Abstract
Flavonoids are important natural compounds with diverse biologic activities. Citrus flavonoids constitute an important series of flavonoids. Naringin and its aglycone naringenin belong to this series of flavonoids and were found to display strong anti-inflammatory and antioxidant activities. Several lines of investigation suggest that naringin supplementation is beneficial for the treatment of obesity, diabetes, hypertension, and metabolic syndrome. A number of molecular mechanisms underlying its beneficial activities have been elucidated. However, their effect on obesity and metabolic disorder remains to be fully established. Moreover, the therapeutic uses of these flavonoids are significantly limited by the lack of adequate clinical evidence. This review aims to explore the biologic activities of these compounds, particularly on lipid metabolism in obesity, oxidative stress, and inflammation in context of metabolic syndrome.
Introduction
Epidemiologic evidence implicates Western-style dietary patterns as 1 of the contributing factors to the development of cardiovascular diseases, dyslipidemia, and diabetes (1, 2). Recent evidence also suggests that a high-fat diet is responsible for the development of metabolic syndrome both in animals (3) and in humans (4, 5). Metabolic syndrome is a cluster of diseases, including hypertension, dyslipidemia, insulin-resistant diabetes, and central (visceral) obesity (6). Pharmacologic agents have been successfully used for the treatment of major risk factors, including hypertension (the angiotensin-converting enzyme inhibitor rimipril, the angiotensin receptor blocker candesertine, the calcium channel blocker amlodipine, etc.), plasma cholesterol (statins), and hyperglycemia (metformin, glybenclamide etc). However, side effects are prominent with those agents, such as cough, dizziness, headaches, flushing sensation, palpitation, angioedema, liver dysfunction, and myositis (7–9). Thus, there is a growing interest in dietary bioactive compounds that protect against or mitigate the severity of chronic diseases without the undesirable side effects. High amounts of fruit and vegetable consumption would be beneficial for the treatment of chronic diseases such as metabolic syndrome (10, 11). Plant-based dietary nutrients such as polyphenolic compounds (e.g., flavonoids, anthocyanines, and phenolic acids) were demonstrated to have potential health benefits for the treatment of obesity, hypertension, cardiovascular diseases, and metabolic syndrome. Among those bioactive compounds, flavonoids constitute a large proportion (12). Citrus plants are a good source of flavonoids. Naringin, naringenin, nobelitin, narirutin, and hesperidin are the most important flavonoids thus far isolated from citrus fruits (13). These flavonoids were found to possess strong antioxidant and anti-inflammatory activities both in vitro and in vivo (13). This review focuses on the biologic activities and molecular mechanisms of naringin and naringenin in the context of obesity and metabolic syndrome. Moreover, the role of citrus flavonoids on human health is still elusive due to lack of clinical evidence. Thus, most of the biologic activities reported thus far originate from animal or in vitro studies.
Citrus Fruit Extracts and Their Beneficial Effect on Metabolic Diseases
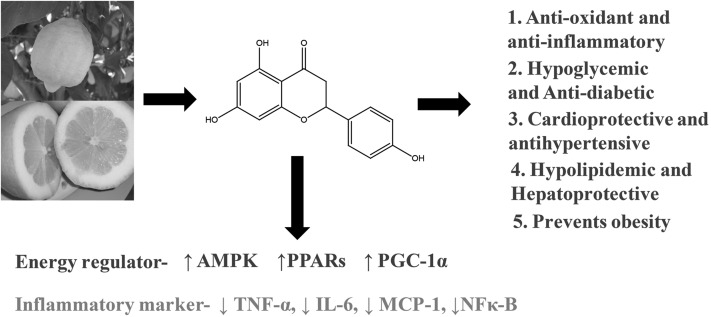
Citrus fruits such as oranges, mandarins, grapefruit, and acid citrus fruits, namely lemons, bergamots, and limes, are notably rich in flavonoid content and possess various bioactivities. Citrus fruit extract also influences the bioavailability of certain drugs by inhibiting cytochrome enzymes or the uptake process of drugs from the gut (14–16). Organic anion transporting polypeptides (OATPs)8 constitute an important family of sodium-independent transport proteins. Grapefruit juice and naringin were found to inhibit organic anion-transporting polypeptide 1A2 (OATP1A2)-mediated fexofenadine uptake and reduce the oral bioavailability of the drug (17, 18).
Many of the bioactivities of citrus flavonoids appear to affect vascular endothelial cells. Bergamot (Citrus bergamia Risso), a less commercialized citrus fruit extract, showed potential antioxidant activity in human umbilical vein endothelial cells, and inhibited the activation of NF-κB (19). Water extracts of sweet orange peel prevented the tert-butyl hydroperoxide–induced cytotoxic effect of HepG2 cells at 50- to 500-μg/mL doses and inhibited TBARS generation, increased mitochondrial membrane potential and Bcl-2:Bax ratio, and decreased caspase-3 activation (20). Citrus fruit extracts were also observed to offer potential health benefit in diabetes and obesity. Mandarin fruit extracts (1% or 3% of the diet) improved the metabolic function of liver and restored the antioxidant enzymes in streptozotocin-induced diabetic rats (21). Citrus unshiu extract (1% or 3% of the diet) administration for 10 wk in type 2 diabetic Goto-Kakizaki rats improved glucose tolerance (22). Insulin resistance in type 2 diabetes may also lead to hepatic steatosis accompanied by progressive inflammation of the liver. The antihyperglycemic effect of Citrus unshiu peel extract (2 g/100 g diet) supplementation in male C57BL/KsJ-db/db mice appeared to be partially mediated through the inhibition of hepatic gluconeogenic phosphoenolpyruvate carboxykinase mRNA expression and its activity and through the induction of insulin/glucagon secretion (23). Mice supplemented with Citrus unshiu peel extract also displayed a significant decrease in body weight gain and body fat mass (23). Citrus unshiu extract also ameliorated hepatic steatosis and hypertriglyceridemia via the inhibition of gene expression and increased activation of lipogenic enzymes and FA oxidation in the liver (23). These beneficial effects of Citrus unshiu might be related to increased concentrations of anti-inflammatory adiponectin and IL-10, and decreased concentrations of proinflammatory markers [IL-6, monocyte chemotactic protein 1 (MCP-1), IFN-γ, and TNF-α] in the plasma or liver (23). Similarly, Citrus sunki extract at a dose of 150 mg · kg−1 · d−1 reduced body weight gain, adipose tissue weight, serum total cholesterol, and TG and serum concentrations of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) in high-fat-diet–fed mice and prevented liver steatosis (24). Hepatic protection and the lipid-lowering effect of dietary supplementation of Citrus sunki extracts are mediated via upregulation of phosphorylation levels of AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC), which are related to FA β-oxidation (24). Citrus fruit extracts also inhibited the advanced glycation end product (AGE)– and H2O2-induced oxidative stress in human adipocytes (25). In a randomized clinical study, fresh grapefruit extracts reduced body weight and improved insulin resistance in obese patients (26). FFA release was used as an indicator of human adipocyte lipolysis. Citrus fruit extract at a dose 1.4 g/d increased human adipocyte lipolysis, probably by inhibiting cAMP-phosphodiesterase (cAMP-PDE) (27) or by enhancing phosphorylation of cAMP-dependent protein kinase A (PKA) and hormone-sensitive lipase (HSL) in mature 3T3-L1 adipocytes (24). These overall beneficial effects are summarized in Fig. 1.
FIGURE 1.
Effect of citrus fruits on various pathologic conditions in human health. MCP-1, monocyte chemotactic protein 1; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α.
Flavonoid Overview and Naringin
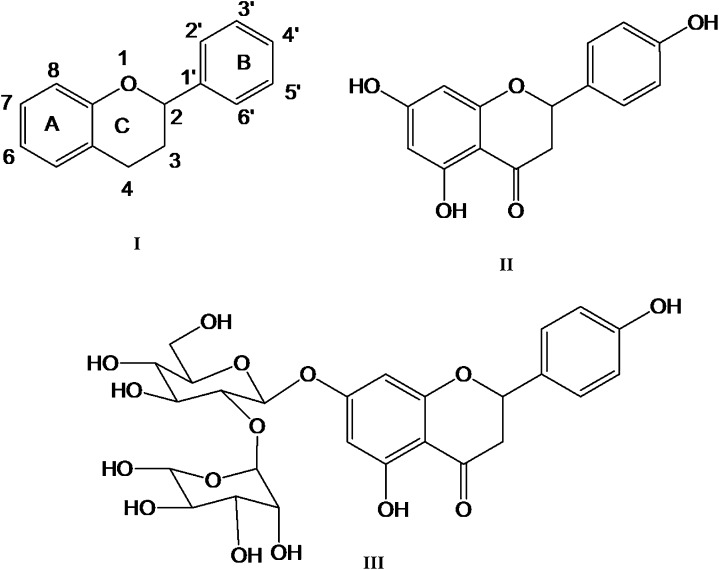
Flavonoids are naturally occurring phenolic compounds with a diverse range of bioactivities. Approximately 4000 flavonoids have so far been discovered, mainly from fruits, vegetables, and herbs (28). The basic flavonoid structure consists of 15 carbon atoms, and 3 rings of which 2 are benzene rings connected with a 3-carbon chain (29). Figure 2 shows the basic structure of flavonoids in which the A ring originates from resorcinol or phloroglucinol synthesized from the acetate pathway and has a characteristic hydroxylation pattern at the 5 and 7 position (29), whereas the B ring originates from the shikimate pathways (29). Several types of flavonoids are found in plants, such as flavones, flavanones, flavonols, isoflavones, anthocyanidins, and flavanols (30). These flavonoids are good scavengers of free radicals and prevent oxidative stress in vivo (29, 31).
