Abstract
Beta-blockers are commonly used during the first trimester of pregnancy. Data regarding risks of congenital anomalies in offspring have not been summarized. We performed a meta-analysis to determine teratogenicity of beta-blockers in early pregnancy. A systematic literature search was performed using PubMed, EMBASE, Cochrane Clinical Trials, and hand search. Meta-analyses were conducted using random-effects models based on odds ratios (ORs). Pre-specified subgroup analyses were performed to explore heterogeneity. Randomized controlled trials or observational studies examining risks of congenital malformations associated with first trimester beta-blocker exposure compared to no exposure were included. Thirteen population-based case-control or cohort studies were identified. Based on meta-analyses, first trimester oral beta-blocker use showed no increased odds of all or major congenital anomalies (OR = 1.00; 95% CI: 0.91 – 1.10, five studies). However, in analyses examining organ-specific malformations, increased odds of cardiovascular (CV) defects (OR = 2.01; 95% CI: 1.18 – 3.42; 4 studies), cleft lip/palate (CL/P) (OR = 3.11; 95% CI: 1.79 – 5.43; 2 studies) and neural tube (NT) defects (OR = 3.56; 95% CI: 1.19 – 10.67; 2 studies) were observed. The effects on severe hypospadias were non-significant (1 study). Causality is difficult to interpret given small number of heterogeneous studies and possibility of biases. Given the frequency of this exposure in pregnancy, further research is needed.
Keywords: Beta-blockers, first trimester, pregnancy, congenital anomalies, heart defects, cleft lip/palate, neural tube defects
INTRODUCTION
There has been a rapid rise in the use of anti-hypertensive medications in pregnancy during the past decade.1,2 Recent data demonstrate that the most common 1st trimester antihypertensive exposure is beta adrenergic blocking agents, with nearly 0.5% of all pregnant women exposed to these medications during this trimester.1,2
The most concerning potential adverse effect of first-trimester medication exposure is teratogenicity. Each year, approximately 3% of infants are born with serious birth defects;3 malformations are the leading cause of infant mortality in the U.S.4 Most beta-blockers are designated by the United States Food and Drug Administration as class C,5 meaning that animal studies have demonstrated adverse fetal effects but there are no adequate or well-controlled studies in humans.
Despite the frequency of this exposure, data regarding risks of fetal congenital anomalies associated with 1st trimester use of oral beta-blockers have not previously been summarized. We, therefore, undertook this systematic review and meta-analysis to combine data from existing randomized controlled trials (RCTs), cohort and case-control studies to answer the hypothesis that first-trimester beta-blocker exposure may be associated with birth defects.
METHODS
Search Strategy
The search engines used included PUBMED (1966-August 2011), EMBASE (1982 to August 2011), Cochrane Clinical Trials, controlled-trials.com, and clinicaltrials.gov to identify all published studies on beta-blocker use and congenital anomalies in all languages. References of selected articles were also hand searched to ensure all possible articles were captured.
Combinations of MeSH and text words in our search string in PubMed and EMBASE included: antihypertensive agent/therapy, beta-adrenergic receptor blocking agent/beta-blocker/beta-antagonist/adrenergic beta-3 receptor antagonists/adrenergic beta-2 receptor antagonists/adrenergic beta-1 receptor antagonists, anti-adrenergic, anti-anxiety agents, generic names of all beta blockers AND pregnancy/pregnant woman/pregnan* AND congenital disorder/congenital abnormality/congenital anomaly/congenital malformation or birth defects/deformit*. The Cochrane Library, clinicaltrials.gov, and controlled-trials.com were searched with similar search strings. No limits were applied to any of these searches. MOOSE guidelines6 were followed.
Inclusion/Exclusion criteria
All available RCTs, cohort, and case-control studies were selected. The inclusion criteria were exposure of pregnant women to one or more oral beta-adrenergic receptor blocking agents during the first trimester of pregnancy versus no use of these drugs in this time frame, and an outcome measure of one or more congenital anomalies. We excluded studies that were cross-sectional, descriptive or case series/reports. Studies examining treatment of hypertensive disorders of late pregnancy including gestational hypertension and preeclampsia/eclampsia were excluded, as late pregnancy exposures are beyond the etiologically relevant gestational period. We also excluded studies in which subjects used beta-blockers to treat thyroid disorders, as these disorders may be independently associated with congenital anomalies.
Selection and Quality Assessment
The titles and abstracts were reviewed independently by two reviewers (R.A.H. and M.Y.Y.), who then retrieved all potential full text manuscripts based on abstracts. Non-English articles meeting eligibility criteria were translated into English using software available online. Authors were contacted for clarification in circumstances where data were not clear or were difficult to interpret (see Results section). The reviewers (R.A.H. and M.Y.Y.) also independently assessed study quality based on criteria determined by all authors. The most important two factors that were thought to potentially influence study validity and quality were 1) whether the study excluded or adjusted for pre-existing diabetes mellitus (DM) (as DM may be associated with beta-blocker use and is independently associated with congenital anomalies); and 2) potential for recall bias (in which women with affected babies could be more likely to recall exposure to drugs). Possible recall bias was assessed based on whether data collection involved retrospective maternal interviews or self-report of beta-blocker exposure after the outcome had occurred, compared to data collection that was prospective or relied on pre-existing prenatal medication records (where recall bias would be unlikely).
