Abstract
Although many human cancers are located in mucosal sites, most cancer vaccines are tested against subcutaneous tumors in preclinical models. We therefore wondered whether mucosa-specific homing instructions to the immune system might influence mucosal tumor outgrowth. We showed that the growth of orthotopic head and neck or lung cancers was inhibited when a cancer vaccine was delivered by the intranasal mucosal route but not the intramuscular route. This antitumor effect was dependent on CD8+ T cells. Indeed, only intranasal vaccination elicited mucosal-specific CD8+ T cells expressing the mucosal integrin CD49a. Blockade of CD49a decreased intratumoral CD8+ T cell infiltration and the efficacy of cancer vaccine on mucosal tumor. We then showed that after intranasal vaccination, dendritic cells from lung parenchyma, but not those from spleen, induced the expression of CD49a on cocultured specific CD8+ T cells. Tumor-infiltrating lymphocytes from human mucosal lung cancer also expressed CD49a, which supports the relevance and possible extrapolation of these results in humans. We thus identified a link between the route of vaccination and the induction of a mucosal homing program on induced CD8+ T cells that controlled their trafficking. Immunization route directly affected the efficacy of the cancer vaccine to control mucosal tumors.
INTRODUCTION
The role of CD8+ T cells to control tumor growth (1) and the correlation found in many human tumors between infiltration of CD8+ T cells and prolonged survival (2, 3) led to the development of therapeutic cancer vaccine based on induction of effective antitumor CD8+ T cells. After recent successes of immunotherapy against melanoma and prostate cancers (4, 5), many cancer vaccines are now also being designed for the treatment of mucosal tumors (such as lung, head and neck, and genital cancers) (6–8). However, cancer vaccines dedicated for mucosal cancers have mostly been tested in preclinical subcutaneous tumors followed by extrapolation to mucosal human tumors, which may explain some contrasts between the potency of these vaccines in mice and their failure in cancer patients. Preliminary studies showed that discrepancies existed between the frequency, phenotype, and function of antitumor T cells in the circulation and in the tumor microenvironment (9–11). To our knowledge, the clinical benefit of preferentially inducing antitumor CD8+ T cells at the anatomic site of mucosal tumors and not only in peripheral blood has never been addressed. Chemokine receptors and integrin molecules expressed by T cells influence their trafficking to mucosal sites (12). We therefore hypothesized that mucosa-specific homing instructions to CD8+ T cells might influence mucosal tumor outgrowth, similar to the way they influence immune control of mucosal pathogens in infectious disease models (13, 14).
Thus, we set up original orthotopic models of head and neck and lung cancers and vaccinated using a nonreplicative delivery system— the B subunit of Shiga toxin as mucosal vector, which has previously been shown to target antigen to dendritic cells (DCs) (15). We demonstrated that only intranasal vaccination elicited mucosal E7-specific CD8+ T cells expressing mucosal integrins (CD49a and CD103). This induction of CD49a was crucial because blockade of CD49a decreased intratumoral CD8+ T cell infiltration and the efficacy of cancer vaccine on mucosal tumors.
RESULTS
STxB-based vaccines elicited comparable CD8+ T cell response in the spleen regardless of the route of immunization
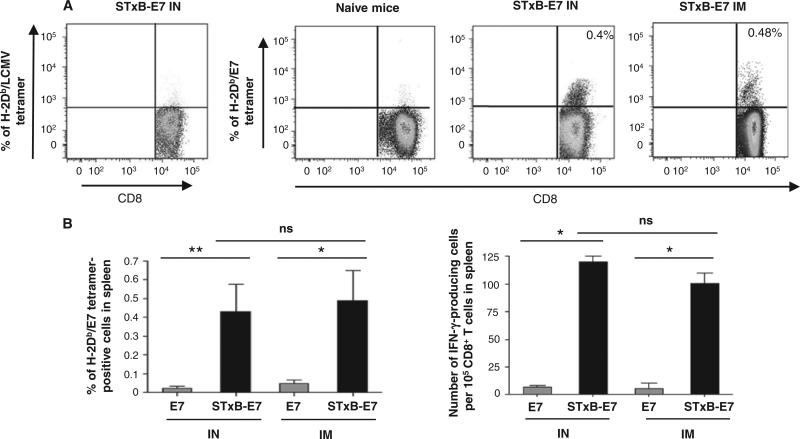
Because our goal was to compare the efficacy of mucosal and systemic vaccination in tumor control, we first selected a vaccine with the ability (i) to be administered by both mucosal (intranasal) and systemic (intramuscular) routes and (ii) to induce antigen-specific CD8+ T cells, because it has been shown that these cells play a major role in the control of tumors (2). We focused on the nonreplicative vector STxB because we previously showed that when coupled to various antigens and administered by a systemic route, antigen-specific humoral and cellular immune responses in the peripheral systemic compartment (blood and spleen) were superior to that elicited by antigen alone (16, 17). Here, we demonstrated that antigen coupled to STxB and administered by the intranasal route was also preferentially targeted to DCs (myeloid CD11b DCs, lymph node–resident CD8α+ DCs, and CD103+ DCs) in mucosal lung-associated mediastinal lymph nodes compared to nonvectorized protein (fig. S1). However, this vector did not deliver antigen to the population of Ly6C+ macrophages expressing CD11c in the lung (fig. S1). We then compared the efficacy of STxB-E7–based vaccines combined with αGalCer—a mucosal adjuvant previously shown to synergize with STxB (18)—to elicit systemic antigen-specific CD8+ T cell responses after intranasal or intramuscular immunization. Administration of STxB-E7 by intranasal or intramuscular routes elicited a similar percentage of Db-E739–47 tetramer–positive and interferon-γ (IFN-γ)–producing antigen-specific CD8+ T lymphocytes in the spleen (Fig. 1, A and B). Vaccination of mice with STxB-E7 induced significantly higher levels of E749–57–specific CD8+ T cells in spleen, as assessed ex vivo by both tetramer assay and IFN-γ enzyme-linked immunospot (ELISpot), than vaccination of mice with the E7 polypeptide, whatever the route of immunization (Fig. 1, A and B). Characterization of E749–57–specific CD8+ T cells elicited by the intra-nasal or intramuscular route by STxB-E7 showed that they were poly-functional because they produced multiple cytokines (IFN-γ and interleukin-2) and were cytotoxic in vivo (Fig. 1B and fig. S2). On the basis of these results, we selected STxB-E7 as a potential mucosal cancer vaccine.
Fig. 1.
Intranasal or intramuscular immunization with STxB-E7 induces E7 antigen-specific CD8+ T cells in spleen. Representative dot plots of spleen E7-specific CD8+ T cells stained by tetramers after two intranasal (IN) or intramuscular (IM) immunizations (days 0 and 14) with STxB-E7 (0.5 nmol = 20 μg). (A) Both vaccines were mixed with αGalCer. (B) E749–57–specific CD8+ T cells from spleen were detected by tetramer assay or IFN-γ ELISpot directly ex vivo. For the tetramer analysis, values shown correspond to means ± SD obtained with specific tetramers. Irrelevant tetramers or tetramer on unimmunized mice gave results <0.05%. For the ELISpot analysis, background obtained with cells not pulsed with the E749–57 peptide was also subtracted (always <10 spots per 105 cells). Spots on unimmunized mice were always <5 spots per 105 cells. Four mice per group were immunized, and these experiments were reproduced three times. *P < 0.05, **P < 0.01. ns, not significant.
