Abstract
Floral morphology determines the pattern of pollen transfer within and between individuals. In hermaphroditic species, the spatial arrangement of sexual organs influences the rate of self-pollination as well as the placement of pollen in different areas of the pollinator's body. Studying the evolutionary modification of floral morphology in closely related species offers an opportunity to investigate the causes and consequences of floral variation. Here, we investigate the recurrent modification of flower morphology in three closely related pairs of taxa in Solanum section Androceras (Solanaceae), a group characterized by the presence of two morphologically distinct types of anthers in the same flower (heteranthery). We use morphometric analyses of plants grown in a common garden to characterize and compare the changes in floral morphology observed in parallel evolutionary transitions from relatively larger to smaller flowers. Our results indicate that the transition to smaller flowers is associated with a reduction in the spatial separation of anthers and stigma, changes in the allometric relationships among floral traits, shifts in pollen allocation to the two anther morphs and reduced pollen : ovule ratios. We suggest that floral modification in this group reflects parallel evolution towards increased self-fertilization and discuss potential selective scenarios that may favour this recurrent shift in floral morphology and function.
Keywords: division of labour, heteranthery, floral evolution, mating system, pollen : ovule ratio, Solanum (Solanaceae)
1. Introduction
Angiosperms display a large amount of variation in the form of their reproductive organs, and the morphological diversification of flowers has attracted the attention of evolutionary biologists since Darwin [1,2]. Correlations between form and function have been demonstrated in many instances including changes in pollinator fauna in closely related species with different floral characteristics (e.g. Aquilegia [3], Mimulus [4], Penstemon [5], Stylidium [6]), visitation by specialized pollinators [7–9] and evolutionary shifts from cross- to self-fertilization [10–13]. Studying the evolution of floral form provides clues about the functional significance of the variation of plants' sexual reproductive structures.
In hermaphroditic species, floral morphology can influence both the pattern of pollen transfer between individuals and the rate of self-pollination. The relative position of male and female organs within a flower affects pollen placement and receipt from the pollinator's body, which in turn influences the rates of pollen transfer between plants [14–16]. In addition, the degree of spatial separation between anthers and stigma within a flower (herkogamy) can reduce sexual interference in self-compatible species [9,17,18] and is functionally related to the extent of within-flower self-pollen deposition, with reduced herkogamy resulting in higher rates of selfing [19,20]. It is therefore expected that changes in flower morphology have the potential to change patterns of pollen transfer, including the relative contribution of self- versus cross-fertilization to offspring production (mating system).
Changes in flower morphology may be determined in part by size-dependent relationships between floral characters. Allometric studies of flower morphology represent a classic and powerful tool to investigate the extent to which changes in flower morphology simply reflect proportional scaling with overall size [21,22]. Moreover, because flowers are formed of developmentally and functionally related modules of phenotypically integrated traits [15], changes in the allometric relationships between traits could reveal developmental or functional variation. Indeed, changes in the allometry of floral traits in closely related species or populations have often been used to identify potential differences in floral function across taxa [23–26].
The evolution of anther dimorphism within a flower, or heteranthery, represents a prime example of the close relationship between flower form and function. Heteranthery has evolved multiple independent times in the history of angiosperms, in at least 12 different orders [27], and is often associated with nectarless flowers that offer pollen as the main or only reward to floral visitors, mirror-image flowers (enantiostyly) and vibratile or buzz pollination [27,28]. Heteranthery is characterized by the morphological and functional differentiation of stamens within flowers into more than one type [27,29–31]. Usually, heterantherous species have two structurally distinct types of stamens that differ in shape, size and/or colour. One stamen type usually has brightly coloured anthers located centrally within the flower. Floral visitors commonly collect pollen from this set of stamens, termed feeding anthers [29,31]. The second set of stamens, known as pollinating anthers [29], consists of one or more anthers that are often larger, cryptically coloured and displaced away from the centre of the flower. The spatial location of this anther set approximately corresponds to the location of the style in the same flower or flowers of the opposite morph in species with mirror-image flowers [27]. Experimental manipulations in Melastoma and Solanum have demonstrated that pollen produced in pollinating anthers is more likely to reach the stigmas of other flowers than pollen from feeding anthers [30,32]. Importantly, these different fates of pollen from the two anther types arise in large part from differences in the morphology and spatial location of stamens within the flower. Pollinating anthers place pollen in areas of the pollinator's body where grains are less prone to be actively removed (groomed), and more likely to contact the stigma [32]. The multiple origins of heteranthery across angiosperms represent an excellent example of pollinator-mediated selection driving the evolution of floral form, and of the functional specialization of anthers into ‘pollinating’ and ‘feeding’ functions partly due to morphological characteristics of the sexual organs.
In order to investigate repeated transitions in floral morphology in a heterantherous group, we have identified species in Solanum section Androceras (Solanaceae) that comprise three pairs of closely related heterantherous taxa with relatively large and small flowers [33,34], suggesting parallel transitions in both floral form and function. Changes in the degree of herkogamy and the level of morphological differentiation between anther types appear to accompany this transition in flower size. However, to date there is no evidence to evaluate the hypothesis that differences in floral characteristics within Solanum section Androceras reflect functional differences.
The six Solanum taxa chosen here (five accepted species and one named variety) represent pairs of closely related or sister taxa [34] in each of the three series in section Androceras [33] (figure 1): S. rostratum Dunal and S. fructo-tecto Cav. (series Androceras); S. grayi var. grayi Whalen and S. grayi var. grandiflorum Whalen (series Pacificum); and S. citrullifolium A. Braun and S. heterodoxum Dunal (series Violaceiflorum). Each of these three pairs contains one taxon with relatively large, strongly heterantherous flowers, and one taxon with smaller, less heterantherous flowers. In one of these pairs (S. grayi var. grayi and S. grayi var. grandiflorum), the transition from large to small flowers closely corresponds with the sympatric presence of a relatively large-flowered sister species (S. lumholtzianum). Populations of S. grayi co-occurring with S. lumholtzianum have significantly smaller flowers (S. grayi var. grayi) than allopatric populations (S. grayi var. grandiflorum) [35]. The reduction of flower size in sympatry appears to allow S. grayi var. grayi to avoid competition with S. lumholtzianum by exploiting smaller pollinators. This case represents a classic example of reproductive character displacement in plants [36,37].
Figure 1.
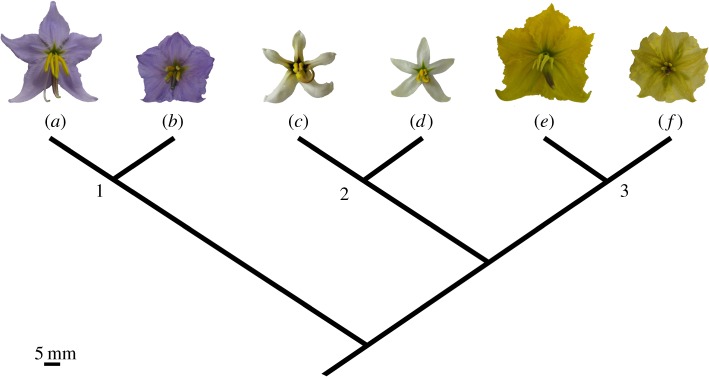
Phylogenetic relationships for the six Solanum taxa studied here, inferred from the analysis of Solanum section Androceras by Stern et al. [34]. Section Androceras is a monophyletic group of 12 species arranged in three series [33,34]. (1) Series Violaceiflorum: (a) S. citrullifolium and (b) S. heterodoxum; (2) series Pacificum: (c) S. grayi var. grandiflorum and (d) S. grayi var. grayi; (3) series Androceras: (e) S. rostratum and (f) S. fructo-tecto. Taxonomic classification follows Whalen [33]. (Online version in colour.)
