Abstract
Insulin-like growth factor 1 (IGF-1) is involved in regulations of reproductive functions in rats and mice. IGF-1 expression is regulated by estrogen in several reproductive organs including the uterus and ovary. Two types of estrogen receptor (ERα and ERβ) are expressed in mouse uteri and ovaries, and it is unclear whether they differently mediate IGF-1 gene transcription. To clarify the roles of ERα and ERβ, mouse endometrial stromal cells and ovarian granulosa cells were treated with ligands specific for individual estrogen receptors. In endometrial stromal cells, propyl-pyrazole-triol (PPT; ERα-selective agonist) increased Igf1 mRNA expression, which was suppressed by methyl-piperidino-pyrazole (MPP, ERα-selective antagonist), while diarylpropionitrile (DPN, ERβ-potency selective agonist) increased Igf1 mRNA expression, which was inhibited by MPP but not by 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-α]pyrimidin-3-yl]phenol (PHTPP; ERβ antagonist). PHTPP enhanced the DPN-induced increase in Igf1 mRNA expression. In ovarian granulosa cells, E2 and DPN decreased Igf1 mRNA expression, whereas PPT did not affect Igf1 mRNA levels. In these cells, PHTPP inhibited the DPN-induced decrease in Igf1 mRNA expression. These results suggest that ERα facilitates Igf1 transcription, whereas ERβ appears to inhibit Igf1 gene transcription in mouse endometrial stromal cells and ovarian granulosa cells.
Keywords: ERα, ERβ, Insulin-like growth factor 1, Mouse
Ovarian steroid hormone estrogens, particularly estradiol-17β (E2), play important roles in the regulation of reproductive functions. Estrogens exert their actions by binding to the estrogen receptor (ER). The activated ERs regulate the transcription of E2-sensitive genes by directly binding to the estrogen responsive element (ERE) or through indirect interaction with other transcription factors, such as activating protein 1 (AP-1) and stimulating protein 1 (Sp-1) [1, 2]. The classic ERs are ERα and ERβ, which are members of the nuclear receptor superfamily of ligand-inducible transcription factors [3, 4]. ERα and ERβ are encoded by two distinct genes, while their DNA-binding domains are highly conserved, but their ligand-binding domains possess a relatively low homology. The N-terminal region, which possesses a hormone-independent activation function (AF-1) domain, is poorly conserved [5,6,7]. E2 can bind to ERα and ERβ with nearly equal affinity [8]. Each receptor has similar or unique roles in estrogen-dependent gene expressions [4]. ERα and ERβ are known to oppositely regulate transcription of the same genes, such as cyclin D1 (Ccnd1) [9]. However, information about specific actions of ERα and ERβ to estrogen-targeted genes has been limited. The present study describes the differential actions of ERα and ERβ on insulin-like growth factor 1 (IGF-1) gene expression.
IGF-1 is involved in regulations of female reproductive functions in rats and mice. In the uterus, the synthesis of IGF-1 is stimulated by E2 [10,11,12]. IGF-1 promotes the proliferation of endometrial epithelial cells [13,14,15]. These findings indicate that IGF-1 is one of the growth factors that regulate the proliferation of endometrial epithelial cells. ERα is reported to be necessary for the IGF-1 signaling cascade controlling endometrial epithelial cell proliferation [16, 17]. It was recently reported that ERα–DNA interaction is necessary for E2-mediated regulation of Igf1 transcription in the mouse uterus [18], and ERα binds to several ERE sites located in the mouse Igf1. On the other hand, Weihua et al. reported the elevation of IGF-1 gene expression in the uterus of ERβ knockout mice, but quantitative analysis of IGF-1 gene expression was not performed in their study [19].
In the ovary, IGF-1 is expressed in granulosa cells in growing and healthy follicles [20, 21] and is required for the proliferation of granulosa cells in early folliculogenesis [22, 23]. Also, IGF-1 augments expression of FSH receptors in preovulatory granulosa cells and regulates responsiveness to FSH in Graafian follicles [24]. In atretic follicles, IGF-1 is not detected. Thus, IGF-1 plays essential roles in the entry of follicles into FSH-dependent stages of follicular development.
IGF-1 expression in the ovary is thought to be regulated by estrogen [25], but it is still not clear how estrogen affects IGF-1 expression in granulosa cells. In mouse granulosa cells, ERβ is more abundant than ERα [26,27,28,29,30]. Hence, it is necessary to clarify which type of ERs is involved in IGF-1 expression in granulosa cells.
The present study was aimed at clarifying the role of ERα and ERβ in the regulation of IGF-1 gene transcription using mouse endometrial stromal cells and ovarian granulosa cells. Using specific agonists as well as antagonists for ERα and ERβ, we analyzed the expression of Igf1 in primary endometrial stromal cells and ovarian granulosa cells. Mammalian IGF-1 genes consist of six exons [31,32,33]. Exons 1 and 2 are the leader exons, and alternative use of these leader exons generates two types of IGF-1 transcripts (class 1 and class 2) [34, 35]. These two types of IGF-1 transcripts have been detected in the mouse uterus and ovary [12]. We determined mainly the expression of class 1 Igf1 transcripts to uncover the role of ERs in the activity of each promoter, since class 1 Igf1 mRNA expression responds more to estrogen than class 2 Igf1 mRNA expression [12].
Materials and Methods
Animals
Immature (21–23 days old) female ICR mice (CLEA Japan, Osaka, Japan) were used in the present study. All animal care and experiments were approved by the Institutional Animal Care and Use Committee at Okayama University (OKU-2012304), and were conducted in accordance with the Guidelines for Animals Experimentation of Okayama University, Japan.
Endometrial stromal cell culture
Endometrial stromal cells were isolated from 21- to 23-day-old mice using previously described methods [13, 36, 37]. Isolated endometrial stromal cells separated from epithelial cells were seeded in poly-L-lysine-coated culture wells at a density of 6.2 × 104 cells/cm2. Endometrial stromal cells were first cultured in a 1:1 mixture of phenol red-free DMEM and Ham’s F-12 medium (DMEM/F12; Sigma-Aldrich, St. Louis, MO, USA) containing 2% dextran-coated charcoal-treated fetal bovine serum (DC-FBS; v/v, Life Technologies, Grand Island, NY, USA). After pre-culture for 1 day, the medium was switched to serum-free DMEM/F12 supplemented with BSA (1 g/l), hydrocortisone (100 µg/l), triiodothyronine (400 ng/l), transferrin (10 mg/l), glucagon (10 ng/l), parathyroid hormone (200 ng/l), sodium selenite (5 µg/l) and insulin (100 µg/l) (all from Sigma-Aldrich). The plates were incubated at 37 C in an atmosphere of 5% CO2 and treated with the indicated hormones/compounds 2 days later.
