Supplemental Digital Content is Available in the Text.
KEY WORDS: Angioscopy, Cerebral vasculature, Coiling, Endoscopy, Endovascular therapy, Flow diverter, Stenting
Abstract
BACKGROUND:
Endoluminal optical imaging, or angioscopy, has not seen widespread application during neurointerventional procedures, largely as a result of the poor imaging resolution of existing angioscopes. Scanning fiber endoscopes (SFEs) are a novel endoscopic platform that allows high-resolution video imaging in an ultraminiature form factor that is compatible with currently used distal access endoluminal catheters.
OBJECTIVE:
To test the feasibility and potential utility of high-resolution angioscopy with an SFE during common endovascular neurosurgical procedures.
METHODS:
A 3.7-French SFE was used in a porcine model system to image endothelial disruption, ischemic stroke and mechanical thrombectomy, aneurysm coiling, and flow-diverting stent placement.
RESULTS:
High-resolution, video-rate imaging was shown to be possible during all of the common procedures tested and provided information that was complementary to standard fluoroscopic imaging. SFE angioscopy was able to assess novel factors such as aneurysm base coverage fraction and side branch patency, which have previously not been possible to determine with conventional angiography.
CONCLUSION:
Endovascular imaging with an SFE provides important information on factors that cannot be assessed fluoroscopically and is a novel platform on which future neurointerventional techniques may be based because it allows for periprocedural inspection of the integrity of the vascular system and the deployed devices. In addition, it may be of diagnostic use for inspecting the vascular wall and postprocedure device evaluation.
ABBREVIATIONS:
CFB, coherent fiber bundle
F, French
SFE, scanning fiber endoscope
Endovascular neurosurgical therapies and technology continue to evolve and have now been developed for a number of intracranial pathologies. However, the lesions or malformations being treated are only visualized indirectly; treatment guidance and assessment are provided by x-ray fluoroscopy, which typically provides an indirect outline or contrast-filled “cast” of the vessel in the form of a 2-dimensional projection. Because fluoroscopy provides minimal information about the endoluminal surface, it cannot detect changes such as atherosclerosis until they cause structural deformation/luminal encroachment, nor can it assess posttherapeutic changes such as stent endothelialization. These limitations of fluoroscopy during “live” imaging can lead to difficulties when attempting to cross complex and tortuous 3-dimensional anatomy, with a concomitant increase in risk and procedure time as well as radiation dose.
To address these issues, direct endoluminal optical visualization or angioscopy has been proposed as an adjuvant imaging modality since the 1980s1 and has been used to visualize the human aorta,2,3 coronary4 and peripheral5 arteries, as well as therapies such as carotid6 and coronary7 angioplasty. Angioscopy has also been used to observe the placement of intravascular occlusion coils and stents in the carotid arteries of porcine and canine animal models,8 as well as to assess the completeness of clip occlusion in a canine aneurysm model.9 Although promising, these early works have all made use of coherent fiber bundle (CFB) endoscopes in which a densely packed array of small-diameter glass fibers relays images from the vessel lumen to an eyepiece or camera mounted on the distal end. CFBs are inherently limited by the restriction that each pixel in an image requires a separate fiber in the bundle, necessitating dense packing for high-resolution imaging; however, additional fibers increase the bundle size and decrease its mechanical flexibility, limiting their utility in the cerebral vasculature. As a result, current angioscopes consist of low-resolution arrays to maintain a small profile and maintain some mechanical flexibility, and thus widespread adoption of angioscopy has been limited to date largely as a result of the mechanical fragility and poor image quality produced by these CFBs.
Scanning fiber endoscopes (SFEs) are an emerging technology that produces images at significantly higher resolution than conventional fiberoptic endoscopes and in a miniature form factor, which is far more robust than a CFB.10 To date, SFEs have been used to image the esophagus11 and pancreatic and bile ducts,12 as well as the respiratory tree,13 but there have been no reports of their use for endovascular imaging. In this work, we demonstrate for the first time the feasibility of using the SFE for endoluminal imaging in a porcine model and demonstrate its utility during typical neurointerventional procedures.
METHODS
Scanning Fiber Endoscope
The details of the SFE design have been previously described elsewhere.10 In brief, the SFE consists of a single optical fiber that is scanned by a piezoelectric drive mechanism to illuminate the entire field of view of the endoscope, and return light is collected by a ring of fibers surrounding the scan head. For standard RGB (red, green, blue) imaging, the illumination spot is scanned in an outwardly expanding spiral, and the reflected light is collected and digitized to construct the image point-by-point in a manner analogous to the operation of a laser scanning microscope. The scan engine is contained within a stainless steel tube, which provides radiopacity, and the entire assembly is packaged into 3.7-French (F) (1.22 mm) low-density polyethylene tubing to provide flexibility while maintaining some pushability. The SFE used in this study is shown in Figure 1 and produces 600-line images at a video rate that is displayed on a monitor adjacent to the fluoroscopy display in our integrated interventional setup. Because the laser illumination light is conveyed through the central scanning fiber, the light source can be considered “cold” and does not actively conduct heat into the surrounding vessel. However, the distal tip of the SFE does contain a heating element that maintains the scan engine at physiological temperatures to avoid lens fogging when used in vivo, eliminating the need for heated saline or gas insufflation systems.8
FIGURE 1.