FIGURE 2.
Basic structure of flavonoids (I), naringin (II), and naringenin (III).
Naringin (with the molecular formula C27H32O14 and a molecular weight of 580.4 g/mol) is a flavanone glycoside found in grapes and citrus fruits (Fig. 1). It possesses the distinct bitter taste of grapefruit juice. Two rhamnose units are attached to its aglycon portion, naringenin, at the 7-carbon position. Naringin contents in various citrus species are summarized in Table 1. Both naringin and naringenin are strong antioxidants (32, 33); however, naringin is less potent compared with naringenin because the sugar moiety in the former causes steric hindrance of the scavenging group. Naringin is moderately soluble in water. The gut microflora breaks down naringin to its aglycon naringenin in the intestine; it is then absorbed from the gut (34).
TABLE 1.
Various citrus species and naringin concentrations found in juice
| Naringin content | Reference | |
| μg/mL | ||
| Citrus (C.) sinensis | 21.3 | (44) |
| C. aurantium | 19.7 | (45) |
| C. reticulata | 3383.6 | (46) |
| C. clementina | 8.0 | (47) |
| C. bergamia | 22.3 | (45) |
| C. paradisi | 230.0 | (45) |
| Phenol Explorer database | (48, 49) | |
| Pure juice (naringenin) | 15.6 | |
| Fruit juices/citrus juices (naringin) | ||
| Orange (blond), pure juice | 7.0 | |
| Grapefruit, juice from concentrate | 37.8 | |
| Pummelo, pure juice | 84.8 | |
| Grapefruit, pure juice | 30.8 | |
| Grapefruit/pummelo hybrid, pure juice | 45.1 | |
| USDA polyphenol database | (50) | |
| Grapefruit, raw (color not specified) C. paradise (naringenin) | 53.0 | |
| Grapefruit, raw, pink and red, all areas (C. paradisi) (naringenin) | 32.6 | |
| Grapefruit, raw, white, all areas (C. paradisi) (naringenin) | 21.3 | |
| Grapefruit juice, white, raw (naringenin) | 18.2 | |
| Grapefruit juice, white, canned, unsweetened (naringenin) | 18.0 | |
| Grapefruit juice concentrate, white, frozen, unsweetened, diluted with 3 volumes of water (naringenin) | 31.2 |
Although the average daily human intake of naringin or flavonoids is not known, the total intake of polyphenols was suggested as ∼1 g/d (35). However, this value is higher than found in some recent findings. Recent evidence suggests that the total daily intake of flavonoids varies among nations and cultures. The consumption of total flavonoids ranges from ∼20 mg/d (United States, Denmark, Finland) to >70 mg/d (The Netherlands) (36). Chun et al. (37) estimated flavonoid intake by combining the USDA flavonoids database and 24-h dietary recall in NHANES 1999–2002 data. The daily mean intake of flavonoids was found from tea (157 mg), citrus fruit juices (8 mg), wine (4 mg), and citrus fruits (3 mg). Another study, conducted in the Australian population, estimated a flavonoid intake of 128 mg/d from 15 flavonoids except for isoflavones (38). The ambiguity of a daily intake value for flavonoids is mainly due to the lack of comprehensive food composition data for ≥1 flavonoid subclasses (36).
There are also very few studies performed with a detailed analysis of the pharmacokinetics of naringin and naringenin. Most of the work reported was mainly conducted in rat models. Naringenin is rapidly metabolized in the liver and converted into glucuronide intermediates (14, 39, 40). This liver metabolism may limit the bioavailability of naringin in plasma in vivo. It was also reported that a single dose of naringenin and naringin, administered to rats via an i.v. bolus and oral route, rapidly underwent conjugation (glucuronoids and sulfates). The serum concentrations of naringenin and naringin sulfates and glucuronides were found almost exclusively in the bloodstream (41). Moreover, the concentration of naringenin sulfates was much higher than that of naringenin glucuronides. A number of other studies also reported the presence of naringenin in urine (14, 39, 40, 42, 43) and plasma (39, 42) after an oral dose of naringin or grapefruit juice.
Effect of Naringin on Obesity
Obesity can be defined as increased energy intake compared with energy expenditure, which ultimately results in fat deposition and weight gain. According to the guidelines from the WHO, overweight in adults is defined as a BMI of 25.0–29.9 kg/m2, and obesity is defined as a BMI of ≥30.0 kg/m2 (51). High body fat also increases the risk of several diseases such as diabetes, hyperlipidemia, and hypertension, which leads to arteriosclerotic disease and metabolic syndrome (52). Body weight gain, fat accumulation, and the development of hyperlipidemia, hyperglycemia, and insulin resistance were significantly suppressed by lemon polyphenols in mice fed a high-fat diet (53). Lemon polyphenols suppressed diet-induced obesity by upregulation of mRNA levels of the enzymes involved in β-oxidation, such as peroxisome proliferator activated receptor (PPAR) α, acyl-CoA oxidase, FA synthase in liver, and white adipose tissue in mice (53). Naringenin supplementation (0.003%, 0.006%, and 0.012% of the diet for 6 wk) lowered adiposity and TG contents in parametrial adipose tissue in rats (54). Naringenin-fed animals had a significant increase in PPARα, carnitine palmitoyltransferase 1 (CPT-1), and uncoupling protein 2 (UCP-2) expression in the liver, which might be responsible for the reduction in adiposity in rats (54).
Adipocyte differentiation is a key regulatory step in fat deposition in adipose tissues. Naringenin promoted gene expression and adiponectin protein secretion from 3T3-L1 adipocytes (55). Naringenin may be useful for ameliorating the inflammatory changes in obese adipose tissue. It was suggested that adipose tissue–derived MCP-1, which exhibits chemotactic properties in inflammatory cells, is the key factor for inducing macrophage infiltration into adipose tissue (56). MCP-1 from hypertrophic adipocytes in obese adipose tissue can also trigger macrophage infiltration into adipose tissue and subsequently activates macrophages to release inflammatory mediators such as TNF-α (57). Naringenin inhibited the production of TNF-α, MCP-1, and nitric oxide (NO) in a dose-dependent manner in RAW264 macrophages and coculture of 3T3-L1 adipocytes and RAW264 macrophages stimulated by LPS (58).
Effect of Naringin on Hyperlipidemia
Hyperlipidemia is a crucial symptom of obesity and related metabolic disorders. Plant flavonoids are capable of lowering increased plasma lipid concentrations (28, 59). Naringin supplementation lowered plasma lipids in experimental models of hyperlipidemia and obesity (Table 2). Naringin supplementation also lowered elevated plasma lipid concentrations in high-fat-diet–fed rats (60) and decreased plasma lipids and cholesterol in high-cholesterol-diet–fed rats (61). The cholesterol-lowering effect of naringin was observed in LDL receptor (LDLR) knockout mice (62). Hepatic 3-hydroxy-3-methyl CoA (HMG-CoA) reductase activity was significantly reduced in the naringin-supplemented (0.02 g/100 g) group, whereas cholesterol acyl transferase (ACAT) activity was unaffected in Ldlr knockout mice (62). A lipid-lowering effect of naringenin was also seen in male Long-Evans hooded rats. PPARα expression in the liver and the expression of CPT-1 and UCP-2, both of which are known to be regulated by PPARα, were markedly enhanced by naringenin supplementation (0.003%, 0.006%, and 0.012% of the diet for 6 wk) (54).
TABLE 2.
Effects of naringin and naringenin on hyperlipidemia, body weight gain, and adipose tissue in metabolic syndrome1
| Derivative and dose | Model | Experimental outcome | Reference |
| Naringin | |||
| 0.02 g/100 g | Cholesterol-fed LDLR-knockout mice | Hepatic HMG-CoA reductase activity was reduced | (62) |
| Increases the excretion of fecal sterol | |||
| 100 mg · kg−1 · d−1 | High-fat/high-carbohydrate–fed Wister rat | Decreased total cholesterol, TGs and NEFAs | (64) |
| Preserved hepatic mitochondrial respiration | |||
| 0.2 g/kg of diet | High-fat-diet–fed C57BL/6 mice | Inhibited the synthesis way and increased FA oxidation | (60) |
| Upregulated AMPK. | |||
| 0.003%, 0.006%, and 0.012% of diet for 6 wk | Male Long-Evans hooded rats | Reduced total TGs and cholesterol in plasma and liver | (54) |
| Increased expression of PPARα, CPT-1, and UCP-2. | |||
| 100 mg · kg−1 · d−1 | High-fat/high-carbohydrate–fed Wister rat | Lowered abdominal fat deposition | (64) |
| Body weight was not affected, probably due to increasing muscle mass | |||
| Naringenin | |||
| 1% or 3% wt:wt of diet | LDLR-null mice | Increased hepatic FA oxidation through a PGC1α/PPARα–mediated transcription program | (63) |
| Prevented SREBP-1c–mediated lipogenesis in both liver and muscle by reducing fasting hyperinsulinemia | |||
| 0.003%, 0.006%, and 0.012% of diet for 6 wk | Male Long-Evans hooded rats | Lowered adiposity and TG contents in parametrial adipose tissue | (54) |
AMPK, AMP kinase; CPT-1, carnitine palmitoyltransferase 1; HMG-CoA, 3-hydroxy-3-methyl coenzyme A; LDLR, LDL receptor; NEFA, nonesterified FA; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α SREBP-1c, sterol regulatory element–binding protein 1c; UCP-2, uncoupling protein 2.