Isolation and culture of ovarian granulosa cells
Ovaries from 21- to 23-day-old mice were dissected free of connective tissues and collected in DMEM/F12 containing 0.3% BSA. The ovaries were punctured with a 27-gauge needle, and a mixture of granulosa cells and oocytes was filtered through cell strainers (40 µm nylon mesh; BD Falcon, Bedford, MA, USA) that allowed the granulosa cells but not the oocytes to pass through. After being centrifuged at 500 × g for 5 min at 4 C, the isolated granulosa cells were seeded in culture wells at a density of 1.3 × 104 cells/cm2. The granulosa cells were first cultured in DMEM/F12 containing 10% DC-FBS. After pre-culture for 1 day, the medium was switched to serum-free DMEM/F12 (phenol red-free). The plates were incubated at 37 C in an atmosphere of 5% CO2, and treated with the indicated hormones/compounds 2 days later.
Cell line culture
Human endometrial adenocarcinoma cells (HEC-1-A cells) were obtained from the Health Science Research Resources Bank (Sennan, Osaka, Japan) and were maintained in DMEM (Sigma-Aldrich) containing 10% FBS at 37 C under 5% CO2. To measure luciferase activity, the cells were grown in phenol red-free DMEM/F12 containing 10% DC-FBS. Cells were seeded into 48-well plates at 0.7 × 105 cells/well and cultured for 24 h before transfection.
Estrogen agonist and antagonist treatment
Endometrial stromal cells and granulosa cells were treated with propyl-pyrazole-triol (PPT), a ERα-selective agonist [38,39,40]; diarylpropionitrile (DPN), a ERβ-potency selective agonist [41,42,43]; methyl-piperidino-pyrazole (MPP), a selective ERα antagonist [42, 44, 45]; or 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-α]pyrimidin-3-yl]phenol (PHTPP), a selective ERβ antagonist [46].
E2 (Sigma-Aldrich) and PHTPP (Tocris Bio, Ellisville, MO, USA) were initially dissolved in sterile ethanol. PPT (Sigma-Aldrich), MPP (Sigma-Aldrich) and DPN (Tocris Bioscience) were initially dissolved in dimethyl sulfoxide (DMSO). Before use, the compounds were diluted in serum-free culture medium. The final concentrations of ethanol or DMSO were < 0.01%.
RNA preparation and cDNA synthesis
Total RNA was prepared from cultured endometrial stromal cells and ovarian granulosa cells using TRIsure reagent (Bioline, London, UK). Total RNA was reverse transcribed using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio, Otsu, Japan) with random hexamers according to the manufacturer’s instructions.
RT-PCR
PCR was performed using Blend Taq (Toyobo, Tokyo, Japan) and a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Branchburg, NJ, USA). The primers used in the present study were designed, and summarized in Table 1. The PCR conditions were as follows: 2 min at 94 C; an appropriate number of cycles of 94 C for 30 sec, 60 C for 30 sec and 72 C for 30 sec; and a final step at 72 C for 10 min. Aliquots (9 µl) of each reaction were electrophoresed on 2% agarose gels, stained with ethidium bromide and photographed under ultraviolet light.
Table 1. Primer sequence used for RT-PCR and Real-time PCR.
| Gene | Sequence (5′–3′) | Length (bp) |
| RT-PCR | ||
| Krt19 | ||
| Forward | GTGTCTGATGGGCTGCTGTCT | 539 |
| Reverse | CTCAGGATCTTGGCTAGGTCG | |
| Vim | ||
| Forward | GGCCGAGGAATGGTACAAGTC | 320 |
| Reverse | GGGCCATCTTAACATTGAGCAG | |
| ERa | ||
| Forward | CTAATTCTGACAATCGACGC | 347 |
| Reverse | GTGCTTCAACATTCTCCCTCCTC | |
| ERb | ||
| Forward | GGTGTCTGGTCCTGTGAAGGATGT | 670 |
| Reverse | CCGTCGTCGCCAGGAGCATGTCAA | |
| RpL19 | ||
| Forward | GAAATCGCCAATGCCAACTC | 406 |
| Reverse | TGAGGCTCGCAGGTCTAAGA | |
| Real-time PCR | ||
| class1 Igf1 | ||
| Forward | GCAGCCTTCCAACTCAATTATTTAA | 89 |
| Reverse | GTAGAAGAGGTGTGAAGACGACATG | |
| class2 Igf1 | ||
| Forward | CACCTGTTCTTAAGTCCTCAGTTTTG | 143 |
| Reverse | GTAGAAGAGGTGTGAAGACGACATG | |
| RpL19 | ||
| Forward | CCGCAGCCATGAGTATGCT | 60 |
| Reverse | CGCAGCGGAGGACACTAGA | |
Real-time PCR
Real-time PCR was performed using SYBR Premix Ex Taq (Perfect Real Time; Takara Bio) with an ABI PRISM 7300 Real Time PCR System (Applied Biosystems). The PCR program was as follows: initial denaturation at 95 C for 10 sec, 40 cycles of 95 C for 5 sec and 60 C for 31 sec and melting-curve analysis (95 C for 15 sec, 60 C for 1 min, 95 C for 15 sec and 60 C for 15 sec). Melting-curve analysis was conducted to confirm the absence of primer dimers. The primers are summarized in Table 1. A standard curve was generated by serial dilution of a preparation of total cDNA. The mRNA expression of each target gene was normalized for the mRNA expression of ribosomal protein L19 (Rpl19).
Plasmid construction
The expression vector for human ERα (pSG5-hERα) was constructed by inserting cDNA encoding human ERα into the EcoRI site of pSG5. The expression vector pSG5-hERβ was constructed by inserting the cDNA encoding human ERβ (530 bp) into the EcoRI/BglII site of pSG5, which was provided by Dr N Fujimoto (Hiroshima University, Hiroshima, Japan). The estrogen-responsive reporter plasmid, (ERE)3-Luc, was constructed as previously described [47]. Briefly, (ERE)3-Luc was generated by inserting three consensus estrogen-responsive elements with the SV40 TATA promoter into the multiple cloning region of the SacI/HindIII site of pGL4.12 (Promega, Madison, WI, USA). The phRL-TK vector (Promega) was used as an internal control in the reporter assay.