Data Extraction
Two authors (R.A.H. and M.Y.Y.) independently extracted data from original full text articles using a standardized data collection form. Data extracted included study type, data source, study location, primary indication for beta-blocker use, timing of exposure, class(es) of malformation(s), confounders adjusted for, sample size, ORs (adjusted if available) and 95% confidence intervals (CIs), study exclusion/adjustment for diabetes, and potential for recall bias. Extracted data were compared and discrepancies resolved by discussion among all authors. Where multiple articles existed from a single database, the one with the most complete and/or recent data was used. Data from studies having multiple datasets using the same control group were adjusted for potential multiple comparison issue7 by performing sensitivity analyses to confirm the robustness of results.
Statistical Analysis
The primary outcome analyzed was all congenital anomalies. Various organ-specific anomalies were also studied, including cardiovascular (CV) defects, cleft lip/palate (CL/P), neural tube (NT) defects, and severe hypospadias. These organ-specific outcomes were decided post hoc based on available data. Odds ratio was the a priori metameter of choice given expectations that most studies would be case-control in design. When stated/available in the manuscripts, adjusted ORs were used. If adjusted ORs were not available, raw numbers were used to compute ORs with 95% CIs. The included studies were meta-analyzed (separately for studies that analyzed all or major malformations overall and studies that specified organ system-specific anomalies, as appropriate). The specific beta-blocker medication varied or was often not stated. DerSimonian-Laird random effects was the a priori model of choice given our assumption of high heterogeneity. Heterogeneity among studies was determined using visual inspection of forest plots and I2 statistic. An I2 value of > 30% was taken to indicate substantial heterogeneity.
Pre-specified sub-group analyses were performed for outcomes where substantial heterogeneity was found. This was based on the main quality criterion of study adjustment or exclusion of diabetics. The studies were also stratified according to potential for recall bias and indication of beta-blocker use. Publication bias was analyzed by visual inspection of funnel plots and the Egger's test. A two-tailed p-value of < 0.05 was considered to indicate publication bias. If such bias was found, a trim-and-fill plot was used to address potential missing studies and to obtain pooled estimates after adjusting for this bias.
We used three data sets from Puho et al.8 for the CL/P analysis (all with the same control group) and two data sets from Medveczky et al.9 for the NT defects analysis (both with the same control group). We, therefore, performed sensitivity analyses, removing two studies at a time for the CL/P analysis and one study at a time for the NT defects analysis to assess overall robustness of the results after accounting for this multiple comparison issue.
Power calculations were performed post-hoc after all studies had been collected using methodology described by Cafri et al.10 The power was 96% to detect an OR of 1.20 for all or major anomalies, 72.1% for an OR of 2.00 for CV defects, 97.9% for an OR of 3.10 for CL/P and 60.9% for an OR of 3.50 for NT defects. For details about the macro and SAS code used, refer to the Supplemental Material.
Meta-analyses were conducted in Review Manager Version 5.0 (RevMan Version 5.0, Cochrane Collaboration, 2008).
