Abstract
Ultrasound and combined optical and ultrasonic (photoacoustic) molecular imaging have shown great promise in the visualization and monitoring of cancer through imaging of vascular and extravascular molecular targets. Contrast-enhanced ultrasound with molecularly targeted microbubbles can detect early-stage cancer through the visualization of targets expressed on the angiogenic vasculature of tumors. Ultrasonic molecular imaging can be extended to the imaging of extravascular targets through use of nanoscale, phase-change droplets and photoacoustic imaging, which provides further molecular information on cancer given by the chemical composition of tissues and by targeted nanoparticles that can interact with extravascular tissues at the receptor level. A new generation of targeted contrast agents goes beyond merely increasing imaging signal at the site of target expression but shows activatable and differential contrast depending on their interactions with the tumor microenvironment. These innovations may further improve our ability to detect and characterize tumors. In this review, recent developments in acoustic and photoacoustic molecular imaging of cancer are discussed.
Keywords: molecular imaging, ultrasound, photoacoustic imaging, microbubble, nanodroplet, nanoparticle, cancer
Ultrasound is a widely used clinical imaging modality that has emerged into molecular imaging of cancer with targeted contrast agents. A related imaging modality, photoacoustic imaging, also shows great potential in the molecular imaging of cancer because of its ability to image optical absorption properties of both intrinsic tissue chromophores and exogenous contrast agents. Photoacoustic imaging uses pulsed laser irradiation to induce localized thermoelastic expansion, generating acoustic waves detectable by a traditional ultrasound transducer. The modalities share acquisition equipment and data processing techniques that can provide the basis for real-time, nonionizing, and cost-effective molecular imaging of focal anatomic areas accessible to ultrasound. This review focuses on the current application of acoustic and photoacoustic imaging for the molecular imaging of cancer in vivo using both exogenous and endogenous contrast agents and sheds light on future developments in both approaches.
ACOUSTIC MOLECULAR IMAGING
Among the most bioneutral and cost-effective of medical imaging modalities, ultrasound imaging typically provides anatomic images based on the reflection and scattering of acoustic waves generated and received by an acoustic transducer. The contrast in ultrasound imaging is based on changes in acoustic impedance between tissues—changes that are dependent on their density and the speed of sound within them. The acoustic impedance of most biologic tissues is relatively similar because of a similar water content, limiting intrinsic contrast. To increase the contrast of ultrasound imaging in clinical practice, shelled, gas-filled microbubbles are routinely injected intravenously to increase the mismatch in acoustic impedance between tissues and thus help detect and characterize focal lesions. These contrast microbubbles also allow ultrasound to be used as a molecular imaging modality by combining contrast enhancement with association with specific molecular targets.
Microbubbles are typically 1–4 µm in diameter and consist of biologically inert gasses such as perfluorocarbons. A shell (made from lipids, albumin, or polymer) is used to stabilize the microbubble in order to increase circulation time. Through covalent and noncovalent techniques, targeting moieties such as antibodies and peptides can be attached to the surface of microbubbles to allow for ultrasound molecular imaging. Some recent reviews have provided a detailed discussion of synthesis and ligand conjugation (1–3). The microbubbles, besides linearly increasing the contrast in B-mode ultrasound images, also display nonlinear behavior. When excited with size-dependent resonance frequencies, typically between 2 and 10 MHz, the microbubbles oscillate, emitting pressure transients at frequencies different from incident waves, unlike the linear response of tissue. Contrast-mode ultrasound imaging listens for these emitted frequencies and creates high-contrast images primarily of microbubble location. Monitoring the wash-in and reperfusion rates after microbubble destruction in diseased tissues is called dynamic contrast-enhanced ultrasound (4), which can provide quantitative information on the tumor vasculature useful for monitoring treatment response during cancer therapy (5). More information on dynamic contrast-enhanced ultrasound and ultrasound molecular imaging can be found in several previous publications (1–5) and in Figure 1.
