SUMMARY
Acute gene inactivation using short hairpin RNA (shRNA, knockdown) in developing brain is a powerful technique to study genetic function, however, discrepancies between knockdown and knockout murine phenotypes have left unanswered questions. For example, doublecortin (Dcx) knockdown but not knockout shows a neocortical neuronal migration phenotype. Here we report that in utero electroporation of shRNA, but not siRNA or miRNA to Dcx demonstrates a migration phenotype in Dcx knockouts akin to the effect in wildtype mice, suggesting shRNA-mediated off-target toxicity. This effect was not limited to Dcx, as it was observed in Dclk1 knockouts, as well as with a fraction of scrambled shRNAs, suggesting a sequence-dependent but not sequence-specific effect. Profiling RNAs from electroporated cells showed a defect in endogenous let7 miRNA levels, and disruption of let7 or Dicer recapitulated the migration defect. The results suggest that shRNA-mediated knockdown can produce untoward migration effects by altering endogenous miRNA pathways.
Keywords: doublecortin, dcx, shRNA, miRNA, off-target, migration
INTRODUCTION
MicroRNAs can regulate nervous system development, survival, function, and plasticity (Fineberg et al., 2009). Recognition of targets by microRNAs generally involves the 5′ end of the microRNA spanning nucleotides 2-8 (the seed sequence) associating with complementary mRNA targets, allows a single microRNA to potentially regulate many mRNA targets. For most miRNAs, the primary RNA polymerase II-mediated transcript (pri-miRNA) is processed in the nucleus by Drosha/DGCR8 to generate a ~70 nt pre-miRNA, then exported to the cytoplasm by Exportin-5. The pre-miRNA is further processed into a 21-23 nt duplex by the cytoplasmic RNase Dicer, and one or both strands (the miRNA guide strand or the miRNA* passenger strand) is then loaded into the Ago-protein-containing RNA-induced silencing complex (RISC). Within RISC, the single-stranded mature miRNA forms partial complementary contacts on target mRNAs, leading to translation inhibition and mRNA degradation (Yates et al., 2013).
RNA interference (RNAi) takes advantage of this pathway for acute gene inactivation, and applied in the context of the mammalian in utero brain electroporation, has opened up the possibility of studying genetic requirements (Takahashi et al., 2002). DNA plasmids introduced into the lateral ventricle allow expression of shRNAs in neuroblasts specifically in one hemisphere, used to study the effects of genetic loss-of-function in hundreds of publications. It is a particularly powerful technique to study migration, because electroporation is specifically targeted to apical progenitors, so that the effect can be assessed directly by quantifying distance that neurons have migrated from the electroporation site (Kerjan and Gleeson, 2007; Marchetti et al., 2010).
In most such shRNA reports, the results complement data from mouse knockout (KO) experiments, but there are also many examples where the germline KO does not show the effect observed in the acute shRNA-mediated knockdown (KD). A good example is doublecortin (Dcx) and doublecortin like kinase I (Dclk1) genes, where the single KO mice show no neocortical defects whereas acute KD of either shows clear defects (Bai et al., 2003; Corbo et al., 2002; Koizumi et al., 2006; Pramparo et al., 2010). Other examples include the beta-amyloid precursor protein (Young-Pearse et al., 2007) and EF-hand domain-containing protein 1 (de Nijs et al., 2009; Suzuki et al., 2009) when compared directly. The evidence that migration phenotypes are evident with two or more shRNAs targeting the same transcript, and that the effects can be rescued by re-introduction of non-targetable expressing plasmid have provided evidence that the effects are gene-specific (Bai et al., 2003; Manent et al., 2009), yet the controversy still exists as to how a KD has a phenotype when the germline KO shows none, especially considering that KD usually preserves some percent of protein expression.
Multiple potential theories, some partially overlapping, have been proposed to explain this discrepancy: i] Cells may respond differently following acute KD compared with a chronic KO gene deletion (Gotz, 2003). ii] Acute KD might not leave enough time to evoke upregulation of compensatory mechanisms. iii] Acute KD may leave some transcripts intact, compared with KO, which might somehow produce a more severe phenotype. iv] Acute KD might induce off-target effects, effects on endogenous siRNA processing, or inflammatory responses. While direct evidence for any of the first three theories is lacking, the effect of off-target or inflammatory reaction to shRNAs has been well documented (Alvarez et al., 2006; Fedorov et al., 2006; Olejniczak et al., 2011). Here we put these models directly to test by evaluating the basis in the Dcx family, where the phenomenon was first described.
RESULTS
Neocortical migration defects in Dcx and Dclk1 knockdown but not knockout
The Dcx KO allele that has exons 2-3 of 7 replaced with LacZ, and thus produces a stable mRNA without the 3′ UTR, whereas the Dclk1 allele removes exon 3, predicting an unstable mRNA. Both result in null mutations with absent protein, and lack neocortical migration phenotype (Corbo et al., 2002; Koizumi et al., 2006). We verified this finding by electroporating a GFP-expression plasmid at E14.5, then assessed cellular distribution at E18.5 (Figure S1A-B), quantitated by: i] measuring the distribution of total GFP signal within either the cortical plate (CP) compared with the intermediate zone/subventricular zone (IZ/SVZ). ii] measuring the percentage of GFP+ cells within either the upper, middle or lower cortical plate (uCP, mCP, loCP). With the first method, wildtype (WT) controls ~30-40% of GFP cells were CP-localized, whereas the remainder localized in the IZ/SVZ (Figure S1E). With the second method, 55-60% of cells were positioned within the uCP, without difference between WT and either KO. Combined with published histology, BrdU birthdating and laminar marker distribution (Corbo et al., 2002; Deuel et al., 2006; Kappeler et al., 2006; Koizumi et al., 2006), we conclude that, with current methodologies in either KO, neocortical migration is not disrupted.