RESULTS
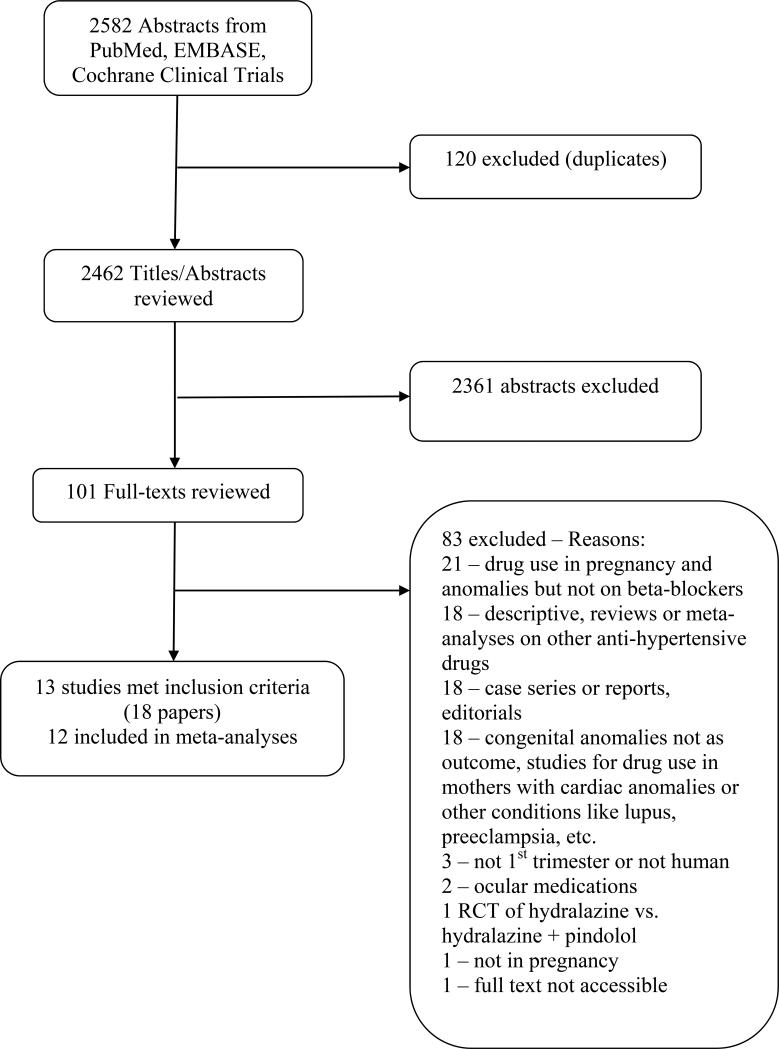
Electronic searches identified 2,582 citations; 2,462 citations remained after duplicates were removed. After title and abstract screening, 101 abstracts were selected for full text review and thirteen (nine case-control8, 9, 11-19 and four cohort20-23) studies met final inclusion criteria. We did not identify any published RCTs. The search flow diagram is given in Figure 1. Details of included studies, including quality grading, are given in Table 1. Sipek et al.19 , written in Czech, was translated into English, but was excluded because the author did not respond to queries regarding numbers and interpretation of study results. Furthermore, the Zagreb-based part of Eric et al.22 was excluded because timing of beta-blocker exposure could not be determined. The exposure comparison in all remaining included studies was use of oral beta-blockers versus none. All studies were conducted in developed countries: three in the US,12, 13, 20 three in Hungary,8, 9, 11, three in Sweden14, 21, 23 and one in Canada,15-17 Germany,18 and Serbia.22 The timing of exposure was first trimester in all studies. In the Puho study8, however, a portion of the data (on posterior cleft palate) reported use in the third and fourth months of pregnancy, just past the first trimester. This study utilized a slightly later definition of the window of teratogenicity due to embryologic timing of palatal fusion (and therefore, cleft palate), and so was included. The indication for beta-blockers was hypertension alone in six studies,8, 12, 13, 15-17, 20, 23 hypertension and other diseases in two studies11, 22 and unspecified/not given in the remaining 4 studies9, 14, 18, 21 (Table 1).
Figure 1.
Flow diagram of literature search.
Table 1.
Characteristics of 12 studies that examine the association between beta-blocker exposure and congenital malformations* (CC=case-control, RC=retrospective cohort, PC= prospective cohort).
| Author, Year | Study design | Country/Data source | Specific beta-blocker | Period of pregnancy of drug use | Indication of drug use | Class of anomalies | Patients with DM excluded/adj usted† | Potential recall bias |
|---|---|---|---|---|---|---|---|---|
| Banhidy 201111 |
CC | Hungary: Hungarian Congenital Abnormality Registry (HCAR), maternal information: prospective medical records, retrospective self-report |
Metoprolol, oxprenolol, pindolol, propranolol |
Included 1st trimester |
Chronic HTN/other indications |
All within 3 months of live birth excluding genetic or chromosomal aberrations |
Not clear | Yes |
| Caton 200912 |
CC | USA: Population-based birth defects surveillance systems; maternal information: interview |
Any | 1 month preconception through the third month of pregnancy |
HTN | CV excluding patent ductus arteriosus (PDA), patent foramen ovale, or recognized single gene or chromosome abnormalities |
Yes | Yes |
| Caton 200813 |
CC | USA: Population- based birth defects surveillance systems; maternal information: interview |
Any | 1 month preconception through month 4 post- conception |
HTN | Severe hypospadias excluding chromosome/gene abnormality or intersex condition |
Yes | Yes |
| Cedergren 2002 14 |
CC | Sweden: Swedish Register Study, Child Cardiology Register, Medical Birth Register, medical records |
Any | 10 - 12 weeks | Not specified |
Cardiac defects referred within one year of birth, excluding PDA and single umbilical artery |
No | No |
| Medveczky 2004a 9 |
CC | Hungary: HCAR; exposure data: retrospective mailed questionnaire, prenatal log/nurse visits |
Oxprenolol | Second month | Not specified |
Non-syndromic NT defects within 3 months of birth or pregnancy termination |
Yes | Yes |
| Medveczky 2004b 9 |
CC | Hungary: HCAR; exposure data: retrospective mailed questionnaire, prenatal log/nurse visits |
