Abstract
Most current theories of human memory are material-general in the sense that they assume that the medial temporal lobe (MTL) is important for retrieving the details of prior events, regardless of the specific type of materials. Recent studies of amnesia have challenged the material-general assumption by suggesting that the MTL may be necessary for remembering words, but is not involved in remembering faces. We examined recognition memory for faces and words in a group of amnesic patients, which included hypoxic patients and patients with extensive left or right MTL lesions. Recognition confidence judgments were used to plot receiver operating characteristics (ROCs) in order to more fully quantify recognition performance and to estimate the contributions of recollection and familiarity. Consistent with the extant literature, an analysis of overall recognition accuracy showed that the patients were impaired at word memory but had spared face memory. However, the ROC analysis indicated that the patients were generally impaired at high confidence recognition responses for faces and words, and they exhibited significant recollection impairments for both types of materials. Familiarity for faces was preserved in all patients, but extensive left MTL damage impaired familiarity for words. These results suggest that face recognition may appear to be spared because performance tends to rely heavily on familiarity, a process that is relatively well preserved in amnesia. The findings challenge material-general theories of memory, and suggest that both material and process are important determinants of memory performance in amnesia, and different types of materials may depend more or less on recollection and familiarity.
Keywords: episodic memory, recollection, familiarity, amnesia, face recognition, word recognition
What types of processes allow us to recognize events that we have experienced in the past, and how are they instantiated in the brain? Since the time of Aristotle, philosophers and scientists have distinguished between two forms of recognition memory: recollection and familiarity. The former reflects retrieval of qualitative information about a study event upon encountering a previously-experienced stimulus, while the latter is a judgment of quantitative strength or fluent processing of an item, without any episodic details coming to mind (see Yonelinas, 2002 for review). In recent years, the field of cognitive neuroscience has made considerable progress in mapping the neural substrates of these two forms of recognition memory. An extensive body of research has indicated that the hippocampus is critical for recollection rather than familiarity, and that regions such as the perirhinal cortex are critical for familiarity (Aggleton & Brown, 1999; Brown & Aggleton, 2001; Bowles et al., 2007; Norman & O’Reilly, 2003; Quamme, Yonelinas, Widaman, Kroll, & Sauve, 2004; Yonelinas et al., 2002; see Eichenbaum, Yonelinas, & Ranganath, 2007 for review).
While distinguishing between recollection and familiarity, dual process theories of recognition memory are material-general, in the sense that they are expected to characterize the processes that support recognition memory for a wide variety of different materials (Aggleton & Brown, 1999; Atkinson & Juola, 1974; Brown & Aggleton, 2001; Eichenbaum et al., 2007; Jacoby & Dallas, 1981; Norman & O’Reilly, 2003; Yonelinas, 1994; see Yonelinas, 2002 for review). This material-general assumption leads to the prediction that memory is supported by the medial temporal lobes in similar ways for words, faces, scenes, or any other stimulus class. Thus, hippocampal damage should lead to impaired recollection, but spared familiarity, for all classes of items. Conversely, perirhinal cortex damage should impair familiarity but spare recollection, regardless of stimulus class. Finally, extensive medial temporal lobe damage should produce material-general deficits in both recollection and familiarity.
The material-general assumption is not isolated to dual process theories, but is also widely adopted in a host of other memory theories (e.g., Damasio, 1989; Davachi & Wagner, 2002; Eichenbaum, Otto, & Cohen, 1994; Giovanello, Schnyer, & Verfaellie, 2004; Squire, Cohen, & Nadel, 1984; Squire, Wixted, & Clark, 2007; Teyler & DiScenna, 1986; Tulving, 1983; Tulving & Markowitsch, 1998; Wickelgren, 1979). For example, the proposal that the medial temporal lobe memory system supports declarative memory in general, with the hippocampus and surrounding parahippocampal gyrus both supporting recollection and familiarity (Squire, et al., 2007), generally assumes that memory is supported in an invariant way for different types of materials. Thus, larger MTL lesions should produce greater memory deficits in both recollection and familiarity, regardless of the type of material (Squire, Zola-Morgan, & Alvarez, 1994).
A number of findings from patients with damage to the medial temporal lobes appear to directly challenge this material-general assumption. For example, Carlesimo, Fadda, Turriziani, Tomaiuolo, and Caltagirone (2001) examined recognition performance in a global amnesic patient with a bilateral volume reduction in the hippocampus. Despite impaired item and associative memory for buildings and words, this patient showed sparing of both item and associative memory for faces. Similarly, Taylor, Henson, & Graham (2007) found that patients with selective hippocampal damage were impaired on recognition of scenes but had spared recognition for faces. When patients had damage that extended to medial temporal lobe structures beyond the hippocampus, however, both scene and face recognition memory were impaired. In addition, Bird & Burgess (2008) reviewed data from 10 amnesic patients for whom Recognition Memory Test (RMT) scores were available, and found that patients were significantly more impaired at word recognition than face recognition. In this case, however, face recognition appeared to be preserved in patients with selective hippocampal damage as well as patients with more extensive MTL damage.
