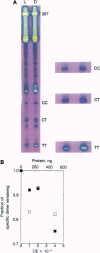
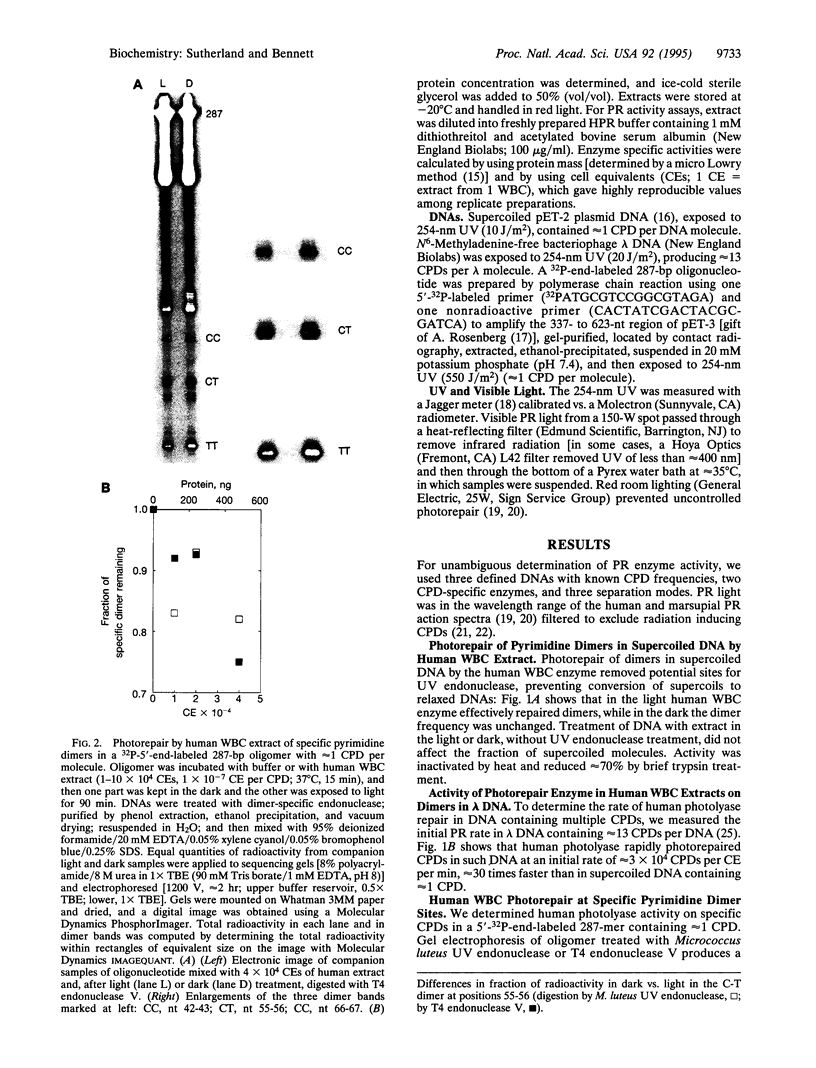
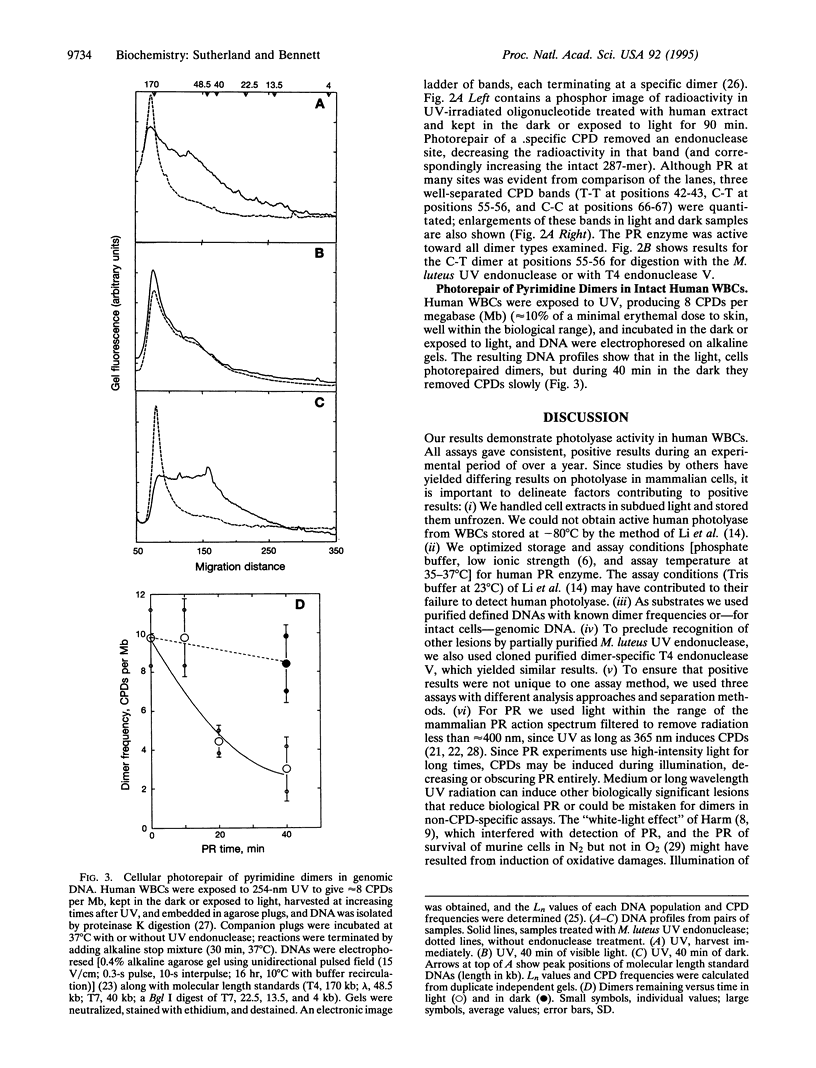
Abstract
Although enzymatic photoreactivation of cyclobutyl pyrimidine dimers in DNA is present in almost all organisms, its presence in placental mammals is controversial. We tested human white blood cells for photolyase by using three defined DNAs (supercoiled pET-2, nonsupercoiled bacteriophage lambda, and a defined-sequence 287-bp oligonucleotide), two dimer-specific endonucleases (T4 endonuclease V and UV endonuclease from Micrococcus luteus), and three assay methods. We show that human white blood cells contain photolyase that can photorepair pyrimidine dimers in defined supercoiled and linear DNAs and in a 287-bp oligonucleotide and that human photolyase is active on genomic DNA in intact human cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. V., Sutherland B. M. Quantitative detection of single-copy genes in nanogram samples of human genomic DNA. Biotechniques. 1993 Sep;15(3):520–525. [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. Photoreactivating enzyme in logarithmic-phase and stationary-phase yeast cells. Biochim Biophys Acta. 1967 Sep 26;145(2):502–505. doi: 10.1016/0005-2787(67)90068-8. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T., Rupert C. S. Action spectrum for photoreactivation of ultraviolet-irradiated marsupial cells in tissue culture. Photochem Photobiol. 1979 Oct;30(4):525–528. doi: 10.1111/j.1751-1097.1979.tb07173.x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Photoraactivation: a radiation repair mechanism absent from mammalian cells. Biochem Biophys Res Commun. 1966 Aug 23;24(4):569–576. doi: 10.1016/0006-291x(66)90359-7. [DOI] [PubMed] [Google Scholar]
- Cook J. S., McGrath J. R. Photoreactivating-enzyme activity in metazoa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1359–1365. doi: 10.1073/pnas.58.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Bisaccia E., Whetstone J. W., Scarborough D. A., Lowney E. DNA repair in skin of lupus erythematosus following in vivo exposure to ultraviolet radiation. J Invest Dermatol. 1983 Nov;81(5):452–454. doi: 10.1111/1523-1747.ep12522651. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Whetstone J. W., Slazinski L., Lowney E. Photorepair of pyrimidine dimers in human skin in vivo. Photochem Photobiol. 1981 Oct;34(4):461–464. [PubMed] [Google Scholar]