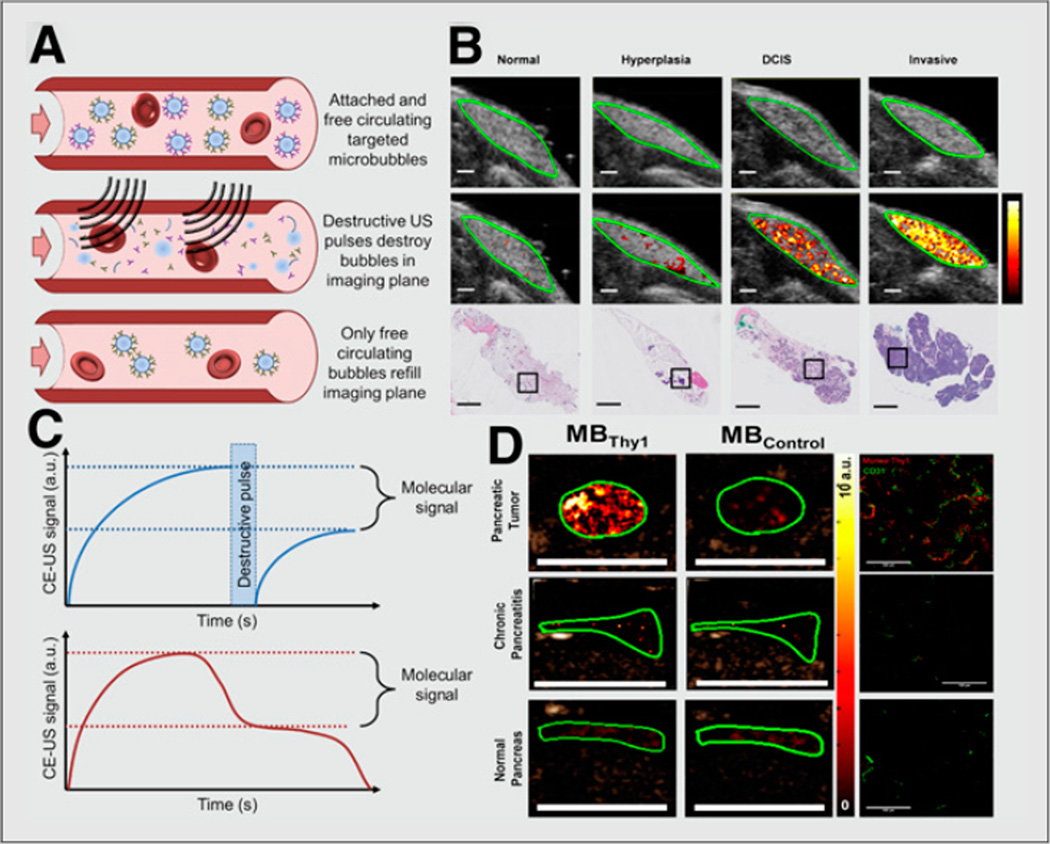
FIGURE 1. Ultrasound molecular imaging of cancer.
(A) Schematic showing quantification of ultrasound molecular imaging signal using molecularly targeted contrast microbubbles. (Top) First, microbubbles attach to targets on vascular surface, and signal amplitude is recorded. (Middle) Next, ultrasound pulses are applied to destroy microbubbles within imaging plane. (Bottom) Finally, free circulating microbubbles perfuse into imaging plane and signal amplitude is again recorded. (B) Molecular ultrasound images using clinical-grade human vascular endothelial growth factor receptor 2 (kinase insert domain receptor)–targeted contrast microbubble in transgenic breast cancer mouse model showing increased signal as tissues progress from normal to hyperplasia, ductal carcinoma in situ, and invasive breast cancer (11). (C, top) Difference in pre- and postdestruction images corresponds to signal from attached microbubbles. (C, bottom) Alternative method for determining molecular signal involves waiting (e.g., 10 min after intravenous injection of microbubbles) to allow clearance of freely circulating microbubbles and measuring steady-state signal corresponding to microbubbles attached to molecular targets. (D) Ultrasound molecular image of 4-mm tumor in transgenic pancreatic cancer mouse model using microbubbles targeted at Thy-1 (novel pancreatic cancer target) compared with normal pancreas and chronic pancreatitis tissues (13). a.u. = arbitrary unit; CE-US = contrast-enhanced ultrasound; DCIS = ductal carcinoma in situ; MBThy1 = microbubbles targeted at Thy-1; MBControl = microbubbles targeted at control tissues. (B and D reproduced with permission of (11,13).)
