Abstract
Pancreatic cancer is the deadliest of all solid malignancies. Early detection offers the best hope for a cure, but characteristics of this disease such as the lack of early clinical symptoms, make the early detection difficult. Recent genetic mapping of the molecular evolution of pancreatic cancer suggests that a large window of opportunity exists for the early detection of pancreatic neoplasia, and developments in cancer genetics offer new, potentially highly specific, approaches for screening for curable pancreatic neoplasia. We review the challenges of screening for early pancreatic neoplasia, as well as opportunities presented by incorporating molecular genetics into these efforts.
Introduction
There are as many as a billion neoplastic cells in a cubic centimeter of a cancer(1). Invasive pancreatic cancers, whose average diameter is 4 cm at the time of diagnosis, therefore contain as many as 25 billion cancer cells. This is clearly a large number of cells, though not much larger than the number of neoplastic cells in other tumor types. The problem with pancreatic cancers is that they have usually metastasized by the time they are diagnosed, and once this occurs, it is virtually impossible to cure most patients(2).
As has been nicely demonstrated for colon cancer, the best hope for reducing the cancer-specific mortality of pancreatic cancer lies in early diagnosis and treatment, ideally at a precancerous stage(3). Six issues, however, need to be addressed for the early detection and cure of pancreatic neoplasia to come to fruition. First, the curable lesions that give rise to advanced, noncurable, pancreatic cancers have to be characterized. Second, there has to be a reasonable window of opportunity to detect these potentially curable lesions. That is, the progression from localized curable lesions to advanced cancers has to be slow enough that there is a reasonable chance that the localized lesions can be detected clinically. In this paper, we define "localized" as tumors which have not yet metastasized or advanced to the point that they can't be removed surgically because of their involvement of other tissues, particularly major arteries. Third, a test has to be developed to detect the compendium of curable localized lesions. This is not a trivial problem for lesions arising in an organ that lies deep in the abdomen. Fourth, because treating lesions in the pancreas is not trivial, there has to be a method to distinguish localized lesions with a reasonable chance of progressing to an advanced cancer from lesions with little or no risk of progressing. Fifth, the prevalence of the disease has to be reasonably high in the population to be screened, such that screening tests with achievable sensitivities and specificities will have a high positive predictive value. Sixth, an evidence base has to be developed which demonstrates that screening actually saves lives.
Using the framework of the six issues described above, this article will review recent developments that bring the early detection of pancreatic cancer closer to clinical practice. Equal emphasis will be placed on the opportunities and on the challenges that lie ahead.
Characterize the curable lesions that give rise to metastatic pancreatic cancer
Pancreatic intraepithelial neoplasia
Pathologists have recognized precursor lesions in the pancreas for more than a century(4). S.P.L. Hulst, in 1905, described small microscopic lesions in the pancreas that he felt were in between normal and invasive cancer, which he called “zwischenformen.” These lesions, which we now call “pancreatic intraepithelial neoplasia (PanIN),” were subsequently shown to be more common in pancreata with an invasive carcinoma than in pancreata without a cancer, to increase with age, and to harbor many of the same genetic alterations as do invasive adenocarcinomas of the pancreas(5). PanINs have been reported in 16–45% of pancreata that do not harbor an invasive cancer(6–8).
Pancreatic intraepithelial neoplasia lesions are noninvasive epithelial proliferations within the smaller pancreatic ducts(5). They can be flat or papillary, and are graded histologically as PanIN-1 (low-grade), PanIN-2 (intermediate-grade), or PanIN-3 (high-grade) based on the degree of architectural and cellular atypia present in the lesion (Figure 1)(5). Paralleling this histologic progression is a genetic progression. The lower grade lesions (PanIN-1 and PanIN-2) often harbor genetic alterations in the KRAS and p16/CDKN2A genes, while the higher grade PanIN-3 lesions and invasive adenocarcinomas, in addition to genetic alterations in KRAS and p16/CDKN2A, also often harbor mutations in TP53 and SMAD4(9–11). Autopsy studies indicate that low-grade PanINs are found in the pancreata of most adults once they reach middle age(8, 12). High-grade PanINs, however, are rarely found, unless there is an associated invasive pancreatic cancer or the patient has a strong family history of pancreatic cancer(8, 12–14). In total, these observations support the hypothesis that PanIN lesions are precursors to invasive adenocarcinoma, and that there is a progression from normal ductal epithelium, to low-grade PanIN, to high-grade PanIN, to localized adenocarcinoma, to metastatic adenocarcinoma(5).