The existing results suggest that the medial temporal lobe is critical for the recognition of materials like words and scenes, but that it is not necessary for the recognition of faces. Whether the pattern differs for patients with focal hippocampal or more extensive MTL lesions is not yet clear. In any case, the existing results directly challenge the prevalent material-general assumption.
How can these results be explained? Dual process models could account for these results if recognition memory for faces relies heavily on familiarity, whereas word recognition relies heavily on both recollection and familiarity. Because recollection is disproportionally disrupted in MTL amnesics (Yonelinas, Kroll, Dobbins, Lazzara, & Knight, 1998), they would be expected to have a greater impairment in word recognition than face recognition.
Unfortunately, the effects of medial temporal lobe damage on recollection and familiarity for faces and words has been examined in only three case studies, and the results are not entirely consistent. In each of these cases, recognition confidence judgments were used to plot receiver operating characteristics (ROCs), which were used to derive estimates of recollection and familiarity (Yonelinas, 1994). For example, patient VC, who suffered from bilateral hippocampal and left parahippocampal volume reductions related to migraine and seizures, was found to have deficits in both recollection and familiarity for words, but did not exhibit significant impairments on either recollection or familiarity for faces (Cipolotti et al., 2006). Patient RH, who suffered damage to the right hippocampus, was found to have preserved recollection and familiarity for both faces and words (Bird, Shallice, & Cipolotti, 2007). In addition, patient Jon, who suffered selective bilateral hippocampal damage in early infancy as a result of hypoxia, exhibited a deficit in recollection but not familiarity for words, but showed normal recollection and familiarity for faces (Bird, Vargha-Khadem, & Burgess, 2008; Brandt, Gardiner, Vargha-Khadem, Baddeley, & Mishkin, 2009). Although these case studies are not definitive, they do appear to challenge the dual process account that face memory is preserved in amnesia because face recognition relies heavily on familiarity.
In the current study, we aimed to determine whether the material-specific deficits seen in amnesia are related to the differential contribution of recollection and familiarity to memory for faces versus words. Specifically, we examined recognition for faces and words in a group of amnesic patients, using the ROC method to estimate the contributions of recollection and familiarity. We included stroke patients who had damage to the hippocampus and surrounding cortex, but restricted to either the left or the right hemisphere, as well as patients who had suffered a hypoxic episode and were expected to have bilateral damage restricted to the hippocampus (see Gadian et al., 2000; Hopkins, Kesner, & Goldstein, 1995; Rempel-Clower, Zola, Squire, & Amaral, 1996). Since severe hypoxia increases the likelihood of damage outside the hippocampus (Kono, Kono, & Shida, 1983; Smith, Auer, & Siesjo, 1984), and leads to exceedingly profound memory impairments (e.g. Manns, Hopkins, Reed, Kitchener, & Squire, 2003), we only included patients who had suffered a mild hypoxic event as a result of cardiac arrest.
A previous study (Taylor et al., 2007) found that only hypoxic patients with selective hippocampal damage showed a greater word than face recognition deficit, while patients with extensive medial temporal lobe damage were impaired at recognition for both words and faces. However, there is also evidence that patients with damage that includes surrounding MTL can show spared face memory and impaired word memory (Bird & Burgess, 2008; Cipolotti et al., 2006). We therefore examined performance for the different patient groups separately, as well as the results for the overall patient group, in order to determine if the pattern of results differs as a result of different etiologies or lesion locations.
On the basis of previous studies, we expected to find that, in general, patients would be less impaired on face recognition than word recognition. The critical question was whether the material-specific dissociation could be attributed to a differential contribution of recollection and familiarity to face and word recognition. One possibility, consistent with dual-process theory, is that face recognition is relatively spared because it relies more heavily on familiarity, which is relatively preserved in amnesia (Yonelinas et al., 1998). Alternatively, both recollection and familiarity for faces may be unaffected by medial temporal lobe damage. Such a finding would directly challenge the material-general assumption commonly adopted by many current theories of MTL amnesia. Finally, it is possible that the dissociations between word and face recognition depend on the specific amnesic group (i.e. hypoxia versus broad MTL damage, or left versus right hemisphere lesions).
Method
Participants
Three patient groups took part in the experiments (see Table 1 for patient characteristics). The hippocampal (H) group consisted of four patients who had suffered a mild hypoxic episode as a result of cardiac arrest. Although we could not confirm that the damage in these patients was restricted to the hippocampus, mild hypoxia is less likely to affect extra-hippocampal structures than more severe hypoxic episodes (Gadian et al., 2000; Hopkins et al., 1995; Kono et al., 1983; Rempel-Clower et al., 1996; Smith et al., 1984).