Because the size of molecularly targeted microbubbles restricts them to the vasculature, molecular ultrasound targets must be expressed on vascular endothelial cells. Neovasculature, a hallmark of cancer, occurs in most solid tumors at an early stage (250-µm tumors) to establish an independent oxygen and nutrient supply. Two commonly researched neovascular targets are vascular endothelial growth factor receptor 2 and αvβ3 integrin. The αvβ3 integrin receptor binds peptides in matrix proteins with the sequence arginineglycine-aspartic acid (RGD). Anderson et al. targeted microbubbles with a cyclic RGD peptide and monitored targeting signal in Met-1 orthotopic breast cancer tumors (6). The group found a 33-fold increase in contrast-enhanced signal over nontargeted control microbubbles. A clinical-grade vascular endothelial growth factor receptor 2–targeted microbubble was designed (7) and showed promising results for imaging expression of the receptor in several animal models of cancer, including an orthotopic hepatic adenocarcinoma model (8), a colon cancer mouse model (9), and an orthotopic breast cancer mouse model (10). Recently, this clinical- grade contrast microbubble enabled assessment of breast cancer development in a transgenic breast cancer mouse model. In vivo ultrasound imaging signal was substantially increased when breast tissue progressed from benign hyperplasia to precursor ductal carcinoma in situ and further increased when mammary glands developed invasive breast cancer (Fig. 1) (11). These findings suggest that ultrasound molecular imaging may be further developed for earlier breast cancer detection—for example, in a breast cancer screening setting—by using quantitative ultrasound molecular imaging information to differentiate clinically action- able from nonactionable lesions (11). This clinical-grade contrast agent has recently entered first-in-human clinical trials to assess safety and efficacy (12).
To find highly specific vascular markers of cancer as potential new molecular imaging targets for ultrasound, tissues from human pancreatic adenocarcinoma tissues, primary chronic pancreatitis, and normal pancreatic tissue have undergone proteomic analysis. Thymocyte differentiation antigen 1 (Thy1) was identified as a highly specific and novel potential molecular imaging target for pancreatic cancer. The feasibility of human Thy1 imaging was shown in a novel orthotopic pancreatic cancer xenograft model expressing human Thy1 on murine neoangiogenic vessels (13). Furthermore, murine Thy1- targeted ultrasound molecular imaging was tested in a transgenic pancreatic cancer mouse model (Pdx1-Cretg/+; KRasLSL G12D/+; Ink4a/Arf−/−); tumors with a diameter of as small as 2 mm could be visualized (Fig. 1) with an approximately 3-fold higher signal than is needed for imaging normal pancreatic tissue or chronic pancreatitis, suggesting that Thy1-targeted ultrasound molecular imaging may be further developed within an algorithm for early detection of pancreatic cancer (13). Targeting multiple molecular targets is possible and, compared with single-targeted microbubbles, can increase signal. An in-depth summary of ultrasound molecular imaging applications in cancer has been provided in recent reviews (1,2,12).
Sized at several micrometers, microbubbles have limited extravasation through the poorly formed vasculature characteristic of many cancers, with cell junction gaps typically ranging between 100 and 800 nm (14). Because many clinically relevant molecular markers are located on cancer cells or on the tumor stroma in the extravascular compartment, ultrasound molecular imaging using nanoscale contrast agents has recently been applied to explore extravascular targets.
NANOSCALE ULTRASOUND CONTRAST AGENTS
Taking advantage of the enhanced permeability and retention (EPR) effects of tumor vasculature (i.e., leaky vasculature combined with poor lymphatic drainage in tumors), liquid nanodroplets of perfluorocarbon have been studied as contrast agents for ultrasound imaging. It is hypothesized that such contrast agents accumulate in the extravascular compartment via the EPR effect after intravenous administration. Once accumulated, they would act as pooled Raleigh scatterers (small scatterers compared with the wavelength of ultrasound causing elastic scattering) to increase the reflection of ultrasonic signals. These agents are similarly synthesized to microbubbles with lipid, protein, or polymer shells (15,16) through emulsification, extrusion, or microfluidic techniques (16). However, because stronger ultrasonic contrast mechanisms are acoustic impedance mismatch and nonlinear interactions, nanoscale liquid agents do not provide sufficient ultrasound contrast compared with their gaseous counterparts. Therefore, liquid nanoscale droplets need to undergo a phase change into gas after reaching their target location.
