Abstract
Echium oil (EO) contains stearidonic acid (18:4), a n-3 polyunsaturated fatty acids (PUFAs), and gamma-linolenic acids (18:3), a n-6 PUFA that can be converted to long chain (LC)-PUFAs. We aimed to compare a safflower oil (SO)-enriched diet to EO- and fish oil (FO)-enriched diets on circulating and tissue PUFAs levels and glycemic, inflammatory, and cardiovascular health biomarkers in insulin resistant African green monkeys. In a Latin-square cross-over study, eight monkeys consumed matched diets for 6 weeks with 3-week washout periods. Monkeys consuming FO had significantly higher levels of n-3 LC-PUFAs and EO supplementation resulted in higher levels of circulating n-3 LC-PUFAs and a significant increase in dihomo-gamma linolenic acid (DGLA) in red blood cells and muscle. Glucose disposal was improved after EO consumption. These data suggest that PUFAs in EO supplementation have the capacity to alter circulating, RBC and muscle LC-PUFA levels and improve glucose tolerance in insulin-resistant monkeys.
Keywords: Echium oil, Fish oil, Stearidonic acid, Polyunsaturated fatty acids, Gamma-Linolenic acid, Diabetes
1. Introduction
Lifestyle interventions, including dietary modulation, have been demonstrated to be a promising approach for the prevention and treatment of type 2 diabetes [1,2]. Currently there is considerable scientific interest in substituting saturated fats with n-3 long-chain polyunsaturated fatty acids (LC-PUFAs), specifically, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are found mainly in fatty fish and fish oils (FO). The scientific literature generally supports the use of fish or fish oils containing EPA and DHA for the prevention and/or treatment of type 2 diabetes and its associated risk for cardiovascular disease [3-7]. However, controversy exists as to FO’s ability to affect insulin sensitivity [8-10] while benefits on oxidation and plasma lipid profiles being more consistently observed[11,12]. Despite the evidence for the preventive and therapeutic benefits of dietary n-3 PUFAs, addition of fish or fish oil supplements to human diets has been less than optimal due to several factors including the fear of contaminants in fish, instability of FO due to the oxidation of highly unsaturated n-3 LC-PUFAs, and some find that FO has objectionable smell and taste properties. Additionally, there are important issues concerning exploited stocks of fish to meet the dramatic increase in future demand for fish and fish oil supplements.
Plant seed oils appear to offer a promising means to increase EPA and DHA levels in humans and have been suggested as an alternative to fish oil. However currently available botanical alternatives (such as flax seed oil) contain the PUFA α-linolenic acid (ALA) as its primary n-3 fatty acid and the conversion of ALA to EPA and DHA is poor in humans [13]. This is believed to be a result of the inefficiency of the initial rate-limiting step (Δ-6 desaturase, FADS2 gene) in LC-PUFA biosynthesis in humans and rodents [14,15]. Seed oils from plants such echium (echium plantageneum; EO) contain PUFAs such as stearidonic acid (18:4n-3; SDA) and gammalinolenic acid (18:3n-6; GLA) which are downstream of Δ-6 desaturase in LC-PUFA biosynthesis. We and others have shown that SDA is converted by humans to EPA[16,17] and James and colleagues [18] demonstrated that SDA is 4–5 times as effective as ALA for increasing tissue EPA concentrations. Consequently, SDA-containing oils have the potential, to impact chronic diseases such as heart disease and diabetes. Echium seed oil (echium plantageneum; EO) has a pleasant odor and taste and contains 12–14% of its fatty acids as SDA (n-3) and 9–11% of its fatty acids as GLA (n-6). The objective of the current study was to compare the impact of diets enriched with PUFA-containing oils (safflower oil [linoleic acid], echium oil [SDA and GLA], or fish oil [EPA and DHA]) on insulin sensitivity and biomarkers of inflammation and cardiovascular disease in a relevant non-human primate model of age-associated insulin resistance and diabetes.
2. Materials and methods
2.1. Animals
All experimental procedures were approved and complied with the guidelines of the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences. Eight middle-aged to aged African green monkeys (Chlorocebus aethiops), that ranged in age from 9 to 21 years (mean 17.1 ± 1.7 year) and in weight from 4.2–11.6 kg (mean 6.2 ± 0.8 kg), were included in study. Monkeys were insulin resistant and had been selected based on repeated documentation of elevated blood glucose and insulin concentrations. All monkeys were initially acclimated to a standard Western diet (17% of calories as protein, 37% calories as fat, and 46% calories as carbohydrate) for at least 2 weeks before beginning a Latin square crossover study design, where each monkey was fed each of three diets (safflower oil [SO], fish oil [FO], or echium oil [EO] enriched) for 6 weeks, with a washout period of 3 weeks between each diet switch. Diets were made from natural ingredients, and constructed to be matched on caloric density, macronutrient content, and percent of monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs) and saturated fatty acids (SFAs) (Table 1). Diet analysis was conducted at each treatment period with mean fatty acid profile presented in Table 2.
Table 1.
The ingredient list and calculated macronutrient and fatty acid breakdown from experimental diets.