Intranasal immunization with STxB-E7 inhibited the growth of orthotopic head and neck and lung tumors in both prophylactic and therapeutic settings
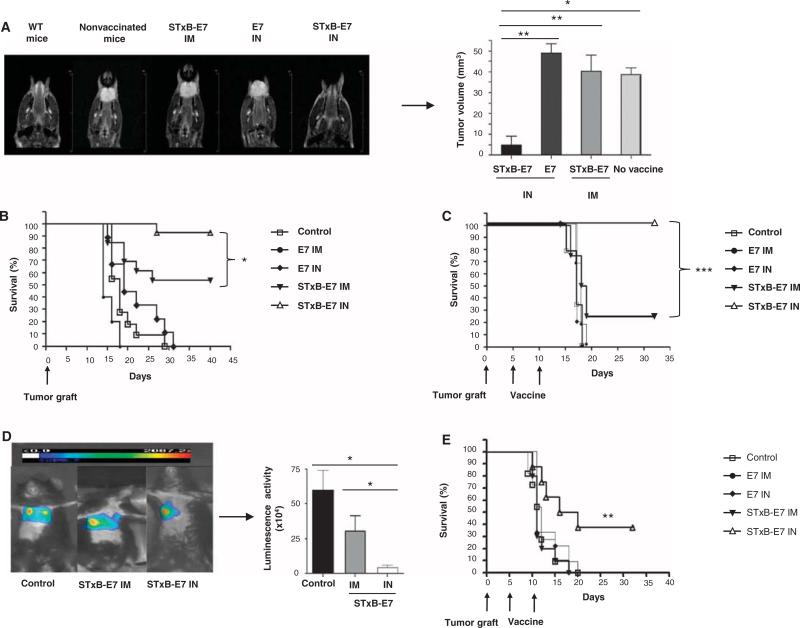
We then set up an orthotopic model of head and neck cancer by grafting the human papillomavirus 16 (HPV16) E7–expressing epithelial TC1 cell line in the submucosal lining of the tongue of B6 mice. Tumor growth was monitored by magnetic resonance imaging (MRI) (Fig. 2A). This provided us with a clinically relevant model because, in humans, 30% of head and neck cancer express HPV16 (8).
Fig. 2.
Intranasal immunization with STxB-E7 inhibits orthotopic head and neck and lung tumors in both prophylactic and therapeutic settings. (A and B) In a prophylactic setting, mice were immunized twice via the intranasal or intramuscular route with STxB-E7 or E7 peptide–based vaccines mixed with the aGalCer adjuvant. Seven days after the second vaccination, mice were grafted with 5 × 104 TC1 cells in the submucosa of the tongue. Representative MRI (coronal T2-weighted image of mouse tongue) (A, left) and tumor volume measured by MRI (A, right) at day 7 after tumor graft in the tongue are shown. (C to E) In the therapeutic setting, mice were immunized 5 and 10 days after grafting TC1 cells in the submucosa of the tongue (C) or 5 and 10 days after grafting TC1 luciferase (105 cells) into the lung (D and E), which was monitored by luminescence. Representative bioluminescence images of tumor-bearing mice in the lung are depicted in (D) (left panel). Luminescence (luciferase activity) in the chest was quantified 4 days after the second immunization with STxB-E7 via the intranasal route (n = 7) or the intramuscular route (n = 4) or in the nonvaccinated control mice (n = 4) (D, right panel). Kaplan-Meier survival curves of prophylactic (B) or therapeutic (C and E) cancer vaccine experiments for head and neck (B and C) and lung tumor (E) are shown. Each experiment was reproduced four times. *P < 0.05, **P < 0.01, ***P < 0.001.
When mice were vaccinated with STxB-E7 by the intranasal route and then challenged with TC1 tumors, more than 90% of mice had survived at day 40, whereas only 54% of mice were still alive when immunized with STxB-E7 by the intramuscular route (P < 0.05) (Fig. 2B). The tumor volume of mice immunized with STxB-E7 by the intranasal route was also significantly reduced compared to mice immunized by the intramuscular route (P < 0.05) (Fig. 2A). Regardless of immunization route, vaccination with E7 polypeptide was ineffective in eliciting protective immunity in this prophylactic experiment (Fig. 2, A and B). In a therapeutic setting, where mice were vaccinated 5 days after tumor implantation, all mice intranasally vaccinated with STxB-E7 were still alive 35 days after tumor graft, whereas 75% of mice vaccinated with STxB-E7 by the intramuscular route had died (Fig. 2C). When vaccinated with nonvectorized E7 polypeptide or not treated, all mice had succumbed less than 20 days after tumor graft (Fig. 2C). Thus, intranasal immunization with STxB-E7 provided better prophylactic and therapeutic efficacy than systemic or peptide vaccination against orthotopic head and neck cancers.
To support these results in a second orthotopic mucosal model, we vaccinated mice engrafted with luciferase-expressing TC1 cells in the lung (Fig. 2D). Intranasal administration of STxB-E7 significantly controlled tumor growth and improved overall survival (Fig. 2D), whereas all mice left untreated or receiving STxB-E7 by the intramuscular route or the E7 polypeptide by the intranasal route died less than 20 days after the graft of TC1 into the lung (Fig. 2E). The vaccine was composed of STxB-E7 mixed with the adjuvant αGalCer. To demonstrate that these results could be reproduced with another vaccine composition, we showed that STxB-E7 combined with CpG instead of αGalCer induced the same tumor protection, as that observed after intranasal administration of the previous vaccine (fig. S3).
Intranasal immunization preferentially induced CD8+ T cells in mucosa-draining lymph node and in the microenvironment of mucosal head and neck cancer
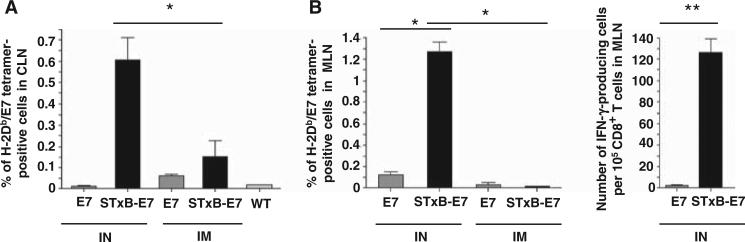
To identify possible mechanisms involved in the superior growth control of head and neck or lung tumors after local intranasal immunization, we analyzed the E739–47–specific CD8+ T cells in mucosa-draining lymph nodes and tumor-infiltrating cells. In contrast to the results observed in the systemic compartment (spleen), E749–57–specific CD8+ T lymphocytes were only detected in the mediastinal and cervical lymph nodes draining mucosal lung and head and neck tissue after two intranasal administrations of STxB-E7 (Fig. 3, A and B). These antigen-specific T cells were functional because they produced IFN-γ ex vivo (Fig. 3B). Neither E7 polypeptide vaccination, regardless of administration route, nor intramuscular administration of STxB-E7 elicited measurable E7-specific T cell responses at the mucosa-draining lymph nodes (Fig. 3, A and B).