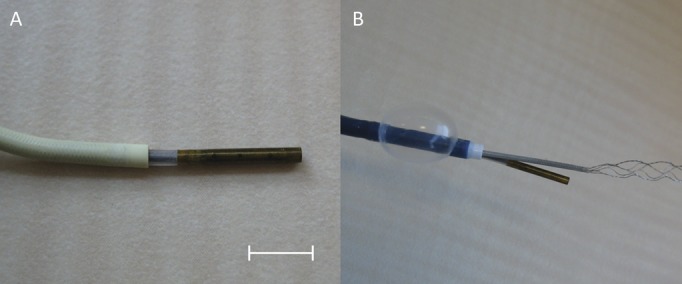
A, a 3.7-French (F) scanning fiber endoscope (SFE) used in this study exiting from a 5-F Envoy guide catheter, demonstrating the 8-mm radiopaque tip. Scale bar = 5 mm. B, the SFE deployed in combination with a 9-F coaxial balloon guide catheter and 2.5-F microcatheter for imaging of mechanical thrombectomy with a stent retriever.
In Vivo Imaging Using a Porcine Model
All animal procedures were carried out with institutional approval (University Health Network, Toronto, Ontario, Canada). Three Yorkshire pigs weighing 35 to 45 kg were sedated using ketamine/atropine/midazolam, and after induction and intubation, general anesthesia was maintained using inhaled isoflurane. An indwelling catheter was placed in an ear vein for fluid maintenance, along with electrocardiography, end-tidal CO2, and SpO2 leads for physiological monitoring. Bilateral femoral artery access was obtained using ultrasound guidance, and the renal, common carotid, and cervical arteries (3-5 mm) were selectively catheterized and assessed using standard fluoroscopic angiography. The catheters were continuously flushed using heparinized physiological saline solution (10 U/mL). Autologous thrombi were prepared by allowing unheparinized blood to clot over 24 hours and then immersing the resultant clot in fluoroscopic contrast media for 48 hours. Thrombi were then cut to size and injected into either a porcine lingual artery or an internal carotid artery as previously described.14 Immediately on completion of the study, the animals were euthanized under anesthesia by pentobarbital sodium overdose.
Endovascular Imaging Arrangement
To minimize any potential risks to the vessel endothelium during testing, the SFE was always used within a 056/070-inch guide catheter (Envoy; Codman, Raynham, Massachusetts/Neuron; Penumbra, Alameda, California). A clear field of view was provided by continuous heparinized saline solution flush through the outer guide catheter from standard pressure bags. The flush flow rate was manually adjusted to provide clear imaging depending on the diameter of the vessel being imaged and local blood flow conditions. Where necessary, proximal occlusion was provided by a coaxial balloon guide catheter (8-/9-F, Merci; Concentric Medical, Mountain View, California) through which the SFE could be placed within its own guide catheter.
Detachable aneurysm coils of various designs were delivered through a microcatheter that was advanced via a second 6-F guide catheter from the contralateral access sheath to allow visualization of coil deployment and detachment in real time. The internal carotid artery immediately proximal to the rete mirabile was embolized using coils to create an area of flow stagnation to test flow-diverter placement. Then, in a manner similar to coil deployment, flow-diverting stents (Pipeline; Covidien, Saint-Laurent, Quebec, Canada) were deployed in the usual fashion through a microcatheter under direct visualization by the SFE in various anatomies including parent vessel bends, straight vessel segments, distally occluded side branches, and overlying patent side branches. Mechanical thrombectomy of injected autologous thrombi was performed using various stent retrievers (Solitaire FR; Covidien, Saint-Laurent/Trevo Provue; Stryker, Hamilton, Ontario, Canada) after lesion crossing with a microwire and microcatheter under SFE observation. Endothelial dissections were created by using a 0.014-inch microwire in reverse with support from the guide catheter to force the rigid tip of the wire under the endothelial surface and advanced to create a dissection flap.
RESULTS
As shown in Figure 1, the SFE was easily integrated with standard guide catheters including flexible distal access catheters used in neurointerventional procedures. The radiopaque distal end (scan engine) of the SFE was clearly visualized during fluoroscopy, and the optical image could be observed to ensure that the SFE did not protrude beyond the orifice of the carrying catheter without the need for x-ray validation. Clear images of portions of the endothelium could be generated using modest saline solution flush rates on the order of 50 mL/min, and with proximal control of blood flow using a coaxial balloon guide catheter, completely clear fields of view could be obtained using minimal saline flow rates as shown in Figure 2 (see Video, Supplemental Digital Content 1-2, which demonstrate renal artery inspection and microwire branch selection, http://links.lww.com/NEU/A627 and http://links.lww.com/NEU/A628). The SFE sheath was found to transmit torque relatively poorly to the distal end; however, rotation of the angled-tip guide catheter containing the SFE allowed for inspection of vessel walls and side braches from multiple angles.
FIGURE 2.
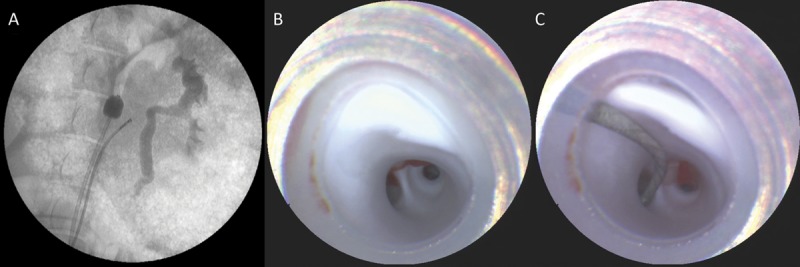
A, fluoroscopic image of the scanning fiber endoscope (SFE) in place in the renal artery with a balloon in the descending aorta for proximal flow control. B, view from the SFE at the end of the guide catheter, showing a smooth white endothelium and the first bifurcation of the renal artery. C, SFE image of renal branch selection using a 0.014-inch microwire.
