Abstract
Accumulating evidence indicates that the gut microbiota, long appreciated to be a key determinant of intestinal inflammation, is also playing a key role in chronic inflammatory disease of the liver. Such studies have yielded a general central hypothesis whereby microbiota products activate the innate immune system to drive pro-inflammatory gene expression thus promoting chronic inflammatory disease of the liver. This article reviews the background supporting this hypothesis, outlines how it can potentially explain classic and newly emerging epidemiological chronic inflammatory liver disease, and discusses potential therapeutic means to manipulate the microbiota so as to prevent and/or treat liver disease.
Keywords: LPS, flagellin, TLR, portal vein, inflammation
Background/Introduction
Microbiota, complex partner of the intestine
The gut microbiota is the collective term for the 100 trillion bacteria, 1–2 kg in mass, that inhabit the gastrointestinal tract. The gut microbiota is a very diverse ecosystem in that it is comprised of over 2,000 distinct species and has a collective genome of 150-fold more genes than the human genome (1). Most of these bacteria cannot be grown as purified cultures and thus much of the study of these bacteria largely consists of identifying bacterial species and their genes (collectively referred to as the microbiome) based on DNA sequencing - a technology in which there has been dramatic advances in recent years - and studying phenotypes of "germfree" mice, which lack a microbiota or germfree mice transplanted with a complex microbiota whose composition has typically been associated with a particular phenotype. Such studies have led to the appreciation that the microbiota is at least as metabolically complex as the liver, and that the microbiota should not be viewed as entirely alien but rather as having coevolved with the intestine. Metabolic activity of the microbiota provides a great benefit to human health both by providing essential nutrients and maximizing the efficiency of energy harvest from ingested food. However, the microbiota also contains numerous potential opportunistic pathogens and thus has the potential to harm its host if this complex microbial community is not well managed. Maintaining the homeostasis of the gut microbiota has necessitated the development of a specialized mucosal immune system, whose development is in fact dependent upon the presence of a microbiota in that it is absent in germ free mice (2).
Microbiota, large source of pro-inflammatory agonists
The mucosal immune system expediently detects and clears most food-borne pathogens, keeps potential opportunists in check without excess harm to beneficial bacteria and host tissues. A central component of the mucosal immune system is the intricate system of receptors that recognize conserved feature of microbial products (3, 4). Primary classes of such receptors include the Toll-like (TLR) and nod-like (NLR) receptors that recognize a variety of bacterial products including lipopolysaccharide (LPS), flagellin, peptidoglycan, and bacterial DNA. The primary consequence of TLR/NLR detecting their cognate agonists is to broadly induce host-defense gene expression that can protect against numerous microbes. This is achieved in large part by activating some of the dominant signaling cascades such as the NF-κB transcriptional pathways that are generally referred to as pro-inflammatory in that they promote immune cell recruitment. While immune cell recruitment plays an important role in containing pathogens, it can also result in host tissue damage. Moreover, it is now well appreciated that cytokines that mediate such classic inflammatory responses such as tumor necrosis factor-alpha (TNF-α), also have a variety of effects on metabolic/growth/differentiation pathways and thus there is a potential for microbial products to have broad effects on host phenotype. Accordingly, as reviewed elsewhere (5), expression and function of intestinal TLR/NLR are normally regulated in a manner that prevents activation of these receptors by the microbiota. However, activation of intestinal TLR/NLR may still drive a variety of inflammatory diseases including liver disease. Moreover, as discussed below, the liver also expresses TLR/NLR that are increasingly appreciated to play a direct role in liver disease.
Role of microbiota in intestinal disease
As study of the microbiota in liver disease is in its infancy, it is useful to first consider lessons from study of how the intestinal microbiota can promote other diseases. The microbiota has long been considered as a central player in inflammatory bowel disease (6). Altered gut microbiota is associated with disease in humans and mice, and gut microbiota is essential for most murine models of colitis. The essential role seems to largely reflect that gut microbial products activating TLR/NLR drive the inflammation that defines disease. But yet, TLR/NLR also play a key role in keeping gut bacteria in-check thus preventing disease. Thus, given that humans would not normally exist in germfree states, the most important lesson from the intestine may be that a properly functioning immune system, which will clearly involve TLR/NLR signaling, can maintain a healthy microbiota such that it does not cause a potentially problematic level of activation of TLR/NLR that would result in clinical indicators of inflammation. Importantly, such problematic, i.e. colitis-associated, levels of TLR/NLR activation can result from an inherently colitogenic microbiota, excessive immune activation, or an underlying immune deficiency that results in a compensatory immune activation that is necessary to clear the bacteria. Intestinal microbiota can also promote metabolic disease by 3 primary mechanisms. First, microbiota can alter the efficiency of energy harvest from ingested food in that microbiotas from obese humans exhibit altered Bacteroidetes/Firmicutes ratios, which promote increased energy harvest and adiposity when transplanted into germ-free mice (7). Another means by which microbial metabolism may negatively influence the host is by generating toxic metabolites from the diet. For example, Wang et al. observed that microbiota converts choline to phosphatidylcholine linked to heart disease (8). Perhaps an overarching means by which altered host-microbiota interactions promotes metabolic disease is by driving low-grade inflammation as several mouse strains that fail to maintain healthy populations of gut microbiota develop metabolic syndrome (9–11). In addition, such metabolic disease may be driven, at least in part, by microbiota-derived TLR/NLR agonists activating pro-inflammatory signaling in organs that control central metabolism. This concept is best studied for the quintessential TLR agonist, LPS, which activates TLR4. In mice, high-fat diets result in increased gut permeability and modest but significantly increased levels of circulating LPS, termed metabolic endotoxemia, that drives metabolic disease (12). The concept that reduced intestinal barrier function can result in gut microbiota products breaching the intestine, sometimes referred to as "leaky gut syndrome", is increasingly thought to play a central role in liver disease.
