Abstract
The pancreas has been the subject of intense research due to the debilitating diseases that result from its dysfunction. In this review, we summarize current understanding of the critical tissue interactions and intracellular regulatory events that take place during formation of the pancreas from a small cluster of cells in the foregut domain of the mouse embryo. Importantly, an understanding of principles that govern the development of this organ has equipped us with the means to manipulate both embryonic and differentiated adult cells in the context of regenerative medicine. The emerging area of lineage modulation within the adult pancreas is of particular interest, and this review summarizes recent findings that exemplify how lessons learned from development are being applied to reveal the potential of fully differentiated cells to change fate.
Introduction
The pancreas originates from the gut endoderm, one of three germ layers that emerge after gastrulation in vertebrates, and through a series of coordinated signaling events and transcriptional regulatory cascades is patterned into the adult organ (Gittes, 2009). Distinct cell types reside in the mature pancreas and perform exocrine or endocrine functions. The exocrine compartment, which accounts for greater than 90% of pancreatic tissue in adult mice, is identified as acinar and ductal cells. Acinar cells synthesize digestive enzymes that aid in nutrient metabolism, and ductal cells line the channels that transport these secretions to the gastrointestinal tract. The endocrine compartment, which forms the remainder of the organ, is a compound structure called the Islet of Langerhans. Multiple cell types populate the islet, including insulin-producing β-cells, glucagon-producing α-cells, somatostatin-producing δ-cells, and pancreatic polypeptide-producing PP cells. Finely tuned regulation of hormone release is achieved by coordinated interactions between the islet cells and the vascular environment, establishing hormonal homeostasis within the animal.
The widespread prevalence of debilitating diseases that result from pancreatic dysfunction underscores the urgent need to strengthen our understanding of the principles governing the formation and function of this organ. Diabetes, resulting from either loss (type 1) or impaired function (type 2) of pancreatic β-cells, is characterized by abnormal regulation of blood glucose levels and significant complications. According to the American Diabetes Association, close to 8% of the population of the United States suffers from diabetes. Pancreatic cancer, specifically pancreatic ductal adenocarcinoma, carries a grim prognosis with a median survival of 6 months, and 5 year survival of 3%–5% (Wescott and Rustgi, 2008). Understanding the etiology of these diseases, and working toward cures, will rely on our comprehension of the development, functional capacity, and cellular plasticity of the organ.
Past research has focused on unraveling the mechanisms involved in normal growth of the pancreas and how these processes go awry upon genetic or physical insult. Classical gene knockout and Cre-based recombination technologies have made great inroads into establishing the molecular hierarchies that operate during pancreatic organogenesis. Translating this information toward disease therapy is a significant goal of ongoing research. In the obvious scenario, dysfunction of insulin-producing β-cells leads to severe defects, and it is particularly valuable to find avenues to either replenish the damaged cells using external sources or trigger a response within the organism to replace the lost cell population. To this end, the pancreatic developmental program can be used as a road map to examine the pliability of both embryonic stem cells and cells resident in the adult pancreas as sources of β-cells. In this review, we summarize current understanding of vertebrate pancreas development, and examine the various attempts made to maneuver cells, both embryonic and adult, toward pancreatic fate.
Pancreas Induction
Morphogenesis
The morphological events that occur during pancreas organogenesis involve a series of complex tissue interactions that are described in extensive detail both in vivo and in explant culture analyses using the mouse as a model organism (Gittes, 2009; Oliver-Krasinski and Stoffers, 2008). Cells fated to form the pancreas arise from the primitive gut tube, derived from definitive endoderm (Wells and Melton, 2000). The first morphological evidence for a pancreatic domain appears as a thickening on the dorsal side of the foregut epithelium at embryonic day 9.5 (e9.5). Simultaneously, two ventral buds originate laterally within the epithelium, in close apposition to the hepatic and bile duct endoderm, although one of them regresses prior to gut rotation. As the dorsal bud thickens, the base of the diverticulum attached to the epithelial sheet thins out to adopt a stalk-like morphology. After gut rotation and fusion of the dorsal and ventral aspects, the pancreatic epithelium branches into the surrounding mesenchyme (Figure 1). Fate specification of all differentiated cell types that form the adult organ occurs by e15.5. As the complex three-dimensional branched structure forms, endocrine precursors, depicted as green cells in Figure 1, delaminate from the developing epithelium and the cells that remain within the epithelium (yellow) adopt exocrine fates. Attempts to elucidate the sequence and molecular requirements for appropriate branching have focused on culture systems that support growth of the embryonic pancreas in vitro (Gittes et al., 1996; Percival and Slack, 1999). Live imaging of tissue explants has revealed that the pattern of branching within the embryonic pancreas is similar to ‘‘domain branching’’ in the embryonic lung, wherein new buds originate lateral to the primary axis of budding, distinct from the terminal bifurcation that occurs in the developing kidney (Morrisey and Hogan, 2010; Puri and Hebrok, 2007). Although such analyses, along with modeling of morphogenetic movements, have begun to forge a path toward improving our understanding of the complex tissue organization that occurs during development, the mechanisms that govern the intricate concert of branching in the pancreas remain poorly understood (Puri and Hebrok, 2007; Setty et al., 2008).
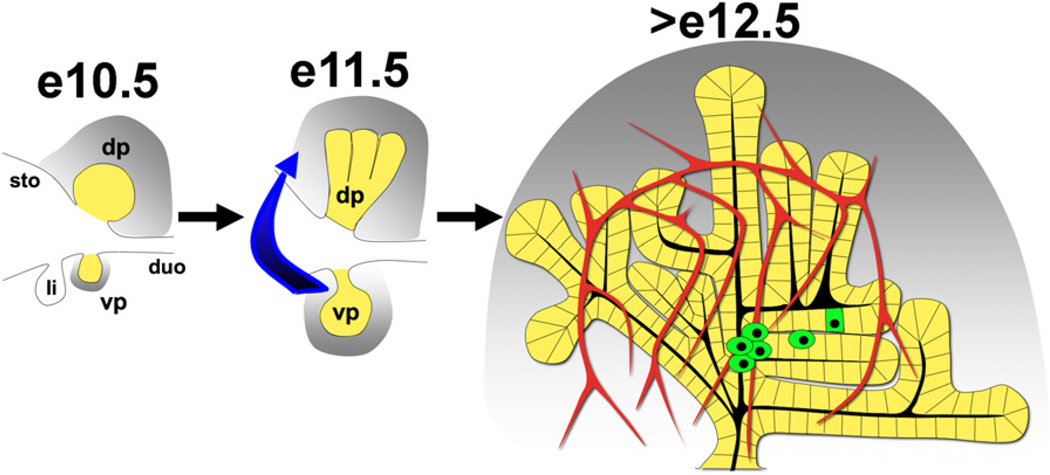
Figure 1. Early Steps during Embryonic Pancreas Development.
Dorsal (dp) and ventral (vp) pancreatic buds can be visualized (yellow) along with the stomach (sto), duodenum (duo), and liver bud (Li) in the posterior foregut at e10.5. The pancreatic mesenchyme (gray) collects around developing buds and provides essential instructive signals. Gut rotation (represented by the blue arrow) at e11.5 leads to fusion of the ventral and dorsal aspects followed by expansion into the surrounding mesenchyme (gray). After e12.5, secondary transition leads to endocrine specification (green) within the epithelium furthest from the mesenchyme, in close apposition to the vasculature (red vessels).
The Developmental Niche
The cellular environment of the growing pancreas plays a crucial instructive role. Prepancreatic patterning of the gut endoderm relies on signals from the surrounding mesodermal tissues— the notochord, lateral plate mesoderm, and the vasculature (Gittes, 2009; Wells and Melton, 2000). The earliest such interaction occurs upon gut closure, when the dorsal face of the epithelial sheet is in close apposition to the notochord. Molecular signals from the notochord establish a permissive environment for dorsal pancreatic specification within the gut endoderm (Hebrok et al., 1998). Patterning of the ventral buds is distinct from that of the dorsal bud, relying on signals from the overlying cardiac mesenchyme and the lateral plate mesoderm (Kumar et al., 2003; Wandzioch and Zaret, 2009). Intriguingly, the default fate of the ventral endoderm is pancreatic, and prohepatic signals are required to promote liver bud formation from the ventral foregut (Wandzioch and Zaret, 2009). Thus, context is a defining factor in patterning the presumptive pancreatic region.
The influence of the vasculature in coordinating pancreas formation is evident at e8 in the mouse embryo when the fused paired aortas displace the notochord adjacent to the dorsal foregut. Using Xenopus embryos, Lammert and colleagues showed that the dorsal aorta is necessary and sufficient for pancreas, and specifically endocrine pancreas, specification (Lammert et al., 2001). The endothelium appears to impact dorsal and ventral growth differently (Yoshitomi and Zaret, 2004). The dorsal bud, although specified, fails to expand in a genetic model that irreversibly depletes the endothelium, but the ventral aspect proliferates successfully, again highlighting inherent differences between the requirements for dorsal and ventral pancreas formation.
Not surprisingly, the vascular niche impacts adult endocrine organization and function as well. Ectopic overexpression of vascular endothelial growth factor (VEGF-A) in the pancreatic epithelium results in islet hyperplasia and the appearance of insulin-expressing cells in the posterior stomach, while loss of VEGF-A depletes capillaries from the islet, resulting in defective insulin secretion (Lammert et al., 2001; Lammert et al., 2003). The interplay between endothelial and endocrine cells is further illustrated by contribution of the endothelium to the islet basement membrane (Nikolova et al., 2006). From a functional perspective, close association of the endocrine compartment with the vasculature is crucial for the ability of β-cells to monitor blood glucose levels (Brissova et al., 2006). The intimate connections between endothelia and endocrine cells, both during development and in the mature organ, argue that endothelial-derived signals might also be useful for guiding the differentiation of stem cell populations toward β-cells in cell culture.
