Abstract
This review summarizes the current literature on cancer-related cognitive impairment (CRCI) with a focus on prevalence, mechanisms, and possible interventions for CRCI in those who receive adjuvant chemotherapy for non-central nervous system tumors and is primarily focused on breast cancer. CRCI is characterized as deficits in areas of cognition including memory, attention, concentration, and executive function. Development of CRCI can impair quality of life and impact treatment decisions. CRCI is highly prevalent; these problems can be detected in up to 30% of patients prior to chemotherapy; up to 75% of patients report some form of CRCI during treatment, and CRCI is still present in up to 35% of patients many years following completion of treatment. While the trajectory of CRCI is becoming better understood, the mechanisms underlying the development of CRCI are still obscure; however, host characteristics, immune dysfunction, neural toxicity, and genetics may play key roles in the development and trajectory of CRCI. Intervention research is limited, though strategies to maintain function are being studied with promising preliminary findings. This review highlights key research being conducted in these areas, both in patient populations and in animals, which will ultimately result in better understanding and effective treatments for CRCI.
Introduction
Cancer treatments, including chemotherapy, radiation therapy, and targeted biological therapies, have greatly led to improved survival; however, their administration is often associated with side effects that greatly reduce quality of life. Cognitive problems are among the most frequently reported symptoms among patients during treatment, especially related to chemotherapy. Longitudinal neuropsychological assessment research studies in cancer patients indicate that up to 30% of patients experience cancer-related cognitive impairment (CRCI; i.e., problems with memory, executive functioning, and attention/concentration) prior to any treatment; up to 75% experience CRCI during treatment, and up to 35% experience CRCI months or years following completion of treatments for cancer (Janelsins et al., 2011). With over 13 million cancer survivors in the United States (ACS, 2013), up to 4.5 million individuals may be living with long-lasting cognitive difficulties resulting from cancer and cancer treatments. CRCI is of considerable concern as this set of problems can influence adherence to treatments, impair quality of life, and lead to long-term cognitive impairments. It is important to note that CRCI can vary in domains affected and may be subtle or dramatic, temporary or permanent, and stable or progressive (Ahles et al., 2012). Recent research also suggests that CRCI in patients who received chemotherapy can have a later onset (Wefel et al., 2010), and may persist for 20 years following treatment (Koppelmans et al., 2012). The impact of CRCI has led to a field of research to better characterize and define the problem areas, understand mechanisms that contribute to its development, and to begin to develop and test putative management strategies.
Characterization of CRCI Associated with Chemotherapy
Systematic research to understand CRCI was first reported during the mid 1990s to early 2000’s (Ahles et al., 2002; Brezden et al., 2000; Paraska & Bender, 2003; van Dam et al., 1998; Wieneke, 1995); these studies initially established CRCI as a phenomenon associated with cancer and chemotherapy treatments. However, these early studies were often cross-sectional in design and did not account for pre-treatment assessment data. The importance of a pre-chemotherapy treatment baseline was first shown in a study conducted by Wefel and colleagues of the effects of 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC) chemotherapy on cognitive function in breast cancer (Wefel et al., 2004). As one of the first prospective, longitudinal studies to assess cognitive function with pre-treatment and post-treatment cognitive measures, this study demonstrated the importance of assessing changes in cognition over time. In this study, there were no overall mean differences in cognitive function between patients and normative data. Within-subject analyses, however, showed that 61% had cognitive declines in learning, attention, and processing speed. If the pre-treatment assessment had been unavailable, 46% would not have had detectable cognitive impairments (because their post-treatment assessment scores were in the normal range). This finding is extremely important, since cognitive dysfunction can be subtle. If a patient scores well on a cognitive test before chemotherapy and less well after treatment (but that score is still within the normal range), the decline can still represent a clinically significant difference. Many studies of CRCI over the past decade have included pre-chemotherapy treatment assessments, so that assessments during and after treatment can be interpreted in the context of baseline status. To date, over 20 longitudinal studies have been conducted (Ahles et al., 2010; Bender et al., 2006; Biglia et al., 2012; Collins et al., 2009; Debess et al., 2010; Fan et al., 2005; Hedayati et al., 2012; Hermelink et al., 2008; Hermelink et al., 2007; Hurria et al., 2006; Jansen et al., 2011; Jenkins et al., 2006; Mehlsen et al., 2009; Quesnel et al., 2009; Schagen et al., 2006; Shilling et al., 2005; Stewart et al., 2008; Tager et al., 2011; Vearncombe et al., 2009; Wefel, et al., 2004; Wefel, et al., 2010).
A majority of longitudinal studies, largely conducted in breast cancer patients, have compared changes in cognitive function pre- and post-chemotherapy with healthy controls or normative data. In these studies in patients receiving chemotherapy, 12-82% demonstrated CRCI in the domains of executive function, memory, psychomotor speed, and attention (Ahles et al., 2008; Ahles, et al., 2010; Collins, et al., 2009; Hermelink, et al., 2007; Hurria, et al., 2006; Jansen, et al., 2011; Jenkins, et al., 2006; Ouimet et al., 2009; Quesnel, et al., 2009; Schagen, et al., 2006; Stewart, et al., 2008; Wefel, et al., 2010). Not all studies revealed significant changes in all domains; changes in memory, executive function, processing speed, and attention appear most frequent. Several of the studies consisted of small sample sizes and, therefore, limited power to significantly detect CRCI. These studies also utilized a variety of cognitive assessments and control groups, and most investigated patients on various treatment regimens. Furthermore, criteria for cognitive impairment cut-offs varied. Additional large-scale, well-powered studies are needed to confirm the findings of previous studies conducted to date, and should include large multi-center setting studies. These will help to further clarify, characterize, and determine reliable estimates of CRCI.
