Abstract
AIM: To study the relation between acoustic parameters and histological structure of biological tissue and to provide the basis for high-resolution image of biological tissues and quantitative ultrasonic diagnosis of liver disease.
METHODS: Ultrasonic imaging and tissue characterization of four normal porcine liver and five cirrhotic liver tissue samples were performed using a high frequency imaging system.
RESULTS: The acoustic parameters of cirrhotic liver tissue were larger than those of normal liver tissue. The sound velocity was 1577 m/s in normal liver tissue and 1631 m/s in cirrhotic liver tissue. At 35 MHz, the attenuation coefficient was 3.0 dB/mm in normal liver tissue and 4.1 dB/mm in cirrhotic liver tissue. The backscatter coefficient was 0.00431 dB/Srmm in cirrhotic liver tissue and 0.00303 dB/Srmm in normal liver tissue. The backscatter coefficient increased with the frequency. The high frequency images coincided with their histological features.
CONCLUSION: The acoustic parameters, especially the sound backscatter coefficient, are sensitive to the changes of liver tissues and can be used to differentiate between the normal and pathological liver tissues. High frequency image system is a useful device for high-resolution image and tissue characterization.
Keywords: Porcine liver tissue, Hepatocirrhosis, High frequency imaging, Tissue characterization, Acoustic parameter
INTRODUCTION
Ultrasonic diagnosis has been widely used in the clinical medicine. However, contemporary ultrasonic diagnosis technique is limited to the qualitative description at frequency ranging between 0.5-10 MHz based on the gray scale presentation in ultrasonic image. Due to the longer wavelength at a low frequency, the image resolution is not very high. Besides, the physical quantity using the ultrasonic image is the amplitude of the echo while the spectrum of the echo is overlooked.
The research on the ultrasound scatter spectrum has begun since 1970s. Shung and Reid[1] conducted an experimental study of ultrasound backscatter in calf liver and muscle using the pulse insert-substitution method. The backscatter coefficients for calf heart, kidney, pancreas and spleen were studied by Ten and Shung[2] at frequency between 2-7 MHz using bandwidth pulse insert-substitution measurement system. The ultrasound backscatter in myocardial muscle of dog was studied by Donnell et al[3]. The anisotropy for ultrasound backscatter in myocardial muscle was reported by O’Mottley and Miller[4], which coincides with the theoretical prediction. The backscatter coefficient for human brain was studied at frequency between 0.5-1.5 MHz by Barger[5], who successfully differentiated the white from the gray matters in the brain. Nicholas[6] studied the backscatter coefficient of human tissues using spectrum technique. However, the frequency used in these researches is below 10 MHz. To increase the resolution of imaging, the frequency of ultrasound must be increased. D’Astous et al[7] and Foster et al[8] have achieved the high frequency imaging of breast. Turnbull[9-12] studied mouse embryos using ultrasound backscatter microscopy. Ye et al[13] and Pavlin et al[14,15] studied the ocular tissue using high frequency imaging. The ultrasonic properties of vascular tissues and blood were measued from 35 to 65 MHz by Lockwood et al[16] and Meyer et al[17]. Yano et al[18] and Liu et al[19] studied the high frequency imaging of skin and thyroid. Ultrasound backscatter microscopy images of the internal structure of living tumor spheroids have been obtained[20,21].
Liver is the important organ for metabolism. Hepatic lobule is the basic element in liver, and is surrounded by connective tissue. Most blood capillaries pass through the boundary of its connective tissues. Hepatic tissue regeneration and connective tissue hyperplasia induce change of the normal structure of hepatic lobule. The liver becomes deformed and stiff, known as hepatocirrhosis. Common liver diseases can be diagnosed by B- ultrasound. However, it is difficult to diagnose diffuse diseases of liver.
The present study was to investigate the relation between acoustic parameters and histological structure of biological tissue and to provide the basis for high-resolution image of biological tissues and quantitative ultrasonic diagnosis of liver disease.
