Summary
Modern medical and hygienic practices have greatly improved human health and longevity; however, increased human lifespan occurs concomitantly with the emergence of metabolic and age-related diseases. Studies over the past decade have strongly linked host inflammatory responses to the etiology of several metabolic diseases including atherosclerosis, type 2 diabetes (T2D), obesity and gout. A common immunological factor to these diseases is the activation of the inflammasome and release of pro-inflammatory cytokines that promote disease progression. Here we review the molecular mechanism(s) of inflammasome activation in response to metabolic damage associated molecular patterns (DAMPs) and discuss potential targets for therapeutic intervention.
Introduction to Inflammasomes and Metabolic Diseases
The incidence of metabolic disorders such as type 2 diabetes (T2D), obesity, gout and cardiovascular disease have dramatically increased. Recent studies provide strong evidence suggesting an essential role of chronic inflammation in the pathogenesis of metabolic disorders. Elevated levels of circulating inflammatory mediators including cytokines and chemokines are hallmarks of chronic inflammation and are now found to promote the initiation and progression of metabolic diseases. The inflammasome complex, which leads to the processing of inactive pro-IL-1β and IL-18 into their mature forms, has been found to regulate chronic inflammation and modify physiological metabolic processes. This area of research provides promise because understanding the mechanism(s) of inflammasome activation should shed light on the development of new therapeutic regimens. This review will focus on the role of inflammasome activation in key metabolic diseases.
The discovery of conserved gene families with structural similarity has led to the revelation of important pathways in innate immunity, including the Toll-like receptors and c-type lectin receptors, which are primarily membrane associated and respond to pathogen-associated products (Takeuchi and Akira, 2010). An important advance is the discovery of the NLRs (nucleotide-binding domain, leucine-rich repeat containing, also known as nucleotide-oligomerization domain-like receptors) that encompass a large gene family encoding intracellular proteins that respond to changes in cellular homeostasis and/or microbial infection. As designated by the name, NLRs have an evolutionarily conserved arrangement of nucleotide binding domain (NBD) followed by a leucine rich region (LRR). These genes are evolutionarily conserved in both plants and animals, but unlike TLRs found in Drosophilia, NLRs are not present in lower organisms including fruit flies and nematodes, and represent a unique family of signaling molecules for higher eukaryotes (Ting and Davis, 2005). However NLRs are abundant in plants, and are classified as disease resistance (R) genes, which represent a major force to combat pathogens (Jones and Dangl, 2006). Plant NLRs also act through an intracellular route and reside in both the cytoplasm and nucleus. In animals, the NLR family includes four subgroups with distinguishing N-terminal domains: acidic transactivation, pyrin, CARD (caspase activation and recruitment domain), baculoviral inhibitory repeat (BIR)-like domain. There are variations where additional domains are found in the C-terminus. An example is the human NLRP1 protein which encodes C-terminal FIIND and CARD domains.
The NLR family includes 22 members with broad and divergent functional roles. These include two that have been found to serve as master transcriptional regulators of class I and II Major Histocompatibilty Complex (MHC) gene transcription, several that are positive or negative regulators of key signaling pathways such as NFκB and MAPK, and multiple that exhibit functions in mechanisms of cell death ranging from pyroptosis, apoptosis, necrosis and autophagy (Wen et al., 2013). Certain NLRs have more than one role in the cell and it is likely that their functional repertoire will expand with further investigation (Lupfer and Kanneganti, 2013).
The most-extensively studied NLR sub-family remains those that trigger the inflammasome leading to the activation of the cysteine protease CASPASE-1 and subsequent cleavage of pro-IL-1β and pro-IL-18 by CASPASE-1 to their mature forms, and this topic has been extensively reviewed (Latz et al., 2013; Strowig et al., 2012; Wen et al., 2013). Although there are ten NLRs with inflammasome function in response to an array of agonists (NLRP1, NLRP2, NLRP3, NLRP6, NLRP7, NLRP12, NOD2, NLRC4/IPAF, NAIP2, NAIP5), the protein NLRP3 which represents a pyrin-containing NLR, remains the most extensively studied due to its broad functional impact in numerous disease models.
NLRP3 variants with gain-of-function mutations were first found to underlie a form of inherited periodic condition characterized by arthritis, fever, skin rashes and increased serum IL-1β/IL-18 (Hoffman and Wanderer, 2010). These mutations are found in a spectrum of auto-inflammatory diseases collectively referred to as FCAS (Familial Cold Auto inflammatory Syndrome) or CAPS (Cryopyrin-associated Periodic Syndrome), which remarkably are successfully treated with the IL-1 receptor antagonist (IL-1Ra), Anakinra/Kineret (Hoffman and Wanderer, 2010). These genetic analyses in patients provided clinical evidence linking NLRP3 to IL-1β. The inflammasome was first defined in vitro though studies of NLRP1, and later NLRP3, using cell free lysates enriched for NLRP1, the adaptor ASC (apoptosis-associated speck-like protein containing CARD) and pro-CASPASE-1/5. The assembly of these components resulted in the proteolytic cleavage of CASPASE-1 and is referred to as the inflammasome complex. Biochemically, the adaptor ASC which contains both pyrin and CARD domains respectively interact with the pyrin domain of NLRP1 and the CARD domain of pro-caspases to form the inflammasome (Martinon et al., 2002). Complex assembly leads to the auto-catalytic cleavage of CASPASE-1, which then processes pro-IL-1β and pro-IL-18 to their mature forms (Hoffman et al., 2001; Sutterwala et al., 2006). Studies of Nlrp3−/− mice indicate that this gene is pivotal for the secretion of IL- 1β and IL-18 by myeloid macrophage cell lineages in response to a spectrum of pathogen or microbialderived products, called pathogen- or microbial-associated molecular patterns (PAMPs or MAMPs) and products from damaged cells, referred to as damage-associated molecular patterns (DAMPs) (Feldmann et al., 2002; Hoffman et al., 2001). Many of the metabolic byproducts associated with metabolic diseases are thought to serve as DAMPs, resulting in sterile inflammation that is not caused by microbial agents. In contrast to NLRP3, other inflammasome NLRs such as NLRC4/NAIP and NLRP1 have a more restricted repertoire of cognate ligands or agonists (Agostini et al., 2004; Mariathasan et al., 2004; Meylan et al., 2006; Miao et al., 2006); (Fink et al., 2008; Hsu et al., 2008; Moayeri et al., 2012; Newman et al., 2010) and the non-NLR inflammasome AIM2 (absence in melanoma2) senses and binds DNA. These have either not been studied in the context of metabolic diseases or have not been found to have a role in metabolic diseases. Consistent with the study in other fields, analysis of metabolic disorders and their link to the inflammasome has focused almost entirely on the NLRP3 protein, with evidence that NLRP6 also plays a role in non-alcoholic fatty liver disease models (Henao-Mejia et al., 2012). In this review, our focus will be on the study of inflammasome in four metabolic disease models in mice, representing atherosclerosis, T2D, obesity and gout. Key findings in humans will be underscored to indicate translational relevance of these findings.
The Inflammasome in Atherosclerotic Disease
Atherosclerosis is the progressive narrowing of arterial vessels due to combinatorial effects of dietary, genetic and immune factors. Disease progression can take decades and occurs through a series of stages in which fatty cholesterol deposits accumulate at branch points along the arterial wall (Weber and Noels, 2011). Macrophages are recruited to these sites and become “foam cells” due to their altered morphology following high cholesterol intake and storage as lipid droplets. The crystalline form of cholesterol is believed to rupture foam cells resulting in extracellular cholesterol deposition and further recruitment of immune cells with continued lesion expansion (Grebe and Latz, 2013). The center of the lesion is comprised of dying cells and particulate cholesterol with a fibrotic cap of collagen and smooth muscle cells. The destabilization of the cap leads to thrombosis and ischemia, resulting in acute myocardial infarction, stroke or other tissue injury (Grebe and Latz, 2013; Weber and Noels, 2011).