A beneficial effect of naringin was observed in LDLR-null mice fed a high-fat diet (63). Mice lacking the LDLR, when fed a Western-style diet, displayed many features of insulin resistance, including VLDL overproduction, dyslipidemia, and obesity (63). In that study, naringenin (1% or 3% wt:wt of diet) prevented hyperinsulinemia, leading to a reduction in hepatic sterol regulatory element binding protein (SREBP) 1c and hepatic lipogenesis in the fasted state (63). The reduction in hepatic TG availability due to naringenin supplementation also contributed to VLDL-TG production and VLDL-apoB secretion and attenuated dyslipidemia (63). A recent investigation also showed that naringin supplementation in high-fat/high-carbohydrate-diet–fed obese rats ameliorated the increase in plasma cholesterol, TGs, and circulating FFAs (64). In a clinical trial, naringin supplementation (400 mg · capsule−1 · d−1) reduced plasma total- and LDL-cholesterol concentrations, whereas plasma TG and HDL-cholesterol concentrations remained unaffected in hypercholesterolemic patients (33).
Effect of Naringin on Hypertension
Naringin supplementation was found to improve hypertension in high-carbohydrate/high-fat-diet–fed obese rats (64) and stroke-prone hypertensive rats (65) (Table 3). Moreover, naringin significantly increased the production of NO metabolites in urine and improved the acetylcholine-mediated endothelium function using thoracic aortic ring preparations by NO production (65). A similar vasodilatation effect was also observed in high-carbohydrate/high-fat-diet–fed obese rats (64) and streptozotocin-induced diabetic rats (66). Calcium-dependent K channels are important regulators of vascular relaxation. Naringenin activated large conductance Ca2+-activated K+ currents in a concentration-dependent manner in rat tail artery myocytes (67).
TABLE 3.
Effect of naringin and naringenin on hypertension and cardiac function in animal and human studies1
| Derivative and dose | Model | Experimental outcome | Reference |
| Hypertension | |||
| Naringin | |||
| 100 mg · kg bw−1 · d−1 | High-fat/high-carbohydrate–fed Wister rats | Decreased blood pressure | (64) |
| Improved endothelial dysfunction, probably by increasing bioavailability of NO | |||
| 250, 500, and 1000 mg/kg diet | Stroke-prone hypertensive rats | Decreased blood pressure, probably by increasing bioavailability of NO | (65) |
| Naringenin | |||
| 10 mg · kg bw−1 · d−1 | Streptozotocin-induced diabetic rats | Endothelium-dependent relaxation to acetylcholine was significantly higher in naringenin-treated diabetic rats | (66) |
| — | VSMC proliferation and migration | Naringenin inhibited TNF-α–induced VSMC proliferation and migration in a dose-dependent manner | (68) |
| Prevented ERK/MAPK and Akt phosphorylation and left p38 MAPK and JNK unchanged | |||
| HO-1 is involved | |||
| Naringin (with narirutin) | |||
| 0.5 L/d (677 mg/L naringin) | In patients with stage I hypertension | Sweetie juice was shown to have a significant beneficial effect in reducing diastolic blood pressure | (69) |
| Cardiac function | |||
| Naringin | |||
| 100 mg · kg bw−1 · d−1 | High-fat/high-carbohydrate–fed Wister rats | Decreased inflammatory cell infiltration and fibrosis | (64) |
| Decreased left ventricular stiffness and improved echocardiographic variables such as fractional shortening, ejection fraction, left ventricular internal diameter in diastole, etc. | |||
| 10, 20, and 40 mg/kg bw, respectively | Isoproterenol-induced myocardial infarction in rats | Significantly decreased the concentrations of lipid peroxidative products and improved antioxidant status by increasing the activities of antioxidant enzymes and nonenzymatic antioxidants | (70) |
| 10 mg/kg bw | Doxorubicin-induced cardiotoxicity in mice | Prior exposure of mice to naringin before doxorubicin administration significantly reduced serum concentrations of AST, ALT, CK-MB, and LDH, indicating that naringin protected against doxorubicin-induced cardiotoxicity | (71) |
| 10, 20, and 40 mg/kg | Isoproterenol-induced myocardial infarction in Wistar rats | Restored the normal mitochondrial function. | (72) |
| Transmission electron microscopic observations confirmed the protection of mitochondrial architecture | |||
| Naringenin | |||
| 10−4–10−5 mol/L | H9c2 cardiomyocyte cells | Naringenin inhibited daunorubicin apoptosis of H9c2 cardiomyocytes cells in vitro | (73) |
Akt, protein kinase B; ALT, alanine transaminase; AST, aspartate transaminase; bw, body weight; CK-MB, creatine kinase-MB; ERK, extracellular signal–regulated kinase; HO-1, heme oxygenase 1; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; NO, nitric oxide; VSMC, vascular smooth muscle cell.
The proliferation and migration of vascular smooth muscle cells (VSMCs) are critical events in the pathogenesis of atherosclerosis and hypertension. Naringenin inhibited TNF-α–induced VSMC proliferation and migration in a dose-dependent manner (68). It also blocked the increased reactive oxygen species (ROS) generation induced by TNF-α. Oxidative stress and TNF-α may also trigger the activation of mitogen-activated protein kinases (MAPKs), which are the key regulatory factors for VSMC proliferation. Naringenin prevented extracellular signal–regulated kinase (ERK)/MAPK and Akt phosphorylation, whereas p38 MAPK and c-Jun N-terminal kinases (JNKs) were unchanged (68). This overall effect is probably mediated via the induction of heme oxygenase 1 (HO-1) and reduction in oxidative stress.
Effect of Naringin on Cardiac Toxicity, Hypertrophy, and Remodeling
The cardioprotective effects of flavonoids are well documented (Table 3) (28, 73, 74). Isoproterenol-induced myocardial infarction was prevented by naringin supplementation (40 mg · kg−1 · d−1) in rats (70). Naringin supplementation also reduced lipid peroxidation, improved antioxidant enzymes, and decreased inflammatory cell and fibrosis in hearts of isoproterenol-treated rats (70). Pretreatment with various doses of naringin inhibited the doxorubicin-induced decline in antioxidant status and reduced the concentrations of 8-OHdG and the activity of poly (ADP-ribose) polymerase (PARP) in heart and liver of mice (71). Oral pretreatment with naringin (10, 20, and 40 mg/kg) in isoproterenol-induced rats daily for a period of 56 d significantly (P < 0.05) minimized the alterations in mitochondrial tricarboxylic acid cycle enzymes (isocitrate dehydrogenase, succinate dehydrogenase, malate dehydrogenase, and α-ketoglutarate dehydrogenase) and respiratory chain enzymes (NADH dehydrogenase and cytochrome c oxidase) (72).
A high-fat diet induces cardiac remodeling and fibrosis in experimental animals. In the early stage of fibrosis, massive inflammatory cells, mainly macrophages, infiltrate into the left ventricle of heart (75). This fibrosis starts from the vascular region of the heart muscle and then disseminates across the left ventricle. Chamber dilation and ballooning have also been observed due to high-fat-diet feeding. Further echocardiographic data confirmed the left ventricular dysfunction and increased left ventricular mass in high-fat/high-carbohydrate-diet–induced obese rats (75). Naringin supplementation in these rats improved the inflammatory state and fibrosis, which ultimately reduced the left ventricular stiffness constant (64). Moreover, naringin supplementation improved many of the left ventricular functional variables, such as percentage of fractional shortening, and prevented the cardiac remodeling in hearts of these rats (64). A recent study showed that naringin (60 and 120 mg/kg) significantly reduced paraquat-induced pulmonary fibrosis by downregulating TNF-α, TGF-β1, matrix metalloproteinase 9 (MMP-9), and tissue inhibitor of metalloproteinase 1 (TIMP-1), which are the key regulators of fibrosis in tissues (76). The mechanism of naringin-induced cardiomyocyte protection has been revealed recently. Naringin (5 μmol/L) attenuated high-glucose–induced (16.7 mmol/L) p38 and p53 phosphorylation, decreased mitochondrial Bax and Bak expression, prevented the release of cytochrome c, and increased Bcl-2 expression in H9c2 cells (77). Moreover, it also prevented high-glucose–induced apoptosis in H9c2 cells followed by inactivation of caspase-3, -8, and -9 (77). In another study, naringin prevented cardiomyocytes due to high-glucose (35 mmol/L glucose) challenge followed by ROS-activated MAPK signal–mediated pathways (78). Naringin (80 μmol/L) prevented the high-glucose–induced phosphorylation of p38 MAPK, ERK1/2, and JNK and reduced apoptosis in H9c2 cardiac cells (78).
