Abstract
Objective(s):
HIV-associated neurocognitive disorders (HAND) remain prevalent in HIV-infected patients on antiretroviral therapy (ART), but the underlying mechanisms are unclear. Some features of HAND resemble those of age-associated cognitive decline in the absence of HIV, suggesting that overlapping mechanisms may contribute to neurocognitive impairment.
Design:
Cross-sectional analysis of cerebrospinal fluid (CSF) from 100 individuals (46 HIV-positive patients and 54 HIV-negative controls).
Methods:
Untargeted CSF metabolite profiling was performed using liquid/gas chromatography followed by mass spectrometry. Cytokine profiling was performed by Bioplex. Bioinformatic analyses were performed in Metaboanalyst and R.
Results:
Alterations in the CSF metabolome of HIV patients on ART mapped to pathways associated with neurotransmitter production, mitochondrial function, oxidative stress, and metabolic waste. Many CSF metabolites altered in HIV overlapped with those altered with advanced age in HIV-negative controls, suggesting a pattern indicative of accelerated aging. Machine learning models identified neurotransmitters (glutamate, N-acetylaspartate), markers of glial activation (myo-inositol), and ketone bodies (beta-hydroxybutyric acid, 1,2-propanediol) as top-ranked classifiers of HAND. These CSF metabolites correlated with worse neurocognitive test scores, plasma inflammatory biomarkers [interferon (IFN)-α, IFN-γ, interleukin (IL)-8, IL-1β, IL-6, IL-2Ra], and intrathecal IFN responses (IFN-γ and kynurenine : tryptophan ratio), suggesting inter-relationships between systemic and intrathecal inflammation and metabolic alterations in CSF.
Conclusions:
Alterations in the CSF metabolome of HIV patients on ART suggest that persistent inflammation, glial responses, glutamate neurotoxicity, and altered brain waste disposal systems contribute to mechanisms involved in HAND that may be augmented with aging.
Keywords: aging, antiretroviral therapy, HIV, HIV-associated neurocognitive disorders, inflammation, metabolomics
Introduction
Despite reduced incidence of severe forms of HIV-associated neurocognitive disorders (HAND) in HIV patients on combination antiretroviral therapy (ART), mild forms including asymptomatic neurocognitive impairment (ANI) and minor neurocognitive disorder (MND) remain prevalent, affecting 20–50% [1–3]. Prior to the introduction of ART, factors associated with HAND included plasma and cerebrospinal fluid (CSF) HIV RNA and inflammation markers [e.g. chemokine (CC motif) ligand 2 (CCL2), tumor necrosis factor (TNF), interleukin (IL)-6, neopterin] [4–8]. In HIV patients on ART, HAND is associated with older age (> age 50), lower nadir CD4+, innate immune activation, chronic inflammation, hepatitis C virus (HCV) coinfection, cardiovascular risk factors, and metabolic disorders [9–16]. The multifactorial nature of factors contributing to HAND suggests these disorders consist of subtypes reflecting distinct pathophysiological mechanisms [3,8,17].
HIV patients on ART have a higher burden of neurological disorders with advancing age compared to HIV-negative controls, and these disorders occur at a younger age [18,19]. The increased prevalence of these disorders, as well as other age-associated comorbidities, including cardiovascular, kidney, liver, and bone disease, is thought to reflect accelerated aging [20–22]. Chronic inflammation plays an important role in this phenotype, termed ‘inflammaging’, and predicts age-associated comorbidities and mortality in HIV patients [23–25]. Markers of inflammation are detected in CSF and brain from HIV patients on ART [26–31]. Neuroinflammation is a prominent feature of age-associated neurodegenerative diseases including Alzheimer's and Parkinson's disease, and has been associated with altered synaptic connectivity and blood–brain barrier (BBB) function and neuronal injury [32–34]. Whether similar mechanisms contribute to HAND is unclear.
Chronic HIV infection is associated with metabolic changes in brain, even among patients on suppressive ART [27,35–37]. Whereas brain tissue is difficult to obtain, CSF is accessible and reflects the biochemical milieu of the central nervous system [38–40]. Early targeted studies of CSF metabolites identified alterations in several neurotoxic metabolites including those associated with the kynurenine (e.g. quinolinic acid) and nitric oxide pathways during HIV and simian immunodeficiency virus (SIV) infection [7,41,42]. CSF lipidomics identified alterations in lipid metabolism, including increased carnitine, acyl-carnitines, fatty acids, and phospholipids in SIV infection [43], and increased ceramides, sphingomyelins, and cholesterol in HIV patients on ART with HAND [44–47]. Here, we performed untargeted metabolomics of CSF from 100 HIV patients and HIV-negative controls to identify altered metabolic pathways associated with HAND. We also examined relationships between these metabolic alterations and those associated with advancing age in HIV-negative controls.
Methods
Study participants
Cerebrospinal fluid samples from HIV patients (n = 46; 36 on ART and 10 not on ART) collected between 1999 and 2009 were from the National NeuroAIDS Tissue Consortium (NNTC) (Manhattan HIV Brain Bank, National Neurological AIDS Bank, California NeuroAIDS Tissue Network, Texas NeuroAIDS Research Center) and CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study. Matched plasma metabolite profiles were available for 20 HIV patients. All HIV patients were enrolled with written informed consent and institutional review board (IRB) approval. Inclusion criteria were advanced disease (nadir CD4+ <300 cells/μl). Exclusion criteria were confounding neurological and psychiatric disorders, systemic opportunistic infection, severe hepatotoxicity (defined as grades 3 or 4 by AIDS Clinical Trials Group [48]), and moderate/severe renal insufficiency [49]. HAND clinical diagnoses were determined using established criteria [50]. Neuropsychological impairment due to other causes (NPI-O) was diagnosed when factors in addition to HIV made significant contributions to neurocognitive impairment (NCI). HIV and HCV-negative control CSF samples from young (<50 years old) and older (≥50 years old) patients collected between 2010 and 2011 were from Bioreclamation (Westbury, New York) and used with Dana-Farber Cancer Institute IRB approval. Samples were de-identified remnants from diagnostic testing, prescreened for sCD14 and CCL2, to exclude those with levels outside normal ranges reported in the literature (>0.25 μg/ml and >1000 pg/ml, respectively).
Neurocognitive testing and neurocognitive impairment classification
All participants were administered a comprehensive test battery designed to assess seven domains of neurocognitive function (Supplemental Digital Content 1). Demographically corrected global T scores were generated from individual-domain T scores as described [51]. HIV patients were classified as impaired if they had a HAND clinical diagnosis (ANI, MND, HAD, or NPI-O) together with global T score less than 40 (or at least two domain T scores <40). Patients were classified as not impaired if they had no clinical diagnosis of HAND and global T score at least 40. Three patients with an ANI diagnosis, global T scores at least 40, and only one domain score less than 40 were classified as not impaired.