Table 1.
Etiology, age, and neuropsychological test scores for the patients in the faces and words experiments.
| Patient | Etiology | Age | Experiments | WMS-R | WAIS-R IQ |
|---|---|---|---|---|---|
| Verb./Vis./Gen./Att./Del | Verb./Perf./Full | ||||
| CC | Hypoxic | 61 | Words | 82/81/77/81/67 | Not available |
| DR | Hypoxic | 61 | Faces | 100/94/97/93/78 | 101 (full) |
| GH | Hypoxic | 40 | Both | 62/130/79/97/83 | 96 (full) |
| RM | Hypoxic | 42 | Both | 94/77/87/96/80 | 94 (full) |
| AL | L PCA | 62 | Both | 73/81/81/85/69 | 96/88/92 |
| EA | L PCA | 54 | Words | Not available | 92/76/84 |
| EM | L PCA | 78 | Both | 82/92/81/93/69 | 113/109/112 |
| MD | L PCA | 85 | Faces | Not available | Not available |
| WM | L PCA | 71 | Both | 71/92/75/88/63 | 118/112/117 |
| WP | L PCA | 37 | Faces | Not available | Not available |
| AR | R PCA | 53 | Both | 98/126/106/98/113 | 96/115/104 |
| CK | R PCA | 33 | Words | 105/122/113/115/118 | 119/113/119 |
| FN | R PCA | 62 | Both | 125/115/126/137/125 | 126 (full) |
| JC | R PCA | 62 | Both | 100/106/101/77/96 | 85 (full) |
The left hippocampal-plus (L H+) group included six patients with lesions to the left medial temporal lobe as a result of left posterior cerebral artery infarcts. An overlay of the lesion reconstructions for the left H+ patients is shown in Figure 1a1. The right hippocampal-plus (R H+) group included four patients with lesions to the right medial temporal lobe as a result of right posterior cerebral artery infarcts. An overlay of the lesion reconstructions for each right H+ patient is shown in Figure 1b. The damage in the infarct patients included the hippocampus, fornix, posterior portion of the parahippocampal gyrus extending up to the posterior surface of the amygdala, and the surrounding fusiform and lingual gyri.
Figure 1.
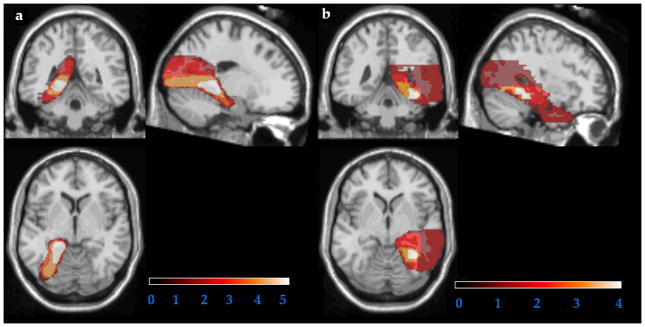
Overlay of lesion reconstructions for the patients with left (a) and right (b) medial temporal lobe damage. Hotter colors indicate greater overlap across subjects.
Data was collected on the face and verbal experiments at the same time, and patients were tested as they became available.
Nine age-matched control participants took part in the faces experiment. A separate group of eleven age-matched control participants took part in the words experiment.
Materials, Design, and Procedure
Memory for faces
Details of this task were described in a previous study examining face recognition in healthy participants (Yonelinas, Kroll, Dobbins, & Soltani, 1999). The participants first studied 120 colored drawings of faces of men, women, and children. To ensure that the faces were well encoded each face was viewed twice—first, at a rate of one face every 4 seconds, and second, after being informed that they were now going to see the same faces for a second study trial, at a rate of 2 seconds per face. The faces were 4–6 inches wide and 5–7 inches high. The recognition test immediately followed the study phase. 60 studied faces were randomly mixed with 60 new faces, and participants rated their confidence that each face was new or old on a 1 (certain it is new) to 6 (certain it is old) scale.
Verbal memory
Details of this task were previously reported in Yonelinas et al. (2002). Results from the hypoxic and left hemisphere stroke patients were previously reported (Yonelinas et al., 2002). We report the data here for comparison to face memory, and include additional data from right hemisphere infarct patients. Participants took part in two sessions. In each session, 80 words were studied under pleasantness encoding instructions and 80 under syllable counting instructions. The recognition test consisted of all studied words randomly mixed with 80 new words. Participants rated the confidence of their recognition response using a 1 (certain it is new) to 6 (certain it is old) scale. Performance was collapsed across the two sessions and encoding instructions because these factors did not change the pattern of results.