Effect of Naringin on Hyperglycemia and Diabetes
Hyperglycemia and insulin resistance are common features of metabolic syndrome. Insulin resistance can be defined as decreased response of the peripheral tissues to insulin action. Certain inflammatory cytokine such as TNF-α may cause insulin resistance in experimental models of obesity (85). Moreover, several studies showed that IL-6 and TNF-α concentrations were increased in individuals with insulin resistance and type 2 diabetes (86, 87). On the other hand, inflammatory cytokines such as TNF-α and IL-6 may also cause dysfunction of peripheral insulin receptor and insulin resistance and ultimately increase glucose concentrations in plasma (88, 89). A high-fat diet increased inflammatory cytokines and caused insulin resistance and hyperglycemia (90, 91). The hypoglycemic effect of naringin is well documented (Table 4) (80, 92). Naringin (30 mg/kg) and vitamin C (50 mg/kg) cotreatment ameliorated streptozotocin-induced diabetes in rats by improving insulin concentration and prevented oxidative stress (79). It also improved insulin concentration and pancreatic architecture in db/db mice at a supplementation dose of 0.2 g/kg of diet (80). Alteration of glucose-regulating enzyme activities also plays a crucial role in the glucose-lowering effect of naringin in experimental animals (80). Naringin markedly lowered the activity of hepatic glucose-6-phosphatase and phosphoenolpyruvate carboxy kinase in db/db mice compared with control mice (80). Recent investigations also suggested that the hypoglycemic activity of naringin is mediated via uptake of glucose in the skeletal muscle (84). Maximum stimulation was seen with 75 μmol/L naringenin for 2 h, which was comparable to maximum insulin response in L6 myotubes (84). Increased glucose uptake is mediated via upregulation of AMPK in skeletal muscle cells (84). AMPK-activated improvement in metabolic syndrome was also reported by Pu et al. (60). In this study, naringin supplementation (0.2 g/kg of diet) improved glucose intolerance and insulin resistance in a model of high-fat-diet–fed mice (60).
TABLE 4.
Effect of naringin and naringenin on insulin resistance and diabetes in animal and cell studies1
| Derivative and dose | Model | Experimental outcome | Reference |
| Naringin | |||
| 15 and 30 mg/ kg bw | STZ-induced diabetic rats | Decreased activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase in liver and kidney | (79) |
| Increased activities of hexokinase | |||
| 0.2 g/kg diet | C57BL/KsJ-db/db mice | Hepatic glucokinase activity and glycogen concentration were both significantly elevated | (80) |
| Lowered the activity of hepatic glucose-6-phosphatase and phosphoenolpyruvate carboxykinase | |||
| 15 and 30 mg/ kg bw | STZ-induced diabetic rats | Increased activity of hexokinase and decreased the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase in liver and kidney | (79) |
| Normalized the concentrations of plasma TBARS, lipid hydroperoxides and vitamin E and reduced glutathione in diabetic rats | |||
| Naringenin | |||
| 50 mg · kg bw · d | Experimental STZ-nicotinamide–induced diabetes | Significantly lowered mean concentrations of fasting blood glucose and glycosylated hemoglobin and significantly elevated serum insulin concentrations | (81) |
| Lowered mean activities of ALT, AST, ALP, and LDH in serum | |||
| 50 mg · kg bw−1 · d−1 | Experimental STZ-nicotinamide–induced diabetes | Naringenin ameliorated hyperglycemia-mediated inflammation | (82) |
| 0.5%, 1%, and 2% of the diet | Diabetic mice | Naringenin treatments at 1% and 2% of diet lowered plasma concentrations of glucose and blood urea nitrogen | (83) |
| Increased insulin concentrations and creatinine clearance | |||
| Decreased production and expression of IL-1β, IL-6, and MCP-1 | |||
| 75 μmol/L | Skeletal muscle cells (L6 myotubes) | No significant effect on insulin-stimulated Akt phosphorylation and significantly increased AMPK phosphorylation/activation | (84) |
| Increased glucose uptake by skeletal muscle cells in an AMPK-dependent manner. |
Akt, protein kinase B; ALP, alkaline phosphatase; ALT, alanine transaminase; AMPK, AMP kinase; AST, aspertate transaminase; bw, body weight; LDH, lactate dehydrogenase; STZ, streptozotocin.
Effect of Naringin on Steatosis and Hepatic Protection
A hepatoprotective action of naringin was reported by several investigators (93–95). Naringin supplementation significantly lowered the elevated plasma transaminase activity in nickel (naringin: 80 mg/kg body weight) and cadmium (naringin: 50 mg/kg)–induced liver toxicity in rats (93, 94). In addition, naringenin significantly reduced lipid peroxidation and restored the levels of antioxidant defense [superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and glutathione S-transferase (GST)] in the liver (93, 94). Naringenin supplementation also restored serum albumin and total protein concentrations and reduced the hepatic concentration of malondialdehyde in dimethylnitrosamine-induced hepatotoxicity in rats (43). Furthermore, dimethylnitrosamine-induced collagen accumulation and α-smooth muscle cell accumulation were reduced by naringenin treatment (doses of 20 and 50 mg/kg) (43).
Fructose feeding is 1 cause of developing oxidative stress and nonalcoholic fatty liver disease (96). Naringin supplementation improved the oxidative and nitrosative stress in livers of fructose-fed rats (96). High-fat-diet feeding is another cause of developing liver steatosis and nonalcoholic fatty liver disease (97). Naringin supplementation (0.2 g/kg diet) reduced the high-fat-diet–induced liver steatosis in rats (60). The hepatoprotective nature of naringin in a high-fat-diet–fed rat model was partially mediated by activating the AMPK, which restored the antioxidant enzymes and prevented inflammation (60). LPS-induced TNF release followed by liver injury was investigated; naringin supplementation decreased release of TNF and improved liver injury (98). Moreover, in diabetic male Wistar albino rats fed a high-fat diet, naringin increased PPARγ expression in liver and decreased expression of liver X receptor (LXR), SREBP-1c, and SREBP-1a in hepatic steatosis (99). In our recent investigation, we also found that naringin prevented the increase in hepatic marker enzyme activities (AST, ALT, and ALP) and reduced the accumulation of lipid deposition and fibrosis in the liver of high-carbohydrate, high-fat-diet–fed obese rats (64). Naringin supplementation also improved the mitochondrial respiration in these rats, suggesting an improvement in mitochondrial compartment dysfunction and rapid energy expenditure by liver tissues (64).
Effect of Naringin on Atherosclerosis
The accumulation of cholesteryl ester (CE) within the arterial intima is an initiating factor in atherogenesis (100). Inflammation of the vessel wall, activation of the vascular endothelium, and increased adhesion of mononuclear cells to the injured endothelial layer are the early manifestations of atherosclerosis in metabolic syndrome. In response to this inflammatory state, LDL can enter into the arterial intima and is able to make plaque followed by foam cell formation (101). Citrus flavonoids including naringin showed an inhibitory action on LDL-cholesterol oxidation (102).
Proliferation and migration of VSMCs are initial events in the atherogenesis process (103). Vascular endothelial growth factor (VEGF) stimulates the proliferation of VSMCs and increases the expression of proinflammatory and prothrombotic molecules in atherosclerotic plaques (104). Naringin (500 mg · kg−1 · d−1) significantly reduced fatty streak formation and neointimal macrophage infiltration in vessel walls of cholesterol-fed rabbits (105). Antiatherogenic activity of naringin in hypercholesterolemic rabbits was induced by inhibiting intercellular adhesion molecule 1 (ICAM-1) expression in endothelial cells (105). Similar antiatherogenic effects of naringin and naringenin were found in high-cholesterol–fed rabbits, downregulating the expression levels of vascular cell adhesion molecule 1 (VCAM-1) and MCP-1 in aortas (106). A recent study showed the consistent antiatherogenic effect of naringin in wild-type mice fed a high-fat/high-cholesterol diet and in apoE-deficient mice fed a purified diet (107). Hepatic overproduction of apoB-containing lipoproteins is a characteristic feature of dyslipidemia associated with insulin resistance. In Ldlr−/− mice, the uptake of lipoprotein particles was impaired; thus, the mice became dyslipidemic and insulin resistant and developed atherosclerosis after being fed a high-fat Western-style diet (108). Naringenin inhibited apoB100 secretion by activating signaling cascades in HepG2 cells (109, 110). Moreover, naringenin supplementation (3% of diet, wt:wt) reduced the infiltration of MOMA-2–positive leukocytes in lesions and reduced extensive collagen deposition in plaques of Western diet–fed Ldlr−/− mice, suggesting antiatherogenic activity (111).