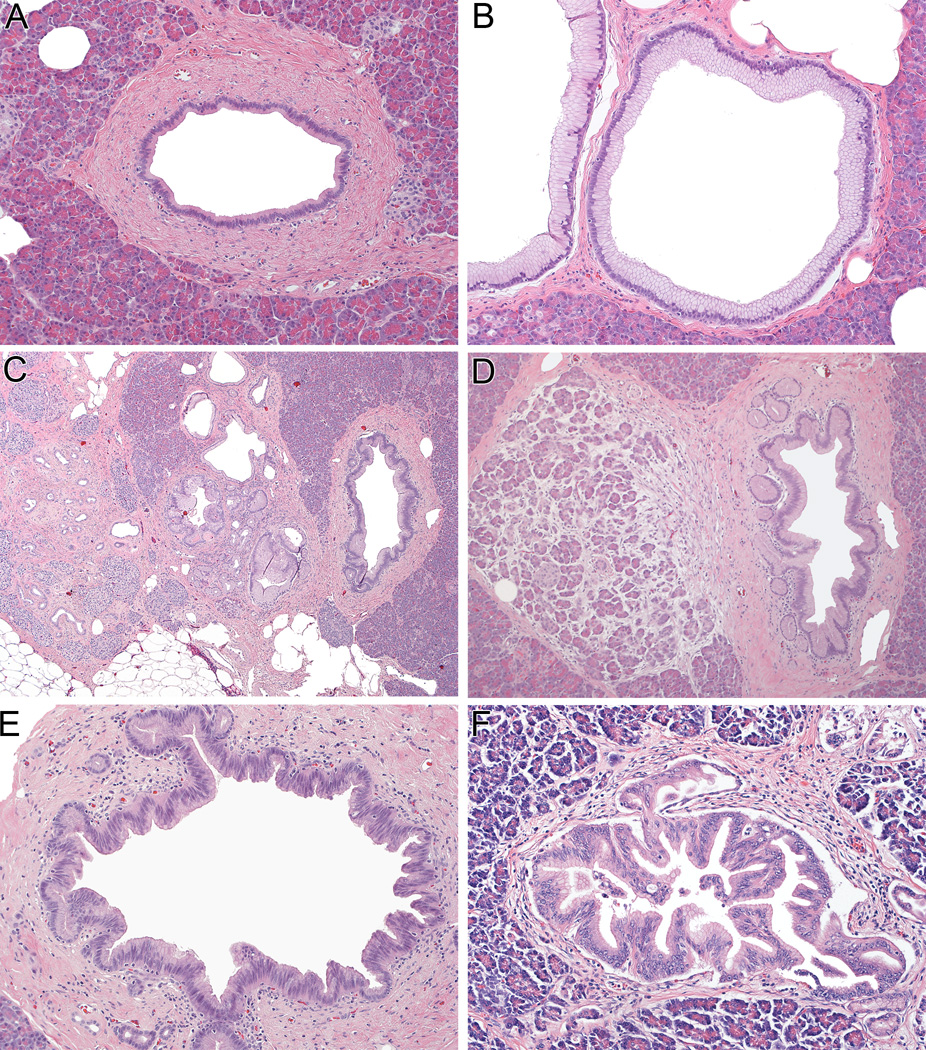
Figure 1.
A normal pancreatic duct (panel A), and multiple pancreatic intraepithelial neoplasia (PanIN) lesions from the pancreas of a single patient with a family history of pancreatic cancer (Panels B-F). These include PanIN-1 (panel B), PanIN with associated lobulocentric atrophy (Panels B and C), PanIN-2 (Panel E), and PanIN-3 (Panel F). (all hematoxylin and eosin). KRAS mutations and telomere shortening occur early in PanIN-1 lesions, p16/CDKN2A loss occurs slightly later in PanIN-2, and SMAD4 and TP53 inactivation are late events (PanIN-3 and invasive carcinoma).
Intraductal papillary mucinous neoplasms
The second major precursor lesion to be identified in the pancreas was the intraductal papillary mucinous neoplasm (IPMN)(5, 15). Pancreatic cysts are very common, being identified in almost 3% of asymptomatic individuals who undergo a computed tomography scan(16), with IPMN accounting for almost 50% of resected pancreatic cysts(17). IPMNs arise in the larger pancreatic ducts and, as the name suggests, they are typically papillary and often produce copious amounts of mucin. IPMNs are, by definition, larger than PanINs. PanINs are usually <0.5 cm, while most IPMNs are ≥ 1.0 cm(5). IPMNs are more prevalent in the elderly than in the young, and up to a third of IPMNs harbor an associated invasive adenocarcinoma(9). As is observed with PanINs, low-grade IPMNs often harbor KRAS and p16/CDKN2A gene mutations, and high-grade IPMNs harbor further mutations in TP53 and SMAD4(5). In addition, the GNAS and RNF43 genes are mutated in a major fraction of IPMNs(18, 19). When an adenocarcinoma arises in association with an IPMN, the IPMN and the invasive carcinoma almost always harbor the same genetic alterations, supporting the hypothesis that IPMNs are a precursor to invasive adenocarcinomas(10).
Mucinous cystic neoplasms
Mucinous cystic neoplasms (MCNs) are large mucin-producing precancerous lesions of the pancreas that almost always arise in the body or tail of the gland and commonly arise in women(15). They are far less common than IPMNs, accounting for only 16% of resected pancreatic cysts in large surgical series(17). In contrast to IPMNs, MCNs do not significantly involve the pancreatic duct system, and, in contrast to IPMNs, MCNs have a distinctive “ovarian-type” stroma(15). Like IPMNs, however, MCNs can progress to adenocarcinoma. The KRAS, p16/CDKN2A, RNF43, TP53 and SMAD4 genes have all been reported to be mutated in MCNs (though GNAS is not, thereby distinguishing IPMNs from MCNs)(15, 19, 20).
Small invasive cancers
Although they are rarely encountered outside of screening trials, there have been several reports of long-term survival (“cures”) of patients with surgically resected small, lymph node negative, pancreatic cancers(21–23). For example, Egawa et al reported that patients with surgically resected stage I pancreatic cancer have a median survival of 78 months and a 5-year survival rate of 58%(22). Similarly, Ishikawa et al reported a 5-year survival rate close to 70% for patients with small (≤1 cm) pancreatic cancers who were not jaundiced and who did not have a mass-forming lesion on imaging(23).
In summary, three distinct lesions, PanINs, IPMNs and MCNs, have each been identified as distinct precursors to ductal adenocarcinomas of the pancreas. These precursor lesions, together with some small invasive cancers, are curable.
Demonstrate a sufficient window of opportunity to detect the curable lesions that give rise to metastatic pancreatic cancer
If early screening is to be effective, curable lesions have to be present for a relatively long period of time before they progress to metastatic carcinoma. If these lesions were fleeting, rapidly progressing to metastatic carcinomas, then screening would have to be performed so frequently as to be impractical.
