Abstract
We have developed an enantiospecific, nickel-catalyzed cross-coupling of unsymmetric 1,3-disubstituted allylic pivalates with arylboroxines. The success of this reaction relies on the use of BnPPh2 as a supporting ligand for the nickel(0) catalyst and NaOMe as a base. This method shows excellent functional group tolerance and broad scope in both the allylic pivalate and arylboroxine, enabling the preparation of 1,3-diaryl allylic products in high yields with excellent levels of regioselectivity and stereochemical fidelity.
Transition-metal-catalyzed allylic substitution provides an important method of C–C bond formation.1 In particular, enantioselective or -specific arylation of readily accessible 1,3-disubstituted secondary allylic electrophiles enables facile construction of enantioenriched products equipped with vinyl-substituted benzylic carbon stereocenters. These products are useful both as synthetic intermediates and for their potential biological activity.2 An ideal cross-coupling method to deliver these valuable enantioenriched products would enable high levels of enantiospecificity (or enantioselectivity) and regioselectivity, wide functional group tolerance, and the use of an air-stable coupling partner (such as an arylboronic reagent).3,4 Toward these goals, Rh- and Pd-catalyzed cross-couplings of allylic electrophiles and arylboronic acids have been developed to deliver highly enantioenriched products.5−10 However, the additional goal of developing nonprecious metal catalysts is also important in lowering the cost, ensuring adequate supplies of catalysts for large-scale applications, and reducing the environmental impact of this chemistry.11 In contrast to the methods developed with precious metal catalysts, the use of nonprecious metal catalysts for enantiospecific or -selective allylic arylations is less developed and often requires use of air-sensitive aryl nucleophiles that may have incompatibilities with some functional groups. For example, formation of enantioenriched products via Cu-catalyzed allylic arylations largely relies on the use of Grignard, organozinc, or organoaluminum reagents;12−16 only a limited number of methods allow the use of arylboronates.17−19
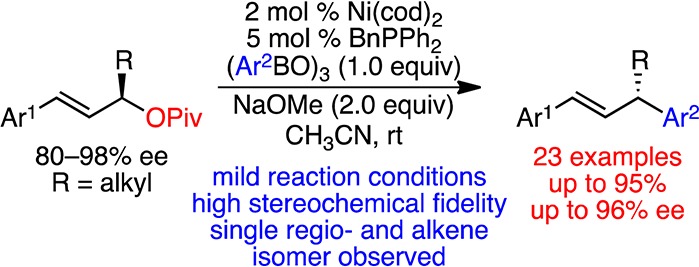
Based on our studies of enantiospecific, Ni-catalyzed couplings of benzylic pivalates and arylboroxines,20 we envisioned that Ni-based catalysts may also serve as efficient, nonprecious metal catalysts for highly enantiospecific and regioselective couplings of 1,3-disubstituted allylic pivalates with aryl boroxines. Prior art in this area has indeed demonstrated that nickel catalysts efficiently catalyze the cross-coupling of allylic electrophiles with aryl Grignard reagents, with both diastereo- and enantioselective variants reported.21−23 Trost and Kobayashi have also shown that more functional group tolerant borate and boronic acid nucleophiles can be used in cross-couplings with allylic amines and carbonates, respectively.24,25 With respect to the stereochemical outcome of these reactions, they have demonstrated that the arylation of cyclic allylic electrophiles occurs with inversion of configuration, and Kobayashi has exploited this in the arylation of cyclopentene diol derivatives to deliver enantioenriched products (Scheme 1A).24a−24e,25 In addition, Uemura has reported a moderately enantioselective, Ni-catalyzed coupling of symmetric and terminal allylic acetates with arylboronic acids (Scheme 1B).26 However, to our knowledge, there are no reports of enantiospecific, Ni-catalyzed cross-couplings of acyclic allylic electrophiles and arylboron reagents to deliver highly enantioenriched products.
Scheme 1. Enantioselective and Enantiospecific Nickel-Catalyzed Allylic Arylation with Arylboron Reagents.
