Abstract
Perceptual learning improves detection and discrimination of relevant visual information in mature humans, revealing sensory plasticity. Whether visual perceptual learning affects motor responses is unknown. Here we implemented a protocol that enabled us to address this question. We tested a perceptual response (motion direction estimation, in which observers overestimate motion direction away from a reference) and a motor response (voluntary smooth pursuit eye movements). Perceptual training led to greater overestimation and, remarkably, it modified untrained smooth pursuit. In contrast, pursuit training did not affect overestimation in either pursuit or perception, even though observers in both training groups were exposed to the same stimuli for the same time period. A second experiment revealed that estimation training also improved discrimination, indicating that overestimation may optimize perceptual sensitivity. Hence, active perceptual training is necessary to alter perceptual responses, and an acquired change in perception suffices to modify pursuit, a motor response.
Keywords: perceptual learning, smooth pursuit eye movements, motion perception, learning transfer, perceptual estimation
Introduction
Perceptual learning (PL), the improvement of perceptual abilities with practice, reflects plasticity in the adult visual system. Behavioral, neuroimaging, and neurophysiological studies show that extensive training in a perceptual task results in specific improvements in the trained task, stimuli, and spatial locations (Astle, Li, Webb, Levi, & McGraw, 2013; Carmel & Carrasco, 2008; Levi & Li, 2009; Sagi, 2011; Sasaki, Nañez, & Watanabe, 2010). One of the hallmarks of PL is its specificity: In most instances, learning does not transfer across stimuli and tasks within a modality (Ahissar & Hochstein, 1993, 1997; Ball & Sekuler, 1987; Fahle & Edelman, 1993; Saffell & Matthews, 2003; but see Sasaki et al., 2010). PL has been investigated in sensory modalities but its effects on motor behavior have not been studied. Here we ask: Does PL affect motor responses?
To address this question we implemented a protocol allowing us to simultaneously examine a perceptual response (visual motion direction estimation) and a motor response (the tracking of motion direction with the eyes). Smooth pursuit eye movements—the voluntary, continuous response of the eyes to moving visual objects—are a model system for studying sensory-motor transformations (Lisberger, 2010; Spering & Montagnini, 2011). Given the lack of consensus regarding the relation between perception and eye movements, it is an open question whether training on a perceptual estimation task would affect smooth pursuit.
On the one hand, several studies support the view that the perception of visual motion and the execution of voluntary smooth pursuit are closely linked both in behavior (Gegenfurtner, Xing, Scott, & Hawken, 2003; Osborne, Lisberger, & Bialek, 2005; Schütz, Braun, & Gegenfurtner, 2011; Spering & Montagnini, 2011; Stone & Krauzlis, 2003) and in terms of the underlying neuronal mechanisms (Lisberger, 2010). Middle temporal area (MT) neuronal activity is correlated with behavioral performance in direction discrimination tasks (Logothetis & Schall, 1989; Newsome, Britten, & Movshon, 1989) and with pursuit direction (Lisberger & Movshon, 1999); lesions to areas MT and middle superior temporal area (MST) impair motion perception (Newsome & Pare, 1988) and lead to pursuit deficits (Dürsteler & Wurtz, 1988). Were both responses controlled by the same neuronal signals, PL could also alter pursuit; that is, effects of training on a perceptual estimation task could transfer to a motor task that observers have not been trained on during the perception task, such as smooth pursuit.
On the other hand, motion perception and pursuit differ in task demands and they may have different temporal dynamics (Spering & Montagnini, 2011). Moreover, dissociations between perception and smooth tracking eye movements have been reported, consistent with the dual-pathway model for perception and action (Goodale, Milner, Jakobson, & Carey, 1991). These dissociations have been found both between perception and smooth tracking in response to larger stimuli, using reflexive gaze-stabilizing movements such as ocular following, and in response to small targets, using gaze-shifting movements such as voluntary smooth pursuit (Blum & Price, 2014; Boström & Warzecha, 2010; Simoncini, Perrinet, Montagnini, Mamassian, & Masson, 2012; Spering & Carrasco, 2012; Spering & Gegenfurtner, 2007; Spering, Pomplun, & Carrasco, 2011; Tavassoli & Ringach, 2010). These studies report perception-pursuit differences in sensitivity (Tavassoli & Ringach, 2010), showing that pursuit can be carried out in the absence of a corresponding visual percept, as well as in response direction (Spering & Carrasco, 2012; Spering & Gegenfurtner, 2007; Spering, Pomplun, & Carrasco, 2011), showing that observers can perceive one motion direction while smoothly tracking another.
Furthermore, PL is generally limited to what was trained: PL does not transfer to untrained stimuli. For example, learning of direction discrimination at one location does not transfer to a stimulus placed at a nearby untrained location (Ball & Sekuler, 1987; Fahle, 2005). Critically, PL is also task specific; after extensive training, learning does not transfer to untrained perceptual tasks even with the same trained stimulus. For instance, learning direction discrimination does not transfer to a speed discrimination task (Saffell & Matthews, 2003) or to a contrast detection task (Ball & Sekuler, 1987). Hence, sensory exposure is not sufficient to produce effects of visual PL (Ahissar & Hochstein, 1993, 1997; Ball & Sekuler, 1987; Fahle & Edelman, 1993; Saffell & Matthews, 2003; but see Sasaki et al., 2010). Taken together these findings suggest that training in a perceptual estimation task should not alter an untrained motor task such as smooth pursuit.
To investigate whether training on a perceptual task can affect a motor task, we trained observers on a direction estimation task (during fixation) and examined its effects on a task in which observers were instructed to track the stimulus (voluntary smooth pursuit eye movements). Visual PL paradigms commonly use discrimination or detection tasks (Ahissar & Hochstein, 1993, 1997; Astle et al., 2013; Ball & Sekuler, 1987; Carmel & Carrasco, 2008; Fahle & Edelman, 1993; Koyama, Harner, & Watanabe, 2004; Law & Gold, 2008; Levi & Li, 2009; Saffell & Matthews, 2003; Sagi, 2011; Sasaki et al., 2010; Wang, Zhou, & Li, 2013), which test sensitivity. In the first experiment, we used an analog estimation task allowing us to directly compare perceptual direction estimation with pursuit eye movement direction.
Previous PL studies have used perceptual sensitivity tasks (e.g., detection or discrimination). In this study, however, to equate pursuit angle to perceived direction responses, we used an analog perceptual direction estimation task (Experiment 1). We also examined whether and how estimation training affects changes in perceptual sensitivity: In Experiment 2, a novel group of observers underwent perceptual estimation training and was tested in a direction discrimination task.