We similarly electroporated published shRNA-expressing constructs, the exact ones used in the key published papers, into WT brains to confirm migration defects (Bai et al., 2003; Koizumi et al., 2006). Two different shRNA-expressing constructs against Dcx and one against Dclk1 were electroporated into WT E14.5 embryos. As published, we found a significant migration defect for each of these vectors compared with control (Figure S1C-D, 12.2 or 18.1 vs. 36.8% of GFP+ in CP, or 27.9 or 22.9 vs. 57.8% of GFP+ cells in uCP, p < 0.01 for each comparison, Figure S1E-F). For the remainder of the study we use only the latter method of quantification.
Acute inactivation does not account for the Dclk1 shRNA phenotype
We tested whether acute gene inactivation using Cre electroporation into Dclk1flox/− E14.5 embryos recapitulates the shRNA migration defect. This method induces recombination occurs within a few hours (Gitton et al., 2009), within roughly the same timeframe as shRNA-mediated silencing, and can report migration defects (Ohtaka-Maruyama et al., 2013). We injected a Cre-GFP plasmid with a DsRed2 expressing Cre-reporter plasmid, mixed in a 1:2 ratio to ensure that nearly every cell with the Cre-GFP plasmid would also carry the Cre reporter plasmid. DsRed2 reporter activity was evident in essentially every GFP+ cell (Figure S1H-K). We found that 79.5% of cells were located in the uCP by E18.5 in controls (either Dclk1flox/+ or Dclk1+/−), not statistically different from the 73.6% of cells in the uCP in Dclk1flox/− embryos. Thus acute inactivation does not recapitulate the shRNA migration defect.
Differences in knockdown and knockout at least partly due to off-target effects but not inflammatory-mediated, nor sequence-specific
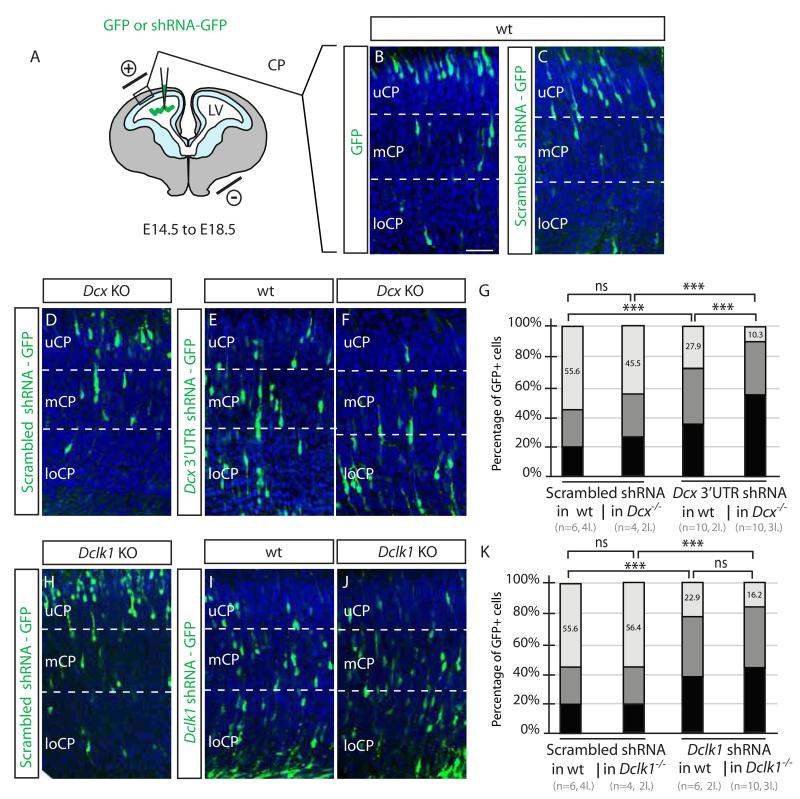
The most direct test whether shRNAs display off-target effects is to perform the KD experiment in the genetic KO background, in the absence of specific targets. We utilized both the Dcx and Dclk1 germline KO for this experiment, electroporating the same plasmids used above to target the Dcx 3′UTR or the Dclk1 open reading frame. We observed that the scrambled shRNAs resulted in 55.6% of GFP+ cells in uCP in control littermates (pooled controls, Figure 1). Similar to results above, scrambled shRNAs resulted in 45.5% and 56.4% of GFP+ cells in uCP in Dcx and Dclk1 KOs, respectively, and Dcx and Dclk1 shRNAs resulted in 27.9% and 22.9% of GFP+ cells in uCP in WT littermates, respectively. The effect of the shRNAs on the KOs was striking, with 10.3% and 16.2% of GFP+ cells in uCP. The fact that the Dclk1 mRNA is unstable suggests that this effect does not require the presence of an intact mRNA. We conclude that the Dcx and Dclk1shRNAs have an effect in the KO lines, supporting an off-target effect.
Figure 1.