| Ingredient (g/100 g diet) | Safflower oil | Echium oil | Fish oil |
|---|---|---|---|
| Casein, USP | 7.00 | 7.00 | 7.00 |
| Lactalbumin | 7.00 | 7.00 | 7.00 |
| Dextrin | 9.00 | 9.00 | 9.00 |
| Sucrose | 7.00 | 7.00 | 7.00 |
| Wheat flour, self-rising | 37.00 | 37.00 | 37.00 |
| Wheat germ | 0.00 | 0.00 | 0.00 |
| Alphacel™ (cellulose) | 8.18 | 8.25 | 8.40 |
| Lard | 3.40 | 3.75 | 3.10 |
| Beef Tallow | 2.50 | 2.80 | 1.30 |
| Butter, lightly salted | 3.10 | 2.40 | 1.50 |
| Safflower oil | 6.10 | 2.00 | 2.28 |
| Menhaden Fish oil | 0.00 | 0.00 | 6.70 |
| Echium oil | 0.00 | 4.08 | 0.00 |
| Dried egg yolk | 1.80 | 1.80 | 1.80 |
| Vitamin mix | 2.50 | 2.50 | 2.50 |
| Modified #2 Ausman-Hayes mineral mix | 5.00 | 5.00 | 5.00 |
| Calcium carbonate | 0.40 | 0.40 | 0.40 |
| Calcium phosphate, monobasic | 0.02 | 0.02 | 0.02 |
| Crystalline cholesterol | 0.004 | 0.004 | 0.000 |
| Calcium/phosphorus | 1.1 | 1.1 | 1.1 |
| Cholesterol (mg/calorie) | 0.18 | 0.18 | 0.18 |
| Protein (% of calories) | 16.7 | 16.6 | 16.6 |
| Lipid (% of calories) | 37.2 | 37.3 | 37.3 |
| Carbohydrate (% of calories) | 46.1 | 46.1 | 46.0 |
| Fatty acids: | % of Fat | % of Fat | % of Fat |
| SFAs (%) | 34.3 | 34.0 | 34.6 |
| MUFAs (%) | 30.2 | 31.5 | 30.8 |
| PUFAs (%) | 35.5 | 34.5 | 34.6 |
| SFAs+MUFAs: PUFAs | 1.8 | 1.9 | 1.9 |
Table 2.
Average fatty acid profiles from experimental diet samples. Diet was measured 3 times at the beginning of each feeding period.
| Fatty acid | Safflower oil | Echium oil | Fish oil |
|---|---|---|---|
| ≤C12:0 | 1.03 | 0.81 | 0.30 |
| C14:0 | 2.43 | 2.50 | 4.78 |
| C15:0 | 0.27 | 0.28 | 0.42 |
| C16:0 | 18.07 | 18.76 | 20.34 |
| C16:1 | 1.62 | 1.52 | 5.59 |
| C17:0 | 0.41 | 0.46 | 0.29 |
| C18:0 | 8.53 | 9.65 | 7.99 |
| C18:1 trans | 1.50 | 1.70 | 1.12 |
| C18:1(n-9) | 24.29 | 25.28 | 20.43 |
| C18:1(n-11) | 1.22 | 1.22 | 1.71 |
| C18:2(n-6) | 37.46 | 20.67 | 18.88 |
| C18:3(n-3) | 0.58 | 8.15 | 0.98 |
| C18:3(n-6) | 0.24 | 2.73 | 0.15 |
| C18:4(n-3) | 0.00 | 3.51 | 1.17 |
| C20:0 | 0.19 | 0.13 | 0.31 |
| C20:1(n-9) | 0.25 | 0.43 | 0.64 |
| C20:2(n-6) | 0.18 | 0.15 | 0.24 |
| C20:3(n-3) | 0.06 | 0.06 | 0.14 |
| C20:4(n-3) | 0.05 | 0.05 | 0.13 |
| C20:4(n-6) | 0.26 | 0.26 | 0.61 |
| C20:5(n-3) | 0.00 | 0.00 | 4.32 |
| C22:0 | 0.10 | 0.07 | 0.13 |
| C22:1(n-9) | 0.00 | 0.08 | 0.00 |
| C22:5(n-3) | 0.05 | 0.04 | 0.82 |
| C22:6(n-3) | 0.06 | 0.06 | 3.36 |
| C24:0 | 0.06 | 0.05 | 0.00 |
| C24:1(n-3) | 0.05 | 0.05 | 0.16 |
| Other | 2.09 | 1.67 | 5.60 |
| Total n-3 | 0.92 | 14.49 | 10.79 |
| Total n-6 | 38.14 | 23.81 | 19.87 |
| n-6:n-3 | 41.36 | 1.55 | 2.00 |
| Total PUFAs | 38.98 | 35.75 | 34.14 |
| Total MUFAs | 29.21 | 30.50 | 29.81 |
| Total SFA | 31.12 | 32.69 | 34.55 |
| PUFAs: MUFA+SFA | 0.65 | 0.57 | 0.53 |
| LC PUFAs | 0.10 | 0.10 | 8.49 |
2.2. Measures in each diet interval
At the end of each diet interval, blood sampling, adiposity, and blood pressure measures were collected; muscle biopsies; and an intravenous glucose tolerance test (IVGTT) conducted. Briefly, under ketamine sedation (15 mg/kg IM, supplemented at 3–5 mg/kg as necessary), two baseline blood samples were collected via percutaneous femoral venipuncture and a 300 mg muscle sample was retrieved from the biceps femoris muscle by open excision. Muscle tissue was selected for assessment as this tissue compartment mediates the bulk of glucose disposal from the circulation. Tissue samples were immediately frozen in liquid nitrogen until further analysis. After the biopsy, a 21 G butterfly catheter was used to infuse 50% dextrose (750 mg/kg) via the saphenous vein, followed by saline flush in seven of the eight monkeys. Blood samples were subsequently collected at 5, 10, 20, 30 and 60 min post-dextrose into EDTA-treated tubes and placed on ice. Samples were centrifuged, and plasma stored at −80 °C until analysis for glucose and insulin concentrations. The rate of disappearance was calculated as the slope of the log transformed glucose values between 5 and 20 min. The acute insulin response (AIR) was calculated as the average of 5 and 10 min insulin concentrations, and a disposition index (DI) was calculated from the maximal glucose excursion divided by AIR.
Blood samples were measured for insulin, adiponectin (Mercodia, Uppsala, Sweden), C-reactive protein (ALPCO Diagnostics, Salem, NH), and interleukin-6 (IL-6; R&D Systems, Minneapolis, MN) concentration by ELISA. Tumor necrosis factor alpha (TNF-α) was measured by ELISA (Life Technologies, Grand Island, NY) after LPS-stimulation (10 ng/mL) of whole blood incubated at 37 °C for 3 h. Fructosamine (Roche Diagnostics, Mannheim, Germany) was measured colorimetrically, according to manufacturer recommendations. Glycation of blood hemoglobin A1c was measured by high performance liquid chromatography (Primus PDQ, Primus Diagnostics, Kansas City, MO) to assess long-term glycemic control. Lipid and lipoprotein parameters measured include total plasma cholesterol (TPC), triglycerides and high-density lipoprotein cholesterol (HDL-C). Plasma lipid analyses were performed on an ACE Alera Clinical Chemistry System (Alfa Wasserman, Inc., West Caldwell, NJ).