Inflammation, a central ingredient in liver disease
In accord with its essential role in a panoply of essential life-sustaining processes, diseases of the liver comprise many of the most vexing public health problems. While diseases affecting the liver are quite complex and, reflecting the liver's central role in metabolism and detoxification, generally involve multiple organs, major classifications of liver disease include alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), cancer, and hepatitis. While the latter refers to the group of diseases defined by overt histopathologically-evident inflammation of the liver (i.e. presence of inflammatory cells), it is now clear that inflammation as defined by elevated pro-inflammatory gene expression plays a central role in all of these common hepatic disorders. While disease development and outcome is dictated by host genetics as well as a variety of environmental/behavioral factors such as diet, infection, and alcohol consumption, the mechanisms by which all of these factors affect disease susceptibility can be viewed from the prism of inflammation. Indeed, most if not all liver diseases are associated with elevated markers of inflammation, especially pro-inflammatory cytokines, which are thought to play a role on driving disease and are increasingly being pharmacologically targeted to treat these disorders. Thus, while it seems reasonable to speculate that microbiota altering energy harvest and/or directly producing toxic metabolites plays a role in liver disease, at present, available evidence primarily supports the notion that the microbiota plays a central role in liver disease by promoting inflammation. Hence, the remainder of this review will focus on this concept.
Microbiota as a potential driver of liver inflammation
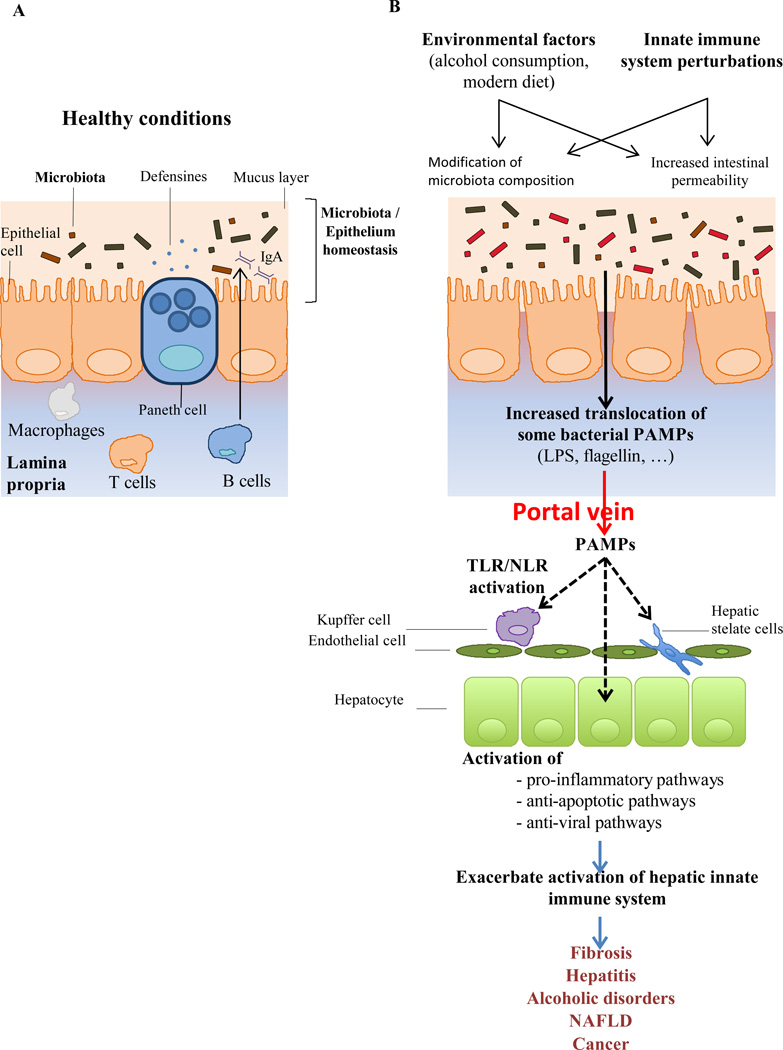
The enormity of the gut microbiota and that portal vein serves as a "super highway" from the intestine to the liver suggests that some gut bacteria and their products might reach the liver on more than just rare occasions. Indeed, although the overwhelming majority of intestinal bacteria are located in the intestinal lumen and outer mucus layer, it seems reasonable to envisage that a very small but perhaps not insignificant minority of bacteria might occasionally breach the gut epithelium and quickly arrive in the liver. In accordance, low levels of some bacterial products can often be detected in systemic circulation in diseased and, to a lesser extent, in healthy persons, further supporting the notion that gut microbiota products might activate TLR/NLR in the liver. Numerous studies indicate that, like most populations of macrophages, Kupffer cells respond to very low concentration of LPS via activation of NF-κB and production of pro-inflammatory cytokines, suggesting that these cells would be responsive to physiologically relevant levels of microbial products that reach the liver (13). Supporting this concept that liver specialized macrophages play a central role in liver inflammation, the use of ischemia/reperfusion as a model of hepatic injury, associated with the use of TLR4 bone marrow chimeras mice, demonstrate that TLR4 pathway plays a central role in actively phagocytic non-parenchymal cells (such as Kupffer cells) for ischemia/reperfusion-induced injury and liver inflammation (14). This hyper-responsiveness of Kupffer cells to LPS is linked to up-regulation of CD14 by a leptin-mediated signaling, and accordingly, up-regulation of CD14 and hyper-responsiveness to low-dose LPS were observed in Kupffer cells in high-fat diet (HFD)-induced steatosis mice, but not chow-fed-control mice (15). Other liver cells that might respond to microbial products include hepatic stellate cells (HSC) (16), which have been observed to exhibit TLR4-mediated NF-κB activation in response to fairly low concentration of LPS and are reported to be the predominant target through which TLR4 ligands promote fibrosis in the liver (17). Hepatocytes have also been observed to respond to TLR agonists and hepatocytes exhibit dynamics regulation of TLR expression. Yet, as such studies typically use relatively high concentration of TLR agonists, the extent to which hepatocytes can directly respond to physiologic TLR/NLR agonists in health and disease has not been extensively investigated. Based upon paradigms gradually emerging from study of intestinal-microbiota interactions, we speculate that activation of TLR on Kupffer, and perhaps other liver cells, might be a common, perhaps even ongoing, occurrence and play a role in liver homeostasis whereas activation of liver NLRs may be more frequent in situations of more unusual danger, such as an infection. A central hypothesis proposed by several other researchers is that increased levels of activation of TLR/NLRs by gut microbiota play a role in chronic inflammatory disease of the liver. The mechanisms by which increased activation of pro-inflammatory signaling might drive liver disease have been reviewed elsewhere. Here, we discuss potential initiating causes of liver disease in terms of how they might result in increased activation of liver TLR/NLR signaling by the microbiota and consider possible therapeutic interventions.
