Abstract
Background
Amongst the risk factors for preterm birth, previous preterm delivery is a strong predictor. Specialised clinics for women with a history of spontaneous preterm delivery have been advocated as a way of improving outcomes for women and their infants.
Objectives
To assess using the best available evidence, the value of specialised antenatal clinics for women with a pregnancy at high risk of preterm delivery when compared with ‘standard’ antenatal clinics.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2011).
Selection criteria
All published, unpublished, and ongoing randomised controlled trials (including cluster-randomised trials) examining specialised compared with standard antenatal clinic care for women with a singleton pregnancy considered at high risk of preterm labour.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
We included three trials with 3400 women, all carried out in the USA. All focused on specialised clinics for women at high risk of preterm birth. Gestational age at delivery, preterm delivery, or both were primary outcomes in all studies. The interventions in the three trials differed.
Overall there was very little data on our prespecified outcomes. For most outcomes a single study provided data, hence there was not the statistical power to detect any possible differences between groups. There was no clear evidence that specialised antenatal clinics reduce the number of preterm births.
Authors’ conclusions
Specialised antenatal clinics are now an accepted part of care in many settings, and carrying out further randomised trials may not be possible. Any future research in this area should include psychological outcomes and should focus on which aspects of service provision are preferred by women. Such research could underpin further service development in this area.
BACKGROUND
Preterm birth occurs in up to 6% to 10% of all births and is associated with perinatal mortality and morbidity (Lumley 2003). Nearly half of all preterm births are due to preterm labour. Preterm birth is associated with emotional and economic costs to both the family and society (Petrou 2001). Once congenital anomalies are excluded, preterm birth is responsible for the majority of neonatal deaths and is the major cause of disability in childhood (Hack 1999). The majority of mortality and morbidity occurs with extremely preterm birth (defined as birth before 28 weeks’ gestation). However, whilst the incidence of death and long-term handicap is relatively low in those born after 32 weeks, they make up over 80% of all preterm deliveries and therefore this group of infants has a significant impact upon the burden that preterm birth makes on public health (Kramer 2000). Despite research over the past few decades, no decrease in the incidence of preterm birth has occurred, although there have been improved survival and outcomes for premature infants.
Amongst the risk factors for preterm birth, previous preterm delivery is a strong predictor and the earlier the birth the more likely it is to be repeated at the same gestation (Hoffman 1984). Other risk factors include cervical weakness, cervical trauma, and stress (El-Bastawissi 1999; Iams 2004; Ruis 2003). Specialised clinics for women with a history of spontaneous preterm delivery have been advocated, with non-randomised cohort data suggesting improved perinatal outcomes with the provision of intensive antenatal education, continuity of carer and individualised care (Bienstock 2001). Such specialised clinics may also see women with previous preterm prelabour rupture of the membranes, previous late miscarriage (16 weeks 0 days to 23 weeks 6 days of gestation), or prior cervical surgery. Prediction of risk may utilise ultrasound assessment of cervical length and the presence of fetal fibronectin in cervico-vaginal secretions. The package of care offered in such clinics may include promising prophylactic interventions for labour prevention including progesterone, clindamycin or cervical cerclage (Althuisius 1998; Da Fonesca 2003; Ugwumadu 2003). However, stress has been associated with enhanced risk of preterm delivery and there is evidence that, whilst some pregnant women welcome referral to a specialist clinic during pregnancy, others experience it as unsettling (Jackson 2006).
The aim of this review is to assess, using the best available evidence, the value of such specialised antenatal clinics for women with a pregnancy at high risk of preterm birth when compared with ‘standard’ antenatal clinic attendance. The primary outcomes relate to maternal and neonatal morbidity, and maternal and perinatal mortality. In women with multiple pregnancy, who have an increased risk of premature delivery, specialised clinics have been advocated in an attempt to improve perinatal outcomes. The value of specialised antenatal care for women with a multiple pregnancy when compared with ‘standard’ antenatal care is the subject of a different Cochrane review (Dodd 2007).
OBJECTIVES
To assess, using the best available evidence, the value of specialised antenatal clinics for women with a pregnancy at high risk of preterm delivery when compared with ‘standard’ antenatal clinics.
METHODS
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing randomised controlled trials including cluster-randomised trials. We have included quasi-randomised controlled trials.
Types of participants
Pregnant women with a singleton pregnancy considered by the trial authors to be at high risk of preterm labour.
Types of interventions
‘Specialised’ antenatal clinics (as defined by trial authors) compared with ‘standard’ antenatal clinic care (as defined by trial authors).
Types of outcome measures
Primary outcomes
Perinatal death (defined as stillbirth after trial entry, or death of a liveborn infant up to seven days of age).
Extremely preterm birth (defined as birth less than 28 weeks’ gestation).
Secondary outcomes
Secondary outcomes relate to pregnancy outcomes, complications, satisfaction and costs
Pregnancy outcomes
Development of antenatal complications (including preterm labour (actual or suspected), preterm prelabour ruptured membranes, intrauterine growth restriction (estimated fetal weight less than 10th centile for gestational age)).
Preterm birth (defined as birth before 37 weeks’ gestation).
Very preterm birth (defined as birth before 34 weeks’ gestation).
Gestation at birth.
Instrumental vaginal birth.
Infection requiring intravenous antibiotics.
Mode of birth.
Postnatal depression.
Breastfeeding.
Complications for infants
Neonatal intensive care unit admission.
Respiratory distress syndrome.
Parameters of birth asphyxia (neonatal irritability, neonatal seizures, neonatal hypotonia, abnormal level of consciousness, neonatal apnoea, tube feeding greater than 48 hours).
Complications of prematurity.
Disability at childhood follow-up (including deafness, blindness, neurodisability or cerebral palsy).
Measures of satisfaction
Women’s satisfaction.
Costs
Costs associated with ‘specialised’ antenatal care versus ‘standard’ care.
Number of antenatal visits.
Number of antenatal admissions and length of admission.
Length of maternal postnatal stay.
Length of stay in neonatal intensive care unit.
Infant length of hospital stay.
We have included outcomes in the analysis if data were available according to original allocation and reasonable measures were taken to minimise observer bias. Only outcomes with available data appear in the analysis tables. In order to minimise the risk of bias, we have based the conclusions solely on the pre-stated outcomes.