Knockdown vs. knockout discrepancy in Dcx and Dclk1 mice is at least partly due to off-target effects of shRNA (A-C) DNA injected into the cerebral lateral ventricle (LV) at E14.5, electroporated into the ventricular zone (light blue), then GFP+ cell positions assessed at E18.5, dividing the cortex into upper, middle and lower cortical plate regions (uCP, mCP, loCP). In WT with empty GFP or Scrambled shRNA-GFP, most labeled cells were located in uCP. Scale bar 100 um. (D-F) Scrambled shRNA into Dcx knockout (KO) showed no defect, whereas Dcx 3′UTR shRNA into either WT or Dcx KO showed a migration defect, with most cells in mCP and loCP. (G) Scrambled shRNA in WT or Dcx KO showed 55.6% vs. 45.5% of cells in UCP. There was a mild though not significant difference in Dcx KO with scrambled shRNA (ns). n = 4-6 mice from 2-4 litters for each condition. p >0.05, Student t-test. Dcx 3′UTR shRNA impaired migration in both the WT and Dcx KO, with 27.9% vs. 10.3% of cells in UCP (***, p < 0.001). (H-K) Scrambled shRNA in WT vs. Dclk1 KO compared with Dclk1 CD shRNA in WT vs. Dclk1 KO showed 55.6% vs. 56.4%, and 22.9 vs. 16.2% of cells in UCP, indicating an effect of the Dclk1 shRNA, irrespective of mouse genotype. n = 6-10 mice from 2-4 litters for each condition. See also Figure S1.
Numerous examples of shRNA off-target phenotypes related to inflammatory responses have been described (Kabilova et al., 2012). Major immunostimulatory triggers include dsRNA oligonucleotides longer than 29-30 bp and the presence of specific U-rich or G-rich sequences (Hornung et al., 2005). Although none of the siRNAs we used satisfy these requirements, we tested for possible activation of the inflammatory sensor protein kinase R (PKR) using a phospho-specific antibody (Gantier and Williams, 2007) and lymphocyte accumulation as reporters, but found none at either E16.5 or E18.5 (Figure S2A-B), suggesting that the off-target effect is not inflammatory-mediated.
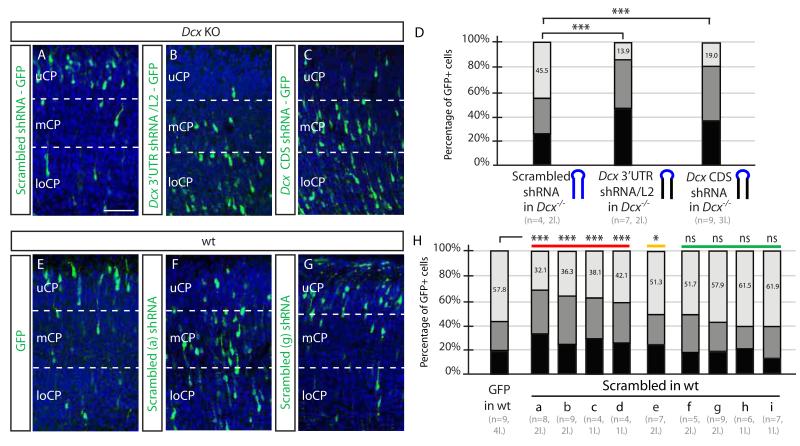
To further investigate which components of the Dcx shRNA mediated the influence on migration, we generated chimeric shRNA constructs consisting of parts of the scrambled and parts of the Dcx shRNAs. A construct containing the same Dcx 3′ UTR shRNA stem sequence but a scrambled hairpin loop sequence induced the same degree of migration defect as the Dcx shRNA (13.9% of GFP+ cells in the uCP, Figure 2A-D), whereas the scrambled stem sequence but the Dcx hairpin loop sequence behaved like scrambled shRNA (not shown). We repeated the experiment with the Dcx CDS shRNA sequence, which induced a very similar migration defect. We conclude that the off-target migration defect can be triggered by multiple shRNA sequences.
Figure 2.
Sequence dependent but not sequence specific off-target effects of shRNAs (A-D) Dcx KO electroporated with swapped loop (blue) maintaining Dcx 3′UTR or CDS stem sequence, showed migration defects (13.9% vs. 19.0% of cells in uCP, compared with 45.5% in Scrambled, ***, p < 0.001). Scale bar 100 um. (E-H) Severe, Moderate or None migration defects induced by a scrambled shRNAs. Nine scrambled shRNAs electroporated into WT mice, ranked from most to least severe effects, compared with GFP alone. The first four (a-d) showed the most dramatic differences (a-d, 32.1-43.1% in uCP, ***, p < 0.001), the next showed moderate difference (e, 51.3% in uCP, * p < 0.01), and last four were not significant (f-i, 51.3-1.9%, ns) compared with GFP alone. n = 4-9 mice from 1-4 litters for each condition. See also Figure S2.
We reasoned that if the off-target effect on migration is not sequence-specific, then some subset of scrambled shRNAs should induce a migration defect as well. We thus cloned 9 additional scrambled shRNAs with purine/pyrimidine ratios similar to Dcx shRNA, not matching any predicted murine mRNA sequences (Figure S2D). We observed that about half of these scrambled shRNAs induced a migration defect in WT brain, in graded severity (Figure 2E-H). Four of the nine scrambled shRNAs showed statistically significant differences in migration. These results confirmed that the observed off-target effect of shRNA on migration is not sequence-specific but is likely sequence-dependent since not all scrambled shRNAs induced a migration phenotype. Overall, these observations indicate that cortical migration is very sensitive to off-target shRNA effects.
shmiRNA knockdown method does not produce the same off-target effects
Artificial RNAi utilizes endogenous miRNA processing pathways to achieve gene silencing. The vector driven shRNA hairpins are exported by Exportin-5 into the cytoplasm where they undergo steps similar to endogenous miRNA processing, including loading onto the RISC complex. Improper design and/or dosage of artificial RNAi tools have been shown to lead to disruptions in endogenous miRNA processing and subsequent off-target effects. For example several shRNAs were show to saturate Exportin-5 and cause downregulation of critical miRNAs, causing off-target toxicity (Grimm et al., 2006), but overexpression of Exportin-5 in Dcx shRNA KD cells did not rescue the migration defect (not shown).