Results
Memory for Faces
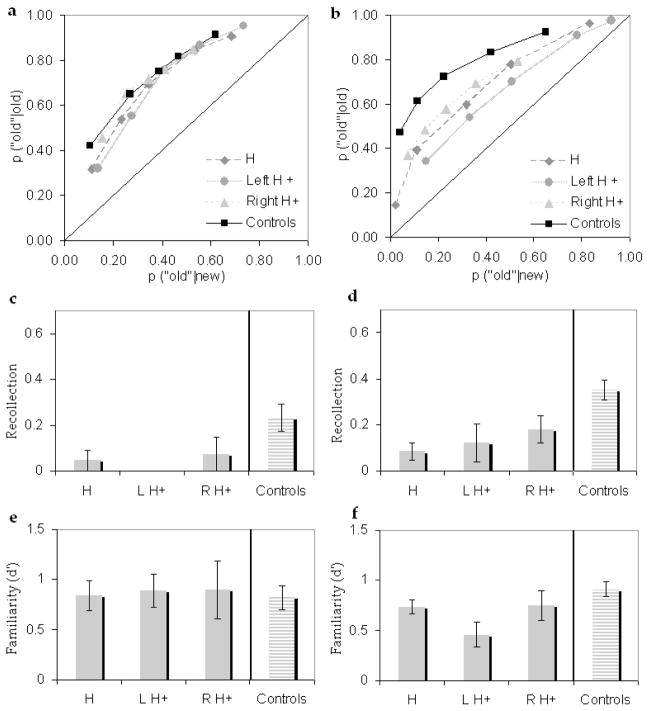
In order to assess recognition performance and estimate the contribution of recollection and familiarity, receiver operating characteristics (ROCs) were examined for each patient and control. The proportion of correct recognitions (hits) was plotted against the proportion of incorrect recognitions (false alarms) as a function of confidence, with the left-most point indicating the most confidently recognized items. The dual-process signal detection (DPSD) model was then fit to the ROCs to derive estimates of recollection and familiarity (Yonelinas, 1994; Yonelinas, 2001). Recollection is measured as the y-intercept of the ROC, and familiarity as the degree of curvilinearity of the ROC.
Figure 2a shows the aggregate face ROC for the controls and each of the patient groups. An examination of that figure shows that the patients and controls did not differ from one another in the middle of the ROCs, indicating that the patients did not show an overall impairment in recognition memory for faces. This was confirmed by an analysis of d′ at the midpoint of the ROC, which showed that the patients did not differ significantly from controls, t<1. This was consistent for all three patient groups (i.e. H, left H+, and right H+), all t<1. However, the patients’ ROCs did fall below that of the controls for the high confidence responses (i.e., the left most points). A d′ analysis on the ‘6’ confidence responses showed that the patients were significantly less accurate in these high-confidence responses than controls, t(18)=2.80, p=.006. Inasmuch as recollection leads to high-confidence responses, this observation suggests that patients were impaired on recollection relative to controls.
Figure 2.
Performance on the face (left column) and verbal (right column) tests for each patient group and the control group.(a,b)Receiver operating characteristics. (c,d)Recollection. (e,f)Familiarity.
This was confirmed by an examination of the recollection estimates (see Figure 2c). Recollection estimates were significantly reduced in amnesics compared to the controls, t(18)=3.69, p<.001. In addition, looking at the patient groups separately, each patient group was significantly impaired relative to the controls (ps<.05). Note that several patients had negative recollection estimates, so the analysis was repeated with the constraint than the recollection parameter remained greater than zero. Although constraining the estimates in this way underestimates the observed impairments, the patients’ recollection estimates were still significantly reduced compared to controls, t(18)=3.47, p=.001).
Thus, the three methods of examining recollection converged in showing that the amnesic patients were impaired at recollection. Analyses of (1) d′ at the high-confidence ‘6’ responses, (2) recollection probability estimates that were constrained to be greater than or equal to zero, and (3) recollection probability estimates that were allowed to become less than zero all suggested that patients were impaired at recollection. Moreover, recollection deficits were seen in each of the patient subgroups, although the impairments were not always statistically significant because of the small sample sizes in the individual patient groups.
In contrast to recollection, the patients showed no evidence of an impairment in familiarity, t<1. In addition, none of the patient groups showed any evidence of impaired familiarity, all t<1, ns (see Figure 2e).
Verbal Memory
As with faces, we plotted ROCs for each patient and control and assessed the contribution of recollection and familiarity to word recognition (see Figure 2b). As can be seen in the figure, the patients’ performance fell below that of the controls across the entire range of confidence responses, indicating that the patients were severely impaired at word recognition. d′ analysis on the ROC midpoints and the high confidence points verified that the amnesics were impaired at both overall recognition, t(20)=5.55, p<.001, and high confidence recognition, t(20)=4.47, p<.001. The d′ impairment at the ROC midpoints and high confidence points was seen for all patient groups, all ps<.01.