There is strong direct evidence that IPMNs and MCNs are present for years before they progress to invasive cancer. Some patients with IPMNs report that they had symptoms caused by their IPMNs for years, and some even had symptoms for decades, before their tumors were diagnosed, suggesting that their lesions had been present for years(15). In addition, the average age of patients with noninvasive IPMNs is three to five years younger than the average age of patients with an IPMN with an associated adenocarcinoma, again suggesting that IPMNs are present for years before they progress to invasive cancer(15). Finally, hundreds of patients with an IPMN have been followed clinically with serial imaging and the vast majority IPMNs are relatively stable over months to years, or at most, grow slowly(24–26). Although the numbers are smaller, similar evidence exists for MCNs(15).
Because of their small size, it is much more difficult to directly observe PanIN lesions. The best estimates of the time-line for the progression of PanIN lesions to invasive carcinoma come from the genetic sequencing studies(27). Iacobuzio-Donahue et al sequenced a series of primary invasive pancreatic cancers and multiple matched metastases from the same patients(27). They applied modelling, similar to modelling used by evolutionary biologists, to the patterns of genetic alterations present in multiple lesions from the same patient, and estimated that it takes at least a decade for a cell with an initiating mutation in the pancreas to progress to what they designated as the parental, nonmetastatic founder cell (a cell likely present in an advanced PanIN lesion, prior to the onset of the invasion required to define the lesion as an adenocarcinoma)(27). This study further estimated that it takes at least another five years for the neoplastic clone to develop the ability to metastasize(27). While the time line for progression calculated is based on several assumptions, it does correspond nicely to the time line observed for other epithelial neoplasms(28). For example, Jones et al sequenced a series of well-characterized adenomas and invasive carcinomas of the colon and calculated that, for those lesions that progress to invasive cancer, it takes almost 17 years for a micro adenoma to progress into an advanced cancer(29).
Clearly, as in other tumor sites, there is a large window of opportunity to detect potentially curable, neoplastic lesions in the pancreas.
Establish a method to detect curable neoplastic lesions
Two broad approaches have been taken to detect curable neoplastic lesions in the pancreas. The first is imaging, particularly with endoscopic ultrasound (EUS). The second involves molecular methods, such as those to detect circulating mutant DNA shed by the neoplastic lesions.
Computed tomography (CT), magnetic resonance imaging (MRI), and EUS have all been used to detect curable lesions in the pancreas. All three imaging modalities have been compared in a prospective study of 225 asymptomatic high-risk individuals by Canto et al(30). EUS detected a pancreatic abnormality in 43% of patients, in contrast with MRI and CT which identified lesions in 33% and 11% respectively. Five of the lesions identified on EUS were resected, of which three were IPMNs with high-grade dysplasia. It is clear that cystic precursor lesions can be detected by existing imaging, that EUS appears to be the most sensitive modality, and that some of these will be curable, high-grade precursor lesions.
Smaller precursor lesions, such as PanINs, are not directly detectable by EUS, but their presence can be inferred indirectly. PanINs produce small areas of fibrosis called “lobulocentric atrophy,” and when multiple PanIN lesions are present, they create multiple areas of fibrosis that can be detected as changes of chronic pancreatitis by EUS(14, 31). Brune et al demonstrated a linear correlation between the number of PanIN lesions present in a pancreas and the EUS score of the severity of chronic pancreatitis(14). The contribution of imaging to the detection of precursor lesions will only increase as the resolution of imaging improves in the coming years.
Two recent advances highlight the enormous potential of molecular-based approaches as tools for the detection of curable lesions in the pancreas. First, whole exome sequencing of well-characterized cyst-forming precursor lesions has defined the genes targeted (mutated) in each of the different precursor lesions in the pancreas (Table 1). Second, new technologies have been developed that can detect rare mutant alleles, even when these mutant alleles are admixed with a thousand-fold more wild-type alleles(18, 19, 32).
Table 1.
Genes targeted (mutated) in the most common precursor and cystic lesions in the pancreas
| Gene | IPMN- LG |
IPMN- HG |
MCN-LG | MCN- HG |
PanIN-1 and -2 |
PanIN- 3 |
SCA | SPN |
|---|---|---|---|---|---|---|---|---|