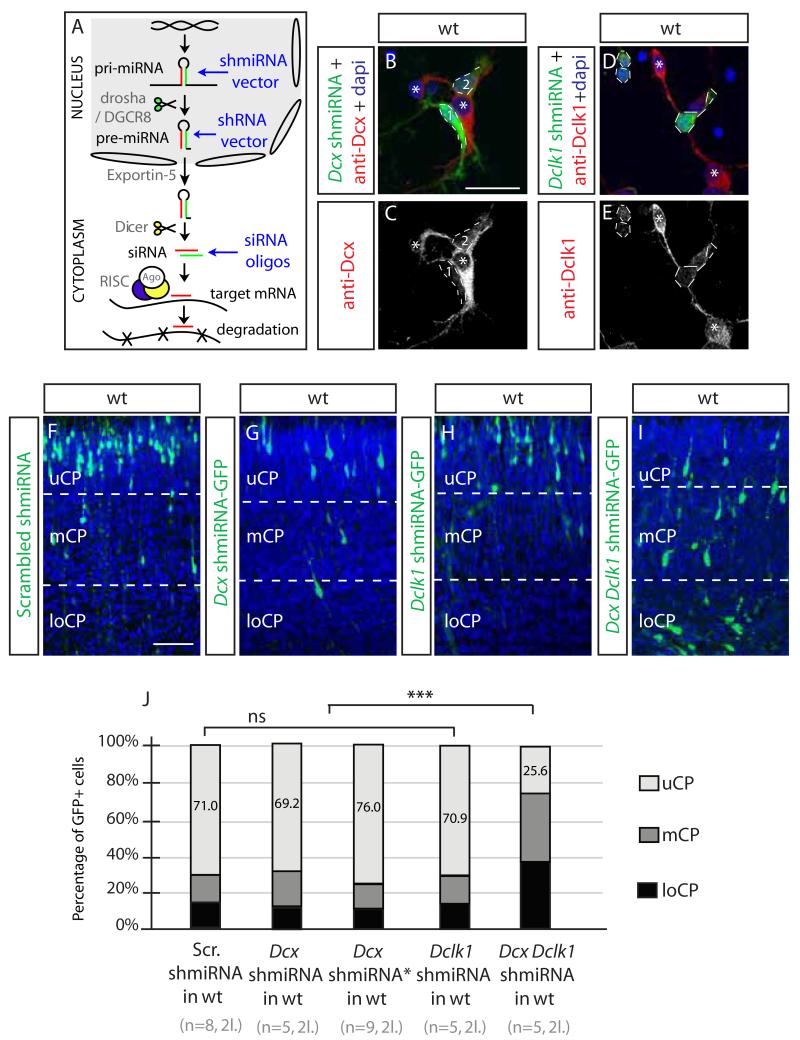
The next-generation of shRNA vectors, named shmiRNAs, drive production of long RNA hairpins (about 200 nt long) with shRNAs embedded into endogenous miRNA loop and flanking sequences (Figure 3A), designed to undergo more natural miRNA processing including processing by Drosha/DGCR8. This method was shown previously to overcome at least some of the off-target effects of shRNA (Bauer et al., 2009). We thus embedded the Dcx 3′ UTR and Dclk1 CDS target sequences into murine miR-155 using the pcDNA6.2 GW vector (Chung et al., 2006), also encoding eGFP within the pre-miRNA expression cassette. The shmiRNA vectors resulted in depleted Dcx and Dclk1 protein levels in a fashion similar to the traditional shRNA vectors in dissociated electroporated neurons (Figure 3B-E). Neither Dclk1 nor either of two different Dcx shmiRNAs demonstrated a phenotype in WT embryos (scrambled 71%, Dcx and Dclk1 69.2%-70.9% GFP+ neurons in uCP, Figure 3F-J).
Figure 3.
shmiRNA or siRNA fail to induce off-target migration defects (A) The pri-mRNA is cleaved in the nucleus by drosha/DGCR8 to yield pre-miRNA, which is akin to products from an shRNA vector, containing an antisense (red) and * (green) strand, exported through the nuclear pore (space between ovals) by Exportin-5, further cleaved in the cytoplasm by Dicer to yield siRNAs, akin to synthetic siRNA duplex oligos, loaded into the RISC complex and then guide strand associates with the target mRNA for silencing. (B-E) Dcx and Dclk1 miRNA are effective against endogenous Dcx or Dclk1 protein in transfected primary cortical neurons (green). Bar 50 um. (F-I) Dcx and Dclk1 shmiRNAs together but neither separately induces migration defect. Bar 100 um. (J) Specificity and sensitivity of shmiRNAs targeting Dcx and Dclk1in WT cortex (71.0% Scrambled vs. 69.2% Dcx shmiRNA1 vs. 76.0% Dcx shmiRNA2 vs. 70.9% Dclk1 shmiRNA vs. 25.6% Dcx;Dclk1 shmiRNA KD of cells in UCP, ***, p 0.001). n = 5-9 mice from each of 2 litters for each condition. See also Figure S3.