Quantification of soluble markers in cerebrospinal fluid and plasma
Interferon (IFN)-α, IFN-γ, IL-8, C-X-C motif chemokine ligand (CXCL)9, CXCL10, IL-1b, IL-6, TNF-α and IL-2 receptor alpha (Ra) were measured using Bioplex (Bio-Plex System; Bio-Rad Laboratories, Hercules, California, USA). Soluble CD14 (sCD14) and CCL2 were quantified by ELISA (R&D Systems, Minneapolis, Minnesota, USA).
Metabolomic profiling
Metabolomic profiling was performed by Metabolon (Durham, North Carolina, USA) using ultra high performance liquid or gas chromatography and tandem mass spectrometry as described in the Supplemental Methods (Supplemental Digital Content 1) [52,53].
Data processing, bioinformatics, and statistical analysis
Metabolite data were normalized by median centering. Missing values were imputed with the lower limit of detection for a given metabolite. Significantly altered metabolites were defined by fold change greater than 1.2, a P-value less than 0.05, and false discovery rate (FDR) 10% or less. Classification analysis [principal component analysis (PCA), partial least-squares discriminant analysis (PLS-DA), random forest, support vector machine (SVM), and unsupervised hierarchical clustering] were performed in Metaboanalyst (http://www.metaboanalyst.ca). Quantitative enrichment analysis was performed in Metabolite Set Enrichment Analysis (MSEA) using the Metabolic Pathways library. Visualization of pathway mapping [Kyoto Encyclopedia of Genes and Genomes (KEGG) and Small Molecule Pathway Database (SMPDB) pathways] was performed in Cytoscape. Additional statistical analyses were performed on log-transformed data in R. Pearson correlations were used to evaluate relationships between metabolites (P < 0.05, FDR ≤0.1). Spearman correlations were used to examine relationships between metabolites, global and domain T scores, and markers of intrathecal [sCD14, CCL2, IL-6, IFN-γ, and kynurenine to tryptophan ratio (K : T)] and systemic inflammation (IFN-α, IFN-γ, IL-8, CXCL9, CXCL10, IL-1b, IL-6, IL-2Ra, sCD14, and CCL2). Multiple hypothesis testing corrections were performed for fold change and correlation analyses by calculating FDR in fdrtool.
Results
HIV and aging cohorts
The HIV cohort was predominately male with late-stage disease (nadir CD4+ <300 cells/μl) and high prevalence of HCV coinfection (64%) (Supplemental Digital Content 2), and included young and older patients (50% <45 years and 50% ≥45 years). Two patients had mild hyperlipidemia, one had lipodystrophy, and one had diabetes. Of those on ART [n = 36; median time on ART 26 months; interquartile range (IQR) 16–49], 72% were on protease inhibitors, 100% were on nucleoside reverse transcriptase inhibitors (NRTIs), and 31% were on drugs associated with mitochondrial toxicity (zidovudine, stavudine and didanosine) HIV patients on ART (n = 36) had lower plasma (mean log10 2.87 vs. 4.99 copies/ml; P < 0.001) and CSF viral loads (mean log10 1.68 vs. 2.87 copies/ml; P = 0.009) than those not on ART (n = 10). HIV patients had normal CSF protein levels and white blood cells counts. The HIV cohort had a high prevalence of NCI (69%), the majority having ANI or MND. The aging cohort was composed of HIV-negative controls. Among them, 44% were young [<age 50; median age 40 (33–44)] and 56% were older [≥ age 50; median age 57 (53–67)].
Characterization of cerebrospinal fluid metabolomes from HIV and aging cohorts
Untargeted metabolomic profiling of 100 CSF samples detected 199 (145 named and 54 unnamed) and 204 (149 named and 55 unnamed) metabolites in the HIV and aging cohorts, respectively (Supplemental Digital Content 3). To reduce noise in the analysis, preprocessing was performed to exclude xenobiotics and metabolites with more than 70% imputed values. One HIV-negative sample was excluded on the basis of outlier analysis in Metaboanalyst. One hundred and seven named metabolites detected across both cohorts met the acceptability criteria. The majority of the detected metabolites were amino acids (49%), followed by carbohydrates (19%), lipids (16%), and nucleotides (8%), and were mapped to biologically relevant pathways including neurotransmitters [glutamate, N-acetylaspartate (NAA), N-acetylaspartylglutamic acid (NAAG), glycine]; pathways associated with neurotransmitter production [phenylalanine and tyrosine metabolites (dopamine), tryptophan metabolites (serotonin), and homocarnosine (gamma-aminobutyric acid (GABA))]; markers of glial activation (choline, myo-inositol, arachidonate); markers of mitochondrial dysfunction (acyl-carnitines and Krebs cycle components); oxidation products (5-oxoproline and homocarnosine) and markers of oxidative stress (purine metabolites); and metabolic waste products (ketone bodies, lactate, creatinine, phenylacetylglutamine, p-cresol sulfate).
Alterations in the cerebrospinal fluid metabolome of HIV patients on antiretroviral therapy map to pathways associated with neurotransmitter production, mitochondrial dysfunction, oxidative stress, and metabolic waste
Fifteen named and 12 unnamed metabolites distinguished between HIV patients on ART (n = 36) and age and sex-matched HIV-negative controls (fold change >1.2, P < 0.01, FDR <10%; Fig. 1a, Supplemental Digital Content 4 and 5). The 15 named metabolites classified HIV vs. control individuals with more than 90% predictive accuracy in random forest. Mapping altered named metabolites to KEGG and SMPDB pathways identified alterations in aspartate and glutamate metabolism, phenylalanine and tyrosine metabolism, GABA synthesis, Krebs cycle, mitochondrial electron transport chain, carnitine metabolism, glutathione metabolism, and ketone body metabolism, corresponding to metabolic alterations associated with altered neurotransmitter production, mitochondrial dysfunction, oxidative stress, and accumulation of metabolic waste products (Fig. 1b). Fourteen of these 15 named metabolites were altered in the subgroup of HIV patients on ART with suppressed viral replication (n = 20, plasma viral load <400 copies/ml, CSF viral load <50 copies/ml) compared to age and sex-matched HIV-negative controls (n = 20; Fig. 1c). Eleven were altered in the subgroup of HIV patients on stable ART (>2 years) with maximally suppressed plasma viral loads (<50 copies/ml) (n = 10 per group; Supplemental Digital Content 6) and 13 were altered in HIV patients not on ART (n = 10 patients per group, median CSF viral load 328 copies/ml, median plasma viral load 76 936 copies/ml) (Supplemental Digital Content 5). An additional 12 metabolites were altered only in HIV patients not on ART, including kynurenine and markers of glial cell activation (choline and myo-inositol) (Supplemental Digital Content 7). Therefore, alterations in these CSF metabolites were detected irrespective of viral replication in CSF and plasma or current ART.
Fig. 1.
Cerebrospinal fluid metabolomics identifies metabolites that distinguish HIV patients on antiretroviral therapy from HIV-negative controls.