Effect of Naringin on Inflammation
Obesity, metabolic syndrome, and diabetes are strongly correlated with inflammatory response. Inflammation is a complex process. In obesity, inflammatory processes are characterized by infiltration of inflammatory cells (generally macrophages, mast cells) in inflamed organ/tissues, especially in adipose tissue (112, 113). Adipose tissue itself is considered an endocrine organ and secretes various adipocytokines such as TNF-α, IL-6, and leptin (114). Various reports also suggest that inflammatory cytokine concentrations are higher in individuals suffering metabolic syndrome and obesity (115–117). Among the cytokines released in obesity, TNF-α is the most common inflammatory cytokine found in plasma of obese individuals. TNF-α is responsible for insulin resistance (118) and β-cell damage in islets of the pancreas (119). TNF-α–mediated impairment of insulin signaling is probably due to activation of Ser/Thr kinases, which act on insulin receptor and insulin receptor substrate (IRS) molecules, making them poor substrates for insulin-mediated tyrosine phosphorylation (114).
Flavonoids are strong anti-inflammatory compounds (120). There is evidence that naringin shows anti-inflammatory activity in an air-pouch model of inflammation in which this flavonoid normalized the elevated TNF-α concentration and normalized inflammatory cell infiltration (121). Liver is known to be affected by the proinflammatory secretion of adipose tissue. Chronic activation of NF-κB by cytokines has been directly linked to the development of insulin resistance (122). LPS-induced TNF release followed by liver injury was investigated in rats. Naringin supplementation decreased TNF release and improved liver injury (98). Enhanced expressions of the cell adhesion molecules in human umbilical vein endothelial cells due to high glucose were significantly attenuated by pretreatment with naringin (10–50 μg/mL). In that study, naringin suppressed a high-glucose–induced increment of NF-κB expression (123, 124). Nuclear factor-erythroid 2–related factor 2 (Nrf2) mediated regulation of cellular antioxidant production, and the anti-inflammatory mechanism plays an important role against various degenerative diseases. Recent evidence suggests that naringin upregulates NAD(P)H:quinone oxidoreductase 1, HO-1, GST P1, and γ-glutamylcysteine ligase mRNA expression followed by activation of Nrf2 and decreased expression of proinflammatory mediators such as TNF-α, cyclooxygenase-2, and inducible NO synthase in 3-nitropropionic acid–induced rats (125).
Effect of Naringin on Oxidative Stress and Free Radical Damage
Citrus fruit extracts possess large amounts of flavonoids and show potent free radical scavenging activity (126). Naringin and naringenin both are strong scavengers of free radicals and prevent lipid peroxidation (127). Both superoxide and hydroxyl radicals are scavenged by these flavonoids in vitro (127). Xanthine oxidase enzymes are physiologic sources of superoxide anions in eukaryotic cells. Naringin was found to significantly inhibit the xanthine oxidase activity in vitro (128). It also showed strong antioxidant activity in vivo in different disease conditions. A protective effect of naringin was seen in diabetic rats; naringin supplementation improved antioxidant enzymes such as SOD, catalase, and GPx in diabetic animals (79, 92, 129). It also improved the antioxidant enzyme status in cholesterol-fed rabbits (129). TBARS concentrations were not altered in high-cholesterol–fed rabbits treated with naringin (129). Similar antioxidant effects were also observed in isoproterenol-induced cardiotoxicity in Wistar rats (70). Naringin supplementation reduced the lipid peroxidation product and hydroperoxides in the plasma and heart of isoproterenol-induced Wistar rats (70).
Clinical Findings for Citrus Fruit Juices and Their Active Component Naringin
Most beneficial effects found for citrus flavonoids were mainly based on animal and in vitro cell culture studies, which may be relevant in explaining mechanisms of the bioactive components present in citrus fruits. However, very limited clinical studies have been conducted on citrus flavonoids or various citrus fruit juices in relation to possible cardiovascular and obesity benefits. In 1 study, bergamot extract given orally for 30 d to diet-induced hyperlipemic Wistar rats and in 237 patients suffering from hyperlipemia that was either associated or not associated with hyperglycemia (130). Bergamot extract reduced concentrations of total and LDL cholesterol (an effect accompanied by elevation of cholesterol bound to high density lipoprotein) and TGs and significantly decreased blood glucose concentration (130). Moreover, bergamot extract inhibited HMG-CoA reductase activity and enhanced reactive vasodilation in hyperlipidemia patients (130). Another randomized controlled trial was conducted to evaluate the role of grapefruit in reducing body weight and blood pressure and in promoting improvements in lipid profile in 74 overweight healthy adults (131). Supplementation of one-half of a fresh Rio Red grapefruit with each meal for 6 wk did not significantly decrease body weight compared with the control condition (131). However, supplementation improved blood pressure and lipids, which warrants further studies conducted on grapefruit in the context of obesity and cardiovascular disease prevention. A similar beneficial effect on lipid variables was also observed in a study that showed that long-term orange juice consumption (480 mL of orange juice/d for at least 12 mo) lowered concentrations of total cholesterol, LDL cholesterol, and apoB and the LDL/HDL ratio in comparison with the nonconsumer counterparts (132). However, the percentage of abdominal obesity among orange juice consumers did not differ from that of nonconsumers (132). In addition, a previous study showed a positive effect of orange juice on HDL-cholesterol concentrations with the consumption of 750 mL of orange juice/d (133). Most of these observed benefits were linked to high amounts of vitamin C and folate present in orange juice. However, the amount of polyphenols, flavonoids, or naringin concentration, cannot be determined from these reports. There is also a significant lack of information regarding clinical studies with pure naringin or naringenin. One clinical study found that naringin treatment (400 mg · capsule−1 · d−1 for 8 wk) lowered plasma total cholesterol by 14% and LDL cholesterol by 17%, whereas plasma TG and HDL-cholesterol concentrations remained unaffected (33). Moreover, naringin significantly increased erythrocyte SOD and catalase activities in the hypercholesterolemic group, whereas GPx activity and plasma TBARS concentrations were not different from baseline measurements (33). Recently, the safety of bitter orange (Citrus aurantium) consumption was assessed in a 60-d double-blind, placebo-controlled trial (134). p-Synephrine, which is the primary protoalkaloid in bitter orange, given alone or in combination with naringin and hesperidin twice daily to 25 healthy subjects per group showed no significant changes in systolic or diastolic blood pressures, blood chemistries, or blood cell counts in the control or p-synephrine–treated groups (134).
Summary and Future Directions
Vitamin C and vitamin E appear to be the most effective antioxidants in biologic systems and prevent oxidative damage (135, 136). Several large trials on supplementary antioxidant vitamin therapy failed to produce clinically relevant beneficial effects. The Heart Outcomes Prevention Evaluation–The Ongoing Outcomes (HOPE-TOO) trial reported that patients with vascular disease or diabetes prescribed 400 IU vitamin E showed a higher risk of heart failure and an increased number of hospitalizations (137). Similarly, in the GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico)-Prevenzione study, vitamin E treatment was associated with a 50% increase in the development of heart failure (138). Vitamin E has been suggested as a pro-oxidant in the absence of coantioxidants and failed to augment antioxidant defenses in vivo (139). Moreover, vitamin E has no effect on certain ROS, such as hypochlorite-induced oxidation (139). Another study was conducted to evaluate the effect of long-term vitamin E or vitamin C supplementation on major risk of cardiovascular events among men. In this randomized, double-blind, placebo-controlled factorial trial, vitamin E or vitamin C supplementation did not reduce the risk of major cardiovascular events (140). These frustrating outcomes of vitamin E or vitamin C studies open up new doors for studying other bioactive flavonoid molecules for the prevention of cancer, diabetes, obesity, and cardiovascular diseases.
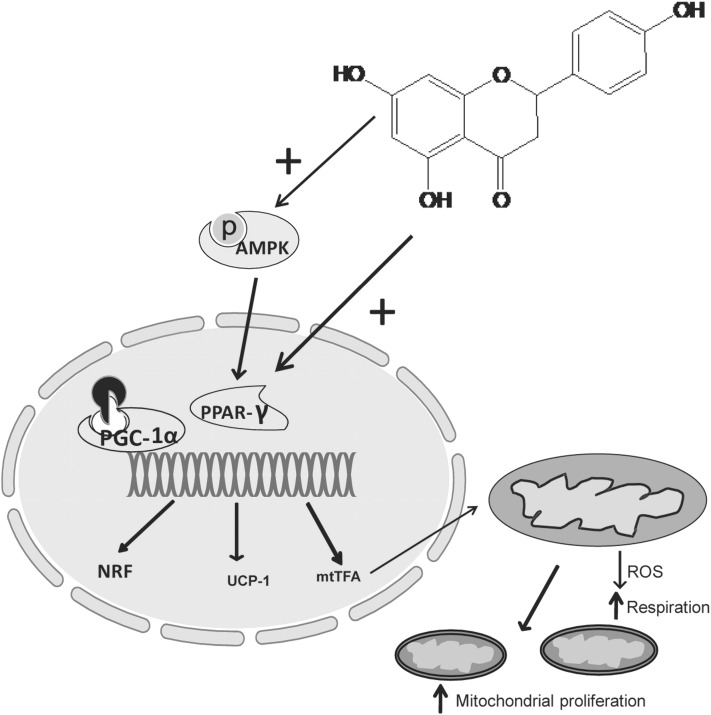
Naringin and naringenin supplementation has proven to be efficacious for the treatment of metabolic syndrome and obesity in animal models (60, 111). The dose used in animal studies may not be achieved in human trials, however, and emphasis should be given to establish a dietary recommendation of citrus fruit consumption or for the pure compound naringin. Most of naringin’s observed biologic activities are related in some part to its potent antioxidant nature. However, several other molecular signaling pathways modified by naringin were also revealed recently. In metabolic syndrome, obesity, and related cardiovascular complications, naringin influences AMPK-, PPARα–, and CPT-1–mediated fat utilization and preserves mitochondrial function. Moreover, naringin also prevents the TNF-α–mediated inflammatory process and tissue damage in liver and vasculature. Figure 3 shows a proposed mechanism for increased mitochondrial biogenesis by naringin. Another consideration for oral administration of naringenin/naringin should be the contraindications of taking certain drugs such as statins and calcium channel blockers because of the inhibitory effects on intestinal CYP450 enzymes. Moreover, further clinical investigations should be carried out with naringin in metabolic diseases.