The impairment at high-confidence recognition was confirmed by an analysis of recollection estimates, which revealed that amnesics were significantly impaired relative to the controls, t(20)=3.78, p<.001. In addition, all three patient groups had significant recollection impairments relative to controls, ps<.05 (see Figure 2d). As a group, the amnesics’ familiarity estimates were reduced compared to controls, t(20)=2.45, p=.01 (see Figure 2f). However, only the left H+ group had a significant familiarity deficit, t(13)=3.066, p=.005, whereas the H and right H+ groups were not significantly different from controls, t(12)=1.14 and t(13)=1.05 respectively, ns.
Discussion
Most dominant theories of recognition memory assume that the function of medial temporal lobe structures is invariant across different types of materials, such that their role does not depend on whether the to-be-remembered items are faces, words, or any other stimulus category. This has been true of the current dual process models as well as other models of MTL function, such as single process models (Aggleton & Brown, 1999; Atkinson & Juola, 1974; Brown & Aggleton, 2001; Damasio, 1989; Eichenbaum et al., 1994; Eichenbaum et al., 2007; Jacoby & Dallas, 1981; Norman & O’Reilly, 2003; Squire et al., 1984; Squire et al., 2007; Teyler & DiScenna, 1986; Tulving, 1983; Tulving & Markowitsch, 1998; Wickelgren, 1979; Yonelinas, 1994).
Contrary to the material-general assumption inherent in these theories, several patient studies have found differential sparing and impairment of memory for different types of materials (Bird et al., 2007, 2008; Carlesimo et al., 2001; Cipolotti et al., 2006; Taylor et al., 2007) suggesting that memory for different materials has different neuroanatomical substrates. A particularly compelling finding has been that medial temporal lobe damage can lead to impaired recognition memory for words, while recognition of faces is spared (e.g., Bird & Burgess, 2008). In line with that research, we found that MTL amnesia is associated with a severe recognition impairment for words, whereas recognition for faces can appear well preserved.
The current results however, indicated that the apparent sparing of face recognition in amnesia arose because performance relied heavily on familiarity. That is, when we examined overall recognition performance by collapsing across confidence, we found little evidence for a face recognition deficit. However, the ROC analysis revealed that recognition accuracy was impaired in the patients for the high confidence responses, and further indicated that the amnesics exhibited a deficit in recollection, but not familiarity-based recognition responses. Because recollection preferentially supports high confidence responses, the memory impairments for faces were largely masked when confidence was ignored.
In contrast to face recognition, the amnesics exhibited pronounced deficits in recognition for words, and these deficits were observed across the full range of the ROCs. Moreover, recollection was found to be significantly disrupted, whereas familiarity was less consistently impaired. That is, the hypoxics and right hemisphere lesion patients showed little evidence of a familiarity impairment, whereas the patients with left hemisphere MTL damage did exhibit a familiarity deficit. Thus, the word recognition deficit was further exaggerated for the left hemisphere MTL patients because of their deficit in word familiarity. The greater deficit in word recognition compared to face recognition appears to be related to the finding that recollection played a greater role in word recognition. That is, because recollection was less likely to occur for faces than for words in the control participants, the recollection impairments in the patients were largely inconsequential in terms of overall accuracy in face recognition.
Three case studies have reported spared recollection and familiarity for faces in amnesia (Bird et al., 2007, 2008; Cipolotti et al., 2006). Why recollection for faces was not significantly impaired in those patients is unclear, but one possibility that cannot be ruled out is that it was related to low statistical power. For example, although patient VC (Cipolotti et al., 2006) did not exhibit a significant impairment in recollection compared to controls, recollection was numerically reduced in the patient (.21 compared to .36), and this reduction may have been significant with a larger sample size. Differences in lesion location may also be important. For example, patient RH (Bird et al., 2007) sustained a unilateral hippocampal lesion, which may not be sufficient to disrupt recollection for faces.