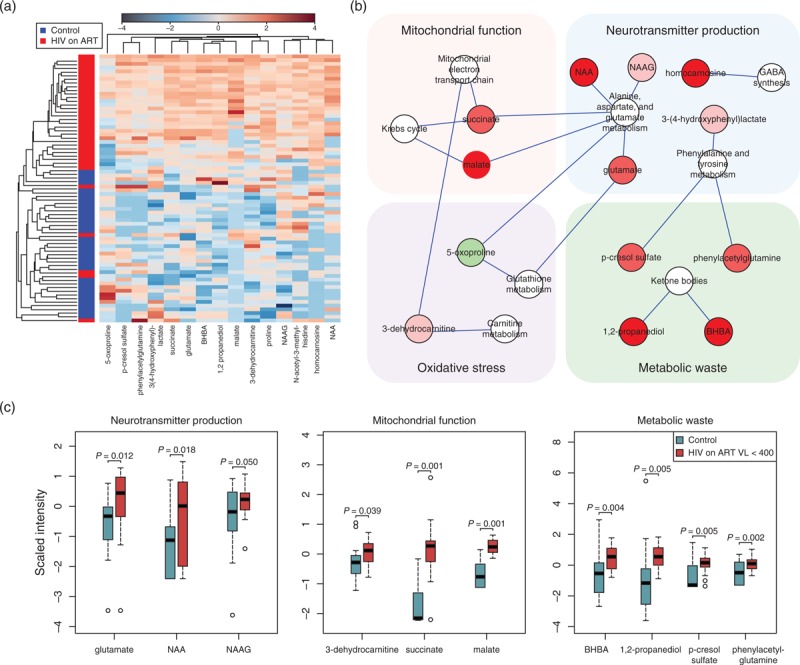
(a) Unsupervised hierarchical clustering of signature metabolites (n = 15; FC >1.2, P < 0.01, FDR <10%) altered in HIV patients on ART (n = 36, red) compared to age and sex-matched HIV-negative controls (n = 36, blue). Red and blue indicate increased and decreased metabolite levels, respectively. FDR was used to correct for multiple hypothesis testing. (b) Metabolites altered in HIV patients on ART compared to HIV-negative controls mapped to biosynthetic pathways linked to production of neurotransmitters, mitochondrial dysfunction, oxidative stress, and metabolic waste products. Altered metabolites (FC >1.2, P < 0.01, FDR <10%) were mapped to metabolite pathways and interaction networks were generated in Cytoscape. Green and red nodes represent metabolites with increased and decreased levels, respectively. White nodes represent pathways. (c) Box plots of metabolites altered in HIV patients on ART with low plasma viral loads (n = 20, plasma VL <400 copies/ml, CSF VL <50 copies/ml) compared to age and sex-matched HIV-negative controls (n = 20) that could be mapped to biological processes associated with NCI. Medians are represented by horizontal bars, boxes span the interquartile range (IQR) and whiskers extend to extreme data points within 1.5 times IQR. Outliers plotted as open circles lie outside 1.5 times the IQR. Blue and red represent controls and HIV patients, respectively. The P-values were calculated using Welch's t-tests. ART, antiretroviral therapy; BHBA, beta-hydroxybutyric acid; CSF, cerebrospinal fluid; FC, fold change; FDR, false discovery rate; GABA, gamma-aminobutyric acid; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; NCL, neurocognitive impairment; VL, viral load.
Alterations in the HIV cerebrospinal fluid metabolome overlap with those associated with normal aging in HIV-negative individuals
Given recent studies suggesting accelerated aging in HIV patients on ART [23,24], we compared alterations in the CSF metabolome of young HIV patients on ART (n = 16, age <50, plasma viral load <1000 copies/ml and CSF viral load <50 copies/ml) to the profile altered with aging in HIV-negative controls (n = 23 per group, young vs. older patients) (Supplemental Digital Content 8). Thirty-four named and 12 unnamed metabolites were altered in older (age ≥50) compared to sex and race-matched young HIV-negative controls (age <50), including metabolites associated with neurotransmitter production [glutamate, homocarnosine, 3-(4-hydroxyphenyl)lactate], markers of glial activation (choline and arachidonate), oxidative products (5-oxoproline) and markers of oxidative stress (urate, hypoxanthine), and metabolic waste products [3-hydroxybutyrate (BHBA), 1,2-propanediol, phenylacetylglutamine, lactate] (Fig. 2a). Many of these metabolites correlated positively with advancing age (P < 0.05; Fig. 2b). Ten named and four unnamed metabolites altered in young HIV patients on ART vs. age and sex-matched HIV-negative controls overlapped with those associated with normal aging in HIV-negative individuals including glutamate, phenylacetylglutamine, succinate, and ketone bodies (BHBA and 1,2 propanediol) (Fig. 3), suggesting a pattern indicative of accelerated aging.
Fig. 2.
Alterations in the cerebrospinal fluid metabolome associated with aging in HIV-negative controls.
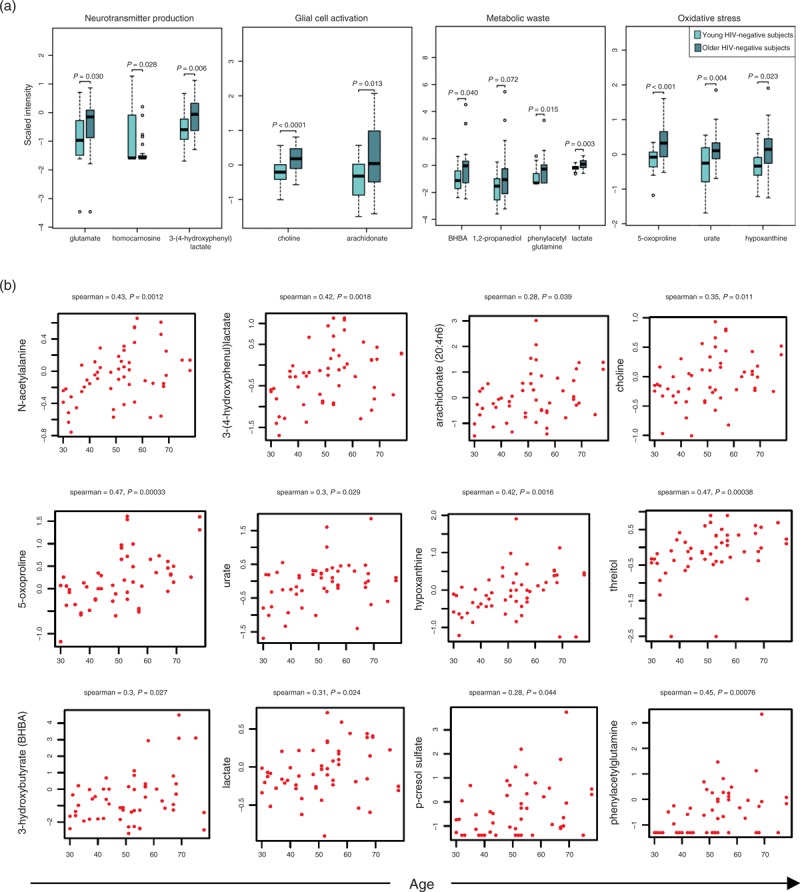
(a) Box plots of metabolites altered in older (n = 23; ≥50 years old) compared to young HIV-negative controls (n = 23; <50 years old). Light and dark blue represent young and older controls, respectively. Medians are represented by horizontal bars, boxes span the interquartile range (IQR) and whiskers extend to extreme data points. The P-values were calculated using Welch's t-tests. (b) Correlation plots of biologically relevant metabolites (log2 scaled intensity) vs. age in HIV-negative controls (n = 53). Spearman correlations were used to examine relationships between metabolite levels and age. The correlation coefficient R and P-value are shown above each plot. False discovery rate below 10% was used to correct for multiple hypothesis testing.