FIGURE 3.
Transcriptional regulation of fat metabolism and mitochondrial biogenesis via AMPK-PGC-1α–mediated pathway. PGC-1α, in combination with the nuclear hormone receptor PPAR-γ, promotes the expression of NRF, UCP-1, mtTFA, and components of the respiratory chain complex. mtTFA translocates to mitochondria where it regulates the expression of mitochondrial genes and mitochondrial DNA replication. AMPK, AMP-activated protein kinase; mtTFA, mitochondrial transcription factor A; NRF, nuclear respiratory; factor; pAMPK, phosphorylated AMPK; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α ROS, reactive oxygen species; UCP-1, uncoupling protein 1.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACAT, cholesterol acyl transferase; ACC, acetyl-CoA carboxylase; AGE, advanced glycation end product; Akt, protein kinase B; ALP, alkaline phosphatase; ALT, alanine transaminase; AMPK, AMP-activated protein kinase; AST, aspertate transaminase; Bak, B-cell lymphoma 2; Bax, B-cell lymphoma 2 associated X protein; Bcl-2, Bcl-2 antagonist; CE, cholesteryl ester; CPT-1, carnitine palmitoyltransferase I; ERK, extracellular signal–regulated kinase; GPx, glutathione peroxidase; GST, glutathione S-transferase; HMG-CoA, 3-hydroxy-3-methyl CoA; HO-1, heme oxygenase 1; HSL, hormone-sensitive lipase; ICAM-1, intercellular adhesion molecule 1; IRS, insulin receptor substrate; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase, LDLR, LDL receptor; LXR, liver X receptor; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein 1; MMP-9, matrix metalloproteinase 9; MOMA-2, monocyte/macrophage marker antibody-2; mtTFA, mitochondrial transcription factor A; NEFA, nonesterified fatty acid; NO, nitric oxide; Nrf2, nuclear factor-erythroid 2–related factor 2; OATP, organic anion transporting polypeptide; PARP, poly (ADP-ribose) polymerase; PDE, phosphodiesterase; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α PKA, protein kinase A; ROS, reactive oxygen species; SOD, superoxide dismutase; SREBP, sterol regulatory element binding protein; TIMP-1, tissue inhibitor of metalloproteinase 1; UCP-2, uncoupling protein 2; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
References
- 1.Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Meigs JB, Jacques PF. The 2005 Dietary Guidelines for Americans and risk of the metabolic syndrome. Am J Clin Nutr 2007;86:1193–201 [DOI] [PubMed] [Google Scholar]
- 2.Kesse-Guyot E, Fezeu L, Galan P, Hercberg S, Czernichow S, Castetbon K. Adherence to French nutritional guidelines is associated with lower risk of metabolic syndrome. J Nutr 2011;141:1134–9 [DOI] [PubMed] [Google Scholar]
- 3.Yang Z-H, Miyahara H, Takeo J, Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr 2012;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce KD, Hanson MA. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr 2010;140:648–52 [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7 [DOI] [PubMed] [Google Scholar]
- 6.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23:469–80 [DOI] [PubMed] [Google Scholar]
- 7.Russell RP. Side effects of calcium channel blockers. Hypertension 1988;11:II42–4 [DOI] [PubMed] [Google Scholar]
- 8.Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet 2000;355:637–45 [DOI] [PubMed] [Google Scholar]
- 9.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. Hypertension 1995;26:869–79 [DOI] [PubMed] [Google Scholar]
- 10.Estaquio C, Castetbon K, Kesse-Guyot E, Bertrais S, Deschamps V, Dauchet L, Péneau S, Galan P, Hercberg S. The French National Nutrition and Health Program Score is associated with nutritional status and risk of major chronic diseases. J Nutr 2008;138:946–53 [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Serdula M, Janket S-J, Cook NR, Sesso HD, Willett WC, Manson JE, Buring JE. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 2004;27:2993–6 [DOI] [PubMed] [Google Scholar]
- 12.Martin K, Appel C. Polyphenols as dietary supplements: A doubleedged sword. Nutrition and Dietary Supplements 2010, 2:1–12 [Google Scholar]
- 13.Tripoli E, Guardia ML, Giammanco S, Majo DD, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem 2007;104:466–79 [Google Scholar]
- 14.Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther 1995;58:365–73 [DOI] [PubMed] [Google Scholar]
- 15.Ameer B, Weintraub RA. Drug interactions with grapefruit juice. Clin Pharmacokinet 1997;33:103–21 [DOI] [PubMed] [Google Scholar]
- 16.Bailey DG. Fruit juice inhibition of uptake transport: a new type of food–drug interaction. Br J Clin Pharmacol 2010;70:645–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther 2007;81:495–502 [DOI] [PubMed] [Google Scholar]
- 18.Dresser GK, Bailey D, Leake B, Schwarz U, Dawson P, Freeman D, Kim R. Fruit juices inhibit organic anion transporting polypeptide–mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther 2002;71:11–20 [DOI] [PubMed] [Google Scholar]
- 19.Trombetta D, Cimino F, Cristani M, Mandalari G, Saija A, Ginestra G, Speciale A, Chirafisi J, Bisignano G, Waldron K, et al. In vitro protective effects of two extracts from bergamot peels on human endothelial cells exposed to tumor necrosis factor-α (TNF-α). J Agric Food Chem 2010;58:8430–6 [DOI] [PubMed] [Google Scholar]
- 20.Chen Z-T, Chu H-L, Chyau C-C, Chu C-C, Duh P-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem 2012;135:2119–27 [DOI] [PubMed] [Google Scholar]
- 21.Sugiura M, Ohshima M, Ogawa K, Yano M. Chronic administration of Satsuma mandarin fruit (Citrus unshiu Marc.) improves oxidative stress in streptozotocin-induced diabetic rat liver. Biol Pharm Bull 2006;29:588–91 [DOI] [PubMed] [Google Scholar]
- 22.Sugiura M, Ogawa K, Yano M. Effect of chronic administration of fruit extract (Citrus unshiu Marc.) on glucose tolerance in GK rats, a model of type 2 diabetes. Biosci Biotechnol Biochem 2006;70:293–5 [DOI] [PubMed] [Google Scholar]
- 23.Park H-J, Jung UJ, Cho S-J, Jung H-K, Shim S, Choi M-S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J Nutr Biochem 2013;24:419–27 [DOI] [PubMed] [Google Scholar]
- 24.Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Kim MH, Ko HC, Kim SJ. Immature Citrus sunki peel extract exhibits antiobesity effects by beta-oxidation and lipolysis in high-fat diet-induced obese mice. Biol Pharm Bull 2012;35:223–30 [DOI] [PubMed] [Google Scholar]
- 25.Ramful D, Tarnus E, Rondeau P, Robert Da Silva C, Bahorun T, Bourdon E. Citrus fruit extracts reduce advanced glycation end products (AGEs) and H2O2-induced oxidative stress in human adipocytes. J Agric Food Chem 2010 [DOI] [PubMed] [Google Scholar]
- 26.Fujioka K, Greenway F, Sheard J, Ying Y. The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J Med Food 2006;9:49–54 [DOI] [PubMed] [Google Scholar]
- 27.Dallas C, Gerbi A, Tenca G, Juchaux F, Bernard F-X. Lipolytic effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (SINETROL) in human body fat adipocytes: mechanism of action by inhibition of cAMP-phosphodiesterase (PDE). Phytomedicine 2008;15:783–92 [DOI] [PubMed] [Google Scholar]
- 28.Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 1996;7:66–76 [Google Scholar]
- 29.Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci 1998;854:435–42 [DOI] [PubMed] [Google Scholar]
- 30.Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr Res 1998;18:1995–2018 [Google Scholar]
- 31.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 2002;22:19–34 [DOI] [PubMed] [Google Scholar]
- 32.Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009;256:128–34 [DOI] [PubMed] [Google Scholar]
- 33.Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, Kim HK, Jeong TS, Choi MS. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr 2003;22:561–8 [DOI] [PubMed] [Google Scholar]
- 34.Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun 1999;265:410–5 [DOI] [PubMed] [Google Scholar]
- 35.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000;130 Suppl:2073S–85S [DOI] [PubMed] [Google Scholar]
- 36.Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 2003;133 Suppl:3248S–54S [DOI] [PubMed] [Google Scholar]
- 37.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr 2007;137:1244–52 [DOI] [PubMed] [Google Scholar]
- 38.Lyons-Wall P, Autenzio P, Lee E, Moss R, Samman S. Catechins are the major source of flavono ids in a group of Australian women. Asia Pac J Clin Nutr. 2004;13:S72 [Google Scholar]
- 39.Ishii K, Furuta T, Kasuya Y. Determination of naringin and naringenin in human urine by high-performance liquid chromatography utilizing solid-phase extraction. J Chromatogr B Biomed Sci Appl 1997;704:299–305 [DOI] [PubMed] [Google Scholar]