Cholesterol is carried through the blood via low density lipoproteins (LDL), which are endocytosed using the LDL-receptor and either immediately utilized by the cell or stored in lipid droplets as cholesterol esters (Grebe and Latz, 2013). Some lipoproteins (e.g. apolipoprotein E (APOE)) are important for clearing cholesterol-rich LDL from the blood. APOE loss-of-function mutations in humans lead to elevated serum cholesterol and targeted Apoe gene deletion in mouse models greatly increases progression of atherosclerosis when combined with cholesterol-rich diets (Cladaras et al., 1987; Zhang et al., 1992). Studies using Apoe−/− and the related Ldlr−/− (LDL-receptor deficient) mice, have implicated innate and adaptive immune cells in both exacerbation and protection from atherosclerosis, and have recently been reviewed (Libby et al., 2013). This section will focus on innate immune responses during atherogenesis with an emphasis on the role of the NLRP3 inflammasome and IL-1 signaling.
Early studies treating Apoe−/− mice with recombinant IL-1R antagonists showed that blocking IL-1 signaling reduces atherosclerosis (Elhage et al., 1998). The complimentary experiment of deleting the endogenous IL-1 receptor antagonist (IL1-Ra) gene resulted in worse disease (Devlin et al., 2002; Isoda et al., 2004; Nicklin et al., 2000). Almost 10 years later, Duewell et al. provided a direct link between cholesterol, IL-1 signaling and atherosclerosis by discovering that cholesterol crystals (CC) activate the NLRP3 inflammasome resulting in robust IL-1β release (Duewell et al., 2010; Rajamäki et al., 2010). Subsequent studies confirmed and expanded the notion that CC activates the NLRP3 inflammasome in both mouse and human (Jiang et al., 2012; Rajamäki et al., 2010; Usui et al., 2012). The mechanism for NLRP3 activation by CC includes a requirement for crystal engulfment, release of lysosomal cathepsins, generation of ROS and K+ efflux (Duewell et al., 2010; Rajamäki et al., 2010).
Autophagy has been implicated in protection from atherosclerosis and may work at multiple levels of the disease. Autophagosomes are double membrane vesicles that form around damaged organelles or intracellular pathogens and proceed to fuse with lysosomes to recycle host components and/or clear infection (Ma et al., 2013). Macrophage-specific deletion of Atg5 (an essential gene for autophagosome formation) exacerbates atherogenesis in the Apoe−/− mice and correlates with increased IL-1 β production (Razani et al., 2012). The authors suggest that autophagy protects from atherosclerosis by multiple mechanisms with one possibility being that autophagy promotes cholesterol efflux and mitigates macrophage foam cell formation, which would reduce CC formation and subsequent triggering of the inflammasome. A second possibility is that autophagic removal of damaged mitochondria and/or lysosomes decreases intracellular ROS and subsequently reduces NLRP3 activation and IL-1β release.
The ROS for inflammasome activation is hypothesized to be mitochondrial derived (mROS) and allows for downstream release of NLRP3 co-activators. A sensor of oxidative stress associated with NLRP3 activation is NFE2-related factor 2 (NRF2), which is a known transcriptional regulator that is activated in response to oxidative stress and upregulates expression of anti-oxidant genes to restore homeostasis (Ma, 2013). Freigang et al. reported that Nrf2−/− mice have decreased atherosclerotic disease correlating with decreases in both IL-1α and IL-1β release by dendritic cells following CC treatment (Freigang et al., 2011). NRF2 is induced by LPS stimulation and is present in both cytosolic and nuclear compartments (Piantadosi et al., 2011). Therefore, one possibility is that NRF2 is directly involved in assembly of NLRP3 inflammasomes in the cytosol following CC treatment. Alternatively, NRF2 may function in the nucleus to prime inflammasome activation by inducing transcription of inflammasome related genes (e.g. Nlrp3, Casp1, Il1b). Cholesterol treatment induced expression of NRF2 target genes, suggesting that NRF2 is activated in response to CC; however, mRNA levels of inflammasome-related genes were not reported (Freigang et al., 2011; Ma, 2013). Further experimentation using Nrf2−/− cells will be necessary to determine its mechanistic role during NLRP3 inflammasome activation.
Cardiolipin was recently identified as a mitochondrial derived phospholipid capable of activating the NLRP3 inflammaosme in response to mitochondrial damage. During normal homeostasis cardiolipin resides on the mitochondrial innermembrane (IM) and re-localizes to the outermembrane (OM) in response to mitochondrial stress where it is suggested to bind NLRP3 via the LRR domain (Iyer et al., 2013). Perturbing cardiolipin synthesis in vitro decreases IL-1β responses and reduces NLRP3 association with the mitochondria. This occurs using both mROS dependent and independent NLRP3 inflammasome activators (e.g. silica and linezolid, respectively)(Iyer et al., 2013). More studies are required to determine if cardiolipin functions exclusively as a scaffold for inflammasome assembly or if it directly activates NLRP3. Due to its prokaryotic origins, cardiolipin is suggested to be an “endogenous PAMP” with the capacity to induce apoptotic and inflammatory signaling following changes in mitochondrial structure. The generation of mROS following phagocytosis of CC is a potential trigger for cardiolipin re-localization and NLRP3 activation. Further studies will be needed to determine if cardiolipin is involved in NLRP3 activation during atherosclerosis and other metabolic diseases.
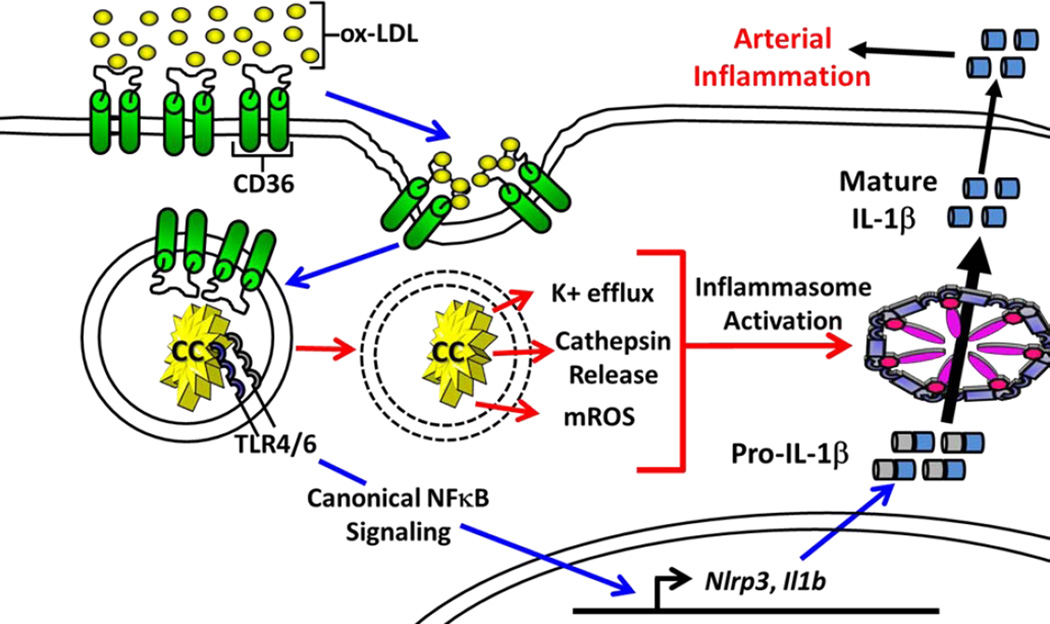
Macrophages have been found to de novo synthesize CC following treatment with oxidized-LDL (ox-LDL), which suggests that innate immune cells not only mount inflammatory responses to pre-formed CC, but are also contributors to arterial cholesterol accumulation (Duewell et al., 2010). Recent studies describe the process by which ox-LDL is recognized by the cell surface receptor CD36 and facilitates intracellular CC formation (Sheedy et al., 2013). The ability to convert ox-LDL to CC was independent of Tlr4, Tlr6 or Nlrp3; however, the ability to sense newly formed crystals via the inflammasome required all of these genes (Sheedy et al., 2013). This is consistent with a previous report that CD36 combined with TLR signaling responds to ox-LDL by inducing NF-κB-mediated transcription of Il1b, which is a key step for inflammasome priming (Stewart et al., 2010).