Fig. 3.
Intranasal immunization with STxB-E7 elicits functional E749–57–specific T cells in cervical and mediastinal lymph nodes. Mice were immunized by the intranasal or the intramuscular route with STxB-E7 or the nonvectorized E7 polypeptide. (A and B) E749–57–specific CD8+ T cells from cervical lymph node (CLN) (A) or mediastinal lymph node (MLN) (B) were detected by tetramer assay or IFN-γ ELISpot directly ex vivo. WT, wild type. For the tetramer analysis, means ± SD obtained with specific tetramer after subtracting values from irrelevant tetramer (always <0.1%) are shown. For the ELISpot analysis, background obtained with cells not pulsed with the E749–57 peptide was also subtracted (always <10 spots per 105 cells). These experiments were reproduced three times with four mice per group. *P < 0.05, **P < 0.01.
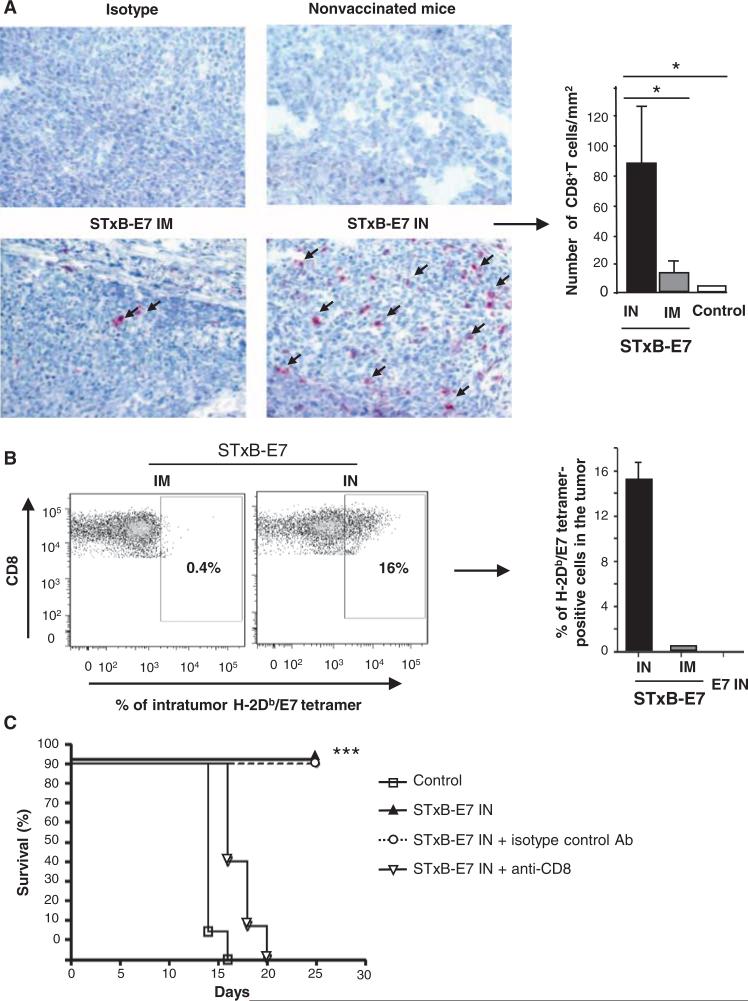
With respect to the orthotopic head and neck tumor micro-environment, we observed a very weak immune cell infiltration in the absence of immunization (Fig. 4A). A greater infiltration of CD8+ T cells was detected 7 days after tumor graft in mice that had been previously intranasally immunized with STxB-E7 than in mice vaccinated by the intramuscular route (Fig. 4A). Indeed, a mean of 80 (±20.8) CD8+ T cells/mm2 or 10 (±4) CD8+ T cells/mm2 cells was observed after immunization by the intranasal or intramuscular routes, respectively (P < 0.05) (Fig. 4A, right). We also found that more than 16% of intratumoral infiltrating CD8+ T cells were specific for E749–57, as detected by tetramer staining, when mice were immunized by the intranasal route compared to 0.4% after intramuscular immunization (Fig. 4B). We detected low levels of infiltration by natural killer (NK) cells or CD4+ T cells after intranasal immunization (fig. S4). We then depleted CD8+ T cells to directly demonstrate their role in tumor protection in our model. As shown in Fig. 4C, CD8+ T cell–depleted mice vaccinated with STxB-E7 by the intranasal route died before 20 days. As a control, we demonstrated again within the same experiment that nondepleted or isotype control–treated mice survived more than 25 days after tumor graft (Fig. 4C). Together, these experiments demonstrate that CD8+ T cells are involved in the ability of STxB-E7 to control tumor growth after mucosal intranasal immunization.
Fig. 4.
CD8+ T cells are required to control the growth of orthotopic head and neck cancer after intra-nasal immunization with STxB-E7. Eight days after the orthotopic graft of TC1 cells in the submucosa of the tongue, tumors were removed and CD8+ T cells were detected by immunoenzymatic staining. (A) (Left) Arrows show CD8+ T cells infiltrating the tumors in mice previously intranasally or intramuscularly immunized with the STxB-E7 vaccine or nonvaccinated B6 mice (original magnification, ×40). (Right) Histograms show the means ± SEM values from pooled data of three to four mice per group. Intratumoral CD8+ T cells were purified from a pool of three to four tumors of mice vaccinated with various E7 vaccines and stained with H-2Db/E7 tetramer. These experiments were reproduced twice with similar results. (B) (Left) Values shown correspond to means ± SD obtained with specific E7 tetramer after subtracting values from irrelevant tetramer (right). B6 mice were intranasally immunized with STxB-E7 at days 0 and 14. At day 20 and then once a week, mice received anti-CD8 mAb (100 mg, intraperitoneally) or isotype-matched control mAb. (C) Seven days after the second vaccination (day 21), mice were grafted with TC1 cells in the submucosa of the tongue and monitored for survival. All these experiments were reproduced three times. *P < 0.05, ***P < 0.001.