MATERIALS AND METHODS
Experimental system
High frequency imaging system was set up in our laboratory. The sample was fixed in the sample-holder between quartz and a thin plastic membrane and then mounted in water bath. The focused ultrasound transducer (center frequency 35 MHz, -3dB bandwidth from 25 MHz to 55 MHz, f-number 1.6, focus depth 1 mm, beam width 70 µm) was excited with a pulse (300 Vpp in amplitude, 15 ns in width). The ultrasonic pulse passing through the membrane, sample reflected from the quartz flat was received by the same transducer. The transducer and the step motor were carefully moved, the region of interest in the sample was located in the focus area and in the focal depth of the transducer. The scan parameters for raster motion were downloaded by MTM2500 pp (Newport Co, USA). The attenuation signal and backscatter signal were recorded by a 400 MHz digital scope (HP54502A, USA). The information about the C-scan imaging was acquired by sampling the backscatter signal after a specified delay corresponding to the focus and stored in a hard disk for software scan conversion after data collection was completed. An IEEE-488 bus transferred the digitized signals to the control computer for further processing
The transducer in a raster fashion over a 3 mm × 5 mm area was removed and a backscatter image of the sample was generated using C-scan mode. The region of homo-genous tissue was selected for quantitative measurement. The incident direction of the sound beam was perpendicular to the surface of the sample during the measurement. The interest region containing 16 points in 4 by 4 grids was selected to measure the velocity of sound, 64 points in 8 by 8 grids were selected to measure the attenuation and backscatter coefficients of the sample and each grid was separated by 80 µm.
Sample preparation
Liver tissue samples were obtained from the Meat Processing Plant. In the experiment, the sample was clamped between plastic membrane and reflector quartz flat in a specially designed sample holder and both sides of the sample were cut at -10 °C using Cryostat 2 700 (Frigocut, Reichert-june, Germany) to make the surface with a homogenous thickness between 0.5-1.5 mm. Then the sample was thawed in saline solution and sealed with a thin plastic membrane. In the study, four normal liver tissue samples and five cirrhotic liver tissue samples (hepatocirrhosis) were used. A slice from each sample was stained. The corresponding histological diagram and ultrasound image were obtained.
RESULTS
Images
The ultrasound imaging of normal and cirrhotic liver tissues and their corresponding pathological slices are shown in Figure 1A-1D. From the images, some microstructures of the tissue could be observed. However, the image of normal liver tissue (Figures 1A, 2B) was different from that of cirrhotic liver tissue (Figures 1C, 1D). The image of normal liver tissue was ultrasonically homogenous while the image of cirrhotic liver tissue was contrary to that of normal liver tissue. From the imaging, we could see the hyperecho of the cirrhotic liver. Some short and strong echoes caused by hyperplasia of connective tissue occurred.
Figure 1.
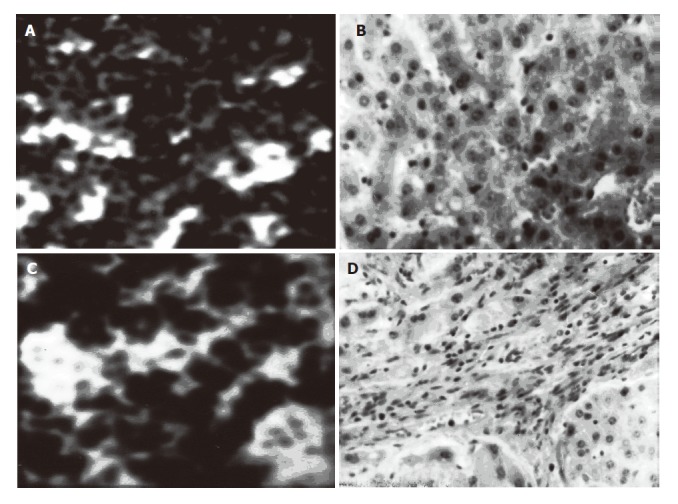
Ultras-ound images of normal and cirr-hotic liver and their corresponding pa-thological slices A: ultrasound imaging of normal liver; B: pathological slice of normal liver; C:ultrasound imaging of cirrhotic liver; D: pathological slice of cirrhotic liver.
Sound velocity, attenuation and backscatter coefficient
A summary of the acoustic parameters measured in the liver are listed in Table 1.The experimental results showed that the acoustic parameters from cirrhotic liver were larger than those from normal liver. The sound velocity in normal liver was 1 577 m/s and 1 631 m/s in cirrhotic liver, which coincides with the sound velocity (1 600 m/s) in bovine liver[22]. At 35 MHz, the attenuation coefficient was 3.0 dB/mm in normal liver and 4.1 dB/mm in cirrhotic liver. However, it has been reported to be 9.73 dB/mm at 70 MHz[22] and 0.89 dB/mm at 10.0 MHz respectively in liver[23]. The backscatter coefficient was 0.00431/Srmm at 35 MHz in cirrhotic liver and 0.00303/Srmm in normal liver. The power-law fits with the backscatter coefficient in normal liver as a function of frequency. However, it has been reported to be (2.66×10-6±1.43×10-6)f1.98/Srmm) and (4.85×10-6±1.52×10-6)f1.91/Srmm in cirrhotic liver[24]. The increase of backscatter coefficient is caused by hyperplasia of the connective tissue in cirrhotic liver (Figure 2). The backscatter occurs when the size of backscatter structure is similar to the wavelength of the sound beam, suggesting that the size of backscatters is within the wavelength of the ultrasound wave and the size of porcine liver lobules may be similar to it. The frequency-dependent decrease in cirrhotic liver reflects the increase of scattering elements in the liver.