The above studies provide a mechanism by which select DAMPs associated with atherosclerosis, e.g. ox-LDL, are sufficient to provide both signal 1 and 2 for inflammasome activation. These data are also consistent with a previous report showing that atherosclerosis in germfree mice occurs independently of microbial colonization or infection (Wright et al., 2000). Together, these studies indicate that microbial PAMPs are not required for atherogenesis and provide a mechanism for how CC alone can trigger NLRP3 inflammasome activation in a sterile environment (Figure 1)(Sheedy et al., 2013). This does not rule out the possibility that infection can aggravate atherosclerotic lesions; in fact some studies have linked atherosclerosis with certain pathogens (e.g. C. pneumoniae, herpes virus infection)(Ross, 1999). It is unknown whether macrophages in vivo respond exclusively to CC formed inside the cell, or if they also respond following phagocytosis of preformed crystals. One of the major pathogenic factors of atherosclerosis is the continuous accumulation of CC in lesions that ultimately pierce through and destabilize the fibrotic cap leading to thrombosis. Therefore, blocking macrophage mediated CC formation may be equally, if not more, important than blocking the downstream inflammatory responses leading to atherogenesis.
Figure 1. Sterile Priming and Activation of the NLRP3 Inflammasome by Ox-LDL and Cholesterol Crystals.
Signal 1, Inflammasome priming (blue arrows); ox-LDL is endocytosed by the scavenger receptor CD36 and is transformed into cholesterol crystals (CC) that are sensed by TLR4 and TLR6, which induce NF-κB dependent transcription of Il1b and inflammasome genes. Signal 2, Inflammasome assembly and activation (red arrows); CC induce phagolysosome destabilization and release of lysosomal cathepsins. Assembly of the NLRP3 inflammasome in response to CC also requires mROS and K+ efflux, however the kinetics of these events are unknown. The activated inflammasome processes pro-IL-1β into its mature form that is subsequently secreted. The example depicted represents a model of sterile inflammation by CC in atherosclerosis; however, CD36 was also shown to facilitate conversion of IAPP into an amyloid form known to activate the NLRP3 inflammasome in T2D.
The discovery that CC activates the NLRP3 inflammasome has favored the hypothesis that IL-1β and IL-18 are major contributors of atherosclerosis (Duewell et al., 2010; Elhage et al., 2003). Some groups have failed to find a role for the NLRP3 inflammasome using both Apoe−/− and Ldlr−/− mouse models of disease and they suggest that IL-1α is the major pathogenic factor in atherogenesis (Freigang et al., 2013; Menu et al., 2011). IL-1α and IL-1β share a common receptor, but their mechanisms of maturation and release differ. Direct processing of IL-1β requires formation of caspase-1 containing inflammasomes, whereas the processing and secretion of IL-1α remains unclear and may occur through multiple mechanisms. One study suggests that release of intracellular IL-1α also requires inflammasome activation and release of processed IL-1β with the hypothesis that mature IL-1β serves as a carrier for intracellular IL-1α through the unconventional secretory pathway (Fettelschoss et al., 2011). A second study suggests that IL-1α release can occur in an inflammasome independent manner that requires calcium influx and calpain-like proteases (Gross et al., 2012). A recent study used chimeric mice in which WT, IL1a−/− or IL1b−/− bone marrow was transplanted into the atherosclerosis susceptible Ldlr−/− mouse strain combined with a cholesterol rich diet. Atheroma lesion size was reduced in mice receiving either IL1a−/− or IL1b−/− bone marrow; however, only the decrease observed with IL1a−/− transplantation achieved statistical significance (Freigang et al., 2013). The authors further showed that IL-1α is produced in response to highly abundant unsaturated fatty acids (uFA) found in atheromas (including oleic, linoleic, and arachidonic acid). Mechanistically their studies suggest that these uFAs trigger mitochondrial uncoupling and release of intracellular Ca2+ stores to promote IL-1α release via calpain proteases. Ucp2−/− macrophages (deficient in mitochondrial uncoupling protein 2) have partial decreases in Ca2+ mobilization and IL-1α release, suggesting that mitochondrial uncoupling is involved; however, the incomplete phenotype suggests other factors contribute to this process (Freigang et al., 2013). Further studies are needed to determine the pathway by which select uFA trigger IL-1α release, in particular which receptors and downstream molecules in conjunction with UCP2 lead to mitochondrial uncoupling and Ca2+ mobilization.
The immune component in atherosclerosis is undeniable; nevertheless, it is only part of disease progression and studies in mice regarding whether IL-1α or IL-1β is the main pathogenic factor remain controversial and ultimately the combinatorial effects of both cytokines likely contribute to disease (Ross, 1999). The discrepancies may be attributed to differences in experimental design; including mouse strain, type of diet, and duration of study. It is clear that treating mouse macrophages with CC activates the NLRP3 inflammasome and simultaneously results in release of IL-1β and IL-1α (Duewell et al., 2010; Freigang et al., 2013). In contrast, experiments treating human monocytes and macrophages with CC show enhanced release of IL-1β, but no change in IL-1α (Rajamäki et al., 2010). Additionally, most patients will likely enter the clinic after atherosclerotic plaques have formed, which will require a multifaceted treatment approach including changes in diet, exercise, cholesterol lowering medication and immune modulating therapeutics. Further studies assessing the cytokine profiles in human atherosclerotic lesions will be instrumental in determining which molecules of the IL-1 axis will be the most effective targets for disease treatment. Perhaps targeting CD36 will be beneficial for combating atherogenesis by reducing CC formation and atherosclerotic plaque expansion through decreased availability of cholesterol and uFA that trigger downstream inflammatory responses.
Inflammasome and Type 2 Diabetes
T2D is among the most common human health problems worldwide and recent studies have demonstrated that chronic inflammation is a key feature of disease. Elevated levels of circulating inflammatory mediators, such as tumor necrosis factor (TNF), interleukins and cytokine-like proteins known as adipokines, are hallmarks of chronic inflammation in T2D (Donath and Shoelson, 2011; Hotamisligil, 2010). Recently, IL-1β has been strongly linked with the pathogenesis of T2D and mechanistically has been shown to work by multiple means. First, IL-1β-induced JNK activation induces serine phosphorylation of insulin receptor substrate-1 (IRS1) and blunts the activity of the insulin-PI3K-Akt signaling pathway in insulin targeted tissues and cells. Second, IL-1β can efficiently evoke the expression of other inflammatory mediators through IL-1R signaling and provides the basis for a self-amplifying cytokine network (Arend et al., 2008 IL-18, and IL-33 families of cytokines). Third, IL-1β induces cell stress, such as ER stress and oxidative stress, both of which have been tightly linked to the pathogenesis of T2D (Cardozo et al., 2005; Verma and Datta, 2010). These findings highlight IL-1β as a potential therapeutic target for the treatment of T2D and small-scale studies employing recombinant IL-1 receptor antagonist (IL-1Ra) provide encouraging data in the treatment of T2D (Larsen et al., 2007).