Gut microbiota and alcoholic liver disease
Potential means by which an environmental factor might cause gut microbiota to activate liver TLR/NLR would be an altered microbiota population and/or altered gut permeability. Indeed, long-appreciated causative factors of liver disease, particularly alcohol, clearly do the latter and are increasingly suggested to do the former. Mounting evidence suggests that alcohol increases gut permeability and liver exposure to gut-derived endotoxin, which activates TLR4, thus driving alcohol-induced tissue injury and organ failure in a subset of alcoholic patients (Figure 1) (18, 19). Several studies in humans and mouse models suggest that endotoxin promotes liver disease by driving Kupffer cell activation (20). Accordingly, endotoxin-mediated liver injury could be prevented by antibiotic treatment (21), by eliminating Kupffer cells (22), or by neutralizing TNF-α with antibody (23) or by using TNF-α knockout mice (24). In a rat model, treatment with polymyxin B, an antibiotic that directly prevents endotoxin from activating TLR4, prevented liver disease induced by ethanol treatment (21). Moreover, absence of the TLR4 gene in bone-marrow cells (including Kupffer cells) derived or somatic cells (including hematopoietic stem cell and hepatocytes) reduced the extent of alcohol-induced steatohepatitis in mice (25). The mechanism by which alcohol increases gut permeability appears to be driven by modification of tight junction protein expression during alcohol exposure, such as zona-occludens protein-1 (ZO-1) (26), as well as cytoskeleton protein, such as microtubule (27). Importantly, this increased intestinal permeability is likely not specific for endotoxin but would likely increase the load of a variety of microbial products that can result in excessive activation of both TLR and NLR mediated pathways, suggesting a broad but central role of gut-derived microbial products in alcohol-induced liver pathology (28). Evidence that such mechanisms are operative in humans include that intestinal permeability and LPS load were largely increased in alcohol-dependent subjects compare to controls (29). Interestingly, a 3-week detoxification program is sufficient to restore normal levels of intestinal permeability and LPS load (29).
Figure 1. Failure to maintain gut microbiota and its products in gut lumen may promote liver disease.
A) A healthful microbiota is maintained in the gut lumen. B) Altered microbiota composition and/or altered barrier function can result in microbial products activating toll-like and nod-like receptors of the innate immune system. Such TLR/NLR activation can drive pro-inflammatory gene expression that promotes liver disease.
Another means by which alcohol can promote activation of liver TLRs/NLRs is by altering microbiota composition. Indeed, the colonic microbiome is altered during alcoholism (30), and alcoholic subjects exhibiting reduced abundances of Bacteroidetes and increased levels of Enterobacteriaceae and Proteobacteria (30, 31). Proteobacteria are elevated in a variety of chronic inflammatory diseases and are thought to be potent activators of innate immunity (32). In accordance, the observed alterations in microbiota composition in alcoholic subjects correlate with endotoxemia in a subgroup of alcoholics (30). In addition to directly promoting increased TLR/NLR activation, the increased in Proteobacteria, and Gram negative bacteria in general, promoted by alcohol also results in accumulation of acetaldehyde, leading to an increased tyrosine phosphorylation of tight junction and adherent junction proteins, that can in turn increase intestinal permeability to bacterial products (33). Importantly, it is very difficult to define the extent to which alterations in microbiota composition associated with alcoholism are a cause of inflammation and/or are a consequence of disease. Indeed, separating the cause/consequence interrelationship between inflammation/microbiota composition remains the great challenge in the microbiome field. Interestingly, the recent report that mice lacking the negative regulator of TLR signaling IRAK-M exhibit increased alcohol-induced TLR4 signaling and microbiota alteration indicate that microbiota composition alteration and inflammation are likely highly intertwined co-regulating events (34). Together, these data support the concept that microbiota products, especially endotoxin, play a central role in alcohol-induced liver disease, and suggest that antagonizing TLR4-mediate recognition of endotoxin might be a means of treating/preventing alcohol-induced liver injury (21).
Microbiota and viral-induced liver disease
Following alcoholism, the most well established classic cause of liver disease is infection, especially with hepatitis viruses. Although less well studied, similar paradigms may be relevant to mechanisms by which alcoholism and viral infection cause chronic inflammatory disease of the liver. For example, hepatitis B virus (HBV), which is thought to be responsible for a considerable portion of the worldwide liver disease burden, is associated with both altered gut permeability and alterations in the gut microbiota composition, either of which can be envisaged to result in increased activation of liver TLR/NLR (35, 36). In a mouse model of viral-induced liver disease, germ-free mice are protected from disease and conventional mice can be protected by the antibiotic/ TLR4 antagonist polymixin B (37). Thus, although the myriad of ways in which microbiota and viruses interact have only begun to be deciphered, it seems likely that mechanisms by which HBV and perhaps hepatitis C virus (HCV) promote liver disease are mediated, in part, via the gut microbiota.