In smaller arteries where the 6-F guide catheters were nearly occlusive, the balloon guide catheter was found to be unnecessary, and clear imaging was obtained using saline solution flush rates less than 50 mL/min. As shown in Figure 3, the SFE was able to provide clear images of a microcatheter delivering aneurysm coils into a side branch and the high-resolution, real-time imaging was extremely sensitive to coil prolapse and microcatheter push-back without the need for continuous fluoroscopy (see Video, Supplemental Digital Content 3, demonstrating coil placement, prolapse, and detachment, http://links.lww.com/NEU/A629). When examining the high-resolution SFE images, it was noted with surprise that despite fastidious preparation, many of the catheters introduced initially contained numerous microbubbles, which could be seen coalescing and being dispersed into the distal circulation during device deployment, examples of which are shown in Figure 4.
FIGURE 3.
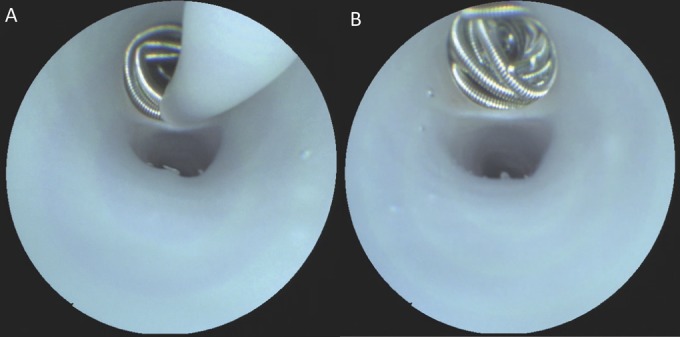
Real-time scanning fiber endoscope images of aneurysm coil delivery (A) into a side branch from a microcatheter having a distal diameter of 2.8-French. B, post-filling inspection of the coil mass demonstrating a hanging detachment zone end and dense packing of coils at the level of the orificium.
FIGURE 4.
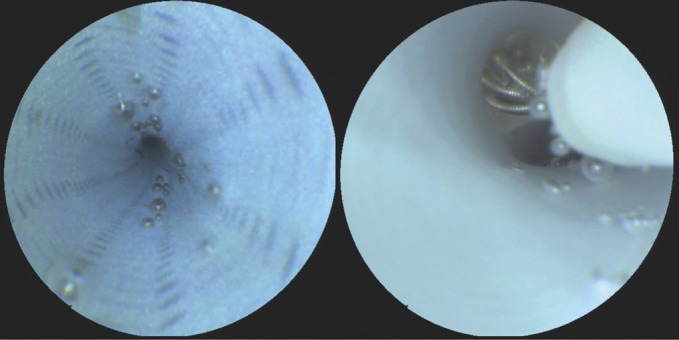
Left, scanning fiber endoscope image demonstrating persistent microbubbles within the lumen of a fastidiously prepared guide catheter that were observed to dislodge randomly with increased saline solution flush rates. Right, image showing the displacement of microbubbles trapped within a flow-diverting stent during device deployment.
Figure 5 demonstrates the ability of the SFE to visualize the key portions of mechanical thrombectomy with a stent retriever, from initial lesion inspection and microwire crossing to the inspection of the stent contents before withdrawal and assessment of residual thrombus and revascularization (see also Video, Supplemental Digital Content 4, demonstrating stent retriever deployment and withdrawal from a microcatheter allowing for visualization of stent tines and incorporated thrombus material, http://links.lww.com/NEU/A630). It was noted that if the SFE and stent retriever were used in the same sheath, then care had to be taken to avoid incorporation of the SFE tip into the tines of the stent during retrieval. Careful withdrawal of both devices under SFE guidance is necessary to avoid interference, which could lead to dislodgment of incorporated thrombus material from the retriever.
FIGURE 5.
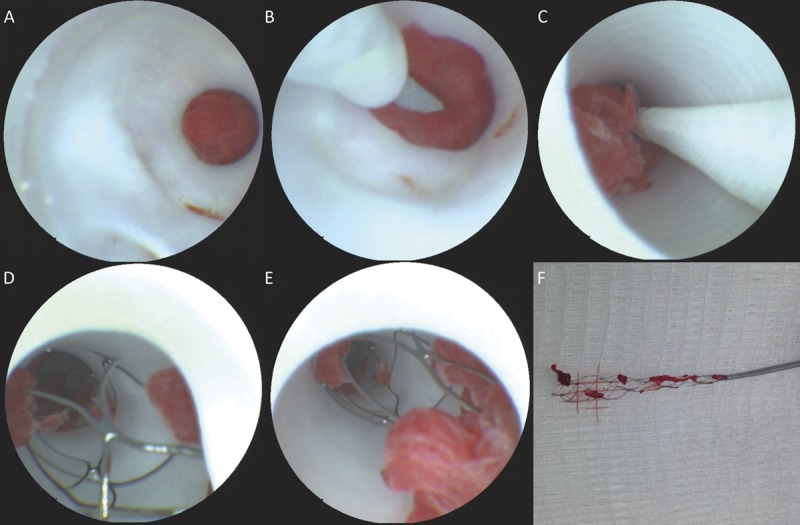
Scanning fiber endoscope images of ischemic stroke thrombectomy from initial lesion inspection (A), microwire crossing (B), initial withdrawal (C), inspection of stent retriever contents in situ (D, E), and ex vivo correlative image of retrieved material (F).