The concept of a phase-change droplet for ultrasound contrast has been applied to nanoscale droplets intended to vaporize into a gaseous, contrast-enhancing state once extravasated to the imaging location. The nanoscale of these liquid droplets quickly proved a challenge for acoustic vaporization due to increased LaPlace pressure (16) and increased boiling temperature of perfluorocarbon at nanoscale volumes (16), providing a hyperstabilized agent. The energy output needed to acoustically vaporize nanodroplets routinely exceeded the mechanical indices of ultrasound that are considered safe for diagnostic purposes (current Food and Drug Administration limit for mechanical index is 1.9; supplemental material [http://jnm.snmjournals.org]). Therefore, several strategies have been developed for vaporizing nanodroplets using a lower mechanical index. Perfluorobutane, with a very low boiling point (−2°C), has been used to synthesize liquid droplets which produce “superheated” droplets that are readily vaporized under bioinert acoustic pressures (16). Perfluorobutane droplets were synthesized by condensing (cooling) gas bubbles after synthesis to make a uniform population of 200- to 300-nm droplets (Supplemental Fig. 2). Another method to create acoustic contrast using nanoscale particles is through pseudovaporizing droplets, which take advantage of the fact that perfluorocarbons solubilize oxygen efficiently (16). Perfluoro-15-crown-5-ether (boiling point, 146°C) was coated with a polymer shell producing a highly stable and long-circulating biostealth droplet (16). These particles undergo a reversible vaporization mediated by the difference between the partial pressure of oxygen solubilized in perfluorocarbon and the partial pressure of the perfluorocarbon itself. Effectively, dissolved oxygen forms bubbles in the perfluorocarbon during peak negative pressures during sonication.
Although these studies are promising, as of the time of publication there has been only one example of ultrasound contrast enhancement after nanodroplet extravasation into a xenograft tumor in vivo after intravenous injection. Williams et al. (17) injected a high concentration (2 × 1012 droplets) of fluorosurfactant-stabilized perfluoropentane droplets with an average size of 200 nm. One hour after injection, focused ultrasound (mechanical index, 1.9) was used to vaporize the extravasated droplets in a tumor, showing contrast enhancement (Supplemental Fig. 2).
Perfluorocarbon nanodroplets could provide many advantages as a contrast agent. First, ultrasound imaging is highly sensitive, being able to detect only a few bubbles and thus allowing for molecular imaging of the most specific extravascular targets. Second, these agents are completely biocompatible, unlike many metal or solid nanoparticle contrast agents used with other imaging modalities, as the perfluorocarbon gas is simply exhaled. With continued research, perfluorocarbon nanodroplets could become a valuable tool for extravascular molecular ultrasound; however, there are a few challenges to overcome. Currently, delivery of contrast agents to tumor interstitium occurs primarily through the EPR effect of tumors. The EPR effect in solid tumors in patients or transgenic animal cancer models recapitulates human diseases more closely and may not always be as pronounced as in the somewhat artificial subcutaneous or orthotopic xenografts in mice with enhanced EPR effects (14). Furthermore, whereas microbubble-mediated enhancement of imaging contrast leverages the nonlinear properties of microbubbles with sizes tuned for the insonation frequency, after vaporization of nanoscale contrast agents they are usually viewed in B-mode ultrasound, in which normal tissues provide high background signal. Although the initial bubble generated from a given size droplet is approximately 5 times the diameter, the predictable size quickly changes because of gas diffusion and bubble coalescence, making these particles suboptimal for nonlinear ultrasound imaging. A recent review provided more details on perfluorocarbon nanodroplets (16).
Another approach to acoustic molecular imaging of intra- and extravascular targets is combination with a complementary imaging modality intrinsically capable of molecular imaging.
PHOTOACOUSTIC MOLECULAR IMAGING
Photoacoustics provide real-time, noninvasive imaging of the optical absorption properties of tissues with optical contrast but at depths of up to 5 cm. Sufficiently short (5–10 ns), pulsed laser irradiation is used to stimulate localized thermoelastic expansion of the tissues, which then emit broadband acoustic waves during contraction that can be detected with traditional ultrasound transducers and processed with similar reconstruction algorithms (18,19). Because photoacoustic imaging contrast is based on optical absorption properties of tissue (which depend on the underlying molecular composition of the tissue), this modality is inherently suited for molecular imaging. Furthermore, traditional ultrasound and photoacoustic imaging processing can be combined because of their complementary information and instrumentation, providing anatomic, functional, and molecular information with a single image acquisition. Photoacoustic molecular imaging can be divided into 2 approaches (described in detail in the supplemental material): photoacoustic imaging of differences in the optical absorption properties of endogenous tissue via spectroscopic (multiwavelength) imaging, and photoacoustic imaging using exogenous contrast agents targeted to a specific molecular marker of interest.