Role of the microbiota in Non-alcoholic fatty liver disease
While alcoholism and infection remain major causes of liver disease, perhaps the most alarming increase in liver disease is occurring in the form non-alcoholic fatty liver disease (NAFLD). Twenty per cent of NAFLD individuals develop chronic hepatic inflammation, i.e. non-alcoholic steatohepatitis, (NASH) associated with cirrhosis, portal hypertension and/or hepatocellular carcinoma. The rapidity of increased incidence of NAFLD over the last half-century, amidst relatively constant human genetics, indicates that environmental and/or lifestyle factors are driving this alarming trend. This is, of course, not occurring in isolation but rather is associated with the constellation of metabolic abnormalities including obesity, insulin resistance/type 2 diabetes, hyperlipidemia, and hypertension collectively referred to as metabolic syndrome. Like NAFLD and other chronic liver diseases, metabolic syndrome is increasingly appreciated to be a chronic inflammatory disease in which gut microbial products are prime suspects to be drivers of inflammation. Thus, it is possible that NAFLD and other aspects of metabolic syndrome are promoting each other and/or that both have related underlying causes. Analogous to the case for alcoholic liver disease, alterations in gut microbiota composition, increases in gut permeability, and serum levels of endotoxin are associated with NAFLD. Recently, intestinal microbiota analysis revealed that patients with NASH had a lower percentage of Bacteroidetes compared to healthy control, consistent with previous observations made in alcoholic patient (38).
Such correlations are strongly suggestive of the notion that gut microbiota products promote NAFLD. In accordance, mice maintained on high-fat/simple carbohydrate, i.e. "modern western", diets exhibit increased intestinal permeability, elevated levels of serum endotoxin, and modestly elevated levels of pro-inflammatory cytokines that correlate with various aspect of metabolic syndrome including NAFLD (39, 40). Increased levels of serum endotoxin may reflect increased permeability and the fairly large shifts in gut microbiota composition that occur in mice in response to diets designed to mimic western diets. Evidence that NAFLD is actually driven by responses to endotoxin and other microbial products include observations that, in mice, diet-induced metabolic syndrome is absent in germ-free conditions and that ablation of innate immune signaling by deleting TLR4 ameliorates disease while absence of MyD88, which plays a central role in TLR/NLR signaling, appears to eliminate it entirely (41–43). Similarly, the suppressor of cytokine signaling 1 (SOCS1) protein, a negative-feedback regulators in cytokine signaling induced upon TLR stimulation, play a protective role in liver injury, since SOCS1 deficient mice display fulminant hepatitis, characterized by hepatic inflammation, fatty degeneration and hepatocyte necrosis (44–46).
Thus, overall, these findings suggest that the dramatically increased incidence of NAFLD may, in part, result from increased consumption of western diets causing increased activation of pro-inflammatory signaling due to increased intestinal permeability and/or changing in microbiota composition. A recent study supports that the former possibility. Specifically, this study examined mice on a high-fat diet that did and did not develop steatosis, and observed changes in microbiota composition that correlated with this phenotypic difference (47). Transfer of the microbiota from the steatotic mice to germfree mice promoted development of HFD-induced steatosis relative to germfree mice given the microbiota of non-steatotic mice. Such steatosis correlated with dysglycemia, suggesting that the altered microbiota was broadly promoting metabolic syndrome. The alterations in gut microbiota involved alterations in numerous bacterial species.
As reviewed elsewhere, increased pro-inflammatory signaling can be a direct cause of liver disease and other aspects of metabolic syndrome (48). Effects of pro-inflammatory signaling on metabolism include dysregulating appetite control thus amplifying events that can drive NAFLD/metabolic syndrome. Inflammation can also alter gut microbiota composition (49). Thus, the cyclical nature of these events thought to drive metabolic syndrome need not necessarily to commence with altered diet but rather suggest that other events that might result in low-grade inflammation might ultimately result in various aspects of metabolic syndrome including NAFLD and deregulated appetite. In other words, it is possible that broad societal changes have altered the gut microbiota in humans in a way that has driven increased incidence of metabolic syndrome, including NAFLD. Evidence to support this possibility comes from studies in mice, in which loss of genes involved in innate immune detection of the microbiota result in altered gut microbiota composition that drives increased activation of compensatory innate immune signaling pathways. These phenomenona are associated with development of various aspects of metabolic syndrome that, in the context of a western diet, result in NAFLD. For example, in the TLR5-deficient mice, an altered microbiota including numerous bacterial species that were over or underrepresented was observed (11). The role of specific species was not evaluated but, overall, such altered microbiotas were shown to be sufficient to cause disease in that they could drive low-grade inflammation and metabolic disease upon transfer to wild-type germ-free mice. Such transfer of microbiota to germ-free mice simulates the acquisition of a microbiota at birth and thus these studies may reflect that acquired alterations in microbiota could be inherited and thus may be playing a role in the increased incidence of metabolic disease.
Potential microbial factors altering host-microbiota dynamic in NAFLD
While the extent to which the human microbiota has actually changed amidst the increased incidence of NAFLD is not clear, one can point to one clear example of an altered microbiota over the last 75 years. Specifically, carriage rates of Helicobacter pylori have dropped dramatically from about 80% to less than 5% of the native born 20 year-olds. While loss of this one specific microbe, which of course has potential to cause disease, may, or may not, have any consequences relating to NAFLD, it may reflect that increased use of antibiotics and/or changes in hygiene/behavioral practices have resulted in broad changes in the microbiota that have played a role in increased incidence of NAFLD and other chronic inflammatory diseases. A related possibility is that the increased incidence of NAFLD may be analogous to a traditional infectious disease in that microbes that promote the disease may not be inherited but can be acquired from other persons. Various aspects of the epidemiology of NAFLD and other aspects of metabolic syndrome, particularly obesity, suggest that these disorders have characteristics of infectious disease and studies have associated carriage of select strains of adenoviruses with obesity (50). Some of the strongest evidence that altered microbiota can promote NAFLD comes from recent mice studies by Flavell and colleagues. They observed that mice lacking either NLRP3 and NLRP6, two NLRs that activate the inflammasome in response to the detection of foreign protein and/or host-derived danger signals, are highly prone to both methionine/choline-deficient diets-induced NAFLD and high fat diet-induced metabolic syndrome (10). Such predisposition to develop NAFLD was, again, driven by pro-inflammatory gene expression driven by other innate immune receptors, since deletion of either TLRs 4 or 9, or the TNF-α receptor, were sufficient to ablate the increased susceptibility to NAFLD conferred by loss of NLRP6. This increased susceptibility to developing NAFLD was associated with altered microbiota composition, namely elevated levels of Prevotella and Porphyromonas species. Such alterations were deemed to play a functional role in driving disease, since co-housing of these genetically altered mice with wild type mice transferred both the elevations in these bacterial populations and the predisposition to develop metabolic syndrome (10). While transfer of bacteria amongst co-housed mice seems likely to be far more efficient than one would expect in cohabitating humans, especially since mice are coprophagic, it nonetheless supports the principal that predisposition to NAFLD may be spreading through the population in a manner analogous to a traditional infectious disease.