Teasing Apart the Impact of Other Cancer Treatments on Cognitive Function
Longitudinal studies assessing effects of chemotherapy have allowed us to better understand the multiple contributing factors of CRCI. While many studies have investigated the impact of chemotherapy, other studies have evaluated the impact of radiation therapy and hormonal therapies on cognitive function and found deficits in similar domains as with chemotherapy treatments (Castellon et al., 2004; Kohli et al., 2007; Schilder et al., 2009; Schilder et al., 2010; Schilder et al., 2012). Studies with comparison groups of patients treated with chemotherapy to those with hormonal therapies both show declines compared to healthy controls suggesting that hormone therapies can also lead to CRCI (Ahles, et al., 2010), and the combined treatment of tamoxifen and chemotherapy leads to greater difficulties than chemotherapy alone (Castellon, et al., 2004) (Palmer et al., 2008). One prospective study found deterioration in verbal memory and executive function in post-menopausal patients taking tamoxifen for at least a year compared to healthy controls; those taking the aromatase inhibitor, exemestane, did not have significant deficits compared to controls (Schilder, et al., 2010). Additional studies in patients receiving radiation suggests that this modality may also contribute to CRCI; however, the effects are not as severe as with chemotherapy (Kohli, et al., 2007). As more large-scale studies are conducted, we will improve our understanding of the effects of other treatment modalities on CRCI.
CRCI Prior to Chemotherapy
Pre-chemotherapy baseline cognitive assessments also suggest that surgery and development of cancer itself may also contribute to CRCI by comparing patients’ performance at these time-points to healthy controls and/or normative data. These studies have revealed that up to 33% of patients exhibit deficits prior to any chemotherapy; including deficits in areas of verbal learning and memory, reaction time (Ahles, et al., 2008), as well as global cognitive function (Jansen, et al., 2011). This finding suggests that cancer itself and/or surgical procedures may alter cognitive function. While a diagnosis of cancer can alter psychological status and cognitive performance just following diagnosis, some psychological factors (e.g. depression, anxiety) and surgery factors (e.g. type) have not been associated with cognitive function in some studies. However, research in this area is still needed and some evidence suggests coping strategies may influence cognitive function during this time (Reid-Arndt & Cox, 2012). Another possibility is that common risk factors for both the development of cancer and mild cognitive impairment associated with aging lead to CRCI. An area of active investigation includes DNA repair mechanisms and oxidative stress that may play a role in both processes (Ahles, et al., 2012).
Defining and Measuring CRCI
A remaining challenge in the cancer and cognition field has been defining and measuring CRCI. Cognition can be measured, analyzed, and interpreted in a variety of ways. Defining CRCI can be challenging because multiple cognitive domains can be affected, and these can vary by individual. Currently, the International Cognition and Cancer Task Force (ICCTF) recommends that the following measures (at minimum) be included in assessing cognitive function in cancer patients: the Hopkins Verbal Learning Test-Revised (HVLT-R), Trail Making Test (TMT), and the Controlled Oral Word Association (COWA) that is part of the Multilingual Aphasia Examination (Wefel et al., 2011).
Self-report measures of perceived cognitive function (Wagner, 2009) correlate with objective assessments, although these correlations are often weak (Lai et al., 2009; J. Vardy et al., 2006). Neuropsychological assessments are a snapshot in time, whereas self-report measures typically ask about experiences over a period of time. Because CRCI symptoms can come and go, they may not always be detectable by objective neuropsychological assessment at the time the assessment is done. Therefore, rates of perceived CRCI may be higher by self-report. Ongoing and future research is aimed at better capturing CRCI with combinations of self-report and optimized objective neuropsychological assessments and methods of analysis.
Subtle cognitive change also poses a unique challenge to detection; many standard neuropsychological tests do not detect subtle change, underscoring the importance of utilizing self-report measures and the need to further refine objective tests to detect subtle CRCI.
Understanding CRCI in Populations Beyond Breast Cancer
Most studies of CRCI have focused on breast cancer. Limited studies have been conducted in testicular cancer (Schagen et al., 2008), lymphoma (Ahles, et al., 2002), colorectal cancer (J. Vardy et al., 2012), ovarian cancer (Correa & Hess, 2012; Correa et al., 2010), and prostate cancer (Nelson et al., 2008). Longitudinal studies are needed in other disease groups to understand whether cognitive difficulties are more or less prevalent and severe in these populations. Having these data will help determine if the effects of chemotherapy are “universal” or more pronounced in specific disease populations. Moreover, studies in other populations will help us to understand whether different treatments (e.g. multi- and single-agent chemotherapies, androgen deprivation therapy, tamoxifen) for different cancers may work by different mechanisms of action to lead to CRCI. Such knowledge will help researchers identify risk factors including disease-specific factors, demographic factors, psychological factors, and genetic factors.
Mechanisms of CRCI Development and Progression
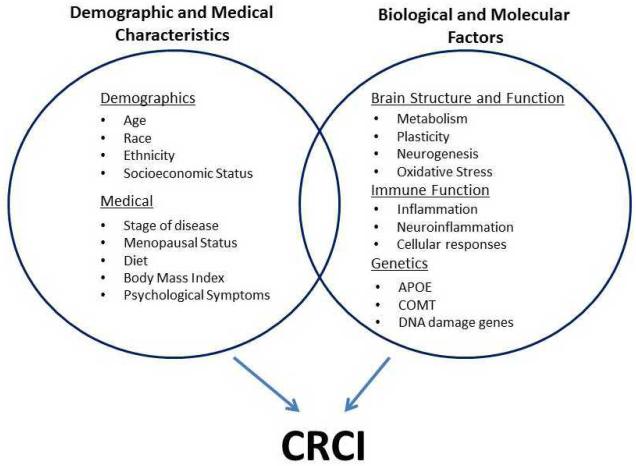
Several predisposing and precipitating factors likely contribute to CRCI and include demographic characteristics, medical and host characteristics, and biological factors (Figure 1).