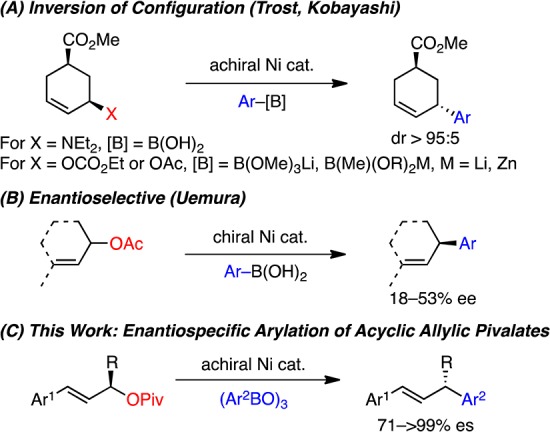
Herein we report the first enantiospecific, Ni-catalyzed cross-coupling of acyclic, 1,3-disubstituted allylic pivalates and arylboroxines (Scheme 1C). This method enables efficient transformation of readily available, highly enantioenriched allylic pivalates to valuable 1,3-diaryl allylic products in good yields with excellent regioselectivities and levels of stereochemical fidelity.
Our initial investigations focused on the coupling of pivalate 1a, easily prepared in 96% ee via a CBS reduction/pivalation sequence, with phenylboroxine (Table 1). Under optimal conditions for the coupling of benzylic pivalates [Ni(cod)2, no additional phosphine ligand, PhMe], desired coupling product 2 was obtained in excellent yield. However, only 18% ee of product was observed (entry 1). Changing the solvent to MeCN resulted in higher enantiomeric excess (ee) of the product (57% ee) in similar yield (entry 2). In an effort to improve the stereochemical fidelity further, we investigated phosphine ligands to support the Ni catalyst. Although bidentate phosphine ligands led to poor ee’s of product (entries 3, 4), the use of monodentate phosphine ligands resulted in significant improvement (entries 5–7). Among the ligands examined, benzyl diphenyl phosphine (BnPPh2) proved to be the best (entry 7). Reducing the reaction temperature gave an increased yield (entry 8). Further increases in yield and ee were obtained when the catalyst loading was lowered to 2 mol % (entries 9, 10). The observation of higher levels of stereochemical fidelity at lower catalyst loadings may suggest a Ni-mediated epimerization pathway.28 Under these optimized conditions, the use of phenylboronic acid in place of phenylboroxine led to a lower yield but with the same ee (entry 11). The reason for this difference is currently unclear, but does not seem to be due to the reaction’s sensitivity to water. Addition of water to the reaction resulted in only a slightly reduced yield (entry 12).
Table 1. Optimization of Reaction Conditionsa.
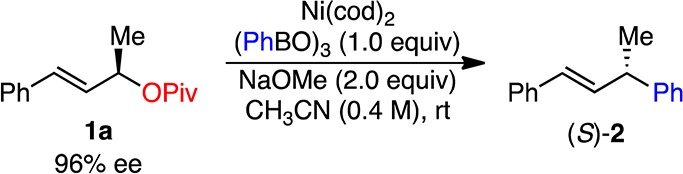
| entry | mol % [Ni] | ligand (mol %) | temp (°C) | yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| 1d | 10 | none | 50 | 95 | 18 |
| 2 | 10 | none | 50 | 96 | 57 |
| 3 | 10 | dppp (11)e | 50 | 90 | 57 |
| 4 | 10 | XantPhos (11)e | 50 | 30 | 27 |
| 5 | 10 | PCy3 (22) | 50 | 80 | 84 |
| 6 | 10 | CyPPh2 (22) | 50 | 83 | 85 |
| 7 | 10 | BnPPh2 (22) | 50 | 85 | 87 |
| 8 | 10 | BnPPh2 (22) | rt | 90 | 87 |
| 9 | 5 | BnPPh2 (11) | rt | 90 | 92 |
| 10 | 2 | BnPPh2 (5) | rt | 92 | 94 |
| 11f | 2 | BnPPh2 (5) | rt | 72 | 93 |
| 12g | 2 | BnPPh2 (5) | rt | 86 | 94 |
Conditions: pivalate 1a (96% ee, 0.1 mmol, 1.0 equiv), (PhBO)3 (1.0 equiv), Ni(cod)2, ligand, NaOMe (2.0 equiv), CH3CN (0.25 mL, 0.4 M), 4 h, unless otherwise noted.
Determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
Determined by HPLC analysis using a chiral stationary phase.
PhMe instead of CH3CN.
dppp = 1,3-bis(diphenylphosphino)propane. XantPhos = 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene.
PhB(OH)2 (3.0 equiv).
H2O (1.5 equiv) added.
Under our optimized conditions (Table 1, entry 10), we observed a broad scope with respect to the arylboroxine (Scheme 2).27 In all cases in Schemes 2 and 3, excellent regioselectivity and E/Z selectivity were observed; only α-aryl products with E-olefins were formed. High yields and excellent levels of stereochemical fidelity were obtained with arylboroxines bearing either electron-donating or -withdrawing groups (2–13). A wide range of functional groups were tolerated including ether (3), thioether (4), vinyl (5), halide (6–8), trifluoromethyl (9), ester (10), ketone (11), nitrile (12), and acetal (13) groups. In particular, the vinyl, bromide, chloride, and thioether groups highlight the mildness of these reaction conditions; undesired Ni-catalyzed reactions of these groups do not occur under our conditions. Notably, the Ni(0) catalyst generated in situ from NiBr2, BnPPh2, and Zn was also effective in this arylation, providing product 2 in the same ee as with Ni(cod)2, albeit in somewhat reduced yield (eq 1). These conditions enable a benchtop setup of the arylation reaction.
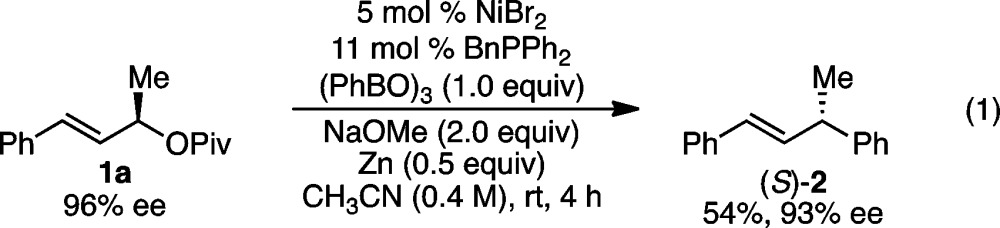 |
1 |
Scheme 2. Scope of Arylboroxine.
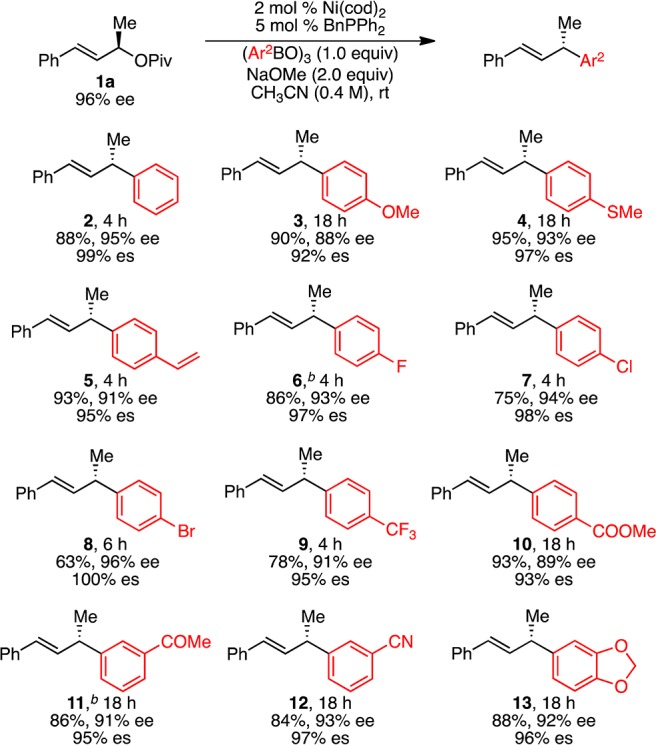
Conditions: pivalate 1a (96% ee, 0.3 mmol, 1.0 equiv), (PhBO)3 (1.0 equiv), Ni(cod)2 (2 mol %), BnPPh2 (5 mol %), NaOMe (2.0 equiv), CH3CN (0.75 mL, 0.4 M), rt, unless otherwise noted. Average yields (±3%) and ee’s (±1%) of isolated products of duplicate reactions. Ee determined by HPLC analysis using a chiral stationary phase. es = (ee of product)/(ee of starting material). b1a (95% ee).