- Freeman S. E., Blackett A. D., Monteleone D. C., Setlow R. B., Sutherland B. M., Sutherland J. C. Quantitation of radiation-, chemical-, or enzyme-induced single strand breaks in nonradioactive DNA by alkaline gel electrophoresis: application to pyrimidine dimers. Anal Biochem. 1986 Oct;158(1):119–129. doi: 10.1016/0003-2697(86)90599-3. [DOI] [PubMed] [Google Scholar]
- Freeman S. E., Hacham H., Gange R. W., Maytum D. J., Sutherland J. C., Sutherland B. M. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5605–5609. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm H. Damage and repair in mammalian cells after exposure to non-ionizing radiations. I. Ultraviolet and visible light irradiation of cells of the rat kangaroo (Potorous tridactylus) and determination of photorepairable damage in vitro. Mutat Res. 1978 Jun;50(3):353–366. doi: 10.1016/0027-5107(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Harm H. Damage and repair in mammalian cells after exposure to non-ionizing radiations. III. Ultraviolet and visible light irradiation of cells of placental mammals, including humans, and determination of photorepairable damage in vitro. Mutat Res. 1980 Jan;69(1):167–176. doi: 10.1016/0027-5107(80)90186-4. [DOI] [PubMed] [Google Scholar]
- Henderson E. E. Host cell reactivation of Epstein-Barr virus in normal and repair-defective leukocytes. Cancer Res. 1978 Oct;38(10):3256–3263. [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- Kato T., Jr, Todo T., Ayaki H., Ishizaki K., Morita T., Mitra S., Ikenaga M. Cloning of a marsupial DNA photolyase gene and the lack of related nucleotide sequences in placental mammals. Nucleic Acids Res. 1994 Oct 11;22(20):4119–4124. doi: 10.1093/nar/22.20.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ley R. D., Applegate L. A., Padilla R. S., Stuart T. D. Ultraviolet radiation--induced malignant melanoma in Monodelphis domestica. Photochem Photobiol. 1989 Jul;50(1):1–5. doi: 10.1111/j.1751-1097.1989.tb04123.x. [DOI] [PubMed] [Google Scholar]