Reporter assay
HEC-1-A cells, seeded in 48-well plates, were transfected with a mixture of 0.2 µg of (ERE)3-Luc reporter plasmid, 1 ng of phRL-TK vector as an internal control and 50 ng of the ERα or ERβ expression vector, using 0.5 µl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in DMEM/F12 supplemented with insulin-transferrin-selenium (Life Technologies). Twenty-four hours after transfection, the cells were placed in fresh medium containing ER agonists and antagonists. Reporter gene activity was assayed 24 h after ligand treatment. For the reporter assay, endometrial stromal cells were plated onto 24-well plates and grown in DMEM/F12 containing 10% DC-FBS for 2 days before transfection. After 1 day of culture, the medium was switched to serum-free DMEM/F12.
Luciferase reporter gene activity was measured using the Dual-Luciferase Reporter Assay System (Promega) with a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA, USA) according to the manufacturer’s instructions. Luciferase activity was normalized to the Renilla luciferase activity (phRL-TK vector) of each sample.
Protein extraction and Western blotting analysis
Endometrial stromal cells and ovarian granulosa cells were grown in 10-cm dishes. After washing with ice-cold PBS, the cells were lysed in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then scraped into a microfuge tube. The cell lysate was passed through a 26-gauge needle, and sonicated twice for 5 min each. After being centrifuged at 9,000 × g for 5 min at 4 C, the protein concentration of the supernatant was measured using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). To detect the proteins, cell lysates (5–60 µg) were separated by 10% SDS-PAGE and transferred to Protran nitrocellulose membranes (0.45 µm pore size; Whatman, Dassel, Germany). The membranes were blocked for 1 h with 1% dried milk (for ERα and ERβ) dissolved in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) at room temperature. After washing once in TBS-T for 10 min at room temperature, the membranes were incubated overnight at 4 C with primary antibodies against ERα (1:2,000; H184; Santa Cruz) and ERβ (1:500; H150; Santa Cruz). The anti-ERα and anti-ERβ antibodies were diluted in TBS-T containing 1% dried milk. After incubation with the primary antibody, the membranes were washed twice in TBS-T and then incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:5,000; Amersham Biosciences, Little Chalfont, UK) in TBS-T for 1 h at room temperature. After washing three times in TBS-T, the protein bands were visualized using an enhanced chemiluminescence detection system (ECL Prime; GE Healthcare, Little Chalfont, UK) and a Lumino-image analyzer (LAS-4000mini; Fujifilm, Tokyo, Japan).
Statistics
Data are expressed as means ± SEM, and they were analyzed by ANOVA followed by Dunnett’s test. Differences were considered significant at P < 0.05.
Results
ER ligand activities of PPT, DPN, MPP and PHTPP
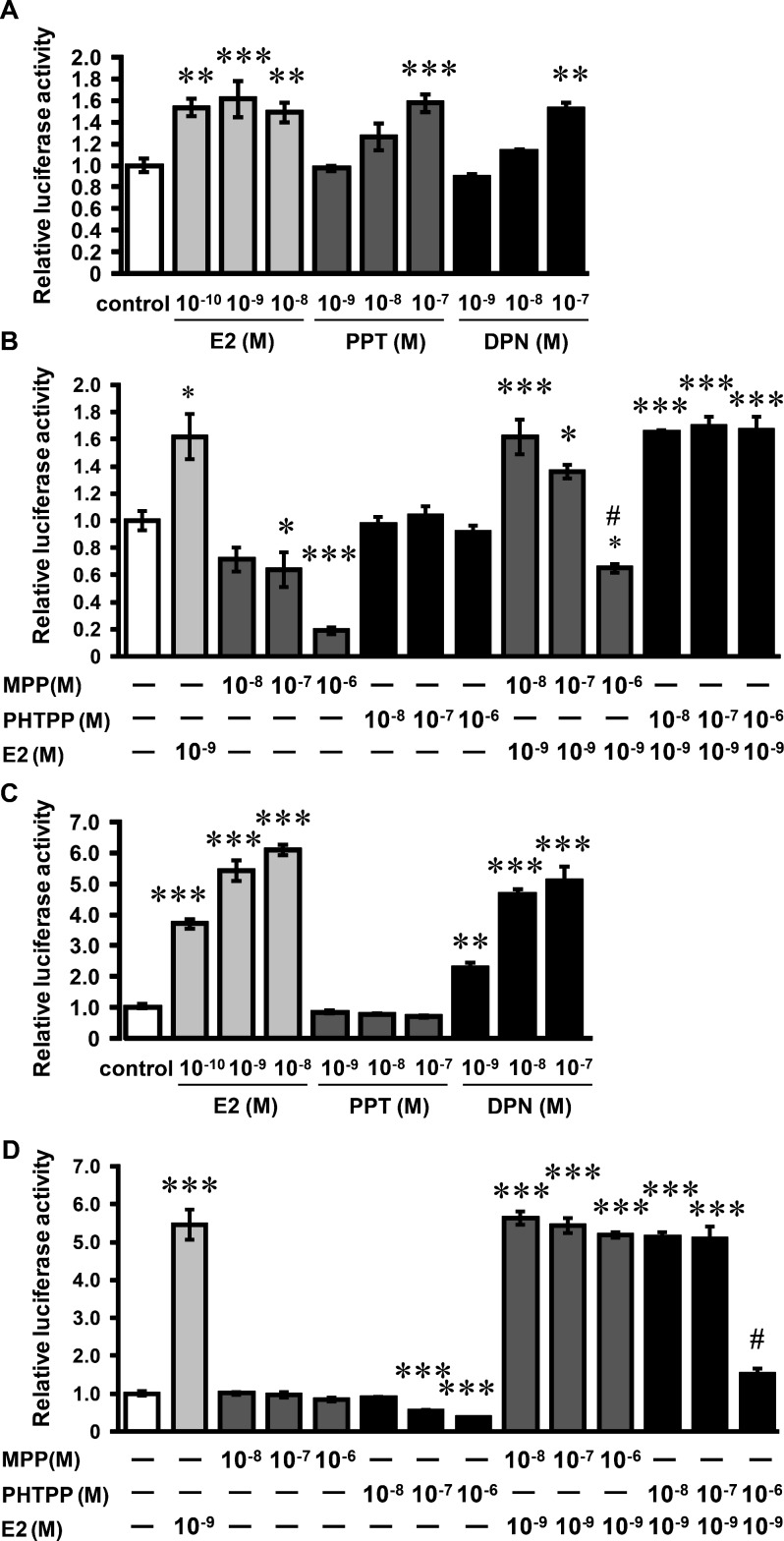
The ER ligand activities of PPT, DPN, MPP and PHTPP were examined using an estrogen-responsive reporter gene plasmid ((ERE)3-Luc) in HEC-1-A cells. HEC-1-A cells were transfected with (ERE)3-Luc and expression plasmids for ERα or ERβ. E2 increased luciferase activity above the basal levels in the presence of ERα or ERβ (Fig. 1A and C). PPT increased luciferase activity in ERα-expressing cells, but not in ERβ-expressing cells (Fig. 1A and C). By contrast, DPN increased luciferase activity in ERα-expressing cells and ERβ-expressing cells (Fig. 1A and C). These results indicate that DPN binds to ERβ and ERα, although DPN is thought to be a ERβ-potency selective agonist. MPP (10–6 M) inhibited ERα activity induced by E2, but not ERβ activity stimulated by E2 (Fig. 1B and D). MPP alone (10–7and 10–6 M) inhibited ERα activity. PHTPP (10–6 M) inhibited E2-stimulated ERβ activity, but did not suppress E2-stimulated ERα activity (Fig. 1B and D). PHTPP alone (10–7 and 10–6 M) inhibited ERβ activity.