The complementary miRNA strand, termed the passenger or * strand, is typically destroyed, but improper recognition can lead to targeting of unintended mRNA targets. Following strand separation by protein components of RISC, usually the strand with the less stably paired 5′ complement serves as the guide (Schwarz et al., 2003). We considered whether incorrect identification of the * strand in the Dcx shRNA vector but not in the mishRNA vector might lead to off-target effect. Although RISC binder software (Ahmed et al., 2009) predicted the correct identity of the intended guide and * strand within the Dcx shRNA hairpin (+0.472 compared with −0.866), to test directly we inverted the position of the guide and * strand within the shmiRNA hairpin. The majority of electroporated cells still migrated normally compared with Dcx shmiRNA construct (Figure S3A-B). We also swapped the shRNA and shmiRNA vector promoters (shRNA uses a U6 promoter while the shmiRNA uses a CMV promoter) and again found that only the shRNA produced the off-target effect (Figure S3C-D). We conclude that the shmiRNA vectors do not produce an off-target effect, despite producing the same antisense nucleotide targeting Dcx or Dclk1.
We wanted to test whether shmiRNA tools are effective to knock down gene function in migration, and while neither Dcx nor Dclk1 KO demonstrated a migration defect, the Dcx/Dclk1double KO showed a robust defect in migration (Deuel et al., 2006; Koizumi et al., 2006). We thus generated a construct ‘chaining’ the two shmiRNA hairpins into the same vector to achieve a Dcx;Dclk1 double shmiRNA, resulting in a dramatic migration defect, with 25.6% GFP+ cells in uCP (Figure 3I-J). The same effect was noted with electroporation of both the Dcx and Dclk1 shmiRNA vectors, but not with single shmiRNA against Dcx or Dclk1 together with the scrambled shmiRNA (not shown). We conclude that shmiRNAs can efficiently inactivate Dcx and Dclk1 without causing off-target migration effects.
We also tested whether a siRNA duplex (i.e. a double stranded RNA rather than a vector-encoded shRNA) induced a migration defect. We first verified that siRNA to Dcx resulted in reduced protein levels by immunofluorescence (Figure S3E-F), then electroporated together with GFP-encoding plasmid into embryos compared with scrambled shRNA or Dcx shRNA as negative- and positive-controls. We found that GFP+ cells migrated well into the cortex, with 45.8% in the uCP compared with 55.6% for scrambled shRNA and 27.9% for Dcx shRNA (Figure S3G-H). We conclude that the off-target effect of shRNAs can be overcome with the use of alternative RNAi methods.
Endogenous miRNAs are dysregulated by Dcx shRNA knockdown
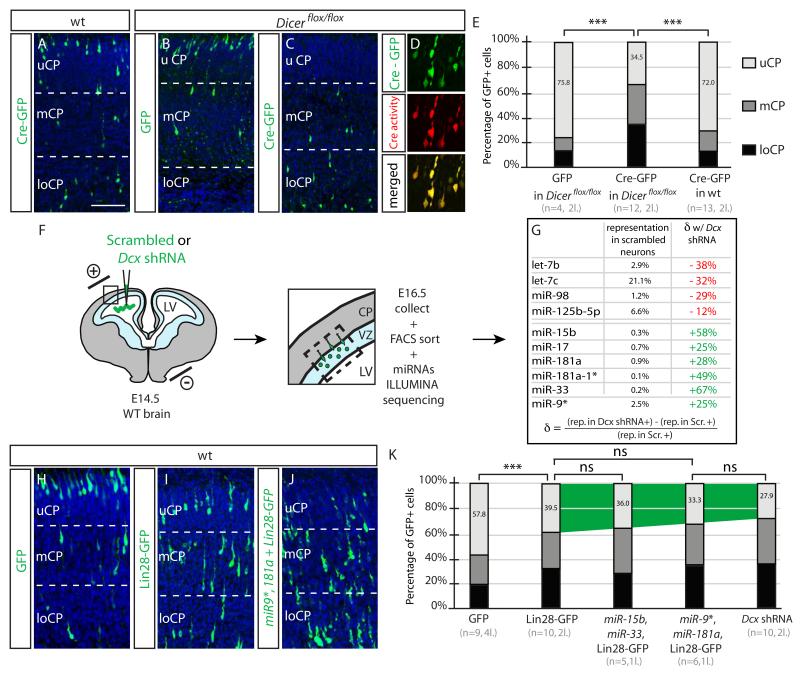
Endogenous miRNAs and processing factors are required in neuronal migration (Pedersen et al., 2013), and conditional Dicer KO mice recombined with Nestin-Cre show fewer BrdU+ cells in uCP (Kawase-Koga et al., 2009). In Dicerflox/flox mice (Harfe et al., 2005) we electroporated GFP-Cre plasmid at E14.5 and observed a striking migration defects, whereas neither GFP-Cre electroporated into WT nor GFP into Dicerflox/flox mice had any effect (Figure 4A-E). We conclude that miRNA processing, dependent upon Dicer, is required for neuronal migration, consistent with a role for endogenous miRNAs in neuronal migration.
Figure 4.
Disruption of endogenous miRNA processing blocks neuronal migration (A-C) Dicerflox/flox Cre-GFP electroporated neurons fail neuronal migration. (D) Co-electroporation with Cre-reporter (DsRed2 expressing). Bar 100 um. (E) Percent GFP+ cells in uCP. Defect only seen for Cre-GFP electroporation into Dicerflox/flox mice. ***, p < 0.001. n = 4-13 mice in 2 litters for each condition. (F) Following electroporation, VZ and loCP was microdissected at E16.5, cells dissociated, FACS isolated, then miRNAs sequenced. (G) miRNAs with most severely dysregulated levels either reduced (red) or increased (green). The percent representation of each miRNA relative to total miRNAs identified (>20,000 reads). Formula to calculate the change (δ) in miRNA. (H-I) WT mice electroporated with GFP alone, Lin28-GFP (to interfere with let-7 family) and combination of miR9*, 181a and Lin28-GFP, showing progressive more severe migration defects. (K) Migration defects of combination electroporations, compared with Dcx shRNA. n = 5-10 mice from each of 1-4 litters for each condition.