To enable unbiased testing of perceived directions, we did not provide observers with feedback in either experiment. Feedback is helpful but unnecessary for visual PL (Ball & Sekuler, 1987; Fahle & Edelman, 1993; Koyama et al., 2004).
Experiment 1
Methods
Observers
Participants were 12 adults (mean age = 23.2 years, SD = 6.9, seven females). All participants had normal or corrected-to-normal visual acuity, were untrained, and were unaware of the purpose of the study. Study procedures were approved by the New York University ethics committee and observers participated with written informed consent. Remuneration was $10/hr.
Visual stimuli and display
Stimuli were random dot kinematograms (RDK) with dots moving at 10°/s in a stationary aperture of 13.5° radius, sparing 2° around the central fixation cross. The black dots (four pixels, 3 cd/m2) were shown on a uniform gray background (26 cd/m2). Seventy-five percent of the dots moved in the signal direction, while the remaining noise dots moved at the same speed but in fixed random directions; dot lifetime was limited to 140 ms. Dot density was 1 dot/deg2. Stimuli were displayed on a calibrated Cathode Ray Tube (CRT) IBM P260 (Armonk, NY) monitor (41 × 30 cm; IBM P260), with a resolution of 1280 × 1024 pixels and a 100-Hz refresh rate. Observers were seated at a 57-cm distance from the screen with their head supported by a combined chin-and-forehead rest.
Testing and training tasks
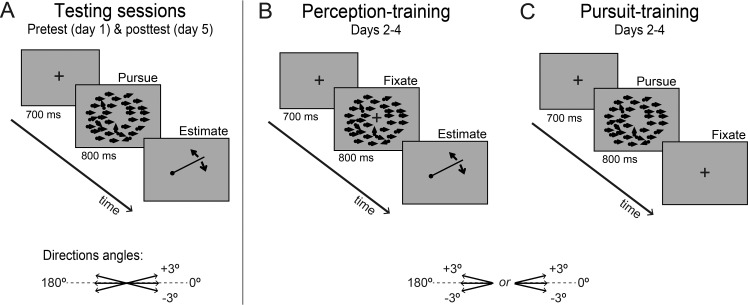
Observers completed five 60-min sessions (one per day over five consecutive days). The first and last sessions (pretest and posttest) were identical and the three intermediate sessions were training sessions. Each trial was initiated by button press and started with a 700-ms fixation period (during which the eye had to be <2° from the fixation mark), followed by an 800-ms stimulus motion (Figure 1).
Figure 1.
Trial sequences for Experiment 1. (A) Trial sequence for testing sessions. For both training groups, the pretest and posttest evaluated pursuit and perception to examine transfer across modalities. (B, C) Between the pretest and posttest, two groups were trained on three consecutive days with either perceptual estimation without pursuit (B) or smooth pursuit without perceptual estimation (C). Each of the three training groups had six observers.
We compared perceptual direction estimates and pursuit angle to moving dots before and after training in two groups of observers (Figure 1). Observers in the perception-training group performed only the perceptual estimation task after fixating on the central fixation cross throughout each trial (Figure 1B). If fixation was broken during the stimulus presentation (eye moved outside the 2° radius from the fixation point), the trial was stopped and repeated. In the pursuit-training group, observers performed only the motor task: They were instructed to smoothly track the dots' motion direction but did not perform the perceptual estimation task (Figure 1C). Pretest and posttest sessions were identical within and across groups and required both the pursuit task and the perceptual estimation task on each trial. In the perceptual estimation task (Figure 1A, B), observers were prompted at the end of the trial to estimate perceived stimulus motion direction as accurately as possible by rotating a line shown on the screen using a mouse. We excluded estimations that deviated more than 100° from horizontal (<0.1% of trials in either experiment). In the pursuit task (Figure 1A, C), observers were required to smoothly track the general motion direction of the dots.
During pre- and posttest sessions, six coherent motion directions were randomly presented: −3°, 0° (horizontal to the right), 3°, 177°, 180° (horizontal to the left), and 183° (Figure 1A, inset). Testing sessions—pretest and posttest—included 408 trials (68 trials per motion direction) presented randomly interleaved in four blocks. Training sessions—the three intermediate sessions—included motion directions to one side only (Figure 1B, C), either to the right (0 ± 3°) or to the left (180 ± 3°), and included 420 trials/day (140 trials per motion direction) presented in four blocks. Observers were randomly assigned a training side.
Eye movement recording and analysis
Eye position signals were recorded during testing and training using a high-resolution eye tracker (EyeLink 1000; SR Research, Kanata, Ontario, Canada) at 1000 Hz. Eye movements were analyzed off-line using custom-made routines in Matlab (The MathWorks, Natick, MA). Saccades in each trace were detected using the standard Eyelink criterion; pursuit onset was detected in individual traces using a piecewise linear function fit to the filtered position trace within the first 300 ms after stimulus onset. To determine eye movement direction (pursuit angle), we computed the mean point (center of gravity, Figure 2A, shown in black) of the two-dimensional eye position trace from pursuit onset to offset of the stimulus, then connected it to the eye position at pursuit onset; the resulting line (Figure 2A, shown in pink) indicates the calculated angle. A pursuit angle was calculated for each valid trial, and then these calculated angles were averaged to produce the mean pursuit angle across trials (separately for pretest and for posttest). We found the same pattern of results regardless of whether the analysis was conducted across the whole trace or across the early (open-loop) and late (closed-loop) phases of pursuit separately.
Figure 2.
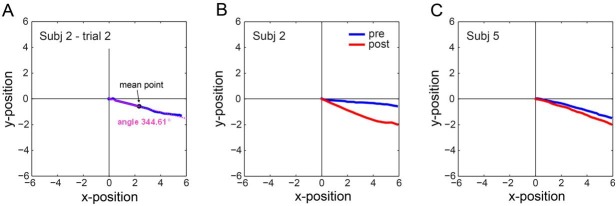
Two-dimensional eye position and direction calculation. (A) Two-dimensional eye position of a single trial (blue) and the calculated eye movement direction (pink). Eye movement direction was calculated using the mean point of the two-dimensional eye position (black dot) and then connecting it to eye position at pursuit onset; the resulting line (pink) indicates pursuit direction. (B, C) The average two-dimensional eye position in the pretest (blue) and the posttest (red) for two observers in the perception-training group. Perception training increased the overestimation in eye direction.
Trials were excluded from further analysis following strict quality criteria (e.g., no pursuit detected, eye changed horizontal direction, blinks) to ensure accurate pursuit angles; exclusion resulted in a minimum of 53.5% trials in either training group. Despite the large number of excluded trials (as can be expected in naïve, untrained observers tracking natural stimuli) each observer had ≥20 valid trials per trained, untrained, and horizontal directions per session. The number of trials rejected during the testing sessions did not differ between groups (pursuit training = 46.2%; perception training = 43.4%).