Possible Predisposing and Prepetuating Contributors to CRCI
Demographic and Medical Characteristics
A number of demographic and medical characteristics have been purported to play a role in the development of CRCI; none of these has been firmly established. Age is the most frequently used explanation for cognitive decline; however, evidence suggests that young and older patients both experience CRCI. Cognitive reserve, assessed by the Wide Range Achievement Test (WRAT) Reading scale, has suggested that lower innate cognitive capacity (taking into account education, environment and occupation) prior to chemotherapy treatments, explains the risk for post-treatment decline in processing speed (Ahles, et al., 2010). However, this result needs to be replicated. Other medical host factors that could influence CRCI that are known to affect cognitive function but have not been critically investigated in cancer patients include race, ethnicity, socioeconomic status, menopausal status, stage of disease, diet and body mass index (Loef & Walach, 2012, 2013; Sherwin, 2012). One recent study suggests that post-treatment cognitive function and brain activation vary by pre-treatment menopausal status (Conroy et al., 2013). Additional research is needed to understand how other symptoms, including fatigue, sleep dysfunction, and nausea/vomiting—all of which correlate with but are distinct processes from CRCI—may moderate or mediate effects on cognitive performance (Morrow et al., 2005; Mustian et al., 2012; Palesh et al., 2012; Ryan et al., 2007; Vearncombe, et al., 2009).
Biological Factors
Brain Structure and Function Alterations
The greatest body of literature on central nervous system (CNS) mechanisms of CRCI comes from neuroimaging studies of breast cancer patients previously treated with chemotherapy. Overall, these cross-sectional studies indicate that patients treated with previous chemotherapy have more gray matter and white matter volume loss than controls, reduced white matter integrity, and altered brain activation. Findings of reduced overall gray matter volume appear to be most pronounced in the prefrontal lobe; however, decreases in parietal and occipital (e.g. precuneus) and temporal lobes (e.g. thalamus, hippocampus, parahippocampal region) have also been found via magnetic resonance imaging (MRI) techniques. Decreased white matter integrity and diffusivity have been noted in widespread brain regions using diffusion tensor imaging (DTI) (Abraham et al., 2008; de Ruiter et al., 2012; Deprez et al., 2011; Inagaki et al., 2007; Swayampakula et al., 2007). These studies have been conducted from 12 months to 20 years following treatment, suggesting that the effects of cancer and cancer treatments are long-lasting, leaving permanent changes at least in a subset of patients. Additionally, chemotherapy appears to produce greater deficits compared to non-chemotherapy treated cancer patients, though results have been inconclusive (Inagaki, et al., 2007; Yoshikawa et al., 2005). Cross-sectional functional MRI (fMRI), MR spectroscopy, and positron emission tomography (PET) studies show altered brain activation, neurochemistry, and metabolism, respectively, in cancer patients post-treatment compared to healthy controls. Additionally those treated with chemotherapy have more pronounced alterations compared to non-chemotherapy treated patients.
However, compensatory mechanisms may help chemotherapy-treated patients preserve their performance during certain tasks, masking cognitive-behavioral impairments (Reuter-Lorenz & Cimprich, 2013). For example, previous fMRI studies have reported hyper-activation and hyper-connectivity during performance of certain cognitive tasks in chemotherapy-treated survivors (Ferguson, Ahles, et al., 2007; Hosseini & Kesler, in press; McDonald et al., 2012). However, studies have also shown hypo-activation during other cognitive tasks (de Ruiter et al., 2011; S. R. Kesler et al., 2011). A recent review of fMRI studies in breast cancer suggested that hyper-activation, or neural compensation, is most apparent at low task difficulty and diminishes as task difficulty increases (Reuter-Lorenz & Cimprich, 2013). Studies showing hypo-activation also tend to involve very long-term survivors (de Ruiter, et al., 2011; S. R. Kesler, et al., 2011) and therefore the capacity for compensation also may diminish as patients age (i.e. become long-term survivors).
Areas most affected by chemotherapy treatment include brain hub regions such as prefrontal cortex, hippocampus and precuneus (de Ruiter, et al., 2011; Ferguson, McDonald, et al., 2007; S. R. Kesler et al., 2009; S. R. Kesler, et al., 2011; S. R. Kesler, C. Watson, et al., 2013). However, recent studies provide increasing evidence that CRCI may be associated with widespread disruption of brain network connectivity rather than regionally specific effects (Bruno et al., 2012; Dumas et al., 2013; Hosseini et al., 2012; S. Kesler, in press; S. R. Kesler, J. S. Wefel, et al., 2013). A few longitudinal studies (including pre-chemotherapy and post-chemotherapy assessments up to 1 year following treatment) provide further evidence for changes in brain structure and function during and after chemotherapy treatments in support of the post-treatment cross-sectional findings (Deprez et al., 2012; McDonald et al., 2010; McDonald, et al., 2012; McDonald et al., 2013). Lastly, while most studies have been conducted in breast cancer, investigating the effects of standard chemotherapy regimens, brain injury has also been observed in advanced breast cancer patients receiving high dose chemotherapy and stem cell transplants as well as in other cancer types (Brown et al., 1998; Hsieh et al., 2013).
Immune Function
Cancer patients have increased levels of circulating cytokines (compared to healthy controls) (Korkaya et al., 2011; Rego et al., 2013), and inflammation has been associated with chemotherapy treatment and cognitive dysfunction(Janelsins et al., 2012), supporting a role for dysregulated immune function in the predisposition or perpetuation of CRCI. It is well established that inflammation plays a role in cognitive dysfunction and “sickness behavior” (e.g. fatigue, sleep dysfunction, loss of appetite, withdrawal and other psychological symptoms: all symptoms experienced by cancer patients) (Dantzer & Kelley, 2007), and this has led several investigators to propose inflammation as central to CRCI etiology (Ahles & Saykin, 2007; Cleeland et al., 2003; Raffa, 2011).