Fig. 3.
Metabolites altered in the cerebrospinal fluid metabolome of young HIV patients on antiretroviral therapy overlap with those altered in older HIV-negative controls.
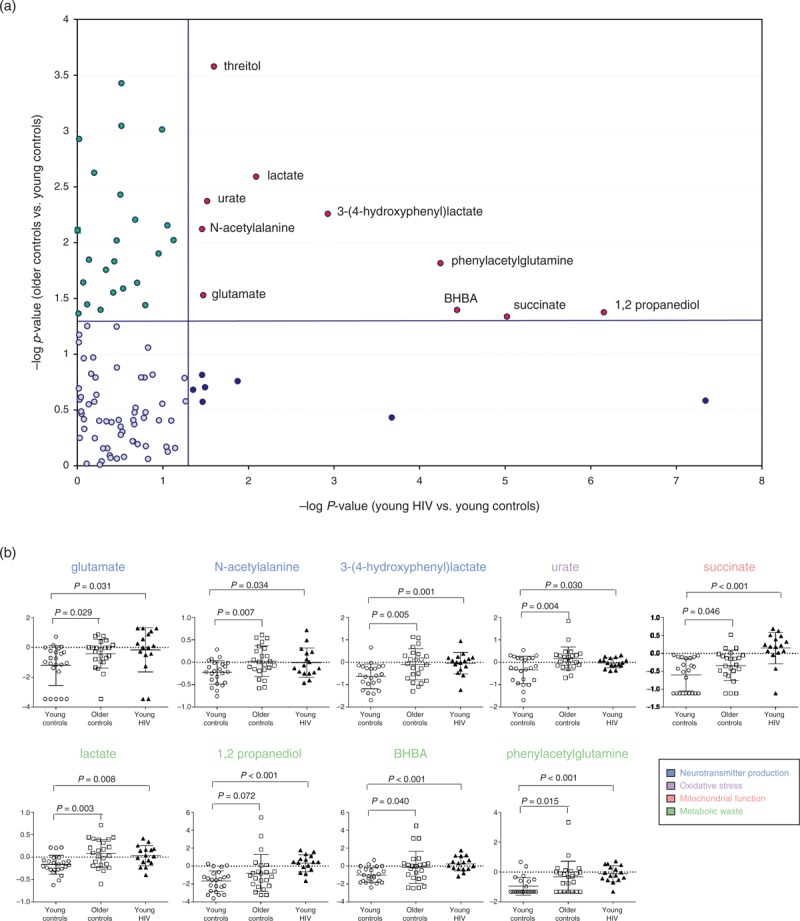
(a) P-value scatter plot of metabolites altered in the HIV CSF metabolome (16 young HIV patients on ART vs. 23 young HIV-negative controls) vs. metabolites altered during normal aging in HIV-negative controls (23 older vs. 23 young HIV-negative controls). The Y-axis shows –log10P-values of metabolites altered in older (≥50 years old) vs. young HIV-negative controls (<50 years old) matched for sex and race. The X-axis shows –log10P-values of metabolites altered in young HIV patients on ART (<50 years old) with viral loads (plasma VL <1000 copies/ml, CSF VL <50 copies/ml) vs. young HIV-negative controls (≥50 years old) matched for age and sex. (b) Beeswarm plots of metabolites altered in young HIV patients on ART (<50 years old, n = 16) compared to young (<50 years old, n = 23) and older HIV-negative controls (≥50 years old, n = 23). Medians are represented by horizontal bars. The P-values were calculated using Welch's t-tests. ART, antiretroviral therapy; BHBA, beta-hydroxybutyric acid; CSF, cerebrospinal fluid; VL, viral load.
Neurocognitive impairment is associated with alterations in cerebrospinal fluid neurotransmitters/neuropeptides, markers of glial activation, and accumulation of metabolic waste in HIV patients on antiretroviral therapy
To identify metabolic pathways associated with HANDs, we compared CSF metabolite profiles between HIV patients on ART with (n = 12) and without (n = 14) NCI for groups matched by age, sex, race, and current and nadir CD4+ (plasma viral load <1000 copies/ml, CSF viral load <50 copies/ml). Seven metabolites differentiated between HIV patients with and without NCI, but these differences did not reach statistical significance following multiple testing correction (Supplemental Digital Content 9). Next, we performed fold-change analysis on HIV patients with or without NCI vs. age and sex-matched HIV-negative controls (n = 14). Ten metabolites distinguished HIV patients with NCI, but not HIV patients without NCI, from HIV-negative controls including neurotransmitters (glutamate, NAA); markers of glial cell activation (myo-inositol, arachidonate); markers of mitochondrial dysfunction (propionylcarnitine, 3-dehydrocarnitine); and metabolic waste products (BHBA, lactate, phenylacetylglutamine) (fold change >1.2, P <0.05, FDR <5%). Twelve metabolite sets were enriched in HIV patients with NCI compared to HIV-negative controls. Five of these sets were enriched only in HIV patients with NCI including ketone body metabolism, aspartate metabolism, and phenylalanine and tyrosine metabolism (P < 0.05, FDR <5%; Supplemental Digital Content 10). Recursive SVM classification models identified eight metabolites as top-ranked classifiers of HANDs; these included neurotransmitters (glutamate, NAA); markers of glial activation (myo-inositol); and ketone bodies (beta-hydroxybutyric acid, 1,2-propanediol) (Fig. 4a and c). These eight metabolites distinguished HIV patients with vs. without HAND with greater than 85% predictive accuracy (Fig. 4b). Further validation in a cohort of HIV patients with plasma viral loads greater than 10 000 copies/ml (n = 15; 9 with NCI and 6 without NCI) showed that six of these metabolites also distinguished HIV patients with vs. without HAND in a separate test cohort (glutamate, NAA, BHBA, 1,2 propanediol, isobutyrylcarnitine, and N-acetylvaline) (Fig. 4d). These CSF metabolite profiles suggest that altered neurotransmitter production, glial cell activation, mitochondrial dysfunction, and accumulation of metabolic waste products are processes that characterize HAND, irrespective of HIV replication.
Fig. 4.
Cerebrospinal fluid metabolites associated with neurocognitive impairment in HIV patients on antiretroviral therapy.