| KRAS | X | X | X | X | X | X | ||
| P16/CDKN2A | X | X | X | X | X | X | ||
| TP53 | X | X | X | |||||
| SMAD4 | X | X | X | |||||
| RNF43 | X | X | X | X | ||||
| GNAS | X | X | ||||||
| CTNNB1 | X | |||||||
| VHL | X |
HG=high grade; IPMN= intraductal papillary mucinous neoplasm; LG=low grade; MCN=mucinous cystic neoplasm; PanIN=pancreatic intraepithelial neoplasia; SCA=serous cystadenoma; SPN=solid-pseudopapillary neoplasm
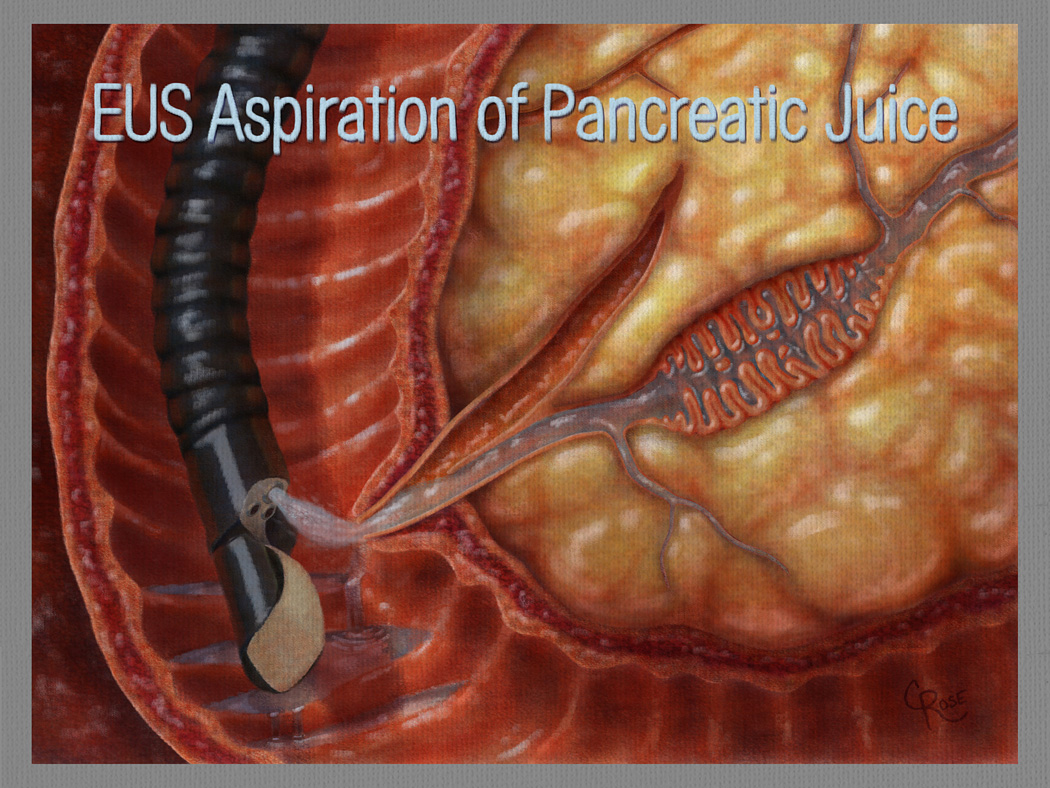
Pancreatic secretion (juice) is a natural place to look for mutant genes shed from precursor lesions in the pancreas and can easily be obtained by stimulating pancreatic juice secretin with secretin, and then collecting the juice with an endoscope (Figure 2). As noted earlier, both IPMNs and PanINs involve the pancreatic duct system, and mutant DNA from both of these lesions is therefore likely to be shed into the pancreatic juice(33, 34). Indeed, Goggins et al have shown that GNAS mutations can be detected in endoscopically obtained pancreatic juice samples in two-thirds of patients with an IPMN(35). Of note, GNAS mutations were also detected in pancreatic juice samples from patients with a clinically normal pancreas who only later developed an IPMN, portending the power of molecular-based tests(35).
Figure 2.
Genetic changes shed by precursor lesions involving the pancreatic duct system can be detected in pancreatic secretions collected at the time of endoscopy. EUS=endoscopic ultrasound. (Illustration by Christian Rose, copyright Johns Hopkins University).
Sequencing pancreatic juice requires endoscopy, which reduces the applicability of juice based approaches. Kinde et al therefore applied a technology that can be used to detect rare mutant alleles, called SafeSeqS, and showed that it is possible to detect KRAS gene mutations in the plasma of 85% of patients with advanced pancreatic cancer (32, 36). Despite this highly sensitive technique, KRAS gene mutations were only detected 45% of patients with surgically resectable pancreatic cancer highlighting the difficulties of early tumour detection using blood. Small non-invasive precursor lesions are unlikely to shed large quantities of DNA, we therefore believe it is unlikely that it will be possible to detect mutant DNA in the plasma of patients with non-invasive precursor lesions such as IPMNs, MCNs, or PanINs(37).
While beyond the scope of this review, it should be noted that a number of other approaches are being developed to detect early pancreatic neoplasia. These include detecting circulating tumor cells, as well as proteins, mucins, and microRNAs shed by the tumors(38, 39).
In sum, curable lesions of the pancreas, both large and small, are detectable with technologies already in clinical practice, including EUS, magnetic resonance imaging (MRI) and computerized tomography (CT). Molecular-based technologies have enormous potential, particularly if they can be applied to biosamples, such as blood or stool, which are obtainable noninvasively. The resolution of imaging and the sensitivity and specificity of molecular-based screening tools are certain to improve in the coming years, and the two technologies may even be combined with molecular-based imaging(38).
Distinguishing between precursor lesions and benign mimics
One of the problems in screening for precursor lesions is that harmless benign lesions can mimic these lesions and lead to overtreatment with just over 20% of pancreatic cysts found to be benign following resection(17, 40–42). While this isn’t a significant problem for superficial organs such as the skin (freezing or surgically removing a small harmless skin lesion that mimics an in situ squamous carcinoma is not a substantial problem), these mimickers can be a real problem when more deeply seated organs are studied. For example, H. G. Welch and H.J. Passow analyzed the literature on screening for breast cancer, and concluded that if 1,000 50-year-old American women are screened annually for a decade, 490 to 670 women will have at least one false alarm (recall mammogram), and that 70 to 100 of the women will have a false positive biopsy recommendation(43, 44).
As troublesome as false positive biopsy recommendations are for breast lesions, such recommendations would be a much greater problem for pancreatic lesions(40–42). The pancreas lies deep in the back of the abdomen and lesions in the pancreas are not easily accessed(2). Furthermore, surgical resection is sometimes the only way to diagnose a lesion definitively, and the surgical resection of pancreatic lesions is associated with significant morbidity and a nontrivial risk of mortality. Tools to distinguish harmless lesions in the pancreas, such as serous cystadenomas (SCAs), from true precursor lesions, such as IPMNs and MCNs, are needed, as are tools to distinguish low-grade PanINs, MCNs and IPMNs from high-grade PanINs, MCNs and IPMNs.