Neural substrates of recollection and familiarity
The current results are largely consistent with models that assume that the hippocampus is critical for recollection, whereas surrounding MTL regions, such as the perirhinal cortex, are important for familiarity (e.g., Aggleton & Brown, 1999; Eichenbaum et al., 2007; Norman & O’Reilly, 2003; Yonelinas et al., 2002). For example, the hypoxic patients were found to exhibit deficits in recollection but not familiarity. This is consistent with previous studies showing this pattern of deficits in word recognition (e.g., Quamme et al., 2004; Yonelinas et al., 2002), and it extends those results by showing that the same holds for faces. In addition, the finding that the left H+ group exhibited deficits in both recollection and familiarity for words is consistent with patient and neuroimaging studies implicating the left perirhinal cortex in familiarity for words (Bowles et al., 2007; Davachi, Mitchell, & Wagner, 2003; Ranganath, Yonelinas, Cohen, Dy, Tom, & D’Esposito, 2003; Uncapher, Otten, & Rugg, 2006; see Eichenbaum et al., 2007 for review). Finally, the deficits in the right H+ group replicate the finding that right MTL damage leads to deficits in recollection but not familiarity for verbal materials (Peters, Thoma, Koch, Schwarz, & Daum, 2008; see also Dobbins, Kroll, Tulving, Knight, & Gazzaniga, 1998).
In contrast to words, face familiarity was not disrupted in either the left or right hemisphere H+ groups. The current results suggest that face familiarity is either represented redundantly in the medial temporal lobe in both hemispheres (such that damage to the MTL in one hemisphere is not sufficient to disrupt it), or it is supported by regions outside the medial temporal lobe that were preserved in the patients (e.g. anterior temporal lobe or fusiform gyrus in one or both hemispheres). Evidence that face recognition may be bilaterally represented comes from imaging studies showing bilateral perirhinal cortex deactivations at retrieval related to familiarity-based memory for faces (Gonsalves, Kahn, Curran, Norman, & Wagner, 2005). Furthermore, Taylor et al. (2007) found that patients with extensive bilateral medial temporal lobe damage were impaired on face recognition. Evidence for a potential role of the fusiform gyrus in face familiarity comes from neuroimaging and patient research that implicates this region in both hemispheres in face processing (Barton, 2008; Goldsmith & Liu, 2001; Kanwisher, McDermott, & Chunn, 1997; Puce, Allison, Asgari, Gore, & McCarthy, 1996). Finally, the anterior temporal lobe has been implicated in the identification and imagery of faces (Barton, 2008; Kriegeskorte, Formisano, Sorger, & Goebel, 2007), and thus may also play a role in familiarity-based face memory. Whether these regions are necessary for familiarity-based face memory remains to be determined.
Theoretical Implications
The current results have important implications for current memory theories of the MTL. First, the results indicate that the proposal that the MTL is not necessary for supporting episodic recognition memory for faces appears to be premature. MTL damage does lead to recollection impairments for both faces and words. Face recognition appears to be spared because it tends to rely more heavily on familiarity, which is relatively preserved in amnesia (Yonelinas et al., 1998). These findings are therefore consistent with dual-process models of recognition (Aggleton & Brown, 1999; Eichenbaum et al., 2007; Norman & O’Reilly, 2003; Yonelinas et al., 2002).
The current results do not fit easily into earlier single process models of the MTL (e.g., Squire et al., 1994). One potential account for the relative sparing of face recognition compared to word recognition is that face recognition is simply easier than word recognition, and it is only the more difficult tasks that rely on the MTL. However, in the current study, control participants performed slightly better in the word memory test than in the face memory test. Thus, it was not the case that the amnesics were simply less impaired on an easier recognition task.
An alternative to the dual-process interpretation of these results is the unequal-variance signal detection model, another two-component model that has sometimes been applied to recognition memory. Although behavioral studies have largely ruled against this model (for review see Yonelinas & Parks, 2007; but also see Bird et al., 2007), it leads to the same general conclusions in the current study as the dual process view. That is, the current results could be described as reflecting the fact that amnesia is associated with a relatively selective reduction in the ‘variance’ component/process in recognition that leaves the ‘strength’ component less affected. If MTL damage reduces the variance component without greatly influencing the strength component of recognition, this could account for the lowering of high confidence hits in the amnesics. In addition, if the variance component was lower for faces than for words, then face recognition should be less impaired than word recognition. Consistent with this interpretation, the variance component was lower for faces than for words (controls’ zROC slope was significantly higher for faces, t(18)=3.17, p=.003). Moreover, the amnesics were impaired at overall discriminability for words (lower zROC intercept, t(20)=4.20, p<.001), but not for faces, t <1. Finally, amnesics had significantly higher zROC slopes compared to controls for both words, t(20)=3.77, p<.001, and faces, t(18)=1.73, p=.01, indicating that the old item variance was lower for amnesics than controls. Thus, both the dual-process and signal-detection approaches propose that amnesics are more reliant on a particular process (familiarity or strength), and are less impaired on face recognition because it relies heavily on this component.
Why do faces rely more on familiarity than words?
