Abstract
The most important risk factor for human aneuploidy is increasing maternal age, but the basis of this association remains unknown. Indeed, one of the earliest models of the maternal-age effect—the “production-line model” proposed by Henderson and Edwards in 1968—remains one of the most-cited explanations. The model has two key components: (1) that the first oocytes to enter meiosis are the first ovulated and (2) that the first to enter meiosis have more recombination events (crossovers) than those that enter meiosis later in fetal life. Studies in rodents have demonstrated that the first oocytes to enter meiosis are indeed the first to be ovulated, but the association between the timing of meiotic entry and recombination levels has not been tested. We recently initiated molecular cytogenetic studies of second-trimester human fetal ovaries, allowing us to directly examine the number and distribution of crossover-associated proteins in prophase-stage oocytes. Our observations on over 8,000 oocytes from 191 ovarian samples demonstrate extraordinary variation in recombination within and among individuals but provide no evidence of a difference in recombination levels between oocytes entering meiosis early in fetal life and those entering late in fetal life. Thus, our data provide a direct test of the second tenet of the production-line model and suggest that it does not provide a plausible explanation for the human maternal-age effect, meaning that—45 years after its introduction—we can finally conclude that the production-line model is not the basis for the maternal-age effect on trisomy.
Main Text
Occurring in as many as 35% of all human pregnancies, aneuploidy is the leading known cause of pregnancy wastage and congenital birth defects in our species (reviewed in Nagaoka et al.1). The vast majority of aneuploidy cases are maternal in origin, suggesting that egg production is inherently error prone in humans.2 Further, this risk increases dramatically with the age of the woman: for women in their twenties, the likelihood of having a clinically recognized trisomic pregnancy is about 2%–3%, but this value increases to over 30% for women in their forties.3
The basis of the effect of maternal age on aneuploidy remains a mystery, but a number of potential mechanisms have been proposed. One of the earliest and most enduring hypotheses is the “production-line model,” initially proposed in 19684 on the basis of apparent age-dependent changes in chiasma frequency in mouse oocytes. It ascribes the age effect to differences in recombination and has two key components. First, it assumes that there is a direct relationship between the timing of meiotic entry in the fetal ovary and the timing of ovulation in the adult (i.e., oocytes that are the first to enter meiosis are the first ovulated and oocytes entering meiosis last are ovulated at the end of the reproductive lifespan). Second, it assumes that meiotic recombination rates vary with gestational age (i.e., the first oocytes entering meiosis have higher recombination levels than oocytes entering later). According to this model, the last oocytes ovulated have the lowest numbers of crossovers and might in fact have an increased frequency of “crossoverless” homologs, greatly increasing the risk of nondisjunction.
In the intervening 45 years, two lines of experimental evidence consistent with the tenets of the production-line model have been produced. First, radiolabeling studies in mice and rats have suggested that there is indeed a production line, i.e., the first oocytes to enter meiosis appear to be the first to be ovulated.5,6 Second, studies in a variety of organisms have demonstrated the importance of recombination abnormalities to the occurrence of meiotic nondisjunction.7–9 The evidence from humans is especially strong, given that abnormal levels or positioning of recombination events has been detected in all trisomies that have been appropriately studied.2,10,11 However, the predicted relationship between recombination levels and maternal age—i.e., that oocytes from older women have lower levels of recombination than oocytes from younger women—has not been demonstrated. Some genetic linkage studies have shown a reduction in recombination in pregnancies involving older women,12,13 but others have either found no effect or reported an increase in recombination levels with increasing maternal age.14 Thus, the data from linkage analyses are equivocal, and moreover, this approach might not be appropriate for a key reason. That is, virtually all linkage studies are based on liveborn individuals, but the vast majority of aneuploid conceptuses terminate in utero. Consequently, traditional linkage analysis of liveborn populations has limited power to assess the relationship between recombination and maternal-age-related aneuploidy.
Accordingly, we decided to directly test the second tenet of the production-line model by examining meiotic recombination in human fetal oocytes. We reasoned that recombination differences in oocytes that initiate meiosis at different times would be evident in a population of fetal ovarian samples as a change in recombination levels with gestational age. Thus, we took advantage of our ongoing analyses of fetal ovarian samples from elective terminations of pregnancy at the San Francisco General Hospital Women’s Options Center,15–17 as well as data from our previous studies at the University of Washington Medical Center in Seattle,16 to ask whether the levels of recombination are affected by the gestational age of the fetus. For these analyses, we collected fetal ovarian samples from elective terminations of pregnancy, as previously described.15–17 Our studies were conducted according to the principles expressed in the Declaration of Helsinki and were approved by the institutional review boards at the University of Washington, Washington State University, and University of California, San Francisco, and informed consent was obtained from all study participants.