2.3. Fatty acid analysis
Plasma, muscle homogenate, red blood cells (RBCs), and diet samples were assayed for fatty acid composition. Samples were analyzed for fatty acids via gas chromatography for fatty acid methyl ester (FAME) residues [19] by gas liquid chromatography on a CP-Select CB for FAME capillary column (100 × 0.25 mm ID, Part number CP7420, ChromPack) with a deactivated guard column (0.53 mm ID) installed in a temperature programmed HP 5890 Series II gas chromatograph equipped with an on-column capillary inlet, flame ionization detector, and HP7673 autosampler/injector. The chromatographic conditions were as follows: H2 carrier gas, 20-psi head pressure, 1.25 mL/min at 90 °C; He makeup gas, 23 mL/min; inlet temperature at 3 °C above the oven temperature; and flame ionization detector at 230 °C. The oven temperature was programmed to begin at 90 °C and hold for 0.5 min, increase at 10 °C/min to 150 °C, increase at 2.5 °C/min to 200 °C, increase at 1.5 °C/ min to the final temperature, 220 °C, and hold at 220 °C for 20 min. Total run time is 60 min plus a 5-min equilibration period between runs. Chromatographic data collection and analysis is via a serial connection to a 300-MHz Intel Pentium II personal computer running Chrom Perfect Spirit Chromatography Data System (Justice Laboratory Software) in Microsoft Windows NT. Each chromatogram is examined for correct identification of constituent fatty acids and quality control.
2.4. Statistical methods
Data are presented as mean ± standard error of the mean (SEM) with significance defined as P≤0.05. Data were analyzed using mixed-effects linear regression modeling, with sequence, period, and treatment as fixed effects and monkey and error as random effects. Covariates were age, body weight, and baseline value if available. Pearson’s correlation coefficients were computed for association. Statistics were examined without the inclusion of data from the one male to examine for potential sex-differences and overall results were unchanged. All statistical analyses were done using SAS v9.1 (SAS Institute, Cary, NC).
3. Results
Great care was taken to ensure that diets were matched in all parameters using ingredients commonly consumed in western populations (Table 1). Fatty acid analyses from each diet are shown in Table 2. The fatty acid distribution matches the construction well with the SO diet enriched in LA, the FO enriched diet in EPA and DHA and the EO containing higher levels of SDA (18:4n-3), ALA (18:3n-3), as well as GLA (18:3n-6).
Table 3 shows the composition of fatty acids in plasma. As expected, the SO group had higher circulating levels of LA, and total n-6 fatty acids than either of the other groups; however, statistical significance was achieved only with the comparison of SO and EO. With regard to n-6 LC PUFAs, EO or FO diets did not significantly alter either circulating DGLA (20:3) or AA (20:4) when compared to the SO control diet. As expected, FO-enriched diets induced a significant increase circulating levels of EPA levels 6 weeks after dietary enrichment. DHA levels in FO-fed monkeys were increased, and EO-fed monkeys had intermediate levels of EPA and DHA, but these fatty acid shifts did not reach statistical significance. The significance for EO fed group in these n-3 LC PUFAs was negatively impacted because two of the monkeys did not respond with increases in EPA and DHA following diet exposure for unknown reasons.
Table 3.
Mean (± SEM; n=8/group) plasma levels of fatty acids measured at the end of each experimental diet period. Overall analysis of variance p-value is shown with group differences indicated by unlike superscripted letters.
| Fatty acid | Plasma (% of total fatty acids) |
p-value | ||
|---|---|---|---|---|
| Safflower oil | Echium oil | Fish oil | ||
| C14:0 | 0.40 (0.03) | 0.56 (0.06) | 0.50 (0.04) | 0.06 |
| C16:0 | 18.08 (0.46)a | 21.9 (0.99)b | 20.03 (1.07)a | 0.02 |
| C16:1 (n-7) | 0.913 (0.07) | 1.38 (0.09) | 1.15 (0.20) | 0.06 |
| C18:0 | 12.5 (0.34) | 13.8 (0.52) | 12.7 (0.31) | 0.07 |
| C18:1trans | 0.538 (0.03) | 0.563 (0.06) | 0.488 (0.03) | 0.52 |
| C18:1 (n-9) | 14.8 (0.42) | 15.6 (0.84) | 13.7 (0.95) | 0.25 |
| C18:1 (n-11) | 1.64 (0.09) | 1.90 (0.13) | 1.68 (0.15) | 0.30 |
| C18:2 (n-6) | 35.2 (1.49)a | 26.7 (1.59)b | 31.2 (2.93)a | 0.03 |
| C18:3 (n-6) | 0.614 (0.15) | 0.514 (0.22) | 0.50 (0.37) | 0.92 |
| C18:3 (n-3) | 0.688 (0.15) | 0.813 (0.27) | 0.60 (0.22) | 0.79 |
| C20:1 (n-9) | 0.117 (0.02) | 0.133 (0.02) | 0.143 (0.03) | 0.74 |
| C20:2 (n-6) | 0.438 (0.03) | 0.30 (0.04) | 0.467 (0.11) | 0.15 |
| C20:3 (n-6) | 4.013 (0.72) | 2.62 (0.78) | 1.90 (0.47) | 0.10 |
| C20:4 (n-6) | 6.063 (0.48) | 5.49 (0.66) | 5.69 (0.44) | 0.75 |
| C20:5 (n-3) | 0.383 (0.05)a | 3.85 (1.09)a | 5.18 (1.66)b | 0.02 |
| C22:5 (n-3) | 0.888 (0.11) | 1.075 (0.21) | 1.16 (0.25) | 0.61 |
| C22:6 (n-3) | 1.86 (0.22) | 2.82 (0.69) | 3.75 (0.88) | 0.15 |
| Sum n-6 | 46.2 (0.83)a | 35.6 (2.66)b | 39.4 (3.29)a | 0.02 |
| Sum n-3 | 3.39 (0.32) | 7.00 (1.78) | 9.01 (2.51) | 0.10 |
| n-3:n-6 | 14.5 (1.23) | 12.8 (5.62) | 9.73 (3.23) | 0.68 |
Table 4 shows the fatty acid composition of RBCs and muscle tissue 6 weeks after supplementation. As expected, the SO-enriched group had higher levels of linoleic acid in RBCs but this trend did not reach statistical significance in muscle tissue. There was a significant increase in DGLA (the GLA elongation product) in both RBCs and muscle tissue after EO supplementation. As expected, FO-enriched diets had higher levels of EPA and DHA in both RBCs and muscle tissue.