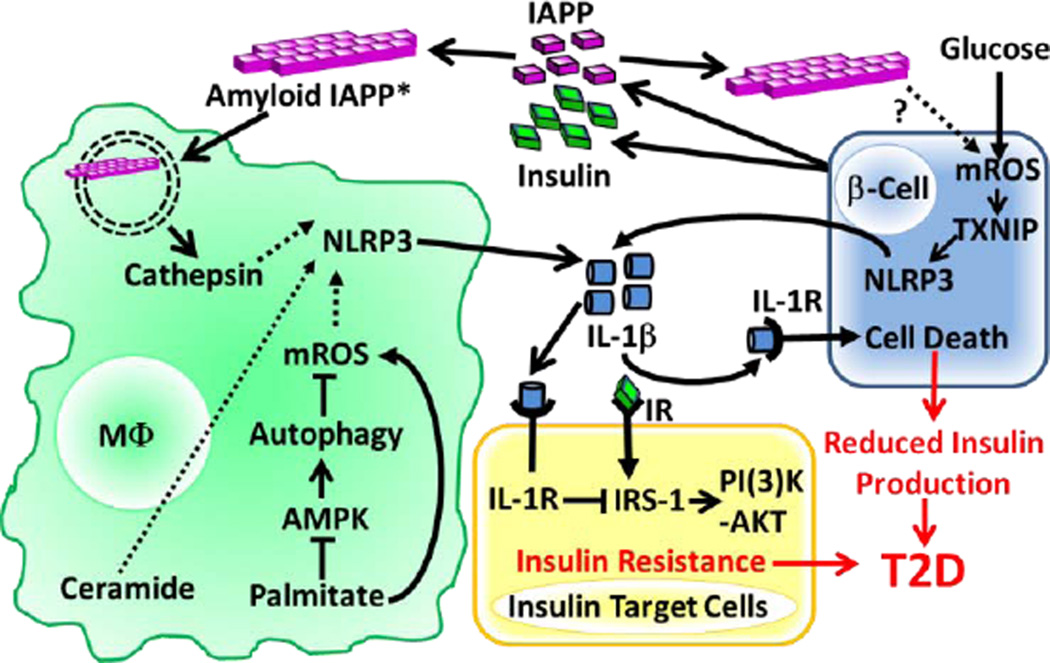
Based on the potential importance of IL-1β in the pathogenesis of T2D, many studies have been focused on the identification of endogenous and exogenous ligands that activate the inflammasome in murine models. Evidence exists for multiple endogenous DAMPs relevant to T2D that activate the NLRP3 inflammasome. For example, islet amyloid polypeptide (IAPP; also known as amylin) is a hormone that is co-secreted with insulin. In patients with T2D, IAPP can form an amyloid structure that is deposited in the islet interstitium. Amyloid IAPP is an inducer of NLRP3 inflammasome in mouse macrophages involving mechanisms that require IAPP phagocytosis and lysosome destabilization (Figure 2)(Masters et al., 2010). Therefore, IAPP has been considered a significant DAMP for T2D (Westermark et al., 2011). Interestingly, the surface receptor CD36 (described in the atherosclerosis section) also facilitates the conversion of soluble IAPP to its amyloid form and may also be a critical target for T2D therapeutics (Sheedy et al., 2013).
Figure 2. NLRP3 Inflammasome Activation in Response to T2D-associated Metabolic DAMPs.
IAPP is co-secreted with insulin from pancreatic β-cells and the amyloid form of IAPP is internalized by macrophages (soluble IAPP can also become the amyloid form via CD36 mediated internalization*) and destabilizes the phagolysosome resulting in cathepsin release. Palimitate was shown to increase mROS by reducing autophagy through blockade of AMPK signaling. The precise mechanisms by which mROS, cathepsins and ceramide activate the NLRP3 inflammasome remain unknown (dashed lines). Glucose and possibly IAPP can also stimulate NLRP3 activation in β cells by a mechanism that involves mROS and TXNIP activation. Release of IL-1β downstream of NLRP3 activation induces β cell death and blocks insulin receptor (IR) signaling in insulin target cells leading to T2D. (High serum glucose levels are hypothesized to contribute to T2D by maximizing Il1b mRNA expression through the ‘Warburg Effect’ downstream of glycolysis/HIF1α signaling (not depicted).
Another significant danger signal in T2D is a high level of circulatory glucose, which has been reported to induce IL-1β production by pancreatic β-cells in vitro and in turn causes functional impairment and apoptosis of β-cells in an autocrine manner (Maedler et al., 2002; Zhou et al., 2010). In myeloid cells glucose is required for NF-κB-dependent, but NLRP3 inflammasome-independent, pro-IL-1β and IL-6 production without altering IAPP-induced IL-1β release (Masters et al., 2010). Therefore, the molecular mechanism underlying the glucose-induced IL-1β release by islets remains to be determined. Recent studies also reported that products of long chain saturated fatty acid metabolism such as palmitate and ceramide can induce NLRP3 inflammasome activation (Vandanmagsar et al., 2011; Wen et al., 2011). Mechanistically, palmitate signals through an AMP-activated protein kinase (AMPK)-autophagy-mROS pathway to activate the NLRP3 inflammasome, and provides an example of a T2D-associated DAMP that might affect NLRP3 inflammasome activation and promote insulin resistance (Figure 2)(Wen et al., 2011). Interestingly, two widely used anti-diabetic drugs also provide a link between insulin resistance and NLRP3 inflammasome activation. For example, glyburide has been shown to inhibit NLRP3 inflammasome activation (Lamkanfi et al., 2009), and metformin may decrease IL-1β levels by activating AMPK (Lee et al., 2013).
Linking insulin resistance to the NLRP3 inflammasome has been extended to gene-deletion mouse strains (Stienstra et al., 2010; Stienstra et al., 2011; Vandanmagsar et al., 2011; Wen et al., 2011; Zhou et al., 2010). Deficiency in Nlrp3 or inflammasome-associated genes such as Asc/Pycard and Casp1 improved glucose tolerance and insulin sensitivity following exposure to a high fat diet (HFD). This was accompanied by lower inflammatory cytokines in the serum and metabolic tissues (e.g. liver and adipose tissue) of inflammasome deficient mice, with increased signaling though the insulin-PI3K-Akt pathway. These studies provide a direct link between the NLRP3 inflammasome, chronic inflammation and insulin resistance.
The mechanism of NLRP3 inflammasome activation downstream of oxidative and ER stress has gained special attention since both of these signals are implicated in the pathogenesis of T2D (Hotamisligil, 2010; Wellen and Thompson, 2010). ROS has been shown to activate the NLRP3 inflammasome by either promoting interactions between NLRP3 and thioredoxin-interacting protein (TXNIP)(Zhou et al., 2010) or by increasing Nlrp3 expression at the transcriptional level (Bauernfeind et al., 2009). ER stress induced by various pharmacological agents results in the activation of the NLRP3 inflammasome; however, none of the known signal transducers downstream of ER stress (PERK, IRE1α and ATF6) seem to be required for ER stress-induced NLRP3 inflammasome activation. Another two recent studies confirmed that ER stress activates the NLRP3 inflammasome and further implicates ER stress induced inflammasome signaling as an essential pathway leading to β-cell death and inflammation (Lerner et al., 2012; Oslowski et al., 2012). Additionally, it is known that ER stress elevates cytosolic Ca2+ concentrations raising the possibility that ER stress activates the NLRP3 inflammasome through a Ca2+- dependent manner. These studies establish a link between cell stress responses and inflammation and suggest the former could be a major driving force in the progression of T2D.