Search methods for identification of studies
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (30 June 2011).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence and assessed whether it should have produced comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non-random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding (checking for possible performance bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. With an intervention such as the provision of specialised clinics blinding women and clinic staff to group allocation is not generally feasible, it may however be possible, for some outcomes, to blind outcome assessors. We considered studies to be at lower risk of bias if outcome assessment was blinded or if we judged that the lack of blinding would have been unlikely to have affected the results. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the methods as:
low, high, or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were likely to be related to outcomes. We assessed methods as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(We had intended to exclude data from the analysis where loss to follow-up for a particular outcome was greater than 20%; however, in two of the included trials information about exclusions and attrition was not clear, so we have included all outcome data as reported by study authors.)
(5) Selective reporting bias
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre-specified outcomes were reported; one or more reported primary outcomes were not pre-specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We have described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses - see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster-randomised trials
We have included one cluster-randomised trial in the analyses along with individually-randomised trials. We adjusted the standard error for results from this study using the methods described in the Handbook (Higgins 2011). No estimate of the intracluster correlation co-efficient (ICC) was provided for this trial and so we used a published ICC from another trial of antenatal care (Piaggio 2001). As we used an ICC from another source, we have conducted sensitivity analyses to investigate the effect of variation in the ICC. Where we identified both cluster-randomised and individually-randomised trials examining the same outcomes, we synthesised the relevant information. We considered it reasonable to combine the results from both, provided that there was little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Dealing with missing data
For included studies, we have noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis; but in this version of the review, with so few studies contributing data, we did not think further analysis would shed any further light on findings. In updates of the review, if more data are added, we will carry out sensitivity analysis excluding data from studies (or for particular outcomes) where there are high levels of missing data.
For all outcomes, we have carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta-analysis using the T2, I2 and Chi2 statistics. We have regarded heterogeneity as substantial if I2 is greater than 30% and either T2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If there were 10 or more studies in the meta-analysis we planned to investigate reporting biases (such as publication bias) using funnel plots. In this version of the review, insufficient studies contributed data and we did not generate funnel plots. In updates, if more data become available we will assess funnel plot asymmetry visually, and use formal statistical tests; for continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If we detect asymmetry in any of these tests or by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We have carried out statistical analysis using the Review Manager software (RevMan 2011). We have used fixed-effect meta-analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where we suspected clinical heterogeneity, or if we detected substantial statistical heterogeneity, we have used random-effects meta-analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful.
Where we have used random-effects analyses, we have presented results as the average treatment effect with 95% confidence interval, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses:
parity (nulliparous versus multiparous women);
type of care received (that is, time that specialised care commenced; number of antenatal visits; use of ultrasound);
use of cervical length measurements (transvaginal ultrasound versus no transvaginal ultrasound).
We planned to restrict subgroup analyses to primary outcomes. We planned to assess differences between subgroups by inspection of the subgroups’ confidence intervals; with non-overlapping confidence intervals suggesting a statistically significant difference in treatment effect between the subgroups. In view of the small number of studies contributing data we did not think there would be sufficient information for any particular subgroup to explore possible subgroup differences and so in this version of the review we did not perform these additional analyses. If further studies are added in updates, we will consider carrying out further analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses to evaluate the effect of trial quality. In this version of the review, so few trials contributed data that we did not think sensitivity analysis would help in the interpretation of results. One outcome included data from a cluster-randomised trial, and for this outcome we carried out a sensitivity analysis where we varied the value of the ICC using a more conservative value (upper 95% confidence interval for a published ICC (Piaggio 2001)).
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Using the search strategy, we identified 15 reports describing the methods and findings from seven randomised trials (several of the studies resulted in multiple publications). We have included three of these trials in the review and have excluded four.
Included studies
All three included trials were carried out in the USA. All trials focused on specialised clinics for women at high risk of preterm birth, and gestational age at delivery, preterm delivery, or both, were primary outcomes in all studies. The interventions in the three trials differed slightly. In a study in Ohio (Iams 1989), women attended clinics and had cervical examinations weekly between 20 and 36 weeks’ gestation and were advised on the signs and symptoms of labour onset. In the Pennsylvania trial (Main 1989), women attended weekly or twice-weekly clinics from 22 weeks’ gestation; and again women were educated on the early signs of labour and had cervical assessment. In the West Los Angeles 1994 trial, clinics rather than women were randomised (five experimental clinics and three control). Women attending experimental clinics had fortnightly visits and had three classes on preterm birth prevention. In addition, in a nested study, women were randomised to one of four secondary interventions (bed rest, psychosocial support, oral progestin or a placebo). Participants in both experimental and control clinics received psychosocial and nutritional screening and were offered crisis intervention. We have set out further details on these studies in the Characteristics of included studies tables.
Excluded studies
We excluded four studies: three because the samples included women with multiple pregnancies and separate results were not reported for singletons (Collaborative Group 1993; Goldenberg 1990; Pittsburgh 1989); finally, the study by Beazley 2001 was reported in a brief abstract, we were not able to assess risk of bias, and it was not clear how many women were randomised to intervention and control groups. We have provided further information on these studies in the Characteristics of excluded studies tables.
Risk of bias in included studies
Allocation
In the study by Iams 1989 there was little information on study methods; the way the randomisation sequence was generated and methods used to conceal allocation were not clear. In the Main 1989 trial, allocation was by a quasi-randomised method. The West Los Angeles 1994 study was a cluster-randomised trial; eight clinics were randomised using a blocked technique.
Blinding
Blinding women and care providers to treatment group is difficult to achieve with an intervention involving different models of care. Women would be aware of their more frequent attendance and so would clinic staff. In the cluster-randomised trial, randomisation at the clinic level would be likely to reduce bias associated with contamination. This study also examined additional interventions, and within the nested study in experimental clinics, it would be difficult to blind women and staff to these additional interventions (e.g. bed rest) although one of the four groups received a placebo. It is not clear whether lack of blinding represents a serious source of bias in these studies.
Incomplete outcome data
Rates of attrition and exclusion in the control and experimental groups were not clearly set out in two studies (Iams 1989; Main 1989). In the Main 1989 study, there appeared to be little loss to follow-up for the primary outcome (preterm birth), although there were relatively high levels of missing data for neonatal outcomes as approximately 20% of infant case-notes were not reviewed. In the West Los Angeles 1994 study, loss to follow up was less than 10%; however, even small losses may be important in studies examining a relatively infrequent outcome, especially if loss is not balanced across groups, as those most vulnerable to poor outcomes may be over-represented amongst those who are not followed up.
Selective reporting
Without access to study protocols, it is difficult assess selective reporting bias. All three studies reported the number of preterm births, although other key outcomes (for example, perinatal death and extreme prematurity) were not reported. It is not clear whether or not this information was collected.