Table 4.
Mean (± SEM; n=8/group) red blood cell and muscle levels of fatty acids measured at the end of each experimental diet period. ND=not detected. Overall analysis of variance p-value is shown with group differences indicated by unlike superscripted letters.
| Fatty acid | Red blood cells (% of total fatty acids) |
p-value | Muscle (ug fatty acid/mg muscle protein) |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Safflower oil | Echium oil | Fish oil | Safflower oil | Echium oil | Fish oil | |||
| C14:0 | 0.26 (0.03) | 0.27 (0.02) | 0.33 (0.04) | 0.19 | 2.35 (1.00) | 1.87 (0.84) | 1.90 (0.72) | 0.91 |
| C16:0 | 23.6 (1.54) | 24.1 (1.41) | 26.8 (1.88) | 0.35 | 33.7 (9.90) | 30.1 (9.85) | 29.8 (9.24) | 0.95 |
| C16:1(n-7) | 0.31 (0.02)a | 0.34 (0.02)a | 0.44 (0.04)b | < 0.001 | 3.45 (1.00) | 3.30 (1.46) | 2.95 (0.84) | 0.94 |
| C18:0 | 20.8 (1.15) | 21.4 (1.24) | 22.5 (1.35) | 0.64 | 14.8 (4.66) | 11.9 (2.52) | 13.2 (3.67) | 0.87 |
| C18:1trans | 0.60 (0.04)ab | 0.69 (0.06)b | 0.50 (0.04)a | 0.01 | 1.09 (0.57) | 0.829 (0.38) | 0.738 (0.35) | 0.84 |
| C18:1(n-9) | 11.9 (0.40) | 12.5 (0.38) | 12.0 (0.42) | 0.50 | 44.9 (17.7) | 39.7 (18.5) | 36.6 (15.4) | 0.94 |
| C18:1(n-11) | 1.33 (0.20) | 1.31 (0.18) | 1.64 (0.09) | 0.32 | 3.84 (0.93) | 3.80 (1.39) | 3.44 (0.97) | 0.96 |
| C18:2(n-6) | 17.2 (1.01)a | 14.4 (0.72)a | 10.6 (0.75)b | < 0.001 | 23.3 (7.42) | 18.9 (5.63) | 16.5 (3.46) | 0.69 |
| C18:3(n-6) | 0.07 (0.005) | 0.20 (0.03) | 0.07 (0.005) | 0.08 | ND | 0.180 (0.04) | ND | |
| C18:3(n-3) | 0.42 (0.01) | 0.37 (0.08) | 0.36 (0.05) | 0.66 | 0.85 (0.62) | 0.74 (0.32) | 0.56 (0.18) | 0.83 |
| C18:4(n-3) | 0.09 (0.04) | 0.10 (0.03) | 0.09 (0.005) | 0.89 | ND | ND | ND | |
| C20:2(n-6) | 0.60 (0.03)a | 0.49 (0.05)ab | 0.38 (0.02)b | 0.004 | 0.40 (0.13) | 0.36 (0.12) | 0.32 (0.07) | 0.91 |
| C20:3(n-6) | 1.57 (0.15)a | 2.74 (0.20)b | 1.15 (0.17)a | < 0.001 | 0.61 (0.06)a | 0.94 (0.06)b | 0.53 (0.06)a | 0.003 |
| C20:4(n-6) | 12.5 (1.07) | 13.3 (1.61) | 10.7 (1.38) | 0.44 | 3.41 (0.39) | 3.69 (0.51) | 3.44 (0.54) | 0.91 |
| C20:5(n-3) | 0.87 (0.24)a | 0.68 (0.10)a | 4.20 (0.34)b | < 0.001 | 0.200a | 0.250 (0.03)a | 1.14 (0.14)b | < 0.001 |
| C22:5(n-3) | 2.07 (0.44) | 2.16 (0.37) | 2.54 (0.38) | 0.67 | 0.813 (0.12) | 0.871 (0.18) | 1.16 (0.12) | 0.19 |
| C22:6(n-3) | 3.32 (0.74) | 1.96 (0.41) | 3.91 (0.54) | 0.05 | 1.23 (0.19)a | 1.12 (0.29)a | 2.67 (0.35)b | 0.002 |
| Sum n-6 | 31.8 (2.02)a | 31.3 (2.40)a | 22.9 (2.29)b | 0.02 | 27.7 (7.26) | 24.03 (5.38) | 20.63 (8.37) | 0.66 |
| Sum n-3 | 6.67 (1.37)ab | 5.27 (0.79)a | 10.5 (1.52)b | 0.02 | 2.37 (0.36)a | 2.71 (0.34)a | 4.92 (0.63)b | < 0.001 |
| Ratio n-3:n-6 | 0.20 (0.03)a | 0.17 (0.02)a | 0.43 (0.05)b | < 0.001 | 0.11 (0.04)a | 0.13 (0.04)a | 0.29 (0.04)b | 0.006 |
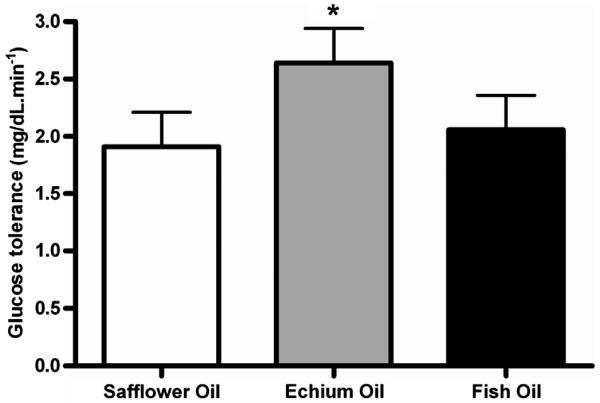
The EO-enriched diet significantly improved the ability to remove blood glucose from the circulation in monkeys with insulin resistance and hyperglycemia after glucose challenge when compared to SO- and FO-enriched groups (Fig. 1). Table 5 shows the glycemic status and insulin sensitivity of monkeys in each of the three diet groups. There were no changes in any of the nine parameters that we measured with the exception of the glucose disappearance rate. Improvement in the disposition index was observed in both EO- and FO-enriched groups as compared to the SO-enriched group but variability precluded statistical significance.