Fig. 1.
Effects of ER agonists/antagonists on ERE activity in HEC-1-A cells. HEC-1-A cells were transiently transfected with expression plasmid vectors for ERα (A, B) or ERβ (C, D) together with a luciferase reporter plasmid ((ERE)3-Luc) containing three copies of tandem-arrayed ERE inserted upstream of a TATA promoter. Transfected cells were treated with E2, PPT and DPN (A, C) or with MPP or PHTPP in the absence or presence of E2 (B, D). The dual luciferase activity was assayed after incubation for 24 h. Values are means ± SEM of four wells. Independent experiments were performed twice, and similar results were obtained. *P < 0.05; **P < 0.01 and ***P < 0.001 compared with the control; #P < 0.001 compared with E2 (10−9 M) alone.
Expression of estrogen receptors in cultured endometrial stromal cells
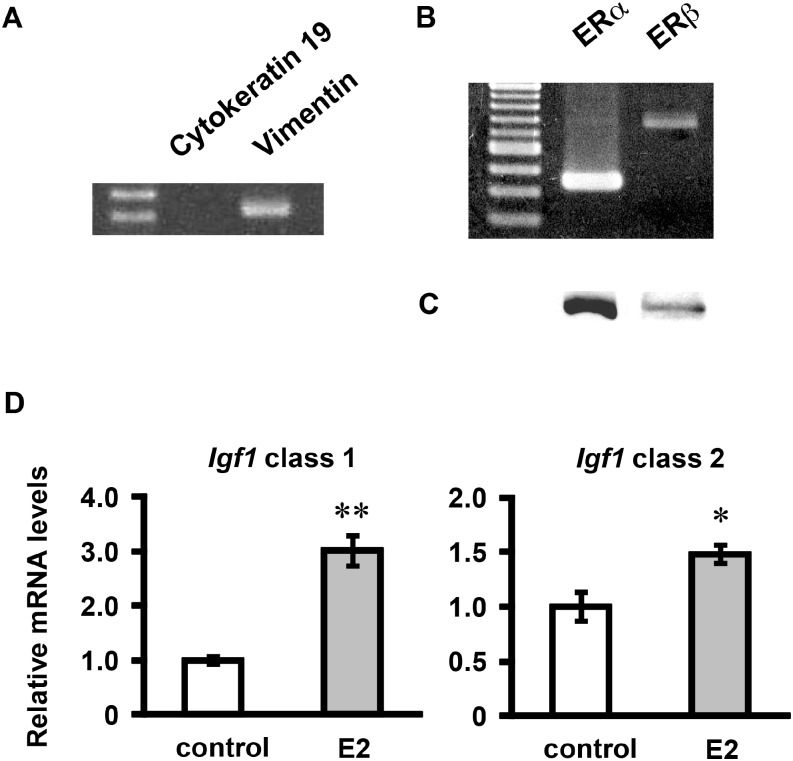
RT-PCR analyses of ER mRNAs and Western blots of ERs were performed using cultured endometrial cells. In the cultured cells, vimentin mRNA (a marker for stromal cells, Vim) was detected, but cytokeratin 19 mRNA (a marker for epithelial cells, Krt19) was not detected (Fig. 2A), showing that cultured cells mostly consisted of endometrial stroma cells. In our previous study, immunocytochemical analysis already revealed that the cultured cells expressed vimentin but not cytokeratin [37]. The mRNAs and protein products of ERa and ERb were detected in the cultured endometrial stromal cells (Fig. 2B and C).
Fig. 2.
Analysis of cell properties of cultured endometrial stromal cells. Total RNA was obtained from the cultured endometrial stromal cells, reverse-transcribed, and amplified by PCR using primers for cytokeratin 19, vimentin, ERα and ERβ (A, B). Each PCR product was electrophoresed and stained with ethidium bromide. Whole-cell extracts were subjected to Western blotting with rabbit polyclonal anti-ERα and anti-ERβ antibodies (C). The cells were treated with E2 (10−9 M) for 24 h. Igf1 mRNA levels were determined by real-time RT-PCR (D). Values are means ± SEM of triplicate wells. The expression of each mRNA was normalized for RpL19 mRNA expression. *P < 0.05; **P < 0.01 compared with the control.
Effects of E2 on class 1 and class 2 Igf1 mRNA expression in cultured endometrial stromal cells
To determine whether Igf1 mRNA expression was regulated by E2 in our culture system, we measured Igf1 mRNA levels by real-time RT-PCR. E2 (10–9 M) significantly increased the mRNA levels of class 1 and class 2 Igf1 in cultured endometrial stromal cells (Fig. 2D). Class 1 Igf1 mRNA increased more in response to E2 treatment than class 2 Igf1 mRNA expression. These results indicate that the cultured endometrial stromal cells were responsive to estrogen in our culture system.