Statistical analysis
Analysis across groups was performed using repeated-measures analysis of variance (ANOVA), with session as the within-subjects factor and group as the between-subjects factor. Within groups, we performed paired t-tests and repeated-measures ANOVA, with session and task as within-subject factors. All statistical analysis used two-tailed tests. Effect sizes are reported as Cohen's d or partial η2 (ηp2). Analysis was performed using SPSS statistics 20.0 (IBM, Armonk, NY).
Results
We compared perceptual estimations and pursuit angles between the pre- and posttest sessions and across groups (perception training and pursuit training) to assess training effects and transfer across modalities.
In all results reported, we did not find systematic differences between responses to upward (+3°) and downward (−3°) motion directions (training session X motion direction X training group) in either perception or pursuit—both F(1, 10) < 1. Thus, responses to upward and downward motion directions were collapsed into one response representing the absolute deviation from the horizontal. We further collapsed responses across trained and untrained sides for all analyses because neither group showed a difference between sides: The session X side interaction was nonsignificant for the perception-training group [perception: F(1, 5) = 2.11, p = 0.21; pursuit: F(1, 5) = 0.03, p = 0.88] and for the pursuit-training group [perception: F(1, 5) = 0.79, p = 0.41; pursuit: F(1, 5) = 0.66, p = 0.45]. Across training groups, there were no differences between trained and untrained directions of ±3° (session X training side X group) in perception or pursuit, both F(1, 10) < 1. Moreover, we divided the pretest perception trials into two halves and found an increase in overestimation from the first half to the second half across both groups, F(1, 10) = 16.607, p = 0.002, ηp2 = .624; importantly, this increase was similar for the two groups: The two-way interaction was not significant, F(1, 10) < 1. Thus, we rule out that any differential effects across groups could be due to initial pretest differences between the groups.
Pretest session
In the pretest, both the perception-training and pursuit-training groups overestimated deviations of the dots' direction from horizontal. This effect was similar between groups in both perceptual estimations and in pursuit angles; both main effects of group, F(1, 10) < 1 (Figure 3A, B).
Figure 3.
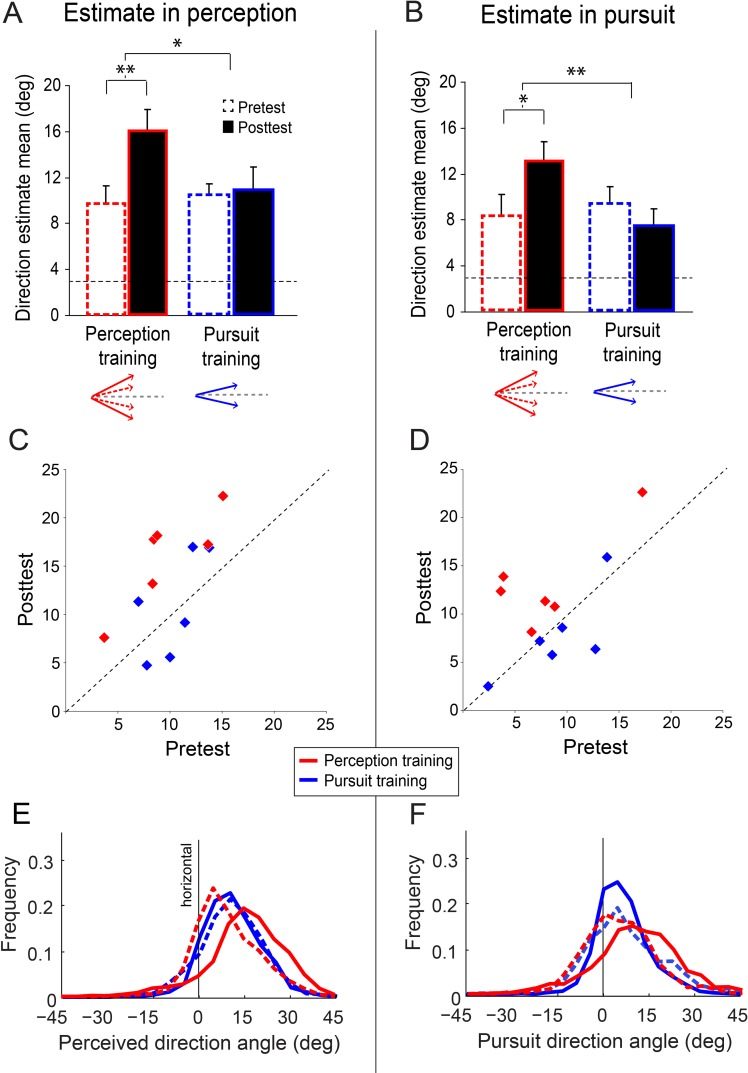
Direction estimates in perception and pursuit angles. Direction estimates in perception (A, C, E) and pursuit (B, D, F) before (dashed) and after (solid) perception training (n = 6, red) and pursuit training (n = 6, blue) in Experiment 1. (A, B) Asterisks indicate significant differences (*p < 0.05, **p < 0.01). Horizontal grey dashed line represents the presented direction (±3°). Perception training significantly affected estimates for both perception (A) and pursuit (B), indicating transfer of PL. Schematic of reported directions below graphs. Values are means ± standard error of the mean (SEM) (C, D) Individual observer data. Only observers in perception training consistently changed their direction estimates and their pursuit angles. (E, F) Frequencies of responses (bin size 5°) in perception (E) and pursuit (F) for 3° motion directions.
Does perception training affect the perceptual domain?
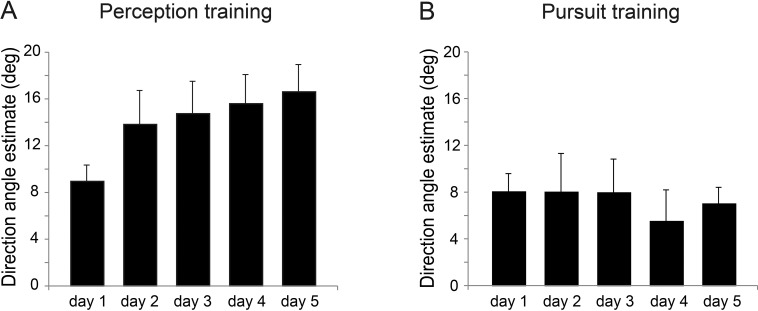
Comparing perceptual estimation for the pretest and posttest sessions across groups revealed a differential effect of training; group X session interaction, F(1, 10) = 8.81, p = 0.02, ηp2 = .47. Perception training significantly increased overestimation, shifting perceptual direction estimates farther from the horizontal; paired t-test t(5) = 5.88, p = 0.002, d = 1.38 (Figure 3A, C, E). This effect was consistent across observers (Figure 3C, red dots). Perceptual estimates increased by 65%, from 9.7° before training to 16° after training (Figure 3A, red bars). Training gradually increased overestimation across training days (Figure 4A). In contrast, perceptual estimates did not change in the pursuit-training group, t(5) = 0.25, p = 0.81 (Figure 3A, blue bars). Perceptual estimates of horizontal motion directions were generally not affected by training; session X group, F(1, 10) < 1 for both perception-training and pursuit-training groups.