Fig. 1.
Average rate of glucose disappearance (±SEM) following intravenous glucose challenge in insulin resistant monkeys (n=8/diet group) following dietary exposure of different fatty acid sources. * indicates statistical significance with p<0.05.
Table 5.
Mean (± SEM) values for endpoints relating to glycemic status and insulin sensitivity in insulin-resistant and diabetic monkeys at baseline and after being fed experimental diets. Overall analysis of variance p-value is shown with group differences indicated by unlike superscripted letters.
| Fatty acid | Safflower oil | Echium oil | Fish oil | p-value |
|---|---|---|---|---|
| Fasting glucose (mmol/L) | 7.99 (2.05) | 9.66 (3.27) | 7.66 (1.99) | 0.60 |
| Fasting insulin (μIU/mL) | 25.7 (9.19) | 24.4 (10.7) | 19.0 (3.14) | 0.59 |
| Fructosamine (mEq/L) | 238 (31) | 256 (37) | 243 (37) | 0.42 |
| HbA1c(%) | 6.5 (0.8) | 6.9 (0.8) | 6.4 (0.7) | 0.18 |
| AUC glucose | 18190 (1879) | 20914 (2768) | 19196 (2189) | 0.38 |
| AUC insulin | 1171 (241) | 1773 (756) | 1263 (405) | 0.28 |
| Glucose disappearance rate (%/min) | 1.91 (0.14)a | 2.64 (0.45)b | 2.06 (0.29)a | 0.04 |
| Acute insulin response (μIU/mL) | 29.9 (8.03) | 34.0 (14.1) | 20.4 (5.81) | 0.18 |
| Disposition Index | 14.7 (3.57) | 30.7 (14.1) | 50.0 (30.6) | 0.23 |
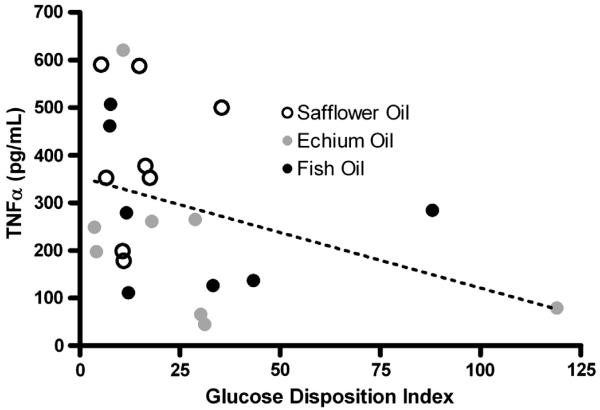
Morphometric measurements, plasma lipids and lipoproteins, blood pressure and inflammatory cytokine measurements are shown in Table 6. There were no changes in morphometric, blood pressure or heart rate endpoints with any diet group. Similarly, there were no changes in total plasma cholesterol and triglyceride levels. However there was a reduction in HDL cholesterol in the FO-enriched group. There were no statistically significant differences between any of the groups in inflammatory and adipokine end-points. However, there was a trend for reduced TNF-α production (~40%) in both the EO and FO enriched diet groups compared to the SO group. Since previous studies have shown that TNF-α reduces insulin sensitivity [20,21], we examined the relationship between TNF-α and the glucose disposition index in these insulin resistant monkeys. Fig. 2 illustrates that TNF-α levels were negatively associated with the glucose disposition index (r=−0.41, p=0.04).
Table 6.
Mean (± SEM) values for endpoints relating to body composition, plasma lipids and lipoproteins, blood pressure, and inflammatory endpoints in insulin-resistant and diabetic monkeys at baseline and after being fed experimental diets. Overall analysis of variance p-value is shown with group differences indicated by unlike superscripted letters.
| Fatty acid | Safflower oil | Echium oil | Fish oil | p-value |
|---|---|---|---|---|
| Bodyweight (kg) | 6.41 (1.01) | 6.36 (1.08) | 6.23 (0.92) | 0.53 |
| Waist Circumference (cm) | 33.5 (2.21) | 32.4 (2.61) | 32.3 (2.24) | 0.40 |
| Sagittal abdominal diameter (cm) | 10.59 (0.55) | 10.10 (0.51) | 10.11 (0.51) | 0.21 |
| Body mass index (kg/m2) | 31.2 (3.31) | 31.4 (3.62) | 27.5 (1.58) | 0.54 |
| TPC (mmol/L) | 5.05 (0.49) | 5.31 (0.57) | 5.34 (0.57) | 0.23 |
| HDLC (mmol/L) | 1.91 (0.09)a | 2.02 (0.10)a | 1.47 (0.10)b | < 0.001 |
| TG (mmol/L) | 0.61 (0.12) | 0.72 (0.23) | 0.56 (0.14) | 0.47 |
| Systolic blood pressure (mmHg) | 101 (9) | 100 (7) | 95 (8) | 0.76 |
| Diastolic blood pressure (mmHg) | 63 (9) | 55 (3) | 58 (6) | 0.75 |
| Heart Rate (bpm) | 156 (13) | 150 (13) | 146 (10) | 0.86 |
| C-reactive protein (ng/mL) | 491 (85) | 559 (75.8) | 526 (86) | 0.77 |
| IL-6 (pg/mL) | 0.85 (0.13) | 1.64 (0.64) | 1.50 (0.35) | 0.27 |
| TNF-α (pg/mL) | 392 (60) | 223 (70) | 245 (64) | 0.13 |
| Adiponectin (ng/L) | 11.68 (1.39) | 12.79 (1.17) | 13.68 (1.70) | 0.31 |
Fig. 2.