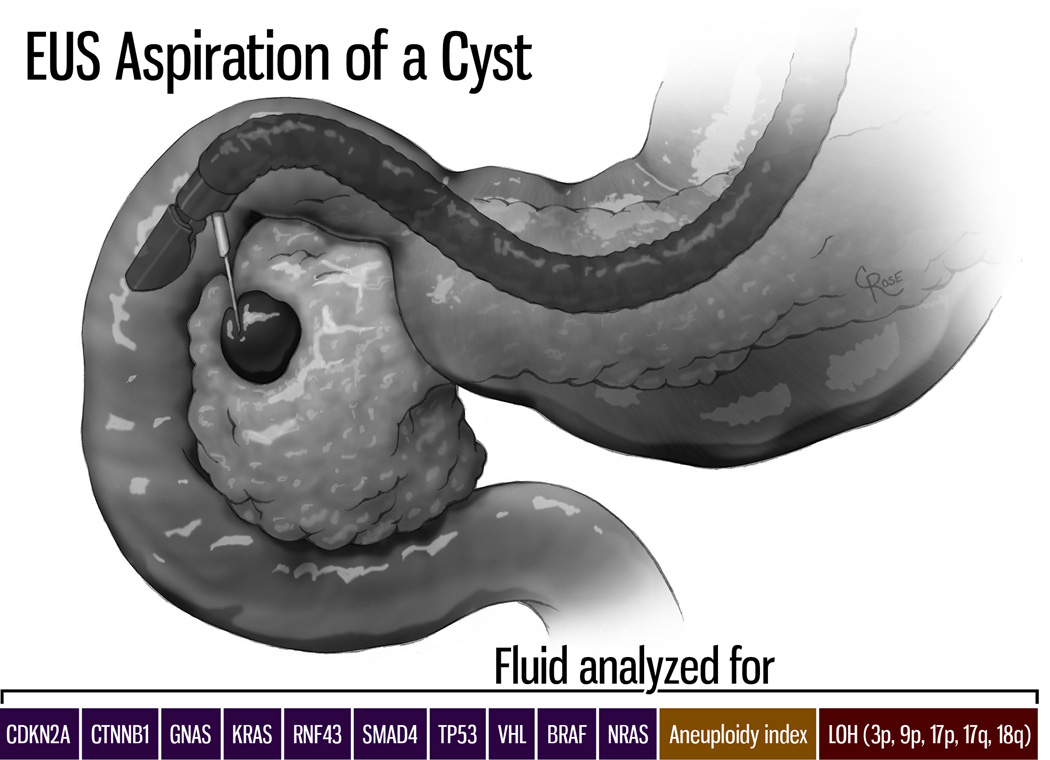
Genetic markers have the potential to distinguish among the various cystic lesions of the pancreas and therefore could help distinguish harmless lesions and precursor lesions. Wu et al sequenced the exomes of the four most common cystic neoplasms of the pancreas (SCA, IPMN, MCN and solid-pseudopapillary neoplasms [SPN]) and found that each of these four tumors was associated with a specific pattern of genetic alterations (Table 1)(18, 19). SCAs are characterized by VHL gene alterations, SPNs by CTNN1 (beta-catenin) gene mutations, IPMNs by KRAS, GNAS, RNF43, TP53, p16/CDKN2A and SMAD4 gene mutations, and MCNs by KRAS, RNF43, TP53, p16/CDKN2A and SMAD4 gene mutations(18, 19). The mutations present in the neoplastic cells in these cystic neoplasms are shed into the cyst fluid and therefore can be detected in cyst fluid (Figure 3). For example, in a study which included analyses of both neoplasms and cyst fluid, 96% of 132 IPMNs were found to harbor a mutation in GNAS and/or KRAS, while mutations in these genes were not observed in 44 SCAs(19). With these advances we can easily envision that harmless cysts of the pancreas will be readily distinguishable from true precursor lesions in the near future.
Figure 3.
Fluid from pancreatic cysts can be aspirated at the time of endoscopic ultrasound (EUS), and the mutations present in the cyst fluid can suggest cyst type. (Illustration by Christian Rose, copyright Johns Hopkins University)
Distinguishing PanINs lesions that are likely to progress from PanIN lesions that are unlikely to progress to invasive cancer is significantly more complex. PanINs, particularly low-grade PanIN lesions, are fairly common in the population, and yet most do not progress to invasive cancer(7, 8, 12, 45). For example, Andea et al reported that 54% of 152 pancreata resected from patients without a pancreatic malignancy harbored at least one PanIN lesion, yet clearly 54% of the population doesn’t develop pancreatic cancer(46). Indeed, Terhune et al, in a “back of the envelope” calculation estimated that less than 1% of PanIN lesions progress to invasive carcinoma(45). While it is assumed that the higher grade PanIN lesions (PanIN-3) are more likely to progress than are lower grade lesions (PanIN-1 and PanIN-2), there is currently no way, other than resection and histological examination, to determine the grade of a PanIN lesion. The challenge posed by PanIN lesions will only grow as imaging and molecular detection technologies improve.
While genetic markers can be used to distinguish harmless cystic lesions (such as SCA) from those that are precursor lesions (IPMNs and MCNs), genetic markers cannot yet be used to determine the grade of a precursor lesion(18, 19).
Identify populations at risk for pancreatic cancer who will benefit from screening
The positive predictive value of any test can be greatly improved by increasing the prevalence of the disease being tested for in the population being tested. The prevalence of pancreatic cancer in the general United States population (all ages) is approximately 9 per 100,000, and it rises to ~68/100,000 in individuals above the age of 55(2). This low prevalence is problematic for screening. For example, if 100,000 people over the age of 55 were screened for pancreatic cancer using a test with a specificity of 98% and a sensitivity of 100%, it would generate 1,999 false positive test results but only 68 true positive results. A specificity of higher than 99% would be required for a more acceptable positive predictive value. Alternatively, populations whose risk is elevated above the general population could derive benefit from screening methods that were inadequately specific for the general population.