The SFE was found to be very useful in imaging the placement and deployment of flow diverters as shown in Figure 6. After deployment, the angioscopic images were able to clearly assess factors such as wall apposition, stent coverage of the aneurysm base, protrusion of aneurysm coils, and side branch coverage as well as flow-diverter properties (complete opening of the device, determination of tine opening, and size of the cells). Because of the unique manner in which the SFE generates an image, it is able to realize nearly diffraction-limited image resolution assuming negligible aberration effects from the objective lens. The current SFE design has a spatial resolution less than 20 μm, which may be further decreased when using dynamic zoom10 to magnify areas of interest. In standard imaging operation, the SFE can resolve features not visible on fluoroscopy, such as the secondary windings of coils as seen in Figure 3, and can differentiate between the 28- to 33-μm15 cobalt-chromium-nickel and platinum-tungsten braided strands in the pipeline as shown in Figure 6.
FIGURE 6.
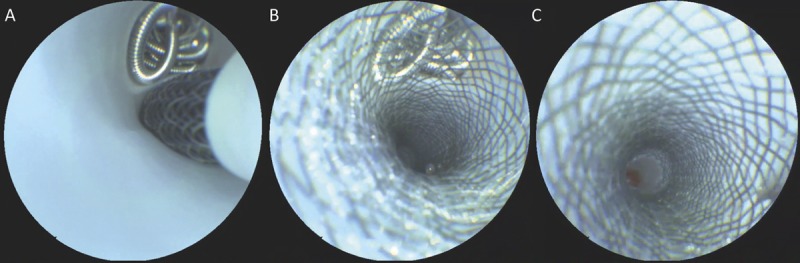
Scanning fiber endoscope imaging of flow-diverter placement over a previously coiled side branch showing placement of the stent during exit from the 2.8-French delivery microcatheter (A), the ability to resolve the number of diverter tines overlying the base of the coiled branch and any coil mass movement (B), and inspection of side-wall apposition, wall kinking, and side-branch patency (C).
Figure 7 illustrates the ability of the SFE to detect endothelial dissections as they are developed and the utility of angioscopic imaging in differentiating the resultant true and false lumen. The SFE was also capable of detecting areas of mild endothelial damage, such as those induced by attempted dissection flap creation, which were not apparent on concurrent fluoroscopy.
FIGURE 7.
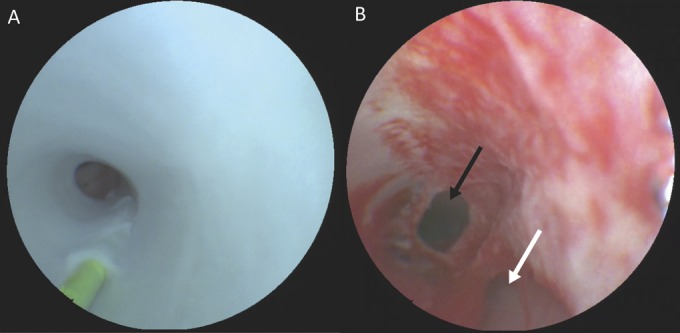
Scanning fiber endoscope images of the development of an endothelial dissection. A, 0.014-inch microwire being applied in reverse showing the proximal entry point of the wire into the subendothelial space as well as the body of the wire tunneling distally. B, fully developed dissection with a damaged endothelium demonstrating the ability to differentiate the true (black arrow) and false (white arrow) lumens using angioscopy.
DISCUSSION
To our knowledge, this is the first report of in vivo, high-resolution (>600 lines) endoluminal imaging during endovascular neurosurgical procedures. In cardiac applications, endoluminal imaging was previously shown to be a useful adjunct to fluoroscopy for detecting vascular pathology and guiding interventions, but the poor image quality produced by conventional fiberoptic angioscopes has greatly limited their use in routine practice.
This study demonstrates the potential of endovascular intracranial imaging with an SFE for research, teaching, and clinical (diagnostic and peri-interventional) applications. From a research point of view, imaging both current and novel endovascular devices in vivo during application and at follow-up would certainly enhance our understanding of both mechanisms of action and biological responses, such as endothelial healing of aneurysms and stents. When used for long-term follow-up in animal experiments, it may be used to monitor this healing process over time without the need to euthanize the animals. The physical properties of the device can be tested in real time in the live animal, and its response to various challenges can be directly imaged.
In this regard, the SFE may also play a role in training physicians in the use of new devices. Currently, training options are based on computer simulations, transparent silicone flow models, or fluoroscopic images of animal models. Although the latter has the inherent problems of only visualizing a 2-dimensional projection of the vessel “cast” or the device deployed, the former models cannot simulate the in vivo properties of vessels, side branches, and so on. Direct inspection of the device and its effects on the vessel will be useful to teach the physician how to use specific devices, what to do, and what to avoid (eg, the effects of torquing a flow diverter can be directly demonstrated).
From a clinical perspective, endoluminal optical imaging may prove useful in diagnostic, peri-interventional, and postinterventional applications.