After the initial signaling from the dorsal aorta, the splanchnic lateral plate mesoderm accumulates around the growing pancreatic buds and secretes factors required for appropriate expansion of the organ (Figure 2; Gittes et al., 1996). Active interaction between the developing mesenchyme and the vasculature exists, and the dorsal mesenchyme, but not the ventral, requires signals from endothelial cells to grow (Edsbagge et al., 2005; Jacquemin et al., 2006). Although the list of known soluble factors produced by the mesenchyme is short, it was generally accepted that increased proximity to the mesenchyme promoted acinar or exocrine differentiation (purple cells, Figure 2) and inhibited endocrine growth (blue cells, Figure 2; Gittes et al., 1996; Miralles et al., 1998). Before endocrine differentiation is initiated, mesenchymal (gray cells, Figure 2) signals promote the expansion of pancreas progenitor cells (yellow cells, Figure 2; Attali et al., 2007; Bhushan et al., 2001). While informative, these explant culture findings also highlight the requirement for in vivo manipulation of the pancreatic mesenchyme, a task that has not been accomplished due to the absence of Cre lines that efficiently target this tissue. Future identification of diffusible factors secreted by the pancreatic mesenchyme should augment our knowledge of how fate restriction occurs within the developing pancreatic epithelium.
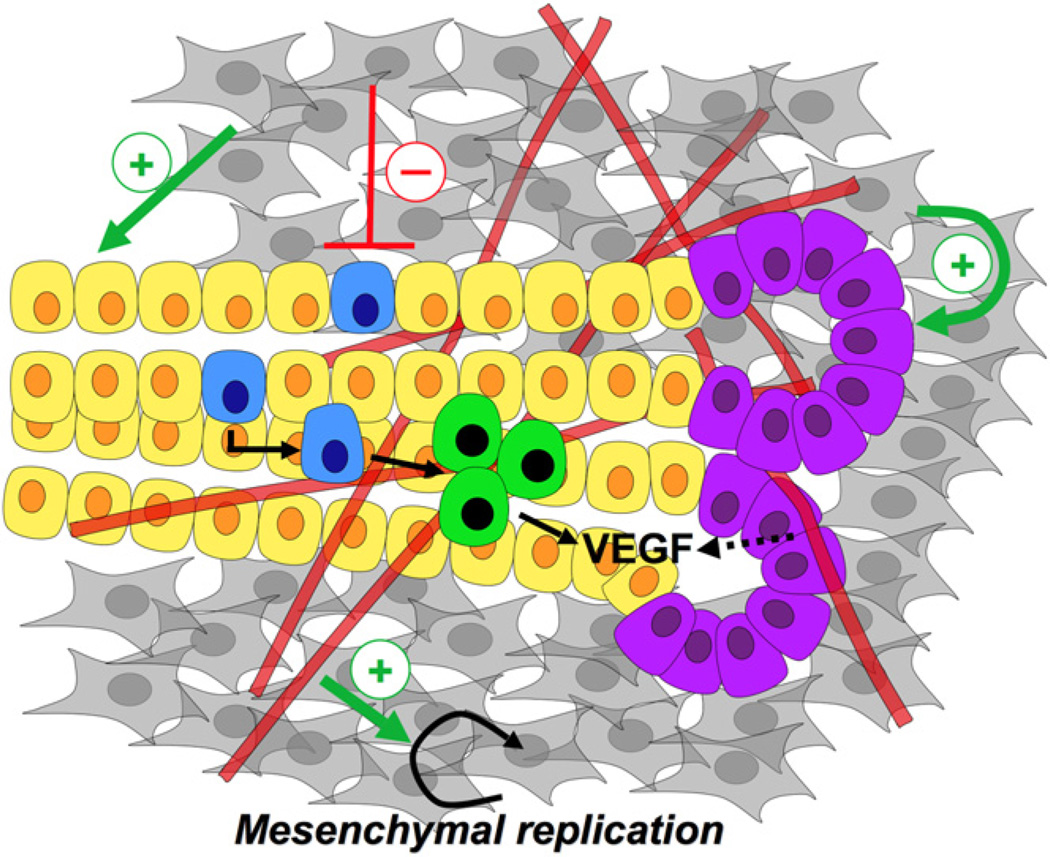
Figure 2. The Developmental Niche of the Embryonic Pancreas.
During the early stages of development (e10.5–e13.5), multipotent progenitor cells within the pancreatic epithelium (yellow) expand into the surrounding mesenchyme (gray) while being populated by the endothelium (red tubes). Epithelium-mesenchyme, epithelium-endothelium, and endothelium-mesenchyme interactions occur simultaneously during pancreas development. The mesenchyme positively promotes the ‘‘proximal’’ tissue (purple cells), while being inhibitory to the more ‘‘distal,’’ endocrine progenitor cells (blue), which have been observed to form closer to the interior of the three-dimensional organ. Interestingly, the mesenchyme has a positive influence on the pancreatic progenitors. Endocrine cells (green) are found in close apposition to the vasculature, and known to secrete VEGF, which may recruit more blood vessels to the site of islet formation. The endothelium also exerts a positive effect on the mesenchyme, as in the absence of the vasculature the mesenchyme fails to develop around the dorsal pancreatic bud.
Signaling within the Developing Pancreas
To date, we have a fairly comprehensive understanding of the signaling events that regulate pancreas development (Gittes, 2009; Oliver-Krasinski and Stoffers, 2008). Two recurring themes emerge during pancreas organogenesis with respect to signaling pathways. First, a pathway may serve temporally distinct functions, and second, dosage of signaling appears critical. Strict control of the level of signaling likely provides the desirable outcome during development. It is notable that the dorsal and ventral aspects of the pancreas receive distinct environmental cues, yet form the same cohort of cell types. This suggests flexibility in terms of the sequence and type of signals that can be applied to differentiating cells, including embryonic stem cells, to enrich for pancreatic fate. The key signaling events that impact pancreas organogenesis have been reviewed extensively in the citations above and are outlined briefly below.
FGF, TGF-β, and BMP Signaling
Patterning of the dorsal foregut by the notochord and dorsal aorta is mediated at least in part by secreted members of the FGF and transforming growth factor β (TGF-β) signaling pathways. FGF10 is required for pancreas development, and loss of signaling leads to a block in epithelial proliferation (Bhushan et al., 2001). At least two functions have been attributed to FGF signals from the pancreatic mesenchyme. First, ectopic expression of FGF10 impacts the size of the epithelial progenitor population via Notch signaling (Hart et al., 2003; Norgaard et al., 2003). In tissue culture, the mesenchyme has an inhibitory effect on endocrine fate via increased Notch signaling, indicated by upregulation of hairy enhancer of split 1 (Hes-1) expression at the point of epithelial-mesenchymal contact (Duvillie´ et al., 2006). However, a recent report suggests that mesenchymal signaling boosts the pancreatic progenitor pool and thereby increases the endocrine population (Attali et al., 2007). These contrasting observations strongly suggest that the mode of culture can have a significant impact on the experimental outcome, and careful interpretation of such approaches is critical. Second, low levels of FGF signaling repress expression of sonic hedgehog (Shh) in the foregut epithelium, thereby blocking Hedgehog (Hh) signaling within the pancreatic domain (Hebrok et al., 1998). Similarly, combined activities of activin, a ligand of the TGF-β family, and retinoic acid signaling originating from the pancreatic mesenchyme regulate appropriate development within the pancreatic epithelium (Kim et al., 2000; Martı´n et al., 2005; Molotkov et al., 2005). Later in embryonic development, TGF-β signaling appears to regulate the endocrine-exocrine fate decision (Miralles et al., 1998).
The ventral pancreas arises from the lateral endoderm domain, adjacent to the presumptive liver. Detailed fate mapping of the early mouse embryo has revealed the existence of bi-potential foregut progenitors that can give rise to hepatic and pancreatic cell types (Tremblay and Zaret, 2005). Retinoic acid signaling from the paraxial mesoderm cells aids in establishing an anterior-posterior positioning of the liver and pancreatic domains within the gut (Kumar et al., 2003). Further patterning of the ventral foregut occurs through FGF signals from the cardiac mesenchyme and bone morphogenetic protein (BMP) signals from the septum transversum mesenchyme, which promote hepatic fate while suppressing pancreatic fate (Deutsch et al., 2001; Rossi et al., 2001; Wandzioch and Zaret, 2009). Upon foregut closure, it is thought that cells that move caudally away from these signals adopt a pancreatic fate, as tissue explant cultures of a mutant defective in caudal migration lacked a ventral pancreas (Bort et al., 2004). Thus, an intricate concert of signaling events impacts the decision to form liver or pancreas in the ventral foregut.