Here, we test the hypothesis that changes in floral characteristics reflect a shift in reproductive strategy. Specifically, we address three questions: (i) Is the reduction in floral size associated with a change in floral architecture (changes in the allometric relationships between floral traits)? (ii) Are smaller flowers less heterantherous? and (iii) Are changes in flower size correlated with shifts in the relative allocation to pollen and ovule production? To address these questions, we used a morphometric analysis of floral traits measured in plants grown in a common garden. Our results suggest that the recurrent transition from larger to smaller flowers in Solanum section Androceras has been accompanied by a disproportionate reduction in herkogamy, the breakdown of heteranthery and lower pollen : ovule ratios, suggesting a shift in mating system towards increased self-fertilization.
2. Material and methods
(a). Study system
Solanum is the largest genus in the Solanaceae, comprising approximately 1500 species with diverse habits and life histories, including annual and perennial herbs, vines and trees [38]. Solanum flowers are commonly hermaphroditic, nectarless, usually radially symmetric, pentamerous, with five equal anthers arranged in a cone at the centre of the flower and a style protruding from the anther cone terminating in a small to minute stigma [34,39,40]. The anthers of Solanum open through small apical pores, and pollinators, usually bees, use high-frequency vibrations to extract the small, dry pollen grains in a phenomenon known as buzz pollination [39,41].
Solanum section Androceras consists of a monophyletic clade of 12 enantiostylous, self-compatible, annual to short-lived perennial species with varying degrees of anther differentiation [33,34]. Solanum section Androceras is distributed across Mexico and North America and is divided into three taxonomic groups: series Androceras, with five species with yellow or rarely white flowers distributed from south-central Mexico to Canada; series Violaceiflorum, with four purple-flowered species located from the Mexican highlands to Texas; and series Pacificum with three species of white- or yellow-flowered plants distributed along the Pacific slope from Mexico to Arizona [33]. Molecular analyses have shown that series Androceras and Violaceiflorum are not monophyletic, and that two species (S. heterodoxum and S. citrullifolium) are polyphyletic [34]. Despite being a small group, species in section Androceras display considerable variation in the colour, size and shape of flowers. Importantly, some shifts in floral characteristics, most prominently a reduction in floral size, seem to have occurred in parallel along different clades, providing an excellent study system to investigate replicate evolutionary transitions in floral traits in a small group of closely related species.
(b). Seed collection and plant growth
Seeds of five of the six studied taxa were collected from natural populations in Mexico between 2007 and 2010 (electronic supplementary material, table S1). Seeds of S. citrullifolium were obtained from the Experimental Garden and GeneBank Solanaceae collection at Radboud University, Nijmegen, The Netherlands (electronic supplementary material, table S1). Seeds were extracted from mature fruits, dried and stored in paper bags at 5–7°C until the experiment commenced. To induce germination, seeds were pre-treated for 24 h with a 2000 ppm aqueous solution of gibberellic acid (GA3; Sigma-Aldrich, Dorset, UK). Seeds were planted in plastic trays containing Modular seed growing medium compost (William Sinclair Horticulture PLC, Lincoln, UK) and kept in a glasshouse at 14–22°C with natural daylight supplemented with compact-fluorescent lamps to provide at least 14 daylight hours. After two to three weeks, seedlings were transplanted to individual 3.5- to 6-inch pots containing All Purpose Growing Medium (Sinclair) with Perlite Standard (Sinclair) in 3 : 1 proportion and fertilized once with slow-release fertilizer (Osmocote 16 : 9 : 12; Scotts Miracle-Gro Co., Marysville, OH, USA).
(c). Floral characteristics
To describe the variation in floral characteristics among the studied species, between 38 and 251 flowers per species were analysed soon after the onset of flowering (586 flowers total). For each individual plant, one to eight flowers (mean = 3.70) were randomly selected and the following measurements were taken: (i) maximum corolla length, (ii) maximum corolla width, (iii) distance between the stigma and the apical pores of the pollinating anther, (iv) distance between the stigma and the apical pore of the closest feeding anther and (v) distance between the apical pores of the pollinating anther and the nearest feeding anther. These measurements were always made directly facing the anther cone in the same plane as a pollinator would normally approach the flower. The flowers were then dissected and an additional set of measurements of individual sexual organs was recorded: (vi) length and (vii) width of the pollinating anther, (viii) length and (ix) width of the feeding anther, (x) style length and (xi) stigma width. Stigma width was not measured for flowers of S. grayi var. grayi and S. grayi var. grandiflorum. All measurements were taken either from digital images (S. rostratum, S. fructo-tecto, S. citrullifolium and S. heterodoxum; Canon Powershot A480, Canon, Surrey, UK) analysed in ImageJ [42], or using digital callipers (S. grayi var. grayi and S. grayi var. grandiflorum; Absolute Digimatic Caliper, Mitutoyo Ltd., Hampshire, UK).
To estimate pollen and ovule numbers, anthers and ovaries were collected from a subset of individuals and stored in 70% ethanol (mean = 18 individuals per species; range = 7–25; 100 flowers total). The four feeding and single pollinating anthers were stored separately in microcentrifuge tubes with 1 ml of 70% ethanol. Ovules were counted in a stereo microscope (MZ6, Leica Microsystems, Milton Keynes, UK). Pollen grains were stained with lactophenol-aniline blue and counted in a haemocytometer. Two counts were performed per flower: one on the single pollinating anther and the other on the four feeding anthers collectively. Multiple flowers were measured per individual, and the average values per plant for each trait were used for further analysis. Pollen : ovule ratios (P : O) were calculated by dividing the total number of pollen grains in both sets of anthers by ovule number.
(d). Statistical analysis
(i). Floral morphology
Overall floral morphology was compared across species using linear discriminant analyses (LDA). LDA is a multivariate technique that simultaneously uses multiple traits to calculate a new set of variables that maximize the differentiation among pre-defined groups, in our case, different taxa. Therefore, it allowed us to determine to what extent species can be differentiated based on quantitative floral measurements alone. The LDA included the first 10 floral traits measured (i.e. except stigma width), and a cross-validation test was performed using the MASS package [43] in R v. 3.0.1 [44]. In addition, a correlation matrix was calculated among floral traits 1–10 and used to perform a principal component analysis (PCA). The PCA was used to simplify the comparison of overall floral morphology across species.
To compare statistically overall flower morphology (PC components) and P : O ratios between small- and large-flowered taxa, we introduced a fixed effect variable for floral type, where S. rostratum, S. grayi var. grandiflorum and S. citrullifolium are coded as ‘large’, and S. fructo-tecto, S. grayi var. grayi and S. heterodoxum were coded as ‘small’. In this model, taxon and individual were included as random effects. This floral-type variable allowed us to estimate whether flower morphology and P : O ratios floral traits are different not only across species (random effect) but between large- and small-flowered taxa. Mixed models were fitted with the lme4 package [45] in programming language R, and p-values were estimated with the car package [46] for fixed effects and with likelihood ratio tests of nested models for random effects.
(ii). Floral allometry
To investigate changes in the relationships among floral characteristics in taxa with smaller flowers compared to those observed in their larger flowered relatives, a series of mixed effects models were analysed. Mixed models were chosen because the dataset collected here included multiple flowers per individual for each taxon, and such nested data structures can be easily accommodated using mixed effect models. Other methods for comparing differences in floral architecture, such as Mantel tests of species-specific correlation matrices [47], may not be suitable for this kind of structured data.
