Abstract
Objective
Previous studies have examined the relationships between structural brain characteristics and early life stress in adults. However, there is limited evidence for functional brain variation associated with early life stress in children. We hypothesized that early life stress and trauma would be associated with increased functional brain activation to negative emotional faces in children with and without a history of depression.
Method
Psychiatric diagnosis and life events in children (starting at ages 3–5) were assessed in a longitudinal study. A follow-up magnetic resonance imaging (MRI) study acquired data (N = 115 at ages 7–12, 51% female) on functional brain response to fearful, sad, and happy faces relative to neutral faces. We used a region of interest (ROI) mask within cortico-limbic areas and conducted regression analyses and repeated-measures analysis of covariance (ANCOVA).
Results
Greater activations to fearful, sad, and happy faces in the amygdala and its neighboring regions were found in children with higher life stress. Moreover, an association between life stress and left hippocampal and globus pallidus activity depended on children's diagnostic status. Finally, all children with higher life trauma showed greater bilateral amygdala and cingulate activity specific to sad faces, but not the other emotional faces, although right amygdala activity was moderated by psychiatric status.
Conclusions
These findings may suggest that limbic hyperactivity is a biomarker of early life stress and trauma in children and may have implications in the risk trajectory for depression and other stress-related disorders. However, this pattern varied based on emotion type and history of psychopathology.
Keywords: early life stress, early life trauma, child, fMRI, emotion
INTRODUCTION
Experiences of chronic stress and trauma have deleterious effects on neurobiological, affective, and behavioral functions.1 For example, animal studies have found that chronic exposure to stress changes molecular and cellular activities, such as dendritic remodeling,2 shifts in the dendritic spine population,3 reduced axon density,4 decreased astroglial cells,5 and altered neuropeptide mRNA expression6 in the cortico-limbic-striatal system, including prefrontal cortex (PFC), amygdala, hippocampus, caudate putamen, and nucleus accumbens. Likewise, human adults who report chronic stress exhibit reduced volume in the amygdala, hippocampus, medial and dorsolateral PFC, anterior cingulate cortex (ACC), and subgenual ACC (sgACC), striatum, and insula,7–10 as well as increased functional response to emotional stimuli in the amygdala, hippocampus, ventromedial PFC, and ACC.10–12
Similar structural and functional brain differences to those found in currently stressed adults may also be present in previously stressed individuals.13 Animal studies have shown that early life stress (e.g., abuse by mother) leads to functional amygdala changes in adolescence, including increased activation of protein kinase14 and increased cFOS-labeled neural activation.15 In humans, adults who retrospectively report child maltreatment show altered volume or functional activity in the hippocampus, amygdala, PFC, ACC, and other limbic regions.8–10, 12 Some additional studies have focused on children who experienced a specific type of stressor (e.g., low maternal support) and found that these children showed altered volume in the hippocampus,16, 17 orbitofrontal cortex,18 and amygdala.19
In contrast to these structural imaging studies, evidence for functional brain changes in children with cumulative stress/trauma is limited. Some functional magnetic resonance imaging (fMRI) studies report that, compared to healthy children, children with a history of institutional care showed increased bilateral amygdala activity in response to fearful faces.20 Although these previous studies provided evidence of a relationship between early life stress/trauma and functional amygdala activity in children, it focused on a unique type of early life stress/trauma (i.e., institutional rearing) in a sample of children both with and without psychopathology. Hence, it still remains unclear whether (1) amygdala hyperactivity occurs in relation to stressful/traumatic life events other than early institutionalization; and (2) the presence or absence of psychopathology in children influences the relationship between early stress and functional amygdala changes.
It is important to investigate the influence of psychopathology, particularly major depressive disorder (MDD), on the relationship between early life stress or trauma and functional brain activity in children because stress is thought to contribute to the development of MDD,21, 22 and high levels of stressful or traumatic life events in childhood increases risk for preschool-onset MDD (PO-MDD).23 Thus, the goal of the current study was to examine the relationships between early life stress/trauma and function brain activation to faces portraying negative emotions in a sample of school-aged children with and without a history of psychiatric disorders.
To achieve this goal, we tested two hypotheses. First, we hypothesized that early life stress/trauma would be associated with increased functional activation to negative emotion stimuli in the amygdala, hippocampus, basal ganglia, medial and dorsolateral PFC, and/or ACC. These regions have been shown to be sensitive to stress in previous animal and human studies,10, 13, 22, 24–28 and functional hyperactivity to negative emotion in some of these brain regions has been associated with a history of child maltreatment.10, 12, 20 To clarify whether these functional brain changes were specific to negatively valenced emotions, the present study examined functional brain response to both negative and positive stimuli in relation to early life stress and trauma. The present study used fearful and sad faces as negative stimuli and happy faces as positive stimuli because these stimuli have been commonly used in neuroimaging research on the effects of maltreatment,20, 29 MDD,30, 31 and mood-congruent brain reactivity,32–34 compared to the other facial stimuli.
Second, we also hypothesized that the effects of life stress/trauma on functional brain activity would be exacerbated in the context of a positive early history of MDD. This hypothesis was based in part on the findings that PO-MDD is associated with neurobiological alterations in stress-responsive brain regions, including reduced hippocampal volume,35 decreased functional connectivity between sgACC and cognitive control regions,36 and greater functional activation to sad faces in the amygdala and hippocampus.30, 31
METHOD
Participants
Between 2003 and 2005, preschoolers between the ages of 3.0 and 5.11 were recruited from the St. Louis metropolitan area, using the Preschool Feelings Checklist,37, 38 and preschoolers with symptoms of MDD were oversampled. With written consent from parents and assent from the children, a sample of 306 children, without head trauma, neurological disease, severe developmental delays, or premature birth, were enrolled into the Validation of Preschool Depression Study, a longitudinal study approved by the Institutional Review Board at the Washington University School of Medicine.