Earlier studies indicated that chemotherapy is associated with increased levels of pro-inflammatory cytokines (e.g. interleukin (IL); IL-1β) in those treated for cancers of the lymphatic system (Ahles & Saykin, 2007; Meyers et al., 2005; Villani et al., 2008). In breast cancer patients receiving paclitaxel, levels of IL-6 increased 3 days after treatment compared to pre-treatment, but not in those who received a combination of fluorouracil, cyclophosphamide and methotrexate (Pusztai et al., 2004). Significant changes in markers of endothelial and platelet activation were found in breast cancer patients receiving anthracycline-based treatment, further supporting the hypothesis that inflammation occurs as a result of chemotherapy (Mills et al., 2008). Another study found a trend between cytokine levels and cognitive performance in breast cancer (J. L. Vardy et al., 2007), and these investigators are currently assessing cytokine levels in relationship to cognitive function (via neuropsychological and computerized tests) in an ongoing observational study of cognitive function in colorectal cancer (J. Vardy, et al., 2012). Another study in breast cancer patients receiving chemotherapy found increases in monocyte chemoattractant protein one (MCP-1), IL-6, and IL-8 in patients receiving anthracycline-based chemotherapies but not non-anthracycline-based regimens; associations between MCP-1 changes and perceived cognitive function were significant (Janelsins, et al., 2012). More recently, in a large, prospective cohort study, increased circulating soluble TNF Receptor 2 (sTNFRII) was associated with higher memory complaints in post-chemotherapy patients (prior to hormone therapy). A decline in sTNFRII levels was also associated with improvements in memory at 1 year post-treatment, suggesting that inflammation plays a role in the progression of CRCI and is reduced as patients regain cognitive abilities (Ganz et al., 2013).
Two published studies have examined the relationship between circulating cytokine expression in breast cancer survivors with neuroimaging. In the first study, investigators assessed regional brain volume and IL-6 and TNF-α in breast cancer survivors compared to controls. Overall, lower hippocampal volume was associated with higher levels of both markers in the entire cohort. In breast cancer survivors, lower volume was associated with higher TNF-α and lower IL-6, with an interaction effect between both cytokines. Both were also associated with memory performance; overall, these results suggest that both molecules play a role in the development of CRCI (S. Kesler, M. Janelsins, et al., 2013). Another pilot study of breast cancer survivors investigated brain metabolism, and cytokines (i.e. CRP and IL-6) and cytokine receptors (i.e. IL-1 receptor antagonist (ra) and sTNF-RII). Significant relationships were found between all markers and metabolism at the baseline timepoint, and additionally, baseline levels of IL1ra and sTNFRII were significantly positively associated with increased frontal lobe metabolism at the 1 year follow-up timepoint (Pomykala et al., 2013).
Further clarification of the role of cytokines, chemokines, and other immune factors on cognitive function in cancer is needed. Large-scale observational studies that correlate changes in cytokines and chemokines with those in cognitive function should help clarify the association between inflammation and cognitive function in cancer patients.
Genetic Factors
Genetic studies not only provide important information on possible mechanisms for CRCI, but they may also have clinical utility in helping to predict those that might be at increased risk for cognitive impairments. Two studies examined the association between apolipoprotein E (APOE) and catechol-o-methyltransferase (COMT) genotypes and CRCI (Ahles et al., 2003; Small). APOE is a complex glycolipoprotein that facilitates uptake, transport, and distribution of lipids, and plays and important role in neuronal repair following an insult (Morley & Montgomery, 2001). The E4 allele has been associated with a variety of disorders with prominent cognitive dysfunction including aging-related cognitive decline, Alzheimer’s disease, and poor cognitive outcomes in stroke and traumatic brain injury (McAllister et al., 2004). Cancer survivors with at least one E4 allele scored significantly lower in the visual memory and spatial ability domains, with a trend to score lower in executive function compared to survivors who did not carry an E4 allele (Ahles, et al., 2003).
The COMT Val158Met single nucleotide polymorphism (SNP) has been associated with dopamine levels in the prefrontal cortex. COMT-Val carriers metabolize dopamine more rapidly than COMT-Met carriers, with less availability of the neurotransmitter that is critical for cognitive function, making this an important SNP for further investigation in cancer. In a cross-sectional study of cancer survivors treated with chemotherapy and healthy controls, COMT-Val carriers performed more poorly on tests of attention, verbal fluency, and motor speed compared to COMT-Met carriers. Breast cancer survivors who were COMT-Val carriers and exposed to chemotherapy performed more poorly on tests of attention than healthy controls who were also carriers. Additionally, COMT-Met carriers treated with chemotherapy performed better on an attention task than COMT-Val carriers also treated with chemotherapy, further supporting that Val carriers have less bioavailable dopamine to support cognitive function.
These studies support the hypothesis that there may be a genetic predisposition to CRCI but these results need to be validated in additional research. Other possible genetic gene targets that warrant future investigation include: genes of neural repair, neuronal plasticity genes, DNA damage and repair genes, and other inflammation genes. Finally, increasing interest in transcriptional changes and epigenetics and cognitive function in cancer patients may lead to improved understanding of the mechanisms involved in CRCI.
Further research on the assessment of various medical, genetic, and biological factors may help us further clarify pathways involved in CRCI development and progression and identify high risk groups for cognitive difficulties or those at highest risk for persistent long-term effects. Although we currently have no clinically recommended treatments for cognitive difficulties in cancer patients, identifying biological markers related to cognitive function would enable us to understand possible mechanisms and aid in the development of targeted interventions.
Impact of CRCI on Quality of Life and Activities of Daily Function
Several studies have investigated the impact of CRCI on daily function and quality of life. CRCI has been associated with a reduction in the ability to return to work at all or to a limited capacity (Bradley et al., 2005; Wefel, et al., 2004). CRCI—specifically executive dysfunction—has also been associated with reduced function in productivity, social role functioning, and community engagement (Reid-Arndt et al., 2009). Patients also report difficulties driving and reading that interfere with quality of life (Myers, 2012).
Management of CRCI
While researchers and clinicians are continuing to understand and characterize CRCI, efforts have begun to develop and test interventions that might alleviate or prevent CRCI development. Based on the clinical research literature, possible approaches with the most promise to date include cognitive behavioral therapy approaches, cognitive brain training, modafinil, and physical activity.