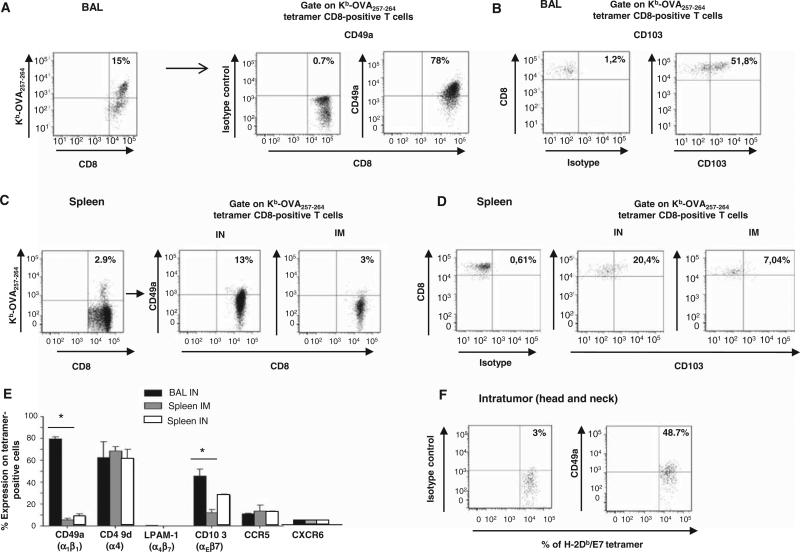
Intranasal immunization up-regulated the expression of the mucosal integrins CD49a and CD103
To explain this preferential recruitment of CD8+ T cells at the mucosal site after intranasal immunization, we performed a comprehensive phenotype of these cells. Analysis of integrin and chemokine receptor expression revealed that the intranasal route of immunization induced a higher expression of the mucosal integrins CD103 and CD49a [the α chain of the type IV collagen receptor VLA-1 (α1β1)] on antigen-specific CD8+ T cells in bronchoalveolar lavage (BAL) than after intramuscular immunization in the spleen (Fig. 5, A to E). Because the α1 integrin only associates with the β1 chain, its expression corresponds to that of VLA-1. In addition, 48.7% of intratumor E7-specific T cells expressed CD49a, within the microenvironment of head and neck cancer (Fig. 5F). We did not find differences in the expression of other integrins (α4β7 and CD49d) or chemokine receptors (CCR5 and CXCR6) in the BAL or in the spleen after intranasal or intramuscular immunization, respectively (Fig. 5E).
Fig. 5.
Expression of CD49a (α1β1) and CD103 (αEβ7) by antigen-specific CD8+ T cells in the BAL, spleen, and tumor microenvironment after immunization of mice with STxB-based vaccine via the intranasal or intramuscular routes. (A to D) OVA-specific CD8+ T cells were detected in BAL in a pool of six to seven mice (A and B) or in the spleen (C and D) 7 days after the second immunization with STxB-OVA. Expression of CD49a [corresponding to the α1 chain of VLA-1 (α1β1), which associates only with β1] (A and C) and CD103 (B and D) was analyzed after gating on tetramer-positive CD8+ T cells. Isotype controls were included in each experiment. Phenotypic analysis of tetramer-positive OVA-specific CD8+ T cells in the BAL after intramuscular immunization is not shown because this route of immunization did not induce OVA-specific CD8+ T cells in this compartment. (E) Values shown correspond to means ± SD for the expression of CD49a, CD49d, α4β7, CD103, and CCR5 analyzed after gating on tetramer-positive Kb-OVA257–264–positive cells in BAL or spleen using the same protocol described for (A) to (D). Isotype controls were included in each experiment. This experiment was reproduced twice with a pool of five to six mice per experiment. Mice were grafted with TC1 cells in the tongue and then immunized twice (days 5 and 10) by the intranasal route with STxB-E7 mixed with αGalCer. At day 15, tumors (n = 5) were pooled and intratumoral CD8+ T cells were purified from the tumors and stained with H-2Db/E7 tetramer, CD8, and CD49a. (F) Dot plots were generated on gated CD8+ T cells.
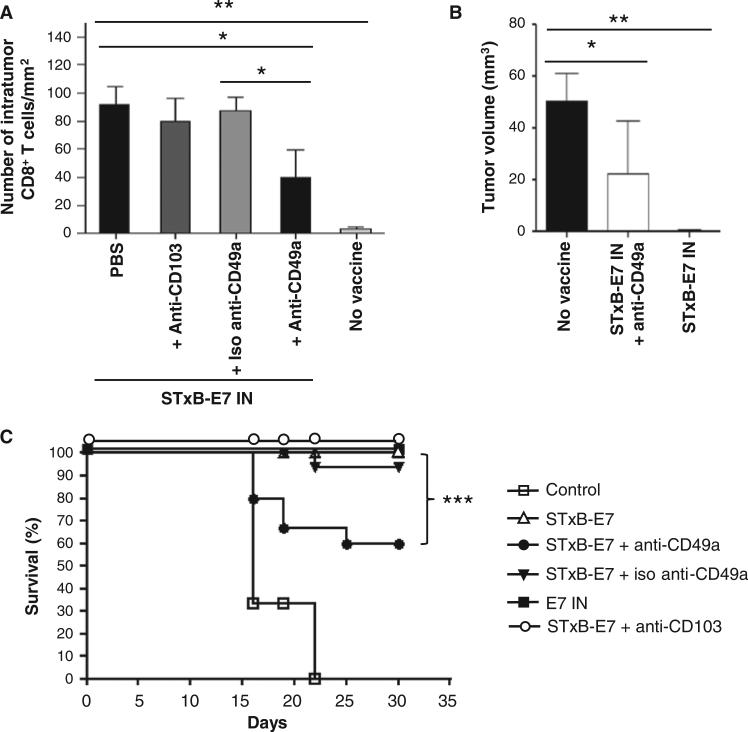
Neutralization of CD49a hampered the recruitment of CD8+ T cells into mucosal tumor beds and reduced the antitumor efficacy of mucosal vaccination
To address the role of CD49a and CD103 in the homing of CD8+ T cells, we used neutralizing antibodies against these molecules. The CD8+ T cells infiltrating head and neck cancer after intranasal STxB-E7 administration significantly decreased when the vaccine was co-administered with anti-CD49a monoclonal antibody (mAb) (Fig. 6A). Moreover, the co-administration of anti-CD49a mAb and vaccine partially inhibited the therapeutic efficacy of the intranasal STxB-E7 vaccine (Fig. 6, B and C). In contrast, anti-CD103 mAb had no effect on the level of tumor infiltration by CD8+ T cells or on the therapeutic efficacy of the intranasal STxB-E7 vaccine (Fig. 6, A to C).
Fig. 6.
Blockade of CD49a inhibits CD8+ T cell infiltration and the activity of cancer vaccine on head and neck tumors. Mice were grafted with TC1 cells in the tongue and then immunized two times with STxB-E7 mixed with αGalCer (days 5 and 10). (A to C) They were also treated with either anti-CD49a (250 μg, intraperitoneally) or anti-CD103 (150 μg, intraperitoneally) or isotype-matched control mAb at days 8, 11, and 14. CD8+ T cell infiltration was detected by immunoenzymatic staining (A), and tumor volume was monitored by MRI at day 20 (B). Kaplan-Meier survival curve of mice bearing a head and neck tumor and treated or not with an intranasal cancer vaccine combined or not with anti-CD49a mAb, anti-CD103 (C), or isotype-matched control. Each experiment included five to six mice per group and was reproduced three times. *P < 0.05, **P < 0.01, ***P < 0.001.