Hh Signaling
Extensive research has established that Hh signaling needs to be minimal within the dorsal and ventral pancreatic anlagen during initiation of organ development. The binding of soluble ligands Sonic (Shh), Desert (Dhh), and Indian (Ihh) Hedgehog to the cell surface receptor Patched (Ptch) relieves inhibition on Smoothened (Smo), resulting in intracellular transduction of the signal. Suppression of Shh, via signaling from the notochord, is critical for pancreas development (Hebrok et al., 1998; Kim et al., 2000). In fact, ectopic expression of Shh in the pancreatic endoderm results in a dysmorphic pancreas surrounded by intestinal tissue (Apelqvist et al., 1997). Furthermore, suppression of Shh in the foregut leads to expansion of the pancreatic field, indicating that Shh likely functions to repress pancreas fate while promoting stomach and duodenal fates (Kim and Melton, 1998). Thus, Shh expression generates a molecular boundary between organs in the fore-midgut area. In contrast, loss of Ihh results in decreased pancreas mass, suggesting that Ihh might be required for certain aspects of pancreas development, perhaps in concert with Dhh (Hebrok et al., 2000). Although it is currently unclear when during development Ihh/Dhh exert their influence, existing evidence points to a complex interplay of ligands in determining the appropriate level of signaling within the pancreas.
Notch Signaling
Notch signaling regulates cell fate decision during pancreas development by maintaining cells in an undifferentiated, progenitor-like state, most likely in conjunction with FGF signaling through epithelial-mesenchymal interactions (Hart et al., 2003; Norgaard et al., 2003). Cell surface binding of ligands such as Delta and Serrate leads to intramembrane cleavage of the Notch receptor, generating an active intracellular domain that interacts with the effector RBPJ to activate the Hes family of transcription factors. The inhibitory effect of Notch signaling on endocrine development is well documented. Embryonic depletion of components of the Notch pathway leads to the pancreas becoming overpopulated with endocrine cells, demonstrating the requirement of Notch in maintaining pancreatic progenitors (Apelqvist et al., 1999; Jensen et al., 2000). The suppression of Ngn-3 by Notch, mediated by Hes-1, also highlights a critical role for the signaling pathway in curbing endocrine development (Gradwohl et al., 2000; Jensen et al., 2000). Predictably, sustained activation of the Notch pathway has a converse effect on the developing pancreas, with persistence of progenitors at the expense of differentiated endocrine and acinar cells (Hald et al., 2003; Murtaugh et al., 2003). These data underscore the involvement of the Notch pathway in maintaining a pool of progenitors that contribute to both endocrine and exocrine lineages.
The role of Notch in the exocrine compartment presents a complex picture. Depletion of RBPJ in exocrine tissue results in a severe loss of pancreatic mass and postnatal death (Nakhai et al., 2008). Precocious differentiation of endocrine cells in this model indicates that cells exit the stem cell state and differentiate into endocrine cells inappropriately. Animals lacking Notch1/2 within the pancreatic epithelium survive with no gross abnormalities, suggesting a Notch-independent role of RBPJ in regulating both endocrine and exocrine growth. It is of interest to note that RBPJ knockout mice do form some acinar cells, and this may be explained by the presence of the RBPJ homolog, RBPJL (Beres et al., 2006). Also, although they are mainly expressed in the pancreatic mesenchyme, one cannot rule out the contribution of Notch 3 and 4 in the Notch1/2 knockout animals (Apelqvist et al., 1999; Lammert et al., 2000). Activation of Notch signaling in pancreatic explant cultures from mouse embryos, either by overexpressing Notch intracellular domain or Hes-1, causes repression of acinar differentiation, further supporting the idea that prolonged Notch activity inhibits exocrine development (Esni et al., 2004). In summary, Notch appears to regulate a stem cell niche that, when perturbed during embryogenesis, leads to precocious differentiation of the endocrine compartment at the expense of the exocrine lineage. However, sustained Notch activation in exocrine precursors blocks acinar differentiation, demonstrating a tightly controlled temporal and spatial requirement for the pathway in guiding pancreas cell differentiation.
Wnt Signaling
Several members of the canonical Wnt signaling pathway are expressed in the developing pancreas (Heller et al., 2002). Conflicting data exist about the role of Wnt signaling during early pancreas development, perhaps because different pathway components have been targeted using distinct Cre lines. Predictably, too much or too little signaling is detrimental to organogenesis and can result in similar phenotypes. Activation (through either stabilized β-catenin, the downstream mediator of canonical Wnt signaling, or loss of function of adenomatous polyposis of the colon [APC]) or inhibition (through either loss of β-catenin or misexpression of a frizzled 8 dominant-negative protein) of the pathway impacts both pancreas size and composition (Murtaugh, 2008). The importance of timing with regard to regulation of Wnt pathway activation has been demonstrated using two different Cre lines that permit temporally distinct stabilization of β-catenin (Heiser et al., 2006). Activation of Wnt signaling using an early Cre line results in pancreatic hypoplasia due to reduction and inappropriate differentiation of progenitor cells, while activation later during embryogenesis leads to increased pancreatic mass. Thus, temporal regulation of signaling during development is critical in regulating organ fate and size. The contribution of non-canonical Wnt signaling to pancreas development, including the planar cell polarity pathway, remains to be elucidated.
Wnt signaling also appears to play a role in the adult endocrine compartment. The effect of postnatal stabilization of Wnt signaling in mouse β-cells may depend on the age of the cells. Increased Wnt activity in younger β-cells produces larger islets and increased insulin secretion, suggesting that Wnt signaling plays a role in β-cell proliferation (Rulifson et al., 2007). In contrast, prolonged Wnt activation causes eventual breakdown of the β-cell differentiation state accompanied by a loss of hormone and marker expression (Heiser et al., 2006). This is reminiscent of ectopic stabilization of β-catenin during early development, which results in profound loss of Pdx-1 expression (Heiser et al., 2006). Finally, the discovery that polymorphisms of TCF7L2, a member of the TCF/LEF family of Wnt effectors, have a strong genetic association with type 2 diabetes further underscores the importance of elucidating the role of this pathway in adult islet function (da Silva Xavier et al., 2009; Grant et al., 2006). Thus, Wnt signaling appears to play multiple roles during pancreas development and in adult tissue, and its effects are strongly dependent on timing and context. These issues need to be kept in mind when designing strategies to promote desired fates in undifferentiated cells ex vivo.
In summary, strict temporal control of signaling events that trigger intracellular cascades is imperative as they direct cell differentiation, tissue morphogenesis, and organ function. Ectopic activation or depletion analyses do not necessarily provide a clear picture of the events guiding pancreas morphogenesis but nevertheless have contributed greatly to our understanding. We have come to appreciate the controlled concert of signaling interactions that occurs during pancreas development and are slowly peeling away the layers of complexity that govern these processes. It is important that we understand the impact of modulating any of these pathways if we are to progress into a therapeutic arena.
Transcriptional Control of Gene Specification
Through intense research over the last several years, a hierarchy of transcription factors regulating pancreas development has emerged (Wilson et al., 2003). Identifying and manipulating master regulatory molecules to generate functional β-cells has become the focus of regenerative medicine aimed at curing diabetes. Pancreas and duodenal homeobox gene-1 (Pdx-1) is a prominent factor in the regulation of pancreas development. First expressed around e8.5 in mouse, Pdx-1 marks a multipotent progenitor population within the pancreatic domain early in development. Although early pancreas buds still form, Pdx-1 knockout mice completely arrest pancreas organogenesis after the initial stages (Ahlgren et al., 1996; Jonsson et al., 1994; Offield et al., 1996). Lineage tracing analysis has revealed contribution of Pdx-1-positive cells to all adult pancreatic fates (Gu et al., 2002). In the adult pancreas, Pdx-1 is most highly expressed in β-cells and δ-cells, with lower expression in exocrine cells. Significantly, Pdx-1 directly modulates expression of the insulin gene, along with other genes required for appropriate β-cell function such as Glut-2 and glucokinase (Ohlsson et al., 1993). An essential role for Pdx-1 has been established in humans as well. Haploinsufficiency of Ipf1, the human homolog of Pdx-1, leads to maturity onset diabetes of the young (MODY4), an autosomal dominant form of diabetes caused by monogenic mutations (Stoffers et al., 1997). Recent reports further emphasize the important role of Pdx-1 in β-cell formation and function (Zhou et al., 2008). In fact, it is one of three factors required to activate β-cell-specific genes within an acinar cell (discussed below). Thus, Pdx-1 provides essential functions for both pancreas development and adult islet function.
Although it is critical for pancreas development, Pdx-1 expression is not restricted to the pancreas and spreads to the distal stomach, common bile duct and the duodenum. A recent report demonstrates that Pdx-1 is co-expressed with another transcription factor, Sox17, in the ventral foregut domain that gives rise to both the biliary and pancreatic primordia. Subsequent ventral pancreas formation requires the downregulation of Sox17, whose expression persists in the adjacent biliary tract and serves to generate a molecular boundary between these tissues (Spence et al., 2009). Also overlapping with Pdx-1 expression and activatedshortlyafterwardsisp48/PTF1a,atran-scription factor whose expression is restricted to the pancreatic anlage in the foregut. As development proceeds, p48 expression is confined to the acinar and ductal compartments. p48 null mice are devoid of all exocrine and most endocrine tissue and exhibit postnatal lethality (Kawaguchi et al., 2002; Krapp et al., 1998). p48 is also known to interact with Notch signaling effectors, thus integrating signaling cues into the transcriptional regulatory network (Fukuda et al., 2006).
Several transcription factors have been identified that pattern the gut endoderm toward a pancreatic lineage before Pdx-1 and p48 expression. These include Hlxb9 (Hb9), Onecut1 (HNF6), FoxA2, Tcf2, and Hhex (Hex) (Gittes, 2009). Identification of these factors has enabled elucidation of the earliest steps in pancreatic formation. Three members of the HNF family of transcription factors, HNF6/onecut1, vHNF1/HNF1β, and FoxA2, impact steps from early development to mature pancreatic function. HNF6, which is expressed in the gut endoderm, is regulated by vHNF1/HNF1β, expressed throughout the early endoderm. HNF6 in turn regulates FoxA2, which, along with FoxA1 (Hnf3α), is a major regulator of Pdx-1 expression during pancreas specification (Gao et al., 2008).