Effects of PPT and MPP on class 1 Igf1 mRNA expression in cultured endometrial stromal cells
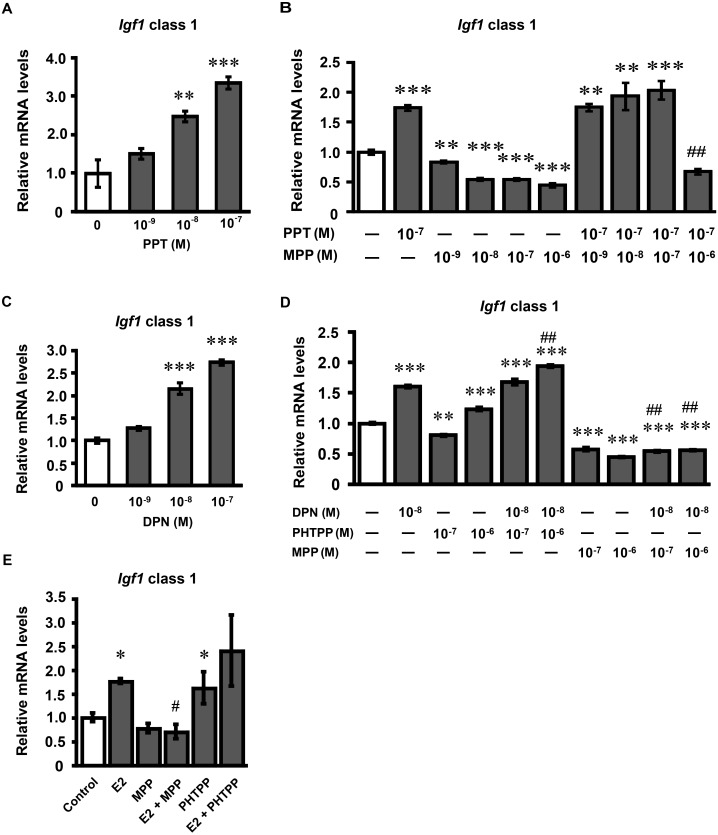
To clarify the role of ERα on Igf1 mRNA expression, we determined the effects of PPT and MPP on Igf1 mRNA expression in cultured endometrial stromal cells. The cultured endometrial stromal cells were treated with PPT for 24 h. PPT increased class 1 Igf1 mRNA expression in a dose-dependent manner (Fig. 3A). Based on these data, PPT was administered at the concentration of 10–7 M in the following studies. MPP (10–6 M) significantly inhibited the PPT-induced and E2-induced increase in Igf1 mRNA expression (Fig. 3B and E). MPP alone decreased class 1 Igf1 mRNA expression as observed in the ERE-mediated reporter gene analysis.
Fig. 3.
Effects of PPT, MPP, DPN and PHTPP on class 1 Igf1 mRNA expression in cultured endometrial stromal cells. (A) Endometrial stromal cells were treated with PPT (0, 10–9, 10–8, 10–7 M) for 24 h. (B) Endometrial stromal cells were treated with MPP (0, 10–9, 10–8, 10–7, 10–6 M) in the absence or presence of PPT (10–7 M) for 24 h. (C) Endometrial stromal cells were treated with DPN (0, 10–9, 10–8, 10–7 M) for 24 h. (D) Endometrial stromal cells were treated with PHTPP (0, 10–7, 10–6 M) or MPP (0, 10–7, 10–6 M) in the absence or presence of PPT (10–8 M) for 24 h. (E) Endometrial stromal cells were treated with MPP (10–6 M) or PHTPP (10–6 M) in the absence or presence of E2 (10–9 M) for 24 h. Igf1 mRNA levels were determined by real-time RT-PCR. Values are means ± SEM of triplicate wells. The expression of each mRNA was normalized for RpL19 mRNA expression. Independent experiments were performed three times, and similar results were obtained. *P < 0.05; **P < 0.01 and ***P < 0.001 compared with the control; #P < 0.05 compared with E2 (10–9 M) alone; ##P < 0.001 compared with PPT (10–7 M) alone or DPN (10–8 M) alone.
Effects of DPN, PHTPP and MPP on class 1 Igf1 mRNA expression in cultured endometrial stromal cells
To clarify the role of ERβ on Igf1 mRNA expression, we examined the effects of DPN, PHTPP and MPP on Igf1 mRNA expression in cultured endometrial stromal cells. Treatment with DPN for 24 h increased class 1Igf1 mRNA expression in a dose-dependent manner (Fig. 3C). Based on these data, DPN was used at the concentration of 10–8 M in the following studies. A high dose of PHTPP (10–6 M) slightly increased class 1 Igf1 mRNA expression, and facilitated the DPN-induced increase in class 1 Igf1 mRNA expression (Fig. 3D). By contrast, MPP decreased class 1 Igf1 mRNA expression, and decreased DPN-induced class 1 Igf1 mRNA expression.