Figure 4.
Mean responses across training days for the trained side in Experiment 1 (directions ± 3°). (A) Results for the perceptual training group. (B) Results for the pursuit training group. Values are means ± SEM.
Does perception training affect the motor domain?
Comparing pursuit angles for the pretest and posttest sessions revealed a training group X session interaction, F(1, 10) = 12.23, p = 0.006, ηp2 = .55. Critically, the increased perceptual overestimation following perception training transferred to the untrained motor domain, leading to greater overestimation in pursuit after perception training, t(5) = 3.34, p = 0.02, d = 0.96 (Figure 3B, D, F). This effect was consistent across observers (Figure 3D, red dots). Figure 2B and C shows the average two-dimensional eye position for each of two individual observers in pretest versus posttest sessions, demonstrating the increase in overestimation after training. Across all observers, pursuit angles increased by 58%, from 8.2° before training to 13° after training (Figure 3B, red bars). The magnitude of the overestimation was similar in the trained domain (perception) and the untrained one (pursuit); session X task interaction, F(1, 5) < 1 (Figure 3A, B). Moreover, we calculated the trial-by-trial correlation between the perceptual response direction and the pursuit angle for each observer in the perception-training group. This correlation increased with training (p = 0.02; d = 1.38) from pretest (r = .55) to posttest (r = .76; Figure 5). This augmented correlation reveals that the coupling between perception and pursuit was strengthened even though observers trained only in the perceptual domain.
Figure 5.
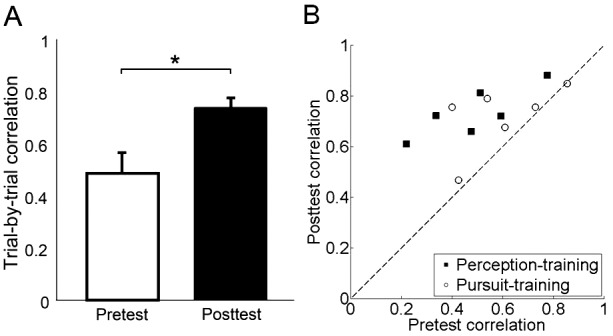
(A) Trial-by-trial correlation between perception and pursuit angle before and after perception training. Values are means ± SEM (B) Individual observer data. Correlation between perception and pursuit angles increased in all observers after perception training (squares), but either did not change or slightly increased after pursuit training (circles).
Contrary to perception training, there was no significant change in pursuit angles following pursuit training, t(5) = 1.44, p = 0.21 (Figure 3B, D; Figure 4B).
Does training affect smooth pursuit?
Neither perception training nor pursuit training significantly improved pursuit accuracy (i.e., latency, position error, and velocity gain; Table 1).
Table 1.
Means and standard deviations (SD) for selected open-loop and closed-loop pursuit measures in Experiment 1. 1 Within-group comparisons: perception training: t(5) = 4.35, p = 0.007; pursuit training: t(5) = 0.94, p = 0.39. 2 Within-group comparisons: perception training: t(5) = 2.49, p = 0.06; pursuit training: t(5) = 1.81, p = 0.13.
| Perception training |
Pursuit training |
Interaction (group X session) |
||||
| Pre |
Post |
Pre |
Post |
F(1,10) |
p |
|
| Latency | 140.96 (13.18) | 135.34 (15.67) | 140.26 (6.36) | 135.77 (12.32) | 0.05 | 0.84 |
| Open-loop velocity | 2.66 (0.55) | 2.58 (0.47) | 2.81 (0.35) | 3.15 (0.71) | 2.87 | 0.12 |
| Closed-loop gain | 0.52 (0.08) | 0.48 (0.07) | 0.52 (0.06) | 0.53 (0.08) | 9.06 | 0.011 |
| Position error | 6.24 (0.51) | 6.47 (0.40) | 6.25 (0.33) | 5.99 (0.59) | 8.30 | 0.022 |
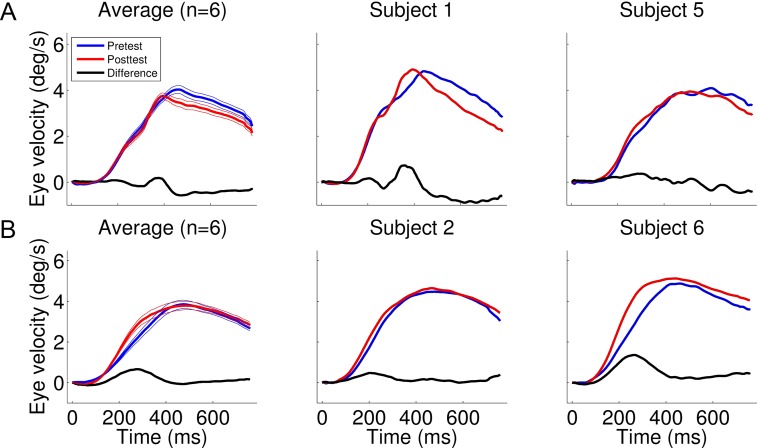
In contrast to perception training (Figure 6A, left panel), pursuit training increased pursuit velocity (Figure 6B, left panel); however, differences in pursuit measures between pretest and posttest sessions were not significant (Table 1). This is consistent with the literature on ocular motor training, showing only marginal improvements in pursuit gain with training (Guo & Raymond, 2010). Perception training had a tendency to impair pursuit, leading to significant group X session interactions for closed-loop gain and position error. These results are likely due to the fact that observers were instructed to strictly fixate during training.
Figure 6.
(A, B) Two-dimensional eye velocity traces in pretest (blue) and posttest (red) sessions and the difference (black) for the perception-training group (A) and the pursuit-training group (B). The left panels depict the means and the standard error of the means, and the middle and right panels show two observers who illustrate individual differences (typical in naïve observers). Target speed was 10°/s. For illustration purposes, data were filtered with an additional 75-ms window average and normalized to the mean fixation position prior to stimulus onset. Filtered individual data were averaged to create the mean across observers.