Given these results, the off-target effects of shRNA may be due to disruptions of endogenous miRNA processing. We thus performed a comprehensive deep sequencing of mature miRNA sequencing of WT neurons electroporated with Dcx 3′UTR at E14.5 compared with scrambled shRNA. We selected E16.5 for FACS sorting because migration is still ongoing and because neurons in both conditions are located in the IZ, thus minimizing location biases into the results (Figure 4F). We detected 124 miRNAs that appeared in >50 reads each, 16 miRNAs accounted for more than 80%, and 58 miRNAs accounted for more than 96% of total miRNA read counts. The miRNA-9 and let-7c were the most highly represented, accounting for 25% and 21% of total counts, respectively (Hohjoh and Fukushima, 2007).
We focused on the 58 miRNAs representing more than 96% of the total miRNAs in control neurons. Noticeably, we observed a diminution of several members of the let-7 family of miRNAs. Mature let-7c, let-7b and miR-98 representations were diminished approximately −12-38% compared with baseline (Figure 4G). On the contrary, several mature miRNAs such as mature miR-15b, miR-17, miR-181a, miR-181a*, miR-33 and miR-9 were increased by +25-67%. The fact that we observed both increase and decrease suggest that this dysregulation is not simply due to saturation of a limiting maturation step, but more likely disrupting the balance of specific miRNA expressions.
We mimicked these dysregulations, individually or collectively, overexpressing the upregulated miRNA or inhibiting the maturation of the downregulated miRNAs. We observed that none of the individually electroporated miRNAs disturbed migration appreciably, and neither did combinations of miR-15b/miR-17/miR-33 or miR-9/miR-181a/miR-33 or miR9/miR-17/miR-181a (not shown), suggesting that individual or combinations of most miRNAs is not sufficient to impact neuronal migration. Next, inhibition of let-7 family maturation was performed by overexpression of Lin28, a well-characterized suppressor of let-7 miRNA biogenesis (Viswanathan et al., 2008). Human Lin-28A (97% similar to mouse Lin-28) fused to GFP (Balzer and Moss, 2007) resulted in defective migration (Fig. 4H-I), although not as strongly as with the Dcx shRNA. We next combined the let-7 family maturation inhibition with the overexpression of some upregulated miRNAs, and observed that this combination compounded the phenotype, specifically for the combination with miR-15b and miR-33, and so further did the co-expression of miR-9* and miR-181a. We conclude that mimicking several features of the miRNA sequencing closely recapitulates the severity of the Dcx off-target phenotype.
DISCUSSION
Here we show that at least some of the effects of shRNAs in neuronal migration are mediated by off-target effects, to account for the discrepancy between KO and KD phenotypes. This result was not entirely surprising, given that a complete loss-of-function induced by KO should display at least as severe phenotype as a partial loss-of-function induced by KD. What was surprising was that the effect was observed so dramatically and with so many different scrambled shRNA constructs tested. These results offer a warning in the interpretation of shRNA results in neuronal migration, and link to other areas of neurobiology (Alvarez et al., 2006).
Nevertheless, simply switching from shRNA to shmiRNA constructs by-passed the toxic effect. In fact, the data supports the model whereby the off-target effect was not so much due to mispairing of shRNA with improper mRNA targets, but most likely due to disruptions in processing of endogenous miRNAs caused by overexpression of unnatural shRNAs. The fact that the same sequences did not induce off-target effects when expressed within the shmiRNA constructs, and that the Dicer conditional mouse showed specific neuronal migration defects, support this conclusion. Further, the finding that the combination of Dcx and Dclk1 KD using these shmiRNA constructs faithfully recapitulates the findings from the KO experiments supports the effectiveness of this approach.
Although Dcx neurons display defective migration in several contexts, the final placement of these cells within the laminated cortex is indistinguishable from WT cortex, suggesting some form of compensation (Kappeler et al., 2006; Pramparo et al., 2010). Studies from double KOs suggest that the Lis1 and Dclk1 genes are partially redundant with Dcx as the double KOs show phenotypes not observed in single KOs. This may also be one reason why Dcx shRNA electroporation in the Dcx KO showed the most severe migration defect of any of the conditions tested, but an alternative hypothesis is that the absence of a target for the shRNA yielded higher off-target effects. Thus our results do not demonstrate that Dcx has no role in murine cortical migration, but prompt a reevaluation of genetic interactions in the in vivo setting.
We utilized Lin28 overexpression to model the effect of downregulation of let-7 family induced by Dcx shRNA. We found that Lin28 overexpression, which inhibits the biogenesis of let-7, impaired neuronal migration. Individual members of the let-7 miR family can regulate neuronal differentiation and migration by targeting signaling of the homologue of the Drosophila tailless gene TLX (Cimadamore et al., 2013; Zhao et al., 2013). While morphology of Lin28 cells was unremarkable in the cortex, they showed defects in migration. Lin-28 is also involved in many other cellular processes, and further experiments will be required to test whether the defect caused by Lin-28 overexpression is mediated through let-7 and TLX signaling.