We utilized immunofluorescence to examine crossover-associated proteins in prophase-stage oocytes from these samples. Specifically, we analyzed the number and distribution of foci for the DNA-mismatch-repair protein MLH1, thought to localize to approximately 90% of crossovers in pachytene-stage cells of mammalian species.18 Given that MLH1 foci occur in the context of the synaptonemal complex (SC), we also visualized the SC by using antibodies against the axial-element protein SYCP3 (Figure 1A). In total, we analyzed 8,518 cells from 191 fetal samples with gestational ages ranging from 14 to 26 weeks, and we typically examined between 25 and 65 cells per case.
Figure 1.
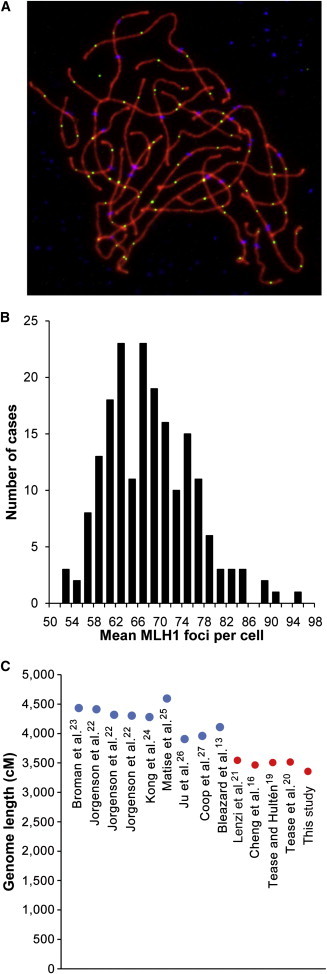
Recombination in Human Oocytes
(A) Representative image from a pachytene-stage human fetal oocyte. Antibodies against SYCP3 (representing the axial-lateral elements of the SC) are visualized in red, those against the crossover-associated DNA-mismatch-repair protein MLH1 are in green, and those against CREST antiserum-positive signals (recognizing centromeric regions) are in blue.
(B) Distribution of mean MLH1 values per cell in 191 fetal ovarian samples.
(C) Estimates of female genetic map lengths from genetic linkage studies (left, in blue) and cytological studies of pachytene oocytes (right, in red). References are indicated beneath each estimate.
Extensive individual variation was evident in the 191 cases. That is, mean MLH1 values (±SE) ranged from 51.1 ± 1.3 to 92.3 ± 2.1, meaning that the level of recombination for the cases with the lowest MLH1 values was only 55% of that for the cases with the highest values (Figure 1B). Pooling the data from all cases, we calculated the overall mean number of MLH1 foci per cell to be 66.3 ± 0.6. If we assume that one MLH1 focus = one crossover = 50 cM, this yields a genome-wide female map length of approximately 3,315 cM. This estimate is consistent with inferred genome-wide estimates (about 3,000–4,000 cM) from previous cytological studies of recombination in human females16,19–21 (Figure 1C). However, these values are consistently lower than those derived from linkage analysis, where estimates range from about 4,000 to 4,500 cM13,22–27 (Figure 1C). Previously, we16,17 suggested two possible reasons for this discrepancy. First, all cytological analyses have involved immunostaining assays of MLH1 and have not assessed the fewer than 10% of crossovers that result from alternative recombination pathways, e.g., noninterfering crossovers associated with the endonuclease MUS81.28,29 Second, localization of MLH1 on SCs appears to be asynchronous in human oocytes, and not all foci are visible in pachytene-stage oocytes.16,21 These caveats aside, the general conclusions from our and other cytological studies of recombination are in good agreement with data from linkage studies and provide evidence of surprising variability in genome-wide recombination levels in human females by comparison with human males.16,17,19,21,24
In subsequent analyses, we tested the effects of gestational age on meiosis. Initially, we examined the mean number of MLH1 foci per cell for each case and sorted cases by gestational age (Figure 2). As is evident from Figure 2, there was no apparent effect of gestational age on genome-wide mean MLH1 values. Figure 2 also shows no change in the range of MLH1 values within individual cases across multiple gestational ages. Subsequently, we analyzed the distribution and mean number of MLH1 foci on four chromosomes known to be nondisjunction prone or to contribute to clinical disorders (i.e., chromosomes 16, 18, 21, and 22). We found no association between gestational age and the placement (data not shown) or number (Figure 3) of MLH1 foci on these chromosomes. Importantly, in contrast to the prediction of the production-line model, there was no increase in “crossoverless” chromosomes with increasing gestational age (Figure 3). Finally, we examined the SC length, a variable known to be directly correlated with genome-wide MLH1 values.19,30 We analyzed the SC lengths for chromosomes 16, 18, 21, and 22 but found no effect of gestational age on SC length for any of the chromosomes (Figure S1, available online). Thus, taken together, our analyses failed to detect any genome-wide or chromosome-specific recombination-associated changes attributable to the gestational age of the sample.