Experiment 2
Visual PL is assessed with discrimination tasks (Ahissar & Hochstein, 1993, 1997; Astle et al., 2013; Ball & Sekuler, 1987; Carmel & Carrasco, 2008; Fahle & Edelman, 1993; Koyama et al., 2004; Law & Gold, 2008; Levi & Li, 2009; Saffell & Matthews, 2003; Sagi, 2011; Sasaki et al., 2010; Wang et al., 2013). In Experiment 1, we used a perceptual estimation task and found that training increased overestimation. In Experiment 2, we investigated on a new set of observers whether and how estimation training affects direction discrimination. To prevent ceiling discrimination performance, we adjusted the paradigm to fit both estimation and discrimination tasks by shortening the presentation time, using a smaller stimulus, and adjusting the noise level for each observer. In pre- and posttests we examined both discrimination and estimation tasks for the same stimuli. In the three intermediate days, observers trained only on perceptual estimation during fixation.
Methods
Observers
Six adults participated (mean age = 23.3 years, SD = 1.8, five females). All participants had normal or corrected-to-normal vision, were untrained, were untrained and naive with regard to the experiment's purpose, and participated with written informed consent.
Visual stimuli and display
Stimuli were RDK with dots moving at 15°/s in a stationary aperture with a 5° radius, sparing 0.75° around the central fixation cross. On each frame each dot was assigned a direction that was either the coherent direction or a different random direction with the probability matching the coherence level found for each observer (Brownian motion) during the pretest. The display was identical to that used in Experiment 1.
Testing and training tasks
As in Experiment 1, Experiment 2 consisted of five 60-min sessions (one per day over five consecutive days); the first and last sessions were identical (pretest and posttest) and the three intermediate sessions were training sessions. The pretest included a short practice on both the estimation and the discrimination tasks. For each observer we tested discrimination coherence thresholds for motion directions of ±4° using randomly interleaved 60-trial three-down-one-up staircases. We estimated coherence thresholds by averaging staircases. This coherence level was then used for all following testing and training.
During pre- and posttest sessions, we tested performance on six randomly presented coherent motion directions (directions relative to horizontal to the right): −8°, −4°, and −2° (downward from horizontal) and 8°, 4°, and 2° (upward from horizontal). Testing sessions (pretest and posttest) included a block of motion discrimination and a block of direction estimation in a counterbalanced order. Each block consisted of 360 trials. Observers were trained on the estimation task. Training sessions consisted of ±4° directions. Each session consisted of 720 trials presented in four blocks.
Each trial started with a 500-ms fixation cross at the center of the screen. Then, the 200-ms RDK appeared, followed by a 700-ms interstimulus interval (ISI), after which an auditory start signal indicated that a perceptual response could be given. In blocks in which a discrimination response was required, observers pressed a key on the keyboard indicating upward or downward motion relative to horizontal. In blocks in which an estimation response was required, a randomly oriented line appeared and, using a mouse, observers had to orient the line according to the motion direction they perceived. No feedback was given for either discrimination or estimation tasks.
Results
As in Experiment 1, estimation training significantly increased overestimation, t(5) = 6.06, p = 0.002, ηp2 = .88 (Figure 7A). Critically, it also led to a significant increase in perceptual discrimination, t(5) = 9.3, p < 0.001, ηp2 = .94 (Figure 7B), indicating enhanced sensitivity following perceptual estimation training.
Figure 7.
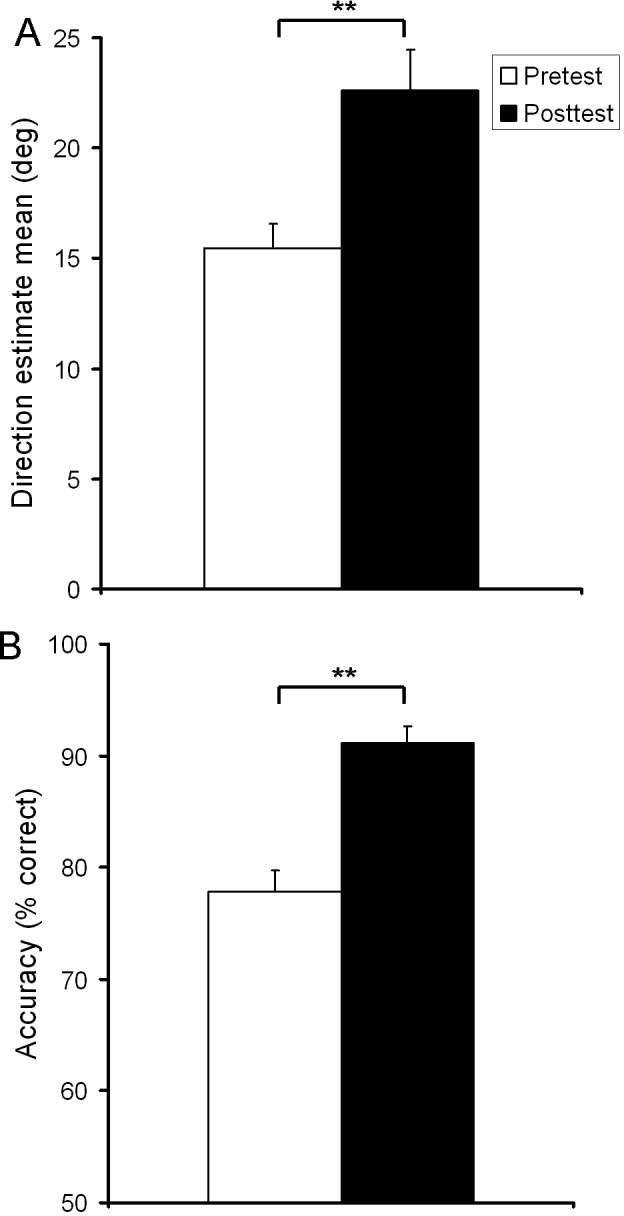
Perceptual estimations and discrimination accuracy for Experiment 2. (A) Direction estimates. (B) Discrimination accuracy after estimation training. Asterisks indicate significant differences (**p < 0.005). Values are means ± SEM.
Discussion
In this study, the first to assess the effects of perceptual training on estimation, we found that perceptual training yielded both stimulus transfer and domain transfer: Training observers with an estimation task led to overestimation of motion direction, both for the trained and untrained directions. Stimulus generalization has been reported with easy discrimination tasks (Ahissar & Hochstein, 1997; Wang et al., 2013) and with low-precision transfer tasks (Jeter, Dosher, Petrov, & Lu, 2009). The present study is the first to evaluate PL for an estimation task. Other studies will help establish whether stimulus generalization is particular to motion estimation or a general characteristic of estimation tasks. It is possible that attention may have played a role in PL and voluntary pursuit. However, given that we neither operationalized nor manipulated attention, we prefer not to speculate about its possible role.