Applications of Photoacoustic Molecular Imaging in Cancer
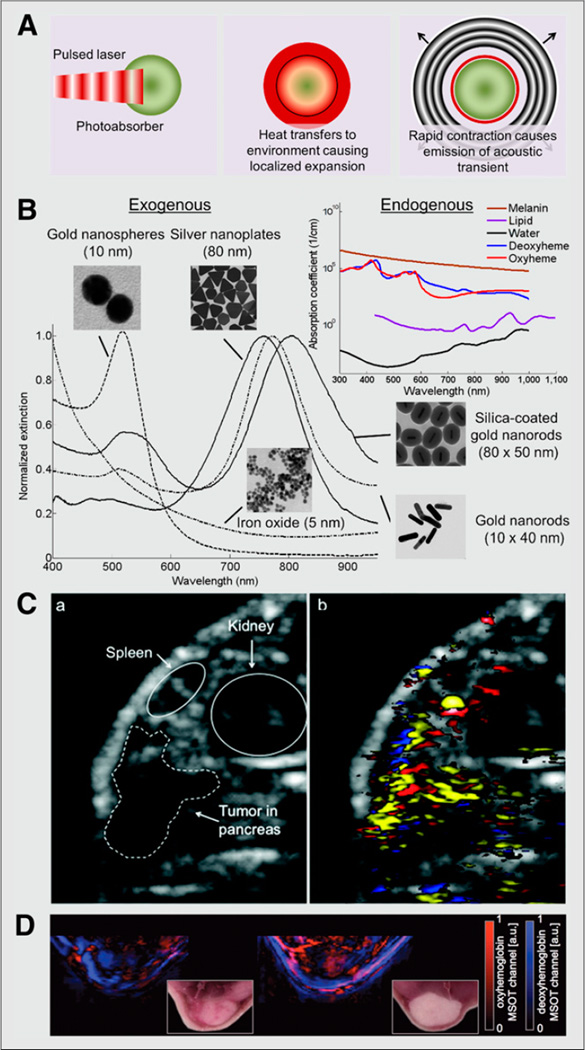
Contrast-enhanced photoacoustic molecular imaging allows background signal suppression via wavelength selection (i.e., minimizing background signal generation from endogenous photoabsorbers, typically within the optical window between 700 and 1,000 nm) and via targeting of specific molecular markers expressed either on the tumor vasculature or in the extravascular compartment. As in ultrasound, studies have shown that the use of photoacoustic molecular imaging is feasible for assessing molecular targets expressed on the neovasculature of cancer. The use of RGD-labeled gold nanoparticles allowed the integrin expression on the neovasculature of glioblastoma to be visualized by photoacoustics (20). Similarly, the use of RGD-labeled single-walled carbon nanotubes conjugated with indocyanine green dyes has allowed photoacoustic molecular imaging of integrins on the neovasculature of glioblastomas with an estimated sensitivity of 170 pM (21). In addition to imaging the neovasculature of cancer, photoacoustic molecular imaging of extravascular targets has been explored using contrast agents that extravasate from the tumor vasculature. For example, gold nanorods, indocyanine green–loaded PEBBLEs (probes encapsulated by biologically localized embedding; i.e., dye-loaded silica shells), and dyes (indocyanine green and methylene blue) have been conjugated with HER-2 antibodies, and the clinically available antibody trastuzumab has been dyeand gold-modified to allow imaging of breast cancer through trastuzumab receptor expression on breast cancer cells (18,19). Similarly, photoacoustic contrast agents have been used to image and identify cancer cells expressing epidermal growth factor receptor, including gold nanorods and nanospheres, silica-coated gold nanorods, and silver nanoplates (18,19). In one study, 80-nm epidermal growth factor receptor–targeted silver nanoplates were intravenously injected and allowed to accumulate in a xenograft tumor model of pancreatic cancer (MPanc96 cells) (20) as shown in Figure 2. The epidermal growth factor receptor–targeted nanoparticles could be spectroscopically resolved over nontargeted nanoparticles and endogenous photoabsorbers. However, gold and silver nanoparticles would have to undergo extensive testing and clinical trials before they would be useable in human practice, making the use of organic dyes (indocyanine green, methylene blue), which are Food and Drug Administration–approved, pertinent to allow for near-term clinical usage.