A variety of factors increase the risk of pancreatic cancer, but after age, a family history of pancreatic cancer appears to increase risk the most(47). For example, Klein et al followed 5,179 individuals from 838 kindreds enrolled in the National Familial Pancreas Tumor Registry (NFPTR, http://pathology.jhu.edu/pc/nfptr/index.php) and found that individuals in families in which at least a pair of first-degree relatives have been diagnosed with pancreatic cancer (designated “familial pancreatic cancer kindreds”) are nine-fold more likely to develop pancreatic cancer than is the general population(48). In the study by Klein et al the risk of pancreatic cancer rose with the number of family members diagnosed with pancreatic cancer, such that individuals with three first-degree relatives with pancreatic cancer had a 32-fold increased risk(48). Thus, individuals with a strong family history of pancreatic cancer represent a well-defined population with a significantly increased risk.
Risk-prediction models aimed at identifying families that carry a high-penetrance pancreatic cancer gene, such as PancPRO, have been developed that can be used to calculate the risk of pancreatic cancer based on disease patterns within a specific pedigree(49). For example, the risk of pancreatic cancer is higher for individuals with two first-degree relatives with pancreatic cancer (e.g. a mother and a brother), than it is for individuals with a first-degree and a second-degree relative (e.g. a sister and an aunt) (49). These models can help identify individuals that may have a greatly elevated risk of developing pancreatic cancer. In contrast, risk models that predict the risk of pancreatic cancer using low-penetrance single nucleotide polymorphisms (SNPs) as well as known pancreatic cancer risk factors (age, diabetes mellitus, heavy alcohol use, body-mass index and presence or absence of a family history) have not been shown to identify individuals with a substantively elevated risk of pancreatic cancer(50).
Risk can be further refined when the causative genes are known and individuals can now undergo genetic testing to see if they carry a familial pancreatic cancer susceptibility gene. A number of genes have been identified that, when mutated in the germline, increase the risk of pancreatic cancer (Table 2)(51, 52). These include BRCA2, ATM, PALB2, p16/CDKN2A, STK11/LKB1, BRCA1, PRSS1, and the genes associated with the hereditary nonpolyposis colorectal cancer syndrome (HNPCC). Importantly, as shown in Table 2, pancreatic cancer risk can often be quantified when the gene is known(52).
Table 2.
Genes associated with familial pancreatic cancer
| Individual/gene | % of Families | Relative risk | Risk by age of 70years |
|---|---|---|---|
| No history | 1 | 0.5% | |
| ATM | <2 | ? | ? |
| BRCA1 | <1 | ? | ? |
| BRCA2 | 6–12 | 3.5–10 | 3.5% |
| HNPCC associated genes | ? | 8 | 3.7% |
| p16/CDKN2A | 1–3 | 20–34 | 10–17% |
| PALB2 | 3 | ? | ? |
| PRSS1 | <1 | 50–80 | 25–40% |
| STK11 | <1 | 132 | 30–60% |
HNPCC= hereditary nonpolyposis colorectal cancer syndrome
Individuals with an inherited genetic abnormality that increases their risk of developing pancreatic cancer, as well as individuals without a known predisposing gene mutation but who are predicted to be at increased risk based on their family history, would therefore be a natural first group to benefit from screening.
Another way to increase the prevalence of a disease in a population is to select members of the population that are known to have a pre-existing condition that predisposes to the disease. As noted earlier, two of the precursor lesions in the pancreas form cysts, and cysts can be detected by CT and by MRI(16, 53). Approximately 70 million CT scans are performed in the United States every year, and 2.6% of the CT scans that include the pancreas will reveal a cystic lesion in the pancreas(16). This fraction is even higher for patients who have MRI exams (53). The prevalence of cysts detectable by either CT or by MRI increases with age, such that cysts can be found in 7 to 12 percent of individuals over the age of 70 years(16, 53). Some of these cysts will be the precursor lesions IPMNs and MCNs. When applied to individuals with a pancreatic cyst, tests for markers such as circulating tumor DNA (ctDNA), will have a significantly higher positive predictive value than when these same tests are applied to the general population(36).
The screening of patients with multiple risk factors, such as elderly individuals with family history and a pancreatic cyst, would lead to even higher positive predictive values for any early screening test.
Develop an evidence-base establishing that screening at-risk individuals is beneficial
Demonstrating that screening actually saves lives is extremely difficult(54, 55). Screening for colonic neoplasia and cervical cancers are examples of screening that has been shown to save lives, but this was only shown through very large population studies carried out years after such screening had become routine. For example, among 88,902 participants followed over a period of 22 years in the Nurses' Health Study and the Health Professionals Follow-up Study, colon cancer mortality was reduced after screening sigmoidoscopy (hazard ratio, 0.59) and screening colonoscopy (hazard ratio, 0.32). By contrast, evidence in support of pancreatic cancer screening is similar to the evidence that was available to justify colorectal cancer screening 20 years ago, i.e. studies suggest that screening can detect asymptomatic precursor lesions, but it is only an expectation that removing these precursor lesions will improve outcomes for patients(56). Ongoing collaborative research enabled by a worldwide Cancer of the Pancreas Screening (CAPS) consortium will provide a larger study population base to determine the yield and clinical outcomes of screening high-risk individuals(56–59). Even so, it will take an extremely large study population followed for many years to show that screening for pancreatic neoplasia actually saves lives(30, 56–60).