Figure 2.
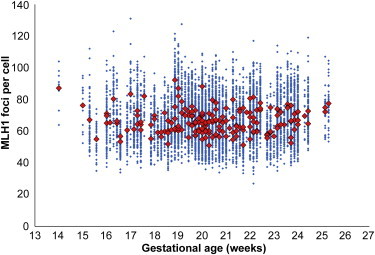
Influence of Gestational Age on Genome-wide Recombination Levels
For each of the 191 cases, the mean number of MLH1 foci per case is represented by red diamonds, and the values for individual cells are represented by blue diamonds. No obvious effect of gestational age on recombination levels was observed.
Figure 3.
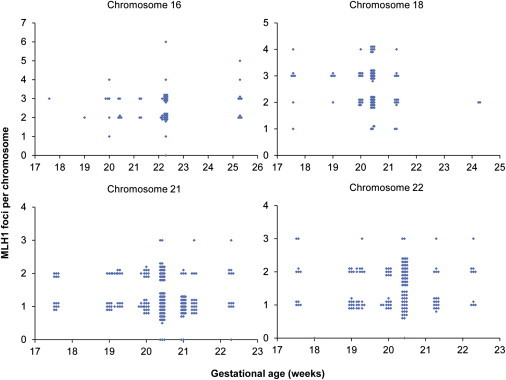
Influence of Gestational Age on the Number of Crossovers on Individual Chromosomes
For a subset of cases, we analyzed the number of MLH1 foci on individual chromosomes, i.e., (A) nine cases for chromosome 16, (B) seven cases for chromosome 18, (C) 11 cases for chromosome 21, and (D) 11 cases for chromosome 22. There was no obvious effect of gestational age on the number of MLH1 foci per chromosome; in particular, the number of chromosomes lacking an MLH1 focus was not affected by gestational age.
In a final set of studies, we asked whether the age of the mother might influence recombination levels in the oocytes of her female fetuses (i.e., a potential grandmaternal-age effect). However, as is evident from Figure 4, we found no indication of such an effect.
Figure 4.
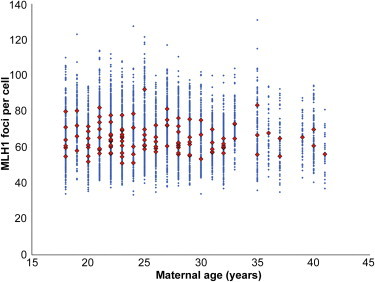
Influence of Maternal Age on Genome-wide Recombination Levels
For each of the 119 cases, the mean number of MLH1 foci per case is represented by red diamonds, and the values for individual cells are represented by blue diamonds. No obvious effect of maternal age on recombination levels was observed.
Two important conclusions derive from our analyses. First, we found no evidence that the gestational age of the fetus influences the level or positioning of crossover events. Thus, the suggestion by Henderson and Edwards4 that a “gradient” in the fetal ovary causes the first-formed oocytes to have more chiasmata than those formed last appears to be incorrect. Accordingly, we conclude that the production-line model as initially proposed is not the cause of the maternal-age effect on human aneuploidy. Nevertheless, the observations that led to the model—i.e., declining numbers of chiasmata with increasing maternal age—can easily be reconciled with our data. That is, recent studies in rodents have indicated an age-related loss of cohesin in oocytes.31,32 In addition to tethering sister chromatids, cohesin serves to link homologous chromosomes together during the first meiotic division. Thus, loss of cohesion with increasing maternal age might cause homologs tethered by single distal exchanges to slip apart from one another and, on cytological examination of diakinesis preparations, would yield an apparent increase in the number of univalents.