One possibility is that since the particular faces studied are not pre-experimentally familiar, each face receives a large boost in familiarity from being studied, and this allows familiarity judgments to be useful in discriminating between studied and unstudied faces at test. In contrast, words are pre-experimentally familiar, so any additional increment to familiarity as a result of study might be too small in some cases to discriminate between targets and lures at test. This becomes particularly apparent when one considers the number of words an individual has been exposed to in just the few minutes before the experiment (i.e. in conversation, reading, etc). The words test, then, becomes akin to a source memory test in which the individual has to decide if the word is familiar because it has just been studied, or because it has been recently encountered but not in the study phase. On the other hand, the concepts associated with the studied words may bring to mind a number of pre-experimental associations, or trigger thoughts, emotions, or mental images related to those concepts, which can be used to support memory based on recollection. Such associations may be less likely to come to mind for unfamiliar faces, leading to a reduced role for recollection (see Bird & Burgess, 2008).
Another reason why face memory may depend largely on familiarity is the high feature overlap among faces, which may make the recollection of an individual feature undiagnostic of previous occurrence. Instead, the conjunction of features, or their configuration, is more diagnostic than individual features themselves. The perirhinal cortex may be involved in representing complex feature conjunctions, and has been implicated in face perception (Bussey, Saksida, & Murray, 2002, 2005; Bussey & Saksida, 2005, 2007; Lee et al., 2005; Lee, Levi, Davies, Hodges, & Graham, 2007; Litman et al., 2009), which might contribute to a familiarity-driven memory for faces.
Material or process?
The current results suggest that amnesia cannot be fully characterized on the basis of selectively affecting memory for particular materials (e.g. words, scenes, or faces) or for particular memory processes (recollection or familiarity). Rather, it is necessary to take into account material, process, and lesion location in order to understand the recognition deficits in amnesia. An important determinant of performance is whether the patient exhibits deficits in recollection and/or familiarity, and how heavily a given recognition task relies on recollection and familiarity. However, it is not the case that recollection and familiarity are entirely domain-general, so it is important to consider how different lesion locations lead to dissociations across materials. For example, the left medial temporal lobe seems to be critical for familiarity for words, as seen from the current study as well as previous patient studies (e.g. Bowles et al., 2007) and neuroimaging studies (Davachi et al., 2003; Ranganath, et al., 2003; Uncapher et al., 2006; see Eichenbaum et al., 2007 for review). Presumably this is because the left parahippocampal gyrus includes regions that are specialized for processing verbal materials. Left MTL damage, however, does not seem sufficient to disrupt familiarity for faces.
In addition, there is some evidence that the hippocampus may be particularly critical for processing complex scene information (e.g. Lee et al., 2005, 2007; Lee, Buckley, Pegman, Spiers, Scahill et al., 2005), and as such, damage to the hippocampus may lead to deficits in the initial encoding or identification of scenes, resulting in both recollection and familiarity-based memory deficits for these materials (for cases studies, see Bird et al., 2007, 2008). There are therefore clear limitations to material-general dual-process theories, and one needs to take into account the contributions of recollection and familiarity as well as the materials being tested and the site of the lesions in the patients in order to predict memory performance.
We propose that the hippocampus is critical for forming the novel associations that support subsequent recollection of both words and faces. Thus, selective hippocampal damage should disrupt recollection but not familiarity for faces and for words. It is possible that left versus right hippocampal damage can be more disruptive for word and face recollection, respectively, although we did not observe this in the current study. Furthermore, left parahippocampal gyrus damage should impair familiarity for words, but not for faces. Finally, bilateral medial temporal lobe damage may be necessary for face familiarity to be impaired.
Conclusions
The current results indicate that in order to determine whether amnesia will impact recognition memory, it is necessary to consider the materials that one is trying to remember as much as the retrieval process that is supporting that memory. An extensive body of research strongly supports the proposal that recollection and familiarity make distinct contributions to recognition. We propose that memory for different types of materials may depend more or less on recollection and familiarity, and these differences can be useful in explaining material-specific effects in amnesia. Nevertheless, differential contributions of recollection and familiarity are not the entire story, and it is necessary to also take into consideration the materials to be remembered as well as the lesions of the patients. It remains for future research to determine the boundaries of material generality and specificity.