Association of glucose disposition index with TNFα levels elicited from whole blood collected from insulin resistant monkeys (n=8/diet group) following dietary exposure of different fatty acid sources. The Pearson’s correlation coefficient was r=0.41, p<0.05.
4. Discussion and conclusions
The current study compares the impact of three diets enriched in either SO, EO or FO on glucose metabolism and inflammatory endpoints in an insulin resistant monkey model. Both EO- and FO-enriched diets were associated with higher circulating levels of n-3 LC-PUFAs and the EO diet group had higher levels of DGLA in RBCs and muscle tissue. Both the FO and EO groups (compared to the SO group) showed a trend toward a reduction in levels of the inflammatory cytokine, TNF-α. However, only the EO group (when compare to SO or FO groups) improved glucose disposal rates indicating improved peripheral ability to uptake glucose from the circulation. These results are in line with a recent meta-analysis of fish or fish oil consumption which did not demonstrate protective effects of intake on diabetes risk, whereas plant sourced fatty acids suggested a trend towards lower diabetes risk [22].
Improved glucose disposal indicates that there is an enhanced peripheral ability to take-up glucose from the circulation in insulin resistant monkeys on EO-enriched diets. While the mechanism behind increased disposal rates is not directly addressed in this manuscript, it is interesting to note that it was not observed in FO-fed monkeys and was associated with levels of TNF-α, a marker of systemic inflammation. A human trial of EO supplementation also saw similar trends towards reduced TNF-α at 3 and 6 weeks [18]. EO has also been associated with a reduction in inflammatory biomarkers and events in animal models. In swine, SDA consumption leads to a reduction of the expression of SCD, a gene associated with obesity and insulin resistance [17]. SDA also up-regulated PONS3, a gene associated with reduced CRP, an important marker of systemic inflammation and cardiovascular disease [17]. Recently, Brown and colleagues used LDLr−/− mice to examine the development of hypercholesterolemia-associated monocytosis and neutrophilia [23]. They demonstrated in palm oil-fed mice that EO and FO markedly reduced trafficking of inflammatory Ly6Chi monocytes and lowered atherosclerosis to the levels observed in low fat chowfed mice. Taken together, these data suggest that SDA-containing oils reduce several key inflammatory parameters that could drive diseases such as diabetes and atherosclerosis.
A key difference between EO and FO is that the former also contains the n-6 PUFA, GLA that has been demonstrated to have it own anti-inflammatory effects. GLA markedly reduces inflammatory cytokines and leukotrienes in healthy humans and asthmatic patients and positively impacts critically ill patients suffering with acute respiratory distress syndrome [24-26]. GLA is efficiently elongated in rodent and human cells and tissues to DGLA, and DGLA is then incorporated into membrane phospholipids. DGLA competes with arachidonic acid for cyclooxygenase-mediated conversion to prostaglandin (PG)H1, which is then converted to PGE1. PGE1 has been illustrated to have anti-inflammatory effect in both animals and diabetic humans [27-29]. PGH1 also suicidally inactivates thromboxane (TX)A synthase, and thus inhibits the generation of TXA2 [30]. PGE1 vasodilates and inhibits platelet aggregation and leukocyte influx [31], and has been used therapeutically in patients with acute lung injury [32]. Alveolar macrophages obtained from rats that were fed a diet enriched in GLA plus EPA showed a marked “shift” in their production of PGE2 to that of PGE1 in response to lipopolysaccharide (LPS) [33], and decreased TXA2 generation.
Importantly, GLA-containing oils, such as borage oil and evening primrose oil, also have clinical efficacy in rheumatoid arthritis and atopic eczema in a several small trials [34-36]. A recent study [3] in diabetic patients comparing plant and fish oil sources of fatty acids found while both improved metabolic control, the plant oil diet was superior to fish oil in reducing fasting and post-prandial glucose, and insulin secretion. Additionally, the supplementation of GLA-containing borage oil improved insulin sensitivity in muscle tissue of diabetic rats [37] and it has been proposed to contribute to a protective effect against insulin resistance through potential activation of the peroxisome proliferator-activated receptor [38]. The current observation that muscle tissue is enriched with the elongation product of GLA, DGLA in monkeys after EO-enriched diets suggests that DGLA is dispersed in tissues where it can impact the biology of glucose uptake. GLA and DGLA levels measured in muscle and erythrocyte membranes from populations of people have reported both positive and negative relationships with insulin sensitivity and diabetes development [9,10].
SDA-containing oils have been shown to have a number of important lipid lowering and anti-inflammatory properties. As mentioned above, SDA is much more efficiently converted to EPA than ALA in humans [16,17]. While the majority of monkeys in the current study responded robustly to the SDA supplementation with the generation of EPA, for reasons we do not understand, two of the monkeys did not. This may reflect unexpected genetic variation between monkeys in the desaturase activity as has recently been described in humans [39]. EO has been demonstrated to reduce triglycerides in hypertriglyceridemic humans and rodents [40,41], however this lipid lowering effect was not observed with SDA-enriched soybean oils consumed by healthy humans and was not observed in the current study with insulin resistant monkeys[18,42]. We are confident that our dietary supplementation levels and duration of consumption were sufficient to modify fatty acids, as n-3 LC-PUFA’s were increased in plasma, and there is even indication that changes appear in RBCs and muscle. We chose our echium oil intake level after a preliminary pharmacokinetic analysis of plasma SDA levels after different dose levels of echium oil in cynomolgus monkeys (data not shown); our dose was selected to match the plasma SDA seen with the 15 g/day human dose [16]. In ruminants dosed with similar preparations and dose levels of echium oil for 4 weeks, muscle tissue showed the accumulation of SDA, GLA, and DGLA fatty acids similar to our findings in these nonhuman primates [43].