Scheme 3. Scope of Pivalates.
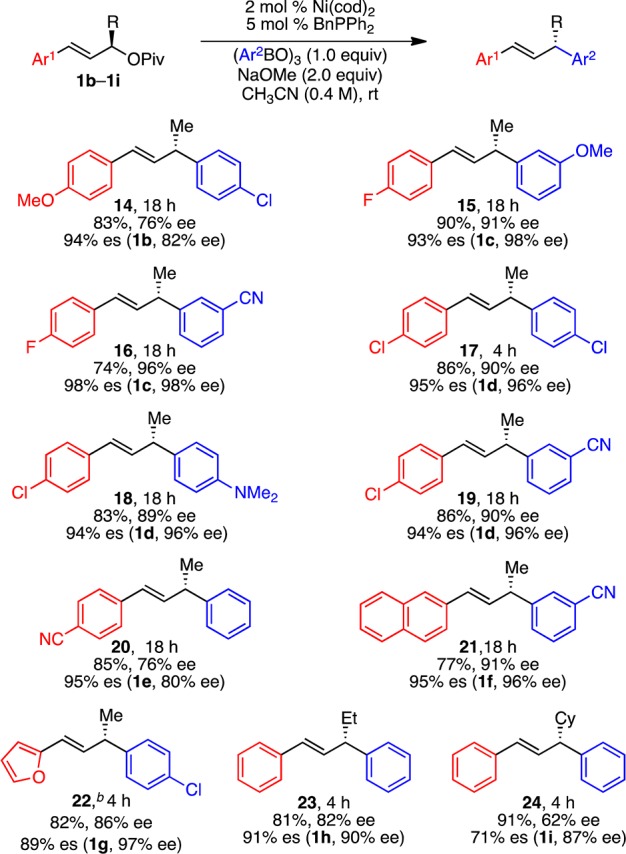
Conditions: pivalates 1b–1i (0.3 mmol, 1.0 equiv), (PhBO)3 (1.0 equiv), Ni(cod)2 (2 mol %), BnPPh2 (5 mol %), NaOMe (2.0 equiv), CH3CN (0.75 mL, 0.4 M), rt, unless otherwise noted. Average yields (±3%) and ee’s (±1%) of isolated products of duplicate reactions. Ee determined by HPLC analysis using a chiral stationary phase. es = (ee of product)/(ee of starting material); corresponding starting materials and their ee’s in parentheses. b Single experiment.
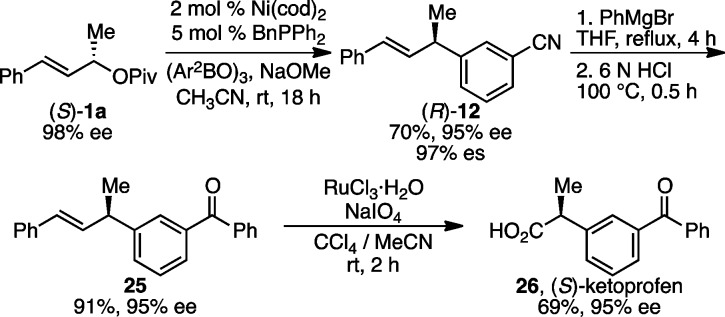
With respect to the allylic pivalate, pivalates with both electron-rich and -deficient aryl substituents efficiently underwent arylation (Scheme 3, 14–20). As had been true of the arylboroxine, aryl chlorides are also well tolerated on the allylic pivalate, again highlighting the orthogonality of this reaction to aryl chlorides (17–19). In addition to the functional groups already demonstrated on the arylboroxine, a tertiary amine was well tolerated (18). Allylic pivalates with naphthyl and heteroaryl substituents were also successfully coupled (21, 22). In terms of the alkyl substituent (R) on the allylic pivalate, increased steric hindrance did not diminish reactivity, but lower levels of stereochemical fidelity were observed with cyclohexyl-substituted 1i (23, 24). The X-ray crystallographic analysis of product 17 shows that its absolute configuration is S,29 which indicates that the reaction proceeds with inversion of configuration. To confirm further the stereochemical outcome and demonstrate the utility of this arylation method, compound (R)-12, prepared in 95% ee via the arylation of (S)-1a, was converted to (S)-ketoprofen (26), an anti-inflammatory drug, via a two-step manipulation (Scheme 4). Comparison of the optical rotation of 26 to previously reported values confirms its absolute configuration is S.30 The absolute configurations of other products were assigned by analogy.