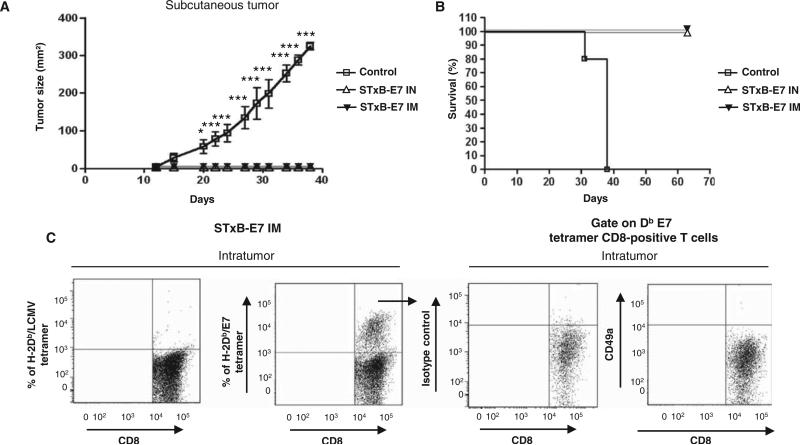
Nonmucosal subcutaneous tumors were efficiently controlled by both routes of immunization
Then, we analyzed whether the better efficacy of the intranasal route was restricted tomucosaltumors. Wethus compared the ability of the intranasal and intramuscular routes of immunization to control the growth of subcutaneous tumors. The superior antitumor activity of the intranasal route was linked to the mucosal location of the tumor because subcutaneous TC1 tumors were efficiently controlled by both intranasal and intramuscular immunizations with a follow-up exceeding 70 days (Fig. 7, A and B). The E7-specific CD8+ T cells infiltrating the subcutaneous tumors did not express the CD49a integrin (Fig. 7C), contrasting with their phenotype in mucosal head and neck tumors (Fig. 5F).
Fig. 7.
The intranasal and intramuscular routes are both efficient to control the growth of subcutaneous grafted TC1 cells. B6 mice were immunized via the intranasal or intramuscular routes with STxB-E7 or E7 vaccines mixed with αGalCer. Seven days after the second vaccination, mice were grafted in the right flank with 105 TC1 cells. (A and B) Tumor growth (A) and survival (B) were monitored every 3 days. Five mice per group were included in each experiment, which was reproduced twice. *P < 0.05, ***P < 0.001. (C) Mice were grafted with TC1 cells in the right flank and then immunized two times (days 5 and 10) by the intranasal route with STxBE7 mixed with αGalCer. At day 15, tumors (n = 5) were pooled, and intratumoral CD8+ T cells were purified from the tumors and stained with H-2Db/E7 tetramer, CD8, and CD49a. Dot plots were generated on gated Db-E7 tetramer CD8+ T cells. Tetramer and isotype control were included in each experiments.
Lung parenchyma DCs induced in vitro CD49a expression on antigen-specific CD8+ T cells
To explain the preferential induction of CD49a on mucosal CD8+ T cells after intranasal immunization, we purified the lung parenchyma CD11c+ DCs and co-cultured them with OVA257–264–specific CD8+ T cells (OT-1). We showed that lung parenchyma DCs purified 2 hours after intranasal vaccination with STxB-E7 induced the expression of CD49a on OT-1 cells (fig. S5A). In contrast, lung DCs from nonvaccinated mice (fig. S5B) or spleen DCs from vaccinated mice (fig. S5C) did not lead to the expression of CD49a on OT-1 cells. Nonactivated OT-1 cells did not express CD49a (fig. S5D).
The combination of anti-CD3 and anti-CD28 without DCs did not induce CD49a on T cells, suggesting that CD49a could not only be considered as an activation marker (fig. S5E).
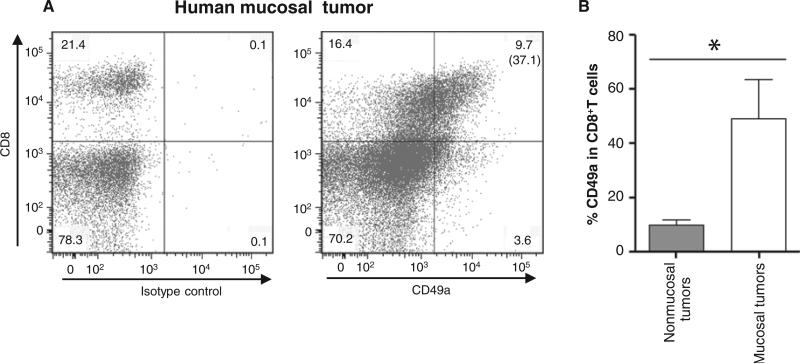
CD8+ T cells from human mucosal tumors express CD49a
To assess the relevance of our results in humans, we analyzed the expression of CD49a in tumor-infiltrating lymphocytes (TILs) from human mucosal tumors (lung and head and neck cancer). We found that about 48.9% of intratumor CD8+ T cells derived from mucosal tumors express CD49a (Fig. 8, A and B), whereas only 14.7% of intratumor CD8+ T cells derived from nonmucosal tumors (renal cell carcinoma) express CD49a.
Fig. 8.
CD49a expression in TILs from mucosal tumors. TILs were obtained from four enzymatically treated mucosal tumors (three human lung and one head and neck cancers) and eight nonmucosal tumors (eight renal cell carcinoma biopsies). They were stained by anti-CD8 and anti-CD49a mAb. Isotype controls were included in each experiment. (A) Representative dot plots of CD49a expression on head and neck TILs after gating on CD45+ T cells. Values in the rectangle shown in each quadrant correspond to percent of cells, and the value in bracket corresponds to the percent of CD49a in the CD8+ T cells. (B) Values shown on the histogram correspond to means ± SD for the expression of CD49a in CD8+ T cells from mucosal and nonmucosal tumors. *P < 0.05.
DISCUSSION
This study emphasizes the need to elicit a potent antitumor response at the anatomic site of the mucosal tumor—at least for head and neck and lung cancers—and not just in the systemic compartment to induce tumor regression. Indeed, we showed that the administration of a cancer vaccine via the intranasal route effectively induced an appreciable antitumor CD8+ T cell response at the mucosal site of tumors, allowing the clinical control of head and neck and lung cancers (Fig. 2). In contrast, both routes of immunization (intranasal and intramuscular) elicited peripheral CD8+ T cell response and inhibited the growth of subcutaneous tumors. The role of CD8+ T cells in controlling the growth of tumors has been demonstrated in other tumor models including spontaneous preclinical mucosal lung cancer (19) and in human cancers (2).
Our results supporting the need of mucosal immunization to elicit mucosal CD8+ T cells are in line with various reports in the field of infectious diseases, showing that mucosal immunization generates a more potent regional humoral and mucosal CD8+ T lymphocyte response compared with systemic immunization (20–23). These mucosal CD8+ T cells were required to protect against a mucosal infectious challenge, whereas systemic (spleen) CD8+ T cell response alone failed to control the same infectious challenge (24). Other studies claimed that systemic immunization can overcome immune compartmentalization (25–28). However, in the latter case, the respective efficiency of mucosal and systemic immunization regimen was rarely compared. The requirement of mucosal immunization to elicit mucosal immunity may be more restrictive with protein-based vaccines than with live viral vectors (21).