More importantly, the present results reveal transfer across domains, from a perceptual estimation task to untrained voluntary smooth pursuit. In contrast, pursuit training did not change either the mean direction in pursuit or the mean estimation in perception. Experiment 2 revealed that estimation training led to both increased overestimation and increased sensitivity. This finding illustrates a beneficial consequence of increased sensitivity and overestimation: enhanced ability to discriminate between categories.
Differences between training and the effect on learning
The difference in direction estimates following perceptual training and pursuit training may reflect different goals of perception and pursuit. Whereas the pursuit system is suited to track motion direction as quickly and accurately as possible, the perceptual system may optimize categorization by overestimating the difference relative to a fixed decision boundary (Goldstone, 1995; Jazayeri & Movshon, 2007).
The pretest perceptual overestimation we observed is consistent with overestimation reported in fine-direction discrimination and estimation tasks (Jazayeri & Movshon, 2007). The authors attributed such overestimation to a preferential weighting of signals from neurons whose responses best discriminate between similar motion directions. A similar mechanism may underlie the augmented overestimation following estimation training.
The perceptual visual system heightens sensitivity around important category boundaries to aid categorical perception (e.g., Harnad, 1987). The presumed narrowed neural tuning responsible for this sensitivity produces repulsive distortions for low- and high-level features. For example, orientation discrimination (Appelle, 1972) and motion discrimination (Ball & Sekuler, 1982; Matthews & Welch, 1997) are best for horizontal and vertical trajectories, and deviations from the cardinals are perceptually exaggerated for orientation of a stimulus (Smith, 1962) and motion direction (e.g., Rauber & Treue, 1998). Likewise, increases in sensitivity at category boundaries produce repulsive distortions of facial identity (McKone, Martini, & Nakayama, 2001) and a person's direction of walking (Sweeny, Haroz, & Whitney, 2012).
Whereas perceptual training led to perceptual overestimation of directions, pursuit training did not. The perceptual overestimation in the perception training group may have been driven by performing the perceptual task during training. Task performance may have led to better categorization of directions, thereby increasing the overestimation. In contrast, for pursuit training, no perceptual task was required and no perceptual overestimation occurred. This finding is consistent with studies showing that task performance during training is required for learning and that supraliminal exposure alone does not facilitate learning (Ahissar & Hochstein, 1993, 1997; Ball & Sekuler, 1987; Fahle & Edelman, 1993; Saffell & Matthews, 2003; Sasaki et al., 2010). This possibility is further strengthened by a small but significant increase in perceptual overestimation during the pretest for both groups, when the perceptual response was required.
Although in some cases pursuit can improve perceptual sensitivity—pursuit increases perceptual sensitivity to color (Schütz, Braun, Kerzel, & Gegenfurtner, 2008) and improves speed judgment of isoluminant stimuli (Braun et al., 2008) as well as the ability to predict motion direction (Spering, Schütz, Braun, & Gegenfurtner, 2011)—pursuit does not improve direction discrimination of the followed target (Krukowski, Pirog, Beutter, Brooks, & Stone, 2003). One possibility is that the increase in perceptual sensitivity for direction tasks is mediated by perceptual overestimation (as suggested by Experiment 2 in which increased overestimation was coupled with increased sensitivity).
During pursuit, eye position relative to image motion is critical. An ongoing error signal is computed between the eye's position and the target's position, and pursuit's goal is to minimize this error. Information about the eyes' position may stem from the proprioceptive signals of the eye's location in the orbit or from the efference copy of the motor command. The proprioceptive signals lag behind the eye movement itself and are unlikely to be used for calculating the ongoing motor commands, but could be used when estimating the overall movement error (Wang, Zhang, Cohen, & Goldberg, 2007; Xu, Wang, Peck, & Goldberg, 2011). In pursuit training the efference copy, signaling the eye's motion, may “override” perceptual goals, thus preventing perceptual overestimation. On the contrary, in perception training, such signals are task irrelevant (i.e., the eye fixates), allowing for overestimation to arise.
Pursuit motion does not lead only to efference copy and proprioceptive movement signals; unlike other motor actions, it also affects the visual scene. The same physical stimulus creates different retinal stimulation during pursuit and fixation. For our two groups, this would lead to different appearance of the same motion stimulus during training. However, perceptual direction discrimination remains similar under fixation and pursuit (Krukowski et al., 2003). In our study, observers in the perception-training group were required to estimate motion direction during fixation and pursuit. Consistent with Krukowski et al. (2003), we found similar direction estimates under different retinal speeds: There was similar perceptual overestimation during training (fixation) and testing (pursuit; Figure 4A). Thus, although our two groups were exposed to different retinal speed during training, the difference in retinal speed cannot be the only factor driving the distinct effects of training.
Could the perceptual overestimation arise from our response method (mouse movement)? Had that been the case, there would also have been overestimation in the pursuit-training group (although to a smaller extent) because observers in that group also performed the mouse movements during the testing sessions. However, those observers did not overestimate; in fact, half of the observers became more accurate (Figure 3C). Moreover, the perceptual overestimation could not have arisen only from an increase in errors judging the direction. Had that been the case, the mean response would have shifted randomly to an either upward or downward direction for each of the four directions (3°, 357°, 183°, 177°); however, for all directions we found a shift away from horizontal.
Our study did not use feedback during training, as it is not necessary for PL to occur (Ball & Sekuler, 1987; Fahle & Edelman, 1993; Koyama et al., 2004). Moreover, providing feedback in the current study, when observers are required to report the perceived direction, may lead observers to bias their responses instead of reporting the appeared direction. A similar approach avoiding explicit feedback has been used during studies of category learning (Goldstone, 1995), of the interaction of subjective and objective perceptual organizations (Carrasco & Chang, 1995), and of the appearance of various perceptual dimensions, such as contrast (e.g., Carrasco, Ling, & Read, 2004; Fuller, Rodriguez, & Carrasco, 2008) and spatial frequency (e.g., Abrams, Barbot, & Carrasco, 2010; Montaser-Kouhsari & Carrasco, 2009).