In the developing pancreas, Sox9 is expressed between e9.0 and e12.5 in cells that co-express Pdx-1, indicating that Sox9 marks a progenitor population (Seymour et al., 2007). Sox9 inactivation during pancreas organogenesis leads to pancreatic hypoplasia, further supporting a critical role for this gene. Binding of Sox9 to the upstream promoter regions of neurogenin-3 (Ngn-3, see below) suggests a regulatory role for Sox9 in endocrine development (Lynn et al., 2007). Furthermore, lineage-tracing analysis in which Sox9-positive cells express GFP provides evidence that Ngn-3-positive cells arise from Sox9 progenitors (Seymour et al., 2008). At e15.5, approximately 75% of Ngn-3-expressing cells display low amounts of GFP, indicative of prior Sox9 expression. As for Pdx-1, gene dosage appears to be critical for Sox9 function, as removal of one allele leads to endocrine defects in mice (Seymour et al., 2008). Thus, Sox9 promotes survival and proliferation of progenitors within the pancreas.
The endocrine program is initiated by one key transcription factor, Ngn-3. Ngn-3 is a basic helix-loop-helix protein, and marks the progenitor population of cells fated to form the endocrine lineage. Animals lacking Ngn-3 are devoid of islets and die shortly after birth due to hyperglycemia (Gradwohl et al., 2000; Gu et al., 2002). Within the embryonic pancreas, Ngn-3 expression peaks at e15.5, shortly after the secondary transition that signifies the burst of endocrine specification, and subsequently declines. Regenerative medicine aimed at generating pancreatic β-cells has focused on Ngn-3 as a central factor as it is positioned at the apex of the transcriptional cascade that determines all endocrine specification.
Given its importance, several studies have attempted forced expression of Ngn-3 during embryogenesis with the goal of enriching for endocrine cells. In general, ectopic expression of Ngn-3 causes cells to exit from the cell cycle and to express some endocrine markers (Apelqvist et al., 1999). However, it is clear that mere overexpression of Ngn-3 does not guarantee expansion into the β-cell lineage. In fact, in the majority of studies, increased glucagon- or somatostatin-expressing cells are observed (Schwitzgebel et al., 2000). Yet again, context and timing seem to be critical for an appropriate cascade of events to occur. Forced expression of Ngn-3 at different time points during development has exposed temporal windows when embryonic epithelial cells are competent to respond to Ngn-3 and differentiate into distinct endocrine cell lineages (Johansson et al., 2007). More recent work has demonstrated a previously unanticipated role for Ngn-3 in islet maturation and maintenance of endocrine function (Wang et al., 2009).
Downstream of Ngn-3 there are several transcription factors that regulate the formation of the various cell types within the islet. The Pax and Nkx genes, NeuroD, Iroquois-type homeobox proteins Irx1 and 2, Isl1, and Arx genes all contribute to maintaining the appropriate balance of the different types of endocrine cells (Wilson et al., 2003). For instance, knockout of Isl1, Pax6 or NeuroD essentially eliminates endocrine formation, and loss of Nkx2.2 leads to a complete absence of β-cells, with a reduction in α and PP cells (Sussel et al., 1998). Similarly, Nkx6.1 depletion results in a significant loss of β-cell mass (Sander et al., 2000). Several of these factors, first expressed during embryonic development, continue to be expressed in adult islets, playing critical roles in both formation of the endocrine compartment and maintenance of function during adulthood.
The Maf family of basic leucine zipper transcription factors has emerged as a critical mediator of β-cell formation and function (Nishimura et al., 2006). MafB is expressed in α- and β-cells early during embryogenesis. As development proceeds, MafB+Nkx6.1+ cells take on a β-cell identity, and MafB+Nkx6.1− cells proceed to synthesize glucagon. In developing β-cells, a switch from MafB to MafA appears to be critical for eliciting full differentiation. This switch is important as MafA directly regulates insulin transcription, thus playing a central role in regulating β-cell maturation (Matsuoka et al., 2003).
Pancreatic Progenitors during Embryogenesis
Although cell lineage tracing experiments have revealed the existence of progenitors within the embryonic pancreas, the exact genetic signature of such multipotent cells remains poorly defined. Generating large quantities of progenitors from embryonic tissue poses an obvious technical challenge, and efforts thus far have focused on better defining the transcriptional blueprint of such a cell. Characterizing a pancreatic stem cell signature could have considerable applications in regenerative medicine aimed at curing diabetes as it might allow specific expansion of such cells from stem cell populations in vitro.
Currently, progenitor cell populations marked by genes such as Pdx-1 display multipotentiality, although it is a safe assumption that not all Pdx-1-positive cells possess the ability to generate multiple lineages. Clearly, temporal regulation of potentiality exists—Pdx-1-positive cells present early in embryogenesis (for instance, at e10.5, shown as red cells in Figure 3) are multipotent while those present later than e12.5 are not. After e12.5, the Pdx-1-positive lineage becomes restricted in its potentiality, losing the ability to give rise to the ductal lineage (Gu et al., 2002). Furthermore, distal tip cells of the expanding epithelium at e12.5 that coexpress Pdx-1, Ptf1a, c-Myc, and Carboxypeptidase A1 (represented by purple cells in Figure 3) are capable of contributing to all cells within the pancreas, including the ductal (blue), endocrine (green), and exocrine (orange) cells (Zhou et al., 2007). The tip cells closely contact the mesenchyme, depicted as a gray ‘‘cap’’ in the schematic, and perhaps an as yet unidentified molecule signals to the epithelium, maintaining the progenitor state of the tip (purple) cells, while the cells more distant from the mesenchyme lose that influence and become restricted in their fate (Figure 3). This idea would be consistent with the observation that the mesenchyme/epithelial ratio is dramatically reversed during the time when progenitor cells lose their multipotency. Further analysis of these cells by isolation at distinct time points and gene expression profiling should provide a clearer picture of the process by which a progenitor cell loses its multipotency and commits to a specific lineage.
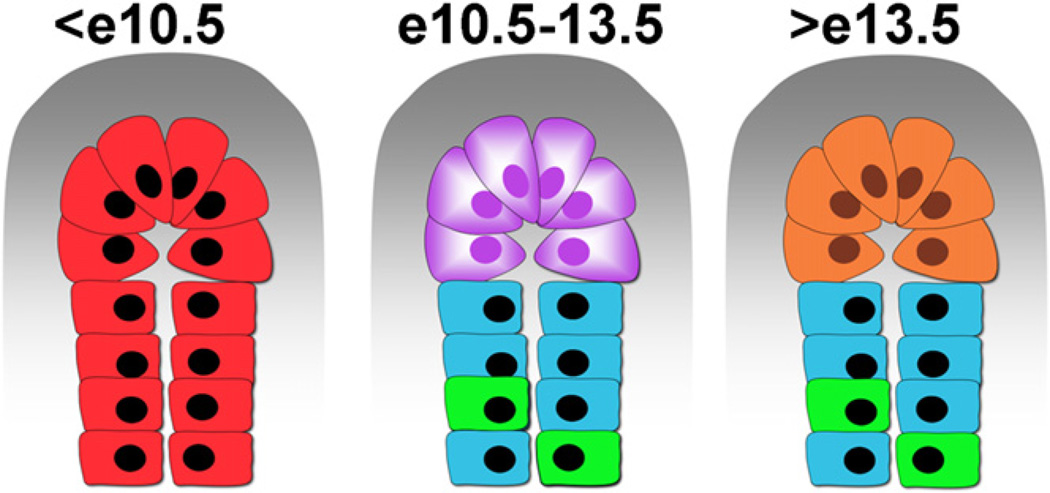
Figure 3. Lineage Restriction within the Pancreatic Epithelium.
Expansion ofthe progenitorpool atthe tip of the epithelium occurs early during development. From an uncommitted pool of progenitors (before e10.5, in red) to multipotent, Cpa-1-positive cells (purple) that give rise to exocrine (orange), ductal (blue), and endocrine cells (green). Endocrine progenitors go on to delaminate from the epithelium and cluster with other endocrine cells. It is believed that distance from the mesenchyme influences the decision of exocrine versus endocrine lineage.
Approaches to Restoring β-Cell Function—Replenish or Regenerate?
β-cell dysfunction is the underlying cause of diabetes (either cell death in type 1 or altered function in type 2). Normalization of blood glucose levels in diabetic patients serves to prevent debilitating complications of the disease. A longstanding objective of regenerative medicine has been to replace healthy functioning β-cells in diabetic patients. Whole pancreas and islet transplants using the Edmonton protocol have reduced the dependence of type 1 diabetics on exogenous insulin (Shapiro et al., 2006). However, the limited availability of cadaver tissue means that transplants are only feasible for a small fraction of patients. Alternative approaches to generate β-cells that can be transplanted to ameliorate hyperglycemia have been actively pursued, including differentiation of embryonic stem cells, expansion of existing β-cells, and finally, directing liver and other pancreatic cells to β-cells.