- Ley R. D. Photoreactivation in humans. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4337–4337. doi: 10.1073/pnas.90.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. F., Kim S. T., Sancar A. Evidence for lack of DNA photoreactivating enzyme in humans. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4389–4393. doi: 10.1073/pnas.90.10.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. S., Haidle C. W., Robberson D. L. Bleomycin-specific fragmentation of double-stranded DNA. Biochemistry. 1978 May 16;17(10):1890–1896. doi: 10.1021/bi00603a014. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Cleaver J. E., Friedberg E. C., Paterson M. C., Smith B. P., Thomas G. H. Photoreactivation of thymine dimers in UV-irradiated human cells: unique dependence on culture conditions. Mutat Res. 1977 Sep;44(3):433–445. doi: 10.1016/0027-5107(77)90101-4. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Harm W. Analysis of photoenzymatic repair of UV lesions in DNA by single light flashes. IX. Excess production of photoreactivating enzyme in E. coli B s-1- 160 under different growth conditions, and its suppression by adenine. Mutat Res. 1972 Oct;16(2):121–131. doi: 10.1016/0027-5107(72)90172-8. [DOI] [PubMed] [Google Scholar]
- Ogut S. E., D'Ambrosio S. M., Samuel M., Sutherland B. M. DNA photoreactivating enzyme from human tissues. J Photochem Photobiol B. 1989 Oct;4(1):47–56. doi: 10.1016/1011-1344(89)80101-0. [DOI] [PubMed] [Google Scholar]
- Quaite F. E., Takayanagi S., Ruffini J., Sutherland J. C., Sutherland B. M. DNA Damage Levels Determine Cyclobutyl Pyrimidine Dimer Repair Mechanisms in Alfalfa Seedlings. Plant Cell. 1994 Nov;6(11):1635–1641. doi: 10.1105/tpc.6.11.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe E. M., Stevens G. H. The effects of ultraviolet and visible radiation on mouse ascites tumour cells. Photochem Photobiol. 1965 Sep;4(4):759–767. doi: 10.1111/j.1751-1097.1965.tb07918.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Roza L., De Gruijl F. R., Bergen Henegouwen J. B., Guikers K., Van Weelden H., Van Der Schans G. P., Baan R. A. Detection of photorepair of UV-induced thymine dimers in human epidermis by immunofluorescence microscopy. J Invest Dermatol. 1991 Jun;96(6):903–907. doi: 10.1111/1523-1747.ep12475429. [DOI] [PubMed] [Google Scholar]