One hundred sixty-eight children completed follow-up annual waves over a 5–8 year period after which an MRI study at school age was conducted. An additional 41 healthy children were recruited for the MRI study to increase the size of the healthy control group (age matched to the children with psychiatric disorders). A total of 209 children, who were between the ages of 7–12, participated in a training sequence using a mock scanner, described previously.35 After applying criteria for image quality (see below for details), 148 children were screened, and 115 medication-free children who did not show structural brain abnormalities (59 females, mean age = 9.88 ± 1.33 years) were included in the present study; 33 subjects were excluded because n = 27 had psychotropic medication prior to scanning, n = 3 had brain abnormalities, n = 1 had both, and n = 2 had missing diagnostic data.
Diagnostic Assessment
Trained interviewers conducted in-person annual interview sessions using an age-appropriate diagnostic interview: the Preschool Age Psychiatric Assessment (PAPA) for preschool-age children39, 40 and the Child and Adolescent Psychiatric Assessment (CAPA) for school-age children.41, 42 Methods to avoid drift and maintain reliability and quality control including review of 20% audiotaped interviews with a master clinician were used.43 The PAPA and CAPA consist of a series of developmentally appropriate questions covering the DSM-IV criteria for Axis-I disorders of childhood. Parent reports were used for children < 8.0 years, and child- and parent reports were combined for children ≥ 8.0 years. Standard DSM-IV algorithms were applied to derive all diagnoses with the exception that the two-week duration of symptoms requirement for MDD was set aside for subjects who were aged younger than 6.0 years based on data suggesting it is not an appropriate threshold for this developmental period.38, 44, 45
Based on diagnoses using the PAPA and CAPA, children were classified into (1) the MDD group (n = 42) if they met DSM-IV criteria for MDD at any annual wave prior to MRI scan, (2) the other psychiatric control (OPC) group (n = 22) if they met DSM-IV criteria for any Axis I disorder (ADHD, ODD, CD, and anxiety disorders) without MDD co-morbidity at any wave prior to scan, and (3) the healthy control group (n = 51) if they did not meet DSM-IV criteria for any Axis I disorder across all waves prior to scan. In addition, PAPA and CAPA items in the MDD module were used to derive a `core' DSM-IV MDD symptom score at each wave.46 Then, the number of core MDD symptoms at the time of scan was summed into a core MDD severity score.
Life Events
The PAPA and CAPA also assessed how frequently children experienced stressful and traumatic life events within the last year, as reported by their caregiver annually at each study wave. A sub-group of healthy children who were newly added in the MRI study retrospectively reported how frequently they had ever experienced any stressful and traumatic events in their lives. The PAPA and CAPA have established test-retest reliability for parental reports of life events47 and defines early life trauma as any type of emotionally harmful life events up to school age (i.e., in the past 7–12 years), including physical and sexual abuse, automobile accidents, natural disaster, death of sibling, etc.; we defined early life stress as any type of less intense but still distressing life event up to school age, including death of a pet, change in daycare/school, birth of a new sibling, etc.
Functional Task and Stimuli
The fMRI task was a facial emotion-processing task using the MacArthur Network Face Stimuli Set, a validated stimulus set that contains 43 different actors from different ethnic/cultural backgrounds.48 Children were shown faces that varied in affective content (fearful, sad, angry, happy, and neutral) from 10 sets of adults and were asked to decide whether the face was male or female. The purpose of this gender judgment was to assure that all subjects were awake and viewed the stimuli, and accuracy was very high. We chose to use a task that did not require explicit attention to the emotional content because of evidence that heightened amygdala responses associated with MDD may be more apparent with tasks that do not explicitly require a focus on the emotional content.49, 50 In addition, we created intermediate affective expressions by morphing the neutral expression for each individual with their emotional expression (MorphAge software). We included these stimuli because behavioral and brain activation biases in depression may be more apparent when viewing emotional expressions that are less intense. Thus, each “actor” in the stimulus set provided a total of 9 facial expressions (neutral, 50% fearful, sad, angry, and happy, 100% fearful, sad, angry, and happy). Children had not previously seen any of these faces and each stimulus was presented once (no repeats) for 2,500 ms, followed by an inter-trial interval (ITI) ranging between 500 and 6,500 ms. The task was programmed in PsyScope, and behavioral responses in the scanner were acquired via a fiber optic button box interfaced with the PsyScope button box. Each run consisted of 45 stimuli, 5 from each of the 9 conditions. Selection of the specific face to be presented on each trial was determined by PsyScope using a random without replacement algorithm, within the constraint of the number of faces of each type to be presented in a run. The image projected on a screen behind the subject's head was viewed by a mirror positioned approximately 8 cm above the subject's face. For the current analyses, activation values for the fear (50% and 100% fear > neutral), the sad (50% and 100% sad > neutral), and the happy (50% and 100% happy > neutral) conditions were used.
Imaging Data Acquisition
Image data acquisition, including structural, functional, and diffusion tensor imaging, was performed on a 3.0 Tesla TIM TRIO Siemens whole body system. The whole MRI session took approximately 1 hour and 15 minutes. During the scan, children completed a film processing task (~5 minutes), two face task runs (~12 minutes), two resting state runs (~12 minutes), and two diffusion tensor imaging runs (~15 minutes). Only functional imaging data acquired during the facial emotion-processing task were used for the present study. Two three-dimensional T1-weighted magnetization prepared rapid gradient echo (MPRAGE) scans (~6 minutes each; TR = 2,400 ms, TE = 3.16 ms, flip angle = 8°, 1 mm3 voxels) were acquired in the sagittal plane. Blood-oxygen-level dependent (BOLD) images during the face task were acquired with a T2*-weighted asymmetric spin-echo echo-planar sequence (~5 minutes; TR = 2500 ms, TE = 27 ms, flip angle = 90°, voxel size = 4 mm3) with a 12-channel head coil; for each functional run, 99 sets of 36 contiguous axial images with isotropic voxels (4 mm3) were acquired parallel to the anterior-posterior commissure plane.