- 40.Lee YS, Reidenberg MM. A method for measuring naringenin in biological fluids and its disposition from grapefruit juice by man. Pharmacology 1998;56:314–7 [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Chao P, Hou Y, Hsiu S, Wen K, Tsai S. Pharmacokinetics and conjugation metabolism of naringin and naringenin in rats after single dose and multiple dose administrations. J Food Drug Anal. 2006;14:247–53 [Google Scholar]
- 42.Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin Pharmacol Ther 1996;60:34–40 [DOI] [PubMed] [Google Scholar]
- 43.Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull 2004;27:72–6 [DOI] [PubMed] [Google Scholar]
- 44.Ooghe WC, Ooghe SJ, Detavernier ClM, Huyghebaert A. Characterization of orange juice (Citrus sinensis) by flavanone glycosides. J Agric Food Chem 1994;42:2183–90 [Google Scholar]
- 45.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from citrus juices. J Agric Food Chem 1999;47:128–35 [DOI] [PubMed] [Google Scholar]
- 46.de Lourdes Mata Bilbao M, Andrés-Lacueva C, Jáuregui O, Lamuela-Raventós RM. Determination of flavonoids in a citrus fruit extract by LC–DAD and LC–MS. Food Chem 2007;101:1742–7 [Google Scholar]
- 47.Dhuique-Mayer C, Caris-Veyrat C, Ollitrault P, Curk F, Amiot MJ. Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J Agric Food Chem 2005;53:2140–5 [DOI] [PubMed] [Google Scholar]
- 48.Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;doi: 10.1093/database/bap024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothwell JA, Urpi-Sarda M, Boto-Ordonez M, Knox C, Llorach R, Eisner R, Cruz J, Neveu V, Wishart D, Manach C, et al. Phenol-Explorer 2.0: a major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database (Oxford). 2015;doi: 10.1093/database/bas031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhagwat S, Haytowitz D, Holden J. USDA database for the flavonoid content of selected foods. Release 3. USDA; 2011 [Google Scholar]
- 51. WHO. The World Health Report: reducing risks promoting healthy life. Geneva: WHO; 2002. [DOI] [PubMed]
- 52.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis A. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukuchi Y, Hiramitsu M, Okada M, Hayashi S, Nabeno Y, Osawa T, Naito M. Lemon polyphenols suppress diet-induced obesity by up-regulation of mRNA levels of the enzymes involved in beta-oxidation in mouse white adipose tissue. J Clin Biochem Nutr 2008;43:201–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho KW, Kim YO, Andrade JE, Burgess JR, Kim YC. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor alpha protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr 2011;50:81–8 [DOI] [PubMed] [Google Scholar]
- 55.Horiba T, Nishimura I, Nakai Y, Abe K, Sato R. Naringenin chalcone improves adipocyte functions by enhancing adiponectin production. Mol Cell Endocrinol 2010;323:208–14 [DOI] [PubMed] [Google Scholar]
- 56.Hirai S, Takahashi N, Goto T, Lin S, Uemura T, Yu R, Kawada T. Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediators Inflamm. 2010;2010:367838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu R, Kim C-S, Kwon B-S, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14:1353–62 [DOI] [PubMed] [Google Scholar]
- 58.Hirai S, Kim Y, II, Goto T, Kang M-S, Yoshimura M, Obata A, Yu R, Kawada T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci 2007;81:1272–9 [DOI] [PubMed] [Google Scholar]
- 59.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1998;56:317–33 [DOI] [PubMed] [Google Scholar]
- 60.Pu P, Gao DM, Mohamed S, Chen J, Zhang J, Zhou XY, Zhou NJ, Xie J, Jiang H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch Biochem Biophys 2012;518:61–70 [DOI] [PubMed] [Google Scholar]
- 61.Shin YW, Bok SH, Jeong TS, Bae KH, Jeoung NH, Choi MS, Lee SH, Park YB. Hypocholesterolemic effect of naringin associated with hepatic cholesterol regulating enzyme changes in rats. Int J Vitam Nutr Res 1999;69:341–7 [DOI] [PubMed] [Google Scholar]
- 62.Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 2004;92:1–21 [DOI] [PubMed] [Google Scholar]
- 63.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA, Huff MW. Naringenin prevents dyslipidemia, apolipoprotein- B overproduction, and hyperinsulinemia in LDL receptor–null mice with diet-induced insulin resistance. Diabetes 2009;58:2198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alam MA, Kauter K, Brown L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients. 2013;5:637–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikemura M, Sasaki Y, Giddings JC, Yamamoto J. Preventive effects of hesperidin, glucosyl hesperidin and naringin on hypertension and cerebral thrombosis in stroke-prone spontaneously hypertensive rats. Phytother Res 2012;26:1272–7 [DOI] [PubMed] [Google Scholar]
- 66.Fallahi F, Roghani M, Moghadami S. Citrus flavonoid naringenin improves aortic reactivity in streptozotocin-diabetic rats. Indian J Pharmacol 2012;44:382–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saponara S, Testai L, Iozzi D, Martinotti E, Martelli A, Chericoni S, Sgaragli G, Fusi F, Calderone V. (+/−)-Naringenin as large conductance Ca2+-activated K+ (BKCa) channel opener in vascular smooth muscle cells. Br J Pharmacol 2006;149:1013–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen S, Ding Y, Tao W, Zhang W, Liang T, Liu C. Naringenin inhibits TNF-α induced VSMC proliferation and migration via induction of HO-1. Food Chem Toxicol 2012;50:3025–31 [DOI] [PubMed] [Google Scholar]
- 69.Reshef N, Hayarib Y, Gorenb C, Boazc M, Madarb Z, Knoblera H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am J Hypertens 2005;18:1360–3 [DOI] [PubMed] [Google Scholar]
- 70.Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: biochemical and histopathological evidences. Toxicology 2006;228:259–68 [DOI] [PubMed] [Google Scholar]
- 71.Reddy TK, Nagaraju I, Kumar KH, Lokanatha V, Reddy CD, Jagetia GC. Cardioprotective effect of naringin in mice treated with doxorubicin. Planta Med 2008;74:49 [Google Scholar]
- 72.Rajadurai M, Prince PS. Preventive effect of naringin on cardiac mitochondrial enzymes during isoproterenol-induced myocardial infarction in rats: a transmission electron microscopic study. J Biochem Mol Toxicol 2007;21:354–61 [DOI] [PubMed] [Google Scholar]
- 73.Mojzisová G, Šarišský M, Mirossay L, Martinka P, Mojžiš J. Effect of flavonoids on daunorubicin-induced toxicity in H9c2 cardiomyoblasts. Phytother Res 2009;23:136–9 [DOI] [PubMed] [Google Scholar]
- 74.Qin CX, Chen X, Hughes RA, Williams SJ, Woodman OL. Understanding the cardioprotective effects of flavonols: discovery of relaxant flavonols without antioxidant activity. J Med Chem 2008;51:1874–84 [DOI] [PubMed] [Google Scholar]
- 75.Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA, Diwan V, Kauter K, Sernia C, Campbell F, Ward L, et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 2011;57:611–24 [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Nie Y-c, Luo Y-l, Lin F, Zheng Y-f, Cheng G-h, Wu H, Zhang K-j, Su W-w, Shen J-g, et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol 2013;58:133–40 [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Wu K, You Q, Huang R, Li S, Wu K. Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int J Mol Med 2013;32:396–402 [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Guo R, Yan H, Tian L, You Q, Li S, Huang R, Wu K. Naringin inhibits ROS-activated MAPK pathway in high glucose-induced injuries in H9c2 cardiac cells. Basic Clin Pharmacol Toxicol 2014;114:293–304 [DOI] [PubMed] [Google Scholar]
- 79.Punithavathi VR, Anuthama R, Prince PSM. Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male Wistar rats. J Appl Toxicol 2008;28:806–13 [DOI] [PubMed] [Google Scholar]
- 80.Jung UJ, Lee M-K, Jeong K-S, Choi M-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db Mice. J Nutr 2004;134:2499–503 [DOI] [PubMed] [Google Scholar]
- 81.Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J Physiol Biochem 2012;68:307–18 [DOI] [PubMed] [Google Scholar]
- 82.Annadurai T, Thomas PA, Geraldine P. Ameliorative effect of naringenin on hyperglycemia-mediated inflammation in hepatic and pancreatic tissues of Wistar rats with streptozotocin- nicotinamide-induced experimental diabetes mellitus. Free Radic Res 2013;47:793–803 [DOI] [PubMed] [Google Scholar]
- 83.Tsai S-J, Huang C-S, Mong M-C, Kam W-Y, Huang H-Y, Yin M-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J Agric Food Chem 2012;60:514–21 [DOI] [PubMed] [Google Scholar]
- 84.Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun 2010;398:178–83 [DOI] [PubMed] [Google Scholar]
- 85.Hotamisligil GS, Shargill N, Spiegelman B. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 86.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor α and blood cytokine production in type 2 diabetes. Life Sci 2000;67:291–300 [DOI] [PubMed] [Google Scholar]
- 87.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol 1999;36:67–72 [DOI] [PubMed] [Google Scholar]