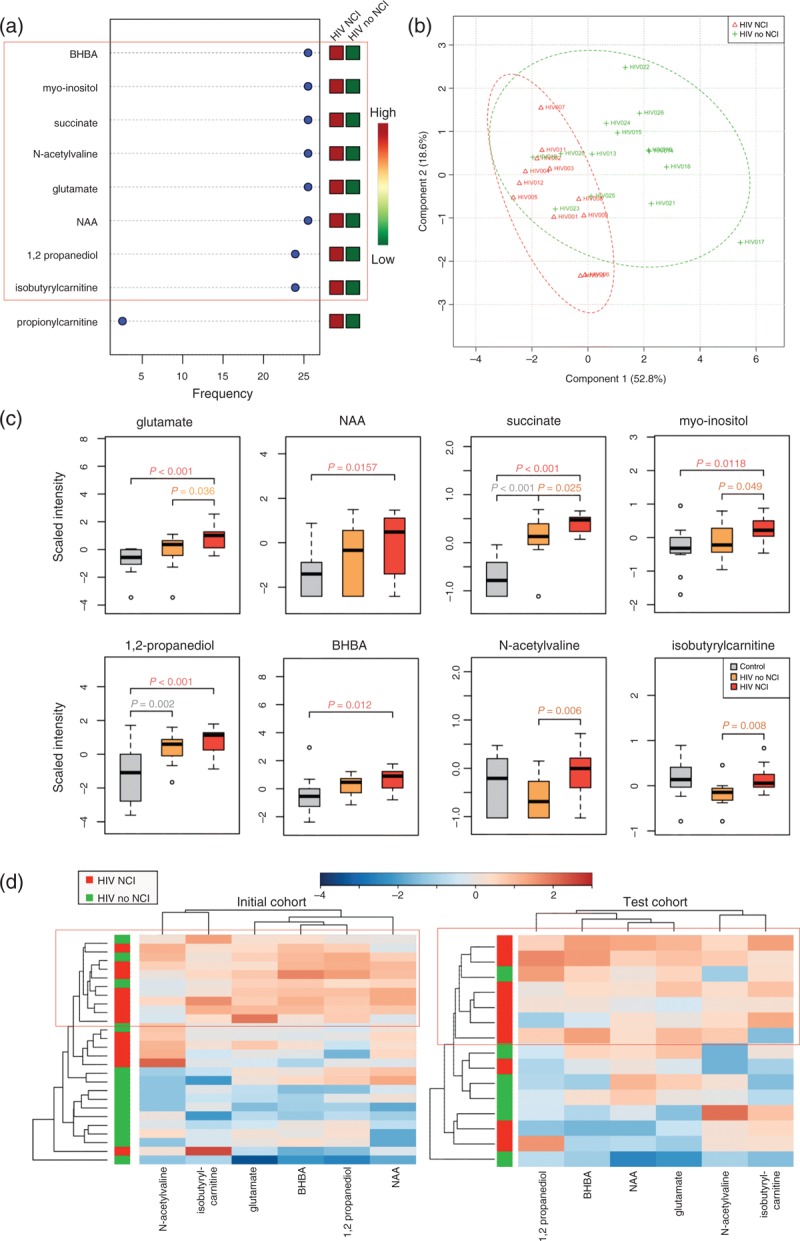
(a) Top classifiers of NCI with expression heatmap from recursive SVM classification models. SVM models were constructed using metabolites identified in FC analysis (FC >1.2, P < 0.05, FDR <10%) comparing HIV patients on ART with NCI (plasma VL <10 000 copies/ml), HIV patients on ART without NCI (plasma VL <10 000 copies/ml), and age and sex-matched HIV-negative controls. Red and green indicate increased and decreased levels, respectively. (b) PLS-DA analysis shows separation of metabolomes from HIV patients on ART with (red, n = 12) and without NCI (green, n = 14). (c) Box plots of top classifiers of NCI identified by SVM in HIV-negative controls (n = 14, gray), HIV patients on ART without NCI (n = 14, orange), and HIV patients on ART with NCI (n = 12, red). Medians are represented by horizontal bars, boxes span the interquartile range (IQR) and whiskers extend to extreme data points. The P-values were calculated using Welch's t-tests. (d) Unsupervised hierarchical clustering of six top-ranked metabolites that distinguish between HIV patients on ART with and without NCI in two independent cohorts. The initial cohort was composed of HIV patients on ART with low viral loads (n = 26, plasma VL <10 000 copies/ml, CSF VL <50 copies/ml). The independent test cohort was composed of HIV patients with high plasma viral loads (n = 15, VL >10 000 copies/ml). Red and blue indicate increased and decreased levels, respectively. ART, antiretroviral therapy; BHBA, beta-hydroxybutyric acid; CSF, cerebrospinal fluid; FC, fold change; FDR, false discovery rate; NAA, N-acetylaspartate; NCL, neurocognitive impairment; PLS-DA, partial least-squares discriminant analysis; SVM, support vector machine; VL, viral load.
Inter-relationships between cerebrospinal fluid and plasma metabolites, neurocognitive test scores, and inflammation in HIV patients on antiretroviral therapy
Next, we examined inter-relationships between CSF metabolites associated with NCI, plasma metabolites, and markers of systemic and intrathecal inflammation. Two distinct subclusters of CSF metabolites were identified by correlation analysis (P < 0.05, FDR <0.1) (Fig. 5). In subcluster 1, isobutyrylcarnitine, myo-inositol, and N-acetylvaline correlated negatively with global and several domain T scores (motor, memory-encoding, memory-retrieval and executive function) and positively with intrathecal IFN responses (CSF IFN-γ and K : T ratio). In subcluster 2, succinate, glutamate, NAA, BHBA, and 1,2 propanediol correlated positively with systemic markers of inflammation (IFN-α, IFN-γ, IL-8, IL-1β, IL-6, IL-2Ra) and negatively with plasma lysophosphocholines (LPCs) and steroids. Plasma LPCs and steroids correlated positively with T scores (global, motor, memory-encoding, memory-retrieval and executive function) and negatively with systemic inflammation. These results suggest inter-relationships between systemic and intrathecal inflammation and plasma or CSF metabolite alterations associated with NCI.
Fig. 5.
Integrative analysis and correlation matrix visualization reveals relationships between cerebrospinal fluid classifiers of neurocognitive impairment, plasma lysophosphocholines and steroids, global and domain T scores, and markers of systemic and intrathecal inflammation in HIV patients on antiretroviral therapy.