Other potential sources of bias
We have noted in the Characteristics of included studies tables other possible sources of bias, and any factors which we thought affected our interpretation of results. One of the included studies was a cluster randomised trial; in the trial report results had not been adjusted for cluster design effect. In order to pool results from this trial with those from trials where women were randomised, where sufficient information was available to allow us to do so, we used the generic inverse variance method and adjusted the standard error of results from the cluster randomised-trial to take account of design effect.
Effects of interventions
Specialised antenatal clinics versus routine care: three studies with 3400 women
Primary outcomes
Perinatal death (defined as stillbirth after trial entry, or death of a liveborn infant up to seven days of age)
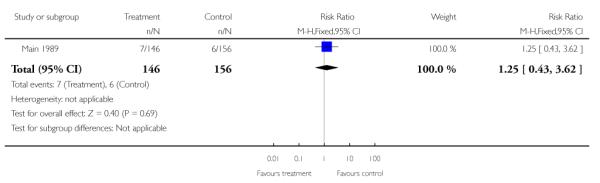
Perinatal death was reported in one of the included studies with outcome data for 302 infants (Main 1989). There was no evidence of a significant difference between groups; overall, there were 13 deaths, seven in the treatment group and six in the control group (risk ratio (RR) 1.25, 95% confidence interval (CI) 0.43 to 3.62).
Extremely preterm birth (defined as birth less than 28 weeks’ gestation)
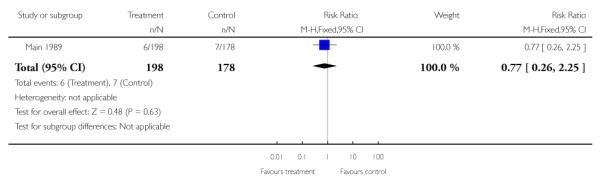
This outcome was reported in one study with data for 376 women (Main 1989). There was no clear evidence of a difference between women attending specialist clinics compared with those receiving routine care (RR 0.77, 95% CI 0.26 to 2.25).
Secondary outcomes
Antenatal complications
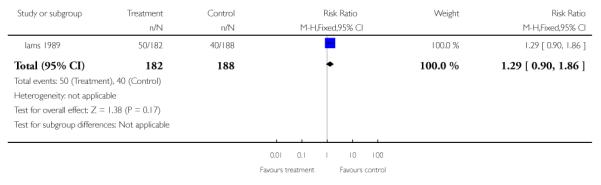
The number of women being admitted with antenatal complications (including preterm labour (actual or suspected), preterm prelabour ruptured membranes, intrauterine growth restriction (estimated fetal weight less than 10th centile for gestational age)) was reported in one study (370 women) (Iams 1989). There was no statistically significant difference between groups (RR 1.29, 95% CI 0.90 to 1.86).
Preterm birth (birth before 37 weeks’ gestation)
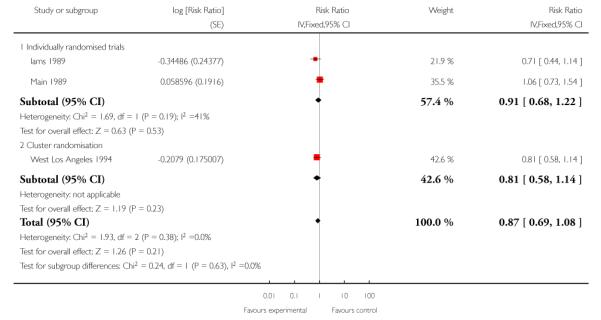
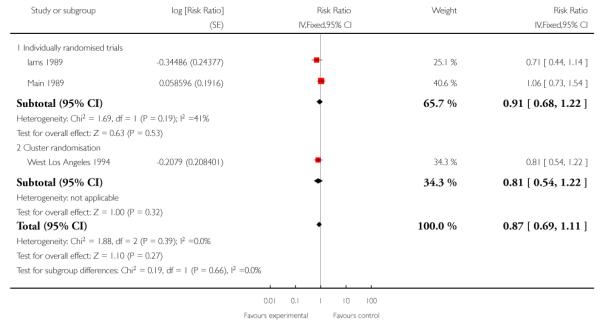
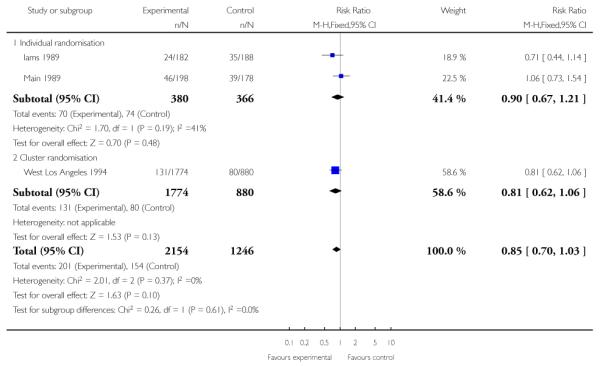
All three included studies provided data for this outcome (3400 women). One of the studies used a cluster-randomisation technique and so the standard error has been adjusted (West Los Angeles 1994). When data from the three studies were pooled, it appeared that there were fewer preterm births in the treatment group compared with controls, but the difference between groups was not statistically significant (RR 0.87, 95% CI 0.69 to 1.08 (adjusted data, inverse variance method Analysis 1.4)). For this outcome, we carried out a sensitivity analysis whereby, for the cluster-randomised trial, we used the upper 95% CI for the ICC (most conservative value); in this analysis, while the weight of the cluster-randomised trial was reduced, this adjustment made very little difference to results (RR 0.87, 95% CI 0.69 to 1.11 (adjusted data, inverse variance method Analysis 1.5 )). (In Analysis 1.27 we have set out the original data for the three studies; again results were similar and although the 95% CI was narrower the difference between groups still did not reach statistical significance (unadjusted RR 0.85, 95% CI 0.70 to 1.03).)
Very preterm birth (before 34 weeks)
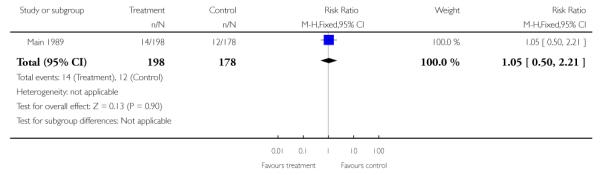
One study contributed data to this outcome and there was no evidence of differences between groups (RR 1.05, 95% CI 0.50 to 2.21) (Main 1989).