Other potential regulatory mechanisms of inflammasome activation during insulin resistance include decreased autophagic activity and altered mitochondrial respiration. Autophagy is linked to T2D through studies with both genetic (ob/ob) and HFD-induced obesity models using mouse strains with impaired autophagy (Yang et al., 2010). Deletion of either the Atg16l1 or Atg7 autophagic genes results in the activation of the NLRP3 inflammasome and is believed to result from disrupted mitochondrial homeostasis (Nakahira et al., 2011; Saitoh et al., 2008; Wen et al., 2011; Zhou et al., 2011). These studies provide evidence that autophagy negatively regulates NLRP3 inflammasome activation by elimination of unhealthy mitochondria, thereby decreasing intracellular ROS. AMPK and/or motor signaling pathways may be upstream modulators of autophagic activity through the direct phosphorylation of ATG1 (Egan et al., 2011; Kim et al., 2011). Therefore, a reasonable possibility is that defective autophagy associated with insulin resistance may promote inflammasome activation resulting in a self-amplifying inflammatory response in insulin target tissues.
Another recent study reported that the increased ratio between glycolysis and mitochondrial oxidative phosphorylation (OXPHOS) is essential for optimal Il1b transcription through the effect of hypoxia-inducible factor-1α (HIF-1) and suggests an indirect regulatory mechanism for IL-1β production. (Tannahill et al., 2013). Activation of macrophages with LPS induces a metabolic reprogramming process known as the “Warburg effect” that increases aerobic glycolysis and decreases mitochondrial OXPHOS, leading to the accumulation of TCA cycle intermediate metabolites (Rodríguez-Prados et al., 2010; Tannahill et al., 2013). Of these, succinate induces the accumulation of HIF-1α and consequently promotes Il1b transcription. Based on the high level of circulating glucose associated with T2D, one could hypothesize that macrophages in T2D patients maintain a heightened state of inflammasome priming due to maximal IL1B transcription downstream of glycolysis and HIF1α. It is tempting to speculate that the inflammasome activation in these highly primed macrophages would occur following exposure to host DAMPs (e.g. IAPP, palmitate, ceramide, glucose) and provides a working model for sterile inflammation associated with T2D.
Obesity and the Inflammasome
The rise of obesity due to over-nutrition and reduced activity is a major underlying cause of multiple metabolic diseases, and a key discovery is the concept that inflammation and obesity interact in a vicious cycle to exacerbate each other’s impact on health (Gregor and Hotamisligil, 2011). The above sections have described many of the studies where HFD induces cholesterol crystal formation, insulin resistance and/or pancreatic changes associated with atherosclerosis or diabetes. This section will focus on the impact of inflammasome activation and its products on obesity per se.
Obesity in mice is studied using either a genetic model (e.g., ob/ob or db/db) or with chow representing a HFD. Extensive studies support the concept that adipose tissue is a source of proinflammatory cytokines and chemokines that exacerbate inflammation (Gregor and Hotamisligil, 2011). Several seminal studies have reinforced this idea by linking inflammasome with obesity in a bidirectional fashion, where metabolic byproducts of obesity activate the inflammasome and inflammasome associated cytokines influence the outcome of obesity. Studies using HFD have shown that the inflammasome components ASC, NLRP3 and CASPASE-1 govern adipocyte differentiation and adipogenic gene expression, which promote obesity. Furthermore, inflammasome expression is not only elevated in the macrophage population found in adipose tissues (Stienstra et al., 2012; Stienstra et al., 2011), but also in isolated adipocytes which can produce IL-1β (Koenen et al., 2011). Thus the inflammasome can be activated in both adipocytes and adipose-associated innate immune cells.
Adipocyte differentiation in both human and mouse is associated with increased CASPASE-1 expression and cleavage, which can induce an insulin resistant status (Stienstra et al., 2012; Stienstra et al., 2011). The major impact of inflammasome activation on obesity has been attributed to influences of IL-1β. Preadipocytes lacking either the gene encoding CASPASE-1 or NLRP3 are more metabolically active with improved adipogenesis. In addition to in vitro cell culture studies, treatment of whole animals with a CASPASE-1 inhibitor increased insulin resistance, and Casp1 deletion changes adipose tissue morphology represented by smaller adipocytes, reduced body fat mass and free fatty acids, and increases in key metabolic regulators of insulin sensitivity (e.g. PPARγ, adiponectin and GLUT4)(Stienstra et al., 2010). A separate study showed that leptin-deficient adipocytes from the visceral abdominal and subcutaneous adipose tissues (VAT and SAT, respectively) of (ob/ob and db/db) obese mice also exhibit increased Asc, Casp1 and Nlrp3 expression in VAT, with less consistent increases in SAT (Giordano et al., 2013). The authors found that adipocytes from obese mice also display morphology consistent with pyroptosis, an inflammatory cell death frequently associated with inflammasome activation, and furthermore Casp1 was not found in a transgenic strain where adipocytes die from apoptosis. These experiments dissociate apoptosis from inflammasome activation, and suggest that inflammasome activation in adipocytes leads to pyroptosis in obese mice. Various inducers of inflammasome activation in fatty tissues have been described in the T2D section, however deletion of the P2X7 gene, which encodes a cell-membrane ion channel receptor for extracellular ATP that causes potassium efflux, had no effect on inflammasome activation in adipose tissues (Sun et al., 2012).
One of the outcomes of a HFD and subsequent obesity is a prevalence of non-alcoholic fatty liver disease (NAFLD) (Marchesini et al., 2003), which can progress to non-alcoholic steatohepatitis (NASH). In mice, the disease can be modeled by using a diet deficient in both methionine and choline or by feeding a HFD. Clinical outcomes include steatosis, cirrhosis, hepatitis and fibrosis. Recently, a link between NLRs that exhibit inflammasome function and NAFLD has been reported. One study found that ASC, NLRP3 and a new inflammasome NLR, NLRP6, can all protect against NAFLD and NASH progression via IL-18 dependent and IL-1β independent mechanism (Henao-Mejia et al., 2012). Interestingly, the tissue origin of the inflammasome that protects against disease symptoms resides in the non-myeloid and non-hepatic cells. Disease is exacerbated in inflammasome-deficient strains and is transmissible to wildtype strains by co-housing, and is attributed to microbiota transfer. In contrast to this study, another report used HFD to induce NASH and found an opposing role for the inflammasome in that Casp1−/− deficient mice showed improved disease outcome and attenuated HFD-induced hepatitis injuries (Dixon et al., 2013). The most likely reason for these opposing findings resides in how NASH was induced in the two studies, the former by a methionine-choline deficient diet, and the latter by HFD.
However the association of inflammasome activation with obesity is complex, and not all inflammasome and CASPASE-1 cleaved products enhance obesity or insulin resistance. A case-in-point is the very intriguing roles of IL-18 in obesity. IL-18 was found to control food intake, and Il18−/− mice and Il18r−/− mice exhibited hyperphagia (increased food intake), insulin resistance and hyperinsulinemia (Netea et al., 2006; Zorrilla et al., 2007). These overt phenotypes of Il18−/− mice were also associated with increased gluconeogenesis gene expression and defective STAT3 phosphorylation (Netea et al., 2006). As proof of principle of the importance of IL-18, administration of recombinant IL-18 into Il18−/− mice reversed hyperglycemia and increased STAT3 phosphorylation. However in addition to hyperphagia, adult Il18−/− mice gained significantly more weight (2- to 3-fold) than control mice based on per unit of energy consumed regardless of the fat content of the diet (Zorrilla et al., 2007), indicating that IL-18 reduced feed efficiency. Another report showed an additional level by which IL-18 could affect metabolism, in that Il18−/− mice exhibited reduced skeletal AMPK, a key metabolic regulator (Lindegaard et al., 2013). Treating skeletal muscle cells with IL-18 activated AMPK and increased fat oxidation, and more important, in vivo introduction of IL-18 into the skeletal muscle resulted in increased AMPK activation and reduced weight gain. These findings collectively indicate multiple roles of IL-18 in regulating food intake, energy expenditure, fat oxidation, obesity and insulin sensitivity (Zilverschoon et al., 2008). Paradoxically, weight loss has been correlated with decreased IL-18 in humans, while increased circulating IL-18 has been found to correlate with enhanced body mass index, adiposity, triglycerides, serum glucose and insulin resistance in a variety of metabolic syndromes (Esposito et al., 2002; Olusi et al., 2003; Trøseid et al., 2010). It was discovered that leukocytes isolated from obese or T2D patients showed defective responses to IL-18, leading the authors to advance a hypothesis that resistance of IL-18 led to increased IL-18 levels in these patients (Trøseid et al., 2010; Zilverschoon et al., 2008).