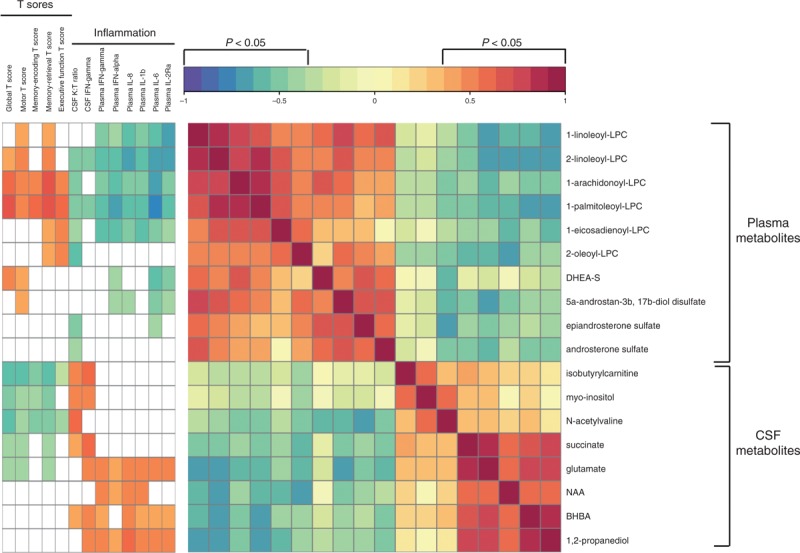
The correlation matrix and clinical covariate bar were constructed in R using the heatmap.2 function. Pearson correlations were used to evaluate relationships between metabolites (P <0.05, FDR ≤0.1). Spearman correlations were used to examine relationships between metabolites, global and domain T scores, and markers of inflammation. Significant correlations had a −0.35 > r > 0.35 (P < 0.05), and FDR below 10%. FDR was used to correct for multiple hypothesis testing. Red and blue indicate positive and negative correlations, respectively (see Color Key). BHBA, beta-hydroxybutyric acid; DHEA-S, dehydroepiandrosterone sulfate; FDR, false discovery rate; LPC, lysophosphocholine; NAA, N-acetylaspartate.
Discussion
Here, we characterized the CSF metabolome in a cohort of HIV patients on ART with advanced disease and identified eight metabolites as top-ranked classifiers of HAND, including neurotransmitters (glutamate, NAA); markers of glial cell activation (myo-inositol); mitochondrial function (succinate); and ketone bodies, suggesting that glutamate excitotoxicity, astrocyte activation, mitochondrial dysfunction, and accumulation of metabolic waste may contribute to NCI. This HAND signature overlapped with a CSF metabolite profile associated with aging in HIV-negative controls, suggesting a pattern indicative of accelerated aging. Correlation analysis identified inter-relationships between plasma inflammation markers and intrathecal IFN responses and metabolic alterations associated with NCI, suggesting that ongoing systemic and intrathecal inflammation may contribute to this accelerated aging phenotype. These results suggest that therapeutic strategies targeting ‘inflammaging’ and associated metabolic abnormalities may be beneficial for treatment of HAND.
Alterations in CSF metabolites identified in our study provide insights into mechanisms that may contribute to HAND. In particular, alterations in neurotransmitters (glutamate and NAA) and markers of glial cell activation (myo-inositol) and mitochondrial dysfunction (succinate) were associated with NCI. Glutamate is neurotoxic at high concentrations and increased levels have been shown in HIV-associated dementia (HAD) and other neurological disorders [54–56]. Decreased NAA, a marker of neuronal density and integrity, has been reported in brain from HIV patients with HAND [27,35–37,57,58]. Here, CSF NAA was increased in patients with HAND, possibly reflecting differences in the sample tested (brain vs. CSF), and may represent leakage into the CSF associated with neuronal damage. Alterations in N-acetylated alpha-linked acidic dipeptidase activity, which converts NAAG to glutamate and NAA, have been shown to correlate with neuronal loss in Alzheimer's disease [59]. Myo-inositol, a marker of astrocyte activation, was also increased in HIV patients, consistent with magnetic resonance spectroscopy studies, showing increased myo-inositol in the brain of HIV patients with HAND, including those on ART [27,35–37,58]. This may be relevant to HAND pathophysiology because astrocyte activation can impair their neuroprotective functions (e.g. BBB integrity and glutamate reuptake) [60]. Another important finding was increased CSF succinate. Increased succinate, a component of the Krebs cycle, can reflect mitochondrial dysfunction [52]. Succinate may also act as a danger signal stabilizing hypoxia-inducible factor (HIF)-1a expression and enhancing IL-1b production during inflammation [61]. More than 80% of the CSF metabolites which were altered in HIV patients on ART were also altered in HIV patients not on ART (Supplemental Digital Content 7), suggesting that these alterations are not directly associated with ART or ongoing HIV replication in plasma or CSF. These results suggest that glutamate excitotoxicity, astrocyte activation, and mitochondrial dysfunction may contribute to HAND.
Another important finding was increased accumulation of metabolic waste, including ketone bodies, phenylacetylglutamine, and p-cresol sulfate. Accumulation of metabolic waste, such as protein aggregates, is a hallmark of Alzheimer's disease and other age-associated neurodegenerative diseases [62–64]. In HIV patients, accumulation of hyperphosphorylated tau, amyloid, and alpha-synuclein has been reported in older patients [65–68]. CSF circulates nutrients filtered from the blood to the brain and removes metabolic waste by active transport or bulk flow. CSF is absorbed into the blood through arachnoid villi or exchanged with brain interstitial fluid via aquaporin 4 (AQP4) channels [69–71]. In reactive astrogliosis, mislocalization of AQP4 results in a loss of interstitial flow and accumulation of extracellular waste products [69–71]. Whereas astrogliosis is common in HIV [30,72] and AQP4 expression is increased in HIV patients with HAD [73], further studies are required to determine if loss of interstitial flow and detrimental effects on the glymphatic system [64,69–71] contribute to accumulation of metabolic waste and development of HAND.
Ketone bodies (BHBA and 1,2 propanediol) were identified as top-ranked CSF classifiers of HAND. Ketone bodies are an energy source for metabolically active tissues under conditions of glucose deficiency [74]. Whereas ketogenic diets have shown therapeutic potential in some neurological diseases [75,76], ketone bodies are toxic at high concentrations and can stimulate insulin release, generate oxygen radicals, and cause lipid peroxidation, contributing to oxidative stress [77,78]. Increased ketone bodies have been reported in metabolic disorders (i.e. diabetes and obesity), inflammatory diseases (i.e. multiple sclerosis and rheumatoid arthritis), and are associated with neurological complications in diabetic ketoacidosis [79–82]. In view of these observations, we predict that increased ketone bodies in CSF from patients with HAND reflect both increased production and decreased clearance. Given that ketone bodies are signaling molecules that play an important role regulating lipid metabolism and mitochondrial function (reviewed in [83]), further studies are warranted to examine their potential role in HAND via neurotoxic or metabolic effects.
Antiretroviral therapy is associated with increased age-associated comorbidities including cardiovascular, liver, kidney, and bone diseases [20,22]. These age-related comorbidities often occur at younger ages than would be expected among HIV-negative individuals, possibly reflecting ‘inflammaging’ along with other mechanisms [23,24]. Here, alterations in the HIV CSF metabolome, including those associated with NCI, showed significant overlap with metabolites altered in aging HIV-negative controls (e.g. glutamate, succinate, BHBA, and 1,2 propanediol), suggesting a pattern of accelerated aging. Consistent with these findings, increased glutamate and succinate were detected in plasma from HIV-negative individuals with advancing age [84,85], and increased glutamate and ketone bodies were detected in CSF from HIV-negative individuals with cognitive impairment and Alzheimer's disease [86]. These alterations likely reflect increased metabolite production, together with decreased metabolite clearance and reduced CSF turnover, which has been reported in aging populations and Alzheimer's disease [87,88]. In the present study, alterations in these CSF metabolites were associated with systemic inflammation (IFN-γ, IFN-α, IL-8, IL-1b, IL-6, and IL-2Ra) and intrathecal IFN responses (IFN-γ and K : T ratio), suggesting ongoing systemic and intrathecal inflammation both contribute to this accelerated aging phenotype. Plasma LPC and steroids correlated positively with neurocognitive test scores, and negatively with markers of systemic inflammation. These findings, together with previous studies, suggest these LPC and steroids may have beneficial roles, such as anti-inflammatory or neuroprotective effects. Phosphatidylcholines represent a class of lipids altered in Alzheimer's and other neurodegenerative diseases [89,90]. Depletion of dehydroepiandrosterone sulfate and related steroids have been implicated in aging, age-related comorbidities, and immune dysfunction [91–93]. These results suggest that metabolic abnormalities and ‘inflammaging’ mechanisms both contribute to HAND.