EXPERIMENTAL PROCEDURES
Histology and immunohistochemistry
Embryos were genotyped and dissected and fixed in 4% PFA in PBS for 20 μm coronal sectioning and immunohistochemistry (Kerjan et al., 2009). Primary antibodies were rabbit anti-Cre (1:1000, Novagen), anti p-PKR (1:200, Biosource), rabbit anti GFP (1:500 Molecular probes). Images were acquired with an Olympus FV1000 confocal microscope. Quantification of percentage of signal in cortical subdomains was performed by drawing a line at the junction between the CP and IZ/SVZ, using Volocity software.
shRNAs, shmiRNAs, and plasmids
Dcx 3′UTR, Dcx CDS, Dclk1 CDS and multiple scrambled shRNAs cloned into pGE2hrGFP II (Stratagene) ensured co-expression of GFP with shRNAs. Scrambled sequences were generated by siRNA Wizard v.3.1 software (InvivoGen). Identical sequences were cloned into the shmiRNA pcDNA6.2 GW/emGFP (Invitrogen). The miR 15b, 17, 9*, 181a, 3 and flanking regions (~150 bp up- and down-stream) were cloned into pmR-ZsGreen1 (Clontech). Lin28-GFP was in the pLT.143 vector utilizing phrGFP II (Stratagene). Cre recombinase was encoded by pBS500 EF1alpha-GFPCre (Addgene #11920), and Cre-reporter plasmid was in pCALNL-DsRed2 (Addgene #13769). All electroporations were with Endofree® preparations. Dcx siRNA duplexes (Qiagen HPP purified) were electroporated at 2 μM together with phrGFP II (Stratagene) at 4:1 molar ratio.
Animal breeding and Electroporations
Dcx, Dclk1, Dicertm1Bdh/J lines were bred as reported in a mixed C57/BL6;129Svj background (Harfe et al., 2005; Koizumi et al., 2006) (Jackson Labs 013170, 006366). C57Bl6 (Charles River) were used when mating did not allow WT littermate controls. All work was in accordance with UCSD IACUC protocols. Surgery and electroporation was performed as described (Koizumi et al., 2006), injecting 1-2 μl with 0.1% Fast Green (Sigma) by pressure (General Valve Picospritzer) through the uterus into the lateral ventricles of anesthetized dams, then BTX Squarewave ECM 830 produced 5-pulses of 30mV.
Cortical neuron preparations
Dissociation was as described (Kerjan et al., 2009), transfected after 24h with Lipofectamine 2000, fixed at 2 days with 4% PFA for 30 min then immunostained with anti-Dcx (1:100, Santa Cruz Biotechnology) or anti-Dclk1 (1:500, gift of Dr. A. Edelman). FACS sorting was performed on a BD FACSAria™ II instrument with empiric parameters.
RNA-Seq and Processing
mRNA and miRNA were extracted using mRNAeasy (Qiagen) without smRNA fraction enrichment, to collect all RNAs, checked by Agilent 2100 Bioanalyzer (RNA Nano Chip, Agilent), prepared by Illumina sequencing via size selection on Novex 15% TBE-Urea gel (Invitrogen), lengths 20-30 bases isolated, ligated with 5′ adapter, size-selected, then ligated with 3′ adapter, size selected and subject to reverse transcription and 15 PCR cycle amplification, size selected to 90-100 bp, and used for cluster generation and 40 bp single end sequencing, called with Casava v1.5, demultiplexing the 5bp barcodes at the 5-prime of the read with 1 mismatch allowed. mirDeep2 was used to annotate miRNA reads (Friedlander et al., 2012), and EdgeR used for statistical analysis (Robinson and Smyth, 2007).
Supplementary Material
HIGHLIGHTS.
The effect of Dcx shRNA is partly mediated by off-target effects.
A substantial subset of scrambled shRNAs produce migration defects.
Dcx shRNA induces disruption of miRNA levels.
Migration defects recapitulated by disrupting let7 or Dicer function.
ACKNOWLEDGEMENTS
We thank Hong-Tuyet Nguyen and Jesus Olivera for technical expertise, G. Yeo and A. Pasquinelli for discussions. This work was supported by the National Institutes of Health R01NS41537. G.K. was supported by an EMBO Long Term Fellowship, S.L.B. by the A.P. Giannini Fellowship, and A.G.F. by the Brain Behavior Research Foundation. We thank the UCSD Neuroscience Microscopy Core P30 NS047101 for imaging support, S.R. Head and L. Schaffer from Scripps Research Institute Genomics Core Facility, J. LoTurco for Dcx shRNA constructs, E. Balzer for Lin28-GFP construct, D. Young at the UCSD Flow Cytometry Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
URLs
RISC binder: http://crdd.osdd.net:8081/RISCbinder/
SUPPLEMENTAL INFORMATION
Supplemental Information includes three figures and can be found with this article online.
AUTHOR CONTRIBUTIONS
G.K. generated figures, S.L.B., A.G.F, G.N., and J.E.L. generated clones, S.T.B. tested genetic perturbations on migration and edited the manuscript. J.G.G. directed the project.
Authors have no conflicts of interest.