Summary and conclusions
It has been established that curable precursor lesions do exist in the pancreas, that these lesions are long-lived before they progress to adenocarcinomas, that curable precursor lesions can be detected, and that groups at high-risk of developing the disease can be identified (Figure 4). Small invasive cancers are also detectable and small pancreatic adenocarcinomas are much more likely to be surgically curable than are larger ones(21–23). Furthermore, as new medical therapies for pancreatic adenocarcinoma are developed, they are likely to be considerably more efficacious against less advanced cancers than against more advanced ones. Thus, screening tests that detect smaller adenocarcinomas may still be useful, as they could increase the chances of success with subsequent therapy.
Figure 4.
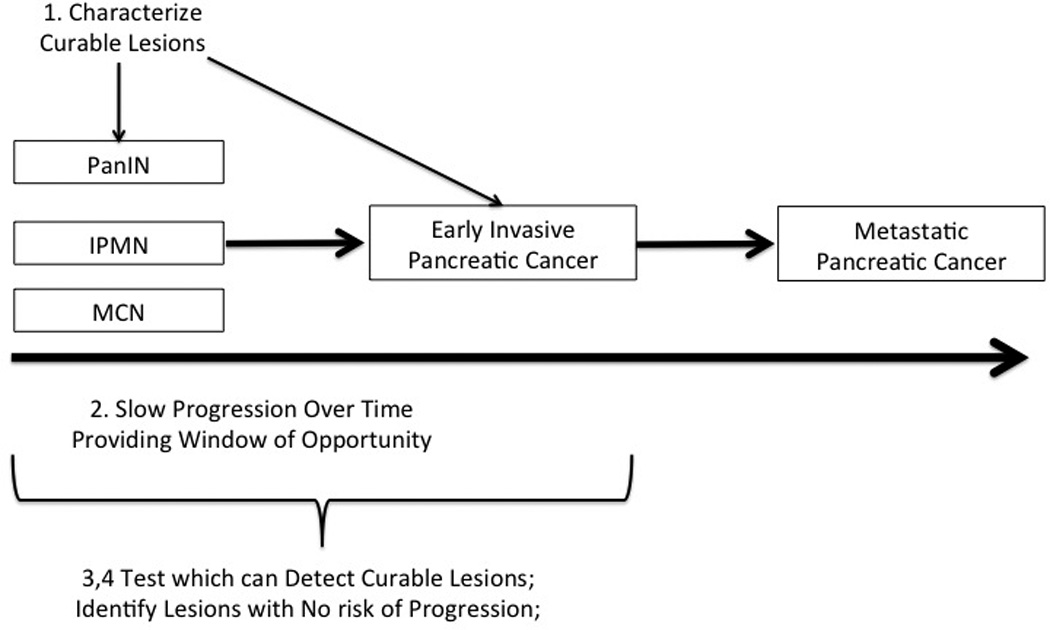
There are a series of steps which need to be overcome to diagnose and treat curable pancreatic cancer. (1) First, the curable lesions that give rise to advanced pancreatic cancers have to be characterized. (2) There has to be a reasonable window of opportunity to detect these potentially curable lesions. (3,4) A test has to be developed to detect the compendium of curable localized lesions, and lesions with a reasonable chance of progressing have to be distinguished from those with little or no risk of progressing. Not shown, the prevalence of the disease has to be reasonably high in the population to be screened, and an evidence base has to be developed which demonstrates that screening actually saves lives.
As technologies advance and the opportunities for the early detection of pancreatic neoplasia grow, it is likely that patients, guided by their physicians, will have to decide whether or not to be screened based on an imperfect understanding of the risks and benefits of screening. This situation is not unprecedented, as it has previously been applied to all other effective screening methods at their inception, such as those for colorectal, cervical, and breast tumors. Hopefully, the principles outlined in this review, as well as additional data and common sense, will guide the development and implementation of these tests, as these tests are sorely needed.
Acknowledgments
Supported by National Institutes of Health grant P50 CA62924, the Lustgarten Foundation for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, and the Michael Rolfe Foundation. Drs. Hruban, Klein, Goggins, Papadopoulos, Kinzler and Vogelstein receive royalty payments from Myriad Genetics. Drs. Vogelstein, Papadopoulos, and Kinzler are on the advisory boards of PGDX and Sysmex Inostics. Drs. Diaz, Kinzler, Papadopoulos and Vogelstein own stock in PGDX. Dr. Lennon has received consulting fees for Boston Scientific.
Footnotes
DISCLOSURES: The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
Contributors: All authors contributed to the writing of the manuscript. All authors have read and approved the manuscript.