Transfer of perceptual overestimation to pursuit
Transfer from perception to pursuit is consistent with behavioral studies showing that pursuit tracks perceived motion (Gegenfurtner et al., 2003; Kowler & McKee, 1987; Schütz et al., 2011; Spering & Montagnini, 2011; Stone & Krauzlis, 2003) and with neurophysiological studies revealing similarities in motion processing for perception and pursuit (Osborne et al., 2005). Any similarities in perception and pursuit may be mediated by key motion-processing pathways in the brain, critically involving area MT (Lisberger, 2010). However, improved behavioral sensitivity due to PL is not necessarily accompanied by changes in motion-sensitive neurons in area MT (Law & Gold, 2008); Law and Gold suggest that such improvements may rather involve areas combining sensory and motor signals, such as the lateral intraparietal area (LIP). Thus, transfer of PL to motor behavior may also be mediated by areas combining sensory and motor signals, such as LIP (Freedman & Assad, 2011; Law & Gold, 2008); the frontal eye fields, which modulate the strength of visual motor transformation for pursuit; and the cerebellum, a key area for pursuit learning (Lisberger, 2010).
To conclude, perception training led to greater overestimation, which transferred to untrained directions. More importantly, it also modified the eye movement response to the stimulus, resulting in a similar overestimation of motion direction in perception and voluntary smooth pursuit. We obtained these novel findings by implementing, for the first time in PL, an estimation task. Moreover, this learning not only changed perceived direction but also enhanced performance in a discrimination task, indicating an increase in sensitivity. This study reveals sensorimotor plasticity across domains, from perception to motor. PL has been shown to be an effective method for improving visual acuity in amblyopia (Levi & Li, 2009) and cortical blindness (Huxlin et al., 2009). Our novel findings could therefore be of clinical relevance for rehabilitation of disorders involving sensorimotor function.
Acknowledgments
This work was supported by a research grant from the NIH RO1 EY016200 to MC. We would like to thank Lu Wang for help with data collection in Experiment 2. We are grateful to the Carrasco lab members, M. Landy, A. Montagnini, and J. A. Movshon for comments on the manuscript.
Commercial relationships: none.
Corresponding author: Sarit F. A. Szpiro.
Email: sarit.szpiro@nyu.edu.
Address: Department of Psychology, New York University, New York, NY, USA.
Contributor Information
Sarit F. A. Szpiro, Email: sarit.szpiro@nyu.edu.
Miriam Spering, Email: mspering@mail.ubc.ca.
Marisa Carrasco, Email: marisa.carrasco@nyu.edu.
References
- Abrams J., Barbot A., Carrasco M. (2010). Voluntary attention increases perceived spatial frequency. Attention, Perception, and Psychophysics , 72 (6), 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M., Hochstein S. (1993). Attentional control of early perceptual learning. Proceedings of the National Academy of Sciences, USA , 90, 5718–5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M., Hochstein S. (1997). Task difficulty and the specificity of perceptual learning. Nature , 387, 401–406 [DOI] [PubMed] [Google Scholar]
- Appelle S. (1972). Perception and discrimination as a function of stimulus orientation: The “oblique effect” in man and animals. Psychological Bulletin , 78, 266–278 [DOI] [PubMed] [Google Scholar]
- Astle A. T., Li R. W., Webb B. S., Levi D. M., McGraw P. V. (2013). A Weber-like law for perceptual learning. Scientific Reports , 3, 1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K., Sekuler R. (1982). A specific and enduring improvement in visual motion discrimination. Science , 218, 697–698 [DOI] [PubMed] [Google Scholar]
- Ball K., Sekuler R. (1987). Direction-specific improvement in motion discrimination. Vision Research , 27, 953–967 [DOI] [PubMed] [Google Scholar]
- Blum J., Price N. S. (2014). Reflexive tracking eye movements and motion perception: One or two neural populations? Journal of Vision , 14 (3): 8 1–14, http://www.journalofvision.org/content/14/3/23, doi:10.1167/14.3.23. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Boström K. J., Warzecha A. K. (2010). Open-loop speed discrimination performance of ocular following response and perception. Vision Research , 50 (9), 870–882 [DOI] [PubMed] [Google Scholar]
- Braun D. I., Mennie N., Rasche C., Schutz A. C., Hawken M. J., Gegenfurtner K. R. (2008). Smooth pursuit eye movements to isoluminant targets. Journal of Neurophysiology , 100, 1287 [DOI] [PubMed] [Google Scholar]
- Carmel D., Carrasco M. (2008). Perceptual learning and dynamic changes in primary visual cortex. Neuron , 57, 799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Chang I. (1995). The interaction of objective and subjective organizations in a localization search task. Perception and Psychophysics , 57 (8), 1134–1150 [DOI] [PubMed] [Google Scholar]
- Carrasco M., Ling S., Read S. (2004). Attention alters appearance. Nature Neuroscience , 7, 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürsteler M. R., Wurtz R. H. (1988). Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. Journal of Neurophysiology , 60, 940–965 [DOI] [PubMed] [Google Scholar]
- Fahle M. (2005). Perceptual learning: Specificity versus generalization. Current Opinion in Neurobiology , 15, 154–160 [DOI] [PubMed] [Google Scholar]
- Fahle M., Edelman S. (1993). Long-term learning in vernier acuity: Effects of stimulus orientation, range and of feedback. Vision Research , 33, 397–412 [DOI] [PubMed] [Google Scholar]
- Freedman D. J., Assad J. A. (2011). A proposed common neural mechanism for categorization and perceptual decisions. Nature Neuroscience , 14, 143–146 [DOI] [PubMed] [Google Scholar]
- Fuller S., Rodriguez R. Z., Carrasco M. (2008). Apparent contrast differs across the vertical meridian: Visual and attentional factors. Journal of Vision , 8 (1): 8 1–16, http://www.journalofvision.org/content/8/1/16, doi:10.1167/8.1.16. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenfurtner K. R., Xing D., Scott B. H., Hawken M. J. (2003). A comparison of pursuit eye movement and perceptual performance in speed discrimination. Journal of Vision , 3 (11): 8 865–876, http://www.journalofvision.org/content/3/11/19, doi:10.1167/3.11.19. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Goldstone R. L. (1995). Effects of categorization on color perception. Psychological Science , 6, 298–304 [Google Scholar]
- Goodale M. A., Milner A. D., Jakobson L. S., Carey D. P. (1991). A neurological dissociation between perceiving objects and grasping them. Nature , 349, 154–156 [DOI] [PubMed] [Google Scholar]
- Guo C. C., Raymond J. L. (2010). Motor learning reduces eye movement variability through reweighting of sensory inputs. Journal of Neuroscience , 30, 16241–16248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnad S. (1987). Psychophysical and cognitive aspects of categorical perception: A critical overview. In Harnad S. (Ed.), Categorical perception: The groundwork of cognition (pp 1–52) New York: Cambridge University Press; [Google Scholar]