We acknowledge certain limitations of our study. We selected HIV patients with advanced disease and high prevalence of HCV coinfection. Although we cannot exclude the possibility that HCV contributed to the results, matched analyses of HIV patients with and without HCV coinfection did not identify significant differences in CSF metabolites or inflammation markers. HIV samples were collected from 1999 to 2009 and therefore may not reflect contemporary populations due to differences in ART regimens. We cannot exclude the possibility that prolonged sample storage could affect the stability of some metabolites. However, recent studies suggest prolonged storage has minimal effects on the majority of CSF metabolites [94,95]. Small sample volumes limited our ability to detect certain lipids in CSF that were detected by others [43,44,46]. Further studies are required to define alterations in larger, recent cohorts with less advanced disease, treated with specific ART regimens, and their possible association with HAND subtypes.
In summary, untargeted metabolomic profiling identified alterations in the CSF metabolome of HIV patients on ART that suggest persistent inflammation, glial responses, glutamate neurotoxicity, and age-dependent effects on brain waste disposal systems contribute to mechanisms involved in HAND that may be augmented by aging. These alterations were not directly associated with ART or ongoing HIV replication in CSF or plasma. This study provides insights into disease mechanisms associated with HAND and represents progress toward identifying biomarkers to predict and monitor neurocognitive outcomes and therapeutic responses.
Acknowledgements
The work was supported by the National Institute of Mental Health (NIMH) grant RO1 MH097659 and NIDA grant DPI DA28994 to D.G. E.C. was supported in part by a fellowship from Canadian Institutes of Health Research (CIHR). A.D. was supported by NIH T32 AG00222. NNTC and CHARTER sites were supported by NIMH and National Institute of Neurological Disorders and Stroke (NINDS) (grants U01MH083501, R24MH59724, U01MH083507, R24NS45491, U01MH083500, R24NS38841, U01MH083506, R24MH59745, U01MH083545, N01MH32002, and N01MH22005). Core facilities were supported by the Harvard Center for AIDS Research grant (P30 AI060354) and Dana-Farber Cancer Institute/Harvard Cancer Center Research grant (P30 CA06516).
E.C. performed the experiments and data analysis, drafted the manuscript, and prepared figures. V.M. and A.D. performed bioinformatics and statistical analysis and prepared figures. S.M. participated in study design, data analysis, and manuscript editing. D.G. conceived the study, supervised its design, coordination, and data analysis, and helped write and edit the manuscript. All authors read, participated in editing the manuscript, and approved the final manuscript.
Conflicts of interest
The authors declare that they have no competing interests.
Supplementary Material
References
- 1.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic?. AIDS 2010; 24:1367–1370 [DOI] [PubMed] [Google Scholar]
- 2.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002; 8:136–142 [DOI] [PubMed] [Google Scholar]
- 3.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 2010; 67:699–714 [DOI] [PubMed] [Google Scholar]
- 4.Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis 2001; 184:699–706 [DOI] [PubMed] [Google Scholar]
- 5.Gartner S, Liu Y. Insights into the role of immune activation in HIV neuropathogenesis. J Neurovirol 2002; 8:69–75 [DOI] [PubMed] [Google Scholar]
- 6.Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol 1997; 42:679–688 [DOI] [PubMed] [Google Scholar]
- 7.Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol 1991; 29:202–209 [DOI] [PubMed] [Google Scholar]
- 8.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med 2010; 18:45–55 [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, et al. Neurocognitive effects of HIV, hepatitis C, and substance use history. J Int Neuropsychol Soc 2012; 18:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendelken LA, Valcour V. Impact of HIV and aging on neuropsychological function. J Neurovirol 2012; 18:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73:1292–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr 2006; 43:405–410 [DOI] [PubMed] [Google Scholar]
- 16.Wright EJ, Grund B, Robertson K, Brew BJ, Roediger M, Bain MP, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 2010; 75:864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassol E, Misra V, Morgello S, Gabuzda D. Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol 2013; 8:1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP, et al. Neurologic disorders incidence in HIV+ vs. HIV- men: multicenter AIDS cohort study, 1996–2011. Neurology 2012; 79:1873–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004; 63:822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–1126 [DOI] [PubMed] [Google Scholar]
- 21.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008; 47:542–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oursler KK, Goulet JL, Crystal S, Justice AC, Crothers K, Butt AA, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS 2011; 25:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009; 17:118–123 [PubMed] [Google Scholar]
- 24.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009; 338:a3172. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and antiinflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128:92–105 [DOI] [PubMed] [Google Scholar]
- 26.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 2012; 60:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011; 25:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 47:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 2007; 196:1779–1783 [DOI] [PubMed] [Google Scholar]
- 30.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 2009; 15:360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 2005; 64:529–536 [DOI] [PubMed] [Google Scholar]
- 32.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 2008; 29:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection?. Lancet Neurol 2009; 8:382–397 [DOI] [PubMed] [Google Scholar]
- 34.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response?. Nat Med 2006; 12:1005–1015 [DOI] [PubMed] [Google Scholar]
- 35.Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, et al. A multicenter 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging 2003; 17:625–633 [DOI] [PubMed] [Google Scholar]
- 36.Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, et al. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage 2004; 23:928–935 [DOI] [PubMed] [Google Scholar]
- 37.Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, et al. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther 2003; 8:17–26 [PubMed] [Google Scholar]
- 38.Wishart DS, Lewis MJ, Morrissey JA, Flegel MD, Jeroncic K, Xiong Y, et al. The human cerebrospinal fluid metabolome. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 871:164–173 [DOI] [PubMed] [Google Scholar]
- 39.Mandal R, Guo AC, Chaudhary KK, Liu P, Yallou FS, Dong E, et al. Multiplatform characterization of the human cerebrospinal fluid metabolome: a comprehensive and quantitative update. Genome Med 2012; 4:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoop MP, Coulier L, Rosenling T, Shi S, Smolinska AM, Buydens L, et al. Quantitative proteomics and metabolomics analysis of normal human cerebrospinal fluid samples. Mol Cell Proteomics 2010; 9:2063–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyes MP, Jordan EK, Lee K, Saito K, Frank JA, Snoy PJ, et al. Relationship of neurologic status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain Res 1992; 570:237–250 [DOI] [PubMed] [Google Scholar]