Other secondary outcomes
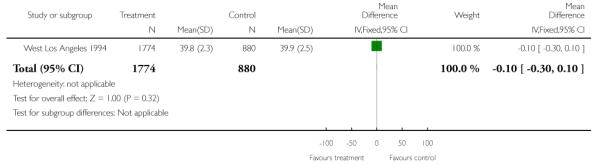
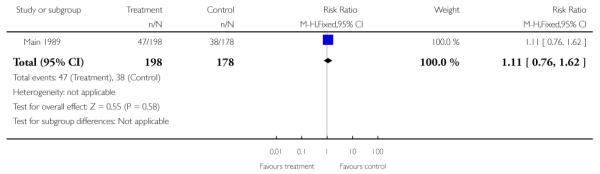
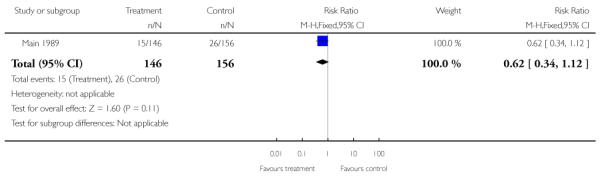
There was no significant evidence of differences between women attending specialised clinics and women receiving routine care for any of the following secondary outcomes: gestational age at birth (Analysis 1.7); caesarean section (Analysis 1.9); or neonatal intensive care unit admission (Analysis 1.13).
There were no data reported for our remaining secondary outcomes.
Costs of care and process variables
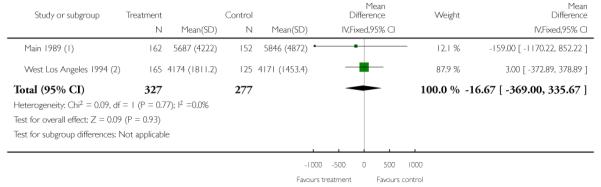
Two studies examined the costs associated with care (Main 1989; West Los Angeles 1994). However, in one of these studies (West Los Angeles 1994), a sub-sample of approximately 10% of the total sample only was included in the cost-analysis (280/2705 women). Further, in this study there was no adjustment for cluster design effect and in the data and analyses tables we have therefore presented unadjusted data for cost outcomes.
When data from the Main 1989 and West Los Angeles 1994 studies were pooled there was no significant evidence that specialised clinics and routine management differed in terms of the total cost of care for women (Analysis 1.19).
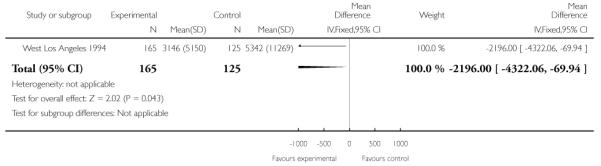
The cost of newborn infant care was examined in the West Los Angeles 1994 trial and appeared to be reduced in infants of women attending the experimental clinics compared with controls. However, results were not simple to interpret. The authors suggest that the cost difference was due to a combination of fewer preterm births in the experimental group, a lower relative rate of delivery at less than 32 weeks’ gestation, and lower mean costs for infants of comparable gestations (Analysis 1.20). They did not comment on what interventions were required by babies in the different groups or whether the two groups had different rates of steroid administration which may have impacted on neonatal condition at delivery.
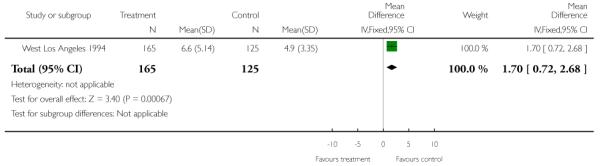
In the West Los Angeles 1994 study women in the control group had fewer antenatal clinic visits with a mean of 4.9 clinic attendances compared with a mean of 6.6 for women in the experimental group (mean difference 1.7, 95% CI 0.72 to 2.68) (Analysis 1.21).
DISCUSSION
Summary of main results
Overall there was very little data on our pre-specified outcomes. For most outcomes a single study provided data, hence there may have been insufficient statistical power to detect any possible differences between groups. There was no clear evidence that specialised antenatal clinics reduce the number of preterm births. Perinatal death was reported in only one of the included studies and there was no evidence of a significant difference between groups.
Overall completeness and applicability of evidence
Interpreting the findings of this review is not straightforward. Preterm birth is a multi-factorial disorder and hence a complex intervention such as a package of antenatal care is a logical solution to preventing its recurrence. The antenatal care needs to be aimed at managing diverse contributing factors such nutritional deficiencies, smoking cessation, dental hygiene, infection, cervical weakness, pro-thrombotic tendency, psycho-social stress and excessive uterine contractility. The review includes only three trials, which tackled potential contributing factors to varying degrees, and the same outcomes were not reported for all trials. Furthermore, the three trials varied in the nature of the services provided as part of the specialised antenatal clinic. Two studies included repeat digital cervical assessment (RDCA) in the treatment arm (Iams 1989; Main 1989I). Whilst there is no evidence that RCDA is effective as a routine intervention in the antenatal clinic as a screening test for the risk of preterm birth in average risk pregnancies, it has not been thoroughly evaluated as a component of a complex intervention such as a specialist antenatal clinic, targeting preterm birth (Alexander 2010). This makes interpretation of our results more difficult. In addition, the trials were all conducted in the 1980s prior to the introduction of many of the screening tests currently offered in specialised antenatal clinics e.g. fetal fibronectin testing and ultrasound assessment of cervical length. There is clear evidence that antenatal steroids improve neonatal survival and reduce neonatal morbidity (Roberts 2006), hence the ability to accurately predict preterm labour in a specialised clinic would ensure that women received the steroids prior to delivery. The problem is that at present the current predictive tests (cervical length monitoring and fetal fibronectin) are not sufficiently specific to make them cost effective (Honest 2009).
Quality of the evidence
The quality of the evidence in these three trials was mixed, and the methods used to conceal allocation were at high or unclear risk of bias in the two trials where women were randomised (Iams 1989; Main 1989). In the cluster-randomised trial findings were not adjusted for cluster design effect; and while we attempted to take account of possible design effects for some outcomes we did not have sufficient information to adjust the cost data reported in the West Los Angeles 1994 trial. Missing data were a concern in two of the included trials; even small losses may be important in studies examining relatively infrequent outcomes, as those most vulnerable to poor outcomes may be over-represented amongst those who are not followed up.