Pindolol | Second month | Not specified |
Non-syndromic NT defects within 3 months of birth or pregnancy termination |
Yes | Yes |
| Nakhai- Pour 201015-17 |
CC | Canada: Quebec Pregnancy Registry |
Selective and non- selective |
First trimester | HTN | Major congenital malformations within first year of life |
Yes | No |
| Puho 2006a8 |
CC | Hungary: HCAR, maternal information: prospective medical records and retrospective self-report |
Metoprolol | Third to fourth months§ |
HTN | Posterior cleft palate within 3 months of birth or pregnancy termination |
Yes | Yes |
| Puho 2006b8 |
CC | Hungary: HCAR, maternal information: prospective medical records and retrospective self-report |
Oxprenolol | Third to fourth months§ |
HTN | Posterior cleft palate within 3 months of birth or pregnancy termination |
Yes | Yes |
| Puho 2006c8 |
CC | Hungary: HCAR, maternal information: prospective medical records and retrospective self-report |
Oxprenolol | Second to third months |
HTN | Cleft lip and palate 3 months of birth or pregnancy termination |
Yes | Yes |
| Quiesser- Luft 1996 18 |
CC | Germany: Mainz birth defect monitoring system, maternal & infant medical records |
Any | First three months |
Not specified |
At least one major malformation within first week of life, excluding spontaneous abortions |
No | No |
| Davis 201120 |
RC | USA: HMO Research Network records |
Any | First trimester | HTN | All congenital anomalies within first year of life |
No | No |
| Eric 2009 22 |
PC | Serbia: Maternal questionnaire, neonatal or fetal physical or pathologic examination |
Propranolol and metoprolol |
First trimester | CV disorders |
Minor or major anomalies at birth |
No | Yes |
| Kallen 200321‡ |
RC | Sweden: Swedish Medical Registry |
Any | first trimester | Not specified |
CV (except PDA and single umbilical artery) |
No | No |
| Lennestal 200923 |
RC | Sweden: Swedish Medical Birth Register, Congenital Malformation Register, Hospital Discharge Register |
Any | Early pregnancy |
HTN | CV | Yes | No |
Sipek et al.19 is excluded from the table.
Whether diabetes was excluded/adjusted in any analyses in the paper. For specific information on beta-blocker use, see Supplemental Table S1.
This study not included in the meta-analysis; more recent study by Lennestal et al. with extended follow-up using the same data set is included.
3rd to 4th months of pregnancy included due to timing of fetal palate formation.
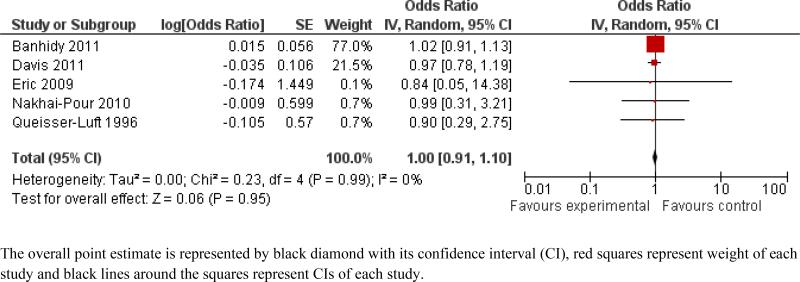
Details of data used for the meta-analyses are given in Supplemental Table S1. Based on the meta-analysis of the five studies that analyzed all or major malformations (not organ-specific), use of beta-blockers during the first trimester of pregnancy was not associated with increased odds in the random effects model (OR = 1.00, 95% CI: 0.91 – 1.10) (Figure 2). There was no evidence of heterogeneity (I2 = 0%) or publication bias (Egger's p = 0.32). None of these studies adjusted or excluded for diabetes (adjustment status of one study11 was not clear), so subgroup analysis with this quality factor could not be performed. Studies with potential for recall bias showed similar results (OR = 1.01; 95% CI: 0.91 – 1.13) to studies that did not have this possibility (OR = 0.96; 95% CI: 0.79 – 1.18) (see Supplemental Figure S1). Studies using “hypertension only” as indication for beta-blocker use showed similar results (OR = 0.97; 95% CI: 0.79 – 1.19) to studies where indication was “hypertension and other cardiovascular disorders” (OR = 1.01; 95% CI: 0.91 – 1.13) and where indication was “unspecified” (OR = 0.90; 95% CI: 0.29 – 2.75) (see Supplemental Figure S2).
Figure 2.
Meta-analysis of the association between beta-blocker exposure in first trimester of pregnancy and all or major congenital anomalies.