Role of dietary factors in altering microbiota in NAFLD
A likely factor in altering gut microbiota composition and consequently playing a role in perturbing the host-microbiota dynamic in NAFLD is diet. Indeed, high-fat diets alter gut microbiota composition by altering phyla ratios and promoting growth of Proteobacteria, both of which can increase the microbiota's pro-inflammatory potential (51). Importantly, such alterations in the microbiota occur quickly and are independent of weight gain, suggesting they are not purely a consequence of inflammation. Considerable suspicion has also focused on the role of fructose, whose consumption has greatly increased in a manner roughly paralleling the rise of NAFLD as a common disease. Fructose consumption, mainly by consumption of added sugars, can represent 10% of total energy intake in developed country (52). Placing mice on a high-fructose diet robustly promotes lipid accumulation in the liver and alters microbiota composition, although the extent to which fructose promotes hepatic lipid accumulation in humans is far from clear (52). It is speculated that, analogous to the case of the role of the high-fat diet in metabolic syndrome, diets high in fructose might alter host-microbiota interactions to promote NAFLD by altering microbial metabolism or promoting low-grade inflammation. The notion that fructose alters the metabolic capability of the microbiota in a manner that promotes lipid uptake and deposition is based on observations that a high fructose diet alters the mouse microbiota by shifting phyla ratios in a way that increases energy harvest (53). That fructose might promote inflammation is based on studies, in mice, that show that high-fructose diets result in rapidly reduced expression of tight junction proteins, thus altering gut barrier function. Such reduction in gut tight junction protein expression correlates with elevated expression of liver TLRs expression that would presumably promote inflammation upon detection of the leaked gut microbiota products (54).
Role of Microbiota in driving advanced liver disease
While much of the above discussion focuses on the role of the microbiota as promoting initiation of disease, there are a number of reports showing that microbiota also plays a role in promoting the transition from moderate to more severe liver disease. While some of the end disease states are quite distinct, there is considerable overlap in the proposed underlying mechanisms and thus we discuss them under a collective heading.
Progression of NAFLD to NASH
The severe clinical consequences of NASH underscore the great importance of discerning the factors that drive the progression from NAFLD to NASH. A recent study described that persons with NASH harbor a modified microbiota that result in endogenous ethanol production, thus suggesting the possibility that microbiota-produced alcohols may drive some portion of NASH and explain some of the communities between NASH and alcoholic liver disease (55). Other observation supporting the hypothesis that TLR4 mediated recognition of LPS play a central role in liver inflammation induced injury is the report showing that TLR4 play a key role in Kupffer cells for the progression of steatosis to NASH, especially by inducing activation of XBP-1 (56). Moreover, it was recently reported that MD-2 and TLR4 deficiency attenuate NASH in mice, strengthen the concept that hepatic LPS recognition by MD-2 and TLR4 play a central role in murine NASH (57). Thus, not only is the microbiota a likely determinant of NAFLD but may also be involved in its potential progression to NASH.
Fibrosis, Cirrhosis, and hepatic encephalopathy
Recent evidence also supports the notion of microbiota involvement in the most severe forms of liver disease, namely fibrosis and cirrhosis. More specifically gut microbiota may play a central role in liver fibrosis as evidenced by findings that, in mice, chemical-induced induction fibrosis from the gut to the liver was associated with increase bacterial translocation (58). Furthermore, antibiotics treatment could delay the development of cirrhosis (58) and, moreover, the protection offered by neomycin is ablated by endotoxin treatment, suggesting that protective effect of neomycin is mediated by an alteration of the intestinal microbiota associated with a decrease of intraluminal endotoxin (59). This hypothesis is further supported by the finding that intestinal microbiota as well as TLR4/CD14 are essential for apparition of hepatic fibrosis, and HSC are found to be the predominant target by which TLR4 ligands promote hepatic fibrosis (17, 60).
In addition, cirrhosis is often associated with complication such as hepatic encephalopathy, characterized by cognitive impairment and poor survival (61). Importantly, there was no difference in stool microbiota between cirrhotic patients with or without hepatic encephalopathy, but yet mucosal microbiomes differ by having lower Roseburia and higher levels of Enterococcus, Veillonella, Megasphaera, and Burkholderia abundance. Such altered microbiomes are associated with poor cognition, endotoxemia, and inflammation (IL-6, TNF-α, IL-2, and IL-13) in hepatic encephalopathy patients compared to cirrhotic patients without hepatic encephalopathy (31, 62). The notion that these events actually drive clinical manifestations of hepatic encephalopathy is supported by findings that antibiotics, especially Rifaximin, can effectively treat acute hepatic encephalopathy (63, 64). Furthermore, Rifaximin treatment is also effective in maintain hepatic encephalopathy remission, suggesting that the gut microbiota may play a role in triggering initial manifestation of and flares of these severe extra-hepatic disease manifestations.