FIGURE 2. Photoacoustic molecular imaging of cancer.
(A) Diagram of photoacoustic effect. Pulsed laser irradiation is absorbed by photoabsorber, causing localized heating and expansion of directly surrounding environment. During rapid contraction, high-frequency acoustic transient (sound wave) is emitted. (B) Absorption spectra of endogenous and exogenous contrast agents. (C) Ultrasound (left) and spectroscopically resolved photoacoustic images (right) (14.5 × 11.8 mm) of epidermal growth factor receptor targeted silver nanoplates (yellow), oxygenated hemoglobin (red), and deoxygenated hemoglobin (blue) in human pancreatic carcinoma (MPanc96 cells) tumor xenograft (21). (D) Spectroscopically resolved photoacoustic images (and corresponding photographs) of oxy- and deoxyhemoglobin in orthotopic murine breast tumor in mice (20). a.u. = arbitrary unit; MSOT = multispectral optoacoustic tomography. (Silicacoated gold nanorods and iron oxide nanoparticle images and spectra in B reproduced with permission of (24,25); C reproduced with permission of (21); D reproduced with permission of (20).)
Clinical applications for molecular photoacoustic imaging are still being explored. Initial applications will likely be correlated to areas in which ultrasound is currently used for diagnosis and detection but has room for improvement. For example, breast cancer screening in patients with dense breast tissues is notoriously ineffective, with low positive predictive rates. The additional use of photoacoustic imaging could be added to provide additional information such as oxygenation saturation and lipid content of suspect lesions, potentially aiding in characterizing focal lesions or spotting previously undetected lesions. Two primary hurdles exist before photoacoustic molecular imaging can become a reality in the clinic. First, an approved clinical system is not yet available, although a few companies and groups are attempting to develop one. Second, photoacoustic imaging will be limited to focal imaging areas by light penetration into tissues. Although current estimates suggest that the depth of imaging is limited to 5–7 cm, there are several methods of working around this limitation, including the use of endoscopy and alternate wavelengths that minimize background signal and allow for higher-fluence use. More details about and applications of photoacoustic molecular imaging have been described in several recent reviews (18–20).
“Smart” Photoacoustic Contrast Agents
Contrast-enhanced photoacoustic imaging is not limited to using basic contrast agents that simply accumulate to provide increased signal but instead can be enhanced by the use of complex smart probes that interact with their molecular targets in the tumor microenvironment, providing activatable forms of contrast. One group of smart probes is activated when it comes into contact with a tumor-specific enzyme found in the tumor microenvironment. For example, a peptide-dye–based photoacoustic agent that activates when exposed to matrix metalloproteases was used in FTC133 thyroid xenograft tumors in nude mice. The activity of matrix metalloproteases in follicular thyroid carcinoma may distinguish it from benign thyroid adenomas, potentially decreasing the need for unnecessary surgical removals (22). Another smart technique, photoacoustic lifetime imaging, uses electron excited-state methylene blue (clinically approved), which has absorption properties that are oxygen concentration–dependent and thus can image the oxygen saturation in tumors. This technique has been demonstrated in a human prostate adenocarcinoma (LNCaP cells) xenograft model in mice in which photoacoustic lifetime imaging and direct oxygenation probe measurements showed a strong correlation (23).
Most preclinical studies using exogenous photoacoustic contrast agents have been performed in vitro or in vivo using subcutaneous or orthotopic xenograft tumor models. Further work is warranted to confirm that these techniques are viable in tumor models that better recapitulate cancer in humans.
CONCLUSIONS AND FUTURE DIRECTIONS
Acoustic and photoacoustic imaging have been shown to have great potential for imaging molecular signatures in cancer, and both techniques are clinically translatable. Currently, molecularly targeted microbubbles to detect disease markers on vascular surfaces for early cancer diagnosis and treatment monitoring are undergoing clinical testing. Ultrasound combined with photoacoustics can provide additional molecular information with or without the addition of exogenous contrast agents. The current direction of research focuses on further exploiting vascular targets by discovery and validation of novel neoangiogenesis-associated molecular markers and on developing nanoscale or even smaller contrast agents for both ultrasound and photoacoustic molecular imaging. Future contrast agents for cancer molecular imaging may also be “smart,” selectively changing their contrast properties and imaging signal on the basis of the tumor environment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Yun-Sheng Chen and Ryan Truby for providing nanoparticle micrographs and absorption spectra.