Potential biases in the review process
We are aware of potential biases in the reviewing process; and we took steps to minimise bias (e.g. two authors independently assessed study eligibility, carried out data extraction and assessed risk of bias).
AUTHORS’ CONCLUSIONS
Implications for practice
Since the publication of the trials included in this review (1989 to 1994) there have been further meta-analyses of trials of individual treatments that were effective at preventing preterm birth: progesterone (Barros 2010; Honest 2009; Rode 2009); smoking cessation (Barros 2010); eradication of asymptomatic bacteriuria (Iams 2010); appropriate use of cerclage (Iams 2010I); and calcium supplementation (Hofmeyr 2010). Implementation of evidence from clinical trials is notoriously slow and incomplete (Bodribb 2010). Hence, it may be that a future trial of specialised antenatal clinic would show evidence of benefit because of improved implementation of these interventions that have been more recently shown to be effective.
Importantly, it is not known whether preventing preterm birth reduces neonatal morbidity and mortality as it may be that retaining a fetus in an unfavourable intrauterine environment has a detrimental effect on in utero development. Hence, any future trials need to report both short and long-term neonatal outcomes (Kenyon 2008).
Implications for research
As specialised antenatal clinics for women with a pregnancy at high risk of preterm delivery are now an accepted part of antenatal services in many areas, it will be difficult to carry out further randomised studies. However, the trials included in the review included no information on psychological outcomes for women. Some studies have suggested that whilst some pregnant women welcome referral to a specialist clinic during pregnancy, others experience it as unsettling (Jackson 2006). Any future research in this area should examine which aspects of service provision are preferred by women. Such research could underpin further service development in this area.
PLAIN LANGUAGE SUMMARY.
Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve outcomes for women and babies
Women who have had a previous preterm birth are at increased risk of having another premature birth. Babies who are born before the 37th week of pregnancy, and particularly those born before the 34th week, are at greater risk of suffering problems at birth and of disability in childhood. ‘Specialised’ antenatal clinics have been suggested for women at high risk of a preterm birth as a way of improving health outcomes for the women and their infants. This review of three randomised controlled trials involving 3400 women in the USA found that there was no reduction in the number of preterm births in women attending specialised antenatal clinics. The results were difficult to interpret, as the trials were conducted in slightly different ways and offered slightly different care. The trials were all conducted in the 1980s, before the introduction of many of the screening tests currently offered in specialised antenatal clinics such as ultrasound assessment of cervical length. There was no information available on the effect of specialised antenatal care on maternal wellbeing or long-term outcome.
ACKNOWLEDGEMENTS
As part of the pre-publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
• The University of Liverpool, UK.
External sources
• National Institute for Health Research (NIHR), UK.
TD is supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS 10/4001/02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial of routine antepartum care versus routine antepartum care plus a preterm birth prevention clinic in which symptoms and signs of labour were taught and the cervix examined at weekly visits between 20 and 36 weeks’ gestation | |
| Participants | 370 pregnant women considered high risk for preterm birth based on use of the Creasey Scoring system (Creasy 1980). Women were selected from 2829 women attending prenatal clinic at the Department of Obstetrics and Gynecology, Ohio State University, Ohio | |
| Interventions | Preterm birth prevention clinic in which symptoms and signs of labour were taught and the cervix examined at weekly visits between 20 and 36 weeks’ gestation | |
| Outcomes | Preterm labour diagnosed before 37 weeks’ gestation when cervical changes accompanied persistent uterine contractions despite bedrest and intravenous hydration, or, if the cervix was already 2 cm dilated and/or 50% effaced, when 8 contractions/hour persisted despite bedrest and parenteral hydration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Insufficient information about the sequence generation process to permit judgement of risk of bias |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No reporting of attrition or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of risk of bias. |
| Other bias | Unclear risk | Does not explicitly state that only singleton pregnancies were included in the study. No comment is made on what cut-off was used for the Creasy scoring system to define women as of high risk of preterm birth (Creasy 1980). |
| Methods | Randomised controlled trial of routine antepartum care versus a preterm labour detection clinic in which women were seen on a weekly or biweekly basis, the cervix examined at each visit and education provided regarding subtle signs of labour | |
| Participants | All women were from the obstetric clinic of the University of Pennsylvania Hospital, USA. 376 black women considered at high risk for preterm birth based on a Papiernik-Creasey Scoring system value of 10 or more (Creasy 1980). 178 control patients and 198 women assigned to preterm labour detection clinic | |
| Interventions | Attendance at a preterm labour detection clinic on a weekly or biweekly basis from 22 weeks’ gestation. Cervical assessment by 1 of 3 physicians at each visit. Education provided by a nurse specialist regarding subtle signs of labour | |
| Outcomes | Gestational age at delivery, birthweight, preterm PROM, inpatient costs, outpatient costs | |
| Notes | Higher rate of accrual into the intervention group due to: (1) high-risk women who participated in the Preterm Labour Detection Clinic in earlier pregnancies being uniformly assigned to it in subsequent pregnancy; (2) higher elective abortion and transfer of care rates in control group | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive numbers of a random numbers table were used to allocate the first 479 participants whilst the second sample of 464 women was divided into groups by birthday (even versus odd day of the month) |
| Allocation concealment (selection bias) | High risk | Date of birth used for allocation of half of participants. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Neither the physicians or the control patients were made aware they were at high risk of preterm birth. Not possible to blind participants attending the preterm labour detection clinic due to the frequency of attendance |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Insufficient reporting of attrition/exclusions: there were missing data (approximately 20%) for neonatal outcomes as not all infant case notes were examined |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of risk of bias |
| Other bias | Unclear risk | Women included in study during more than one pregnancy. |