Hepatocellular carcinoma
Chronic inflammatory disease of the liver can eventuate in hepatocellular carcinoma (HCC) and/or ultimately require liver transplant. Consistent with the central role of the microbiota in driving inflammation, recent research suggests a key role for the microbiota in determining the outcomes of these processes. Specifically, recent pioneering work by Dapito et al. found that both TLR4 and intestinal microbiota were not required for HCC initiation but, rather, plays a key role in HCC promotion (66). Interestingly, the authors reported that both innate immune pathway mediated by TLR4 and intestinal microbiota are involved in an increased hepatocyte proliferation, an increased expression of the hepatomitogen epiregulin, and the prevention of apoptosis. By using germ free animals, a reduction of HCC was observed, suggesting that both intestinal microbiota and TLR4 pathway represent therapeutic targets for HCC prevention in advanced liver disease. Another study demonstrates that the circulating levels of LPS were elevated in animal models of hepatocarcinogenesis, and that the reduction of LPS-induced signaling by using antibiotics or TLR4KO mice prevented excessive tumor growth and multiplicity (67). These data indicate that LPS-induced signaling pathway plays a central role in inflammation-associated hepatocellular carcinoma, and that manipulation of the gut flora to decrease endotoxin absorption may be of interest in liver disease patients.
Transplant rejection
Liver transplantation is often the only long-term therapeutic option for patients with severe liver disease. Factors that govern the success rate of transplantation, particularly whether the engrafted organ will function properly and not be attached by the host's immune system, remain poorly defined. Recent studies indicate that microbiota composition, perhaps in both donor and recipient may play a role. It was recently demonstrated that the abundance of various gut bacteria were altered after liver transplantation, such as Bifidobacterium spp., Faecalibacterium prausnitzii (an anti-inflammatory bacteria, (68)), and Lactobacillus spp. that were significantly lower in the liver transplantation recipients, while Enterobacteriaceae and Enterococcus spp. were significantly higher (69). Interestingly, these bacteria showed a tendency to restore to a normal level along with the time after liver transplantation, demonstrating that microbiota composition is altered during liver injury and revert to the normal when liver normal function is restored. Consistent with these findings, it was also reported that alteration in gut microbiota was associated with the elevation of plasma endotoxin and with a higher rate of bacterial translocation to the liver in rats during acute liver rejection. Acute rejection was accompanied by the shifts of gut microbiota towards members of Bacteroides and Ruminococcus family (70). These findings support the notion that gut microbiota plays a role in the progression of liver carcinogenesis and that major composition modifications occur during liver transplantation and rejection.
Potential Therapeutics
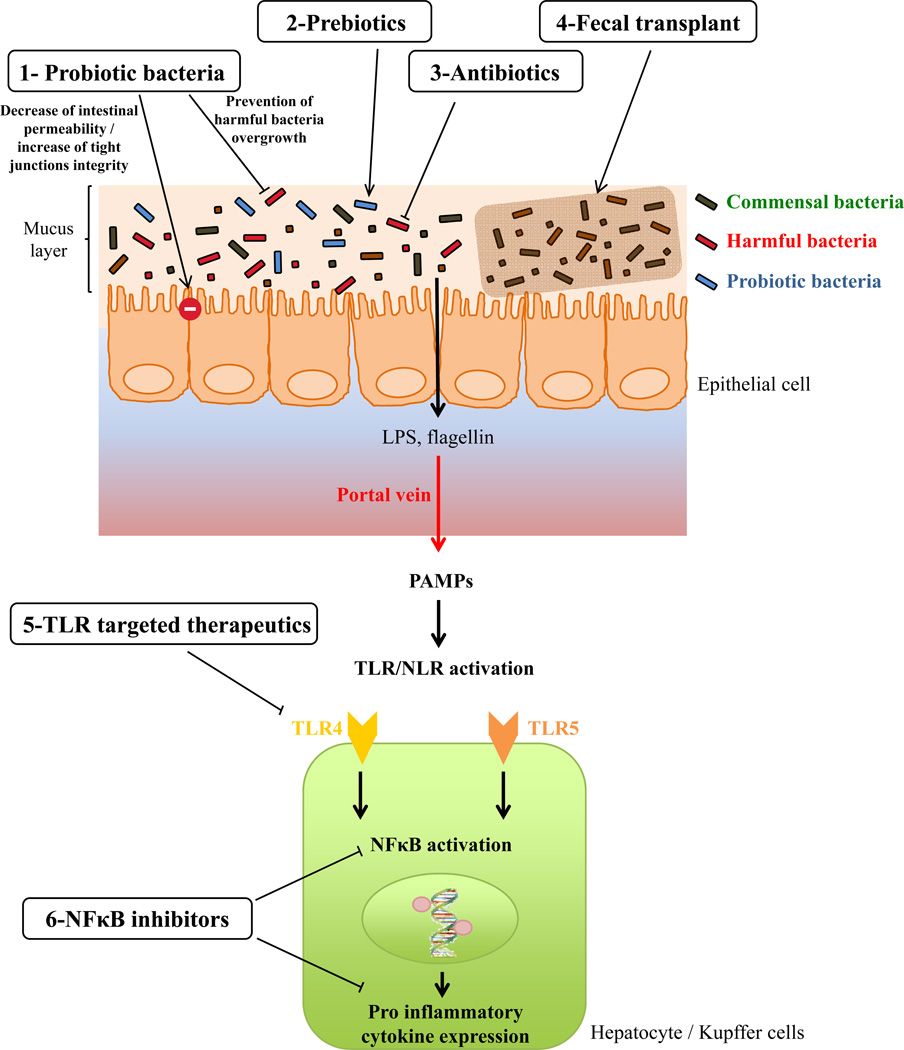
As discussed herein, in both classic and modern liver disease, accumulating evidence from animal models and human studies suggests that microbial product-induced pro-inflammatory gene expression plays a central role in liver disease (Figure 2). Consequently, it might be logical to seek to manipulate these pathways to treat and/or prevent liver disease. On the one hand it might be logical to directly antagonize some of the receptors that detect microbial products. Indeed, it has long been suggested that antagonizing TLR4 signaling might be a reasonable means to treat a variety of inflammatory disorders. Approaches to antagonize NLR signaling and or NLR-produced cytokines, particularly IL-1β, have been proposed as means of treating metabolic syndrome (71). Another possible approach might be to reduce gut epithelial permeability thus reducing effective exposure to gut microbial products. An important caveat to consider in this endeavor is that, sometimes, antagonizing innate immune signaling can result in greater bacterial dysbiosis and ultimately drive enhanced pro-inflammatory gene expression via other innate immune receptors. Thus, it might be more effective to directly target the gut microbiota to restore it to a more healthful state, which would presumably invoke reduced pro-inflammatory gene expression in the host. Manipulating the microbiota could be done with pre-biotics (i.e. dietary