A brief CBT-I intervention, Memory and Attention Adaptation Training, has been tested for feasibility and preliminary results in a small randomized waitlist trial. The results of this study suggest that this intervention is safe, feasible, and results in high patient satisfaction, and preliminarily improves working memory and quality of life (Ferguson et al., 2012). Cognitive training, used in a breast cancer survivor waitlist trial with a novel, online, home-based program targeting executive function, significantly improved multiple executive function skills by objective and self-report measures (S. Kesler, S. M. Hadi Hosseini, et al., 2013). A second cognitive training trial, focused on memory training and speed of processing training, showed that both types of training produced improvements in objective and perceived cognitive function (Von Ah et al., 2012). A single-arm pilot trial of tai chi in cancer survivors demonstrated significant improvements in objective and subjective cognitive function tests as well as on psychological and physical health (Reid-Arndt et al., 2012). Further evidence of improvements in memory produced by exercise came from a secondary analyses of a large study in which older cancer patients who exercised reported less memory loss than non-exercisers (Sprod et al., 2012). Lastly, medical Qigong, a practice consisting of meditation and gentle exercise, improved perceived cognitive function in a pilot randomized controlled trial (Oh et al., 2012). These behavioral interventions all show promise; however, larger efficacy trials are needed to confirm these preliminary positive findings.
Pharmacological interventions are also being tested for alleviating CRCI. Modafinil produced improvements in memory, speed of memory, and attention in breast cancer patients with fatigue compared to those who had received modafinal but switched to a placebo in a secondary data analysis of a completed randomized controlled trial (Kohli et al., 2009). A pilot placebo-controlled trial of modafinil in advanced cancer showed improvements in psychomotor speed and attentional function (Lundorff et al., 2009). Further research on modafinil needs to be confirmed in Phase II and III trials. Clinical trials have also investigated the effects of methylphenidate on cognitive function. To date, results with this psychostimulate have not been promising for patients with non-CNS tumors in placebo-controlled studies, although at least one of these may have been underpowered (Lower et al., 2009; Mar Fan et al., 2008). Ginkgo biloba, an herbal supplement, was tested for prevention of CRCI in a 10 week randomized clinical trial, but did not show any improvement in CRCI by subjective or objective measures (Barton et al., 2013).
Animal Models Aid Clinical Research
Animal models continue to aid future directions of clinical research by allowing researchers to further elucidate mechanisms that cannot easily be assessed in humans (Seigers & Fardell, 2011). Rodent model studies investigating the effects of chemotherapy administration on molecular and biological processes reveal reduced neurogenesis (Dietrich et al., 2006; Janelsins et al., 2010), indicators of reduced metabolism and oxidative stress (Tangpong et al., 2007), reduced myelination (Han et al., 2008), increased inflammation (Tangpong et al., 2006), and cognitive dysfunction. Healthy mice and rats given chemotherapy display deficits in multiple cognitive domains including spatial memory, object recognition, and associative tasks (Fardell et al., 2013; Fardell et al., 2010; Reiriz et al., 2006).
Chemotherapy animal models also provide a platform to preclinically investigate interventions for CRCI (Fardell et al., 2011). Mice receiving chemotherapy that are exposed to voluntary running show less cognitive dysfunction in spatial memory and object recognition compared to mice receiving chemotherapy alone (Fardell et al., 2012). Future research with animal models could help determine dose and intensity needed for translation into human trials. Preclinical studies of fluoxetine also may have promising results for future human trial translation; fluoxetine mitigates chemotherapy-induced memory impairments and reverses neurogenesis impairments in rats (Lyons et al., 2012; Lyons et al., 2011). Lastly, antioxidants have also been tested in animal models, exhibiting improvements in cognitive function, oxidative stress, and neurogenesis (Helal et al., 2009; Joshi et al., 2007; Konat et al., 2008).
Summary
CRCI is a prevalent side effect of cancer treatments that can persist for years following treatment and negatively affect quality of life. Current research, supported by human and preclinical animal model studies, demonstrates that chemotherapy can cause immediate or delayed cognitive dysfunction in areas of memory, attention, concentration, and executive function. The underlying etiology of CRCI is not well understood; however, a combination of host characteristics, genetic factors, and biological factors all likely play a role in the predisposition and perpetuation of CRCI. Interventions, both behavioral and pharmacological, are currently being developed and tested for alleviating or preventing CRCI.
Acknowledgements
This review was supported by NCI K07CA168886. We thank Raven Shah for contributing to a literature search in preparation for this article.
Footnotes
The authors do not have any financial disclosures or conflicts of interest.