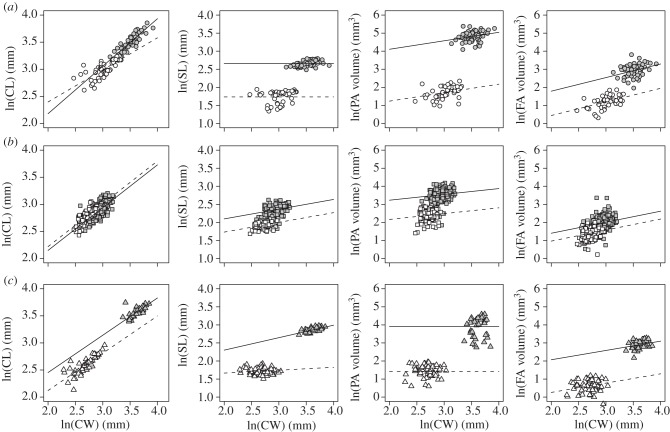
We investigated whether the allometric relationships between floral traits differed between the two taxa in each of the three small- and large-flowered taxon pairs (series Androceras, Pacificum and Violaceiflorum). We analysed the allometric relationships between corolla width—as an overall estimate of flower size—and other floral characters. Corolla width is positively correlated with all floral traits measured here (corolla length, pollinating and feeding anther width and length, style length and distance between stigma and anthers; Pearson's r: mean = 0.75, min = 0.28, max = 0.96), except stigma size (r = −0.74; all correlations calculated at the flower level). We analysed the following linear mixed effects (LMEs) model for a given floral character y: yijk = Bi + Ai × xijk + cij + eijk, where i represents the identity of a taxon (two taxa per model), j represents the individual plant, k represents a flower, xijk is the value of floral character x (corolla width), cij is a normally distributed random variable and eijk are the residuals. Each of the three pairs of taxa was analysed separately. When analysing log-transformed variables, the coefficients Ai and Bi are easily comparable, respectively, to the allometric slope and intercept of the familiar allometric regression [48]: log(y) = log(b) + α[log(x)]. Differences in the intercept between species in a pair indicate different relative sizes of character y with respect to flower size, while differences in the allometric slope indicate changing scaling trajectories [48].
Fitted models included taxon, corolla width and taxon × corolla width as fixed effects, and plant identity as a random effect. We selected the simplest model by backward elimination of fixed effects using likelihood ratio tests. All analyses were done with log-transformed variables. Anther volume was estimated from the linear measurements (i.e. (anther width)2 × anther length) as it has previously been shown to positively correlate with pollen production in S. rostratum [32]. Mixed models were fitted with the lme4 package [45] in programming language R, and p-values for the main effects and the interaction were estimated with the car package [46].
3. Results
(a). Floral morphology
The linear discriminant analysis (LDA) showed that the six taxa studied here can be clearly distinguished based on the characteristics of their floral morphology alone (figure 2). The cross-validation error of the LDA was 3.24% (19/586), indicating strong confidence in the assignment of individuals to their respective taxon. The first two linear discriminant axes explained 90% of the trace, and differentiated large- from small-flowered taxa. However, small-flowered taxa showed much less separation in these first two axes (figure 2).
Figure 2.
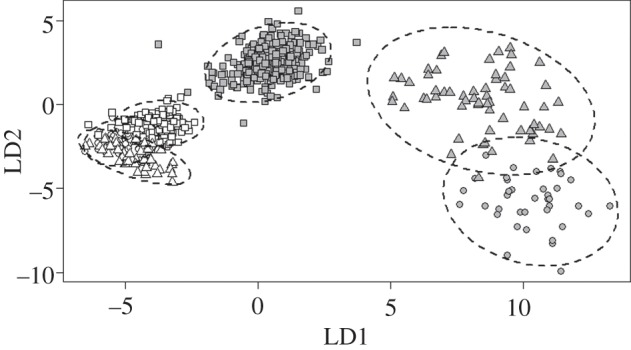
The first two axes of an LDA of 10 floral traits in Solanum section Androceras. Grey triangles: S. citrullifolium; open triangles: S. heterodoxum; grey squares: S. grayi var. grandiflorum; open squares: S. grayi var. grayi; grey circles: S. rostratum; open circles: S. fructo-tecto. Ellipses enclose 95% of the observations.
Table 1 presents the summary statistics for the 11 floral characteristics used in this study. In general, all floral traits were relatively smaller in the corresponding small-flowered taxon of each of the three pairs (table 1). An exception to this pattern was stigma size. In the four species for which this trait was measured, stigma width was larger in species with smaller flowers than in the sister taxa with larger flowers (0.30 ± 0.02 versus 0.65 ± 0.01 for S. citrullifolium/S. heterodoxum, and 0.20 ± 0.01 versus 0.74 ± 0.02 for S. rostratum/S. fructo-tecto; electronic supplementary material, figure S1). The PCA on 10 floral variables (excluding stigma width) indicated that the first principal component (PC1) explains 88% of the variation in floral traits and is positively correlated with all traits (electronic supplementary material, table S2). Therefore, PC1 represents an overall estimate of floral size. A LMEs analysis on PC1 showed that ‘flower type’ (large/small) is statistically significant after accounting for taxon and plant as random effects (figure 3; regression coefficients: ‘large’ type = 15.07 ± 6.3, ‘small’ type = −7.5 ± 6.3, p < 0.05). A likelihood ratio test of nested models confirmed that both taxon and plant significantly contribute to explaining variation in PC1 (p < 0.001).
Table 1.
Summary statistics for 10 floral traits measured in six taxa of Solanum section Androceras. N, number of flowers measured per taxon; PA, pollinating anther; FA, feeding anther. All measurements in millimetres. Mean ± s.e.
| Taxon | N | corolla width | corolla length | PA width | PA length | FA width | FA length | distance PA–FA | distance FA–stigma | distance PA–stigma | style length |
|---|---|---|---|---|---|---|---|---|---|---|---|
| series Androceras | |||||||||||
| S. rostratum | 61 | 34.19 ± 0.66 | 33.94 ± 0.66 | 3.23 ± 0.05 | 12.18 ± 0.16 | 1.59 ± 0.03 | 7.62 ± 0.12 | 7.67 ± 0.27 | 6.49 ± 0.25 | 7.06 ± 0.25 | 14.27 ± 0.14 |
| S. fructo-tecto | 50 | 20.68 ± 0.55 | 20.20 ± 0.45 | 1.15 ± 0.02 | 4.25 ± 0.07 | 0.99 ± 0.02 | 3.49 ± 0.07 | 2.65 ± 0.09 | 1.12 ± 0.08 | 1.92 ± 0.10 | 5.80 ± 0.13 |
| series Pacificum | |||||||||||
| S. grayi var. grandiflorum | 251 | 19.21 ± 0.13 | 18.33 ± 0.14 | 2.22 ± 0.01 | 7.03 ± 0.05 | 1.15 ± 0.01 | 5.58 ± 0.04 | 3.27 ± 0.04 | 3.91 ± 0.06 | 6.89 ± 0.07 | 10.59 ± 0.08 |
| S. grayi var. grayi | 130 | 15.12 ± 0.14 | 16.25 ± 0.15 | 1.47 ± 0.01 | 5.19 ± 0.06 | 0.96 ± 0.01 | 4.52 ± 0.04 | 2.05 ± 0.06 | 0.72 ± 0.03 | 2.64 ± 0.06 | 6.87 ± 0.05 |
| series Violaceiflorum | |||||||||||
| S. citrullifolium | 38 | 37.79 ± 0.70 | 35.79 ± 0.53 | 1.98 ± 0.08 | 13.20 ± 0.11 | 1.42 ± 0.02 | 9.14 ± 0.09 | 7.88 ± 0.24 | 7.40 ± 0.37 | 3.82 ± 0.28 | 17.57 ± 0.18 |
| S. heterodoxum | 56 | 14.87 ± 0.33 | 13.52 ± 0.28 | 1.05 ± 0.02 | 3.95 ± 0.05 | 0.78 ± 0.01 | 3.15 ± 0.04 | 2.71 ± 0.09 | 1.04 ± 0.05 | 2.12 ± 0.09 | 5.59 ± 0.07 |
Figure 3.