Functional Data Preprocessing
The fMRI data were preprocessed using the following steps: (1) compensation for slice-dependent time shifts; (2) removal of the first four images of each run to allow BOLD signal to reach steady state; (3) elimination of odd/even slice intensity differences due to interpolated acquisition; (4) realignment of data acquired in each subject within and across runs to compensate for rigid body motion51; (5) intensity normalization to a whole brain mode of 1,000; (6) registration of the 3-D structural volume (T1) to the atlas representative template in the Talairach coordinate system52 using a 12-parameter affine transform and resampling to 1 mm cubic representation51, 53; (7) coregistration of the 3-D fMRI volume to the T2, and the T2 to the participants structural image; (8) transformation of the fMRI to atlas space using a single affine 12-parameter transform that included resampling to a 3 mm cubic representation; and (9) spatial smoothing using a 6 mm full-width half-maximum Gaussian filter. Prior research has validated the use of this approach with children in our age range.54, 55
Further processing of fMRI data was performed using in-house software (FIDL analysis package, http://www.nil.wustl.edu/labs/fidl/index.html) utilized in previously published studies.30, 31, 35, 56–61 Estimates of BOLD response to each face type within each subject were obtained using fixed effects general linear models (GLM) incorporating regressors for linear trend and baseline shifts. A hemodynamic response shape was assumed (Boynton function) and used to derive magnitude estimates relative to fixation baseline. These single subject estimates were then entered into group-level analyses that treated subjects as random effects.
Image Quality Criteria
We applied stringent criteria for image data quality. First, the signal-to-noise ratio (SNR) was calculated on the face task runs, and subjects with an SNR above 200 were initially screened (n = 174 out of 209). This is a loss rate of approximately 17%, which is typical for developmental neuroimaging studies. Second, a `motion scrubbing' procedure, previously validated corrections for head motion used for functional connectivity analysis,62 was applied to the present task-related fMRI analysis by assessing frame-wise displacement based on the movement parameters.63 In this procedure, frame-wise displacement detects the differential head motion from the previous frame summing across linear (x, y, z) and rotational displacements (yaw, pitch, roll, where degrees of rotation are converted to millimeters of movement by calculating displacement on the surface of a sphere with a radius of 50 mm) for any given frame (i.e., time point). A temporal mask was created to remove any frame with a sum displacement greater than 0.9 mm. This threshold was selected to be stringent and remove any spikes in head motion while still maintaining the majority of the data. We retained 148 subjects who had more than 100 frames of data that survived “scrubbing.” Details on the validity and efficacy of this procedure have been reported previously.64
Data Analysis
Demographic and clinical differences (age, gender, handedness, family income at scan, average core MDD severity score across the assessments, and co-morbidity) between the MDD, healthy, and OPC groups were tested using one-way ANOVAs or χ2 tests. If the ANOVAs revealed significant group differences, post hoc tests with a Bonferroni correction were used. The total number of each of stressful and traumatic life events up to the scan was also compared between the groups. Each variable had 1–3 outliers with a standard score ≥ 3, thus Winsorising was performed such that these extreme values were set to the closest non-extreme value (with a standard score < 3). If any demographic variable was related to diagnostic group or life events, we included that variable as covariate in subsequent analyses.
To examine the relationship between life events and functional brain activity, we used a priori region of interest (ROI) mask. As described above, the selected ROIs were those demonstrated to be relevant in affective processing associated with MDD: amygdala, hippocampus, basal ganglia, medial and dorsolateral PFC, and ACC. Anatomical templates for our mask were derived from prior work65–67 (see Figure S1, available online). The PFC mask was defined on an atlas-representative image using the boundaries described by Rajkowska and Goldman-Rakic.68, 69 The cingulate mask used centroids of activation identified in prior studies49, 70, 71 around which we drew 25 mm diameter spherical ROIs edited to respect gray matter boundaries on an atlas-representative image.
We computed six sets of voxel-wise regression analyses within the mask described above. For the dependent variables, we subtracted the magnitude of BOLD response to neutral faces from the magnitude of BOLD response to (1) fearful faces, (2) sad faces, and (3) happy faces for each participant. The predictor variables were MDD status, OPC status, either cumulative stressful or traumatic life events, and their interactions. These regression analyses were conducted with correction for multiple comparisons using simulations to generate a p-value and cluster size criterion that provide a false positive rate of p < .05 for the whole ROI mask.72, 73 This threshold/cluster-size requirement provides protection against type I error and was chosen based on Monte-Carlo simulations via AlphaSim (Ward, 2000) and was set at a threshold of z = 2.6 at p < .0094 and 20 voxels. If any effect was found significant, multivariate outliers were evaluated, assessing the probability of Mahalanobis distance. If any multivariate outliers were detected (i.e., Mahalanobis D2 with p ≤ .001), we removed them and tested the effect again. All results reported below were those that survived when multivariate outliers were removed. For exploratory purposes, we additionally ran whole-brain analyses of life stress and trauma relationships to fearful, sad, and happy faces (presented in Tables S2 and S3, available online).
Moreover, for some regions, we found significant relationships between life events and brain activity in response to one facial emotion type, but not for another emotion type. To determine whether such relationships were significantly stronger for one emotion type versus another, we used repeated-measures analyses of covariance (ANCOVAs) for each identified region. These follow-up ANCOVAs added emotional face type and all possible interactions with emotional face type as within-subjects variables to each original regression model. When the interaction with emotional face type was significant, we computed partial correlations between an identified predictor and BOLD response (covarying for the other predictors) for each emotional face type to confirm the pattern of relationships.
RESULTS
Demographic and Clinical Characteristics
Table 1 shows that there were no differences in age, sex, handedness, or family income between the MDD, healthy, and OPC groups. However, the groups differed in stressful (F(2,112) = 9.15, p < .01) and traumatic life events (F(2,112) = 14.16, p < .01). Post hoc contrasts indicated that the MDD group experienced more stressful and traumatic life events than the healthy control group (p < .01). Moreover, the OPC group showed more traumatic life events than the healthy control group (p < .05). Within the healthy control group, those whose life events were assessed retrospectively reported less stressful (F(1,49) = 16.02, p < .01) and traumatic life events (F(1,49) = 37.62, p < .01) than the others who were enrolled since the baseline assessment.
Table 1.