References
- Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clinical breast cancer. 2008;8(1):88–91. doi: 10.3816/CBC.2008.n.007. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- ACS . Cancer Facts and Figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. doi: nrc2073 [pii] 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(2):485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast cancer research and treatment. 2008;110(1):143–152. doi: 10.1007/s10549-007-9686-5. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Kaufman PA. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 doi: 10.1200/JCO.2009.27.0827. doi: JCO.2009.27.0827 [pii]10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-oncology. 2003;12(6):612–619. doi: 10.1002/pon.742. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- Barton DL, Burger K, Novotny PJ, Fitch TR, Kohli S, Soori G, Loprinzi CL. The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(4):1185–1192. doi: 10.1007/s00520-012-1647-9. doi: 10.1007/s00520-012-1647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15(5):422–430. doi: 10.1002/pon.964. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D'Alonzo M, Torta R. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. European journal of cancer care. 2012;21(4):485–492. doi: 10.1111/j.1365-2354.2011.01320.x. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Neumark D, Bednarek HL, Schenk M. Short-term effects of breast cancer on labor market attachment: results from a longitudinal study. J Health Econ. 2005;24(1):137–160. doi: 10.1016/j.jhealeco.2004.07.003. doi: S0167-6296(04)00098-0 p. 10.1016/j.jhealeco.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(14):2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- Brown MS, Stemmer SM, Simon JH, Stears JC, Jones RB, Cagnoni PJ, Sheeder JL. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. AJNR. American journal of neuroradiology. 1998;19(2):217–221. [PMC free article] [PubMed] [Google Scholar]
- Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. Journal of clinical and experimental neuropsychology. 2004;26(7):955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18(2):134–143. doi: 10.1002/pon.1379. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Ahles TA, West JD, Saykin AJ. Chemotherapy-induced amenorrhea: a prospective study of brain activation changes and neurocognitive correlates. Brain imaging and behavior. 2013 doi: 10.1007/s11682-013-9240-5. doi: 10.1007/s11682-013-9240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Hess LM. Cognitive function and quality of life in ovarian cancer. Gynecologic oncology. 2012;124(3):404–409. doi: 10.1016/j.ygyno.2011.11.005. doi: 10.1016/j.ygyno.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Correa DD, Zhou Q, Thaler HT, Maziarz M, Hurley K, Hensley ML. Cognitive functions in long-term survivors of ovarian cancer. Gynecologic oncology. 2010;119(2):366–369. doi: 10.1016/j.ygyno.2010.06.023. doi: 10.1016/j.ygyno.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, behavior, and immunity. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Human brain mapping. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human brain mapping. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debess J, Riis JO, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast cancer research and treatment. 2010;121(1):91–100. doi: 10.1007/s10549-010-0756-8. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human brain mapping. 2011;32(3):480–493. doi: 10.1002/hbm.21033. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. doi: jbiol50 [pii] 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Makarewicz J, Schaubhut GJ, Devins R, Albert K, Dittus K, Newhouse PA. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9244-1. doi: 10.1007/s11682-013-9244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, Tannock IF. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(31):8025–8032. doi: 10.1200/JCO.2005.01.6550. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Johnston IN. The short and long term effects of docetaxel chemotherapy on rodent object recognition and spatial reference memory. Life sciences. 2013 doi: 10.1016/j.lfs.2013.05.006. doi: 10.1016/j.lfs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Johnston IN, Winocur G. Chemotherapy and cognitive impairment: treatment options. Clinical pharmacology and therapeutics. 2011;90(3):366–376. doi: 10.1038/clpt.2011.112. doi: 10.1038/clpt.2011.112. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Logge W, Johnston I. Single high dose treatment with methotrexate causes long-lasting cognitive dysfunction in laboratory rodents. Pharmacology, biochemistry, and behavior. 2010;97(2):333–339. doi: 10.1016/j.pbb.2010.08.019. doi: 10.1016/j.pbb.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology. 2012;220(1):183–193. doi: 10.1007/s00213-011-2466-2. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Mott LA. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, Saykin AJ. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psycho-oncology. 2012;21(2):176–186. doi: 10.1002/pon.1878. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, Cole SW. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain, behavior, and immunity. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. doi: jbiol69 [pii]10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati E, Alinaghizadeh H, Schedin A, Nyman H, Albertsson M. Effects of adjuvant treatment on cognitive function in women with early breast cancer. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2012;16(3):315–322. doi: 10.1016/j.ejon.2011.07.006. doi: 10.1016/j.ejon.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Helal GK, Aleisa AM, Helal OK, Al-Rejaie SS, Al-Yahya AA, Al-Majed AA, Al-Shabanah OA. Metallothionein induction reduces caspase-3 activity and TNFalpha levels with preservation of cognitive function and intact hippocampal neurons in carmustine-treated rats. Oxidative medicine and cellular longevity. 2009;2(1):26–35. doi: 10.4161/oxim.2.1.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer. 2008;113(9):2431–2439. doi: 10.1002/cncr.23853. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Munzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–1913. doi: 10.1002/cncr.22610. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Kesler SR. Multivariate pattern analysis of fMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc. doi: 10.1017/S1355617713001173. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12(1):28. doi: 10.1186/1471-2377-12-28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TC, Wu YC, Yen KY, Chen SW, Kao CH. Early Changes in Brain FDG Metabolism during Anticancer Therapy in Patients with Pharyngeal Cancer. J Neuroimaging. 2013 doi: 10.1111/jon.12006. doi: 10.1111/jon.12006. [DOI] [PubMed] [Google Scholar]
- Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS, Holland J. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. Journal of the American Geriatrics Society. 2006;54(6):925–931. doi: 10.1111/j.1532-5415.2006.00732.x. doi: JGS732 [pii] 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Seminars in oncology. 2011;38(3):431–438. doi: 10.1053/j.seminoncol.2011.03.014. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, Morrow GR. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20(4):831–839. doi: 10.1007/s00520-011-1158-0. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Roscoe JA, Berg MJ, Thompson BD, Gallagher MJ, Morrow GR, Gross RA. IGF-1 partially restores chemotherapy-induced reductions in neural cell proliferation in adult C57BL/6 mice. Cancer investigation. 2010;28(5):544–553. doi: 10.3109/07357900903405942. doi: 10.3109/07357900903405942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011 doi: 10.1007/s00520-010-0997-4. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British journal of cancer. 2006;94(6):828–834. doi: 10.1038/sj.bjc.6603029. doi: 6603029 [pii] 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Hardas S, Sultana R, St Clair DK, Vore M, Butterfield DA. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. Journal of neuroscience research. 2007;85(3):497–503. doi: 10.1002/jnr.21158. doi: 10.1002/jnr.21158. [DOI] [PubMed] [Google Scholar]