Scheme 4. Synthesis of (S)-Ketoprofen.
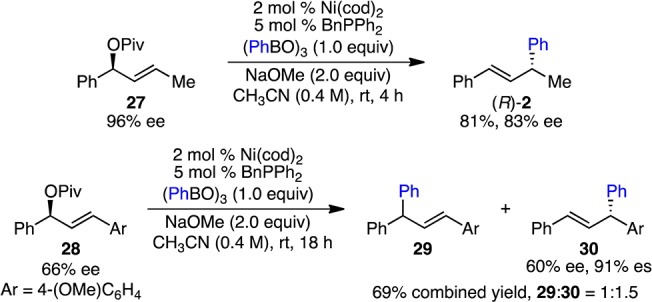
Consistent with previous Ni-catalyzed allylations, we propose that this reaction proceeds through a π-allylnickel intermediate.21−26,31 To test this hypothesis, we subjected pivalate 27 (96% ee), a regioisomer of 1a, to the reaction conditions. Product (R)-2 was formed in 81% yield and 83% ee with inversion of the absolute stereochemistry (Scheme 5). The reason for the mild erosion of ee is unclear, but may be due to epimerization of a faster formed π-allylnickel intermediate. The observation that both regioisomers of starting material (1a and 27) lead to the same product (2) supports the intermediacy of an η3-allylnickel complex. Also consistent with an η3-allylnickel intermediate, the arylation of 1,3-diaryl-substituted 28 resulted in a 1:1.5 ratio of regioisomers 29 and 30 (Scheme 5). Despite the modest regioselectivity, it is notable that 30 was obtained with 91% es.
Scheme 5. Regioselectivity Studies.
In conclusion, we have developed an enantiospecific, Ni-catalyzed cross-coupling of enantioenriched secondary allylic pivalates and arylboroxines to deliver 1,3-diaryl allyl products. The method features mild conditions that allow broad functional group tolerance on both the allylic pivalate and arylboroxine. In all cases, good yields and excellent levels of regioselectivity, E/Z selectivity, and stereochemical fidelity were observed. In addition, air-stable, less expensive NiBr2 can be used as an alternative to Ni(cod)2. Studies exploring other substrate classes as well as details of the mechanism are currently ongoing and will be reported in due course.
Acknowledgments
NSF (CAREER CHE 1151364) is gratefully acknowledged. We thank AstraZeneca for donated boronic acids. Data were acquired at UD on instruments obtained with the assistance of NSF and NIH funding (NSF CHE0421224, CHE 1229234, and CHE0840401; NIH P20 GM103541 and S10 RR02692).