De Novo Generation of β-Cells
As the adult stem cell population within the pancreas is poorly defined despite extensive investigation, a more reasonable approach to generating sufficient quantities of β-cells for transplantation may be differentiation of embryonic stem (ES) cells. ES cells offer a significant theoretical advantage given their potential to generate large amounts of a desired cell type. Not surprisingly, much of the information used to direct human ES cell differentiation comes from early developmental biology studies. Application of principles gleaned from early embryological signaling events has yielded immature β-cells from human ES cells in culture (D’Amour et al., 2005, 2006; Kroon et al., 2008). More recently, small chemical compounds have been identified that increase the efficiency of definitive endoderm formation from human and mouse ES cells (Borowiak et al., 2009). However, even the best-case scenario currently produces immature, β-like cells, and mature β-cells that express the entire glucose sensing and insulin-producing machinery have not been generated. Perhaps what is missing is the significant contribution that the cellular environment provides during β-cell development. Coculture of cells expressing markers of the definitive endoderm with mesenchymal cells and/or endothelial cells in order to mimic the developmental niche during embryogenesis remains to be explored.
In 2006, groundbreaking research demonstrated that four key transcription factors (Oct3/4, Sox2, Klf4, and c-myc) could reprogram embryonic and adult fibroblasts into pluripotent stem cells that resembled but were not identical to ES cells (Takahashi and Yamanaka, 2006). The generation of induced pluripotent stem (iPS) cells has suggested tremendous potential for reprogramming patient-specific cells for replacement therapy, thereby circumventing ethical considerations surrounding human ES cell research (Park et al., 2008; Takahashi et al., 2007; Takahashi and Yamanaka, 2006). Remarkable progress has been accomplished since the original report describing iPS cells. While the original study used four transcription factors mentioned above, the recipe for reprogramming as well as the methods of introducing factors into cells have rapidly evolved (O’Malley et al., 2009). Modifications include the elimination of transcription factors such as c-myc and Sox2, identification of small chemical compounds that significantly improve the reprogramming efficiency by inhibiting DNA methyltransferases and histone deacetylases, and the use of adenoviruses and plasmids to circumvent the unsuitability of viral integrations into host DNA. In an attempt to eliminate viral sequences from reprogrammed cells, a Cre-based excision method has also been devised that generates iPS cells that are factor-free. More recently, proteins have been used to directly reprogram human fibroblast cells in the absence of any exogenous genetic material, albeit inefficiently. The obvious advantage of developing such approaches is to eliminate any genetic manipulation of the host cell. Using three transcription factors, type 1 diabetic patient-specific lines have been generated, providing a model to further investigate pancreatic disease (Maehr et al., 2009).
While stem cell based therapies promise to usher in a new era of regenerative medicine, one cannot ignore the hurdles that such approaches face. One of the largest, and most alarming, is that of oncogenic potential of stem cells. iPS cells pose a similar threat, as they have been subjected to extensive reprogramming that may lead to malignant growth when introduced into patients even after differentiation. Realistically, it is unlikely that any protocol developed would convert 100% of a stem cell population into the desired cell type. Thus, it is critical to develop tools that allow purification of the required cell type to homogeneity. Genetically modifying stem cells to express fluorescent markers facilitating cell purification is helpful as an experimental tool and for work with animal models of diabetes but would not be applicable in a human disease scenario. Isolation of desired cells via fluorescent activated cell sorting with antibodies directed against novel cell surface markers, or new combinations of existing antibodies against cell surface proteins might be used to exclude undifferentiated cells with tumorigenic potential. Encapsulation of stem cell-derived cells before transplantation might be another avenue to inhibit expansion and distribution of unwanted cells. Finally, there needs to be an emphasis on ascertaining that stem cell-derived insulin-producing cells truly function identical to endogenous β-cells. At a minimum, newly generated β-cells need to replicate the many intricate mechanisms that guide secretion of appropriate amounts of insulin in response to changes in blood glucose levels, ranging from glucose sensing, insulin production, storage and secretion, to timely cessation of insulin release. Thus, although embryonic stem and iPS cells do represent great opportunity, significant work needs to be done before transplantation of stem cell-derived tissue into human patients suffering from diabetes can become an accepted therapy.
β-Cell Proliferation and Regeneration
Recent work in rodents has indicated a previously unappreciated proliferative capacity for β-cells, an ability that might be explored to counteract the cell loss or malfunction that occurs during diabetes. In particular, altered metabolic demand, such as pregnancy or obesity, leads to adaptive changes in β-cell mass (Figure 4; Gupta et al., 2007; Karnik et al., 2007; Parsons et al., 1992). Genetic lineage-tracing experiments point to β-cells as the source of new β-cells in adult animals (Dor et al., 2004; Teta et al., 2007). However, the age of the animal appears to play a central role in determining the proliferative capacity of β-cells, and older animals display a significant decline in cellular expansion (Figure 4; Georgia and Bhushan, 2004; Rankin and Kushner, 2009; Teta et al., 2005; Tschen et al., 2009). In mice, β-cells are believed to be long-lived, and in one-year-old animals, only 1 in 1400 β-cells divide in a 24 hr period (Teta et al., 2005). Ectopic expression of growth-promoting factors in β-cells does allow adult cells to enter the cell cycle, and such expansion translates into functional regulation of glucose levels, resulting in hypoglycemia in animals (Garcia-Ocaña et al., 2000; Vasavada et al., 2000). Adult β-cells have a limited lifespan in culture and undergo dedifferentiation, with loss of insulin expression. Attempts have been made to manipulate the culture of these cells to block dedifferentiation (Bar et al., 2008). However, it has not yet been possible to identify culture conditions that allow proliferation of β-cells while maintaining their full functional state.
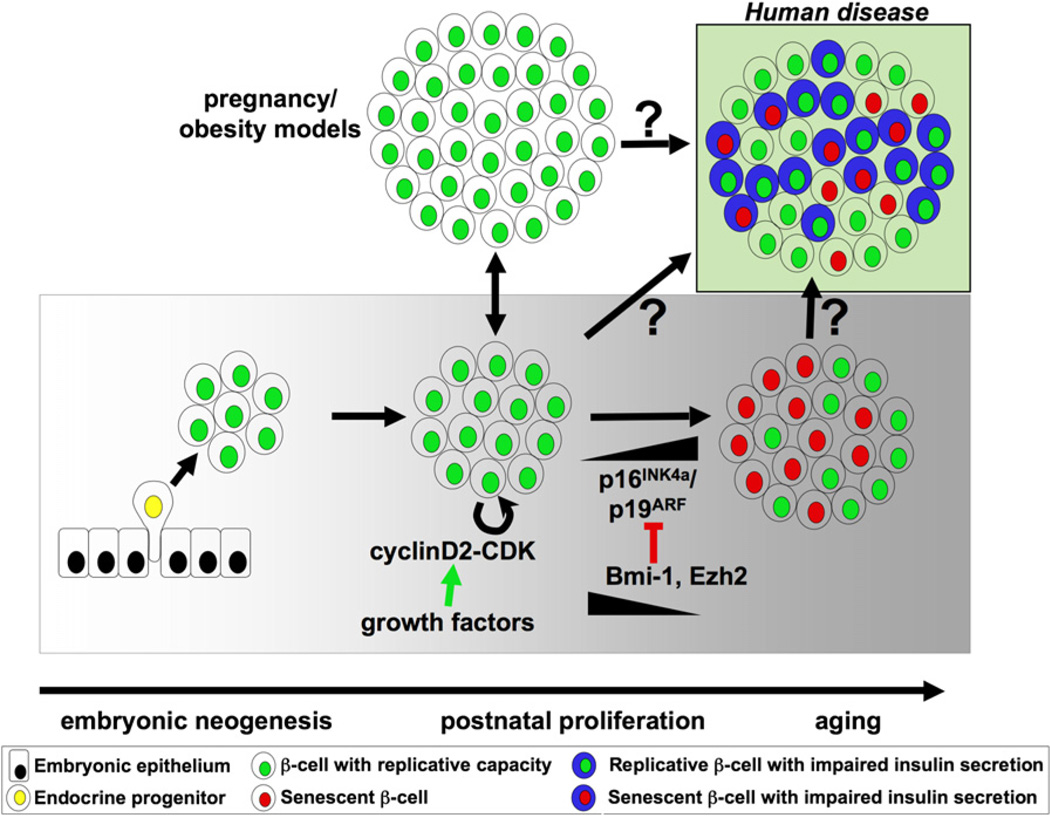
Figure 4. β-Cell Expansion.
During embryonic pancreas development, β-cell (green) expansion occurs through neogenesis from progenitors (yellow) within the pancreatic epithelium (black). In postnatal life, under normal conditions, β-cell proliferation through the cyclinD2-CDK complex is the predominant mode to adjust for changes in insulin demand, e.g., during pregnancy or development of obesity. With age, however, accumulation of tumor suppressors p16INK4a/p19ARF leads to a block in the replication potential of β-cells (red) concomitant with a reduction in the epigenome modifiers Bmi-1 and Ezh2. Although it is unclear what the role of β-cell replication and senescence is in the development ofhuman disease suchas type 2diabetes, one can envision an islet populated by cells competent to divide and respond to glucose and those lacking these abilities (Weir et al., 2009). Other endocrine cells are not shown for simplicity.
Significant progress has also been made in detailing the molecular machinery that regulates cell cycle progression in β-cells (Cozar-Castellano et al., 2006). The cyclin d2-CDK4 complex is important for β-cell mitotic entry, as mice depleted of either component develop severe hyperglycemia accompanied by a failure of β-cell expansion (Georgia and Bhushan, 2004; Kushner et al., 2005; Rane et al., 1999). Inhibitors of cyclin-CDKs, including p27, also impact β-cell proliferation (Georgia and Bhushan, 2006). Recently, epigenetic modifiers have been identified that block inhibitors of β-cell replication early in life. The p16Ink4a/p19Arf locus has emerged as a repressor of β-cell replication (Krishnamurthyetal., 2006). Bmi-1 and Ezh2, both of which form part of histone modification polycomb complexes, inhibit activity of p16Ink4a/p19Arf leading to active β-cell replication (Chen et al., 2009; Dhawan et al., 2009).