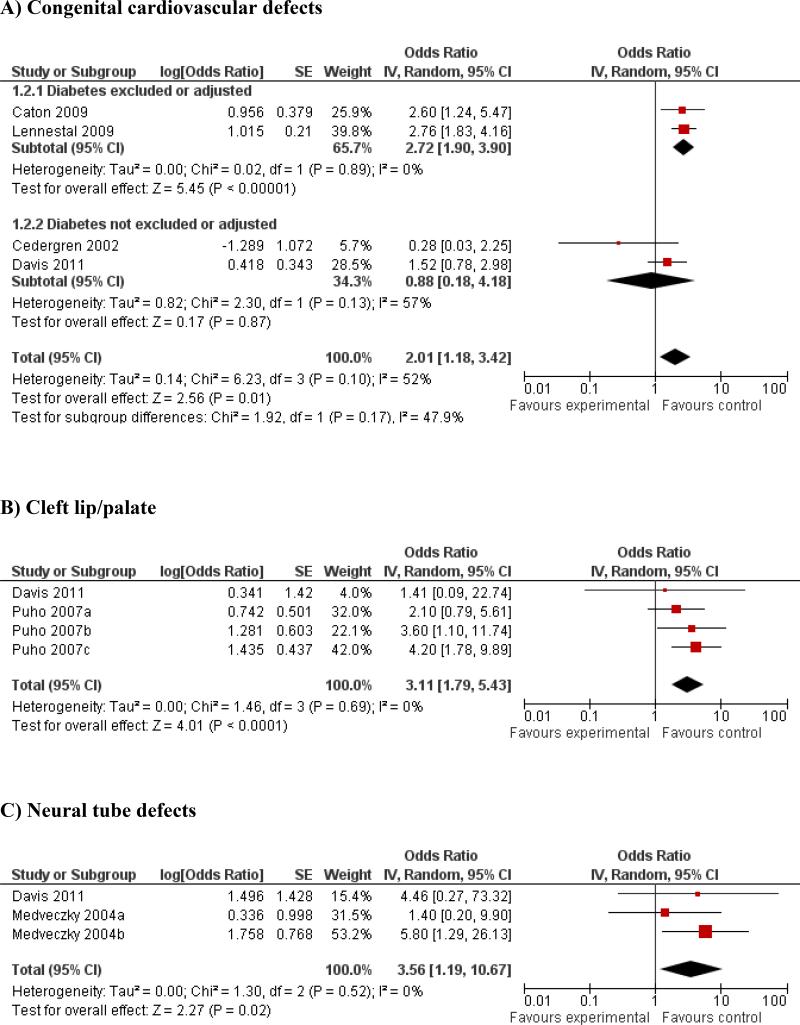
Studies that detailed organ-specific malformations were each meta-analyzed by organ system. In these post hoc secondary analyses, there was a statistically significant increase in odds of cardiovascular abnormalities (OR = 2.01; 95% CI: 1.18 – 3.42; 4 studies). There was evidence of substantial heterogeneity (I2=52%), but no evidence of publication bias (Egger's p = 0.13). Subgroup analyses of studies that excluded/adjusted for diabetes remained significant (OR = 2.72; 95% CI: 1.90 – 3.90) (Figure 3A), and this quality factor accounted to some extent for heterogeneity. However, there was no association with beta-blocker use and cardiovascular anomalies noted among studies that did not exclude/adjust for diabetes (OR = 0.88; 95% CI: 0.18 – 4.18) (Figure 3A). Studies with potential for recall bias showed significant association with CV anomalies (OR = 2.60; 95% CI: 1.24 – 5.47), while studies that used prospectively collected data showed no association (OR = 1.67; 95% CI: 0.75 – 3.71) (see Supplemental Figure S3). To further explore this heterogeneity, we stratified studies according to indication of beta-blocker use. Studies where “hypertension” was the main indication showed statistical significance (OR = 2.36; 95% CI: 1.67 – 3.34), compared with “unspecified” indications (OR = 0.28; 95% CI: 0.03 – 2.25), with reduced heterogeneity in sub-groups (see Supplemental Figure S4).
Figure 3.
Meta-analyses of the association between beta-blocker exposure in first trimester of pregnancy and A) congenital cardiovascular defects; B) cleft lip/palate; C) neural tube defects.
The results for CL/P (OR = 3.11; 95% CI: 1.79 – 5.43; 2 studies) and for NT defects (OR = 3.56; 95% CI: 1.19 – 10.67, 2 studies) were also statistically significant (Figure 3 B & C). There was no evidence of heterogeneity or publication bias. The association of beta-blockers with severe hypospadias (OR = 2.27; 95% CI: 0.69 – 7.46) was statistically non-significant based on a single study.13 No subgroup analyses could be performed for these outcomes because of very few studies.
Sensitivity analyses
After removing two studies at a time for the CL/P analysis, retaining only either Puho (b) or Puho (c) data sets with Davis et al., the results were still statistically significant. However, when analyzing only the Puho (a) data set with Davis et al. the results became statistically non-significant. This indicates that two of three data sets were influential and that the results largely remained significant even after accounting for double counting of controls. However, for NT defects, removal of the Medveczky (a) data set made the results non-significant, while removing the Medveczky (b) data resulted in retained significance. This analysis is susceptible to the multiple comparison issue, and findings are less robust than that of CL/P.