Supporting Information Available
Full experimental data, details on methods and starting materials, and copies of spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
‡ These authors contributed equally.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Tsuji J. Acc. Chem. Res. 1969, 2, 144. [Google Scholar]; b Trost B. M. Tetrahedron 1977, 33, 2615. [Google Scholar]; c Trost B. M.; Van Vranken D. L. Chem. Rev. 1996, 96, 395. [DOI] [PubMed] [Google Scholar]; d Trost B. M.; Crawley M. L. Chem. Rev. 2003, 103, 2921. [DOI] [PubMed] [Google Scholar]; e Kazmaier U.; Pohlman M. In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere A., Diederich F., Eds; Wiley-VCH: Weinheim, 2004; Vol. 2, pp 531–583. [Google Scholar]; f Helmchen G.; Kazmaier U.; Forster S. In Catalytic Asymmetric Synthesis, 3rd ed.; Ojima I., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2010. [Google Scholar]
- a Belloni P. N.; Jolidon S.; Klaus M.; Lapierre J.-M.. Rar Selective Retinoid Agonists. U.S. Patent 2002/0026060 A1, Feb. 28, 2002.; b Klaus M.; Lapierre J.-M.. Heterocyclic Retinoid Compounds. U.S. Patent 2003/0158178A1, Aug. 21, 2003.; c Horibe H.; Fukuda Y.; Kondo K.; Okuno H.; Murakami Y.; Aoyama T. Tetrahedron 2004, 60, 10701. [Google Scholar]
- Pigge F. C. Synthesis 2010, 1745. [Google Scholar]
- For representative reviews of Suzuki cross-couplings, see:; a Miyaura N.; Suzuki A. Chem. Rev. 1995, 95, 2457. [Google Scholar]; b Suzuki A. J. Organomet. Chem. 1999, 576, 147. [Google Scholar]; c Suzuki A. Angew. Chem., Int. Ed. 2011, 50, 6722. [DOI] [PubMed] [Google Scholar]
- For rhodium-catalyzed desymmetrizations of allylic diol derivatives, see:; a Lautens M.; Dockendorff C.; Fagnou K.; Malicki A. Org. Lett. 2002, 4, 1311. [DOI] [PubMed] [Google Scholar]; b Menard F.; Chapman T. M.; Dockendorff C.; Lautens M. Org. Lett. 2006, 8, 4569. [DOI] [PubMed] [Google Scholar]; c Menard F.; Perez D.; Sustac Roman D.; Chapman T. M.; Lautens M. J. Org. Chem. 2010, 75, 4056. [DOI] [PubMed] [Google Scholar]; d Miura T.; Takahashi Y.; Murakami M. Chem. Commun. 2007, 595. [DOI] [PubMed] [Google Scholar]; e Yu B.; Menard F.; Isono N.; Lautens M. Synthesis 2009, 853. [Google Scholar]
- For enantioselective, rhodium-catalyzed arylations of nitroallyl acetates, see:; a Dong L.; Xu Y.-J.; Cun L.-F.; Cui X.; Mi A.-Q.; Jiang Y.-Z.; Gong L.-Z. Org. Lett. 2005, 7, 4285. [DOI] [PubMed] [Google Scholar]; b Dong L.; Xu Y.-J.; Yuan W.-C.; Cui X.; Cun L.-F.; Gong L.-Z. Eur. J. Org. Chem. 2006, 4093. [Google Scholar]
- Enantioselective, rhodium-catalyzed arylations have also been accomplished with arylzinc reagents. See:Evans P. A.; Uraguchi D. J. Am. Chem. Soc. 2003, 125, 7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For enantiospecific, palladium-catalyzed arylations of allylic alcohols and derivatives, see:; a Ohmiya H.; Makida Y.; Tanaka T.; Sawamura M. J. Am. Chem. Soc. 2008, 130, 17276. [DOI] [PubMed] [Google Scholar]; b Li D.; Tanaka T.; Ohmiya H.; Sawamura M. Org. Lett. 2010, 12, 3344. [DOI] [PubMed] [Google Scholar]; c Ohmiya H.; Makida Y.; Li D.; Tanabe M.; Sawamura M. J. Am. Chem. Soc. 2010, 132, 879. [DOI] [PubMed] [Google Scholar]; d Makida Y.; Ohmiya H.; Sawamura M. Chem.—Asian J. 2011, 6, 410. [DOI] [PubMed] [Google Scholar]; e Li C.-G.; Xing J.-X.; Zhao J.-M.; Huynh P.; Zhang W.-B.; Jiang P.-K.; Zhang Y.-J. Org. Lett. 2012, 14, 390. [DOI] [PubMed] [Google Scholar]; f Zhao J.-M.; Ye J.; Zhang Y.-J. Adv. Synth. Catal. 2013, 355, 491. [Google Scholar]; g Wu H.-B.; Ma X.-T.; Tian S.-K. Chem. Commun. 2014, 50, 219. [DOI] [PubMed] [Google Scholar]