As additional correlates to the murine studies, several groups have linked inflammasome activation or expression with obesity in humans and revealed a complex scenario. Although the human studies were restricted to correlative analyses and primarily focused on mRNA expression, these reports showed evidence of heightened inflammasome activity in obese humans, with caveats described below. Experiments in animals have found that mice fed an ad libitum diet showed significantly higher Il1b, Nlrp3 and Asc expression in both VAT and SAT when compared to mice placed on caloric restriction diet. Impressively a correlating pattern was observed in human SAT from ten obese T2D patients undergoing weight loss that showed significant reductions in IL1B, NLRP3 and ASC expression (Vandanmagsar et al., 2011). This change was accompanied by a reduction in CD4+ and CD8+ effector T cells in adipose tissue. Another study of twenty one patients who underwent laparoscopic adjustable gastric banding to achieve weight loss showed decreased Il1b mRNA in SAT six months after the procedure following significant weight loss, but Il18 and Nlrp3 expression in SAT was unaffected. Weight loss also affected liver inflammasome gene expression with a reduction of IL1B and IL18 expression accompanied by elevated IL37. IL-37 is generally found to be anti-inflammatory, thus suggesting that weight loss results in an anti-inflammatory state. Another study revealed extra layers of complexity by examining inflammasome components and products in VAT versus SAT in ten overweight individuals (Koenen et al., 2011). VAT contained significantly higher ASC and CASPASE-1 activity and increased proinflammatory cytokines/chemokines (e.g. IL-1β, IL-18, IL-8, IL-6), accompanied by increased CD8 T cell infiltration. A recent study of ten obese men with insulin resistance and impaired glucose tolerance showed that adipose tissue transcripts for CASP1, the TH1 marker TBX21 and TH17 marker RORC were significantly elevated compared to normal weight controls. Whether elevated inflammasome expression precedes changes in inflammatory T effectors, or vice versa, will be important in establishing a causal relationship between inflammasome and T cell activation (Goossens et al., 2012).
Another analysis reinforces the association of inflammasome components with the heterogeneous outcome of obesity in humans. This study analyzed inflammasome expression in VAT, but differentiated between obese individuals with metabolic complications versus those who are metabolically normal and healthy. The study showed that IL1B and NLRP3 mRNA expression and IL-1β protein secretion were increased in VAT of obese individuals with metabolic abnormalities compared to those without metabolic complications (Esser et al., 2013). Increased inflammasome gene expression in the unhealthy population was accompanied by increased adipose tissue macrophages which also showed increased CASP-1 and IL1B expression. The exact agonist(s) leading to VAT inflammasome gene expression and activation remains unclear and further definition of these agonist(s) in unhealthy obese individuals will provide crucial information for delineating why obesity does not lead to metabolic abnormalities in all individuals. Thus, further study of the inflammasome in obese humans will provide a first step towards translating murine studies to clinical applications. Larger cohorts grouped by gender, age, weight and the existence of metabolic complications coupled with refined analysis of different adipose and inflammatory components are needed to understand the relationship between inflammasome activation and obesity in humans. Furthermore, a holistic picture including changes in inflammasome activity combined with other key inflammatory cytokines (e.g. TNF, IL-6) and T effector populations is ultimately necessary to provide a more complete understanding of obesity-related inflammation (Moschen et al., 2010).
Inflammasomes and Gouty Arthritis
Gout has been recognized for centuries as a metabolic and inflammatory disease; however, it was only 50 years ago that monosodium urate (MSU) was identified as the etiological agent (Shi et al., 2010). Recent studies have focused on the immunological aspects of this disease, specifically on how MSU is recognized as a DAMP (Rock et al., 2013). Formation of MSU crystals is believed to occur when levels of uric acid (UA) in serum (sUA) reach a saturation point (~6-7mg/dl) and combine with Na+ ions to form crystals. Alternatively, these crystals form following the robust release of UA during cell death (Ghaemi-Oskouie and Shi, 2011; Rock et al., 2013). In either case, the crystallization process is likely facilitated by serum antibodies that bind UA and form nucleating crystals that eventually expand and accumulate in the articular/peri-articular tissue of peripheral joints (Ghaemi-Oskouie and Shi, 2011). Elevated sUA levels are associated with rich diet, alcohol consumption and genetic background (Rider and Jordan, 2010). Interestingly, elevated sUA/MSU crystals are not sufficient for gout development as many individuals with high sUA are asymptomatic, therefore other host and/or pathogen factors combined with MSU deposition fuel the inflammation associated with this debilitating disease (Tao et al., 2013).
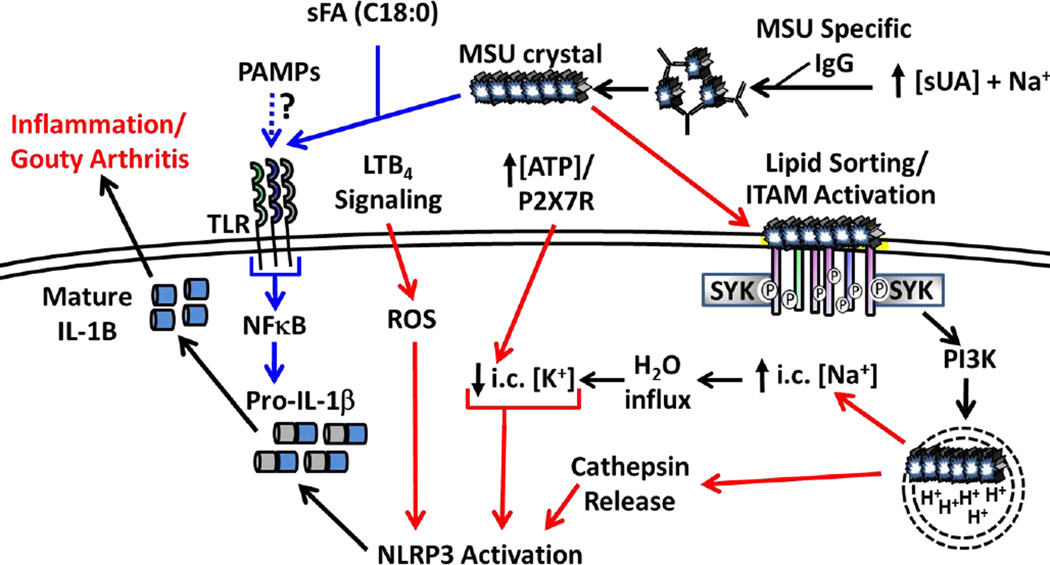
Cellular recognition of MSU occurs through variety of non-mutually exclusive mechanisms that likely depend on how and where the crystals form. MSU specific antibody is detectable in many gout patients and it has been reported to bind complement proteins, which have the capacity to enhance MSU uptake by phagocytic cells (Ghaemi-Oskouie and Shi, 2011). Cell surface receptor binding of MSU to CD14, TLR2 and TLR4 results in inflammatory signaling through p38 phosphorylation and NF-κB signaling, in particular enhanced transcription of Il1b, which is required for priming inflammasome responses (Figure 3)(Scott et al., 2006). More recent studies indicate that MSU can bind the surface of dendritic cells and activate the SYK/PI3K signaling cascade in a receptor independent manner (Figure 3) (Ng et al., 2008). This occurs through a process called “lipid sorting” in which cholesterol rich lipid rafts interact with MSU electrostatically and order the membrane in such a way to activate downstream signaling events through immunoreceptor tyrosine-based activation motif (ITAM) containing receptors. Clustering triggers ITAM phosphorylation and provides docking sites for kinases (e.g. SYK) to propagate downstream signals resulting in enhanced phagocytosis (Figure 3)(Ng et al., 2008). Other studies have shown that select saturated fatty acids (C18:0) can significantly augment MSU induced IL-1β release in a TLR2 dependent manner and further strengthens the notion that metabolic by-products can synergize to instigate and/or amplify inflammatory diseases (Joosten et al., 2010).