Diagnostic Utility
In the case of atherosclerosis, a significant body of work already exists describing the utility of angioscopic imaging in assessing plaque risk factors such as evidence of hemorrhage and lipid content,4,16,17 which has been collected primarily with crude angioscopes in the coronary arteries. The greatly increased resolution of the SFE may allow for better definition of the marginal extent of an atherosclerotic plaque within the cervical carotid arteries and intracranial vasculature and, with future human studies, may identify features associated with embolic risk beyond the basic size and color metrics known today. From vessel wall imaging, it is known that the vulnerable plaque has specific features including a thin or ruptured fibrous cap, a lipid-rich core, and intraplaque hemorrhage. Endoluminal imaging with an SFE may allow for improved evaluation of at least some of the salient features compared with current angiography. In the intracranial circulation, it will not only be important to visualize the plaque components but also the proximity of the plaque to perforating vessels.
As shown in the porcine model, dissections and the surrounding normal structures can be visualized directly using the SFE. Arterial intra-/extracranial dissections may become symptomatic with ischemic symptoms due to various pathomechanisms: distal reopening of the endothelial damage will lead to a false lumen that can lead to arterial thromboembolic events due to slow flow and contact with the subendothelial wall. This false lumen may persist and eventually become endothelialized. False aneurysms can form at the level of the dissection with thrombus formation due to recirculating blood, or the true lumen may be compromised to a degree that hemodynamic infarctions occur. If the dissection (ie, the intramural hematoma) extends intracranially, perforating branches may be occluded. Depending on the pathomechanism of stroke in arterial dissections, different therapies may be used to treat these patients, and the SFE may be able to be of diagnostic use in differentiating between the aforementioned cases in addition to angiography. Endoluminal optical imaging may also allow for improved detection of the type of aneurysms (eg, blister, dissection, saccular) and in cases of SAH with aneurysm multiplicity, detection of the culprit lesion.
Periprocedural Applications
Because of its small size, wide-angle field of view, and high resolution, we were able to use the SFE in a coaxial arrangement with a number of endovascular tools. The SFE was extremely useful in guiding the selection of branches with a microwire, as real-time imaging of tip position was often easier to correlate with proximal end manipulations than the fluoroscopic projection imaging, especially in cases of tortuous anatomy. Following a standard fluoroscopic roadmap, it was frequently the case that branches of interest could be selected extremely quickly under SFE guidance alone, with fluoroscopy being used only for confirmation of final position instead of constant use during the procedure. Although the arteries in the porcine model were largely normal, imaging with the SFE could be of significant benefit in guiding attempts to cross complex stenotic lesions such as those of the carotid bifurcation, where the 3-dimensional structure of the lesion and path of the wire may not be readily apparent on 2-dimensional projection images.
As an extension of the navigation assistance previously alluded to, we have shown that endovascular imaging with the SFE is helpful in cases in which anatomic pathologies such as a dissection may make lumen selection with fluoroscopy alone challenging. The high-resolution images provided by the SFE allowed for straightforward diagnosis of a dissection, even in early stages where they were not readily apparent on angiography. The SFE aided significantly in the differentiation between true and false lumens. SFE angioscopy could also be useful in cases of chronic dissection to determine whether the dissected portion has become endothelialized or whether it contains small amounts of thrombus not yet apparent on other imaging modalities. Considering the ability of the SFE to visualize endothelial damage directly, concurrent imaging may also aid in the prevention of iatrogenic dissection creation by allowing the operator to see when devices may be beginning to tunnel under the endothelium far before the lumen changes are visible on fluoroscopy.
In cases of ischemic stroke as modeled here, the SFE is limited to en face imaging of the thrombus and does not provide any depth-resolved imaging as is possible with optical coherence tomography or intravascular ultrasound. As such, we found the greatest utility of angioscopy to be in the inspection of stent retriever integration into the clot and in monitoring the contents of the stent as they were withdrawn from the body for possible detachment and distal embolization. After thrombectomy, even in cases in which flow was returned to normal by angiographic assessment, the SFE was able to detect small areas of residual thrombus that could be removed to prevent future ischemic events, although these were not observed in this initial feasibility study. Combining the SFE with vacuum-based thrombectomy systems may allow for more complete removal of thrombus in such situations. Endoluminal imaging may also prove useful for detecting cases in which the thrombus lodges on underlying intracranial atherosclerotic plaque. Finally, the SFE can be used after stent retriever removal to determine the integrity of the wall, for example, if the endothelium was stripped during the clot retrieval, acetylsalicylic acid treatment may be considered.
Perhaps the most promising initial applications suggested by this work is the use of the SFE in guiding aneurysm therapy using both coils and flow diverters. In the case of aneurysmal rupture, angioscopy could allow the identification of the rupture point and guide the placement of coils to avoid intraprocedural rupture. Similarly, it was noted in our tests that the real-time imaging could identify coil loops on the verge of prolapsing into the vessel lumen and the microcatheter could be adjusted so as to direct those coils back into the body of the aneurysm. As the SFE was generally held outside the lumen of the aneurysm after framing with coils to avoid incorporation with coils, fluoroscopic inspection was generally preferred for the assessment of coil packing inside the aneurysm body. The ability of the SFE to inspect the density of coil packing in the neck of the aneurysm at the completion of coiling, however, far exceeds the limited detail available with fluoroscopy, and the high-resolution imaging is able to detect subtle coil prolapse or loose detachment zone ends that are not readily visible on fluoroscopy.