- For enantiospecific, palladium-catalyzed coupling of allylic boronates with aryl iodides, see:Chausset-Boissari L.; Ghozati K.; LaBine E.; Chen J. L.-Y.; Aggarwal V. K.; Crudden C. M. Chem.—Eur. J. 2013, 19, 17698. [DOI] [PubMed] [Google Scholar]
- For an enantioselective, iridium-catalyzed arylation with an organozinc, see:Polet D.; Rathgeb X.; Falciola C. A.; Langlois J.-B.; El Hajjaji S.; Alexakis A. Chem.—Eur. J. 2009, 15, 1205. [DOI] [PubMed] [Google Scholar]
- For the importance of developing nonprecious metal catalysts, see:; a Bullock R. M.Catalysis without Precious Metals; Wiley-VCH: Weinheim, 2010. [Google Scholar]; b Rosen B. M.; Quasdorf K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Chem. Rev. 2011, 111, 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Han F.-S. Chem. Soc. Rev. 2013, 42, 5270. [DOI] [PubMed] [Google Scholar]; d Yamaguchi J.; Muto K.; Itami K. Eur. J. Org. Chem. 2013, 19. [Google Scholar]
- Falciola C. A.; Alexakis A. Eur. J. Org. Chem. 2008, 2008, 3765. [Google Scholar]
- For enantioselective, copper-catalyzed arylations with Grignard reagents, see:; a Selim K. B.; Yamada K.-i.; Tomioka K. Chem. Commun. 2008, 5140. [DOI] [PubMed] [Google Scholar]; b Selim K. B.; Matsumoto Y.; Yamada K.-i.; Tomioka K. Angew. Chem., Int. Ed. 2009, 48, 8733. [DOI] [PubMed] [Google Scholar]; c Lopez F.; van Zijl A. W.; Minnaard A. J.; Feringa B. L. Chem. Commun. 2006, 409. [DOI] [PubMed] [Google Scholar]
- For enantiospecific, copper-catalyzed arylations with Grignard reagents, see:; a Norinder J.; Bäckvall J.-E. Chem.—Eur. J. 2007, 13, 4094. [DOI] [PubMed] [Google Scholar]; b Norinder J.; Bogár K.; Kanupp L.; Bäckvall J.-E. Org. Lett. 2007, 9, 5095. [DOI] [PubMed] [Google Scholar]; c Thalén L. K.; Sumic A.; Bogár K.; Norinder J.; Persson A. K. Å.; Bäckvall J.-E. J. Org. Chem. 2010, 75, 6842. [DOI] [PubMed] [Google Scholar]; d Li M.-B.; Tang X.-L.; Tian S.-K. Adv. Synth. Catal. 2011, 353, 1980. [Google Scholar]; e See also: Lauer A. M.; Mahmud F.; Wu J. J. Am. Chem. Soc. 2011, 133, 9119. [DOI] [PubMed] [Google Scholar]
- For enantioselective, copper-catalyzed arylations using organozinc reagents, see:Kacprzynski M. A.; May T. L.; Kazane S. A.; Hoveyda A. H. Angew. Chem., Int. Ed. 2007, 46, 4554. [DOI] [PubMed] [Google Scholar]
- For enantioselective, copper-catalyzed arylations and vinylations using organoaluminum reagents, see:; a Gao F.; Lee Y.-M.; Mandai K.; Hoveyda A. H. Angew. Chem., Int. Ed. 2010, 49, 8370. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee Y.; Akiyama K.; Gillingham D. G.; Brown M. K.; Hoveyda A. H. J. Am. Chem. Soc. 2008, 130, 446. [DOI] [PubMed] [Google Scholar]
- For enantioselective, copper-catalyzed arylations with arylboronates, see:Shintani R.; Takatsu K.; Takeda M.; Hayashi T. Angew. Chem., Int. Ed. 2011, 50, 8656. [DOI] [PubMed] [Google Scholar]
- For enantiospecific, copper-catalyzed arylations with arylboronates, see:Ohmiya H.; Yokokawa N.; Sawamura M. Org. Lett. 2010, 12, 2438. [DOI] [PubMed] [Google Scholar]
- See also:Whittaker A. M.; Rucker R. P.; Lalic G. Org. Lett. 2010, 12, 3216. [DOI] [PubMed] [Google Scholar]