In addition, several in vivo models of β-cell regeneration have been developed to investigate the ability of β-cells to expand in adult animals. In one model, c-Myc overexpression results in synchronous cell death in greater than 90% of β-cells, followed by complete regeneration, presumably through β-cell neogene-sis (Cano et al., 2008). Similarly, a model that expresses the diphtheria toxin to induce cell death depletes close to 70% of β-cells, which subsequently regenerate by replication to restore normo-glycemia (Nir et al., 2007). Although thorough analyses of these models of β-cell regeneration strongly point to apoptosis of β-cells followed by regeneration, it is difficult to rule out that insulin expression may be downregulated in β-cells during such manipulations and thus a dedifferentiated state might be overlooked. This possibility is further suggested by the presence of Glut2+/insulin− cells in a model of β-cell regeneration that activates caspase 8 in β-cells, which could be construed as evidence for either dedifferentiated β-cells or progenitor cells within islets (Wang et al., 2008).
The current systems used to stimulate β-cell regeneration are clearly artificial. However, if we can mimic the cellular state that permits β-cell entry into the cell cycle in a robust manner, we may be able to use β-cells as source for generating more insulin-producing cells. Type 2 diabetes is a progressive disease, and as the ability of β-cells to expand diminishes over time, understanding the cell cycle machinery and how to manipulate it may be critical for increasing the likelihood of success for future therapies.
Manipulating Adult Tissue to Generate β-Cells
It is now evident that terminal differentiation, long thought to be an irreversible process, can be reversed in cells from an adult animal. Consequently, the field of regenerative medicine has seen several attempts to coax differentiated cells toward pancreatic fates. Cellular plasticity, in the context of a fully differentiated cell, is defined as the ability of that cell to change its epigenome and adopt a novel, functionally distinct fate. An adult cell can in principle dedifferentiate into a progenitor-like state with broader fate potential or switch fate to another lineage without going through a multipotent state. Terms such as transdifferentiation or transdetermination have been employed to fit scenarios where adult cells are coerced into expressing genes that mark a different fate, depending on how distinct the new fate is from the original one. Identifying cells amenable to such types of manipulations continues to be a much sought after goal, even though the precise mechanism of these changes remains murky.
Liver to Pancreas
Not surprisingly, one organ that has been explored as a source of reprogrammable cells is the liver. The liver and pancreas share their endodermal origin, and the existence of a bipotential progenitor cell with the ability to give rise to hepatic or pancreatic fate is now generally accepted (Chung et al., 2008; Deutsch et al., 2001; Wandzioch and Zaret, 2009). Several attempts have been made to generate pancreatic cell types from adult liver cells by overexpression of pancreas-specific transcription factors (Ferber et al., 2000; Kaneto et al., 2005; Wang et al., 2007). Adenoviral delivery of Pdx-1 via systemic infection, either alone or in combination with additional soluble factors (EGF and nicotinamide) activates insulin expression in liver cells both in vivo and in hepatocytes isolated from fetal or adult liver. Such treatment alleviates the hyperglycemia induced by streptozotocin (STZ) treatment that abolishes β-cells, providing evidence for insulin expression and secretion in hepatocytes (Ber et al., 2003; Ferber et al., 2000; Sapir et al., 2005). However, manipulating a gene as far upstream in the regulatory network as Pdx-1 is likely to lead to adverse effects. Thus, it is not surprising that other groups report the induction of the exocrine lineage within the liver, or complications such as fulminant hepatitis (Kojima et al., 2003; Miyatsuka et al., 2003).
Until recently, the identity of the hepatic cell that adopted a pancreatic fate had remained elusive. Yechoor et al. (2009) now report that Ngn-3 overexpression in the liver induces expression of several islet hormones concomitant with decrease of albumin, which indicates loss of hepatic identity. Significantly, lineage-tracing analysis strongly suggests that the cells that possess this capacity are the oval cells. The authors propose that the oval cells within the liver play the role of facultative stem cells that retain the ability to adopt pancreatic endocrine fates. Rescue of STZ-induced hyperglycemia in animals injected with adenoviruses expressing Ngn-3 further indicates at least some functional relevance of this approach. Ectopic expression of other more downstream factors such as NeuroD, in combination with factors promoting β-cell growth such as betacellulin, also appears to alleviate the hyperglycemia induced by STZ treatment (Kojima et al., 2003). Thus, modulating factors more specific to the endocrine lineage rather than Pdx-1 may decrease the possibility of inducing the formation of cells of the exocrine compartment (Ferber et al., 2000; Kojima et al., 2003).
Pancreatic Plasticity
The inherent plasticity of the adult pancreatic organ is an area of great interest. A common approach to identifying progenitor cells within the adult pancreas is to induce damage to trigger regeneration. Although pancreatic ducts are believed to harbor precursors for endocrine and exocrine lineages during embryonic development, whether such precursors are present in adult ducts is unclear. One model to investigate this question involves depriving rats and hamsters of copper, which leads to a greater than 80% loss of the acinar compartment, and results in subsequent repopulation of the pancreas with hepatocytes (Rao et al., 1989). A possible explanation for this could be the existence of a pancreatic ‘‘stemlike’’ cell capable of giving rise to hepatocytes and originating from duct-like structures. Alternatively, the origin of these cells might lie within the biliary tree, which is known to harbor cells that can contribute to the endocrine lineage (Dutton et al., 2007; Eberhard et al., 2008). The biliary epithelium possesses the ability to form endocrine cells in the absence of Hes-1, which represses Ngn-3 expression (Sumazaki et al., 2004). It would be interesting to investigate whether the cells that appear in the pancreas after copper deprivation have the ability to form pancreatic endocrine cells, for instance by introducing factors such as Ngn-3, instead of hepatocytes.
A large body of data exists suggesting that pancreatic ductal cells can give rise to insulin-producing cells in vitro (Bonner-Weir et al., 2000; Gao et al., 2003; Ramiya et al., 2000). In culture, pancreatic ductal cell lines can be induced to express islet hormones upon exposure to exendin-4/GLP1, activin A, and HGF or betacellulin. Infecting pancreatic ductal cell lines with Ngn-3-expressing adenoviruses also initiates the endocrine program, pointing once again to the ability of these cells to change fate (Gasa et al., 2004; Heremans et al., 2002). Evidence is accumulating that a ‘‘normal’’ duct cell can dedifferentiate and assume the expression profile of embryonic progenitors (Figure 5). Bonner-Weir and colleagues propose that sustained proliferation of the adult ductal epithelium after injury yields a pool of less differentiated cells that can be coaxed toward an endocrine fate. In the absence of additional cues, perhaps these cells revert back to the ductal phenotype. A carbonic anhydrase II-Cre line marking duct cells in a pancreatic duct ligation (PDL) model of injury labels islets and acinar cells (Inada et al., 2008). Although these data support the idea that duct cells can give rise to both acinar and endocrine cells, the possibility of misexpression in other cell types cannot be ruled out, as a human fragment of the CAII promoter was used to direct Cre expression. In another duct ligation model of pancreatic injury, the ability of cells located in the duct lining to differentiate into islet cells has been reported (Xu et al., 2008). Ligation of the duct leading to the tail of the pancreas, while leaving the head of the organ untouched, leads to acinar degeneration along with duct expansion. Increased Ngn-3 expression within duct cells (schematized as purple cells with elevated Ngn-3 expression in Figure 5, top panel) concomitant with islet hyperplasia indicate that β-cell expansion following such a manipulation progresses, at least in part, through Ngn-3-positive intermediates that originate from the ducts (yellow cell) or cells within the duct lining (orange cell). Furthermore, Pdx-1 expression is induced in ducts after injury through partial pancreatectomy (Sharma et al., 1999). Altogether, these data support two hypotheses—either the ducts harbor a pool of cells that behave as stem cells, or the ducts are capable of dedifferentiating into a more progenitor-like state in culture or upon injury. To develop this type of approach as a treatment option, it would be critical to be able to expand the duct progenitor population in the absence of injury, perhaps using either a gene therapy approach or small chemical compounds.
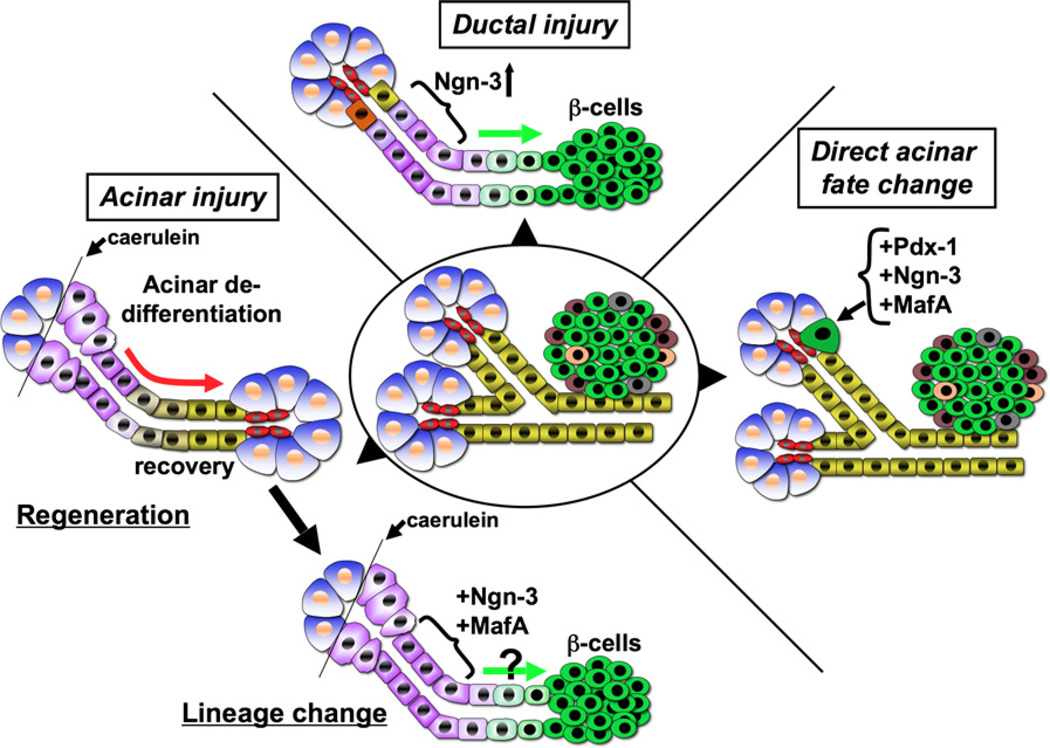
Figure 5. Fate Manipulation within the Adult Pancreas.