The addition of endovascular imaging during flow-diverter placement was found to be especially helpful in assessing the degree of coverage of the target lesion, which in turn can inform the need for telescoping multiple-flow diverters to achieve sufficient coverage. The resolution of the images was sufficient to visualize all of the tines of the flow diverter after deployment to the point that a metric such as the number of tine intersections over the aneurysm base could be calculated, perhaps allowing for a more quantitative approach to deciding how much coverage is “enough,” especially in the case of wide-necked aneurysms. In cases in which re-entry was necessary for diverter overlay, the SFE imaging was found to be much more sensitive than fluoroscopy to interference between the delivery catheter and tines of a previously deployed stent. The SFE was also able to assess features such as stent wall apposition, kinking when deployed around a curve, and side-branch patency, which are not possible with fluoroscopy alone. In cases in which an undersized stent was placed in a vessel, angioscopic imaging could demonstrate migration of the stent if pressure was applied using the microcatheter. From a technical standpoint, the SFE proved to be useful in visualizing and guiding the resheathing of the capture coil of the Pipeline device, a procedure that may sometimes be difficult due to tortuous anatomy and large bore of the delivery catheter with kinking of the capture coil at its distal opening. If the flow diverter had anchor tines, the SFE would also have the ability to assess their embedding into the endothelium.
Postprocedural Applications
Endoluminal imaging at the resolution afforded by the SFE may also be useful at follow-up for both coil and flow-diverter therapy assessment. We have demonstrated the ability of the system to image the endothelium in normal and traumatic vessels, and it could similarly be used to assess re-endothelialization of stented or coiled lesions to guide the withdrawal of antiplatelet therapy. In the same way, SFE angioscopy could be used to assess complications such as in-stent restenosis or anchor tine damage in the hope of improving management or identification of possible mechanisms of pathogenesis.
Limitations and Future Applications
Although we believe that these results have demonstrated a number of potential uses for high-resolution angioscopy with an SFE in endovascular applications, the SFE probe design used for these tests is the same general-purpose device used in previous gastrointestinal imaging studies and, as such, could be specifically modified for optimal intracranial use. The major limiting factor is the necessity to carry the SFE, a 3.7-F device, inside a 6-F carrying catheter to provide an atraumatic tip and saline solution flush lumen. Repackaging the device into an integrated assembly specifically designed for endovascular use would greatly reduce the diameter and allow the SFE to access even more distal regions of the circulation and provide more opportunities for use with other devices without the need for bilateral access. The rigid tip portion of the SFE can be redesigned to a 30% reduction in size (0.85-mm diameter and 6.0-mm length) without any sacrifice in performance from the current design. The angle of viewing of the endoscope may also be adjusted from the present 0° arrangement to 30°/45°/90° to allow for oblique viewing of the wall of a vessel or the contents of an aneurysm without needing to exit the lumen of the vessel.
High-quality angioscopic imaging necessitates an essentially blood-free field, and, as such, many of the images presented in this paper were acquired with near-total proximal occlusion to permit inspection of the entire vessel surface simultaneously. In practice, this may be difficult to achieve in regions of intracranial circulation with large collateral supply while keeping flush volumes low. We have observed, however, that laminar blood flow in vessels tends to deflect around the tip of the guide catheter, allowing inspection of a small portion of the vessel wall with the SFE without the need for flow arrest. By rotating the guide catheter, the entire circumference of the vessel can be examined in this way, and a revised catheter tip design may allow large areas to be imaged rapidly in this manner.
Endovascular imaging as proposed here carries with it risks associated with the invasiveness of the procedure and standard endovascular procedure risks, as well as the need for blood flow disruption for clear imaging. The current SFE has a shaft stiffness roughly equivalent to that of a microcatheter and, as such, is not anticipated to increase the possibility of vessel damage when introduced through a guide catheter into the intracranial circulation. However, this will need to be conclusively shown with ex vivo force testing and in vivo histopathological examination before use in humans. The perceived utility of the SFE for tasks such as postcoiling follow-up assessment will require more rigorous comparison with other approaches such as high-resolution computed tomography to evaluate the potential risk-benefit ratio.
It is also worthwhile to note that the SFE represents a novel optical platform in endovascular applications and is not restricted to white-light imaging alone. Simultaneous fluorescence imaging and spectroscopy have previously been demonstrated,18,19 as have SFE probes that are capable of optical coherence tomography scanning.20 In addition to the possibility of multimodal imaging, the central scanning fiber may also be used for the delivery of light for endoluminal photodynamic therapy or higher power applications such as laser plaque ablation or cautery.10 Further technical developments, such as a steerable tip end and integrated working channel, are planned to address particular endovascular tasks.
CONCLUSION
We have demonstrated the feasibility of real-time, high-resolution endovascular imaging during common neurointerventional procedures using a novel SFE. We have shown that the SFE can detect endothelial changes and common procedural complications with much more sensitivity than is possible with fluoroscopy and that the SFE can add useful information during challenging interventional tasks. This work has only begun to demonstrate what may be possible with the fusion of fluoroscopy and next-generation angioscopy, and we anticipate future applications beyond those shown here to leverage the strengths of both modalities to improve patient care.
Disclosure
This work is supported in part by funding from the NIH including grant R01EB016457 and the Canadian Institutes of Health Research grant RMF111623. Dr Seibel is a co-inventor on a number of patents in SFE technology. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgment
The authors acknowledge the engineering expertise of C.D. Melville and R.S. Johnston for the SFE fabrication and technical support.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).