Expression of estrogen receptors in culture granulosa cells
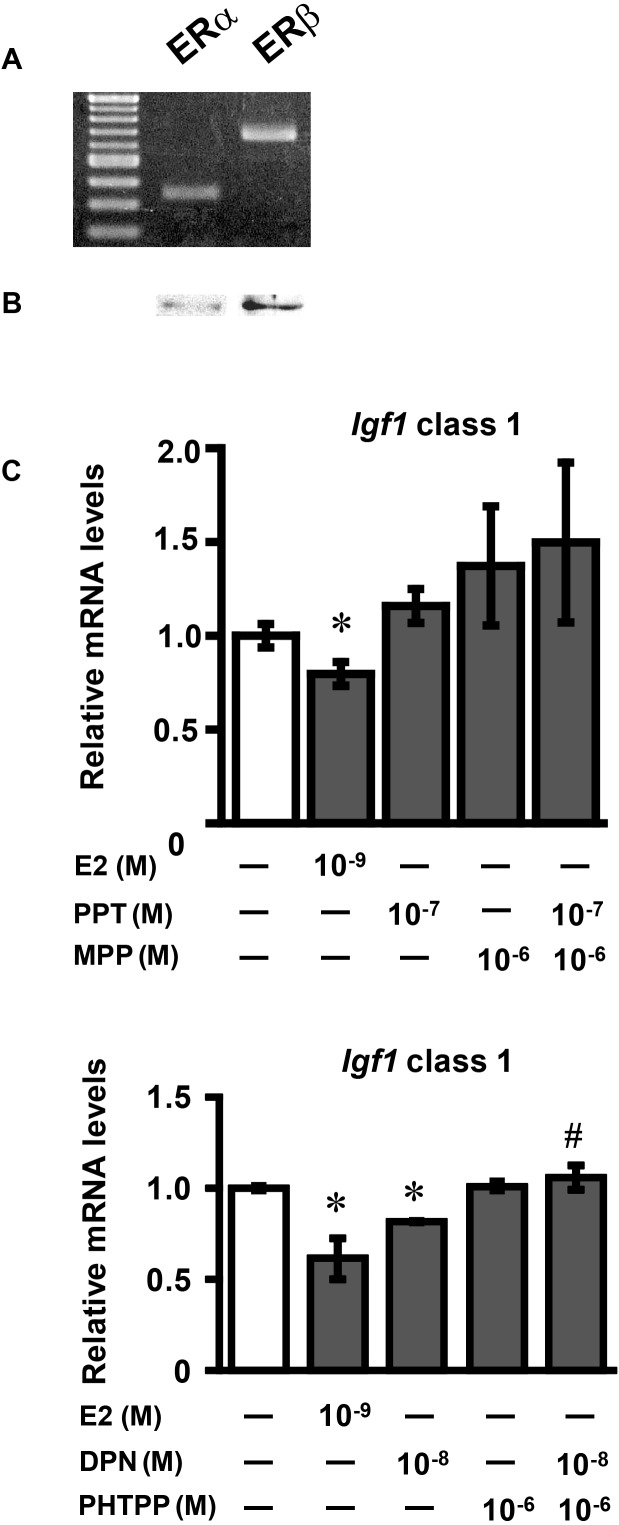
RT-PCR analyses of ER mRNAs and Western blots of ERs were performed using cultured granulosa cells. Dissociated ovarian cells obtained from immature mouse ovaries, expressed FSH receptor and aromatase mRNA expression (data not shown), indicating that the cultured cells consisted of granulosa cells. The mRNAs and protein products of ERa and ERb were detected in the cultured granulosa cells (Fig. 4A and B).
Fig. 4.
Effects of E2, PPT, DPN, MPP and PHTPP on class 1Igf1 mRNA expression in cultured ovarian granulosa cells. (A) Total RNA was obtained from the cultured ovarian granulosa cells, reverse-transcribed, and amplified by PCR using primers for ERα and ERβ. Each PCR product was electrophoresed and stained with ethidium bromide. (B) Whole-cell extracts were subjected to Western blotting using rabbit polyclonal anti-ERα and anti-ERβ antibodies. (C) Ovarian granulosa cells were treated with E2 (10–9 M), PPT (10–7 M), MPP (10–6 M), DPN (10–8 M) and PHTPP (10–6 M) for 24 h. Igf1 mRNA levels were determined by real-time RT-PCR. Values are means ± SEM of triplicate wells. The expression of each mRNA was normalized for RpL19 mRNA expression. Independent experiments were performed three times, and similar results were obtained. *P < 0.05 and **P < 0.01 compared with the control; #P < 0.05 compared with DPN (10–8 M) alone.
Effects of E2, PPT, DPN, MPP and PHTPP on class 1 Igf1 mRNA expression in cultured ovarian granulosa cells
The granulosa cells were then treated with E2, PPT, DPN, MPP and PHTPP for 24 h. E2 and DPN both decreased class 1 Igf1 mRNA expression, whereas no change occurred in cells treated with PPT (Fig. 4C). PHTPP significantly inhibited the decrease in Igf1 mRNA expression induced by DPN, while MPP did not induce significant changes in Igf1 mRNA expression.
Discussion
We investigated roles of ERα and ERβ in the regulation of Igf1 transcription in the murine endometrial stromal cells and ovarian granulosa cells. The nuclear receptors ERα and ERβ are expressed in these cellular systems, with ERα being a major receptor in endometrial cells [27] and ERβ being a major receptor in granulosa cells [28, 29], The present results clearly suggest that Igf1 transcription in mouse endometrial stromal cells is regulated mainly through ERα, which is consistent with previous reports, and that Igf1 transcription in granulosa cells was regulated through ERβ in an inhibitory manner. Thus, ERα and ERβ differentially regulate Igf1 transcription depending upon the types of IGF-1-expressing cells.
The cultured endometrial stromal cells, obtained from immature mouse uteri, expressed the two ERs. E2 treatment significantly increased Igf1 mRNA expressions in the endometrial cells. Ovarian granulosa cells also expressed the two ERs, and E2 treatment significantly decreased Igf1 mRNA. These results altogether indicate that the cultured endometrial stromal cells and granulosa cells retained their functional properties in vitro. Hence, our primary culture system is suitable for analysis of the regulation of Igf1 transcription. Accordingly, we used these two types of cells as an experimental model system in this study.
To determine the effects of selective ER agonists and antagonists, an estrogen-responsive reporter gene plasmid ((ERE)3-Luc) was transfected into HEC-1-A cells together with ERα or ERβ expression vectors. The transiently transfected cells were then treated with PPT, DPN, MPP and PHTPP. PPT showed agonistic activity via ERα, and this was highly selective for ERα, as PPT did not activate the luciferase gene via ERβ [38]. On the other hand, although it is thought that DPN is an ERβ-potency selective agonist, it actually showed agonistic activity via both ERα and ERβ, which was similar to that described in a previous report [42]. MPP and PHTPP prevented luciferase gene activation promoted by E2 via ERα and ERβ, respectively. Interestingly, MPP (10−7, 10−6 M) appeared to decrease ERα activity, which is consistent with a previous report [42], and PHTPP (10−6 M) also appeared to decrease ERβ activity. The reasons for these effects are not clear, but these results may suggest that MPP and PHTPP bind to ERα and ERβ, respectively, and exert inverse agonist activities. From these results, it seems that MPP is highly selective for ERα, whereas PHTPP is highly selective for ERβ [44, 46].
