape the membrane, and build the clathrin pits. The increasing membrane curvature of the clathrin-coated vesicle will then produce the dissociation of the Sec7/N-BAR complex, releasing the endophilin, which can then assume its functions in endocytic vesicle fission and uncoating.
In addition, it is intriguing that EFA6 was found to interact directly or indirectly (via adaptors) with cargos and regulate their intracellular distribution. We previously demonstrated that EFA6 binds to the stimulated β2AR through β-arrestin to control its intracellular fate (23). We have also established that EFA6 interacts directly with TWIK-1, a K+ channel, to govern its distribution between the plasma membrane and the recycling endosomes (36). From these observations, we propose that EFA6 acts as a platform to connect the cargo to the endocytic molecular machinery, notably by controlling directly different steps from coat assembly to vesicle scission.
We observed that EFA6 can interact with the three different isoforms that we have tested, namely endophilin A1, A2, and B1. Endophilins A1 and A2 are known to act in the final stages of endocytosis by recruiting dynamin and synaptojanin for clathrin vesicle fission and uncoating, respectively. This suggests that EFA6 and Arf6 may be involved in the control of clathrin-dependent endocytosis. A role that is in agreement with their principal localization at the plasma membrane, with the effect of their overexpression on transferrin internalization, and with the capability of Arf6 to stimulate PIP2 formation and AP-2 recruitment. Endophilin B1 (which shares approximately 30% identity with A1) also exhibits lipid binding and liposome tubulation properties. However, in contrast to the A isoforms, B1 is found associated with intracellular organelles, and particularly with early endosomes, in which it colocalizes and forms a complex with EEA1. In addition, B1 has been involved in the recycling of the neurotrophin nerve growth factor (i.e., NGF/TrkA; reviewed in ref. 49). Although we have never found EFA6 localized to early endosomes, there is much evidence for involvement of Arf6 in receptor recycling. Arf6 has been involved in the recycling of different receptors (e.g., Tfn receptor, β2AR, IL-2 receptor) and intracellular EEA1-positive staining has also been reported for activated Arf6 (23, 27). One cannot exclude that EFA6 participates in endophilin B1 function at the early endosome level, and further studies are required to analyze such a role in receptor recycling.
In summary, our results indicate that EFA6 and endophilin act as key regulators of receptor-mediated endocytosis, and cooperate to regulate clathrin-dependent endocytosis.
Methods
DNA Constructs.
Sequences encoding residues 1–125 (1/2-N-BAR), 1–256 (N-BAR), and 290–352 (SH3) of mouse endophilin A1 were obtained by PCR and cloned into pGEX-3X (GE Healthcare) for in-frame fusion with GST at the N terminus. Sequences encoding residues 1–256 (N-BAR) and 36–256 (ΔN-BAR) of mouse endophilin A1 were obtained by PCR and cloned into pET16b (Novagen) for in-frame fusion with hexa-His tag at the N terminus. Plasmids encoding vsv-g–tagged EFA6A, EGFP-EFA6A, His-EFA6A, His-EFA6A-Sec7, GST-EFA6A, GST-EFA6A-Sec7, and His-Arf6 have been described elsewhere (30, 33, 50). pcDNA3 myc-tagged mouse endophilin A2, pcDNA3 myc-tagged human endophilin B1, and pGEX-6p-mouse endophilin A1 were provided by A. Schmidt (Institut Jacques Monod, Paris, France). pGEX-human arfaptin-2 and pGEX-human amphiphysin II were provided by P. De Camilli (Yale University, New Haven, CT).
Expression and Purification of Recombinant Proteins.
For the in vitro binding assays, recombinant myristoylated Arf6 WT with a C-terminal hexa-his tag (MyrArf6) was prepared as described elsewhere (25, 51, 52). Recombinant his-tagged EFA6A (His-EFA6A) was prepared as previously described (31). The different GST fusion proteins were produced in Escherichia coli and purified by affinity chromatography on glutathione–Sepharose beads (GE Healthcare). After elution with glutathione, the purified proteins were dialyzed against 20 mM Tris⋅HCl, pH 8.0, 100 mM NaCl, 1 mM MgCl2, 1 mM DTT, and 10% glycerol (dialysis buffer), and stored at −20 °C. Recombinant His-tagged N-BAR and ΔN-BAR domains were expressed in E. coli and purified according to the manufacturer’s instructions (Qiagen).