Footnotes
Scans were not available for patient MD.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–489. [PubMed] [Google Scholar]
- Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Luce RD, Suppes P, editors. Contemporary developments in mathematical psychology: Vol.1. Learning, memory & thinking. San Francisco: Freeman; 1974. [Google Scholar]
- Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: Implications for the acquisition of semantic memory? Journal of Cognitive Neuroscience. 2001;13:357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- Barton JJS. Structure and function in acquired prosopagnosia: Lessons from a series of 10 patients with brain damage. Journal of Neuropsychology. 2008;2:197–225. doi: 10.1348/174866407x214172. [DOI] [PubMed] [Google Scholar]
- Benton AL. The neuropsychology of face recognition. American Psychologist. 1980;35:176–186. doi: 10.1037//0003-066x.35.2.176. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess The hippocampus supports recognition memory for familiar words but not unfamiliar faces. Current Biology. 2008;18:1932–1936. doi: 10.1016/j.cub.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Bird CM, Shallice T, Cipolotti Fractionation of memory in medial temporal lobe amnesia. Neuropsychologia. 2007;45:1160–1171. doi: 10.1016/j.neuropsychologia.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Bird CM, Vargha-Khadem F, Burgess N. Impaired memory for scenes but not faces in developmental hippocampal amnesia: A case study. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cerebral Cortex. 2006;16:183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Köhler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences of the USA. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt KR, Gardiner JM, Vargha-Khadem F, Baddeley AD, Mishkin M. Impairment of recollection but not familiarity in a case of developmental amnesia. Neurocase. 2009;15:60–65. doi: 10.1080/13554790802613025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–60. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: An alternative approach. Current Opinion in Neurobiology. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. The Quarterly Journal of Experimental Psychology. 2005;58B:269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Fadda L, Turriziani P, Tomaiuolo F, Caltagirone C. Selective sparing of face learning in a global amnesic patient. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:340–346. doi: 10.1136/jnnp.71.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L, Bird C, Good T, Macmanus D, Rudge P, Shallice T. Recollection and familiarity in dense hippocampal amnesia: A case study. 2006 doi: 10.1016/j.neuropsychologia.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Computation. 1989;1:123–132. [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple learning mechanisms: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Perani D, Carlesimo GA, Silveri MC, Fazio F. Prosopagnosia can be associated with damage confined to the right hemisphere: An MRI and PET study and a review of the literature. Neuropsychologia. 1994;32:893–902. doi: 10.1016/0028-3932(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Kroll NEA, Tulving E, Knight RT, Gazzaniga MS. Unilateral medial temporal lobe memory impairment: type deficit, function deficit, or both? Neuropsychologia. 1998;36:115–127. doi: 10.1016/s0028-3932(97)00094-8. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behavioral and Brain Sciences. 1994;17:449–472. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Goldsmith ZG, Liu GT. Facial recognition and prosopagnosia: Past and present concepts. Neuro-Ophthalmology. 2001;25:177–192. [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–61. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain and Cognition. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Jacoby LR, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;11:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono E, Kono R, Shida K. Computerized tomographies of 34 patients at the chronic stage of acute carbon monoxide poisoning. Archiv fur Psychiatrie und Nervenkrankheiten. 1983;233:271–278. doi: 10.1007/BF00345797. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Levi N, Davies RR, Hodges JR, Graham KS. Differing profiles of face and scene discrimination deficits in semantic dementia and Alzheimer’s disease. Neuropsychologia. 2007;45:2135–2146. doi: 10.1016/j.neuropsychologia.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Hodges JR, Graham KS. Perceptual deficits in amnesia: Challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia. 2005;43(1):1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19:308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;27:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3:12–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Peters J, Thoma P, Koch B, Schwarz M, Daum I. Impairment of verbal recollection following ischemic damage to the right anterior hippocampus. Cortex. 2009;45:592–601. doi: 10.1016/j.cortex.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Widaman KF, Kroll NEA, Sauve MJ. Recall and recognition in mild hypoxia: Using covariance structural modeling to test competing theories of explicit memory. Neuropsychologia. 2004;42 (5):672–691. doi: 10.1016/j.neuropsychologia.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates for recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. Journal of Neuroscience. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathologica. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Squire LR, Cohen NJ, Nadel L. The medial temporal region and memory consolidation: A new hypothesis. In: Weingartner H, Parker E, editors. Memory consolidation. Hillsdale, NJ: Erlbaum; 1984. pp. 185–210. [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Alvarez P. Functional distinctions within the medial temporal lobe memory system: What is the evidence? Behavioral & Brain Sciences. 1994;17:495–496. [Google Scholar]
- Taylor KJ, Henson RNA, Graham KS. Recognition memory for faces and scenes in amnesia: Dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45:2428–2438. doi: 10.1016/j.neuropsychologia.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behavioral Neuroscience. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford: Clarendon Press; 1983. [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: Role of the hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: An fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. Chunking and consolidation: A theoretical synthesis of semantic networks, configuring, S-R versus cognitive learning, normal forgetting, the amnesic syndrome, and the hippocampal arousal system. Psychological Review. 1979;86:44–60. [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operator characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. The Philosophical Transactions of the Royal Society of London, Biological Sciences. 2001;356:1364–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins IG, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: Convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins IG, Soltani M. Recognition memory for faces: When familiarity supports associative recognition judgments. Psychonomic Bulletin & Review. 1999;6:654–661. doi: 10.3758/bf03212975. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauve MT, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: A review. Psychological Bulletin. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]