REFERENCES
- Ahmed F, Ansari HR, Raghava GP. Prediction of guide strand of microRNAs from its sequence and secondary structure. BMC Bioinformatics. 2009;10:105. doi: 10.1186/1471-2105-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26:7820–7825. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA biology. 2007;4:16–25. doi: 10.4161/rna.4.1.4364. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kinkl N, Meixner A, Kremmer E, Riemenschneider M, Forstl H, Gasser T, Ueffing M. Prevention of interferon-stimulated gene expression using microRNA-designed hairpins. Gene therapy. 2009;16:142–147. doi: 10.1038/gt.2008.123. [DOI] [PubMed] [Google Scholar]
- Chung KH, Hart CC, Al-Bassam S, Avery A, Taylor J, Patel PD, Vojtek AB, Turner DL. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 2006;34:e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013;110:E3017–3026. doi: 10.1073/pnas.1220176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, Wynshaw-Boris A, Walsh CA. Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neurosci. 2002;22:7548–7557. doi: 10.1523/JNEUROSCI.22-17-07548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nijs L, Leon C, Nguyen L, Loturco JJ, Delgado-Escueta AV, Grisar T, Lakaye B. EFHC1 interacts with microtubules to regulate cell division and cortical development. Nat Neurosci. 2009;12:1266–1274. doi: 10.1038/nn.2390. [DOI] [PubMed] [Google Scholar]
- Deuel TA, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49:41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantier MP, Williams BR. The response of mammalian cells to double-stranded RNA. Cytokine & growth factor reviews. 2007;18:363–371. doi: 10.1016/j.cytogfr.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitton Y, Tibaldi L, Dupont E, Levi G, Joliot A. Efficient CPP-mediated Cre protein delivery to developing and adult CNS tissues. BMC Biotechnol. 2009;9:40. doi: 10.1186/1472-6750-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M. Doublecortin finds its place. Nat Neurosci. 2003;6:1245–1247. doi: 10.1038/nn1203-1245. [DOI] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohjoh H, Fukushima T. Expression profile analysis of microRNA (miRNA) in mouse central nervous system using a new miRNA detection system that examines hybridization signals at every step of washing. Gene. 2007;391:39–44. doi: 10.1016/j.gene.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Kabilova TO, Meschaninova MI, Venyaminova AG, Nikolin VP, Zenkova MA, Vlassov VV, Chernolovskaya EL. Short double-stranded RNA with immunostimulatory activity: sequence dependence. Nucleic acid therapeutics. 2012;22:196–204. doi: 10.1089/nat.2011.0328. [DOI] [PubMed] [Google Scholar]
- Kappeler C, Saillour Y, Baudoin JP, Phan Dinh Tuy F, Alvarez C, Houbron C, Gaspar P, Hamard G, Chelly J, Metin C, et al. Branching and nucleokinesis defects in migrating interneurons derived from doublecortin knockout mice. Hum Mol Genet. 2006;15:2183. doi: 10.1093/hmg/ddl062. [DOI] [PubMed] [Google Scholar]
- Kawase-Koga Y, Otaegi G, Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev Dyn. 2009;238:2800–2812. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan G, Gleeson JG. Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 2007;23:623–630. doi: 10.1016/j.tig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kerjan G, Koizumi H, Han EB, Dube CM, Djakovic SN, Patrick GN, Baram TZ, Heinemann SF, Gleeson JG. Mice lacking doublecortin and doublecortin-like kinase 2 display altered hippocampal neuronal maturation and spontaneous seizures. Proc Natl Acad Sci U S A. 2009;106:6766–6771. doi: 10.1073/pnas.0812687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Manent JB, Wang Y, Chang Y, Paramasivam M, LoTurco JJ. Dcx reexpression reduces subcortical band heterotopia and seizure threshold in an animal model of neuronal migration disorder. Nat Med. 2009;15:84–90. doi: 10.1038/nm.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G, Escuin S, van der Flier A, De Arcangelis A, Hynes RO, Georges-Labouesse E. Integrin alpha5beta1 is necessary for regulation of radial migration of cortical neurons during mouse brain development. Eur J Neurosci. 2010;31:399–409. doi: 10.1111/j.1460-9568.2009.07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaka-Maruyama C, Hirai S, Miwa A, Heng JI, Shitara H, Ishii R, Taya C, Kawano H, Kasai M, Nakajima K, et al. RP58 regulates the multipolar-bipolar transition of newborn neurons in the developing cerebral cortex. Cell reports. 2013;3:458–471. doi: 10.1016/j.celrep.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Olejniczak M, Polak K, Galka-Marciniak P, Krzyzosiak WJ. Recent advances in understanding of the immunological off-target effects of siRNA. Current gene therapy. 2011;11:532–543. doi: 10.2174/156652311798192770. [DOI] [PubMed] [Google Scholar]
- Pedersen ME, Snieckute G, Kagias K, Nehammer C, Multhaupt HA, Couchman JR, Pocock R. An epidermal microRNA regulates neuronal migration through control of the cellular glycosylation state. Science. 2013;341:1404–1408. doi: 10.1126/science.1242528. [DOI] [PubMed] [Google Scholar]
- Pramparo T, Youn YH, Yingling J, Hirotsune S, Wynshaw-Boris A. Novel embryonic neuronal migration and proliferation defects in Dcx mutant mice are exacerbated by Lis1 reduction. J Neurosci. 2010;30:3002–3012. doi: 10.1523/JNEUROSCI.4851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Smyth GK. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics. 2007;23:2881–2887. doi: 10.1093/bioinformatics/btm453. [DOI] [PubMed] [Google Scholar]
- Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miyamoto H, Nakahari T, Inoue I, Suemoto T, Jiang B, Hirota Y, Itohara S, Saido TC, Tsumoto T, et al. Efhc1 deficiency causes spontaneous myoclonus and increased seizure susceptibility. Hum Mol Genet. 2009;18:1099–1109. doi: 10.1093/hmg/ddp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Sato K, Nomura T, Osumi N. Manipulating gene expressions by electroporation in the developing brain of mammalian embryos. Differentiation. 2002;70:155–162. doi: 10.1046/j.1432-0436.2002.700405.x. [DOI] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Scientific reports. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.