Next, we treated endometrial stromal cells with the same agonists and antagonists. In endometrial stromal cells, Igf1 mRNA expression was increased by PPT in a dose-dependent manner. This result led us to conclude that ERα mediates promotion of Igf1 transcription, because PPT is highly selective for ERα [38,39,40]. Additionally, the ERα-selective antagonist MPP decreased PPT-induced Igf1 mRNA expression, indicating that ERα is involved in estrogen-induced upregulation of Igf1 mRNA expression in endometrial stromal cells. These results are consistent with previous reports showing that ERα is required for the activation of IGF-1 receptors and IGF-1-induced proliferation of epithelial cells [16,17,18]. DPN, which activated ERα and ERβ, increased Igf1 mRNA expression in endometrial stromal cells, which was similar to the effects of PPT. To elucidate whether these effects of DPN were mediated by ERα or ERβ, we co-treated cells with ERα and ERβ antagonists. MPP, an ERα-selective antagonist, inhibited the DPN-induced increase in Igf1 mRNA expression, whereas PHTPP, an ERβ-selective antagonist, did not affect DPN-induced Igf1 mRNA expression. These results suggest that DPN increases Igf1 mRNA expression via ERα but not via ERβ. Treatment with PHTPP increased Igf1 mRNA expression, and promoted DPN-induced Igf1 mRNA expression. These findings suggest that, unlike ERα, ERβ may have an inhibitory role in the regulation of Igf1 mRNA expression. Alternatively, ERβ may not directly affect Igf1 transcription but does compete with ERα, since PHTPP exerts its antagonistic action on ERβ without suppressing ERα activity. It was previously reported that Igf1 mRNA expression was increased in the uterus of ERβ knockout mice [19]. Hence, it is highly probable that this was due to the lack of ERβ, which may be able to suppress Igf1 transcription.
PHTPP was shown to have an inverse agonist activity on ERβ in the present and previous studies [42]. PHTPP alone increased Igf1 mRNA expression. Therefore, it seems reasonable that ligand-activated ERβ is involved in inhibitory regulation of IGF-1 expression. It was previously reported that E2 does not elicit estrogenic responses in ERα-knockout mice, such as DNA synthesis in endometrial epithelial cells, even though ERβ is expressed in endometrial stromal cells [48]. Taken together, these results may also suggest the possibility that Igf1 expression is regulated by ERβ in an inhibitory manner in endometrial cells.
The regulatory mechanism of ovarian IGF-1 expression seems to be still unclear. ERb mRNA, which encodes ERβ, is primarily detected in the granulosa cells of the mouse ovary [27]. Therefore, to further analyze the involvement of ERβ in the regulation of Igf1 transcription, we used ovarian granulosa cells. Of note, E2 and DPN decreased Igf1 mRNA expression in the granulosa cells unlike their effects in endometrial stromal cells. In granulosa cells, the effects of DPN are most likely to be mediated by ERβ rather than ERα, because the ERα agonist PPT and MPP did not affect Igf1 mRNA expression. Furthermore, the ERβ antagonist PHTPP inhibited the effects of DPN. From these results, we think that ERβ has an inhibitory effect rather than no effect on Igf1 transcription in granulosa cells.
Hernandez et al. [25] reported that diethylstilbestrol increased Igf1 mRNA levels in the ovary of hypophysectomized rats. On the other hand, we found an inhibitory effect of estrogen on Igf1 mRNA expression in granulosa cells through ERβ using a culture system of granulosa cells. The reason for this discrepancy is not clear, but may be partly explained by the difference in the sampling methods (whole ovary or granulosa cells). If estrogens decrease IGF-1 expression thorough ERβ in developing follicles, the proliferation of granulosa cells and responsiveness to FSH in such follicles will be reduced or nullified. It is possible that estrogen regulates follicular development and functions in an autocrine and paracrine manner through the IGF-1 system.
In mouse ovaries, ERβ promoted the proliferation of granulosa cells in early folliculogenesis [49] and also follicular maturation from the early antral to preovulatory stages [50]. In Igf1 null mouse ovaries, the proliferation of granulosa cells was decreased, and E2 stimulated the proliferation of granulosa cells, although the increase was less compared with that of wild-type mice [23]. These findings suggest that estrogen directly stimulates the proliferation of granulosa cells, and that IGF-1 enhances the estrogen-induced proliferation of granulosa cells.
In conclusion, the present results led us to consider that the nuclear receptors ERα and ERβ differentially regulate Igf1 mRNA expression, as ERα promotes its expression and ERβ inhibits its expression. Further studies are needed to determine the precise role of ERβ in the regulation of Igf1 transcription.
Acknowledgments
The authors would like to thank Dr F Otsuka and Dr K Inagaki for their kind guidance on granulosa cell culture, and K Masuda for his help with endometrial stroma cell culture. This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to Sumio Takahashi (Nos. 19370025, 22570066)
References
- 1.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997; 277: 1508–1510 [DOI] [PubMed] [Google Scholar]
- 2.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005; 19: 833–842 [DOI] [PubMed] [Google Scholar]
- 3.Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988; 240: 889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 2007; 87: 905–931 [DOI] [PubMed] [Google Scholar]
- 5.Tremblay GB, Tremblay A, Labrie F, Giguère V. Dominant activity of activation function 1 (AF-1) and differential stoichiometric requirements for AF-1 and -2 in the estrogen receptor α-β heterodimeric complex. Mol Cell Biol 1999; 19: 1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettersson K, Grandien K, Kuiper GGJM, Gustafsson J-A. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol 1997; 11: 1486–1496 [DOI] [PubMed] [Google Scholar]
- 7.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 2004; 11: 537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 1997; 138: 863–870 [DOI] [PubMed] [Google Scholar]
- 9.Liu M-M, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Jr, Pestell RG, Kushner PJ. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J Biol Chem 2002; 277: 24353–24360 [DOI] [PubMed] [Google Scholar]
- 10.Murphy LJ, Murphy LC, Friesen HG. Estrogen induces insulin-like growth factor-I expression in the rat uterus. Mol Endocrinol 1987; 1: 445–450 [DOI] [PubMed] [Google Scholar]
- 11.Inoue A, Takeuchi S, Takahashi S. Insulin-like growth factor-I stimulated DNA replication in mouse endometrial stromal cells. J Reprod Dev 2005; 51: 305–313 [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuki T, Otsuki M, Murakami Y, Hirata K, Takeuchi S, Takahashi S. Alternative leader-exon usage in mouse IGF-I mRNA variants: class 1 and class 2 IGF-I mRNAs. Zoolog Sci 2007; 24: 241–247 [DOI] [PubMed] [Google Scholar]