COMMENTS
Recent developments in endoscope technology are about to afford neurointerventionalists with versatile wide-angle, high-resolution endovascular optical imaging instruments. A marked improvement over conventional fiberoptic endoscopes, the scanning fiber endoscope (SFE) achieves greatly reduced device diameters by scanning the laser-illuminated field of view with a single-mode optical fiber,1 which brings microscopic endoluminal imaging modalities into the realm of endovascular neurosurgery and radiology. In a first in vivo exploration, Krings et al have highlighted the potential of the SFE for catheter guidance, identification of arterial dissection, thrombus characterization, and assessment of flow-diverter apposition and coil prolapse.
Intravascular optical and ultrasound probes have found practical use in conjunction with percutaneous coronary angiography,3 but several drawbacks precluded their adoption in the neurovascular domain. Large-diameter probes with rigid material properties impose a prohibitively excessive bending radius for deployment in small and tortuous brain vessels, and their spatial resolution has hitherto been insufficient for adequate image interpretation.4
In contrast to fluoroscopic interrogation, the SFE enables direct visualization of the site of interest, whether in a diagnostic configuration or coaxial with interventional devices, at an unprecedented level of detail, without the need for ionizing radiation and radiographic dyes. This underscores 2 of the foremost reasons to pursue optical angioscopy in conjunction with conventional percutaneous fluoroscopic interventions. The SFE may reduce the need for radiographic guidance of wires and catheters, thereby limiting radiation exposure of patients and clinicians. Moreover, in 2-dimensional fluoroscopy, radiopaque contrast medium generates a lumen cast only. Endovascular probes permit cross-sectional imaging of and beyond the arterial wall, improving detection of diffuse stenosis and compensatory mechanisms that work to maintain luminal integrity.5 Here, the SFE provides an additional view of the intimal surface with a superior level of detail compared with intravascular ultrasound (IVUS). Additionally, modified SFE designs open up opportunities for subsurface tissue imaging,1 although the tissue penetration depth of IVUS probably remains unmatched. The SFE thus provides a novel optical imaging platform that may complement fluoroscopy.
Nevertheless, several technological challenges remain to be solved to ensure safe and practical use that is necessary to facilitate widespread adoption. Feasibility and safety of endoluminal neurovascular imaging are critically contingent on the diameter and flexibility of the endoscope and its sheathing material. Improvements in device sheathing and packaging are needed to ensure atraumatic deployment without the protection afforded by a guide catheter, which in turn would offer possibilities to carry other devices concurrently and enable distal intracranial access to smaller vessels. Furthermore, good visibility requires a blood-free field of view that is achieved with a blood-displacing flush with saline solution or a more viscous fluid, possibly in combination with proximal occlusion using a balloon catheter. These procedures may carry the risk of ischemic events, especially in cases of prolonged flow arrest and in vascular territories that have a limited capacity for collateral flow compensation. For the SFE to move forward into a reliable and safe neurovascular imaging modality, care needs to be taken to prove both patient safety and superior diagnostic value. Although Krings et al have demonstrated the exciting capabilities of current endoscope technology and the advantages of the SFE when used during neurovascular interventions, it seems that with minor adjustments, most practical concerns can be addressed to establish its clinical value for identification and assessment of cerebrovascular pathologies. Multimodal vascular imaging, combining angioscopy and angiography, may then become a versatile strategy to provide a safe and robust platform for maximal image guidance and diagnostic potential with minimal radiation exposure.
Kajo van der Marel
Matthew J. Gounis
Worcester, Massachusetts
- 1.Lee CM, Engelbrecht CJ, Soper TD, Helmchen F, Seibel EJ. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J Biophotonics. 2010;3(5-6):385-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.High resolution angioscopic imaging during endovascular neurosurgery. Neurosurgery. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida Y. Recent advances in coronary angioscopy. J Cardiol. 2011;57(1):18-30 [DOI] [PubMed] [Google Scholar]
- 4.Massoud TF, Murayama Y, Viñuela F, Utsumi A. Laboratory evaluation of a microangioscope for potential percutaneous cerebrovascular applications. AJNR Am J Neuroradiol. 2001;22(2):363-365 [PMC free article] [PubMed] [Google Scholar]
- 5.Regar E, Weissman NJ, Muhlestein JB. Intravascular ultrasound, optical coherence tomography, and angioscopy of coronary circulation. UpToDate.com. Available at: http://www.uptodate.com/contents/intravascular-ultrasound-optical-coherence-tomography-and-angioscopy-of-coronary-circulation. Accessed April 17, 2014
Today neuroendovascular therapy is based on roentgen fluoroscopy, which allows the control of microcatheters, wires, and various devices and implants to a level that is mostly sufficient from a clinical standpoint. Although the performance of many procedures is possible with the use of high-resolution 2-dimensional digital subtraction angiography (DSA) and fluoroscopic imaging, significant progress of interventional imaging has been achieved during the past 2 decades. Biplane DSA, high-quality road map, rotational angiography with 3-dimensional rendering and flat-panel computed tomography are today considered standard of care and have become a source of valuable information. However, all these are roentgen imaging modalities leaving the operator blind to everything that is not radiopaque.
The interaction between thrombus and stent retriever is still poorly understood. High-resolution angioscopy has the potential to provide better insight into this process and may help to improve devices for mechanical thrombectomy.