The avidity of cytotoxic T lymphocyte (CTL) is a critical parameter in their efficacy to protect against viral infection (29). The avidity of CTL at various sites has been shown to depend on the mucosal or systemic site of immunization (30). The intrarectal mucosal route of immunization induced higher CTL avidity in the gut mucosa than the systemic subcutaneous route. This point could not be addressed in our model because the systemic route of immunization did not induce any detectable anti-E7 CTL at the mucosal site (lung and head and neck) (Fig. 3).
At the molecular levels, we found that intranasal immunization preferentially up-regulated CD49a and CD103 on CD8+ T cells at mucosal site (broncholaveolar lavage). Various arguments strongly suggest that the VLA-1 integrin was involved in the homing-retention of CD8+ T cells to the head and neck tumor because (i) intratumoral E7-specific T cells expressed high levels of CD49a (Fig. 5F) and (ii) the CD8+ T cells infiltrating head and neck cancer after intranasal STxB-E7 administration significantly decreased when the vaccine was co-administered with anti-CD49a mAb (Fig. 6A). Moreover, the co-administration of anti-CD49a mAb and vaccine partially inhibited the therapeutic efficacy of the intranasal STxB-E7 vaccine (Fig. 6C). This role of CD49a is supported by previous data showing that VLA-1 enables both retention and survival of influenza-specific CD8+ T cells in the lung via attachment to the extracellular matrix (31, 32). We found that CD8+ T cells derived from lung tumor biopsies also express CD49a, supporting the clinical relevance of our results (Fig. 8).
We demonstrated a preferential recruitment of CD8+ T cells in mucosal head and neck tumors after intranasal immunization, which may be explained by the role of DCs at the site of initial priming and in regional lymph nodes to imprint homing receptor expression onto T cells favoring their homing to the site of their initial activation (33, 34). In infectious models, intranasally primed CD8+ T cells led to a preferential recruitment of memory CD8+ T cells to the lung airway after antigen challenge compared to other routes of immunization (35, 36). We showed that lung parenchyma DCs induced in vitro the expression of CD49a on antigen-specifc cocultured T cells, which may program them for their homing and retention into the lung. Up until now, it was unclear whether lung DCs dictated a particular instruction pattern for migration into the lung or whether T cell activation or inflammation was sufficient to drive infiltration (34, 37, 38). Our results as well as human studies in which CD8+ T cells specific for respiratory, but not systemic, viruses were selectively enriched in human lungs argue for a lung and upper respiratory tract T cell imprinting (39).
The influence of tumor location and immune compartmentalization on tumor immunity has been rarely addressed. The site of tumor implantation and the route of immunization have been reported to determine the pattern of phenotypic imprinting on tumor-specific CD8+ T cells (40, 41). In vivo transfer of antitumor CD8+ T cells induced by subcutaneous DC vaccination was efficient to induce the regression of subcutaneous tumors, but not gastric tumors from the same origin (42), which also supports the influence of the site of initial priming for the programming of CD8+ T cell homing.
For genital tumors, the requirement of local immunization to control vaginal tumors or HPV infection remains a matter of debate (43, 44). In a general way, the intravaginal route is less efficient to elicit CD8+ T cells, possibly due to the absence of organized lymphoid structures at this site. Wedemonstratedthatintranasalimmunizationisefficienttoprotectagainst both mucosal (head and neck and lung) and systemic (subcutaneous) cancer. Intrarectal mucosal immunization was also efficient to protect against the growth of colorectal cancer and subcutaneous tumor (45), conferring another advantage for the mucosal route of vaccination.
With respect to the efficiency of our cancer vaccine, it has to be mentioned that targeting of antigen to mucosal DCs by STxB-based vaccines was required to elicit local mucosal CD8+ T cells, because nonvectorized antigens were not efficient. Other vectors targeting DCs (adenylate cyclase toxin–hemolysin of Bordetella pertussis, anti-DEC205, DC SIGN receptor, and anti-Clec9a) have been described (46–49), but with the exception of derivatives of cholera toxin associated with potential toxicity (50), they have not been validated for their development as mucosal vectors with the ability to induce mucosal CD8+ T cells, as demonstrated in this study for STxB.
Some limitations of this study have to be mentioned: (i) The immune compartmentalization that we have demonstrated, applied to CD8+ T cells, did not seem to occur for prophylactic vaccines due to possible mucosal transudation of neutralizing antibody induced by systemic vaccination (21). (ii) Although the interaction of αE(CD103)β7 integrin expressed by CD8+ T cells with E-cadherin on epithelial cells has been shown to potentiate the lytic function and to favor the retention of CD8+ T cells in the lung (12, 51), in our model, anti-CD103 mAb had no effect on the level of tumor infiltration by CD8+ T cells or the therapeutic efficacy of the intranasal STxB-E7 vaccine (Fig. 6, A and C). This was possibly due to the absence of the expression of E-cadherin on the tumor cells selected in our various models (fig. S6). (iii) It has been claimed that intranasal immunization elicited immunity at distant mucosal sites such as genital mucosal sites (13). In our model, we found that mucosal immunization induced a response only compartmentalized to the regional mucosal site for the CD8+ T cell response because we did not detect antigen-specific CD8+ T cells in genital draining lymph node after intranasal immunization. However, we could detect an immunoglobulin A (IgA) response in the BAL and vaginal lavage after intranasal immunization (fig. S7). Discrepancies between a strong genital tract antibody response and an absence of genital T cell response have already been reported after intranasal immunization (50).
This study reports a link between the route of vaccination and a mucosal molecular homing program on induced CD8+ T cells controlling their trafficking with a direct application on the control of mucosal tumors. It may explain the differential efficacy of mucosal or systemic vaccines depending on the localization of pathogens or tumors to be eliminated. Immunotherapy protocols were initially restricted to melanoma patients but recently moved toward the therapy of mucosal tumors, such as HPV-associated cancers (6) or lung cancers (52). The present study may lead to modifications of medical practice in the design of therapeutic vaccines against mucosal tumors to tailor them with the ability to elicit an efficient immune response at the anatomic site of the tumor to improve their clinical efficacy.
MATERIALS AND METHODS
Mice
Six- to 8-week-old female C57BL/6 (H-2b) (B6) or OT-1 mice were purchased from Charles River Laboratories. All mice were kept under specific pathogen–free conditions at the INSERM U970 animal facility. Experiments were performed according to institutional guidelines and acceptance by the Veterinary School of Maisons-Alfort ethics committee.
Patients and tumor samples
Biopsy of human cancers was provided by the Department of Pathology of the Hôpital Européen Georges Pompidou, Assistance Publique– Hôpitaux de Paris (Paris, France). Patients signed an informed consent after approval of the study by a local ethics committee (ID RCB 2007-A01128-45).
Proteins, peptides, and adjuvants
Purified chicken ovalbumin (OVA) (grade V) was purchased from Sigma-Aldrich. Synthetic OVA-derived peptide OVA257–264 (SIINFEKL) and HPV16 E7–derived peptide E749–57 (RAHYNIVTF) or E743–57 (GQAEPDRAHYNIVTF) were obtained from PolyPeptide Laboratories, reconstituted in phosphate-buffered saline (PBS), and stored at −20°C.