Demographic and Clinical Characteristics of the Sample (N = 115)
| Variables | MDD (n = 42) | Healthy (n = 51) | Other psychiatric (n = 22) | F or X2 | |
|---|---|---|---|---|---|
| aAge (years) | 9.81(1.23) | 9.80(1.36) | 10.18(1.47) | 0.70 | |
| Sex (female/male) | 22/20 | 24/27 | 13/9 | 0.92 | |
| Handedness (left/right/both) | 2/40/0 | 7/43/1 | 2/20/0 | 3.51 | |
| Family income at scan | ≤ $20,000 | 13 | 7 | 3 | |
| $20,001–$40,000 | 8 | 11 | 3 | ||
| $40,001–$60,000 | 6 | 11 | 2 | 8.69 | |
| ≥ $60,000 | 15 | 22 | 14 | ||
| aCumulative life events | Stress | 11.71(6.32) | 6.45(5.34) | 9.41(6.46) | 9.15** |
| Trauma | 8.21(7.80) | 2.47(2.00) | 6.36(4.52) | 14.16** | |
| aAverage core MDD severity across assessments | 3.13(1.14) | 1.10(0.92) | 1.77(0.84) | 48.42** | |
| Co-morbidity | Externalizing | b24(17) | 0 | b8(5) | 38.44** |
| Non-MDD Internalizing | b26(17) | 0 | b17(5) | 56.19** | |
Note: MDD = major depressive disorder.
Data presented as mean (standard deviation).
A value within a parenthesis indicates the number of subjects who showed both externalizing and non-MDD internalizing co-morbidities.
p < .01
We also assessed whether age, sex, or family income was associated with stressful or traumatic life events, respectively. Age was not related to stressful life events, but it was related to traumatic life events (r(113) = .23, p < .05). There were no gender differences in stressful or traumatic life events. Finally, family income was related to stressful life events (r(113) = −.35, p < .01), but not traumatic life events. Therefore, we subsequently controlled for (1) family income in the analysis of stressful life events and (2) age in the analysis of traumatic life events.
Table 1 also indicates that co-morbidity rates within the MDD group were 57% for any externalizing disorders and 62% for non-MDD internalizing disorders; 40% of the MDD group showed both externalizing and internalizing comorbid disorders. Table S1 (available online) illustrates a comparison of each Axis I disorder between the MDD and OPC groups. Within the OPC group, there were relatively high proportions of anxiety disorders. However, there were no significant differences in the proportion of each specific Axis I disorder, except for MDD (by definition), between the MDD and OPC groups.
Stressful Life Events
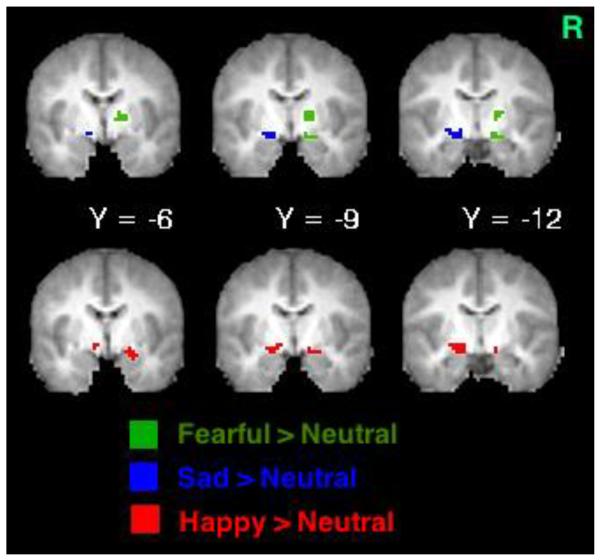
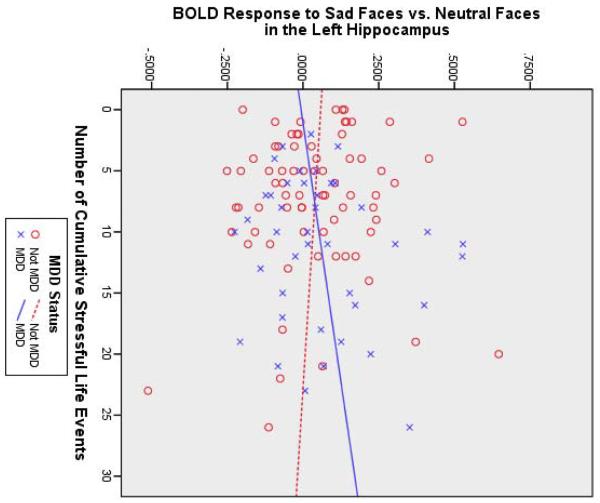
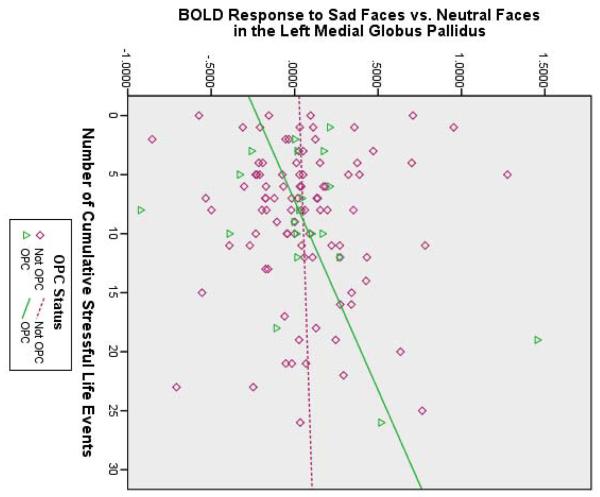
Table 2 shows that stressful life events predicted (1) increased BOLD response to fearful faces in the right amygdala/posterior entorhinal cortex and lateral globus pallidus, (2) increased BOLD response to sad faces in the left amygdala, and (3) increased BOLD response to happy faces in the left medial globus pallidus and right amygdala (also see Figure 1A). Furthermore, stressful life events significantly interacted with (1) MDD status to predict BOLD response to sad faces in the left hippocampus and (2) OPC status to predict BOLD response to sad faces in the left medial globus pallidus. Figure 1B shows that the MDD group tended to show a positive relationship between stressful life events and left hippocampal activity, whereas the non-MDD groups tended to show a negative relationship. For sad faces, there was also a main effect of OPC status; children with other psychiatric disorders showed less activation in the left perigenual ACC (pgACC) than the other children, despite their stressful experience. Figure 1C shows that the OPC group demonstrated a positive relationship between stressful life events and left medial globus pallidus activity, compared to the non-OPC groups.