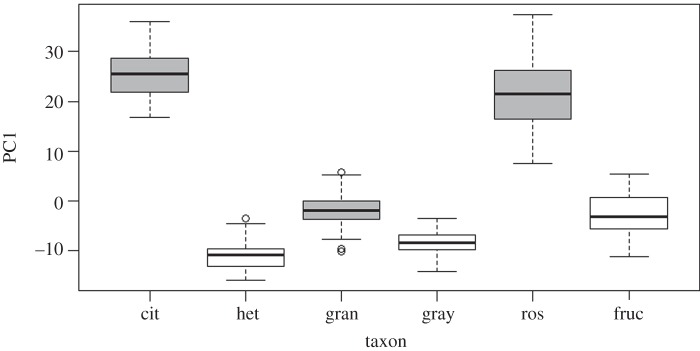
Boxplot of species values of the first principal component (PC1) of a PCA of 10 floral traits in six taxa in Solanum section Androceras. PC1 is positively correlated with all floral traits and is thus a measure of overall size. cit, S. citrullifolium, het, S. heterodoxum, gran, S. grayi var. grandiflorum, gray, S. grayi var. grayi, ros, S. rostratum, fruc, S. fructo-tecto. Taxa classified as large flowered in each pair are shown in grey.
(b). Allometric relationships
Given that small-flowered taxa show an overall reduction in flower size, we were then interested in determining whether allometric relationships between floral traits differed between small- and large-flowered taxa in each species pair. Differences in scaling relationships would indicate that a small-flowered taxon is not simply a proportionally reduced version of the large-flowered one.
Our allometric analysis indicated changes in at least some of the allometric coefficients (intercept and/or slope) of most floral traits within all species pairs (table 3 and figure 4). For series Androceras (S. rostratum/S. fructo-tecto), corolla length and corolla width differed slightly but significantly in both the intercept and slope of the allometric coefficients (table 3), suggesting changes in the length/width ratio of the corolla. However, this species pair showed marked differences in the relative sizes of their style and anthers. For example, S. fructo-tecto displayed proportionately shorter styles relative to average flower size compared with S. rostratum (figure 4). Although the scaling relationship between anther volume and flower size was maintained in this species pair (no statistical evidence for a difference in allometric slope between taxa, table 3), we found a significant reduction in their allometric intercepts, indicating that anther size is proportionately much smaller in S. fructo-tecto than in S. rostratum (table 3 and figure 4).
Table 3.
Allometric relationships between corolla width and corolla length, style length, pollinating anther (PA) volume and feeding anther (FA) volume in three pairs of taxa of Solanum section Androceras. Superscripts indicate the nominal statistical significance (type III Wald likelihood ratio tests) in the taxon-specific intercept (i.e. different intercepts between taxa) and slope (corolla width); a significant interaction term taxon × corolla width indicates a difference between taxa in the allometric slope (interaction coefficient measured relative to the large taxon). Coefficient ± s.e.
| corolla length (mm) | style length (mm) | PA volume (mm3) | FA volume (mm3) | |
|---|---|---|---|---|
| series Androceras (S. rostratum/S. fructo-tecto) | ||||
| taxon (S. rostratum) | 0.43 ± 0.31*** | 2.65 ± 0.03*** | 3.17 ± 0.58*** | 0.28 ± 0.63*** |
| taxon (S. fructo-tecto) | 1.21 ± 0.21*** | 1.73 ± 0.03*** | 0.29 ± 0.49*** | −1.06 ± 0.54*** |
| corolla width | 0.88 ± 0.09*** | n.s. | 0.47 ± 0.16** | 0.75 ± 0.18*** |
| taxon × corolla width | −0.28 ± 0.11* | n.s. | n.s. | n.s. |
| series Pacificum (S. grayi var. grandiflorum/S. grayi var. grayi) | ||||
| taxon (S. grayi var. grandiflorum) | 0.57 ± 0.13*** | 1.56 ± 0.15*** | 2.59 ± 0.41*** | 0.17 ± 0.50*** |
| taxon (S. grayi var. grayi) | 0.64 ± 0.11*** | 1.19 ± 0.14*** | 1.52 ± 0.38*** | −0.25 ± 0.46*** |
| corolla width | 0.79 ± 0.04*** | 0.27 ± 0.05*** | 0.32 ± 0.14* | 0.61 ± 0.17*** |
| taxon × corolla width | n.s. | n.s. | n.s. | n.s. |
| series Violaceiflorum (S. citrullifolium/S. heterodoxum) | ||||
| taxon (S. citrullifolium) | 1.07 ± 0.21*** | 1.61 ± 0.37*** | 3.90 ± 0.12*** | 1.04 ± 0.78*** |
| taxon (S. heterodoxum) | 0.74 ± 0.16*** | 1.50 ± 0.17*** | 1.41 ± 0.08*** | −0.77 ± 0.57*** |
| corolla width | 0.69 ± 0.06*** | 0.34 ± 0.10*** | n.s. | 0.51 ± 0.21* |
| taxon × corolla width | n.s. | −0.26 ± 0.12* | n.s. | n.s. |
***p < 0.001; **p < 0.01; *p < 0.05
n.s. denotes p > 0.05.
Figure 4.
Allometric relationships between corolla width (CW) and corolla length (CL), style length (SL), pollinating anther volume (PA volume) and feeding anther volume (FA volume) in three pairs of taxa of Solanum section Androceras. Panels (a), series Androceras (S. rostratum/S. fructo-tecto); panels (b), series Pacificum (S. grayi var. grandiflorum/S. grayi var. grayi); panels (c), series Violaceiflorum (S. citrullifolium/S. heterodoxum). Symbols as in figure 2. Large-flowered species in each pair are shown in grey symbols (continuous regression line) and small-flowered taxa in open symbols (dashed regression lines). Regression lines were obtained using the coefficients shown in table 3. All values are natural log transformed.