Figure 3. NLRP3 Activation Downstream of MSU Crystal Formation.
High serum UA levels combined with Na+ ions allows for MSU crystallization, which can be facilitated by MSU specific IgG. MSU crystals prime the inflammasome (signal 1, blue arrows) though PRRs (TLR2, TLR4, CD14) and is enhanced in the presence of select sFA (e.g. C18:0). Induction of pro-IL1β may also occur in response to transient PAMP exposure during infection. MSU binding directly to the plasma membrane activates ITAM-containing receptors in a process called “lipid sorting” followed by binding of adaptor molecules (e.g. SYK) that trigger enhanced MSU uptake. Break down of MSU in acidified endosomes is hypothesized to increase intracellular Na+ levels, draw H2O into the cell and effectively lower the apparent intracellular K+ concentration resulting in NLRP3 inflammasome activation (signal 2, red arrows). High ATP concentrations correlating with high sUA levels may enhance K+ efflux via the P2RX7 receptor. MSU crystals can also rupture the phagolysosome resulting in cathepsin release combined with ROS downstream of LTB4 signaling leading to inflammasome activation. The combined effects result in processing and secretion of mature IL-1β, which promotes local inflammation associated with gouty arthritis.
Research in the last decade has elucidated the molecular mechanisms that make MSU such an inflammatory substance through release of cytokines/chemokines (e.g. IL-1β, IL-6, CXCL1 and CXCL2) resulting in inflammatory cell recruitment. Studies by Martinon et al. demonstrated that IL-1β and IL-18 maturation and release in response to MSU is dependent on NLRP3, ASC, CASPASE-1 and MyD88 using both human and mouse ex-vivo monocyte/macrophage cultures (Martinon et al., 2006). In vivo studies demonstrated that inflammasome deficient and Il1r−/− mice had significantly less neutrophil recruitment compared to WT controls in response to peritoneal MSU injection, suggesting the importance of inflammasome and IL-1β in responses to MSU (Martinon et al., 2006). The importance of IL-1 in MSU mediated inflammation is also highlighted by an experiment in which WT BM was adoptively transferred into Il1r−/− mice and failed to recruit neutrophils following peritoneal MSU injection, whereas the reciprocal transfer (Il1r−/− BM into WT recipient) resulted in normal inflammation. These data support a model in which IL-1β is produced initially by innate immune cells; however, it is the signaling through IL-1R on non-hematopoietic cells that ultimately leads to secretion of chemotactic factors and neutrophil influx following MSU challenge. The above studies have led to the hypothesis that NLRP3 is a predominant sensor of MSU and that IL-1β is the major effector cytokine produced in gout. In contrast, another report suggests that in vivo the combination of fatty acid C18:0 and MSU triggers local IL-1β release in manner that appears to bypass the need for NLRP3, but still requires CASPASE-1 and ASC, suggesting that inflammasomes other than NLRP3 could be involved (Joosten et al., 2010).
The sensing of MSU requires the LRR–domain of NLRP3, but has not been shown to occur through direct interaction with MSU crystals, similar to other findings with NLRP3 agonists (Hoffman et al., 2010). Rather, the prevailing hypothesis is that NLPR3 activation results from changes in cellular homeostasis (e.g. intracellular redox state, or potassium levels). One study suggests that leukotriene B4 (LTB4) is an intermediary molecule linking MSU and NLRP3 inflammasome activation. Mice deficient in the enzyme that synthesizes LTB4 are impaired for IL-1β and CXCL-1 production and have reduced neutrophil infiltration following MSU challenge in mouse model of knee joint inflammation. Further in vitro studies showed that LTB4 is sufficient to induce CASPASE-1 cleavage and IL-1β secretion, and that this activity is dependent on the ability of LTB4 to generate ROS (Figure 3). The mechanism by which ROS activates the inflammasome is still unclear, but the origin of ROS appears to be mitochondrial as mutating a major subunit of the phagosomal NADPH oxidase does not affect inflammasome activation by MSU (Hornung et al., 2008; Zhou et al., 2011).
Another proposed mechanism for NLRP3 activation in response to MSU is depletion of intracellular potassium (K+). One group suggests that low pH in the phagolysosome dissociates MSU crystals back into Na+ ions and UA. The resultant rise of intracellular Na+ forces water influx into the cell and reduces the apparent K+ levels to the threshold for NLRP3 activation (Figure 3)(Schorn et al., 2011). Indeed activation of the inflammasome was reduced with inhibitors of lysosomal acidification and aquaporins, and the inflammasome activation by MSU required sodium based uric acid crystals while crystals generated with K+ (MKU) did not induce IL-1β secretion (Schorn et al., 2011). These studies were extended in vivo using a subcutaneous air-pouch model measuring local IL-1β release after MSU injection with or without chloroquine to block lysosomal acidification. The authors reported that chloroquine treated mice had significantly reduced levels of local IL-1β compared to PBS injected controls (Schorn et al., 2011). Another study suggests that the NLRP3 inflammasome senses the attenuation of protein synthesis downstream of K+ efflux from a variety of NLRP3 agonists including MSU. These authors discuss the possibility that NLRP3 antagonistic proteins are produced constitutively and that global blockage of translation leads to rapid loss of these inhibitors in a proteasome dependent manner with subsequent activation of NLRP3 (Vyleta et al., 2012).
NLRP3 inflammasome activation has also been shown to occur following lysosomal rupture after uptake of particulate matter, including MSU. The release of IL-1β required lysosomal acidification, the lysosomal cathepsin proteases, as well as NLRP3 and ASC (Figure 3)(Hornung et al., 2008). It is still unclear how cathepsins mediate the full potential of the NLRP3 inflammasome, but one hypothesis is that active cathepsins in the cytosol allow for cleavage of an NLRP3 inhibitory protein resulting in inflammasome activation (Latz, 2010). Ultimately, NLRP3 inflammasome activation in response to MSU could occur using any combination of the above mechanisms and may be context dependent.
Initial studies suggest that MSU mediated activation of the NLRP3 inflammasome is independent of the P2RX7 receptor and ATP sensing (Martinon et al., 2006). A recent review provides a different perspective where ATP sensing may be synergistic with MSU regarding inflammasome activation. The authors argue that many of the risk factors for increasing UA levels (e.g. excessive food or alcohol consumption, temperature change, vigorous exercise) also affect ATP release which when sensed by P2RX7 could augment NLRP3 inflammasome activation by MSU (Tao et al., 2013). This reinforces the idea that multiple inflammasome signals likely contribute to the sum of disease symptoms and should be considered when developing therapeutics.
In most cases MSU acts as a second signal to activate the inflammasome after priming with a TLR ligand such as LPS or Pam3Cys (Giamarellos-Bourboulis et al., 2009; Martinon et al., 2006; Mylona et al., 2012). Therefore, gout may be a disease in which a chronic signal II for inflammasome activation (MSU crystals) is always present and that disease flare ups result from transient priming of innate immune cells by signal I (low-grade TLR stimulation or abundant release of saturated fatty acids). Periodic microbial exposure that induces signal I could explain the unpredictable and transient nature of acute gout, and may also explain why some individuals are asymptomatic even if they have high levels of sUA or MSU deposition. Current prophylactic treatments for gout are aimed at lowering the levels of sUA or enzymatic break down MSU crystals in affected areas, which would be an effective way to eliminate the chronic signal II and reduce NLRP3 inflammasome activation. Interestingly, the drug colchicine, which inhibits microtubule assembly, is a common treatment for both acute and chronic gout (Rider and Jordan, 2010). The mechanism of action for colchicine was initially attributed to blocking MSU uptake by phagocytic cells; however, a recent study determined that colchicine also blocks NLRP3 inflammasome assembly by preventing microtubule mediated shuttling of mitochondrial-associated ASC to NLRP3 at the endoplasmic reticulum (Misawa et al., 2013). Therefore, colchicine not only works at level of MSU uptake, but is also critical for preventing NLRP3 inflammasome assembly and downstream release of IL-1β.