- Kesler S. Default mode network as a potential biomarker of chemotherapy-related brain injury and altered brain aging. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2014.03.036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, Morrow G. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clinical breast cancer. 2013;13(4):299–306. doi: 10.1016/j.clbc.2013.02.004. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, behavior, and immunity. 2013;30(Suppl):S109–116. doi: 10.1016/j.bbi.2012.05.017. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(21):6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O'Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Archives of neurology. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Watson C, Koovakkattu D, Lee C, O'Hara R, Mahaffey ML, Wefel JS. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain imaging and behavior. 2013 doi: 10.1007/s11682-013-9228-1. doi: 10.1007/s11682-013-9228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A. 2013;110(28):11600–11605. doi: 10.1073/pnas.1214551110. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S, Fisher SG, Tra Y, Adams MJ, Mapstone ME, Wesnes KA, Morrow GR. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115(12):2605–2616. doi: 10.1002/cncr.24287. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S, Griggs JJ, Roscoe JA, Jean-Pierre P, Bole C, Mustian KM, Morrow GR. Self-reported cognitive impairment in patients with cancer. Journal of oncology practice / American Society of Clinical Oncology. 2007;3(2):54–59. doi: 10.1200/JOP.0722001. doi: 10.1200/JOP.0722001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konat GW, Kraszpulski M, James I, Zhang HT, Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008;23(3):325–333. doi: 10.1007/s11011-008-9100-y. doi: 10.1007/s11011-008-9100-y. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(10):1080–1086. doi: 10.1200/JCO.2011.37.0189. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. The Journal of clinical investigation. 2011;121(10):3804–3809. doi: 10.1172/JCI57099. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS, Butt Z, Wagner L, Sweet JJ, Beaumont JL, Vardy J, Cella D. Evaluating the dimensionality of perceived cognitive function. Journal of pain and symptom management. 2009;37(6):982–995. doi: 10.1016/j.jpainsymman.2008.07.012. doi: 10.1016/j.jpainsymman.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef M, Walach H. Fruit, vegetables and prevention of cognitive decline or dementia: a systematic review of cohort studies. The journal of nutrition, health & aging. 2012;16(7):626–630. doi: 10.1007/s12603-012-0097-x. [DOI] [PubMed] [Google Scholar]
- Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity. 2013;21(1):E51–55. doi: 10.1002/oby.20037. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- Lower EE, Fleishman S, Cooper A, Zeldis J, Faleck H, Yu Z, Manning D. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. Journal of pain and symptom management. 2009;38(5):650–662. doi: 10.1016/j.jpainsymman.2009.03.011. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Lundorff LE, Jonsson BH, Sjogren P. Modafinil for attentional and psychomotor dysfunction in advanced cancer: a double-blind, randomised, cross-over trial. Palliative medicine. 2009;23(8):731–738. doi: 10.1177/0269216309106872. doi: 10.1177/0269216309106872. [DOI] [PubMed] [Google Scholar]
- Lyons L, ElBeltagy M, Bennett G, Wigmore P. Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PloS one. 2012;7(1):e30010. doi: 10.1371/journal.pone.0030010. doi: 10.1371/journal.pone.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L, ElBeltagy M, Umka J, Markwick R, Startin C, Bennett G, Wigmore P. Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology. 2011;215(1):105–115. doi: 10.1007/s00213-010-2122-2. doi: 10.1007/s00213-010-2122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, Tannock IF. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16(6):577–583. doi: 10.1007/s00520-007-0341-9. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Ahles TA, Saykin AJ, Ferguson RJ, McDonald BC, Lewis LD, Rhodes CH. Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psychiatry Rep. 2004;6(5):364–371. doi: 10.1007/s11920-004-0023-y. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast cancer research and treatment. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain, behavior, and immunity. 2013;30(Suppl):S117–125. doi: 10.1016/j.bbi.2012.05.007. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlsen M, Pedersen AD, Jensen AB, Zachariae R. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psycho-oncology. 2009;18(3):248–257. doi: 10.1002/pon.1398. doi: 10.1002/pon.1398. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. doi: 10.1002/cncr.21234. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Ancoli-Israel S, Parker B, Natarajan L, Hong S, Jain S, von Kanel R. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain, behavior, and immunity. 2008;22(1):98–104. doi: 10.1016/j.bbi.2007.07.001. doi: S0889-1591(07)00162-6 [pii] 10.1016/j.bbi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KI, Montgomery GW. The genetics of cognitive processes: candidate genes in humans and animals. Behav Genet. 2001;31(6):511–531. doi: 10.1023/a:1013337209957. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K. Management of cancer-related fatigue. Cancer investigation. 2005;23(3):229–239. doi: 10.1081/cnv-200055960. [DOI] [PubMed] [Google Scholar]
- Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Mohile S. Exercise Recommendations for Cancer-Related Fatigue, Cognitive Impairment, Sleep problems, Depression, Pain, Anxiety, and Physical Dysfunction: A Review. Oncology & hematology review. 2012;8(2):81–88. doi: 10.17925/ohr.2012.08.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncology nursing forum. 2012;39(1):E31–40. doi: 10.1188/12.ONF.E31-E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113(5):1097–1106. doi: 10.1002/cncr.23658. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, Vardy J. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20(6):1235–1242. doi: 10.1007/s00520-011-1209-6. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. Journal of clinical and experimental neuropsychology. 2009;31(1):73–89. doi: 10.1080/13803390801992725. doi: 793717043 [pii] 10.1080/13803390801992725. [DOI] [PubMed] [Google Scholar]
- Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, Mustian K. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nature and science of sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of Tamoxifen in premenopausal women with breast cancer compared to healthy controls. Journal of cancer survivorship : research and practice. 2008;2(4):275–282. doi: 10.1007/s11764-008-0070-1. doi: 10.1007/s11764-008-0070-1. [DOI] [PubMed] [Google Scholar]
- Paraska K, Bender CM. Cognitive dysfunction following adjuvant chemotherapy for breast cancer: two case studies. Oncology nursing forum. 2003;30(3):473–478. doi: 10.1188/03.ONF.473-478. doi: 10.1188/03.ONF.473-478. [DOI] [PubMed] [Google Scholar]
- Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, Silverman DH. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain imaging and behavior. 2013 doi: 10.1007/s11682-013-9243-2. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Hortobagyi GN. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast cancer research and treatment. 2009;116(1):113–123. doi: 10.1007/s10549-008-0114-2. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- Raffa RB. A proposed mechanism for chemotherapy-related cognitive impairment ('chemo-fog') Journal of clinical pharmacy and therapeutics. 2011;36(3):257–259. doi: 10.1111/j.1365-2710.2010.01188.x. doi: 10.1111/j.1365-2710.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego SL, Helms RS, Dreau D. Tumor necrosis factor-alpha-converting enzyme activities and tumor-associated macrophages in breast cancer. Immunologic research. 2013 doi: 10.1007/s12026-013-8434-7. doi: 10.1007/s12026-013-8434-7. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA, Cox CR. Stress, coping and cognitive deficits in women after surgery for breast cancer. Journal of clinical psychology in medical settings. 2012;19(2):127–137. doi: 10.1007/s10880-011-9274-z. doi: 10.1007/s10880-011-9274-z. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA, Matsuda S, Cox CR. Tai Chi effects on neuropsychological, emotional, and physical functioning following cancer treatment: a pilot study. Complementary therapies in clinical practice. 2012;18(1):26–30. doi: 10.1016/j.ctcp.2011.02.005. doi: 10.1016/j.ctcp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J Psychosoc Oncol. 2009;27(4):415–434. doi: 10.1080/07347330903183117. doi: 10.1080/07347330903183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiriz AB, Reolon GK, Preissler T, Rosado JO, Henriques JA, Roesler R, Schwartsmann G. Cancer chemotherapy and cognitive function in rodent models: memory impairment induced by cyclophosphamide in mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(16):5000. doi: 10.1158/1078-0432.CCR-06-0138. author reply 5000-5001. doi: 10.1158/1078-0432.CCR-06-0138. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137(1):33–43. doi: 10.1007/s10549-012-2266-3. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. The oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Boogerd W, Muller MJ, Huinink WT, Moonen L, Meinhardt W, Van Dam FS. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta oncologica. 2008;47(1):63–70. doi: 10.1080/02841860701518058. doi: 10.1080/02841860701518058. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. Journal of the National Cancer Institute. 2006;98(23):1742–1745. doi: 10.1093/jnci/djj470. doi: 98/23/1742 [pii] 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta oncologica. 2009;48(1):76–85. doi: 10.1080/02841860802314738. doi: 902183905 p. 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(8):1294–1300. doi: 10.1200/JCO.2008.21.3553. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Linn SC, Boogerd W, Beex LV, Gundy CM, Schagen SB. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psycho-oncology. 2012;21(5):479–487. doi: 10.1002/pon.1928. doi: 10.1002/pon.1928. [DOI] [PubMed] [Google Scholar]
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neuroscience and biobehavioral reviews. 2011;35(3):729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behavioral neuroscience. 2012;126(1):123–127. doi: 10.1037/a0025539. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer--preliminary results of an observational longitudinal study. Breast. 2005;14(2):142–150. doi: 10.1016/j.breast.2004.10.004. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Walsh E, et al. Catechol-O-Methyltransferase (COMT) genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. doi: 10.1002/cncr.25685. In Press. [DOI] [PubMed] [Google Scholar]
- Sprod LK, Mohile SG, Demark-Wahnefried W, Janelsins MC, Peppone LJ, Morrow GR, Mustian KM. Exercise and Cancer Treatment Symptoms in 408 Newly Diagnosed Older Cancer Patients. Journal of geriatric oncology. 2012;3(2):90–97. doi: 10.1016/j.jgo.2012.01.002. doi: 10.1016/j.jgo.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17(2):122–130. doi: 10.1002/pon.1210. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- Swayampakula AK, Alkhouri N, Haut MW, Abraham J. Cognitive impairment with significant brain parenchymal volume loss following standard adjuvant chemotherapy in a patient with breast cancer. Clinical advances in hematology & oncology : H&O. 2007;5(12):985–987. discussion 987-988. [PubMed] [Google Scholar]
- Tager FA, McKinley PS, Schnabel FR, El-Tamer M, Cheung YKK, Fang YX, Hershman DL. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study (vol 123, pg 25, 2010) Breast cancer research and treatment. 2011;126(1):271–272. doi: 10.1007/s10549-009-0606-8. doi: DOI 10.1007/s10549-009-0684-7. [DOI] [PubMed] [Google Scholar]
- Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair W, Butterfield DA. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100(1):191–201. doi: 10.1111/j.1471-4159.2006.04179.x. doi: JNC4179 p. 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, Butterfield DA. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiology of disease. 2006;23(1):127–139. doi: 10.1016/j.nbd.2006.02.013. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. Journal of the National Cancer Institute. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Vardy J, Dhillon H, Pond GR, Rourke S, Xu W, Renton C, Tannock IF. Cognitive function in colorectal cancer patients: Interim analysis of a longitudinal prospective study. J Clin Oncol Supp. abs. 9021. 2012 Abstract. [Google Scholar]
- Vardy J, Wong K, Yi QL, Park A, Maruff P, Wagner L, Tannock IF. Assessing cognitive function in cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2006;14(11):1111–1118. doi: 10.1007/s00520-006-0037-6. doi: 10.1007/s00520-006-0037-6. [DOI] [PubMed] [Google Scholar]
- Vardy JL, Booth C, Pond GR, Zhang H, Dhillon S, Clarke SJ, Tannock IF. Cytokine levels in patients with colorectal cancer and breast cancer and their relationship to fatigue and cognitive function. Supplement to Journal of Clinical Oncology. 2007;18S:9070. Abstract. [Google Scholar]
- Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G. Predictors of cognitive decline after chemotherapy in breast cancer patients. Journal of the International Neuropsychological Society : JINS. 2009;15(6):951–962. doi: 10.1017/S1355617709990567. doi: S1355617709990567 p. 10.1017/S1355617709990567. [DOI] [PubMed] [Google Scholar]
- Villani F, Busia A, Villani M, Vismara C, Viviani S, Bonfante V. Serum cytokine in response to chemo-radiotherapy for Hodgkin's disease. Tumori. 2008;94(6):803–808. doi: 10.1177/030089160809400605. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M, Unverzagt F. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast cancer research and treatment. 2012;135(3):799–809. doi: 10.1007/s10549-012-2210-6. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L, Sweet J, Butt Z, Lai J, Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. Journal of Supportive Oncology. 2009;7:32–39. [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Wieneke M. H. a. D. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-oncology. 1995;4:61–66. E.R. [Google Scholar]
- Yoshikawa E, Matsuoka Y, Inagaki M, Nakano T, Akechi T, Kobayakawa M, Uchitomi Y. No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast cancer research and treatment. 2005;92(1):81–84. doi: 10.1007/s10549-005-1412-6. doi: 10.1007/s10549-005-1412-6. [DOI] [PubMed] [Google Scholar]