We also detected significant allometric changes in series Pacificum (S. grayi var. grayi/S. grayi var. grandiflorum), although the magnitude of these differences was smaller. The scaling relationship between corolla length and width was very similar between the two taxa (intercept = 0.57 and 0.64, for S. grayi var. grandiflorum and S. grayi var. grayi, respectively; slope = 0.79; interaction term non-significant; table 3) indicating small differences in corolla traits (figure 4). For style length and anther size, S. grayi var. grayi was consistently smaller for a given flower size compared with its sister taxon S. grayi var. grandiflorum (i.e. the allometric intercept of S. grayi var. grandiflorum was significantly larger than for S. grayi var. grayi; table 3), although the scaling relationship between each of these organs and corolla width was conserved across these two taxa (no statistically significant difference in allometric slope; taxon × corolla width term, p > 0.05 in all cases). Finally, in series Violaceiflorum, we found a statistically significant relative reduction in corolla length, style and both pollinating and feeding anther size in S. heterodoxum compared with S. citrullifolium as evidenced by smaller allometric intercepts for all traits (table 3 and figure 4). Moreover, S. heterodoxum showed a weaker effect of changes in flower size on style length compared with S. citrullifolium (allometric slope = 0.08 versus 0.34, respectively; taxon × corolla width interaction, p < 0.05; table 3 and figure 4). Taken together, our allometric analysis clearly shows that the flowers of S. fructo-tecto, S. grayi var. grayi and S. heterodoxum are not simply proportionately scaled down versions of their larger flowered counterparts.
(c). Functional traits
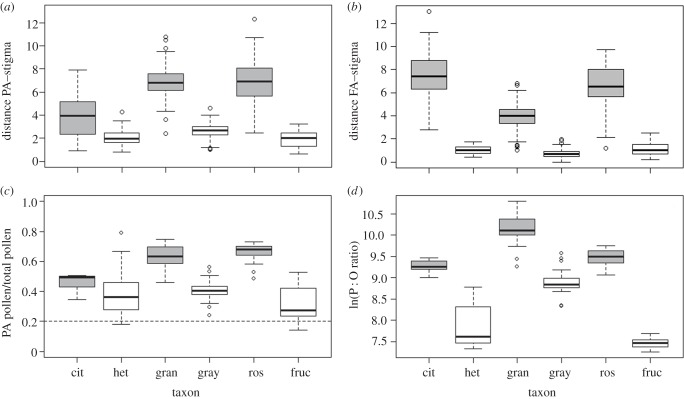
Small-flowered taxa had shorter distances between the stigma and the anther pores of both feeding and pollinating anthers than larger flowered taxa across all taxa pairs (figure 5a,b). This reduction in anther–stigma distance was accompanied by a less marked deflection of the pollinating anther and the style to opposite sides of the floral axis (level of enantiostyly) in small-flowered taxa compared with their larger flowered relatives, although the level of enantiostyly was not quantitatively estimated here. Surprisingly, in the species pair S. citrullifolium/S. heterodoxum, the reduction in anther–stigma distance was less marked in pollinating anthers than in feeding anthers. In part, this may be a consequence of the fact that the flowers of S. citrullifolium studied here displayed a relatively low extent of enantiostyly compared with other large-flowered species (M Vallejo-Marín 2012, personal observation). In general, a reduction both in the size of floral organs and in the level of enantiostyly accompanies the loss of spatial separation between male and female structures.
Figure 5.
Comparison of functional traits in three pairs of taxa in Solanum section Androceras. Panels (a,b): anther–stigma separation (mm) within flowers. (a) Distance between the apical pores of the pollinating anther (PA) and the stigma. (b) Distance between the stigma and the apical pores of the nearest feeding anther (FA). Panels (c,d): (c) proportion of pollen produced in the single pollinating anther (PA pollen), relative to the total pollen production per flower. The dashed line shows the expected proportion of pollen produced if pollen production was equal among the five anthers. (d) Natural logarithm of the pollen : ovule ratio (ln(P : O ratio)). Species names as in figure 3.
Changes in floral morphology in small-flowered taxa were accompanied by a shift in the relative allocation of pollen to pollinating versus feeding anthers. In large-flowered species, the single pollinating anther produced 50% (S. citrullifolium), 64% (S. grayi var. grandiflorum) and 67% (S. rostratum) of the total pollen per flower (table 2 and figure 5c), indicating that heteranthery in these species incorporates a strong asymmetry in the allocation towards pollen to each of the five anthers within a flower. By contrast, in small-flowered taxa, the pollen production of the pollinating anther dropped to 39% in S. heterodoxum, 41% in S. grayi var. grayi and 27% in S. fructo-tecto, with the latter species approaching the allocation when pollen is distributed equally across the five anthers (20%; figure 5c).
Table 2.
Pollen and ovule production in six taxa of Solanum section Androceras. Pollen count estimates are given separately for each of the two anther sets. FA pollen count, total pollen production in all four feeding anthers; PA, pollen production in the single pollinating anther; P : O, pollen : ovule ratio; N, number of individual plants used in the estimation. Mean ± s.e.
| taxon | N | FA pollen count (×103) | PA pollen count (×103) | total pollen (×103) | N | ovule mean | P : O ratio |
|---|---|---|---|---|---|---|---|
| series Androceras | |||||||
| S. rostratum | 18 | 276 ± 13 | 546 ± 34 | 822 ± 42 | 14 | 63.2 ± 2.4 | 13149 ± 680 |
| S. fructo-tecto | 11 | 100 ± 6 | 51 ± 11 | 151 ± 14 | 10 | 79.6 ± 3.2 | 1754 ± 76 |
| series Pacificum | |||||||
| S. grayi var. grandiflorum | 24 | 450 ± 28 | 800 ± 70 | 1250 ± 90 | 27 | 47.2 ± 1.7 | 27528 ± 2144 |
| S. grayi var. grayi | 25 | 167 ± 9 | 118 ± 7 | 285 ± 14 | 28 | 38.8 ± 1.5 | 7612 ± 477 |
| series Violaceiflorum | |||||||
| S. citrullifolium | 7 | 318 ± 26 | 269 ± 21 | 587 ± 37 | 7 | 55.0 ± 2.3 | 10727 ± 642 |
| S. heterodoxum | 15 | 47 ± 10 | 29 ± 4 | 75 ± 13 | 13 | 28.8 ± 2.2 | 2929 ± 479 |
Finally, the comparison of P : O ratios showed that small-flowered taxa produce fewer pollen grains per ovule than large-flowered species (figure 5d and table 2). In small-flowered S. heterodoxum and S. grayi var. grayi, the P : O ratio was 3.6 times lower than in their paired large-flowered relatives S. citrullifolium and S. grayi var. grandiflorum. The shift was even more marked in S. fructo-tecto, which had a 7.4-fold reduction in P : O ratio compared with its sister species S. rostratum (table 2). In S. heterodoxum and S. grayi var. grayi, both pollen and ovule number were reduced compared with their large-flowered relatives, but the larger decrease in pollen grain number relative to ovules resulted in an overall reduction in P : O ratio. By contrast, the reduction in P : O ratio in S. fructo-tecto resulted from producing fewer pollen grains as well as from an increase in the number of ovules per flower compared with S. rostratum (table 2). The differences in P : O ratios between small- and large-flowered taxa were statistically significant after accounting for the effects of taxon (regression coefficients: ‘large’ type = 9.63 ± 0.36, ‘small’ type = 8.07 ± 0.35; p < 0.001).
4. Discussion
We found that the transition from relatively large to small flowers in three species pairs in Solanum section Androceras is accompanied by shifts in both floral allometry (table 3 and figure 4) and the patterns of resource allocation to pollen and ovules. The reduction in anther–stigma distance in small-flowered taxa and the joint evolution of lower P : O ratios strongly suggest a transition towards increased self-fertilization (figure 5a,b,d and table 2). Moreover, the reduction in the relative proportion of pollen allocated to the pollinating versus feeding anthers (figure 5c) and the reduced morphological differentiation between the anther types (table 1) suggest a breakdown of heteranthery in these small-flowered taxa. Our results provide a crucial first step in the study of the recurrent breakdown of heteranthery, a complex floral morphology, where the spatial arrangement of sexual organs within a flower and their interaction with bee pollinators result in functional specialization of pollen into fertilizing and feeding functions.