Table 2.
Region of Interest Analysis of Stressful Life Events. (N = 115)
| Regression Coefficient | Talairach Coordinate | Interact w/emotion | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Brain region | B | SE B | β | t | X | Y | Z | Cluster Size | F |
| Outcome Variable: Fearful > Neutral | |||||||||
| Predictor Variable: Main Effect of Cumulative Stressful Life Events (SLE) | |||||||||
| R PEC (BA28)/Amygdala | .033 | .009 | .464 | 3.61** | 19 | −14 | −10 | 25 | 3.14* |
| R Lateral GP | .011 | .003 | .518 | 4.17** | 16 | −7 | 5 | 27 | 1.00 |
|
No Other Significant Main Effects or Interactions
| |||||||||
| Outcome Variable: Sad > Neutral | |||||||||
| Predictor Variable: Main Effect of Cumulative SLE | |||||||||
| L Amygdala | .016 | .008 | .293 | 2.15* | −19 | −12 | −9 | 32 | .36 |
| Predictor Variable: Main Effect of MDD Status (MDD) | |||||||||
| No Main Effect | |||||||||
| Predictor Variable: Main Effect of Other Psychiatric Control Status (OPC) | |||||||||
| L pgACC (BA32) | −.404 | .108 | −.624 | 3.76** | −6 | 42 | 0 | 20 | 2.12 |
| Predictor Variable: Interaction of SLE × MDD | |||||||||
| L Hippocampus | .016 | .007 | .469 | 2.38* | −25 | −14 | −9 | 20 | 1.04 |
| Predictor Variable: Interaction of SLE × OPC | |||||||||
| L Medial GP | .041 | .016 | .541 | 2.61* | −18 | −11 | −7 | 31 | 1.19 |
|
Outcome Variable: Happy > Neutral | |||||||||
| Predictor Variable: Main Effect of Cumulative SLE | |||||||||
| L Medial GP | .025 | .007 | .463 | 3.46** | −17 | −11 | −8 | 31 | 1.31 |
| R Amygdala | .020 | .010 | .318 | 2.06* | 19 | −6 | −12 | 22 | 2.74 |
| No Other Significant Main Effects or Interactions | |||||||||
Note: Multivariate outliers were excluded; the column labeled as “Interact w/ emotion” represents the F-ratio for the interaction between emotional face type and the variable indicated in each row. B = unstandardized coefficient; β = standardized coefficient; BA = Brodmann area; GP = globus pallidus; L = left hemisphere; MDD = major depressive disorder; PEC = posterior entorhinal cortex; pgACC = perigenual anterior cingulate cortex; R = right hemisphere; SE B = standard error of B.
p < .05;
p < .01
Figure 1.
(A) Brain activity to negative faces (green areas activated by fearful faces; blue areas activated by sad faces) and happy faces (red areas) increased as a function of cumulative stressful life events and scatterplot illustrating interactions between (B) cumulative stressful life events and major depressive disorder (MDD) status and (C) cumulative stressful life events and other psychiatric control (OPC) status. Note: BOLD = blood oxygen level-dependent; R = right side.
Even after accounting for family income as a covariate, stressful life events continued to predict left amygdala activity to sad faces (β = .25, t(105) = 1.75, p = .083) and right amygdala activity to happy faces (β = .27, t(106) = 1.72, p = .089) at a marginal level. The other effects and interactions remained significant at p < .05. Finally, as described above, some regions showed relationships between stressful life events and responses to one emotional face type. Follow-up ANCOVAs showed that the right amygdala identified in the regression for fearful faces showed a significant interaction with emotion type (see Table 2). The relationship between stressful life events and right amygdala activity was significant only when children viewed fearful faces (p < .01) and happy faces (p < .01), but not sad faces.
Traumatic Life Events
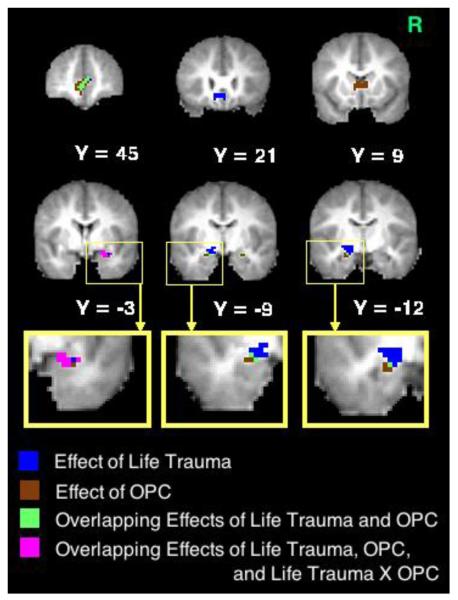
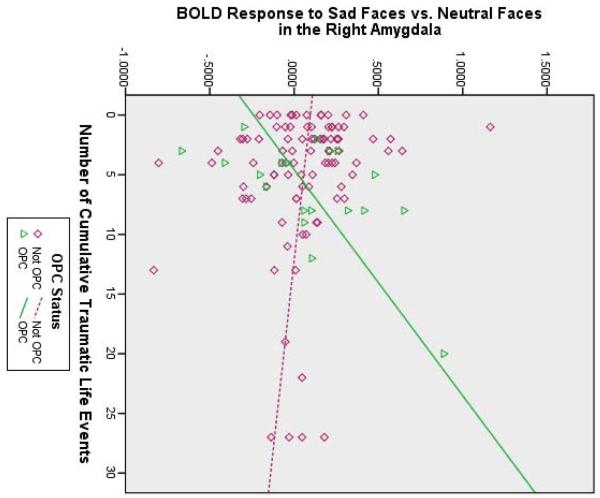
Table 3 revealed that none of the variables predicted BOLD response to fearful faces. However, when processing sad faces (see Figure 2A), traumatic life events were associated with greater functional activation in the left amygdala/posterior entorhinal cortex, the right amygdala, and the bilateral sgACC and pgACC. Moreover, there was a main effect of OPC status, such that, despite their traumatic experience, the OPC group showed less activity in the bilateral amygdala, pgACC, and caudate head, compared to the non-OPC groups. Finally, there was a significant interaction between traumatic life events and OPC status predicting right amygdala activation to sad faces; Figure 2B shows that the positive relationship between traumatic life events and right amygdala activity to sad faces was only present in the children with other psychiatric disorders. Thus, traumatic life events influenced left amygdala activity to sad faces among all children, but the effects of traumatic life events on right amygdala activity to sad faces depended on OPC status (see Figure 2A). All of the above effects remained significant at p < .05 when controlling for age.