- a Zhou Q.; Srinivas H. D.; Dasgupta S.; Watson M. P. J. Am. Chem. Soc. 2013, 135, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]; b See also: Harris M. R.; Hanna L. E.; Greene M. A.; Moore C. E.; Jarvo E. R. J. Am. Chem. Soc. 2013, 135, 3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a phosphine-directed, diastereoselective arylation of allylic ethers with Grignard reagents, see:Didiuk M. T.; Morken J. P.; Hoveyda A. H. Tetrahedron 1998, 54, 1117. [Google Scholar]
- For seminal work in enantioselective, nickel-catalyzed arylations using aryl Grignard reagents, see:; a Consiglio G.; Morandini F.; Piccolo O. J. Chem. Soc., Chem. Commun. 1983, 112. [Google Scholar]; b Hiyama T.; Wakasa N. Tetrahedron Lett. 1985, 26, 3259. [Google Scholar]; c Consiglio G.; Piccolo O.; Roncetti L.; Morandini F. Tetrahedron 1986, 42, 2043. [Google Scholar]; d Consiglio G.; Waymouth R. M. Chem. Rev. 1989, 89, 257. [Google Scholar]
- Hayashi T.; Konishi M.; Yokota K.-I.; Kumada M. J. Organomet. Chem. 1985, 285, 359. [Google Scholar]
- a Kobayashi Y.; Tokoro Y.; Watatani K. Tetrahedron Lett. 1998, 39, 7537. [Google Scholar]; b Kobayashi Y.; Mizojiri R.; Ikeda E. J. Org. Chem. 1996, 61, 5391. [Google Scholar]; c Kobayashi Y.; Watatani K.; Kikori Y.; Mizojiri R. Tetrahedron Lett. 1996, 37, 6125. [Google Scholar]; d Kobayashi Y.; Takahisa E.; Usmani S. B. Tetrahedron Lett. 1998, 39, 597. [Google Scholar]; e Kobayashi Y.; Tokoro Y.; Watatani K. Eur. J. Org. Chem. 2000, 3825. [Google Scholar]; f For the related coupling of allylic acetates with vinyl borates, see: Usmani S. B.; Takahisa E.; Kobayashi Y. Tetrahedron Lett. 1998, 39, 601. [Google Scholar]
- Trost B. M.; Spagnol M. D. J. Chem. Soc., Perkin Trans. 1 1995, 2083. [Google Scholar]
- a Chung K.-G.; Miyake Y.; Uemura S. J. Chem. Soc., Perkin Trans. 1 2000, 15. [Google Scholar]; b For the related coupling of alkynyl borates with allylic carbonates, including a modestly enantioselective example, see: Chen H.; Deng M.-Z. J. Organomet. Chem. 2000, 603, 189. [Google Scholar]
- A similar effect was observed in the nickel-catalyzed coupling of benzylic ammonium triflates; see:Maity P.; Shacklady-McAtee D. M.; Yap G. P. A.; Sirianni E. R.; Watson M. P. J. Am. Chem. Soc. 2013, 135, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- We use “% es” (or % enantiospecificity) to indicate the percentage of the reaction that proceeds via the enantiospecific pathway. es = (ee of product)/(ee of starting material).
- CCDC 973168 contains the supplementary crystallographic data for 17. The data can be obtained from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif free of charge. These data are also included in the Supporting Information.
- a Allen A. E.; MacMillan D. W. C. J. Am. Chem. Soc. 2011, 133, 4260. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shiina I.; Nakata K.; Onda Y. Eur. J. Org. Chem. 2008, 5887. [Google Scholar]; c Fadel A. Synlett 1992, 1992, 48. [Google Scholar]
- a Felkin H.; Swierczewski G. Tetrahedron Lett. 1972, 13, 1433. [Google Scholar]; b Consiglio G.; Morandini F.; Piccolo O. J. Am. Chem. Soc. 1981, 103, 1846. [Google Scholar]; c Kochi J. K.Organometallic Mechanisms and Catalysis; Academic Press: New York, 1978; p 404. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.