The unique strengths of this study include the monkey model, controlled intake of diets matched in all components aside from fatty acids, and dietary composition confirmed by analyses. We also evaluated both circulating and tissue fatty acid profiles to assure the key fatty acids in the oils were bioavailable. Limitations to study include the use of a cross-over design. Some carryover in fatty acid profile may have occurred between diet periods. However, this design facilitated the statistical handling of individual monkey effects and controlling for baseline covariates. As monkeys were spontaneously insulin-resistant, their phenotypes varied widely, thus making a cross-over study the optimal design.
In conclusion, this study provides evidence that EO contains both a combination of n-3 and n-6 PUFAs that may play an important role in improving glucose tolerance in older, insulin resistant non-human primates. We report that echium oil elevates LC-PUFAs in circulation, may increase DGLA content in tissues, and several studies including this one suggest that the health effects of EO may be mediated in part through the attenuation of inflammatory processes. Thus, this study provides evidence that further attention should be given to testing plant-sourced PUFAs for the prevention and management of insulin resistance and diabetes.
Acknowledgments
Funding for these studies came from grants P50 AT002782, K01AG 033641, and T32HL07115 as well as Wake Forest School of Medicine. The authors gratefully acknowledge the advice and input from Drs. Lawrence L. Rudel and John S. Parks.
Footnotes
Sources of support: Grants P50 AT002782, P40 RR019963, K01AG 033641, and T32HL07115 as well as Wake Forest School of Medicine.
No conflicts of interest are present for any of the authors.
References
- [1].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McAuley KA, Williams SM, Mann JI, Goulding A, Chisholm A, Wilson N, Story G, McLay RT, Harper MJ, Jones IE. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care. 2002;25:445–452. doi: 10.2337/diacare.25.3.445. [DOI] [PubMed] [Google Scholar]
- [3].Karlstrom BE, Jarvi AE, Byberg L, Berglund LG, Vessby BO. Fatty fish in the diet of patients with type 2 diabetes: comparison of the metabolic effects of foods rich in n-3 and n-6 fatty acids. Am. J. Clin. Nutr. 2011;94:26–33. doi: 10.3945/ajcn.110.006221. [DOI] [PubMed] [Google Scholar]
- [4].Djousse L, Biggs ML, Lemaitre RN, King IB, Song X, Ix JH, Mukamal KJ, Siscovick DS, Mozaffarian D. Plasma omega-3 fatty acids and incident diabetes in older adults. Am. J. Clin. Nutr. 2011;94:527–533. doi: 10.3945/ajcn.111.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meyer KA, Kushi LH, Jacobs DR, Jr., Folsom AR. dietary fat and incidence of type 2 diabetes in older iowa women. Diabetes Care. 2001;24:1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- [6].Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care. 2000;23:1407–1415. doi: 10.2337/diacare.23.9.1407. [DOI] [PubMed] [Google Scholar]
- [7].Villegas R, Xiang YB, Elasy T, Li HL, Yang G, Cai H, Ye F, Gao YT, Shyr Y, Zheng W, Shu XO. Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am. J. Clin. Nutr. 2011;94:543–551. doi: 10.3945/ajcn.111.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Giacco R, Cuomo V, Vessby B, Uusitupa M, Hermansen K, Meyer BJ, Riccardi G, Rivellese AA. Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n-6 and n-3 fatty acids? Nutr. Metab. Cardiovasc. Dis. 17:572–580. doi: 10.1016/j.numecd.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [9].Iggman D, Arnlov J, Vessby B, Cederholm T, Sjogren P, Riserus U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia. 2010;53:850–857. doi: 10.1007/s00125-010-1669-0. [DOI] [PubMed] [Google Scholar]
- [10].Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2008;18:503–510. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [11].Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- [12].Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic. Biol. Med. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- [13].Mantzioris E, James MJ, Gibson RA, Cleland LG. Dietary substitution with an alpha-linolenic acid-rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. Am. J. Clin. Nutr. 1994;59:1304–1309. doi: 10.1093/ajcn/59.6.1304. [DOI] [PubMed] [Google Scholar]
- [14].Huang YS, Smith RS, Redden PR, Cantrill RC, Horrobin DF. Modification of liver fatty acid metabolism in mice by n-3 and n-6 delta 6-desaturase substrates and products. Biochim. Biophys. Acta. 1991;1082:319–327. doi: 10.1016/0005-2760(91)90208-y. [DOI] [PubMed] [Google Scholar]
- [15].Yamazaki K, Fujikawa M, Hamazaki T, Yano S, Shono T. Comparison of the conversion rates of alpha-linolenic acid (18:3(n-3)) and stearidonic acid (18:4(n-3)) to longer polyunsaturated fatty acids in rats. Biochim. Biophys. Acta. 1992;1123:18–26. doi: 10.1016/0005-2760(92)90166-s. [DOI] [PubMed] [Google Scholar]
- [16].Surette ME, Edens M, Chilton FH, Tramposch KM. Dietary echium oil increases plasma and neutrophil long-chain (n-3) fatty acids and lowers serum triacylglycerols in hypertriglyceridemic humans. J. Nutr. 2004;134:1406–1411. doi: 10.1093/jn/134.6.1406. [DOI] [PubMed] [Google Scholar]
- [17].Whelan J, Gouffon J, Zhao Y. Effects of dietary stearidonic acid on biomarkers of lipid metabolism. J. Nutr. 2012;142:630S–634S. doi: 10.3945/jn.111.149138. [DOI] [PubMed] [Google Scholar]
- [18].James MJ, Ursin VM, Cleland LG. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am. J. Clin. Nutr. 2003;77:1140–1145. doi: 10.1093/ajcn/77.5.1140. [DOI] [PubMed] [Google Scholar]
- [19].Metcalfe LDSA, Pelka JR. Rapid preparation of fatty acid esters from lipid gas chromatography analysis. Anal. Chem. 1966;38:514–515. [Google Scholar]