STxB-OVA and STxB-E743–57 were obtained by chemical coupling, as previously described (18). After purification, endotoxin concentrations determined by the Limulus assay test (Lonza) were <0.5 endotoxin unit/mg.
The invariant NKT cell ligand αGalCer (KRN7000) was purchased from Funakoshi.
Cells
TC1 cells expressing the HPV16 E6-E7 proteins and the TC1 cells expressing the firefly luciferase gene (TC1 luc) were developed in the laboratoryof T.-C.W.(Department of Pathology, School of Medicine,Johns Hopkins University, Baltimore, MD) (53). The mouse thymoma cell line EL4 (H-2b) was provided by K. Rock (University of Massachusetts Medical School, Worcester, MA). Cells were cultured as previously described (15).
Immunization of mice
C57BL/6 mice were immunized by intranasal or intramuscular routes at days 0 and 14 with the various vaccines. αGalCer (2 μg) was associated as an adjuvant during the first immunization. Total volume injected was 30 μl by the intranasal route and 100 μl by the intramuscular route. All mice were anesthetized before immunization.
In vivo tumor protection
For the subcutaneous tumor model, 105 TC1 tumor cells were injected subcutaneously in the right flank of B6 mice. Mice were monitored every 48 to 72 hours for tumor growth.
For the orthotopic models, 5 × 104 TC1 tumor cells were injected at the submucosal area of the tongue of B6 mice, and 105 TC1 cells were grafted into the lungs by intercostal injection at the median axillary line.
In the prophylactic setting, tumors were grafted 7 days after the second immunization, whereas for the therapeutic setting, mice were vaccinated at days 5 and 10 after tumor graft.
In vivo CD8+ T cell depletion
To deplete CD8+ T cells, we injected by the intraperitoneal route 100 μg per mice of anti-CD8 mAb (rat IgG2b mAb, clone 2.43; BioXCell) or isotype control mAb on day 6 after the second immunization of mice and then once a week.
Total and specific anti-OVA IgA measurement
Total IgA was measured with a commercial kit from Bethyl, and specific anti-OVA IgA measurement was performed as previously described (54).
Blocking experiments with anti-CD49a or anti-CD103
The nondepleting anti-CD49a mAb (Armenian hamster IgG2, λ1; Becton Dickinson) or isotype control mAb was injected by the intraperitoneal route (250 μg per mice) on days 8, 11, and 14 after the graft of the TC1 tumor on the tongue. For in vivo blocking of CD103, 150 mg of anti-mouse CD103 antibody (clone M290; BioXCell) was injected by the intraperitoneal route.
Ex vivo ELISpot assays
CD8+ T lymphocytes were enriched from spleen or mediastinal lymph nodes with the CD8α+ T cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. Purified CD8+ cells were incubated in ELISpot plates in the presence of medium or EL4 cells charged with the KbOVA257–264–restricted peptide or DbE749–57–restricted peptide. Plates were incubated for 18 to 21 hours at 37°C with 5% CO2. The IFN-γ spots were revealed following the manufacturer's instructions (Gen-Probe Diaclone). Spot-forming cells were counted with the C.T.L. Immunospot system (Cellular Technology Ltd.).
Flow cytometry analysis
List of tetramer and antibodies used is detailed in Supplementary Materials and Methods.
TIL isolation from human cancer specimens
Tumor-infiltrating CD8+ T cells were isolated by slicing the tumor samples and treating the fragments by enzymatic digestion with deoxyribonuclease I/collagenase D for 1 hour at 37°C in Mg+ and Ca2+ containing PBS and then passed through a 40-μm cell strainer (BD Biosciences). Cells were washed and red blood cells were lysed. The cells were then preincubated with anti-CD16/CD32 antibody (eBioscience) and stained with anti-human CD49a phycoerythrin mAb (BioLegend clone TS2/7), anti-human CD103 allophycocyanin mAb (eBioscience), anti-human CD8 fluorescein isothiocyanate mAb (eBioscience), and LiveDead (Invitrogen) to exclude dead cells.
Imaging techniques
MRI images and in vivo optical imaging of tumor cell luciferase activity are detailed in Supplementary Materials and Methods.
Immunohistochemistry
For immunoenzymatic staining, tongue tumor specimens from immunized or control mice were collected at day 8 after tumor challenge, then frozen, and kept at −80°C. CD8, CD4, and NK cell stainings were performed on 6- to 7-mm cryosections with rat anti-mouse CD8 mAb (eBioscience), rat anti-mouse CD4 mAb (eBioscience), or goat polyclonal anti-mouse NKp46 (R&D Systems). As secondary antibodies, alkaline phosphatase–conjugated F(ab′)2 fragment donkey anti-rat IgG (Jackson ImmunoResearch) for CD8+ and CD4+ staining and alkaline phosphatase–conjugated F(ab′)2 fragment donkey anti-goat IgG (Jackson ImmunoResearch) for NKp46 were used. Slides were revealed with Liquid Permanent Red Substrate (Dako). Isotype-matched control antibodies were systematically used to exclude non-specific staining.
Statistical analyses
Statistical analyses were performed with GraphPad Prism software (GraphPad Software Inc.). Data were expressed as means ± SD and are representative of at least three independent experiments. Significance was assessed with the Mann-Whitney test to compare two different groups and Kaplan-Meier curves to compare the survival of the different mice groups.
Supplementary Material
Acknowledgments
We thank K. Rock (University of Massachusetts Medical School, Worcester, MA) for providing us the EL4 thymoma cell line. Funding: This work was supported by grants from Université Paris Descartes, the Ligue contre le Cancer, the Agence Nationale de la Recherche, the Association pour la Recherche contre le Cancer, the Canceropole and Region Ile de France, the Institut National du Cancer, and the Labex Immuno-Oncology. Author contributions: Study concept and design: F.S., L.J., and E.T.; acquisition of data: F.S., M. Terme, M.N., C. Badoual, M.-F.B., L.F., E.M., A.G., G.F., C. Bouguin, N.M., E.D., T.T., G.A., M. Thiebaud, M.S., S.R., and J.R.; analysis and interpretation of data: F.S., M. Terme, M.N., C. Badoual, O.C., E.M., A.G., C. Bouguin, N.M., E.D., T.T., F.Q.-C., T.-C.W., O.L., C.D., J.T., J.R., L.Z., W.H.F., L.J., and E.T.; statistical analysis: F.S., M. Terme, and M.N.; inclusion of patients: C. Badoual, O.L., J.T., and C.D.; drafting of the manuscript: F.S. and E.T.
Footnotes
Competing interests: No patent was filed on the basis of the current study. However, in this work, a vaccine based on STxB technology has been used. The STxB technology is protected by several delivered patent filings, on which L.J. and E.T. are co-inventors. These patents have been licensed to a biotech company, which was co-founded by L.J. who is a non-majority shareholder. This company has not been involved in the current work and has not funded this study. The vaccine product that was used in the current study will not be developed by the company. The other authors declare that they have no competing interests.