Second, and equally important, our observations suggest extraordinary variation in genome-wide crossover levels among different fetal samples. Clearly, individual variation in recombination rates has been documented previously, e.g., in an analysis of different CEPH families, Broman et al. reported female maps as low as 3,300 cM and as high as 4,700 cM.23 Our observations suggest even greater variability in that genome-wide maps ranged from approximately 2,500 to over 4,600 cM among the different samples. Intriguingly, this level of variation is not evident in the human male,17,23 suggesting that recombination is less tightly controlled in human oogenesis than in spermatogenesis. Although the basis of this sex-specific difference remains unclear, it seems unlikely that it is can be explained by the asynchrony of the process in females. However, by combining SNP analyses of recombination-associated loci (e.g., see Kong et al.33) with direct studies of recombination levels, it might be possible to illuminate the genetic underpinnings of this surprising difference in variation between human males and females.
Acknowledgments
We thank the staff and faculty at San Francisco General Hospital’s Women’s Options Center and at the University of Washington Medical Center for assistance in the collection of tissues. We also thank Tracey Woodruff, Carrie Dickenson, Katie Stevenson, Dylan Atchley, Mei-Lani Bixby, Cynthia Megloza, Jody Steinauer, Edith Cheng, Theresa Naluai-Cecchini, Terah Hansen, Elizabeth Pascucci, Changqing Zhou, Chris Small, and Heather Hagen for their assistance in recruitment and data collection. This work was supported by NIH grants HD21341 (to T.H.) and ES013527 (to P.A.H.) and by an NIH training grant awarded to the School of Molecular Biosciences at Washington State University (T32 GM083864 to R.R. and J.G.).
Supplemental Data
References
- 1.Nagaoka S.I., Hassold T.J., Hunt P.A. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassold T., Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 3.Hassold T., Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum. Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 4.Henderson S.A., Edwards R.G. Chiasma frequency and maternal age in mammals. Nature. 1968;218:22–28. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- 5.Polani P.E., Crolla J.A. A test of the production line hypothesis of mammalian oogenesis. Hum. Genet. 1991;88:64–70. doi: 10.1007/BF00204931. [DOI] [PubMed] [Google Scholar]
- 6.Hirshfield A.N. Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol. Reprod. 1992;47:466–472. doi: 10.1095/biolreprod47.3.466. [DOI] [PubMed] [Google Scholar]
- 7.Ward J.O., Reinholdt L.G., Motley W.W., Niswander L.M., Deacon D.C., Griffin L.B., Langlais K.K., Backus V.L., Schimenti K.J., O’Brien M.J. Mutation in mouse hei10, an e3 ubiquitin ligase, disrupts meiotic crossing over. PLoS Genet. 2007;3:e139. doi: 10.1371/journal.pgen.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koehler K.E., Hawley R.S., Sherman S., Hassold T. Recombination and nondisjunction in humans and flies. Hum. Mol. Genet. 1996;5(Spec No):1495–1504. doi: 10.1093/hmg/5.supplement_1.1495. [DOI] [PubMed] [Google Scholar]
- 9.Ross L.O., Maxfield R., Dawson D. Exchanges are not equally able to enhance meiotic chromosome segregation in yeast. Proc. Natl. Acad. Sci. USA. 1996;93:4979–4983. doi: 10.1073/pnas.93.10.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb N.E., Yu K., Shaffer J., Feingold E., Sherman S.L. Association between maternal age and meiotic recombination for trisomy 21. Am. J. Hum. Genet. 2005;76:91–99. doi: 10.1086/427266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassold T., Hall H., Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum. Mol. Genet. 2007;16 Spec No. 2:R203–R208. doi: 10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- 12.Hussin J., Roy-Gagnon M.H., Gendron R., Andelfinger G., Awadalla P. Age-dependent recombination rates in human pedigrees. PLoS Genet. 2011;7:e1002251. doi: 10.1371/journal.pgen.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleazard T., Ju Y.S., Sung J., Seo J.S. Fine-scale mapping of meiotic recombination in Asians. BMC Genet. 2013;14:19. doi: 10.1186/1471-2156-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong A., Barnard J., Gudbjartsson D.F., Thorleifsson G., Jonsdottir G., Sigurdardottir S., Richardsson B., Jonsdottir J., Thorgeirsson T., Frigge M.L. Recombination rate and reproductive success in humans. Nat. Genet. 2004;36:1203–1206. doi: 10.1038/ng1445. [DOI] [PubMed] [Google Scholar]