DISCUSSION
The rate of antihypertensive use in pregnancy is rapidly escalating. Beta-blockers are the most common antihypertensive used during the 1st trimester, with approximately 1 in 200 pregnant women exposed to these agents.1,2 Our systematic search found 13 case-control and cohort studies that examine this issue. Our meta-analyses incorporating 12 of these studies showed that use of beta-blockers during the first trimester of pregnancy was associated with increased odds of CV anomalies, CL/P, and NT defects, although the primary outcome of all or major congenital anomalies was non-significant.
There are a few explanations for our findings of positive organ-specific associations without an overall increase in odds of all anomalies. The first is that these organ-specific effects are real and get diluted when we pool other anomalies that are not increased. This seems unlikely to fully account for our findings given that these organ-specific anomalies form a significant proportion of all anomalies. An alternative explanation is publication bias for organ-specific anomalies. Although the formal publication bias statistical tests for this outcome were non-significant, these tests are severely underpowered given small numbers of studies. It is also notable that the studies included in the analysis of overall malformations and organ-specific anomalies are, with the exception of two studies,8, 9, 11, 20 different. One of these two studies20 did not show significant results for either overall or organ-specific anomalies. Additional potential explanations include differences in the populations studied, type and dose of beta blockers used, potential differential misclassification of exposure in retrospective case-control studies, or the accuracy with which malformations are detected. Irrespective of the cause, the findings of strong associations with particular malformations provide a powerful incentive to conduct more research in this area.
The sub-group analyses for CV defects highlight some of the factors that may explain heterogeneity and potential sources of bias. The studies that excluded diabetes remained significant, while the subgroup with no adjustment/exclusion of diabetes was non-significant. It is difficult to explain these non-significant findings given the known association between diabetes and malformations; chance may play some role in the patterns we observe here. In other sub-group analyses, there were statistically significant results for studies with potential recall bias compared to non-significant results for studies that did not have a possibility of this bias. This might be due to an element of differential misclassification, shifting the point estimate away from null in retrospective studies. However, we do not actually know if there truly was recall bias, just that there was a possibility; it could also be non-differential misclassification in prospective studies attenuating the results towards null. The CV studies that had hypertension as indication showed significant results, compared to non-significant result of study where indication was not specified. But we assume that even when indication was unspecified, the majority of subjects would still actually be using these drugs for hypertension, so this analysis is likely somewhat artificial. Furthermore, absence of statistical significance in any of these analyses could also be due to lack of power.
To the best of our knowledge, this is the first meta-analysis in the literature examining the association of oral beta-blocker usage in the first trimester of pregnancy on congenital anomalies. The strengths of this analysis include a comprehensive search strategy designed to identify all pertinent data on this subject, careful extraction of study data by multiple authors, and rigorous statistical methodology. An additional strength is our careful attention to the potential confounding role of diabetes. This is important given the known association of poorly controlled maternal diabetes and congenital anomalies in offspring 24-28 and the co-existence of chronic hypertension and diabetes as part of the “metabolic syndrome”. 29 Further, the studies upon which we based these analyses were generally population-based with large sample sizes.
This review, however, does have some limitations. Six included studies were potentially subject to recall bias, in which exposure information and prenatal medication usage was collected through maternal interviews after delivery. Because the outcome had already occurred, mothers of infants with congenital malformations may be more likely to recall the exposure (the use of beta-blockers), thus introducing potential recall bias and differential misclassification. Recording bias would have the same effect. Retrospective interview-based studies tended to find associations while prospective prescription-based data did not, that can also be because women in the prescription database stopped using their prescriptions, leading to non-differential misclassification and attenuating the estimate towards the null. Most studies did not include miscarriages, thus introducing an element of survivor bias that may affect generalizability of the results.
In addition, most studies do not clearly report the indication for beta-blocker use. Hypertension, which is the leading indication for beta-blockers, may itself be associated with congenital malformations30 and act as a confounder. None of the studies compared the risk of malformations in beta-blockers to alternative treatments for hypertension such as methyldopa, calcium channel blockers, or diuretics; this is an important limitation to the available literature and an important focus for future research. There is a small significant increased risk shown in some studies of hypertension itself (41% for CV defects and 43% for NT defects),30 but our estimates are much stronger than these reported estimates, which may indicate risk over and above that of underlying hypertension. Women taking beta-blockers may be taking concurrent medications, but this information was not available from the included studies.