Citation: F. Sandoval, M. Terme, M. Nizard, C. Badoual, M.-F. Bureau, L. Freyburger, O. Clement, E. Marcheteau, A. Gey, G. Fraisse, C. Bouguin, N. Merillon, E. Dransart, T. Tran, F. Quintin-Colonna, G. Autret, M. Thiebaud, M. Suleman, S. Riffault, T.-C. Wu, O. Launay, C. Danel, J. Taieb, J. Richardson, L. Zitvogel, W. H. Fridman, L. Johannes, E. Tartour, Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci. Transl. Med. 5, 172ra20 (2013).
REFERENCES AND NOTES
- 1.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 3.Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J. Clin. Oncol. 2011;29:601–603. doi: 10.1200/JCO.2010.32.9078. [DOI] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 7.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, Debieuvre D, Lena H, Buyse M, Chenard MP, Acres B, Lacoste G, Bastien B, Tavernaro A, Bizouarne N, Bonnefoy JY, Limacher JM. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: A controlled phase 2B trial. Lancet Oncol. 2011;12:1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 8.Badoual C, Sandoval F, Pere H, Hans S, Gey A, Merillon N, Van Ryswick C, Quintin-Colonna F, Bruneval P, Brasnu D, Fridman WH, Tartour E. Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head Neck. 2010;32:946–958. doi: 10.1002/hed.21346. [DOI] [PubMed] [Google Scholar]
- 9.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, Lienard D, Guillaume P, Krieg AM, Cerottini JC, Romero P, Leyvraz S, Rufer N, Speiser DE. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J. Immunol. 2006;177:1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan BS, Lefrançois L. Regional and mucosal memory T cells. Nat. Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czerkinsky C, Holmgren J. Topical immunization strategies. Mucosal Immunol. 2010;3:545–555. doi: 10.1038/mi.2010.55. [DOI] [PubMed] [Google Scholar]
- 14.Fujkuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, Yuki Y, Kiyono H, Fujihashi K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev. Vaccines. 2012;11:367–379. doi: 10.1586/erv.11.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vingert B, Adotevi O, Patin D, Jung S, Shrikant P, Freyburger L, Eppolito C, Sapoznikov A, Amessou M, Quintin-Colonna F, Fridman WH, Johannes L, Tartour E. The Shiga toxin B-subunit targets antigen in vivo to dendritic cells and elicits anti-tumor immunity. Eur. J. Immunol. 2006;36:1124–1135. doi: 10.1002/eji.200535443. [DOI] [PubMed] [Google Scholar]
- 16.Haicheur N, Bismuth E, Bosset S, Adotevi O, Warnier G, Lacabanne V, Regnault A, Desaymard C, Amigorena S, Ricciardi-Castagnoli P, Goud B, Fridman WH, Johannes L, Tartour E. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J. Immunol. 2000;165:3301–3308. doi: 10.4049/jimmunol.165.6.3301. [DOI] [PubMed] [Google Scholar]
- 17.Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, Batteux F, Sandoval F, Adotevi O, Chiu C, Garcia S, Tanchot C, Lone YC, Ferreira LC, Nelson BH, Hanahan D, Fridman WH, Johannes L, Tartour E. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118:4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 18.Adotevi O, Vingert B, Freyburger L, Shrikant P, Lone YC, Quintin-Colonna F, Haicheur N, Amessou M, Herbelin A, Langlade-Demoyen P, Fridman WH, Lemonnier F, Johannes L, Tartour E. B subunit of Shiga toxin-based vaccines synergize with α-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J. Immunol. 2007;179:3371–3379. doi: 10.4049/jimmunol.179.5.3371. [DOI] [PubMed] [Google Scholar]
- 19.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, Crowley D, Chen J, Jacks T. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 22.Lemiale F, Kong WP, Akyürek LM, Ling X, Huang Y, Chakrabarti BK, Eckhaus M, Nabel GJ. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 2003;77:10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, Berzofsky JA. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belyakov IM, Ahlers JD, Brandwein BY, Earl P, Kelsall BL, Moss B, Strober W, Berzofsky JA. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Invest. 1998;102:2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 2008;181:4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal R, Clements JD, Lewis MG, Strober W, Franchini G, Berzofsky JA. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J. Immunol. 2007;178:7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- 31.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 32.Richter MV, Topham DJ. The α1β1 integrin and TNF receptor II protect airway CD8+ effector T cells from apoptosis during influenza infection. J. Immunol. 2007;179:5054–5063. doi: 10.4049/jimmunol.179.8.5054. [DOI] [PubMed] [Google Scholar]
- 33.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL. The route of priming influences the ability of respiratory virus–specific memory CD8+ T cells to be activated by residual antigen. J. Exp. Med. 2010;207:1153–1160. doi: 10.1084/jem.20090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim TS, Sun J, Braciale TJ. T cell responses during influenza infection: Getting and keeping control. Trends Immunol. 2011;32:225–231. doi: 10.1016/j.it.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 38.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 39.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Rüegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourquin C, von der Borch P, Zoglmeier C, Anz D, Sandholzer N, Suhartha N, Wurzenberger C, Denzel A, Kammerer R, Zimmermann W, Endres S. Efficient eradication of subcutaneous but not of autochthonous gastric tumors by adoptive T cell transfer in an SV40 T antigen mouse model. J. Immunol. 2010;185:2580–2588. doi: 10.4049/jimmunol.0903231. [DOI] [PubMed] [Google Scholar]
- 43.Decrausaz L, Domingos-Pereira S, Duc M, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Parenteral is more efficient than mucosal immunization to induce regression of human papillomavirus-associated genital tumors. Int. J. Cancer. 2011;129:762–772. doi: 10.1002/ijc.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, Stadler K, Schiller JT, Anjuère F, Czerkinsky C. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J. Immunol. 2009;183:7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim-Schulze S, Kim HS, Wainstein A, Kim DW, Yang WC, Moroziewicz D, Mong PY, Bereta M, Taback B, Wang Q, Kaufman HL. Intrarectal vaccination with recombinant vaccinia virus expressing carcinoembronic antigen induces mucosal and systemic immunity and prevents progression of colorectal cancer. J. Immunol. 2008;181:8112–8119. doi: 10.4049/jimmunol.181.11.8112. [DOI] [PubMed] [Google Scholar]
- 46.Berraondo P, Nouzé C, Préville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–8855. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- 47.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 48.Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, Steinman RM. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lycke N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 51.Le Floc'h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012;13:e301–e310. doi: 10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D, Hung CF, Wu TC. Monitoring the trafficking of adoptively transferred antigen-specific CD8-positive T cells in vivo, using noninvasive luminescence imaging. Hum. Gene Ther. 2007;18:575–588. doi: 10.1089/hum.2007.038. [DOI] [PubMed] [Google Scholar]
- 54.Haicheur N, Benchetrit F, Amessou M, Leclerc C, Falguières T, Fayolle C, Bismuth E, Fridman WH, Johannes L, Tartour E. The B subunit of Shiga toxin coupled to full-size antigenic protein elicits humoral and cell-mediated immune responses associated with a Th1-dominant polarization. Int. Immunol. 2003;15:1161–1171. doi: 10.1093/intimm/dxg118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.