- Huxlin K. R., Martin T., Kelly K., Riley M., Friedman D. I., Burgin W. S., et al. (2009). Perceptual relearning of complex visual motion after V1 damage in humans. Journal of Neuroscience , 29, 3981–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M., Movshon J. A. (2007). A new perceptual illusion reveals mechanisms of sensory decoding. Nature , 446, 912–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter P. E., Dosher B. A., Petrov A., Lu Z. L. (2009). Task precision at transfer determines specificity of perceptual learning. Journal of Vision , 5 (9): 8 1–13, http://www.journalofvision.org/content/5/9/3, doi:10.1167/5.9.3. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E., McKee S. P. (1987). Sensitivity of smooth eye movement to small differences in target velocity. Vision Research , 27, 993–1015 [DOI] [PubMed] [Google Scholar]
- Koyama S., Harner A., Watanabe T. (2004). Task-dependent changes of the psychophysical motion-tuning functions in the course of perceptual learning. Perception , 33, 1139–1148 [DOI] [PubMed] [Google Scholar]
- Krukowski A. E., Pirog K. A., Beutter B. B., Brooks K. R., Stone L. S. (2003). Human discrimination of visual direction of motion with and without smooth pursuit eye movements. Journal of Vision , 3 (11): 8 831–840, http://www.journalofvision.org/content/3/11/16, doi:10.1167/3.11.16. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Law C. T., Gold J. I. (2008). Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nature Neuroscience , 11, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., Li R. W. (2009). Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research , 49, 2535–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger S. G. (2010). Visual guidance of smooth-pursuit eye movements: Sensation, action, and what happens in between. Neuron , 66, 477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger S. G., Movshon J. A. (1999). Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. Journal of Neuroscience , 19, 2224–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N. K., Schall J. D. (1989). Neuronal correlates of subjective visual-perception. Science , 245, 761–763 [DOI] [PubMed] [Google Scholar]
- Matthews N., Welch L. (1997). Velocity-dependent improvements in single-dot direction discrimination. Perception and Psychophysics , 59 (1), 60–72 [DOI] [PubMed] [Google Scholar]
- McKone E., Martini P., Nakayama K. (2001). Categorical perception of face identity in noise isolates configural processing. Journal of Experimental Psychology: Human Perception and Performance, 27 (3), 573 [DOI] [PubMed] [Google Scholar]
- Montaser-Kouhsari L., Carrasco M. (2009). Perceptual asymmetries are preserved in short-term memory tasks. Attention, Perception, and Psychophysics , 71 (8), 1782–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W. T., Britten K. H., Movshon J. A. (1989). Neuronal correlates of a perceptual decision. Nature , 341, 52–54 [DOI] [PubMed] [Google Scholar]
- Newsome W. T., Pare E. B. (1988). A selective impairment of motion perception following lesions of the middle temporal visual area (MT). Journal of Neuroscience , 8, 2201–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L. C., Lisberger S. G., Bialek W. (2005). A sensory source for motor variation. Nature , 437, 412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauber H. J., Treue S. (1998). Reference repulsion when judging the direction of visual motion. Perception , 27, 393–402 [DOI] [PubMed] [Google Scholar]
- Saffell T., Matthews N. (2003). Task-specific perceptual learning on speed and direction discrimination. Vision Research , 43, 1365–1374 [DOI] [PubMed] [Google Scholar]
- Sagi D. (2011). Perceptual learning in Vision Research. Vision Research , 51, 1552–1566 [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Nañez J. E., Watanabe T. (2010). Advances in visual perceptual learning and plasticity. Nature Reviews Neuroscience , 11, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz A. C., Braun D. I., Gegenfurtner K. R. (2011). Eye movements and perception: A selective review. Journal of Vision , 11 (5): 8 1–30, http://www.journalofvision.org/content/11/5/9, doi:10.1167/11.5.9. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Schütz A. C., Braun D. I., Kerzel D., Gegenfurtner K. R. (2008). Improved visual sensitivity during smooth pursuit eye movements. Nature Neuroscience , 11, 1211–1216 [DOI] [PubMed] [Google Scholar]
- Simoncini C., Perrinet L. U., Montagnini A., Mamassian P., Masson G. S. (2012). More is not always better: Adaptive gain control explains dissociation between perception and action. Nature Neuroscience , 15, 1596–1605 [DOI] [PubMed] [Google Scholar]
- Smith S. L. (1962). Angular estimation. Journal of Applied Psychology, 46 (4), 240–246 [Google Scholar]
- Spering M., Carrasco M. (2012). Similar effects of feature-based attention on motion perception and pursuit eye movements at different levels of awareness. Journal of Neuroscience , 32, 7594–7601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M., Gegenfurtner K. R. (2007). Contrast and assimilation in motion perception and smooth pursuit eye movements. Journal of Neurophysiology , 98, 1355–1363 [DOI] [PubMed] [Google Scholar]
- Spering M., Montagnini A. (2011). Do we track what we see? Common versus independent processing for motion perception and smooth pursuit eye movements: A review. Vision Research , 51, 836–852 [DOI] [PubMed] [Google Scholar]
- Spering M., Pomplun M., Carrasco M. (2011). Tracking without perceiving: A dissociation between eye movements and motion perception. Psychological Science , 22, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M., Schütz A. C., Braun D. I., Gegenfurtner K. R. (2011). Keep your eyes on the ball: Smooth pursuit eye movements enhance prediction of visual motion. Journal of Neurophysiology , 105, 1756–1767 [DOI] [PubMed] [Google Scholar]
- Stone L. S., Krauzlis R. J. (2003). Shared motion signals for human perceptual decisions and oculomotor actions. Journal of Vision , 3 (11): 8 725–736, http://www.journalofvision.org/content/3/11/7, doi:10.1167/3.11.7. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Sweeny T. D., Haroz S., Whitney D. (2012). Reference repulsion in the categorical perception of biological motion. Vision Research , 64, 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli A., Ringach D. L. (2010). When your eyes see more than you do. Current Biology , 20, R93–R94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang M., Cohen I. S., Goldberg M. E. (2007). The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nature Neuroscience , 10, 640–646 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhou Y., Liu Z. (2013). Transfer in motion perceptual learning depends on the difficulty of the training task. Journal of Vision , 13 (7): 8 1–9, http://www.journalofvision.org/content/13/7/5, doi:10.1167/13.7.5. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Xu Y., Wang X., Peck C., Goldberg M. E. (2011). The time course of the tonic oculomotor proprioceptive signal in area 3a of somatosensory cortex. Journal of Neurophysiology , 106, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]