Figure 2.
Brain activity to sad faces predicted by each effect. (A) Voxels predicted by each effect and (B) scatterplot illustrating an interaction between cumulative traumatic life events and other psychiatric control (OPC) status. Note: R = right side.
When processing happy faces, despite their traumatic experience, the OPC group showed less brain activity in the left dorsal ACC and right amygdala/posterior entorhinal cortex. Again, these main effects remained significant at p < .05 when controlling for age.
As with stressful life events, specificity between traumatic life events and responses by emotion type was also found in some regions. Follow-up ANCOVAs identified that (1) traumatic life events significantly interacted with emotional face type in predicting right amygdala activity, bilateral sgACC activity, and bilateral pgACC activity and (2) OPC status significantly interacted with emotional face type in predicting left amygdala, right amygdala, bilateral pgACC, and bilateral caudate head activity (see Table 3). Subsequent partial correlations confirmed that the relationships between trauma and activity in the right amygdala, bilateral sgACC, and bilateral pgACC were present for sad (p < .01) and happy faces (p < .05), but not for fearful faces. Moreover, the relationships to OPC status indicated that children with other psychiatric disorders showed reduced activation in the left amygdala and bilateral pgACC on sad faces (p < .01), as well as the right amygdala and caudate on sad (p < .01) and happy faces (p < .05).
DISCUSSION
The aim of the present study was to examine our hypotheses that a greater number of stressful and traumatic life events would predict greater cortico-limbic activation in children, particularly in response to fearful or sad faces, depending on the child's history of MDD. Three major findings were obtained.
First, our results revealed that both stressful and traumatic life events show similar relationships to amygdala reactivity. That is, an increase in the number of life events, including both stress and trauma, was associated with increased functional activation to emotional faces in the amygdala, particularly the centromedial subregion and even extending dorsally. These results demonstrate that increased amygdala reactivity can occur not only in individuals with a history of child deprivation and maltreatment10, 20 but also in individuals who have experienced a broader array of life stressors and traumas. However, the pattern of effects across face types was somewhat different, depending on whether children experienced stressful or traumatic life events. Children exposed to stressful life events showed increased functional reactivity to emotional face processing across a number of facial emotion types (see Figure 1A). Although the effect of stressful life events on right amygdala activity was exceptionally specific to fearful and happy faces, no other valence-specific effects were found (see Table 2). Thus, exposure to stressful life events increases reactivity to emotional faces relatively generally. In contrast, children exposed to traumatic life events demonstrated increased amygdala and ACC activity only to sad faces (see Table 3), suggesting a more specific effect of this experience. It was somewhat surprising that amygdala reactivity to fearful faces was not related to traumatic life events. This could be consistent with desensitization or “burn-out” to fearful stimuli in those experiencing early trauma, although directed study of this issue is needed. Interestingly, there is mixed evidence regarding the effects of stressors on amygdala reactivity, as relationships are sometimes found across emotional valence74 but also sometimes only found with sad stimuli.75 Our findings suggest that the pattern of effects across emotion types may vary as a function of stressor severity, and more research is needed to examine this issue.
Another interesting finding about stress/trauma effects was that early life trauma was associated with increased functional activity in the bilateral sgACC (close to ventral medial PFC) and pgACC, and this relationship was present only during viewing sad faces; in contrast, emotional ACC reactivity was not related to early life stress. The sgACC and pgACC are involved in the neurocircuitry of anxiety, sadness, and MDD.76–78 For example, a high proportion (46%) of neuroimaging studies have found that functional activation in the ACC is associated with the induction of sad mood,79 and structural differences in the ACC have been consistently reported in adults and adolescents with MDD.80, 81 Moreover, it has been reported that electrical stimulation of the sgACC helps recovery in patients with treatment-resistant MDD.80, 82, 83 Our results suggest that the ACC function during sadness processing may be altered as a function of early life trauma, which may provide a neurobiological explanation for why early life trauma is a risk factor for increasing depression severity.13, 23, 38, 84
The second major finding was that there was an interaction between early life stress and MDD status in predicting left hippocampal activity (see Table 2). Specifically, children with a history of MDD tended to show greater functional activation in the hippocampus to sad faces as the amount of early life stress increased. A few studies have reported that adults and children with MDD show increased right (though not left) hippocampal activity when processing sad faces.31, 85 Interestingly, MDD is frequently associated with a bias towards sad faces,86, 87 and, in prior work, patients with MDD showed increased left hippocampal activity during viewing sad faces relative to happy faces.88 In contrast, healthy individuals typically show a bias towards happy faces,87 and, in prior work, healthy individuals showed increased left hippocampal activity during viewing happy faces relative to sad faces.88 These studies (i.e., the differing face conditions during which MDD versus healthy controls showed increased left hippocampal activity) suggest the possibility (although speculative) that the left hippocampus may be involved in subjects' attentional bias. However, the present study did not measure an attentional bias, and additional work will be needed to test this hypothesis more directly. Further, we did not find any other main effect or interaction relating specifically to MDD. For instance, there was no interaction between traumatic life events and MDD status in predicting amygdala or ACC reactivity. One speculative hypothesis is that interactions between MDD status and life events may become more apparent as these children pass through puberty and into adulthood, but this hypothesis needs to be tested through longitudinal follow-up.