In a ductal injury model such as pancreatic ductal ligation (top schematic), normal duct cells (yellow), or duct-associated cells (orange) adopt a progenitor state (purple) and upregulate Ngn-3 expression that correlates with increased β-cell formation, depicted by the green arrow. In an acinar cell injury model (left schematic), acinar cells (blue) undergo dedifferentiation (purple) (for e.g., upon caerulein treatment) followed by recovery (red arrow) and thus regeneration. Introduction of key transcription factors such as Ngn-3 and MafA into such dedifferentiated cells, shown in the bottom schematic, might promote β-cell fate. A third scenario is of direct acinar fate change (right panel). Ectopic expression of Pdx-1, Ngn-3, and MafA in the adult pancreas redirects acinar cells to adopt an identity resembling an endocrine β-cell (green), although these cells fail to cluster with other endocrine cells.
Directly challenging the models discussed above, Solar et al. (2009) report that mature pancreatic duct cells do not contribute to either the endocrine compartment or the acinar lineage. The authors carry out lineage tracing using an HNF1bCreER mouse model that labels duct cells in both embryonic and adult tissues. Using two distinct approaches that allow for regeneration of β-cells (either using PDL or depleting endogenous β-cells using alloxan followed by treatment with EGF and gastrin), the report demonstrates no contribution of duct cells, as defined by HNF1b–positive cells, toward the β-cells that repopulate the pancreas. Although the analysis is convincing and the data support the conclusions, there are, as usual, some caveats with the experimental set up. The authors acknowledge that the efficiency of cells expressing the Cre recombinase is not 100%, possibly due to resistance of a subset of ductal cells to labeling. The rapid emergence of β-cells after PDL argues against a small stem cell niche or simply β-cell expansion. Furthermore, Xu et al. (2008) provide evidence that Ngn-3 progenitors, and therefore the process of neogenesis, does play a part upon injury related regeneration. Although lineage-tracing was not conducted in this study, the proximity of the Ngn-3 expressing cells to the ducts point to duct or duct associated cells as the culprit for the source of progenitors. Identifying more markers that allow labeling of the various types of duct cell populations should aid in deciphering the source of cells that contribute toward the different lineages in the pancreas.
By far the largest component of the pancreas is the acinar cell compartment, and this therefore provides a sizable source of cells for potential manipulation toward the endocrine lineage. Acinar cells have shown the potential to express β-cell or duct cell markers, for example, in studies that involve exposing them to EGF and nicotinamide, or TGF-α, but these analyses are currently restricted to cell culture manipulations (Means et al., 2005; Minami et al., 2005). Upon induction of exocrine damage via treatment with caerulein, a cholecystokinin analog that elicits precocious secretion of digestive enzymes, acinar cells suppress exocrine gene expression and reactivate a progenitor program by expressing genes like Pdx-1, Sox9, FoxA2, and members of the Notch pathway including Hes-1, suggestive of a progenitor-like state (conversion of normal acinar cells in blue into a dedifferentiated state shown in purple in Figure 5, left panel; Jensen et al., 2005; Morris et al., 2010). This apparent dedifferentiation is transient, as acinar cells regenerate within three to seven days, with no contribution toward a ductal lineage (recovery indicated by the red arrow in Figure 5, left panel). The ability of acinar cells to change fate toward an endocrine lineage has been challenged by lineage tracing in different injury models that demonstrate no contribution to either duct or endocrine lineage (Desai et al., 2007). Under these conditions, an acinar cell only begets an acinar cell, and no β-cell or ductal cell scores positive for the lineage-tracing marker. It cannot be ruled out, however, that Hes-1 expression in the dedifferentiated acinar cells blocks the initiation of the endocrine differentiation program under these conditions. Introduction of Ngn-3, for instance, might enable bypass of such interference and allow for fate change to occur (Figure 5, lower panel). In fact, inhibition of Notch signaling in rat acinar cultures promotes β-cells neogenesis, emphasizing the role of this pathway as an inhibitor of endocrine differentiation (Baeyens et al., 2009).
A recent paper reported exciting new data unveiling the ability of acinar cells to be remodeled into insulin-producing β-cells in vivo (Zhou et al., 2008). Using adenoviral injections directly into the pancreas, a set of three transcription factors, Pdx-1, Ngn-3, and MafA, appear sufficient to induce β-cell formation (Figure 5, right panel). Remarkably, the new β-cells express several β-cell-specific genes including NeuroD, Nkx2.2, and Nkx6.1, possess morphologic characteristics of β-cells, and significantly, correlate with a lowering of blood glucose in diabetic mice. Most interestingly, the insulin-expressing cells appear to arise from acinar cells, without cell replication or transitioning through a dedifferentiated state, thus undergoing direct lineage alteration. One caveat is the absence of physiological analysis of function in the newly formed β-cell. The correction of induced hyperglycemia is partial, and thus these cells might not represent fully functional β-cells. In addition, the acinar-derived β-cells do not aggregate to form the classical islet morphology. It is also possible that the percent of acinar cells that adopt the β-cell fate is too low, i.e., the process of fate change is inefficient. Of course, this approach is far from being ready for human application, but it does suggest the exciting possibility of manipulating the relatively abundant acinar cell population from cadaver donors to generate β-cells for transplantation.
There are several obvious differences between the ‘‘injury’’ and ‘‘viral induction’’ models. It is likely that the transient Pdx-1-expressing population generated upon caerulein treatment requires additional cues for efficient lineage modification toward endocrine cell fates. The absence of such cues would allow the dedifferentiated cells to revert back to an acinar lineage (Figure 5, left panel). Using viral infection of the pancreas in vivo to ectopically express regulatory factors yields a low rate of acinar derived β-cells. One explanation could be that the pancreas harbors a population of ‘‘less’’ differentiated acinar cells. The centroacinar cell is of interest, as it lies at the interface of the acinar and ductal system and exhibits active Notch signaling, indicative of maintaining a progenitor status (Miyamoto et al., 2003). Generating and using a Hes-1-CreER line to label centroacinar cells for lineage-tracing analysis would shed light on the lineage pliability of these cells. Furthermore, increasing the population of acinar cells that are ‘‘pliable,’’ perhaps through injury models such as caerulein followed by ectopic expression of important transcription regulators, might lead to greater success in revealing their potential to form β-cells. The obvious advantage of expanding this approach is to circumvent the ethical issues that haunt human embryonic stem cell research. However, in situ dedifferentiation or lineage maneuvering of acinar cells is not feasible for therapy, as injection of viral vectors into the pancreas is likely to result in pancreatitis phenotypes or potentially even pancreatic cancer. Thus, ex vivo manipulation of acinar cells is more likely the process to be pursued for therapeutic potential.
Differentiation is characterized as a progressive restriction in developmental potential of a cell. Complex regulatory networks ensure the transition of a multi-potent progenitor cell to a differentiated cell with a unique functional identity. Thus, at face value, it seems naive to assume that manipulating individual molecules should result in fate redirection. Also, in the absence of stringent lineage tracing, the identity of the cell population being modified remains obscure. For true lineage alteration to occur, the original identity of an adult cell needs to be repressed and the entire complement of epigenetic changes associated with the desired lineage put in place. In case of β-cells, merely expressing insulin is not sufficient. The glucose sensing and insulin-secreting machineries also need to be active. Furthermore, a significant concern is achieving regulated expression rather than overexpression. This is illustrated by the effect of sustained Pdx-1 expression in the pancreas (in all cell types), which leads to acinar-to-ductal metaplasia (Miyatsuka et al., 2006). Thus, uncontrolled activation of factors that define and maintain the progenitor state during development may not be a successful strategy for adult tissue manipulation. Identifying a subset of regulatory factors that could be manipulated in a controlled manner within the organ seems a more rational approach to remodeling cells within a tissue.