We found Figure 4 most interesting. The authors demonstrate the displacement of air bubbles trapped within a flow-diverting stent during deployment. This observation might be related to the infrequent but potentially fatal parenchymal hemorrhages, which have been encountered after flow-diverter implantation. One of the explanations for this disaster is as follows. Small air bubbles are trapped between the struts of the flow diverter. During the deployment of the implant, these bubbles are released into the bloodstream. In the dependent vasculature, small vessels and capillaries are blocked by these bubbles, which may cause small infarcts, and these infarcts may trigger the hemorrhage. This hypothesis is partly supported by the observation of the authors. As a consequence, efficacious ways to avoid a shower of air bubbles during the deployment of a flow diverter might help to prevent subsequent parenchymal hemorrhages. The obvious challenge is how to incorporate such technology into daily clinical practice and the justification of its use given its cost/benefit implications. One possibility is that the understanding provided by this technology will lead to evolution in device development and/or use so as to avoid such complications.
Hans Henkes
Stuttgart, Germany
REFERENCES
- 1.Stonebridge P, Murie J. Angioscopy—a new light on peripheral vascular-disease. Eur J Vasc Surg. 1992;6(4):346-353 [DOI] [PubMed] [Google Scholar]
- 2.Tokuhiro K, Uchida Y, Kawamura K, et al. Evaluation of annuloaortic ectasia by angioscopy and IVUS “report of 2 cases”. Diagn Ther Endosc. 2000;7(1):35-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsagakis K, Kamler M, Benedik J, Jakob H. Angioscopy—a valuable tool in guiding hybrid stent grafting and decision making during type A aortic dissection surgery. Eur J Cardiothoracic Surg. 2010;38(4):507-509 [DOI] [PubMed] [Google Scholar]
- 4.Uchida Y. Recent advances in coronary angioscopy. J Cardiol. 2011;57(1):18-30 [DOI] [PubMed] [Google Scholar]
- 5.Ishihara T, Iida O, Awata M, Nanto K, Nanto S, Uematsu M. Angioscopic assessment of early phase arterial repair after paclitaxel-coated nitinol drug-eluting stent implantation in the superficial femoral artery. Circ J. 2013;77(7):1838-1843 [DOI] [PubMed] [Google Scholar]
- 6.Tanemura H, Hatazaki S, Asakura F, et al. Angioscopic observation during carotid angioplasty with stent placement. AJNR Am J Neuroradiol. 2005;26(8):1943-1948 [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Yoshimura T, Nakamura D, et al. Comparison of angioscopic findings and three-year cardiac events between sirolimus-eluting stent and bare-metal stent in acute myocardial infarction. Am J Cardiol. 2011;108(9):1238-1243 [DOI] [PubMed] [Google Scholar]
- 8.Miskolczi L, Flaherty J, Guterman L, Hopkins L. Carbon dioxide column angioscopy: a new endovascular imaging technique. AJNR Am J Neuroradiol. 2001;22(10):1849-1853 [PMC free article] [PubMed] [Google Scholar]
- 9.Lanzino G, Miskolczi L, Guterman L, Hopkins L. Angioscopy-assisted aneurysm clipping. Neurosurgery. 1999;45(3):609-613 [DOI] [PubMed] [Google Scholar]
- 10.Lee CM, Engelbrecht CJ, Soper TD, Helmchen F, Seibel EJ. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J Biophotonics. 2010;3(5-6):385-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seibel EJ, Carroll RE, Dominitz JA, et al. Tethered capsule endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett’s esophagus. IEEE Trans Biomed Eng. 2008;55(3):1032-1042 [DOI] [PubMed] [Google Scholar]
- 12.Templeton AW, Webb K, Hwang JH, Seibel EJ, Saunders M. Scanning fiber endoscopy: a novel platform for cholangioscopy. Gastrointest Endosc. 2014. (In press). [DOI] [PubMed] [Google Scholar]
- 13.Soper TD, Haynor DR, Glenny RW, Seibel EJ. In vivo validation of a hybrid tracking system for navigation of an ultrathin bronchoscope within peripheral airways. IEEE Trans Biomed Eng. 2010;57(3):736-745 [DOI] [PubMed] [Google Scholar]
- 14.Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2006;27(6):1357-1361 [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011;32(1):34-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiruta N, Uchida Y, Maezawa Y, Shimoyama E, Uchida Y. Molecular imaging of apolipoprotein B-100 in human coronary plaques by color fluorescent angioscopy and microscopy. Int Heart J. 2013;54(2):68-74 [DOI] [PubMed] [Google Scholar]
- 17.Uchida Y, Maezawa Y, Uchida Y, Hiruta N, Shimoyama E. Molecular imaging of low-density lipoprotein in human coronary plaques by color fluorescent angioscopy and microscopy. Plos One. 2012;7(11):e50678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Hou V, Nelson LY, Seibel EJ. Mitigating fluorescence spectral overlap in wide-field endoscopic imaging. J Biomed Opt. 2013;18(8):086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Kim AS, Ridge JS, Nelson LY, Berg JH, Seibel EJ. Trimodal detection of early childhood caries using laser light scanning and fluorescence spectroscopy: clinical prototype. J Biomed Opt. 2013;18(11):111412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo L, Xi J, Wu Y, Li X. Forward-viewing resonant fiber-optic scanning endoscope of appropriate scanning speed for 3D OCT imaging. Opt Express. 2010;18(14):14375-14384 [DOI] [PMC free article] [PubMed] [Google Scholar]