The studies had variability in timing of exposure within the first trimester (Table 1). Also, it was not reported whether the exposure was continued in subsequent trimesters. There were little to no data describing dosages or frequency of beta-blocker use and many papers did not report which specific beta-blockers were utilized. The small number of studies in each category precluded sub-group analyses according to beta-blocker type. It is also noteworthy that the significant CL/P and NT defect results were primarily driven by the use of oxprenolol. This non-selective lipophilic beta-blocker is no longer frequently used, and it should be noted that all papers documenting its use were from Hungary, with data prior to 1997. Therefore, the inclusion of this drug may limit applicability of the findings. It is not known if it had differential association with CL/P and NT defects compared to other beta-blockers, particularly lipophilic ones such as metoprolol, pindolol, propranolol or labetolol; although there is no evidence that its mechanism of action is different from other beta-blockers. Finally, all studies were performed in developed countries with largely Caucasian populations. However, there is no reason to postulate that racially/ethnically dissimilar populations would have different teratogenic responses to beta-blockers.
Our meta-analysis suggests an increased risk of cardiovascular, orofacial, and NT defects with oral beta-blocker exposure during the first trimester of pregnancy. The strength and causality of this association is difficult to ascertain due to the limited number of published studies, heterogeneity between studies, and potential biases, particularly confounding by indication and/or publication bias. In the future, more accurate and complete data should be collected regarding beta-blocker use, timing of exposure, and confounders to further study this, preferably in the setting of large scale observational studies, if possible. Future studies should also compare beta-blockers with other anti-hypertensives, and to dissociate the effect of underlying hypertension from beta-blocker use, by incorporating untreated hypertensive controls as a comparison group.
PERSPECTIVES
In conclusion, our meta-analysis of available data showed no increase in overall congenital malformations associated with first-trimester exposure to beta-blockers. However, in organ-specific analyses, a two-fold increase in the risk of cardiovascular defects and more than three-fold increase in oral clefts and NT defects were found. These organ-specific findings may either be true associations given their magnitude, or be attributable to publication bias or potential differential misclassification of exposure, for which further research is warranted given the frequency of exposure to these medications in early pregnancy. The current literature assessing risk is limited by lack of comparisons with alternative anti-hypertensives and untreated hypertension, and future research should address this deficit. Given the increasing incidence of hypertension, more information is needed to ensure that healthcare providers treat hypertensive pregnant women, as well as those with the potential to become pregnant, with the least teratogenic anti-hypertensive available.
Supplementary Material
Novelty and Significance.
- What is New?
- Data regarding teratogenic risks of 1st trimester use of oral beta-blockers have not previously been summarized.
- What is Relevant?
- In organ-specific meta-analyses, increased odds of CV defects, cleft lip/palate, and NT defects were observed.
- Summary
- This meta-analysis suggests beta-blockers may be associated with organ-specific teratogenicity.
- Given the increasing incidence of hypertension, more information is needed to ensure that healthcare providers use the least teratogenic anti-hypertensives available.
Acknowledgments
None.
Sources of Funding
No sources of funding to disclose.
Footnotes
Author contributions: All authors made substantial contributions to article design, analysis, and interpretation of data, and drafting and revising the article. All approve of the final version to be published.
Ethics/Consent Statement: IRB approval/informed consent is not applicable as this manuscript involves a meta-analysis only of previously published manuscripts involving de-identified data. This was an unfunded study.
MOOSE guidelines for meta-analysis: performed and attached.
Conflict(s) of Interest/Disclosures(s)
Sonia Hernandez-Diaz is the Principal Investigator (PI) of NIH R01 HD046595-01A1 on teratogenicity of drugs commonly used in pregnancy and NIH R01 HD059861-01A1 grant on phthalates in medications and risk of male genital malformations. She receives sponsorships from Agency of Healthcare Research and Quality (AHRQ) (Grant RO1 HS018533) on anti-depressants use in pregnancy and postpartum, and is involved in projects funded by pharmaceutical companies related to North American Antiepileptic Drug (AED) Pregnancy Registry. Other authors have no conflicts of interest.