- [20].Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. Irs-1-mediated inhibition of insulin receptor tyrosine kinase activity in tnf-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- [22].Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, Djousse LFBH, Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(2):S214–227. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown AL, Zhu X, Rong S, Shewale S, Seo J, Boudyguina E, Gebre AK, Alexander-Miller MA, Parks JS. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler. Thromb. Vasc. Biol. 2012;32:2122–2130. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- [25].Surette ME, Koumenis IL, Edens MB, Tramposch KM, Chilton FH. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin. Ther. 2003;25:948–971. doi: 10.1016/s0149-2918(03)80116-9. [DOI] [PubMed] [Google Scholar]
- [26].Weaver KL, Ivester P, Seeds M, Case LD, Arm JP, Chilton FH. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J. Biol. Chem. 2009;284:15400–15407. doi: 10.1074/jbc.M109.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kapoor R, Huang YS. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006;7:531–534. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
- [28].Arisaka M, Arisaka O, Yamashiro Y. Fatty acid and prostaglandin metabolism in children with diabetes mellitus. II. The effect of evening primrose oil supplementation on serum fatty acid and plasma prostaglandin levels. Prostaglandins Leukot. Essent. Fatty Acids. 1991;43:197–201. doi: 10.1016/0952-3278(91)90169-6. [DOI] [PubMed] [Google Scholar]
- [29].Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T, Kakutani S, Tanaka K, Kiso Y, Miyazaki M. Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in apoe-deficient mice. J. Atheroscler. Thromb. 2009;16:480–489. doi: 10.5551/jat.no430. [DOI] [PubMed] [Google Scholar]
- [30].Jones DA, Fitzpatrick FA. Suicide Inactivation of thromboxane a2 synthase. Characteristics of mechanism-based inactivation with isolated enzyme and intact platelets. J. Biol. Chem. 1990;265:20166–20171. [PubMed] [Google Scholar]
- [31].Chopra J, Webster RO. Pge1 inhibits neutrophil adherence and neutrophil-mediated injury to cultured endothelial cells. Am. Rev. Respir. Dis. 1988;138:915–920. doi: 10.1164/ajrccm/138.4.915. [DOI] [PubMed] [Google Scholar]
- [32].Abraham E, Park YC, Covington P, Conrad SA, Schwartz M. Liposomal prostaglandin e1 in acute respiratory distress syndrome: a placebo-controlled, randomized, double-blind, multicenter clinical trial. Crit. Care Med. 1996;24:10–15. doi: 10.1097/00003246-199601000-00005. [DOI] [PubMed] [Google Scholar]
- [33].Palombo JD, DeMichele SJ, Boyce PJ, Lydon EE, Liu JW, Huang YS, Forse RA, Mizgerd JP, Bistrian BR. Effect of short-term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit. Care Med. 1999;27:1908–1915. doi: 10.1097/00003246-199909000-00032. [DOI] [PubMed] [Google Scholar]
- [34].Andreassi M, Forleo P, Di Lorio A, Masci S, Abate G, Amerio P. Efficacy of gamma-linolenic acid in the treatment of patients with atopic dermatitis. J. Int. Med. Res. 1997;25:266–274. doi: 10.1177/030006059702500504. [DOI] [PubMed] [Google Scholar]
- [35].Henz BM, Jablonska S, van de Kerkhof PC, Stingl G, Blaszczyk M, Vandervalk PG, Veenhuizen R, Muggli R, Raederstorff D. Double-blind, multicentre analysis of the efficacy of borage oil in patients with atopic eczema. Br. J. Dermatol. 1999;140:685–688. doi: 10.1046/j.1365-2133.1999.02771.x. [DOI] [PubMed] [Google Scholar]
- [36].Zurier RB, Rossetti RG, Jacobson EW, DeMarco DM, Liu NY, Temming JE, White BM, Laposata M. Gamma-linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheum. 1996;39:1808–1817. doi: 10.1002/art.1780391106. [DOI] [PubMed] [Google Scholar]
- [37].Khamaisi M, Rudich A, Beeri I, Pessler D, Friger M, Gavrilov V, Tritschler H, Bashan N. Metabolic effects of gamma-linolenic acid-alpha-lipoic acid conjugate in streptozotocin diabetic rats. Antioxid. Redox Signal. 1999;1:523–535. doi: 10.1089/ars.1999.1.4-523. [DOI] [PubMed] [Google Scholar]
- [38].Takahashi Y, Ide T, Fujita H. Dietary gamma-linolenic acid in the form of borage oil causes less body fat accumulation accompanying an increase in uncoupling protein 1 mrna level in brown adipose tissue. Comp. Biochem. Physiol. B Biochem. Mol Biol. 2000;127:213–222. doi: 10.1016/s0305-0491(00)00254-6. [DOI] [PubMed] [Google Scholar]
- [39].Sergeant S, Hugenschmidt CE, Rudock ME, Ziegler JT, Ivester P, Ainsworth HC, Vaidya D, Case LD, Langefeld CD, Freedman BI, Bowden DW, Mathias RA, Chilton FH. Differences in arachidonic acid levels and fatty acid desaturase (fads) gene variants in african americans and european americans with diabetes or the metabolic syndrome. Br. J. Nutr. 2012;107:547–555. doi: 10.1017/S0007114511003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Forrest LM, Boudyguina E, Wilson MD, Parks JS. Echium oil reduces atherosclerosis in apob100-only LDLrKO mice. Atherosclerosis. 2012;220:118–121. doi: 10.1016/j.atherosclerosis.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang P, Boudyguina E, Wilson MD, Gebre AK, Parks JS. Echium oil reduces plasma lipids and hepatic lipogenic gene expression in apob100-only LDL receptor knockout mice. J. Nutr. Biochem. 2008;19:655–663. doi: 10.1016/j.jnutbio.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lemke SL, Vicini JL, Su H, Goldstein DA, Nemeth MA, Krul ES, Harris WS. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010;92:766–775. doi: 10.3945/ajcn.2009.29072. [DOI] [PubMed] [Google Scholar]
- [43].Kitessa SM, Young P, Nattrass G, Gardner G, Pearce K, Pethick DW. When balanced for precursor fatty acid supply echium oil is not superior to linseed oil in enriching lamb tissues with long-chain n-3 pufa. Br. J. Nutr. 2011:1–9. doi: 10.1017/S0007114511005411. [DOI] [PubMed] [Google Scholar]