- 88.Krogh-Madsen R, Plomgaard P, Møller K, Mittendorfer B, Pedersen BK. Influence of TNF-α and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab 2006;291:E108–14 [DOI] [PubMed] [Google Scholar]
- 89.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 90.Terra X, Montagut G, Bustos M, Llopiz N, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola L, et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem 2009;20:210–8 [DOI] [PubMed] [Google Scholar]
- 91.Lee IS, Shin G, Choue R. Shifts in diet from high fat to high carbohydrate improved levels of adipokines and pro-inflammatory cytokines in mice fed a high-fat diet. Endocr J 2010;57:39–50 [DOI] [PubMed] [Google Scholar]
- 92.Ali MM, El Kader MA. The influence of naringin on the oxidative state of rats with streptozotocin-induced acute hyperglycaemia. Z Naturforsch C 2004;59:726–33 [DOI] [PubMed] [Google Scholar]
- 93.Pari L, Amudha K. Hepatoprotective role of naringin on nickel-induced toxicity in male Wistar rats. Eur J Pharmacol 2011;650:364–70 [DOI] [PubMed] [Google Scholar]
- 94.Renugadevi J, Prabu SM. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol 2010;62:171–81 [DOI] [PubMed] [Google Scholar]
- 95.Guan L-P, Nan J-X, Jin X-J, Jin Q-H, Kwak K, Chai K-y, Quan Z-S. Protective effects of chalcone derivatives for acute liver injury in mice. Arch Pharm Res 2005;28:81–6 [DOI] [PubMed] [Google Scholar]
- 96.Kannappan S, Palanisamy N, Anuradha CV. Suppression of hepatic oxidative events and regulation of eNOS expression in the liver by naringenin in fructose-administered rats. Eur J Pharmacol 2010;645:177–84 [DOI] [PubMed] [Google Scholar]
- 97.Bravo E, Palleschi S, Aspichueta P, Buque X, Rossi B, Cano A, Napolitano M, Ochoa B, Botham KM. High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids Health Dis 2011;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawaguchi K, Kikuchi S, Hasegawa H, Maruyama H, Morita H, Kumazawa Y. Suppression of lipopolysaccharide-induced tumor necrosis factor-release and liver injury in mice by naringin. Eur J Pharmacol 1999;368:245–50 [DOI] [PubMed] [Google Scholar]
- 99.Sharma AK, Bharti S, Ojha S, Bhatia J, Kumar N, Ray R, Kumari S, Arya DS. Up-regulation of PPARgamma, heat shock protein-27 and -72 by naringin attenuates insulin resistance, beta-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr 2011;106:1713–23 [DOI] [PubMed] [Google Scholar]
- 100.St Clair RW, Yancey PG, Leight MA. Macrophage cholesterol balance: a potential site of genetic control of susceptibility to atherosclerosis. Ann N Y Acad Sci 1995;748:264–75 [PubMed] [Google Scholar]
- 101.Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol 2001;12:41–8 [DOI] [PubMed] [Google Scholar]
- 102.Naderi GA, Asgary S, Sarraf-Zadegan N, Shirvany H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol Cell Biochem 2003;246:193–6 [PubMed] [Google Scholar]
- 103.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oak M-H, El Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem 2005;16:1–8 [DOI] [PubMed] [Google Scholar]
- 105.Choe S-C, Kim H-S, Jeong T-S, Bok S-H, Park Y-B. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol 2001;38:947–55 [DOI] [PubMed] [Google Scholar]
- 106.Lee C-H, Jeong T-S, Choi Y-K, Hyun B-H, Oh G-T, Kim E-H, Kim J-R, Han J-I, Bok S-H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun 2001;284:681–8 [DOI] [PubMed] [Google Scholar]
- 107.Chanet A, Milenkovic D, Deval C, Potier M, Constans J, Mazur A, Bennetau-Pelissero C, Morand C, Bérard AM. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J Nutr Biochem 2012;23:469–77 [DOI] [PubMed] [Google Scholar]
- 108.Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD. Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol 1999;19:1223–30 [DOI] [PubMed] [Google Scholar]
- 109.Allister EM, Mulvihill EE, Barrett PHR, Edwards JY, Carter LP, Huff MW. Inhibition of apoB secretion from HepG2 cells by insulin is amplified by naringenin, independent of the insulin receptor. J Lipid Res 2008;49:2218–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borradaile NM, de Dreu LE, Huff MW. Inhibition of Net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes 2003;52:2554–61 [DOI] [PubMed] [Google Scholar]
- 111.Mulvihill EE, Assini JM, Sutherland BG, DiMattia AS, Khami M, Koppes JB, Sawyez CG, Whitman SC, Huff MW. Naringenin decreases progression of atherosclerosis by improving dyslipidemia in high-fat–fed low-density lipoprotein receptor–null mice. Arterioscler Thromb Vasc Biol 2010;30:742–8 [DOI] [PubMed] [Google Scholar]
- 112.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res 2005;96:1042–52 [DOI] [PubMed] [Google Scholar]
- 115.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–80 [DOI] [PubMed] [Google Scholar]
- 116.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–28 [DOI] [PubMed] [Google Scholar]
- 117.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15:2792–800 [DOI] [PubMed] [Google Scholar]
- 118.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-α induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–6 [DOI] [PubMed] [Google Scholar]
- 119.Lin C-Y, Ni C-C, Yin M-C, Lii C-K. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine 2012;59:65–71 [DOI] [PubMed] [Google Scholar]
- 120.García-Lafuente A, Guillamón E, Villares A, Rostagno M, Martínez J. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res 2009;58:537–52 [DOI] [PubMed] [Google Scholar]
- 121.Jain M, Parmar HS. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm Res 2011;60:483–91 [DOI] [PubMed] [Google Scholar]
- 122.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-κB. Nat Med 2005;11:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee E-J, Kim D-I, Kim W-J, Moon S-K. Naringin inhibits matrix metalloproteinase-9 expression and AKT phosphorylation in tumor necrosis factor-α-induced vascular smooth muscle cells. Mol Nutr Food Res 2009;53:1582–91 [DOI] [PubMed] [Google Scholar]
- 124.Xiong Y, Wang GF, Zhang JY, Wu SY, Xu W, Zhang JJ, Wu SG, Rao JJ. Naringin inhibits monocyte adhesion to high glucose-induced human umbilical vein endothelial cells. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:321–5 [PubMed] [Google Scholar]
- 125.Gopinath K, Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of Nrf2 signalling pathway. Neuroscience 2012;227:134–43 [DOI] [PubMed] [Google Scholar]
- 126.Guimarães R, Barros L, Barreira JCM, Sousa MJ, Carvalho AM, Ferreira ICFR. Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange. Food Chem Toxicol 2010;48:99–106 [DOI] [PubMed] [Google Scholar]
- 127.Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric 2010;90:1238–44 [DOI] [PubMed] [Google Scholar]
- 128.Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G, Barcellona ML, Vanella A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol Toxicol 2000;16:91–8 [DOI] [PubMed] [Google Scholar]
- 129.Jeon S-M, Bok S-H, Jang M-K, Kim Y-H, Nam K-T, Jeong T-S, Park YB, Choi M-S. Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta 2002;317:181–90 [DOI] [PubMed] [Google Scholar]
- 130.Mollace V, Sacco I, Janda E, Malara C, Ventrice D, Colica C, Visalli V, Muscoli S, Ragusa S, Muscoli C, et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia 2011;82:309–16 [DOI] [PubMed] [Google Scholar]
- 131.Dow CA, Going SB, Chow HH, Patil BS, Thomson CA. The effects of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metabolism 2012;61:1026–35 [DOI] [PubMed] [Google Scholar]
- 132.Aptekmann NP, Cesar T. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis 2013;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piche LA, Serratore P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr 2000;72:1095–100 [DOI] [PubMed] [Google Scholar]
- 134.Kaats GR, Miller H, Preuss HG, Stohs SJ. A 60 day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem Toxicol 2013;55:358–62 [DOI] [PubMed] [Google Scholar]
- 135.Haramaki N, Stewart DB, Aggarwal S, Ikeda H, Reznick AZ, Packer L. Networking antioxidants in the isolated rat heart are selectively depleted by ischemia-reperfusion. Free Radic Biol Med 1998;25:329–39 [DOI] [PubMed] [Google Scholar]
- 136.Nagel E, Meyer zu Vilsendorf A, Bartels M, Pichlmayr R. Antioxidative vitamins in prevention of ischemia/reperfusion injury. Int J Vitam Nutr Res 1997;67:298–306 [PubMed] [Google Scholar]
- 137.The HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293:1338–47 [DOI] [PubMed] [Google Scholar]
- 138.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–55 [PubMed] [Google Scholar]
- 139.Brown BG, Crowley J. Is there any hope for vitamin E? JAMA 2005;293:1387–90 [DOI] [PubMed] [Google Scholar]
- 140.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2008;300:2123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]