REFERENCES
- 1.Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Dal Pan GJ, Scott PE, Platt R. Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiol Drug Saf. 2008;17:240–247. doi: 10.1002/pds.1550. [DOI] [PubMed] [Google Scholar]
- 2.Bateman BT, Hernandez-Diaz S, Huybrechts KF, Palmsten K, Mogun H, Ecker JL, Fischer MA. Patterns of outpatient antihypertensive medication use during pregnancy in a medicaid population. Hypertension. 60:913–920. doi: 10.1161/HYPERTENSIONAHA.112.197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Update on overall prevalence of major birth defects--atlanta, georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 4.Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2008. Natl Vital Stat Rep. 59:1–126. [PubMed] [Google Scholar]
- 5.Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–969. doi: 10.1161/HYPERTENSIONAHA.106.075895. [DOI] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 7.Senn SJ. Overstating the evidence: Double counting in meta-analysis and related problems. BMC Med Res Methodol. 2009;9:10. doi: 10.1186/1471-2288-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puho EH, Szunyogh M, Metneki J, Czeizel AE. Drug treatment during pregnancy and isolated orofacial clefts in hungary. Cleft Palate-Craniofacial Journal. 2007;44:194–202. doi: 10.1597/05-208.1. [DOI] [PubMed] [Google Scholar]
- 9.Medveczky E, Puho E, Czeizel EA. The use of drugs in mothers of offspring with neural-tube defects. Pharmacoepidemiol Drug Saf. 2004;13:443–455. doi: 10.1002/pds.900. [DOI] [PubMed] [Google Scholar]
- 10.Cafri G, Kromrey JD, Brannick MT. A sas macro for statistical power calculations in meta-analysis. Behav Res Methods. 2009;41:35–46. doi: 10.3758/BRM.41.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Banhidy F, Cs N, Puho EH, Czeizel AE. Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: A population-based study. Hypertension Research. 2011;34:257–263. doi: 10.1038/hr.2010.227. [DOI] [PubMed] [Google Scholar]
- 12.Caton AR, Bell EM, Druschel CM, Werler MM, Lin AE, Browne ML, McNutt LA, Romitti PA, Mitchell AA, Olney RS, Correa A. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54:63–70. doi: 10.1161/HYPERTENSIONAHA.109.129098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caton AR, Bell EM, Druschel CM, Werler MM, Mitchell AA, Browne ML, McNutt LA, Romitti PA, Olney RS, Correa A. Maternal hypertension, antihypertensive medication use, and the risk of severe hypospadias. Birth Defects Research Part A - Clinical and Molecular Teratology. 2008;82:34–40. doi: 10.1002/bdra.20415. [DOI] [PubMed] [Google Scholar]
- 14.Cedergren MI, Selbing AJ, Kallen BA. Risk factors for cardiovascular malformation--a study based on prospectively collected data. Scand J Work Environ Health. 2002;28:12–17. doi: 10.5271/sjweh.641. [DOI] [PubMed] [Google Scholar]
- 15.Nakhai-Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Pharmacoepidemiology and Drug Safety (PDS) 2009;18:S76. doi: 10.1002/bdrb.20238. [DOI] [PubMed] [Google Scholar]
- 16.Nakhai-Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Research Part A - Clinical and Molecular Teratology. 2009;85:416. doi: 10.1002/bdrb.20238. [DOI] [PubMed] [Google Scholar]
- 17.Nakhai-Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Research Part B - Developmental and Reproductive Toxicology. 2010;89:147–154. doi: 10.1002/bdrb.20238. [DOI] [PubMed] [Google Scholar]
- 18.Queisser-Luft A, Eggers I, Stolz G, Kieninger-Baum D, Schlaefer K. Serial examination of 20,248 newborn fetuses and infants: Correlations between drug exposure and major malformations. Am J Med Genet. 1996;63:268–276. doi: 10.1002/(SICI)1096-8628(19960503)63:1<268::AID-AJMG45>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Sipek A, Gregor V, Velebil P, Horacek J, Masatova D, Svetnicova K. incidence of congenital defects in the children of mothers who used medications in the first trimester of pregnancy in the czech republic 1996-2001. Ceska Gynekol. 2003;68:401–419. [PubMed] [Google Scholar]
- 20.Davis RL, Eastman D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Dublin S, Platt R. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiology and Drug Safety. 2011;20:138–145. doi: 10.1002/pds.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol. 2003;17:255–261. doi: 10.1016/s0890-6238(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 22.Eric M, Leppee M, Culig J, Krivokuca D. Pregnancy and drugs for cardiovascular diseases. Acta Cardiol. 2009;64:23–28. doi: 10.2143/AC.64.1.2034357. [DOI] [PubMed] [Google Scholar]
- 23.Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65:615–625. doi: 10.1007/s00228-009-0620-0. [DOI] [PubMed] [Google Scholar]
- 24.Aberg A, Westbom L, Kallen B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev. 2001;61:85–95. doi: 10.1016/s0378-3782(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 25.Jensen DM, Damm P, Moelsted-Pedersen L, Ovesen P, Westergaard JG, Moeller M, Beck-Nielsen H. Outcomes in type 1 diabetic pregnancies: A nationwide, population-based study. Diabetes Care. 2004;27:2819–2823. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 26.Kitzmiller JL, Wallerstein R, Correa A, Kwan S. Preconception care for women with diabetes and prevention of major congenital malformations. Birth Defects Res A Clin Mol Teratol. 2010;88:791–803. doi: 10.1002/bdra.20734. [DOI] [PubMed] [Google Scholar]
- 27.Ray JG, O'Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: A meta-analysis. QJM. 2001;94:435–444. doi: 10.1093/qjmed/94.8.435. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Cummings EA, O'Connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108:644–650. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 29.Levit RD, Reynolds HR, Hochman JS. Cardiovascular disease in young women: A population at risk. Cardiol Rev. 2011;19:60–65. doi: 10.1097/CRD.0b013e31820987b5. [DOI] [PubMed] [Google Scholar]
- 30.Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: A retrospective cohort study. BMJ. 2011;343:d5931. doi: 10.1136/bmj.d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.