- 42.Giovannoni G, Miller RF, Heales SJ, Land JM, Harrison MJ, Thompson EJ. Elevated cerebrospinal fluid and serum nitrate and nitrite levels in patients with central nervous system complications of HIV-1 infection: a correlation with blood-brain-barrier dysfunction. J Neurol Sci 1998; 156:53–58 [DOI] [PubMed] [Google Scholar]
- 43.Wikoff WR, Pendyala G, Siuzdak G, Fox HS. Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. J Clin Invest 2008; 118:2661–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 2004; 55:257–267 [DOI] [PubMed] [Google Scholar]
- 45.Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology 2007; 68:1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mielke MM, Bandaru VV, McArthur JC, Chu M, Haughey NJ. Disturbance in cerebral spinal fluid sphingolipid content is associated with memory impairment in subjects infected with the human immunodeficiency virus. J Neurovirol 2010; 16:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandaru VV, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, et al. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology 2013; 81:1492–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.AACTG Table for grading severity of adult adverse experiences. Bethesda, MD:National Institute of Allergy and Infectious Diseases (NIAID); 1992 [Google Scholar]
- 49.Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician 2004; 70:869–876 [PubMed] [Google Scholar]
- 50.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004; 26:759–778 [DOI] [PubMed] [Google Scholar]
- 52.Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 2013; 13:203–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009; 81:6656–6667 [DOI] [PubMed] [Google Scholar]
- 54.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 2001; 57:671–675 [DOI] [PubMed] [Google Scholar]
- 55.Ferrarese C, Riva R, Dolara A, De Micheli A, Frattola L. Elevated glutamate in the cerebrospinal fluid of patients with HIV dementia. JAMA 1997; 277:630. [PubMed] [Google Scholar]
- 56.Espey MG, Ellis RJ, Heaton RK, Basile AS. Relevance of glutamate levels in the CSF of patients with HIV-1-associated dementia complex. Neurology 1999; 53:1144–1145 [DOI] [PubMed] [Google Scholar]
- 57.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007; 81:89–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart JL, Payan C, et al. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology 2001; 56:112–115 [DOI] [PubMed] [Google Scholar]
- 59.Passani LA, Vonsattel JP, Carter RE, Coyle JT. N-acetylaspartylglutamate, N-acetylaspartate, and N-acetylated alpha-linked acidic dipeptidase in human brain and their alterations in Huntington and Alzheimer's diseases. Mol Chem Neuropathol 1997; 31:97–118 [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005; 5:69–81 [DOI] [PubMed] [Google Scholar]
- 61.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013; 496:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 2007; 8:101–112 [DOI] [PubMed] [Google Scholar]
- 63.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med 2004; 10 Suppl:S10–17 [DOI] [PubMed] [Google Scholar]
- 64.Nedergaard M. Neuroscience. Garbage truck of the brain. Science 2013; 340:1529–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active antiretroviral therapy. Acta Neuropathol 2006; 111:529–538 [DOI] [PubMed] [Google Scholar]
- 66.Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, et al. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol 2009; 15:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 2009; 4:190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology 2009; 73:1982–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system?. Stroke 2013; 44:S93–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013; 123:1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol 2003; 13:195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.St Hillaire C, Vargas D, Pardo CA, Gincel D, Mann J, Rothstein JD, et al. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. J Neurovirol 2005; 11:535–543 [DOI] [PubMed] [Google Scholar]
- 74.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999; 15:412–426 [DOI] [PubMed] [Google Scholar]
- 75.Balietti M, Casoli T, Di Stefano G, Giorgetti B, Aicardi G, Fattoretti P. Ketogenic diets: an historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res Rev 2010; 9:273–279 [DOI] [PubMed] [Google Scholar]
- 76.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 2009; 59:293–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain SK, McVie R, Jaramillo JJ, Chen Y. Hyperketonemia (acetoacetate) increases the oxidizability of LDL + VLDL in Type-I diabetic patients. Free Radic Biol Med 1998; 24:175–181 [DOI] [PubMed] [Google Scholar]
- 78.Jain SK, McVie R, Jackson R, Levine SN, Lim G. Effect of hyperketonemia on plasma lipid peroxidation levels in diabetic patients. Diabetes Care 1999; 22:1171–1175 [DOI] [PubMed] [Google Scholar]
- 79.Fery F, Balasse EO. Ketone body production and disposal in diabetic ketosis. A comparison with fasting ketosis. Diabetes 1985; 34:326–332 [DOI] [PubMed] [Google Scholar]
- 80.Hall SE, Wastney ME, Bolton TM, Braaten JT, Berman M. Ketone body kinetics in humans: the effects of insulin-dependent diabetes, obesity, and starvation. J Lipid Res 1984; 25:1184–1194 [PubMed] [Google Scholar]
- 81.Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism 1975; 24:665–679 [DOI] [PubMed] [Google Scholar]
- 82.Fitzpatrick M, Young SP. Metabolomics: a novel window into inflammatory disease. Swiss Med Wkly 2013; 143:w13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2013; 25:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, et al. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008; 9:383–397 [DOI] [PubMed] [Google Scholar]
- 85.Menni C, Kastenmuller G, Petersen AK, Bell JT, Psatha M, Tsai PC, et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol 2013; 42:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trushina E, Dutta T, Persson XM, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PLoS One 2013; 8:e63644–e63656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.May C, Kaye JA, Atack JR, Schapiro MB, Friedland RP, Rapoport SI. Cerebrospinal fluid production is reduced in healthy aging. Neurology 1990; 40:500–503 [DOI] [PubMed] [Google Scholar]
- 88.Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer's type. Neurology 2001; 57:1763–1766 [DOI] [PubMed] [Google Scholar]
- 89.Whiley L, Sen A, Heaton J, Proitsi P, Garcia-Gomez D, Leung R, et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging 2014; 35:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oresic M, Hyotylainen T, Herukka SK, Sysi-Aho M, Mattila I, Seppanan-Laakso T, et al. Metabolome in progression to Alzheimer's disease. Transl Psychiatry 2011; 1:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA): a precursor steroid or an active hormone in human physiology. J Sex Med 2011; 8:2960–2982quiz 2983 [DOI] [PubMed] [Google Scholar]
- 92.Daynes RA, Araneo BA, Ershler WB, Maloney C, Li GZ, Ryu SY. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol 1993; 150:5219–5230 [PubMed] [Google Scholar]
- 93.Vermeulen A. Dehydroepiandrosterone sulfate and aging. Ann N Y Acad Sci 1995; 774:121–127 [DOI] [PubMed] [Google Scholar]
- 94.Rosenling T, Slim CL, Christin C, Coulier L, Shi S, Stoop MP, et al. The effect of preanalytical factors on stability of the proteome and selected metabolites in cerebrospinal fluid (CSF). J Proteome Res 2009; 8:5511–5522 [DOI] [PubMed] [Google Scholar]
- 95.Levine J, Panchalingam K, McClure RJ, Gershon S, Pettegrew JW. Stability of CSF metabolites measured by proton NMR. J Neural Transm 2000; 107:843–848 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