(a). Replicate transitions in floral morphology
The replicate evolutionary transitions from large to small flowers in Solanum section Androceras could be caused by at least three non-mutually exclusive hypotheses: (i) a shift in pollinator assemblages, (ii) a change in the mating system towards increased self-fertilization and (iii) reproductive character displacement driven by selection to reduce interspecific mating (i.e. reinforcement). Below, we discuss each of these hypotheses.
(i). Pollinator shift hypothesis
The functional division of labour in heterantherous species relies on a close interaction between floral and pollinator morphology and behaviour [32,49,50]. Moreover, the correspondence between flower and pollinator size can potentially affect the likelihood that sexual organs contact the pollinator's body to transfer pollen [35]. For example, a small bee visiting a relatively large flower may fail to contact the stigma while collecting pollen from the feeding anthers, and thus effectively act as a pollen thief [35,51]. An increase in the reliance on or availability of smaller pollinators during the speciation process could have favoured a reduction in floral size. To date, there is limited information on the pollinator assemblages of the species studied here. Solanum rostratum is visited by bees and other insects of diverse sizes, but seems to be effectively pollinated mostly by medium- to large-size bees ([52,53], L Solís-Montero and M Vallejo-Marín 2013, unpublished data). The pollinator shift hypothesis would predict that small-flowered species, such as S. fructo-tecto, should be effectively pollinated by relatively smaller bees than their large-flowered relatives. However, this hypothesis alone would not explain why heteranthery breaks down in smaller flowers.
(ii). Mating-system shift hypothesis
The observed changes in flower size, anther differentiation and resource allocation to pollen and ovules may simply reflect selection for autonomous self-fertilization, reducing the reliance on pollinators for reproduction. Large-flowered, heterantherous Solanum have the capacity to both self- and cross-fertilize, but estimates of outcrossing rates in S. rostratum indicate that this species is mostly outcrossing in both natural populations (t = 0.70 ± 0.03; [54]) and experimental arrays (t = 0.74 ± 0.06; [53]). Unfortunately, there are currently no genetic estimates of the outcrossing rates of any of the small-flowered taxa. However, empirical studies in other closely related plant species pairs have repeatedly demonstrated that reductions in flower size and anther–stigma separation often accompany the evolution of increased self-fertilization [11,12,24,55,56]. The increase in stigma size in two of the transitions towards smaller flowers may also aid in capturing self-pollen, as it is the case in wind-pollinated species [57]. In addition, a transition from outcrossing to autonomous self-pollination is expected to be followed by a reduction in pollen production due to both increased local mate competition and because the number of pollen grains necessary to successfully reach and fertilize ovules is reduced relative to outcrossing species [58–60]. Pollen production per flower is significantly reduced in S. heterodoxum, S. grayi var. grayi and S. fructo-tecto compared with their corresponding large-flowered taxa (table 2). In nectarless, buzz-pollinated species such as Solanum, total pollen production may initially need to be particularly high because pollen is directly sought and consumed by floral visitors [39,41,61], but a loss of reliance on insect visitation for reproduction may select for lower pollen production. Moreover, to the extent that heteranthery functions as a strategy to maximize pollen export during visits by pollen-eating visitors [27], the loss of differentiation between anther sets is also consistent with a diminishing role for pollinators in reproduction.
Probably the strongest single piece of evidence that replicated shifts from large- to small-flowers among the species studied here reflect a shift in mating system comes from the observed changes in P : O ratios. A reduction in P : O ratios accompanying increased self-fertilization has been demonstrated across many groups [58,59]. In particular, P : O ratios track the breeding system of other Solanum species, as has been shown in section Basarthrum, where dioecious and self-incompatible taxa have significantly higher P : O ratios than autogamous, self-compatible ones [61]. However, even the P : O ratios observed here in some of the small-flowered taxa are relatively high compared with angiosperms in general [58], particularly for S. grayi var. grayi (table 2), which suggests that pollinators may still be required for either facilitated selfing or facultative outcrossing in these nectarless species. Determining to what extent shifts in floral morphology and P : O ratios reflect different outcrossing rates will require estimating outcrossing rates in the field.
(iii). Reproductive character displacement hypothesis
The evolution of heteranthery allows precise pollen placement on the pollinator's body, and if accuracy and precision in pollen deposition are high [62], populations can specialize in placing pollen in different areas of the pollinator's body [15,63,64]. As proposed by Whalen [33,35], the transition from large to small flowers in Solanum section Androceras could be the result of interactions between sympatric species causing reproductive character displacement in areas where closely related species co-occur. Indeed, the transition from large to small flowers in S. grayi closely matches the zone of sympatry with its sister species S. lumholtzianum [35]. Differences in flower morphology, and more specifically in the relative size and position of pollinating and feeding anthers within a flower, could serve as pre-zygotic barriers by placing and collecting pollen from different areas of the pollinator's body, or by exploiting different pollinators [35,65]. Because hybrids between species in Solanum section Androceras often fail to develop [33,35], selection should favour reproductive character displacement as divergence in floral morphology in sympatric populations could reduce pollen and ovule wastage [37,66].
Reinforcement may have played a role in the transitions in floral morphology in S. grayi var. grandiflorum and S. grayi var. grayi, although this has yet to be demonstrated. The contribution of interspecific hybridization to floral divergence in the species pairs of S. citrullifolium/S. heterodoxum and S. rostratum/S. fructo-tecto is, however, less obvious although it cannot be ruled out. The distribution of S. heterodoxum var. heterodoxum is allopatric from S. citrullifolium [33], but the geographical context during the establishment of floral differences between these two taxa is unknown. By contrast, S. fructo-tecto is distributed mostly in the highlands of central Mexico, nested within the much larger historic distribution of S. rostratum from southern Mexico to the central USA [33], and these two species can occur in sympatry. Studies of sympatric populations, either natural or experimental, could help in determining whether differences in floral morphology among species pairs contribute to reducing hybridization either via pollinator partitioning [65] or through increased selfing [67].
The hypotheses described above to explain the causes and consequences of transitions in flower size are not mutually exclusive and instead could act in concert with each other. For example, divergence in floral morphology caused by selection acting against hybridization may instigate the evolution of higher selfing rates [67]. Alternatively, a shift to visitation by smaller pollinators may negate the fitness benefits of anther dimorphism for pollen transfer as it may not be possible to compartmentalize pollen from feeding and pollinating anthers on the body of small pollinators.
5. Conclusion
Our results indicate that the replicate transitions from large to small flowers in Solanum section Androceras have been accompanied by non-scaling but predictable changes in anther–stigma separation, the extent of anther dimorphism within flowers and allocation of resources to pollen and ovules. Together these changes in morphology reveal a shift in floral function towards increased self-fertilization, and a breakdown of heteranthery. Heteranthery is a reproductive strategy based on a relatively complex floral morphology and a close interaction between plant and pollen-eating insects. Although rare across angiosperms, it has evolved multiple independent times in disparate groups with varied floral characteristics, perhaps in response to similar selective pressures imposed by pollinators [27,28]. Here, we show, for the first time, that heteranthery has also been lost multiple times within a group of closely related species. Elucidating the selective forces involved in the loss of anther dimorphism will help understanding the demise of complex floral phenotypes.