The third major finding was that there were interactions between life events and other psychiatric status in predicting functional brain activity. Children with psychiatric disorders other than MDD (mostly anxiety disorders, as shown in Table S1, available online) showed increased left medial globus pallidus activity to sad faces when they had experienced a greater number of stressful life events (see Table 2). They also demonstrated increased right amygdala activity to sad faces with an increased number of traumatic life events (see Table 3). In contrast, children with a history of MDD and healthy children did not show a change in functional response in these regions as a function of life events. Hence, while traumatic life events had a main effect on left amygdala activity to sad faces in children with/without any psychiatric disorder, the functional change in the right amygdala was more pronounced in children with a history of non-MDD psychiatric disorders. This may be consistent with prior findings that children with a history of institutional care, who exhibit any externalizing and/or internalizing problem, showed bilateral amygdala hyperactivity to negative stimuli.20 Furthermore, early life trauma and altered right amygdala activity for sadness could be important in understanding the developmental trajectory of these disorders.
Some limitations in our study should be mentioned. First, although we found significant associations between stress/trauma and functional brain changes, inferences about causal relationships cannot be made. Second, approximately half of the healthy control group was recruited at the scan wave (28 of 51 children) and reported stress and trauma retrospectively. Because their retrospective reporting showed less stressful and traumatic life events than the other healthy children (discussed above), the occurrence of life events might be underestimated in this group. Third, our MDD group showed high levels of co-morbid anxiety or other psychiatric disorders (common in childhood MDD), making it difficult to test stress/trauma effects between the MDD and OPC groups. Nevertheless, Table S1 (available online) illustrates that there was no group difference in the proportion of any Axis I disorder except for MDD. Hence, our results suggest that differential effects of stress/trauma on brain activity between the MDD and OPC groups are present. Fourth, while the present study followed a common procedure in the literature where neutral faces were used as baseline, functional brain activity to neutral faces themselves could vary in relation to life events and/or diagnostic status.89 If this is the case, then neutral faces may not be an ideal control condition. Future research would benefit from administering other types of control conditions (e.g., non-face images) in addition to neutral faces in order to examine any stress/MDD effects on functional brain activity to neutral faces relative to non-facial stimuli.
In summary, our data extend the previous literature and highlight the critical neurobiological effects of cumulative experience of stress and trauma during early childhood. Overall, not only children with MDD or other psychiatric disorders, but also healthy children showed enhanced functional limbic activity in areas such as the amygdala and ACC based on past experiences of stress and trauma. Moreover, MDD status interacted with life stress in predicting left hippocampal activity, and other psychiatric diagnostic status interacted with stress and trauma in predicting left medial globus pallidus activation and right amygdala activation. These data suggest that there may be unique developmental trajectories of alterations in emotion processing in response to early life stress and trauma informing risk pathways for childhood psychiatric outcomes.
Supplementary Material
Figure S1. Region of interest (ROI) mask used in the present study (shown in blue).
Clinical Guidance.
Children with a history of high life stress, whether or not they had a psychiatric disorder, showed increased functional reactivity to both negative and positive emotional faces; such increased activity was found in the amygdala and globus pallidus.
Children with a history of high life trauma, whether or not they had a psychiatric disorder, showed increased functional reactivity to only sad faces; such increased activity was found in the amygdala and subgenual and perigenual anterior cingulate cortex.
Children with a history of MDD showed increased left hippocampal reactivity to sad faces if their life stress was high, whereas this relationship was not present in the other children.
Children with a history of non-MDD psychiatric disorder showed increased left globus pallidus reactivity (when their life stress was high) or right amygdala reactivity (when their life trauma was high) to sad faces, but the other children did not show this relationship.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants MH064769 (J.L.L.) and MH090786 (J.L.L., D.M.B., K.N.B.).
The authors would like to thank the families and children who participated in these studies, and special thanks to staff members at the Early Emotional Development Program (EEDP) at Washington University in St. Louis for their assistance.
Dr. Luby has received grant or research support from the National Institute of Mental Health (NIMH), the Communities Healing Adolescent Depression and Suicide (CHADS) Coalition, and the Sydney R. Baer Foundation. Dr. Botteron has received grant or research support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Biomedical Imaging and Bioengineering, NIMH, and Autism Speaks. Dr. Barch has received grant or research support from NIMH, the Brain & Behavior Research Foundation, and NIH. She has also served as a consultant for Pfizer, Roche, and Angen on schizophrenia-related work.