- 13.Shiraga M, Takahashi S, Miyake T, Takeuchi S, Fukamachi H. Insulin-like growth factor-I stimulates proliferation of mouse uterine epithelial cells in primary culture. Proc Soc Exp Biol Med 1997; 215: 412–417 [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Wang G, Hardy MP, Kurita T, Cunha GR, Cooke PS. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology 2002; 143: 2673–2679 [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Pollard JW. Estradiol-17β regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA 2007; 104: 15847–15851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klotz DM, Hewitt SC, Korach KS, Diaugustine RP. Activation of a uterine insulin-like growth factor I signaling pathway by clinical and environmental estrogens: requirement of estrogen receptor-α. Endocrinology 2000; 141: 3430–3439 [DOI] [PubMed] [Google Scholar]
- 17.Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem 2002; 277: 8531–8537 [DOI] [PubMed] [Google Scholar]
- 18.Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor α to estrogen-responsive elements. J Biol Chem 2010; 285: 2676–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) β, a modulator of ERα in the uterus. Proc Natl Acad Sci USA 2000; 97: 5936–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver JE, Aitman TJ, Powell JF, Wilson CA, Clayton RN. Insulin-like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology 1989; 124: 2671–2679 [DOI] [PubMed] [Google Scholar]
- 21.Adashi EY, Resnick CE, Payne DW, Rosenfeld RG, Matsumoto T, Hunter MK, Gargosky SE, Zhou J, Bondy CA. The mouse intraovarian insulin-like growth factor I system: departures from the rat paradigm. Endocrinology 1997; 138: 3881–3890 [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Refuerzo J, Bondy C. Granulosa cell DNA synthesis is strictly correlated with the presence of insulin-like growth factor I and absence of c-fos/c-jun expression. Mol Endocrinol 1995; 9: 924–931 [DOI] [PubMed] [Google Scholar]
- 23.Kadakia R, Arraztoa JA, Bondy C, Zhou J. Granulosa cell proliferation is impaired in the Igf1 null ovary. Growth Horm IGF Res 2001; 11: 220–224 [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Kumar TR, Matzuk MM, Bondy C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol 1997; 11: 1924–1933 [DOI] [PubMed] [Google Scholar]
- 25.Hernandez ER, Roberts CT, Jr, LeRoith D, Adashi EY. Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinology 1989; 125: 572–574 [DOI] [PubMed] [Google Scholar]
- 26.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 1998; 95: 15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 1999; 20: 358–417 [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick SL, Funkhouser JM, Sindoni DM, Stevis PE, Deecher DC, Bapat AR, Merchenthaler I, Frail DE. Expression of estrogen receptor-β protein in rodent ovary. Endocrinology 1999; 140: 2581–2591 [DOI] [PubMed] [Google Scholar]
- 29.Sar M, Welsch F. Differential expression of estrogen receptor-β and estrogen receptor-α in the rat ovary. Endocrinology 1999; 140: 963–971 [DOI] [PubMed] [Google Scholar]
- 30.Cheng G, Weihua Z, Mäkinen S, Mäkelä S, Saji S, Warner M, Gustafsson JA, Hovatta O. A role for the androgen receptor in follicular atresia of estrogen receptor β knockout mouse ovary. Biol Reprod 2002; 66: 77–84 [DOI] [PubMed] [Google Scholar]
- 31.Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci USA 1986; 83: 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimatsu A, Rotwein P. Sequence of two rat insulin-like growth factor I mRNAs differing within the 5′ untranslated region. Nucleic Acids Res 1987; 15: 7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamai Y, Mikawa S, Endo K, Sakai H, Komano T. Regulation of insulin-like growth factor-I expression in mouse preadipocyte Ob1771 cells. J Biol Chem 1996; 271: 9883–9886 [DOI] [PubMed] [Google Scholar]
- 34.Adamo M, Lowe WLJ, Jr, LeRoith D, Roberts CTJ., JrInsulin-like growth factor I messenger ribonucleic acids with alternative 5′-untranslated regions are differentially expressed during development of the rat. Endocrinology 1989; 124: 2737–2744 [DOI] [PubMed] [Google Scholar]
- 35.Simmons JG, Van Wyk JJ, Hoyt EC, Lund PK. Multiple transcription start sites in the rat insulin-like growth factor-I gene give rise to IGF-I mRNAs that encode different IGF-I precursors and are processed differently in vitro. Growth Factors 1993; 9: 205–221 [DOI] [PubMed] [Google Scholar]
- 36.Ross AK, Nelson KG, Sakai Y, Fukamachi H, Burroughs CD, McLachlan JA. Isolation and culture of mouse uterine epithelial and stromal cells. In: Heindel JJ, Chapin RE (eds.) Methods in Toxicology. San Diego: Academic Press, Inc.; 1993: 371–385.
- 37.Komatsu N, Maekawa T, Takeuchi S, Takahashi S. Epidermal growth factor and transforming growth factor-α stimulate the proliferation of mouse uterine stromal cells. Zoolog Sci 2003; 20: 639–645 [DOI] [PubMed] [Google Scholar]
- 38.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 2000; 43: 4934–4947 [DOI] [PubMed] [Google Scholar]
- 39.Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS. Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-α and estrogen receptor-β: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology 2000; 141: 3534–3545 [DOI] [PubMed] [Google Scholar]
- 40.Harris HA. Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol 2007; 21: 1–13 [DOI] [PubMed] [Google Scholar]
- 41.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 2001; 44: 4230–4251 [DOI] [PubMed] [Google Scholar]
- 42.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 2003; 206: 13–22 [DOI] [PubMed] [Google Scholar]
- 43.Montgomery S, Shaw L, Pantelides N, Taggart M, Austin C. Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility. Br J Pharmacol 2003; 139: 1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor α. Endocrinology 2002; 143: 941–947 [DOI] [PubMed] [Google Scholar]
- 45.Zhou HB, Carlson KE, Stossi F, Katzenellenbogen BS, Katzenellenbogen JA. Analogs of methyl-piperidinopyrazole (MPP): antiestrogens with estrogen receptor α selective activity. Bioorg Med Chem Lett 2009; 19: 108–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor β antagonist activity. J Med Chem 2004; 47: 5872–5893 [DOI] [PubMed] [Google Scholar]
- 47.Kudoh M, Susaki Y, Ideyama Y, Nanya T, Mori M, Shikama H, Fujikura T. Inhibitory effect of a novel non-steroidal aromatase inhibitor, YM511 on the proliferation of MCF-7 human breast cancer cell. J Steroid Biochem Mol Biol 1996; 58: 189–194 [DOI] [PubMed] [Google Scholar]
- 48.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 1997; 94: 6535–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Müller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci USA 2004; 101: 5129–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 2005; 146: 2817–2826 [DOI] [PubMed] [Google Scholar]