- 15.Rowsey R., Kashevarova A., Murdoch B., Dickenson C., Woodruff T., Cheng E., Hunt P., Hassold T. Germline mosaicism does not explain the maternal age effect on trisomy. Am. J. Med. Genet. A. 2013;161:2495–2503. doi: 10.1002/ajmg.a.36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng E.Y., Hunt P.A., Naluai-Cecchini T.A., Fligner C.L., Fujimoto V.Y., Pasternack T.L., Schwartz J.M., Steinauer J.E., Woodruff T.J., Cherry S.M. Meiotic recombination in human oocytes. PLoS Genet. 2009;5:e1000661. doi: 10.1371/journal.pgen.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruhn J.R., Rubio C., Broman K.W., Hunt P.A., Hassold T. Cytological studies of human meiosis: sex-specific differences in recombination originate at, or prior to, establishment of double-strand breaks. PLoS ONE. 2013;8:e85075. doi: 10.1371/journal.pone.0085075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillon H., Baudat F., Grey C., Liskay R.M., de Massy B. Crossover and noncrossover pathways in mouse meiosis. Mol. Cell. 2005;20:563–573. doi: 10.1016/j.molcel.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Tease C., Hultén M.A. Inter-sex variation in synaptonemal complex lengths largely determine the different recombination rates in male and female germ cells. Cytogenet. Genome Res. 2004;107:208–215. doi: 10.1159/000080599. [DOI] [PubMed] [Google Scholar]
- 20.Tease C., Hartshorne G.M., Hultén M.A. Patterns of meiotic recombination in human fetal oocytes. Am. J. Hum. Genet. 2002;70:1469–1479. doi: 10.1086/340734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenzi M.L., Smith J., Snowden T., Kim M., Fishel R., Poulos B.K., Cohen P.E. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis i in human oocytes. Am. J. Hum. Genet. 2005;76:112–127. doi: 10.1086/427268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgenson E., Tang H., Gadde M., Province M., Leppert M., Kardia S., Schork N., Cooper R., Rao D.C., Boerwinkle E., Risch N. Ethnicity and human genetic linkage maps. Am. J. Hum. Genet. 2005;76:276–290. doi: 10.1086/427926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broman K.W., Murray J.C., Sheffield V.C., White R.L., Weber J.L. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am. J. Hum. Genet. 1998;63:861–869. doi: 10.1086/302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong A., Gudbjartsson D.F., Sainz J., Jonsdottir G.M., Gudjonsson S.A., Richardsson B., Sigurdardottir S., Barnard J., Hallbeck B., Masson G. A high-resolution recombination map of the human genome. Nat. Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 25.Matise T.C., Chen F., Chen W., De La Vega F.M., Hansen M., He C., Hyland F.C., Kennedy G.C., Kong X., Murray S.S. A second-generation combined linkage physical map of the human genome. Genome Res. 2007;17:1783–1786. doi: 10.1101/gr.7156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju Y.S., Park H., Lee M.K., Kim J.I., Sung J., Cho S.I., Seo J.S. A genome-wide Asian genetic map and ethnic comparison: the GENDISCAN study. BMC Genomics. 2008;9:554. doi: 10.1186/1471-2164-9-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coop G., Wen X., Ober C., Pritchard J.K., Przeworski M. High-resolution mapping of crossovers reveals extensive variation in fine-scale recombination patterns among humans. Science. 2008;319:1395–1398. doi: 10.1126/science.1151851. [DOI] [PubMed] [Google Scholar]
- 28.de los Santos T., Hunter N., Lee C., Larkin B., Loidl J., Hollingsworth N.M. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics. 2003;164:81–94. doi: 10.1093/genetics/164.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holloway J.K., Booth J., Edelmann W., McGowan C.H., Cohen P.E. MUS81 generates a subset of MLH1-MLH3-independent crossovers in mammalian meiosis. PLoS Genet. 2008;4:e1000186. doi: 10.1371/journal.pgen.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynn A., Koehler K.E., Judis L., Chan E.R., Cherry J.P., Schwartz S., Seftel A., Hunt P.A., Hassold T.J. Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science. 2002;296:2222–2225. doi: 10.1126/science.1071220. [DOI] [PubMed] [Google Scholar]
- 31.Chiang T., Duncan F.E., Schindler K., Schultz R.M., Lampson M.A. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 2010;20:1522–1528. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lister L.M., Kouznetsova A., Hyslop L.A., Kalleas D., Pace S.L., Barel J.C., Nathan A., Floros V., Adelfalk C., Watanabe Y. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Kong A., Thorleifsson G., Frigge M.L., Masson G., Gudbjartsson D.F., Villemoes R., Magnusdottir E., Olafsdottir S.B., Thorsteinsdottir U., Stefansson K. Common and low-frequency variants associated with genome-wide recombination rate. Nat. Genet. 2014;46:11–16. doi: 10.1038/ng.2833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