Footnotes
Disclosure: Drs. Suzuki, McAvoy, and Ms. Dietrich report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010 Sep;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002 Aug 1;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radley JJ, Rocher AB, Rodriguez A, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008 Mar 1;507(1):1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuramochi M, Nakamura S. Effects of postnatal isolation rearing and antidepressant treatment on the density of serotonergic and noradrenergic axons and depressive behavior in rats. Neuroscience. 2009 Sep 29;163(1):448–455. doi: 10.1016/j.neuroscience.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006 Aug;31(8):1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 6.Lucas LR, Celen Z, Tamashiro KL, et al. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012 Jul 1;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008 Summer;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmiston EE, Wang F, Mazure CM, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med. 2011 Dec;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012 Feb 15;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Admon R, Lubin G, Stern O, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med. 2012 Oct 18;:1–10. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011 Mar;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang TY, Lin CH. Role of amygdala MAPK activation on immobility behavior of forced swim rats. Behav Brain Res. 2006 Oct 2;173(1):104–111. doi: 10.1016/j.bbr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012 May 30;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luby JL, Barch DM, Belden A, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between Income and the Hippocampus. PLoS One. 2011 May 4;6(5) doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson JL, Chung MK, Avants BB, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010 Jun 2;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tottenham N, Hare TA, Quinn BT, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010 Jan 1;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin J, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011 Mar;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001 Jun 15;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010 Feb;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luby JL, Belden AC, Spitznagel E. Risk factors for preschool depression: the mediating role of early stressful life events. J Child Psychol Psychiatry. 2006 Dec;47(12):1292–1298. doi: 10.1111/j.1469-7610.2006.01672.x. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005 Dec;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012 Jan;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress Anxiety. 2008;25(6):514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- 28.Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. J Pediatr Psychol. 2010 Jun;35(5):559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff B, Gee DG, Telzer EH, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2012 Dec 20; doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: An fMRI study. J Affect Disorders. 2011 Mar;129(1–3):364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biol Psychiatry. 2012 Dec 15;72(12):1035–1042. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuhrmann A, Dohm K, Kugel H, et al. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci. 2013 Jul;38(4):249–258. doi: 10.1503/jpn.120060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suslow T, Konrad C, Kugel H, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiat. 2010 Jan 15;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010 Nov;67(11):1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Botteron KN, Luby JL, et al. Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cogn Affect Behav Neurosci. 2013 Mar;13(1):135–151. doi: 10.3758/s13415-012-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaffrey MS, Luby JL, Repovs G, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010 Dec 29;21(18):1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luby J, Heffelfinger A, Mrakrotsky C, Hildebrand T. Preschool Feelings Checklist. St. Louis, Missouri: 1999. [Google Scholar]
- 38.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009 Aug;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egger H, Ascher B, Angold A. The Preschool Age Psychiatric Assessment. Version 1.4 Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; Durham, NC: 1999. p. 2003. [Google Scholar]
- 40.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006 May;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 41.Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA) Psychol Med. 1995 Jul;25(4):739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- 42.Angold A, Costello EJ. A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C) Psychol Med. 1995 Jul;25(4):755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- 43.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disorders. 2009 Jan;112(1–3):111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 2002 Aug;41(8):928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Gaffrey MS, Belden AC, Luby JL. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. J Affect Disorders. 2011 Oct;133(3):537–545. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiat. 2004 Nov;161(11):1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- 47.Costello EJ, Angold A, March J, Fairbank J. Life events and post-traumatic stress: the development of a new measure for children and adolescents. Psychol Med. 1998 Nov;28(6):1275–1288. doi: 10.1017/s0033291798007569. [DOI] [PubMed] [Google Scholar]
- 48.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009 Aug 15;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiat. 2008 Feb 15;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiat. 2008 Jan;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 51.Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 52.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 53.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004 Oct;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002 Sep;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 55.Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003 May;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 56.Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI - II. Analysis. Neuroimage. 2001 Jan;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- 57.Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI - I. The method. Neuroimage. 2001 Jan;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- 58.Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. P Natl Acad Sci USA. 2009 May 5;106(18):7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. P Natl Acad Sci USA. 2010 May 11;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savine AC, Braver TS. Motivated Cognitive Control: Reward Incentives Modulate Preparatory Neural Activity during Task-Switching. J Neurosci. 2010 Aug 4;30(31):10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Becerril KE, Repovs G, Barch DM. Error processing network dynamics in schizophrenia. Neuroimage. 2011 Jan 15;54(2):1495–1505. doi: 10.1016/j.neuroimage.2010.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012 Feb 1;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagliaccio D, Luby JL, Gaffrey MS, et al. Functional brain activation to emotional and nonemotional faces in healthy children: Evidence for developmentally undifferentiated amygdala function during the school-age period. Cogn Affect Behav Neurosci. 2013 May 1; doi: 10.3758/s13415-013-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel JS, Power JD, Dubis JW, et al. Statistical improvements in fMRI analyses produced by censoring high motion datapoints. Hum Brain Mapp. doi: 10.1002/hbm.22307. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mamah D, Wang L, Csernansky JG, Rice JP, Smith M, Barch DM. Morphometry of the hippocampus and amygdala in bipolar disorder and schizophrenia. Bipolar Disord. 2010 May;12(3):341–343. doi: 10.1111/j.1399-5618.2010.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Mamah D, Harms MP, et al. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol Psychiatry. 2008 Dec 15;64(12):1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res. 2007 Jan;89(1–3):59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the talairach coordinate system. Cerebral Cortex. 1995;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 69.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonid definition of prefrontal areas in normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 70.Fales CL, Barch DM, Rundle MM, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009 Jan;112(1–3):206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009 Jan 26; doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage. 2001 Jun;13(6):S198–S198. [Google Scholar]
- 73.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic-Resonance-Imaging (Fmri) -Use of a Cluster-Size Threshold. Magnet Reson Med. 1995 May;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 74.van Harmelen AL, van Tol MJ, Demenescu LR, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci. 2013 Apr;8(4):362–369. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dannlowski U, Kugel H, Huber F, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013 Nov;34(11):2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011 Jan;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000 Jun;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 78.Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- 79.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004 Apr;9(4):258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 80.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011 Feb 15;69(4):301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 81.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002 Feb 15;51(4):342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 82.Lozano AM, Giacobbe P, Hamani C, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012 Feb;116(2):315–322. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 83.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005 Mar 3;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Hopkins J, Lavigne JV, Gouze KR, Lebailly SA, Bryant FB. Multi-domain Models of Risk Factors for Depression and Anxiety Symptoms in Preschoolers: Evidence for Common and Specific Factors. J Abnorm Child Psychol. 2013 Mar 17; doi: 10.1007/s10802-013-9723-2. [DOI] [PubMed] [Google Scholar]
- 85.Fu CH, Williams SC, Cleare AJ, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008 Sep 15;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 86.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004 Feb;113(1):121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 87.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007 Feb;116(1):80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 88.Victor TA, Furey ML, Fromm SJ, Bellgowan PS, Ohman A, Drevets WC. The extended functional neuroanatomy of emotional processing biases for masked faces in major depressive disorder. PLoS One. 2012;7(10):e46439. doi: 10.1371/journal.pone.0046439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mourao-Miranda J, Oliveira L, Ladouceur CD, et al. Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PLoS One. 2012;7(2):e29482. doi: 10.1371/journal.pone.0029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Region of interest (ROI) mask used in the present study (shown in blue).