- Saito N., Werbin H. Evidence for a DNA-photoreactivating enzyme in higher plants. Photochem Photobiol. 1969 Apr;9(4):389–393. doi: 10.1111/j.1751-1097.1969.tb07304.x. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Grist E., Thompson K., Woodhead A. D. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B. M., Bennett P. V., Conlon K., Epling G. A., Sutherland J. C. Quantitation of supercoiled DNA cleavage in nonradioactive DNA: application to ionizing radiation and synthetic endonuclease cleavage. Anal Biochem. 1992 Feb 14;201(1):80–86. doi: 10.1016/0003-2697(92)90176-8. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Harber L. C., Kochevar I. E. Pyrimidine dimer formation and repair in human skin. Cancer Res. 1980 Sep;40(9):3181–3185. [PubMed] [Google Scholar]
- Sutherland B. M., Oliver R. Culture conditions affect photoreactivating enzyme levels in human fibroblasts. Biochim Biophys Acta. 1976 Sep 6;442(3):358–367. doi: 10.1016/0005-2787(76)90310-5. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Oliver R., Fuselier C. O., Sutherland J. C. Photoreactivation of pyrimidine dimers in the DNA of normal and xeroderma pigmentosum cells. Biochemistry. 1976 Jan 27;15(2):402–406. doi: 10.1021/bi00647a025. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M. Photoreactivating enzyme from human leukocytes. Nature. 1974 Mar 8;248(5444):109–112. doi: 10.1038/248109a0. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Runge P., Sutherland J. C. DNA photoreactivating enzyme from placental mammals. Origin and characteristics. Biochemistry. 1974 Nov 5;13(23):4710–4715. doi: 10.1021/bi00720a005. [DOI] [PubMed] [Google Scholar]
- Sutherland J. C., Lin B., Monteleone D. C., Mugavero J., Sutherland B. M., Trunk J. Electronic imaging system for direct and rapid quantitation of fluorescence from electrophoretic gels: application to ethidium bromide-stained DNA. Anal Biochem. 1987 Jun;163(2):446–457. doi: 10.1016/0003-2697(87)90247-8. [DOI] [PubMed] [Google Scholar]
- Sutherland J. C., Sutherland B. M. Human photoreactivating enzyme action spectrum and safelight conditions. Biophys J. 2009 Jan 1;15(5):435–440. doi: 10.1016/S0006-3495(75)85828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Rice M., Sutherland B. M. Photoreactivation of herpes simplex virus in human fibroblasts. Nature. 1975 Apr 17;254(5501):627–628. doi: 10.1038/254627a0. [DOI] [PubMed] [Google Scholar]
- Yasui A., Eker A. P., Yasuhira S., Yajima H., Kobayashi T., Takao M., Oikawa A. A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J. 1994 Dec 15;13(24):6143–6151. doi: 10.1002/j.1460-2075.1994.tb06961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]