| Methods | Eight West Los Angeles County prenatal clinics randomised to be either experimental or control clinics. Women in experimental clinics offered a programme of more frequent visits, preterm birth prevention education, psychosocial and nutritional screening and crisis intervention. Women in control clinics offered routine county care plus psychosocial and nutritional screening and crisis intervention | |
| Participants | 1774 high-risk patients in experimental clinics and 880 control clinic patients. Majority of patients were Hispanic | |
| Interventions | Women in control clinics offered visits at 4-week intervals up to 30 weeks’ gestation, 2-week intervals from 30-35 weeks’ gestation, and then weekly until delivery. Psychosocial and nutritional screening and crisis intervention also offered. Women in experimental clinics offered visits at 2-week intervals, 3 classes regarding preterm birth prevention, psychosocial and nutritional screening and crisis intervention. Participants were randomised to 1 of 4 secondary interventions (bed rest, social work, Provera or Provera-matched control) or to no additional intervention | |
| Outcomes | Preterm birth rate, costs of antenatal care, inpatient preterm labour, delivery and postpartum care, and newborn care. For continuous outcomes (costs and number of antenatal visits) means and standard errors were reported. We calculated standard deviations in order to include these data in the data and analyses tables | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomised therefore not applicable; “these clinics were randomized with a restricted block (grouped by size and percent black) randomized scheme to identify the clinic as either experimental (five clinics) or control (three clinics)” |
| Allocation concealment (selection bias) | Unclear risk | Cluster randomised therefore not applicable. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Women randomised based on which clinic they attended. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | All high-risk women accounted for in a flow chart. 5%-7% of women assessed as high risk lost to follow-up |
| Other bias | Low risk |
PROM: preterm rupture of membranes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beazley 2001 | Study design: randomised prospective trial. Intervention: weekly contact with a nurse versus routine care. Participants: 218 women at high risk of preterm birth Outcomes: preterm birth before 37 weeks’ gestation and spontaneous preterm birth before 37 weeks’ gestation Published in abstract form only. Not clear how many patients were in control and intervention groups therefore analysis not possible. No decrease in the incidence of preterm birth or spontaneous preterm birth in the intervention group (43% vs 41%, 35% vs 30% respectively) |
| Collaborative Group 1993 | Study design: multi-centre randomised controlled trial. Intervention: preterm labour prevention programme with additional visits, education about the signs of preterm labour and cervical assessment at routine visits vs routine care. Participants: 2395 women considered at high risk of preterm delivery. Outcomes: spontaneous preterm birth by 36 and 37 completed weeks with and without PPROM, very low birthweight (< 1500 g at birth), low birthweight (< 2500 g at birth). Women with multiple pregnancies included in all the analyses. Creasy publication of data froma cohort of the women included in the Collaborative Group on Preterm Birth Prevention publication |
| Goldenberg 1990 | Study design: multi-centre randomised controlled trial. Intervention: preterm labour prevention programme with weekly visits, education about the signs of pretermlabour and cervical assessment at routine visits vs routine care. Control women seen in different clinics from intervention patients. Participants: 678 women considered at high risk of preterm delivery. Outcomes: spontaneous preterm birth at less than 37 completed weeks, very low birthweight (< 1500 g at birth), low birthweight (1500-2499 g at birth), spontaneous preterm PROM rate. Women with multiple pregnancies included in all the analyses |
| Pittsburgh 1989 | Study design: randomised controlled trial. Intervention: preterm labour prevention programme with weekly visits, education about the signs of preterm labour and cervical assessment at each visit vs routine care Participants: 831 women considered at high risk of preterm delivery out of 5457 women screened. Outcomes: spontaneous preterm birth at less than 37 completed weeks. Women with multiple pregnancies included in all the analyses |
vs: versus
DATA AND ANALYSES
Comparison 1.
Specialised antenatal care versus routine care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 1 | 302 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.43, 3.62] |
| 2 Extremely preterm birth (< 28 weeks’ gestation) | 1 | 376 | Risk Ratio (M-H, Fixed, 95% CI) | 0.77 [0.26, 2.25] |
| 3 Development of antenatal complications - preterm labour | 1 | 370 | Risk Ratio (M-H, Fixed, 95% CI) | 1.29 [0.90, 1.86] |
| 4 Preterm birth - adjusted for cluster design effect (ICC 0.002) | 3 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.69, 1.08] | |
| 4.1 Individually randomised trials | 2 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.68, 1.22] | |
| 4.2 Cluster randomisation | 1 | Risk Ratio (Fixed, 95% CI) | 0.81 [0.58, 1.14] | |
| 5 Sensitivity analysis. Preterm birth (adjusted for cluster design effect using upper CI, ICC 0.0041) | 3 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.69, 1.11] | |
| 5.1 Individually randomised trials | 2 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.68, 1.22] | |
| 5.2 Cluster randomisation | 1 | Risk Ratio (Fixed, 95% CI) | 0.81 [0.54, 1.22] | |
| 6 Very preterm birth (birth before 34 weeks’ gestation) | 1 | 376 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.50, 2.21] |
| 7 Gestation at birth | 1 | 2654 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−0.30, 0.10] |
| 8 Infection requiring intravenous antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Caesarean section | 1 | 376 | Risk Ratio (M-H, Fixed, 95% CI) | 1.11 [0.76, 1.62] |
| 10 Postnatal depression | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Instrumental vaginal birth | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Neonatal intensive care unit admission | 1 | 302 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.34, 1.12] |
| 14 Respiratory distress syndrome | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Parameters of birth asphyxia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Complications of prematurity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Disability at childhood follow-up | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Costs associated with care | 2 | 604 | Mean Difference (IV, Fixed, 95% CI) | −16.67 [−367.00, 335.67] |
| 20 Costs associated with newborn inpatient care (US $) | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | −2196.0 [−4322.06, −69.94] |
| 21 Number of antenatal visits | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.72, 2.68] |