This work is supported by R01 CA155289-01A1, NIH R01DK092509-01A1, and R25 CA118681.
Footnotes
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: concepts and contrast agents. Curr Med Chem. 2009;16:627–642. doi: 10.2174/092986709787458470. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin Radiol. 2010;65:567–581. doi: 10.1016/j.crad.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unnikrishnan S, Klibanov AL. Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. AJR. 2012;199:292–299. doi: 10.2214/AJR.12.8826. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich CF, Averkiou MA, Correas J-M, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344–351. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 5.Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53–58. doi: 10.1007/s11523-010-0136-7. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CR, Hu X, Zhang H, et al. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest Radiol. 2011;46:215–224. doi: 10.1097/RLI.0b013e3182034fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol. 2010;45:89–95. doi: 10.1097/RLI.0b013e3181c5927c. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto K, Moriyasu F, Negishi Y, et al. Quantification in molecular ultrasound imaging a comparative study in mice between healthy liver and a human hepatocellular carcinoma xenograft. J Ultrasound Med. 2012;31:1909–1916. doi: 10.7863/jum.2012.31.12.1909. [DOI] [PubMed] [Google Scholar]
- 9.Pysz MA, Foygel K, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55) Radiology. 2010;256:519–527. doi: 10.1148/radiol.10091858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bzyl J, Palmowski M, Rix A, et al. The high angiogenic activity in very early breast cancer enables reliable imaging with VEGFR2-targeted microbubbles (BR55) Eur Radiol. 2013;23:468–475. doi: 10.1007/s00330-012-2594-z. [DOI] [PubMed] [Google Scholar]
- 11.Bachawal SV, Jensen KC, Lutz AM, et al. Earlier detection of breast cancer with ultrasound molecular imaging in a transgenic mouse model. Cancer Res. 2013;73:1689–1698. doi: 10.1158/0008-5472.CAN-12-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg. 2012;2:87–97. doi: 10.3978/j.issn.2223-4292.2012.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foygel K, Wang H, Machtaler S, et al. Detection of pancreatic ductal adenocarcinoma in mice by ultrasound imaging of thymocyte differentiation antigen 1. Gastroenterology. 2013;145:885–894. doi: 10.1053/j.gastro.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect (EPR) for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson K, Homan K, Emelianov S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nat Commun. 2012;3:618. doi: 10.1038/ncomms1627. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport N. Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:492–510. doi: 10.1002/wnan.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams R, Wright C, Cherin E, et al. Characterization of ubmicron phasechange perfluorocarbon droplets for extravascular ultrasound imaging of cancer. Ultrasound Med Biol. 2013;39:475–489. doi: 10.1016/j.ultrasmedbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Luke GP, Yeager D, Emelianov SY. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann Biomed Eng. 2012;40:422–437. doi: 10.1007/s10439-011-0449-4. [DOI] [PubMed] [Google Scholar]
- 19.Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011;29:213–221. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1:602–631. doi: 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Zerda A, Liu Z, Bodapati S, et al. Ultrahigh sensitivity carbon nanotube agents for photoacoustic molecular imaging in living mice. Nano Lett. 2010;10:2168–2172. doi: 10.1021/nl100890d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi J, Kothapalli S-R, Bohndiek S, et al. Molecular photoacoustic imaging of follicular thyroid carcinoma. Clin Cancer Res. 2013;19:1494–1502. doi: 10.1158/1078-0432.CCR-12-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao Q, Morgounova E, Jiang C, Choi J, Bischof J, Ashkenazi S. In vivo photoacoustic lifetime imaging of tumor hypoxia in small animals. J Biomed Opt. 2013;18:076019. doi: 10.1117/1.JBO.18.7.076019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y-S, Frey W, Kim S, Kruizinga P, Homan K, Emelianov S. Silica-coated gold nanorods as photoacoustic signal nanoamplifiers. Nano Lett. 2011;11:348–354. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truby RL, Emelianov SY, Homan KA. Ligand-mediated self-assembly of hybrid plasmonic and superparamagnetic nanostructures. Langmuir. 2013;29:2465–2470. doi: 10.1021/la3037549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.