manipulation/supplementation), pro-biotics, antibiotics, or microbiota transplant. Some antibiotics (Polymyxin B and neomycin) were shown to fully protect mice against fructose-induced liver damage and interestingly prevent endotoxin overload induced by fructose consumption (72), and Rifaximin was found to be effective in the treatment of acute hepatic encephalopathy (65, 66), and in maintaining hepatic encephalopathy remission (57). Clinical trials are currently investigating the effects of Rifaximin in fatty liver disease, liver cirrhosis. Concerning probiotic as potential therapy, Lactobacilli administration reduces endotoxemia as well as alcohol-induced liver injury in rat, supporting the concept that probiotic is a potential therapy for both endotoxemia and alcoholic liver disease (18). Another successful example of probiotics from mouse studies is a report that Lactobacillus casei strain Shirota is able to protect mice against the onset of NAFLD via an attenuation of the TLR-4-signalling cascade in the liver and an increased peroxisome proliferator-activated receptor (PPAR)-γ activity (73). Another interesting candidate for the probiotic management of liver disease is Bifidobacterium pseudocatenulatum CECT 7765, seems a recent study demonstrate that the administration of this probiotic bacteria improves various metabolic alterations in the high-fat diet fed mouse model, and is interestingly able to reduce liver steatosis (74). Of note, the changes in gut microbiota composition induced by Polyunsaturated fatty acids-depletion and prebiotics administration (fructo-oligosaccharides) is able to modulate hepatic steatosis by changing gene expression in the liver, suggesting that prebiotic approach could be conceivable in the management of liver disease (75). Probiotics may also be promising way to restore the “leaky gut” state observe in numerous patients with liver disease as Escherichia coli strain Nissle 1917 is able to restore normal mucosal permeability in the murine Dextran Sulfate Sodium (DSS)-induced colitis model, as well as induce up-regulation of zonula occludens 1 expression in vitro (76). Similarly, probiotic mixture VSL#3 is able to prevent the increased intestinal permeability induced by a DSS treatment, phenomena associated with a prevention of decreased expression and redistribution of the tight junction proteins occludin, zonula occludens-1, and claudin-1, -3, -4, and -5 normally observed in DSS treated mice (77). Antibiotics, by themselves, seem unlikely to be an effective means of promoting a healthful host-microbiota relationship, but could be part of approaches to transplant microbiotas. While, at present, the beneficial effects of microbiota transplant are only proven to prevent recurrent Clostridium difficile colitis (78), a recent study found that microbiota transplant ameliorated insulin resistance (79), suggesting that the approach might be broadly applicable to various aspects of metabolic syndrome, including NAFLD. Thus, while further studies are warranted, it is the opinion of the authors that manipulation of the gut microbiota will ultimately be a helpful means of treating and/or preventing liver disease.
Figure 2. Potential therapeutic strategies to prevent or treat liver disease.
1) Probiotics could maintain intestinal permeability by increasing the integrity of tight junctions or by preventing the overgrowth of harmful bacteria. 2) Prebiotics may act by improving the effect of probiotic bacteria or by impeding the growth of harmful bacteria. 3) Antibiotics could directly act by inhibition of harmful bacteria growth and 4) fecal transplant could lead to the restoration of a healthy microbiota. 5) Some therapeutics may act on the innate immune system by targeting TLRs signaling pathways or 6) NFκB inhibitors leading to lower expression of pro-inflammatory cytokine expression.
Concluding Thoughts
It has long been appreciated that environmental factors, including diet and infection, are major determinants of liver disease. Herein, we have reviewed evidence supporting the more recently appreciated concept that another important environmental factor is the large microbial biomass living in the intestine. Being close the liver, the gut microbiota can influence liver phenotype by a number of ways. Yet, herein, we discuss the hypothesis that an overarching mechanism by which the gut microbiota can be a detriment to liver function is by driving chronic inflammation that promotes liver disease. Thus, we envision that future approaches to treating and preventing liver disease will consider the liver-microbiota axis.