| 22 Number of antenatal admissions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Length of antenatal admissions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Length of maternal postnatal stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Length of stay in neonatal intensive care unit | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Infant length of stay in hospital | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Preterm birth - all studies, unadjusted data | 3 | 3400 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.70, 1.03] |
| 27.1 Individual randomisation | 2 | 746 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.67, 1.21] |
| 27.2 Cluster randomisation | 1 | 2654 | Risk Ratio (M-H, Fixed, 95% CI) | 0.81 [0.62, 1.06] |
Analysis 1.1. Comparison 1 Specialised antenatal care versus routine care, Outcome 1 Perinatal death
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 1 Perinatal death
 |
Analysis 1.2. Comparison 1 Specialised antenatal care versus routine care, Outcome 2 Extremely preterm birth (< 28 weeks’ gestation)
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 2 Extremely preterm birth (< 28 weeks’ gestation)
 |
Analysis 1.3. Comparison 1 Specialised antenatal care versus routine care, Outcome 3 Development of antenatal complications - preterm labour
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 3 Development of antenatal complications - preterm labour
 |
Analysis 1.4. Comparison 1 Specialised antenatal care versus routine care, Outcome 4 Preterm birth -adjusted for cluster design effect (ICC 0.002)
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 4 Preterm birth - adjusted for cluster design effect (ICC 0.002)
 |
Analysis 1.5. Comparison 1 Specialised antenatal care versus routine care, Outcome 5 Sensitivity analysis. Preterm birth (adjusted for cluster design effect using upper CI, ICC 0.0041)
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 5 Sensitivity analysis. Preterm birth (adjusted for cluster design effect using upper CI, ICC 0.0041)
 |
Analysis 1.6. Comparison 1 Specialised antenatal care versus routine care, Outcome 6 Very preterm birth (birth before 34 weeks’ gestation)
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 6 Very preterm birth (birth before 34 weeks’ gestation)
 |
Analysis 1.7. Comparison 1 Specialised antenatal care versus routine care, Outcome 7 Gestation at birth
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 7 Gestation at birth
 |
Analysis 1.9. Comparison 1 Specialised antenatal care versus routine care, Outcome 9 Caesarean section
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 9 Caesarean section
 |
Analysis 1.13. Comparison 1 Specialised antenatal care versus routine care, Outcome 13 Neonatal intensive care unit admission
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 13 Neonatal intensive care unit admission
 |
Analysis 1.19. Comparison 1 Specialised antenatal care versus routine care, Outcome 19 Costs associated with care
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 19 Costs associated with care
 |
Maternal charges
Hospital costs for delivery and postpartum care (maternal)
Analysis 1.20. Comparison 1 Specialised antenatal care versus routine care, Outcome 20 Costs associated with newborn inpatient care (US $)
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 20 Costs associated with newborn inpatient care (US $)
 |
Analysis 1.21. Comparison 1 Specialised antenatal care versus routine care, Outcome 21 Number of antenatal visits
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 21 Number of antenatal visits
 |
Analysis 1.27. Comparison 1 Specialised antenatal care versus routine care, Outcome 27 Preterm birth - all studies, unadjusted data
Review: Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes
Comparison: 1 Specialised antenatal care versus routine care
Outcome: 27 Preterm birth - all studies, unadjusted data
 |
HISTORY
Protocol first published: Issue 4, 2007
Review first published: Issue 9, 2011
| Date | Event | Description |
|---|---|---|
| 21 February 2008 | Amended | Converted to new review format. |
| 20 July 2007 | New citation required and major changes | Substantive amendment |
Footnotes
DECLARATIONS OF INTEREST None known.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW The methods section has been updated to reflect the Pregnancy and Childbirth Group’s updated methods text and the latest Handbook (Higgins 2011).
INDEX TERMS
Medical Subject Headings (MeSH)
*Pregnancy Outcome; *Pregnancy, High-Risk; Patient Education as Topic; Premature Birth [diagnosis; *prevention & control]; Prenatal Care [organization & administration; *standards]; Randomized Controlled Trials as Topic; Recurrence [prevention & control]
MeSH check words
Female; Humans; Pregnancy
Editorial group: Cochrane Pregnancy and Childbirth Group.
Publication status and date: New, published in Issue 9, 2011.
Review content assessed as up-to-date: 24 July 2011.
REFERENCES
* Indicates the major publication for the study
References to studies included in this review
- Iams 1989.Iams JD, Johnson FF. Effect of a preterm birth prevention program on the diagnosis and treatment of preterm labor in high risk patients. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; New Orleans, Louisiana, USA. 1989 Feb 1-4.1989. p. 387. [Google Scholar]
- Main 1989.* Main DM, Gabbe SG, Richardson D, Strong S. Can preterm deliveries be prevented? American Journal of Obstetrics and Gynecology. 1985;151:892–8. doi: 10.1016/0002-9378(85)90667-2. [DOI] [PubMed] [Google Scholar]; Main DM, Richardson DK, Hadley CB, Gabbe SG. Controlled trial of a preterm labor detection program: efficacy and costs. Obstetrics & Gynecology. 1989;74:873–7. [PubMed] [Google Scholar]
- West Los Angeles 1994.Hobel CJ, Bemis RL. West Area Los Angeles prematurity prevention demonstration project. Prevention of Preterm Birth. 1986;138:205–22. [Google Scholar]; Hobel CJ, Bragonier R, Ross M, Bear M, Bemis R, Mori B. West Los Angeles premature prevention program: significant impact. Journal of Perinatal Medicine. 1987;15:112. [Google Scholar]; * Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Bear M, Mori B. West Los Angeles preterm birth prevention project (LAPPP): program impact. American Journal of Obstetrics and Gynecology. 1992;166:363. doi: 10.1016/s0002-9378(94)70384-1. [DOI] [PubMed] [Google Scholar]; Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Nessim S, Sandhu M, et al. The West Los Angeles preterm birth prevention project: I. program impact on high-risk women. American Journal of Obstetrics and Gynecology. 1994;170:54–62. doi: 10.1016/s0002-9378(94)70384-1. [DOI] [PubMed] [Google Scholar]; Ross MG, Sandhu M, Bemis R, Nessim S, Bragonier JR, Hobel C. The West Los Angeles preterm birth prevention project: II. cost-effectiveness analysis of high-risk pregnancy interventions. Obstetrics and Gynecology. 1994;83:506–11. doi: 10.1097/00006250-199404000-00004. [DOI] [PubMed] [Google Scholar]; Ross MG, Sandhu M, Bemis R, Nessim S, Bragonier JR, Mori B, et al. West Los Angeles preterm birth prevention project (LAPPP): cost benefit of high risk pregnancy interventions. American Journal of Obstetrics and Gynecology. 1992;166:367. [Google Scholar]
References
References to studies excluded from this review
- Beazley 2001.Beazley D, Mercer B, Meyer N, Carr T. The Memphis preterm birth project: prediction and prevention of preterm birth in extremely high risk women [abstract] American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S86. [Google Scholar]