Supplementary Material
Acknowledgements
We thank S. Armbruster for the invitation to participate in the Royal Society discussion group that gave rise to this special issue, C. Dominguez and J. Fornoni for assistance with fieldwork in Mexico and B. Glover for discussions on floral evolution in Solanum. We are grateful to D. Boles, L. Mitchell and the many undergraduate volunteers that helped with data collection and plant maintenance. We are extremely thankful for the careful and detailed suggestions of the three reviewers and the Editor.
Funding statement
This research was partially supported by a Horizon PhD studentship from the University of Stirling to L.S.M., a Royal Society of London Research grant (RG2010R1) and a Scottish Plant Health License (PH/38/2010–2013) to M.V.M.
References
- 1.Darwin C. 1877. The different forms of flowers on plants of the same species. London, UK: John Murray. [Google Scholar]
- 2.Lloyd DG, Barrett SCH. 1996. Floral biology. Studies on floral evolution in animal-pollinated plants, p. 410 New York, NY: Chapman and Hall. [Google Scholar]
- 3.Whittall JB, Hodges SA. 2007. Pollinator shifts drive increasingly long nectar spurs in columbine flowers . Nature 447, 706–712. ( 10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw HD, Otto KG, Frewen BE, McKay JK, Schemske DW. 1998. Quantitative trait loci affecting differences in floral morphology between two species of monkeyflower (Mimulus) . Genetics 149, 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson P, Castellanos MC, Wolfe AD, Thomson JD. 2006. Shifts between bee and bird pollination in Penstemons. In Plant–pollinator interactions: from specialization to generalization (eds Waser NM, Ollerton J.), pp. 47–68. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Armbruster WS, Edwards ME, Debevec EM. 1994. Floral character displacement generates assemblage structure of Western-Australian triggerplants (Stylidium) . Ecology 75, 315–329. ( 10.2307/1939537) [DOI] [Google Scholar]
- 7.Nilsson L. 1992. Orchid pollination biology . Trends Ecol. Evol. 7, 255–259. ( 10.1016/0169-5347(92)90170-G) [DOI] [PubMed] [Google Scholar]
- 8.Muchhala N, Thomson JD. 2009. Going to great lengths: selection for long corolla tubes in an extremely specialized bat–flower mutualism . Proc. R. Soc. B 276, 2147–2152. ( 10.1098/rspb.2009.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fetscher AE. 2001. Resolution of male-female conflict in an hermaphroditic flower . Proc. R. Soc. Lond. B 268, 525–529. ( 10.1098/rspb.2000.1395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stebbins GL. 1955. Variation and evolution in plants. New York, NY: Columbia University Press. [Google Scholar]
- 11.Anderson IA, Busch JW. 2006. Relaxed pollinator-mediated selection weakens floral integration in self-compatible taxa of Leavenworthia (Brassicaceae) . Am. J. Bot. 93, 860–867. ( 10.3732/ajb.93.6.860) [DOI] [PubMed] [Google Scholar]
- 12.Vallejo-Marín M, Barrett SCH. 2009. Modification of flower architecture during early stages in the evolution of self-fertilization . Ann. Bot. 103, 951–962. ( 10.1093/aob/mcp015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicard A, Lenhard M. 2011. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants . Ann. Bot. 107, 1433–1443. ( 10.1093/aob/mcr023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett SCH. 1992. Evolution and function of heterostyly. Berlin, Germany: Springer. [Google Scholar]
- 15.Armbruster WS, Pelabon C, Hansen TF, Mulder CPH. 2004. Floral integration, modularity and accuracy: distinguishing complex adaptations from genetic constraints. In Phenotypic integration: studying the ecology and evolution of complex phenotypes (eds Pigliucci M, Preston K.), pp. 23–49. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Barrett SCH. 2002. The evolution of plant sexual diversity . Nat. Rev. Gen. 3, 274–284. ( 10.1038/nrg776) [DOI] [PubMed] [Google Scholar]
- 17.Barrett SCH. 2002. Sexual interference of the floral kind . Heredity 88, 154–159. ( 10.1038/sj.hdy.6800020) [DOI] [PubMed] [Google Scholar]
- 18.Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand J. Bot. 24, 163–178. ( 10.1080/0028825X.1986.10409726) [DOI] [Google Scholar]
- 19.Elle E, Hare JD. 2002. Environmentally induced variation in floral traits affects the mating system in Datura wrightii . Funct. Ecol. 16, 79–88. ( 10.1046/j.0269-8463.2001.00599.x) [DOI] [Google Scholar]
- 20.Barrett SCH, Shore JS. 1987. Variation and evolution of breeding systems in the Turnera ulmifolia L complex (Turneraceae) . Evolution 41, 340–354. ( 10.2307/2409143) [DOI] [PubMed] [Google Scholar]
- 21.Niklas KJ. 1994. Plant allometry: the scaling of form and process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 22.Diggle PK. 1991. Labile sex expression in the andromonoecious Solanum hirtum: pattern of variation in floral structure . Can. J. Bot. 69, 2033–2043. ( 10.1139/b91-256) [DOI] [Google Scholar]
- 23.Ushimaru A, Nakata K. 2002. The evolution of flower allometry in selfing species . Evol. Ecol. Res. 4, 1217–1227. [Google Scholar]
- 24.Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H. 2002. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae sl) . Am. J. Bot. 89, 37–49. ( 10.3732/ajb.89.1.37) [DOI] [PubMed] [Google Scholar]
- 25.Barrett SCH, Harder LD, Cole WW. 2004. Correlated evolution of floral morphology and mating-type frequencies in a sexually polymorphic plant . Evolution 58, 964–975. ( 10.1111/j.0014-3820.2004.tb00431.x) [DOI] [PubMed] [Google Scholar]
- 26.Delesalle VA, Mazer SJ. 2009. Size-dependent pollen: ovule ratios and the allometry of floral sex allocation in Clarkia (Onagraceae) taxa with contrasting mating systems . Am. J. Bot. 96, 968–978. ( 10.3732/ajb.0800039) [DOI] [PubMed] [Google Scholar]
- 27.Vallejo-Marín M, Da Silva EM, Sargent RD, Barrett SCH. 2010. Trait correlates and functional significance of heteranthery in flowering plants . New Phytol. 188, 418–425. ( 10.1111/j.1469-8137.2010.03430.x) [DOI] [PubMed] [Google Scholar]
- 28.Jesson LK, Barrett SCH. 2003. The comparative biology of mirror-image flowers . Int. J. Plant Sci. 164, S237–S249. ( 10.1086/378537) [DOI] [Google Scholar]
- 29.Müller F. 1883. Two kinds of stamens with different functions in the same flower . Nature 27, 364–365. ( 10.1038/027364b0) [DOI] [Google Scholar]
- 30.Luo ZL, Gu L, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers . J. Syst. Evol. 47, 43–56. ( 10.1111/j.1759-6831.2009.00002.x) [DOI] [Google Scholar]
- 31.Vogel S. 1978. Evolutionary shifts from reward to deception in pollen flowers. In The pollination of flowers by insects (ed. Richards AJ.). London, UK: Academic Press. [Google Scholar]
- 32.Vallejo-Marín M, Manson JS, Thomson JD, Barrett SCH. 2009. Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates . J. Evol. Biol. 22, 828–839. ( 10.1111/j.1420-9101.2009.01693.x) [DOI] [PubMed] [Google Scholar]
- 33.Whalen MD. 1979. Taxonomy of Solanum section Androceras . Gentes Herbarum 11, 359–426. [Google Scholar]
- 34.Stern SR, Weese T, Bohs LA. 2010. Phylogenetic relationships in Solanum section Androceras (Solanaceae) . Syst. Bot. 35, 885–893. ( 10.1600/036364410X539934) [DOI] [Google Scholar]
- 35.Whalen MD. 1978. Reproductive character displacement and floral diversity in Solanum section Androceras . Syst. Bot. 3, 77–86. ( 10.2307/2418533) [DOI] [Google Scholar]
- 36.Grant V. 1994. Modes and origins of mechanical and ethological isolation in angiosperms . Proc. Natl Acad. Sci. USA 91, 3–10. ( 10.1073/pnas.91.1.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins R. 2013. Reinforcement in plants . New Phytol. 197, 1095–1103. ( 10.1111/nph.12119) [DOI] [PubMed] [Google Scholar]
- 38.Weese TL, Bohs L. 2007. A three-gene phylogeny of the genus Solanum (Solanaceae) . Syst. Bot. 32, 445–463. ( 10.1600/036364407781179671) [DOI] [Google Scholar]
- 39.De Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination . Curr. Opin. Plant Biol. 16, 429–435. ( 10.1016/j.pbi.2013.05.002) [DOI] [PubMed] [Google Scholar]
- 40.Knapp S. 2010. On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae . Phil. Trans. R. Soc. B 365, 449–460. ( 10.1098/rstb.2009.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchmann SL. 1983. Buzz pollination in angiosperms. In Handbook of experimental pollination biology (eds Jones CE, Little RJ.), pp. 73–113. New York, NY: Scientific and Academic Editions. [Google Scholar]
- 42.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis . Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York, NY: Springer. [Google Scholar]
- 44.R Development Core Team. 2013. R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/2.15.0) [Google Scholar]
- 45.Bates D, Maechler M, Bolker B. 2013. lme4: linear mixed-effects models using S4 classes. R package version 0.999999–2 See http://CRAN.R-project.org/package=lme4.