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 3.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nature reviews. Immunology. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nature reviews. Immunology. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annual review of physiology. 2012;74:177–198. doi: 10.1146/annurev-physiol-020911-153330. [DOI] [PubMed] [Google Scholar]
- 6.Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–1728. e1723. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, Hirabara SM, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 13.Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, et al. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932–936. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- 14.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. Journal of immunology. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 15.Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell metabolism. 2012;16:44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 17.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature medicine. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 18.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 19.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. The American journal of pathology. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 20.French SW. Mechanisms of alcoholic liver injury. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2000;14:327–332. doi: 10.1155/2000/801735. [DOI] [PubMed] [Google Scholar]
- 21.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 22.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 23.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 24.Yin M, Kono H, Bradford BU, Thurman RO. Development of a new enteral mouse model using knock out technology to study alcohol-induced liver injury: involvement of TNF-a. Hepatology. 1998;28:321. [Google Scholar]
- 25.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Digestive diseases. 2010;28:737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, et al. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcoholism, clinical and experimental research. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best practice & research. Clinical gastroenterology. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 29.Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain, behavior, and immunity. 2012;26:911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, et al. Colonic microbiome is altered in alcoholism. American journal of physiology. Gastrointestinal and liver physiology. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2012;303:G675–G685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nature reviews. Gastroenterology & hepatology. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. American journal of physiology. Gastrointestinal and liver physiology. 2001;280:G1280–G1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Hu Y, Chao C, Yuksel M, Colle I, Flavell RA, Ma Y, et al. Role of IRAK-M in Alcohol Induced Liver Injury. PLoS One. 2013;8:e57085. doi: 10.1371/journal.pone.0057085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. 1230, e1221–e1223. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microbial ecology. 2011;61:693–703. doi: 10.1007/s00248-010-9801-8. [DOI] [PubMed] [Google Scholar]
- 37.Gut JP, Schmitt S, Bingen A, Anton M, Kirn A. Probable role of endogenous endotoxins in hepatocytolysis during murine hepatitis caused by frog virus 3. The Journal of infectious diseases. 1984;149:621–629. doi: 10.1093/infdis/149.4.621. [DOI] [PubMed] [Google Scholar]
- 38.Mouzaki M, Comelli E, Arendt B, Bonengel J, Fung S, Fischer S, McGilvray I, et al. Intestinal microbiota in patients with non-alcoholic fatty liver disease. Hepatology. 2013 doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 39.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101. e1102. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee IS, Shin G, Choue R. Shifts in diet from high fat to high carbohydrate improved levels of adipokines and pro-inflammatory cytokines in mice fed a high-fat diet. Endocrine journal. 2010;57:39–50. doi: 10.1507/endocrj.k09e-046. [DOI] [PubMed] [Google Scholar]
- 41.Rabot S, Membrez M, Bruneau A, Gerard P, Harach T, Moser M, Raymond F, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 42.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 43.Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell metabolism. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto M, Naka T. SOCS1, a Negative Regulator of Cytokine Signals and TLR Responses, in Human Liver Diseases. Gastroenterology research and practice. 2010;2010 doi: 10.1155/2010/470468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naka T, Tsutsui H, Fujimoto M, Kawazoe Y, Kohzaki H, Morita Y, Nakagawa R, et al. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4 signaling in vivo. Immunity. 2001;14:535–545. doi: 10.1016/s1074-7613(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 46.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, et al. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2012 doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 48.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 49.Rawls JF. Enteric infection and inflammation alter gut microbial ecology. Cell host & microbe. 2007;2:73–74. doi: 10.1016/j.chom.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obesity research. 1997;5:464–469. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 51.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1711–e1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tappy L, Le KA. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clinics and research in hepatology and gastroenterology. 2012;36:554–560. doi: 10.1016/j.clinre.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13:799–809. doi: 10.1111/j.1467-789X.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 54.Wagnerberger S, Spruss A, Kanuri G, Volynets V, Stahl C, Bischoff SC, Bergheim I. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. The British journal of nutrition. 2012;107:1727–1738. doi: 10.1017/S0007114511004983. [DOI] [PubMed] [Google Scholar]
- 55.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 56.Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, et al. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058–1067. doi: 10.1136/gutjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- 57.Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. American journal of physiology. Gastrointestinal and liver physiology. 2011;300:G433–G441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez-Hurtado I, Santacruz A, Peiro G, Zapater P, Gutierrez A, Perez-Mateo M, Sanz Y, et al. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS ONE. 2011;6:e23037. doi: 10.1371/journal.pone.0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broitman SA, Gottlieb LS, Zamcheck N. Influence of Neomycin and Ingested Endotoxin in the Pathogenesis of Choline Deficiency Cirrhosis in the Adult Rat. The Journal of experimental medicine. 1964;119:633–642. doi: 10.1084/jem.119.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, McKim SE, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. American journal of physiology. Gastrointestinal and liver physiology. 2006;290:G1318–G1328. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 61.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Alimentary pharmacology & therapeutics. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 62.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. American journal of physiology. Gastrointestinal and liver physiology. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. European journal of gastroenterology & hepatology. 2008;20:1064–1070. doi: 10.1097/MEG.0b013e328302f470. [DOI] [PubMed] [Google Scholar]
- 64.Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Current medical research and opinion. 1993;13:109–118. doi: 10.1185/03007999309111539. [DOI] [PubMed] [Google Scholar]
- 65.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. The New England journal of medicine. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 66.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 68.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu ZW, Ling ZX, Lu HF, Zuo J, Sheng JF, Zheng SS, Li LJ. Changes of gut bacteria and immune parameters in liver transplant recipients. Hepatobiliary & pancreatic diseases international : HBPD INT. 2012;11:40–50. doi: 10.1016/s1499-3872(11)60124-0. [DOI] [PubMed] [Google Scholar]
- 70.Xie Y, Luo Z, Li Z, Deng M, Liu H, Zhu B, Ruan B, et al. Structural shifts of fecal microbial communities in rats with acute rejection after liver transplantation. Microbial ecology. 2012;64:546–554. doi: 10.1007/s00248-012-0030-1. [DOI] [PubMed] [Google Scholar]
- 71.Doherty TA, Brydges SD, Hoffman HM. Autoinflammation: translating mechanism to therapy. Journal of leukocyte biology. 2011;90:37–47. doi: 10.1189/jlb.1110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. Journal of hepatology. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 73.Wagnerberger S, Spruss A, Kanuri G, Stahl C, Schroder M, Vetter W, Bischoff SC, et al. Lactobaccilus casei Shirota protects from fructose-induced liver steatosis: A mouse model. The Journal of nutritional biochemistry. 2012 doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 74.Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet fed mice. Obesity. 2013 doi: 10.1002/oby.20330. [DOI] [PubMed] [Google Scholar]
- 75.Pachikian BD, Essaghir A, Demoulin JB, Catry E, Neyrinck AM, Dewulf EM, Sohet FM, et al. Prebiotic approach alleviates hepatic steatosis: implication of fatty acid oxidative and cholesterol synthesis pathways. Molecular nutrition & food research. 2013;57:347–359. doi: 10.1002/mnfr.201200364. [DOI] [PubMed] [Google Scholar]
- 76.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PloS one. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. American journal of physiology. Gastrointestinal and liver physiology. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 78.Brandt LJ, Reddy SS. Fecal microbiota transplantation for recurrent clostridium difficile infection. Journal of clinical gastroenterology. 2011;45(Suppl):S159–S167. doi: 10.1097/MCG.0b013e318222e603. [DOI] [PubMed] [Google Scholar]
- 79.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]