Concluding Remarks
The development of vaccines and modern hygienic practices combined with the discovery of antibiotics has nearly doubled human life expectancy since the 1700’s (Finch, 2010). The consequence of increased longevity is the emergence of age related metabolic diseases, including atherosclerosis, T2D, obesity-related diseases and gout. Currently, circulatory disease and diabetes are the leading causes of hospitalization and are estimated to affect over 60 million people in the in the United States alone, with a collective cost of approximately 500 billion dollars annually. These trends are likely to continue with the incidence of metabolic diseases on the rise. According to the CDC, coronary heart disease accounts for 1 in 4 deaths making it the leading cause of mortality in the United States. Obesity and T2D are major risk factors for developing heart disease and currently 35% of American adults and 15% of children are considered obese. Additionally, the estimated number of people over 65 years of age suffering from T2D is 27% with 35% of Americans over 20 years of age considered as pre-diabetic (50% for people over 65). T2D is also the leading cause of kidney failure, non-traumatic lower-limb amputation and blindness in adults (Association, 2013). Gout incidence has also increased over the past two decades (estimated to affect 8.3 million Americans) and is also correlated with obesity, T2D and atherosclerosis related illness with 53%, 26% and 35% co-morbidity, respectively (Zhu et al., 2011).
The rising frequency of metabolically related diseases makes them of utmost importance for development of novel therapeutic and preventative treatments with the consideration that each has dietary, genetic and immunological aspects. Effective treatment regimens will likely require changes in diet and exercise, as well as novel therapeutics that ameliorate the associated immunopathogy. The NLRP3 inflammasome and its products, IL-1β and IL-18, are leading candidates for development of novel therapeutics since they are generated in response to a diverse array of DAMPs associated with metabolic disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Association AD. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- Cladaras C, Hadzopoulou-Cladaras M, Felber BK, Pavlakis G, Zannis VI. The molecular basis of a familial apoE deficiency. An acceptor splice site mutation in the third intron of the deficient apoE gene. J Biol Chem. 1987;262:2310–2315. [PubMed] [Google Scholar]
- Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci U S A. 2002;99:6280–6285. doi: 10.1073/pnas.092324399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, Giugliano D. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- Esser N, L'homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, Piette J, Legrand-Poels S, Paquot N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487–2497. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, Contassot E, Bachmann MF, French LE, Oxenius A, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc Natl Acad Sci U S A. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. 2011;13:160–166. doi: 10.1007/s11926-011-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ, Mouktaroudi M, Bodar E, van der Ven J, Kullberg BJ, Netea MG, van der Meer JW. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann Rheum Dis. 2009;68:273–278. doi: 10.1136/ard.2007.082222. [DOI] [PubMed] [Google Scholar]
- Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, Barazzoni R, Scherer PE, Cinti S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423–2436. doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, Theunissen R, Duijvestijn AM, Clément K, Tervaert JW, Thewissen MM. Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol. 2012;50:142–149. doi: 10.1016/j.molimm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15:313. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Scott P, Mueller JL, Misaghi A, Stevens S, Yancopoulos GD, Murphy A, Valenzuela DM, Liu-Bryan R. Role of the leucine-rich repeat domain of cryopyrin/NALP3 in monosodium urate crystal-induced inflammation in mice. Arthritis Rheum. 2010;62:2170–2179. doi: 10.1002/art.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Wanderer AA. Inflammasome and IL-1beta-mediated disorders. Curr Allergy Asthma Rep. 2010;10:229–235. doi: 10.1007/s11882-010-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y, Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein Edeficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang M, Huang K, Zhang Z, Shao N, Zhang Y, Wang W, Wang S. Oxidized low-density lipoprotein induces secretion of interleukin-1β by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation. Biochem Biophys Res Commun. 2012;425:121–126. doi: 10.1016/j.bbrc.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos-Bourboulis EJ, et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010;62:3237–3248. doi: 10.1002/art.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen TB, Stienstra R, van Tits LJ, Joosten LA, van Velzen JF, Hijmans A, Pol JA, van der Vliet JA, Netea MG, Tack CJ, et al. The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology. 2011;152:3769–3778. doi: 10.1210/en.2010-1480. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard B, Matthews VB, Brandt C, Hojman P, Allen TL, Estevez E, Watt MJ, Bruce CR, Mortensen OH, Syberg S, et al. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62:3064–3074. doi: 10.2337/db12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupfer C, Kanneganti TD. Unsolved Mysteries in NLR Biology. Front Immunol. 2013;4:285. doi: 10.3389/fimmu.2013.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Sastalla I, Leppla SH. Anthrax and the inflammasome. Microbes Infect. 2012;14:392–400. doi: 10.1016/j.micinf.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- Mylona EE, Mouktaroudi M, Crisan TO, Makri S, Pistiki A, Georgitsi M, Savva A, Netea MG, van der Meer JW, Giamarellos-Bourboulis EJ, et al. Enhanced interleukin-1β production of PBMCs from patients with gout after stimulation with Toll-like receptor-2 ligands and urate crystals. Arthritis Res Ther. 2012;14:R158. doi: 10.1186/ar3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 2010;6:e1000906. doi: 10.1371/journal.ppat.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusi SO, Al-Awadhi A, Abraham M. Relations of serum interleukin 18 levels to serum lipid and glucose concentrations in an apparently healthy adult population. Horm Res. 2003;60:29–33. doi: 10.1159/000070824. [DOI] [PubMed] [Google Scholar]
- Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE, Suliman HB. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem. 2011;286:16374–16385. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider TG, Jordan KM. The modern management of gout. Rheumatology (Oxford) 2010;49:5–14. doi: 10.1093/rheumatology/kep306. [DOI] [PubMed] [Google Scholar]
- Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Prados JC, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, Gaipl US, Voll RE, Springer E, Munoz LE, et al. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 2011;286:35–41. doi: 10.1074/jbc.M110.139048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–6378. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev. 2010;233:203–217. doi: 10.1111/j.0105-2896.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Sun S, Xia S, Ji Y, Kersten S, Qi L. The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue. Diabetes. 2012;61:1471–1478. doi: 10.2337/db11-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JH, Zhang Y, Li XP. P2X7R: A potential key regulator of acute gouty arthritis. Semin Arthritis Rheum. 2013 doi: 10.1016/j.semarthrit.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Ting JP, Davis BK. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol. 2005;23:387–414. doi: 10.1146/annurev.immunol.23.021704.115616. [DOI] [PubMed] [Google Scholar]
- Trøseid M, Seljeflot I, Arnesen H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc Diabetol. 2010;9:11. doi: 10.1186/1475-2840-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2012;425:162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma G, Datta M. IL-1beta induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;15:864–876. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- Vyleta ML, Wong J, Magun BE. Suppression of ribosomal function triggers innate immune signaling through activation of the NLRP3 inflammasome. PLoS One. 2012;7:e36044. doi: 10.1371/journal.pone.0036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- Wright SD, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card DJ, Hermanowski-Vosatka A, Bergstrom JD, et al. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein. E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- Zilverschoon GR, Tack CJ, Joosten LA, Kullberg BJ, van der Meer JW, Netea MG. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:1407–1414. doi: 10.1038/ijo.2008.109. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]