- 46.Fox J, Weisberg S. 2010. An R companion to applied regression. Beverley Hills, CA: Sage; See http://CRAN.R-project.org/package=car. [Google Scholar]
- 47.Perez F, Arroyo MTK, Medel R. 2007. Phylogenetic analysis of floral integration in Schizanthus (Solanaceae): does pollination truly integrate corolla traits? J. Evol. Biol. 20, 1730–1738. ( 10.1111/j.1420-9101.2007.01393.x) [DOI] [PubMed] [Google Scholar]
- 48.Shingleton AW, Frankino WA. 2013. New perspectives on the evolution of exaggerated traits . Bioessays 35, 100–107. ( 10.1002/bies.201200139) [DOI] [PubMed] [Google Scholar]
- 49.Ushimaru A, Watanabe T, Nakata K. 2007. Colored floral organs influence pollinator behavior and pollen transfer in Commelina communis (Commelinaceae) . Am. J. Bot. 94, 249–258. ( 10.3732/ajb.94.2.249) [DOI] [PubMed] [Google Scholar]
- 50.Luo Z, Zhang D, Renner SS. 2008. Why two kinds of stamens in buzz-pollinated flowers? Experimental support for Darwin's division-of-labour hypothesis . Funct. Ecol. 22, 794–800. ( 10.1111/j.1365-2435.2008.01444.x) [DOI] [Google Scholar]
- 51.Hargreaves AL, Harder LD, Johnson SD. 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft . Biol. Rev. 84, 259–276. ( 10.1111/j.1469-185X.2008.00074.x) [DOI] [PubMed] [Google Scholar]
- 52.Bowers KAW. 1975. The pollination ecology of Solanum rostratum (Solanaceae) . Am. J. Bot. 62, 633–638. ( 10.2307/2441943) [DOI] [Google Scholar]
- 53.Jesson LK, Barrett SCH. 2005. Experimental tests of the function of mirror-image flowers . Biol. J. Linn. Soc. 85, 167–179. ( 10.1111/j.1095-8312.2005.00480.x) [DOI] [Google Scholar]
- 54.Vallejo-Marín M, Solis-Montero L, Souto Vilaros D, Lee MYQ. 2013. Mating system in Mexican populations of the annual herb Solanum rostratum Dunal (Solanaceae) . Plant Biol. 15, 948–954. ( 10.1111/j.1438-8677.2012.00715.x) [DOI] [PubMed] [Google Scholar]
- 55.Goodwillie C, Ritland C, Ritland K. 2006. The genetic basis of floral traits associated with mating system evolution in Leptosiphon (Polemoniaceae): an analysis of quantitative trait loci . Evolution 60, 491–504. ( 10.1111/j.0014-3820.2006.tb01131.x) [DOI] [PubMed] [Google Scholar]
- 56.Ritland C, Ritland K. 1989. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae) . Am. J. Bot. 76, 1731–1739. ( 10.2307/2444472) [DOI] [Google Scholar]
- 57.Friedman J, Barrett SCH. 2011. The evolution of ovule number and flower size in wind-pollinated plants . Am. Nat. 177, 246–257. ( 10.1086/657954) [DOI] [PubMed] [Google Scholar]
- 58.Cruden RW. 2000. Pollen grains: why so many? Plant Syst. Evol. 222, 143–165. ( 10.1007/BF00984100) [DOI] [Google Scholar]
- 59.Cruden RW. 1977. Pollen-ovule ratios: conservative indicator of breeding systems in flowering plants . Evolution 31, 32–46. ( 10.2307/2407542) [DOI] [PubMed] [Google Scholar]
- 60.Mazer SJ, Dudley LS, Delesalle VA, Paz H, Galusky P. 2009. Stability of pollen–ovule ratios in pollinator-dependent versus autogamous Clarkia sister taxa: testing evolutionary predictions . New Phytol. 183, 630–648. ( 10.1111/j.1469-8137.2009.02886.x) [DOI] [PubMed] [Google Scholar]
- 61.Mione T, Anderson GJ. 1992. Pollen-ovule ratios and breeding system evolution in Solanum section Basarthrum (Solanaceae) . Am. J. Bot. 79, 279–287. ( 10.2307/2445016) [DOI] [Google Scholar]
- 62.Armbruster WS, Hansen TF, Pelabon C, Perez-Barrales R, Maad J. 2009. The adaptive accuracy of flowers: measurement and microevolutionary patterns . Ann. Bot. 103, 1529–1545. ( 10.1093/aob/mcp095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armbruster WS, Herzig AL. 1984. Partitioning and sharing of pollinators by 4 sympatric species of Dalechampia (Euphorbiaceae) in Panama . Ann. Missouri Bot. Gard. 71, 1–16. ( 10.2307/2399053) [DOI] [Google Scholar]
- 64.Miyake T, Inoue K. 2003. Character displacement in style length between pollinator-sharing Clerodendrum trichotomum and C. izuinsulare (Verbenaceae). Plant Syst. Evol. 243, 31–38. ( 10.1007/s00606-003-0059-1) [DOI] [Google Scholar]
- 65.Muchhala N, Potts MD. 2007. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern . Proc. R. Soc. B 274, 2731–2737. ( 10.1098/rspb.2007.0670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Servedio MR, Noor MAF. 2003. The role of reinforcement in speciation: theory and data . Annu. Rev. Ecol. Evol. Syst. 34, 339–364. ( 10.1146/annurev.ecolsys.34.011802.132412) [DOI] [Google Scholar]
- 67.Smith RA, Rausher MD. 2008. Experimental evidence that selection favors character displacement in the ivyleaf morning glory . Am. Nat. 171, 1–9. ( 10.1086/523948) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.