Internalization of Texas Red-Conjugated Transferrin.
TRVb-1 cells plated on 11-mm round glass coverslips were transiently transfected with plasmids encoding EGFP-tagged EFA6A and myc-tagged endophilin A2 as indicated, by using the Jet Pei transfection reagent as described by the manufacturer. Twenty-four hours after transfection, cells were preincubated in serum-free medium containing 1% BSA for 30 min at 37 °C, and then incubated for 3 or 30 min in the same medium supplemented with 50 µg/mL of Texas red-conjugated human transferrin (Molecular Probes/Fisher Scientific). Cells were then washed twice in ice-cold PBS solution, fixed in 3% (wt/vol) paraformaldehyde, and processed for immunofluorescence analysis as described previously (53). The confocal images obtained from the laser scanning confocal microscope (TCS SP5; Leica Microsystems) were analyzed to quantify the transferrin uptake by using a homemade ImageJ (http://imagej.nih.gov/ij/) macro program (W. S. Rasband). Briefly, each cell contour was determined on images corresponding to EGFP-EFA6A–expressing cells, and all the others on Texas red transferrin images by segmentation (filtering and thresholding followed by a watershed on the binary image and detection of the objects). The cumulated surface and intensities of the intracellular transferrin granules and their number were determined in both populations on each cell by using the region of interest defined by the contour.
Supplementary Material
Acknowledgments
The authors thank Drs. Anne Schmidt (Institut Jacques Monod) and Pietro De Camilli (Yale University) for advice and the gift of various DNA constructs, and Dr. D. Debayle for mass spectrometry analysis. This work was supported by the Centre National de la Recherche Scientifique and the National Research Agency (ANR) through the “Investments for the Future” LABEX SIGNALIFE Program Reference ANR-11-LABX-0028-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401186111/-/DCSupplemental.
References
- 1.D’Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7(5):347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89(14):6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer DJ, Helms JB, Beckers CJ, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268(16):12083–12089. [PubMed] [Google Scholar]
- 5.Zhao L, et al. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit beta. Proc Natl Acad Sci USA. 1997;94(9):4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73(5):999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 7.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123(3):561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105(1):93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 9.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75(6):1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft S, et al. Phospholipase D: A downstream effector of ARF in granulocytes. Science. 1994;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 11.Godi A, et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1(5):280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 12.Massenburg D, et al. Activation of rat brain phospholipase D by ADP-ribosylation factors 1,5, and 6: separation of ADP-ribosylation factor-dependent and oleate-dependent enzymes. Proc Natl Acad Sci USA. 1994;91(24):11718–11722. doi: 10.1073/pnas.91.24.11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caumont AS, Galas MC, Vitale N, Aunis D, Bader MF. Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J Biol Chem. 1998;273(3):1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- 14.Béglé A, Tryoen-Tóth P, de Barry J, Bader MF, Vitale N. ARF6 regulates the synthesis of fusogenic lipids for calcium-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2009;284(8):4836–4845. doi: 10.1074/jbc.M806894200. [DOI] [PubMed] [Google Scholar]
- 15.Krauss M, et al. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol. 2003;162(1):113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda A, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99(5):521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 17.Paleotti O, et al. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J Biol Chem. 2005;280(22):21661–21666. doi: 10.1074/jbc.M503099200. [DOI] [PubMed] [Google Scholar]
- 18.Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol. 2002;4(12):929–936. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139(1):49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houndolo T, Boulay PL, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J Biol Chem. 2005;280(7):5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- 21.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154(5):1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altschuler Y, et al. ADP-ribosylation factor 6 and endocytosis at the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1999;147(1):7–12. doi: 10.1083/jcb.147.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macia E, Partisani M, Paleotti O, Luton F, Franco M. Arf6 negatively controls the rapid recycling of the β2 adrenergic receptor. J Cell Sci. 2012;125(pt 17):4026–4035. doi: 10.1242/jcs.102343. [DOI] [PubMed] [Google Scholar]