Pancreatic Cancer—the Dark Side of Plasticity
Plasticity in adult pancreatic cells not only provides a unique opportunity to expand the number of ‘‘desired’’ cell types, e.g., insulin-producing β-cells, but also carries risks with regard to initiation of neoplastic transformation. Injury to pancreas results in exocrine damage and inflammation, hallmarks of acute or chronic pancreatitis. Pancreatitis has been realized as one of the risk factors for developing pancreatic adenocarcinoma (PDA), a lethal disease and the fourth leading cause of cancer death in the United States (Jemal et al., 2009). Short-term pancreatic injury via caerulein treatment results in temporary dedifferentiation of acinar cells that is quickly resolved with cells reassuming acinar identity (Jensen et al., 2005). However, a similar injury in tissues expressing activating mutations in Kras, the predominant oncogene in PDA, changes the redifferentiation course of the dedifferentiated acinar cells. Under these conditions, acinar to ductal metaplasia occurs, followed by the formation of pancreatic intraepithelial neoplasia (PanIN), lesions that lead to PDA over time (Carriere et al., 2009; Guerra et al., 2007). Although the exact mechanisms by which activated Kras signaling converts benign acinar regeneration into neoplastic transformation have not been fully elucidated, recent studies suggest that β-catenin/canonical Wnt signaling is a critical modulator of acinar regeneration that is not engaged in Kras-mediated acinar-ductal metaplasia (Morris et al., 2010). Furthermore, other embryonic signaling pathways implicated in pancreas development, including Notch and Hedgehog signaling, also appear to play crucial roles during acinar regeneration and neoplastic transformation (De La O et al., 2008; Miyamoto et al., 2003; Pasca di Magliano et al., 2006). Thus, while pancreatic injury might provide a previously unexplored opportunity to convert acinar cells into functional β-cells, it is important to place strong emphasis on ensuring that this regenerative response cannot be hijacked to result in the formation of cancer progenitors.
Perspective
Although the discovery of insulin transformed diabetes into a manageable chronic condition, insulin replacement therapy is proving insufficient as a means of preventing all of the side effects associated with long-term irregularities in glucose levels. Our search for large supplies of β-cells is far from over, and our quest is complicated by the fact that the mature β-cell is a highly specialized cell in which glucose sensing and insulin secretion functions are finely balanced. New data are rapidly emerging that point to the acinar cell as a source of tissue that may be open to manipulation for production of β-cells. Clearly, the many years of research that have been invested in understanding the pancreatic developmental program provided a knowledge base that was crucial for such research to take place. Development of new technologies that might aid, for example, in improving efficient and regulated expression of key transcription factors in the acinar cell of a pancreas that lacks functional β-cells will be an encouraging step toward eliminating the dependence of diabetic patients on exogenous insulin.
ACKNOWLEDGMENTS
We would like to thank Drs. J. Lau, S. Cervantes, A. Folias, and T. Guo for critical reading of the review. Research in pancreas development and stem cells in M.H.’s laboratory is supported by funds from the NIH (DK60533), the Brehm Coalition, The Leona M. and Harry B. Helmsley Charitable Trust, and the Juvenile Diabetes Research Foundation. S.P. was supported by funds from the Klein Family Foundation, the JDRF, and The Leona M. and Harry B. Helmsley Charitable Trust.
REFERENCES
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 1997;7:801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Attali M, Stetsyuk V, Basmaciogullari A, Aiello V, Zanta-Boussif MA, Duvillie B, Scharfmann R. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes. 2007;56:1248–1258. doi: 10.2337/db06-1307. [DOI] [PubMed] [Google Scholar]
- Baeyens L, Bonne S, Bos T, Rooman I, Peleman C, Lahoutte T, German M, Heimberg H, Bouwens L. Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology. 2009;136:1750–1760. doi: 10.1053/j.gastro.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Bar Y, Russ HA, Knoller S, Ouziel-Yahalom L, Efrat S. HES-1 is involved in adaptation of adult human beta-cells to proliferation in vitro. Diabetes. 2008;57:2413–2420. doi: 10.2337/db07-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J. Biol. Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loophelix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol. Cell. Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R, Martinez-Barbera JP, Beddington RS, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, et al. Pancreatic islet production of vascular endothelial growth factor—a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Cano DA, Rulifson IC, Heiser PW, Swigart LB, Pelengaris S, German M, Evan GI, Bluestone JA, Hebrok M. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes. 2008;57:958–966. doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res. Commun. 2009;82:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev. Cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocaña A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr. Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Productionof pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- da Silva Xavier G, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, Barg S, Rutter GA. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes. 2009;58:894–905. doi: 10.2337/db08-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La O, J P, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. USA. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lo´ra J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/ Arf locus to control pancreatic beta-cell proliferation. Genes Dev. 2009;23:906–911. doi: 10.1101/gad.1742609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Dutton JR, Chillingworth NL, Eberhard D, Brannon CR, Hornsey MA, Tosh D, Slack JM. Beta cells occur naturally in extrahepatic bile ducts of mice. J. Cell Sci. 2007;120:239–245. doi: 10.1242/jcs.03330. [DOI] [PubMed] [Google Scholar]
- Duvillie´ B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–589. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- Eberhard D, Tosh D, Slack JM. Origin of pancreatic endocrine cells from biliary duct epithelium. Cell. Mol. Life Sci. 2008;65:3467–3480. doi: 10.1007/s00018-008-8427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsbagge J, Johansson JK, Esni F, Luo Y, Radice GL, Semb H. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development. 2005;132:1085–1092. doi: 10.1242/dev.01643. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, et al. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J. Clin. Invest. 2006;116:1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc. Natl. Acad. Sci. USA. 2004;101:13245–13250. doi: 10.1073/pnas.0405301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes. 2006;55:2950–2956. doi: 10.2337/db06-0249. [DOI] [PubMed] [Google Scholar]
- Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439–447. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pe´rez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert CJ, Jr., Kaestner KH. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev. Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Hart A, Papadopoulou S, Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 2003;228:185–193. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133:2023–2032. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, Madsen OD, Serup P. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev. Dyn. 2002;225:260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- Heremans Y, Van De Casteele M, Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J. Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev. Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoterfactor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreaticbeta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kim SK, Melton DA. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc. Natl. Acad. Sci. USA. 1998;95:13036–13041. doi: 10.1073/pnas.95.22.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. Activin receptor patterning of foregut organogenesis. Genes Dev. 2000;14:1866–1871. [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- Krapp A, Kno¨fler M, Ledermann B, Bu¨rki K, Berney C, Zoerkler N, Hagenbu¨chle O, Wellauer PK. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucoseresponsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol. Cell. Biol. 2005;25:3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Brown J, Melton DA. Notch gene expression during pancreatic organogenesis. Mech. Dev. 2000;94:199–203. doi: 10.1016/s0925-4773(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc. Natl. Acad. Sci. USA. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martı´n M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle´ P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev. Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr., Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. USA. 2005;102:15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, et al. Ectopically expressed PDX-1 inliver in itiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem. Biophys. Res. Commun. 2003;310:1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev. Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Morris JP, Cano DA, Sekine S, Wang SC, Hebrok M. Betacatenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BLM. Preparing for the first breath: genetic and cellular mechanisms in the development of the vertebrate respiratory system. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC. The what, where, when and how of Wnt/beta-catenin signaling in pancreas development. Organogenesis. 2008;4:81–86. doi: 10.4161/org.4.2.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algu¨l H, Radtke F, Strobl L, Zimber-Strobl U, Schmid RM. Conditional ablation of Notch signaling in pancreatic development. Development. 2008;135:2757–2765. doi: 10.1242/dev.013722. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fa¨ R, Gu G, Gerber HP, Ferrara N, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev. Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, ElKhattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev. Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- O’Malley J, Woltjen K, Kaji K. New strategies to generate induced pluripotent stem cells. Curr. Opin. Biotechnol. 2009;20:516–521. doi: 10.1016/j.copbio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomaincontaining transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival AC, Slack JM. Analysis of pancreatic development using a cell lineage label. Exp. Cell Res. 1999;247:123–132. doi: 10.1006/excr.1998.4322. [DOI] [PubMed] [Google Scholar]
- Puri S, Hebrok M. Dynamics of embryonic pancreas development using real-time imaging. Dev. Biol. 2007;306:82–93. doi: 10.1016/j.ydbio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Dwivedi RS, Yeldandi AV, Subbarao V, Tan XD, Usman MI, Thangada S, Nemali MR, Kumar S, Scarpelli DG, et al. Role of periductal and ductular epithelial cells of the adult rat pancreas in pancreatic hepatocyte lineage. A change in the differentiation commitment. Am. J. Pathol. 1989;134:1069–1086. [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc. Natl. Acad. Sci. USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, et al. Cell-replacement therapy for diabetes: Generating functional insulinproducing tissue from adult human liver cells. Proc. Natl. Acad. Sci. USA. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Setty Y, Cohen IR, Dor Y, Harel D. Four-dimensional realistic modeling of pancreatic organogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:20374–20379. doi: 10.1073/pnas.0808725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. USA. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev. Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martı´n M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat. Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat. Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58:1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol. Ther. 2007;15:255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- Wang ZV, Mu J, Schraw TD, Gautron L, Elmquist JK, Zhang BB, Brownlee M, Scherer PE. PANIC-ATTAC: a mouse model for inducible and reversible beta-cell ablation. Diabetes. 2008;57:2137–2148. doi: 10.2337/db07-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc. Natl. Acad. Sci. USA. 2009;106:9715–9720. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes. Metab. 2009;11(Suppl 4):82–90. doi: 10.1111/j.1463-1326.2009.01113.x. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Wescott MP, Rustgi AK. Pancreatic cancer: translating lessons from mouse models and hereditary syndromes. Cancer Prev. Res. 2008;1:503–506. doi: 10.1158/1940-6207.CAPR-08-0195. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech. Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Xu X, D’Hoker J, Stange´ G, Bonne´ S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, Chan L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev. Cell. 2009;16:358–373. doi: 10.1016/j.devcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogen-esis. Dev. Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]