References
- 1.Del Monte U. Does the cell number 10(9) still really fit one gram of tumor tissue? Cell Cycle. 2009;8:505–506. doi: 10.4161/cc.8.3.7608. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA: a cancer journal for clinicians. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England journal of medicine. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulst SPL. Zur kenntnis der Genese des Adenokarzinoms und Karzinoms des Pankreas. Virchows Arch (B) 1905;180:288–316. [Google Scholar]
- 5.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 6.Andea A, Sarkar F, Adsay NV. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Modern Pathology. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 7.Kozuka S, Sassa R, Taki T, Masamoto K, Nagasawa S, Saga S, et al. Relation of pancreatic duct hyperplasia to carcinoma. Cancer. 1979;43:1418–1428. doi: 10.1002/1097-0142(197904)43:4<1418::aid-cncr2820430431>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Cubilla ALFP. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer research. 1976;36:2690–2698. [PubMed] [Google Scholar]
- 9.Murphy SJ, Hart SN, Lima JF, Kipp BR, Klebig M, Winters JL, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098–1109. e1. doi: 10.1053/j.gastro.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. Journal of hepato-biliary-pancreatic surgery. 2007;14:224–232. doi: 10.1007/s00534-006-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 13.Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, et al. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7737–7743. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. The American journal of surgical pathology. 2006;30:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 15.Hruban RH, Pitman MB, Klimstra DS. Atlas of tumor pathology. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. Tumors of the pancreas. [Google Scholar]
- 16.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR American journal of roentgenology. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–S12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science translational medicine. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733. e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung KW, Kim MH, Lee TY, Kwon S, Oh HC, Lee SS, et al. Clinicopathological aspects of 542 cases of pancreatic cancer: a special emphasis on small pancreatic cancer. J Korean Med Sci. 2007;22(Suppl):S79–S85. doi: 10.3346/jkms.2007.22.S.S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egawa S, Takeda K, Fukuyama S, Motoi F, Sunamura M, Matsuno S. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28:235–240. doi: 10.1097/00006676-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa O, Ohigashi H, Imaoka S, Nakaizumi A, Uehara H, Kitamura T, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter--collective review of Japanese case reports. Hepatogastroenterology. 1999;46:8–15. [PubMed] [Google Scholar]
- 24.Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Wu BU, Sampath K, Berberian CE, Kwok KK, Lim BS, Kao KT, et al. Prediction of Malignancy in Cystic Neoplasms of the Pancreas: A Population-Based Cohort Study. The American journal of gastroenterology. 2013 doi: 10.1038/ajg.2013.334. [DOI] [PubMed] [Google Scholar]
- 26.Kang MJ, Jang JY, Lee KB, Chang YR, Kwon W, Kim SW. Long-term Prospective Cohort Study of Patients Undergoing Pancreatectomy for Intraductal Papillary Mucinous Neoplasm of the Pancreas: Implications for Postoperative Surveillance. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 27.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 29.Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. ModPathol. 2006;19:1019–1026. doi: 10.1038/modpathol.3800613. [DOI] [PubMed] [Google Scholar]
- 32.Kinde I, Papadopoulos N, Kinzler KW, Vogelstein B. FAST-SeqS: a simple and efficient method for the detection of aneuploidy by massively parallel sequencing. PloS one. 2012;7:e41162. doi: 10.1371/journal.pone.0041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilentz RE, Chung CH, Sturm PDJ, Musler A, Sohn TA, Offerhaus GJ, et al. K- ras mutations in the duodenal fluid of patients with pancreatic carcinoma. Cancer. 1998;82:96–103. doi: 10.1002/(sici)1097-0142(19980101)82:1<96::aid-cncr11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Shi C, Fukushima N, Abe T, Bian Y, Hua L, Wendelburg BJ, et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol Ther. 2008;7:353–360. doi: 10.4161/cbt.7.3.5362. [DOI] [PubMed] [Google Scholar]
- 35.Kanda M, Knight S, Topazian M, Syngal S, Farrell J, Lee J, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2012 doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Science translational medicine. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142:248–256. doi: 10.1053/j.gastro.2011.10.031. quiz e25-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold DV, Newsome G, Liu D, Goldenberg DM. Mapping PAM4 (clivatuzumab), a monoclonal antibody in clinical trials for early detection and therapy of pancreatic ductal adenocarcinoma, to MUC5AC mucin. Molecular cancer. 2013;12:143. doi: 10.1186/1476-4598-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA : the journal of the American Medical Association. 2014;311:392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 40.Cho CS, Russ AJ, Loeffler AG, Rettammel RJ, Oudheusden G, Winslow ER, et al. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013;20:3112–3119. doi: 10.1245/s10434-013-2986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jong K, Nio CY, Mearadji B, Phoa SS, Engelbrecht MR, Dijkgraaf MG, et al. Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas. 2012;41:278–282. doi: 10.1097/MPA.0b013e31822899b6. [DOI] [PubMed] [Google Scholar]
- 42.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernandez-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10:144–150. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch HG, Passow HJ. Quantifying the Benefits and Harms of Screening Mammography. JAMA internal medicine. 2013 doi: 10.1001/jamainternmed.2013.13635. [DOI] [PubMed] [Google Scholar]
- 44.Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmo mammographic screening trial: follow-up study. Bmj. 2006;332:689–692. doi: 10.1136/bmj.38764.572569.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terhune PG, Phifer DM, Tosteson TD, Longnecker DS. K-ras mutation in focal proliferative lesions of human pancreas. Cancer Epidemiol Biomarkers Prev. 1998;7:515–521. [PubMed] [Google Scholar]
- 46.Andea AA, Cheng J, Lauwers GY, Klimstra DS, Adsay NV. Pancreatic intraepithelial neoplasia (PanIN) in pancreata involved by mucinous cystic neoplasms. Modern Pathology. 2002;15:282A. [Google Scholar]
- 47.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, Sulem P, Kristjansson K, Arnason S, et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 2004;1:e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Research. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. PancPRO: risk assessment in individuals with a family history of pancreatic cancer. Journal of Clinical Oncology. 2007;25:1417–1422. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein AP, Lindstrom S, Mendelsohn JB, Steplowski E, Arslan AA, Bueno-de-Mesquita HB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PloS one. 2013;8:e72311. doi: 10.1371/journal.pone.0072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, et al. ATM Mutations in Patients with Hereditary Pancreatic Cancer. Cancer Discovery. 2012;2:41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Advances in surgery. 2010;44:293–311. doi: 10.1016/j.yasu.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. Bmj. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curiel-Lewandrowski C, Chen SC, Swetter SM. Screening and prevention measures for melanoma: is there a survival advantage? Curr Oncol Rep. 2012;14:458–467. doi: 10.1007/s11912-012-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Annals of internal medicine. 1999;131:247–255. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 58.Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:5028–5037. doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 59.Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. The American journal of gastroenterology. 2011;106:946–954. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. The American journal of gastroenterology. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]