- 24.Montagnac G, et al. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19(3):184–195. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Prigent M, et al. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163(5):1111–1121. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann P, et al. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9(3):377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14(2):417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powelka AM, et al. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5(1):20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 29.Casanova JE. Regulation of Arf activation: The Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8(11):1476–1485. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 30.Franco M, et al. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18(6):1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macia E, et al. The pleckstrin homology domain of the Arf6-specific exchange factor EFA6 localizes to the plasma membrane by interacting with phosphatidylinositol 4,5-bisphosphate and F-actin. J Biol Chem. 2008;283(28):19836–19844. doi: 10.1074/jbc.M800781200. [DOI] [PubMed] [Google Scholar]
- 32.Sakagami H, et al. Somatodendritic localization of EFA6A, a guanine nucleotide exchange factor for ADP-ribosylation factor 6, and its possible interaction with alpha-actinin in dendritic spines. Eur J Neurosci. 2007;25(3):618–628. doi: 10.1111/j.1460-9568.2007.05345.x. [DOI] [PubMed] [Google Scholar]
- 33.Derrien V, et al. A conserved C-terminal domain of EFA6-family ARF6-guanine nucleotide exchange factors induces lengthening of microvilli-like membrane protrusions. J Cell Sci. 2002;115(Pt 14):2867–2879. doi: 10.1242/jcs.115.14.2867. [DOI] [PubMed] [Google Scholar]
- 34.Luton F, et al. EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement. Mol Biol Cell. 2004;15(3):1134–1145. doi: 10.1091/mbc.E03-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein S, Partisani M, Franco M, Luton F. EFA6 facilitates the assembly of the tight junction by coordinating an Arf6-dependent and -independent pathway. J Biol Chem. 2008;283(44):30129–30138. doi: 10.1074/jbc.M803375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decressac S, et al. ARF6-dependent interaction of the TWIK1 K+ channel with EFA6, a GDP/GTP exchange factor for ARF6. EMBO Rep. 2004;5(12):1171–1175. doi: 10.1038/sj.embor.7400292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjaerulff O, Brodin L, Jung A. The structure and function of endophilin proteins. Cell Biochem Biophys. 2011;60(3):137–154. doi: 10.1007/s12013-010-9137-5. [DOI] [PubMed] [Google Scholar]
- 38.Peter BJ, et al. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 39.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761(8):897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Sundborger A, et al. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J Cell Sci. 2011;124(PT 1):133–143. doi: 10.1242/jcs.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ringstad N, et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24(1):143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 42.Micheva KD, Kay BK, McPherson PS. Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J Biol Chem. 1997;272(43):27239–27245. doi: 10.1074/jbc.272.43.27239. [DOI] [PubMed] [Google Scholar]
- 43.Farsad K, et al. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155(2):193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987;105(1):207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneko T, et al. Rho mediates endocytosis of epidermal growth factor receptor through phosphorylation of endophilin A1 by Rho-kinase. Genes Cells. 2005;10(10):973–987. doi: 10.1111/j.1365-2443.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- 46.Gallop JL, et al. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25(12):2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarricone C, et al. The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways. Nature. 2001;411(6834):215–219. doi: 10.1038/35075620. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura K, et al. Structural basis for membrane binding specificity of the Bin/Amphiphysin/Rvs (BAR) domain of Arfaptin-2 determined by Arl1 GTPase. J Biol Chem. 2012;287(30):25478–25489. doi: 10.1074/jbc.M112.365783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung ZH, Ip NY. Endophilin B1: Guarding the gate to destruction. Commun Integr Biol. 2009;2(2):130–132. doi: 10.4161/cib.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macia E, Chabre M, Franco M. Specificities for the small G proteins ARF1 and ARF6 of the guanine nucleotide exchange factors ARNO and EFA6. J Biol Chem. 2001;276(27):24925–24930. doi: 10.1074/jbc.M103284200. [DOI] [PubMed] [Google Scholar]
- 51.Franco M, Chardin P, Chabre M, Paris S. Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J Biol Chem. 1995;270(3):1337–1341. doi: 10.1074/jbc.270.3.1337. [DOI] [PubMed] [Google Scholar]
- 52.Chavrier P, Franco M. Expression, purification, and biochemical properties of EFA6, a Sec7 domain-containing guanine exchange factor for ADP-ribosylation factor 6 (ARF6) Methods Enzymol. 2001;329:272–279. doi: 10.1016/s0076-6879(01)29088-0. [DOI] [PubMed] [Google Scholar]
- 53.Franco M, et al. ARNO3, a Sec7-domain guanine nucleotide exchange factor for ADP ribosylation factor 1, is involved in the control of Golgi structure and function. Proc Natl Acad Sci USA. 1998;95(17):9926–9931. doi: 10.1073/pnas.95.17.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.