- Collaborative Group 1993.Collaborative Multicenter randomized, controlled trial of a preterm birth prevention program. American Journal of Obstetrics and Gynecology. 1993;169:352–66. doi: 10.1016/0002-9378(93)90087-y. [DOI] [PubMed] [Google Scholar]; Creasy RK. United States multicenter preterm birth prevention program. Prevention of Preterm Birth. 1986;138:187–204. [Google Scholar]
- Goldenberg 1990.Goldenberg RL, Davis RO, Copper RL, Corliss DK, Andrews JB, Carpenter AH. The Alabama Preterm Birth Prevention Project. Obstetrics & Gynecology. 1990;75:933–9. [PubMed] [Google Scholar]
- Pittsburgh 1989.Mueller-Heubach E. Results of a three-year prospective controlled randomized trial of preterm birth prevention at the University of Pittsburgh. Advances in the Prevention of Low Birthweight: an International Symposium; Cape Cod, Massachusetts, USA. 1988 May 8-11.1988. pp. 61–6. [Google Scholar]; Mueller-Heubach E, Reddick D, Barnett B, Bente R. Preterm birth prevention: evaluation of a prospective controlled randomized trial. American Journal of Obstetrics and Gynecology. 1989;160:1172–8. doi: 10.1016/0002-9378(89)90183-x. [DOI] [PubMed] [Google Scholar]
References
Additional references
- Alexander 2010.Alexander S, Boulvain M, Ceysens G, Haelterman E, Zhang WH. Repeat digital cervical assessment in pregnancy for identifying women at risk of preterm labour. Cochrane Database of Systematic Reviews. 2010;(Issue 6) doi: 10.1002/14651858.CD005940.pub2. [DOI: 10.1002/14651858.CD005940.pub2] [DOI] [PubMed] [Google Scholar]
- Althuisius 1998.Althuisius S, Dekker G, Hummel P, Bekedam D, Kuik D, Van Giejn HP. Cervical incompetence prevention randomisation cerclage trial (CIPRACT); effect of therapeutic cerclage with bed rest v bed rest only on cervical length. Ultrasound in Obstetrics & Gynecology. 1998;12:312–7. doi: 10.1046/j.1469-0705.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- Barros 2010.Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens CE, GAPPS Review Group Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S3. doi: 10.1186/1471-2393-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienstock 2001.Bienstock JL, Ural SH, Blakemore K, Pressman EK. University hospital-based prenatal care decreases rate of preterm delivery and costs, when compared to managed care. Journal of Maternal-Fetal Medicine. 2001;10(2):127–30. doi: 10.1080/714904309. [DOI] [PubMed] [Google Scholar]
- Bodribb 2010.Brodribb W. Barriers to translating evidence-based breastfeeding information into practice. Acta Paediatrica. 2010;100(4):486–90. doi: 10.1111/j.1651-2227.2010.02108.x. [DOI: 10.1111/j.1651-2227.2010.02108.x] [DOI] [PubMed] [Google Scholar]
- Creasy 1980.Creasy RK, Gummer BA, Liggins GC. System for predicting spontaneous preterm birth. Obstetrics & Gynecology. 1980;55:692–5. [PubMed] [Google Scholar]
- Da Fonesca 2003.Da Fonesca EB, Bittar RE, Carvalho MHB, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomised placebo-controlled double blind study. American Journal of Obstetrics and Gynecology. 2003;18(4):419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- Dodd 2007.Dodd JM, Crowther CA. Specialised antenatal clinics for women with a multiple pregnancy to improve maternal and infant outcomes. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD005300.pub2. [DOI: 10.1002/14651858.CD005300.pub2] [DOI] [PubMed] [Google Scholar]
- Egger 1997.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bastawissi 1999.El-Bastawissi AY, Becker TM, Daling JR. Effect of cervical carcinoma in situ and its management on pregnancy outcome. Obstetrics & Gynecology. 1999;93:207–12. doi: 10.1016/s0029-7844(98)00386-x. [DOI] [PubMed] [Google Scholar]
- Hack 1999.Hack M. Consideration of the use of health status, functional outcome, and quality-of-life to monitor neonatal intensive care practice. Pediatrics. 1999;103(1 Suppl E):319–28. [PubMed] [Google Scholar]
- Harbord 2006.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Higgins 2011.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Hoffman 1984.Hoffman HJ, Bakketeig lS. Risk factors associated with the occurrence of preterm birth. Clinical Obstetrics and Gynecology. 1984;27(3):539–52. doi: 10.1097/00003081-198409000-00004. [DOI] [PubMed] [Google Scholar]
- Hofmeyr 2010.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews. 2010;(Issue 8) doi: 10.1002/14651858.CD001059.pub3. [DOI: 10.1002/14651858.CD001059.pub3] [DOI] [PubMed] [Google Scholar]
- Honest 2009.Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technology Assessment. 2009;13(43):1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- Iams 2004.Iams J. Maternal-Fetal Medicine. 5th Edition Saunders; Philadelphia, US: 2004. Cervical incompetence. [Google Scholar]
- Iams 2010.Iams JD, Berghella V. Care for women with prior preterm birth. American Journal of Obstetrics and Gynecology. 2010;203(2):89–100. doi: 10.1016/j.ajog.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson 2006.Jackson CJ, Bosio P, Habiba M, Waugh J, Kamal P, Dixon-Woods M. Referral and attendance at a specialist antenatal clinic: qualitative study of women’s views. BJOG: An International Journal of Obstetrics and Gynaecology. 2006;113:909–13. doi: 10.1111/j.1471-0528.2006.01016.x. [DOI] [PubMed] [Google Scholar]
- Kenyon 2008.Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372(9646):1319–27. doi: 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- Kramer 2000.Kramer M, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;284:843–9. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- Lumley 2003.Lumley J. Defining the problem: epidemiology of preterm birth. BJOG: an international journal of obstetrics and gynaecology. 2003;110(Suppl 20):3–7. [PubMed] [Google Scholar]
- Petrou 2001.Petrou S, Sach T, Davidson L. The long-term costs of preterm birth and low birth weight: results of a systematic review. Child Care, Health and Development. 2001;27:97–115. doi: 10.1046/j.1365-2214.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- Piaggio 2001.Piaggio G, Carroli G, Villar J, Pinol A, Bakketeig L, Lumbiganon P, et al. Methodological considerations on the design and analysis of an equivalence stratified cluster randomization trial. Statistics in Medicine. 2001;20:401–16. doi: 10.1002/1097-0258(20010215)20:3<401::aid-sim801>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- RevMan 2011.The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan) 5.1 The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Roberts 2006.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD004454.pub2. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
- Rode 2009.Rode L, Langhoff-Roos J, Andersson C, Dinesen J, Hammerum MS, Mohapeloa H, et al. Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies. Acta Obstetricia et Gynecologica Scandinavica. 2009;88(11):1180–9. doi: 10.3109/00016340903280982. [DOI] [PubMed] [Google Scholar]
- Ruis 2003.Ruis RJ, Fullerton J, Dudley DJ. The interrelationships of maternal stress, endocrine factors and inflammation on gestational length. Obstetrical & Gynecological Survey. 2003;58:415–28. doi: 10.1097/01.OGX.0000071160.26072.DE. [DOI] [PubMed] [Google Scholar]
- Ugwumadu 2003.Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet. 2003;361:983–8. doi: 10.1016/S0140-6736(03)12823-1. [DOI] [PubMed] [Google Scholar]