Table 1.
Sound speed and attenuation and acoustic backscatter coefficient (mean ±SD)
| Normal liver | Cirrhotic liver | ||
| Sound speed (m/s) | 1577 ± 7 | 1631 ± 5 | |
| Attenuation | α1 | 0.121 ± 0.045 | 0.082 ± 0.023 |
| (dB/mm) | α35 | 3.0 ± 0.6 | 4.1 ± 0.8 |
| m | 0.9 | 1.1 | |
| Backscatter coefficient | μ1 | 2.66 × 10-6 ± 1.43 ×10-6 | 4.85 × 10-6 ± 1.52 ×10-6 |
| (1/Srmm) | μ35 | 0.00303 ± 0.00015 | 0.0043 ± 0.00025 |
| n | 1.98 | 1.91 | |
Figure 2.
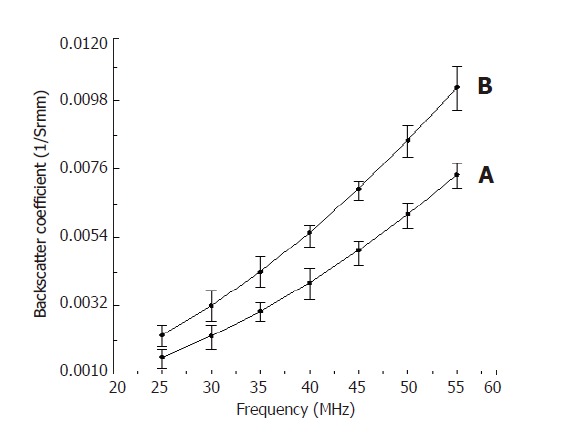
Backscatter coefficients changing with frequency in normal liver (A) and cirrhotic liver (B).
DISCUSSION
Ultrasonic tissue characterization based on spectrum analysis of backscattered radio frequency (RF) echo signals provides information regarding the acoustic properties of tissue, such as the size, concentration, and acoustic impedance of scatterers that are not available by conventional imaging methods[25,26]. The ultrasonic backscatter coefficient in human liver was measured in the range of 2.0-4.0 MHz[24]. The ultrasonic propagation properties at 100 MHz in excessively fatty rat liver were studied[27]. Strong correlation of ultrasonic speed with both water concentration and fat concentration in the liver was observed. The ultrasonic attenuation and velocity in rat liver as a function of fat concentration at 100 MHz were investigated[28] and the acoustic properties of freshly excised bovine liver were characterized at the frequency range between 20-200 MHz by the ultrasonic spectroscope system[22].
On the other hand, ultrasonic imaging systems in medicine depend fundamentally upon analysis of the ultrasonic scattering properties of the soft tissues investigated. However, the mechanism governing ultrasonic scattering within most soft tissues have not been delineated. In many cases the absolute magnitude of the scattering process has not been accurately quantified. The relationship between collagen and ultrasonic backscatter in myocardial tissue was evaluated[3]. The dependence of the ultrasonic scatter coefficient on collagen concentration in mammalian tissues was addressed[29]. And the mean-scatter spacing estimated with spectral correlation was used for tissue characterization[30]. However, there is little report on tissue characterization of hepatic tissues in the range between 25-55 MHz.
In this study, ultrasound images and sound parameters for hepatic tissues were studied by using C-scan ultrasound system at the frequency between 25-55 MHz. The above experimental results indicate that the resolution of high frequency image is much better than that obtained at low ultrasound frequency because some microstructure of the tissue can be seen. The information obtained from the ultrasound image is coincided with the histological feature of the sample. Furthermore, the samples need not to be stained. The acoustic parameters especially the sound backscatter coefficient are sensitive to the changes of the tissues and can be used to differentiate between the normal and pathological tissues, the frequency dependence of backscatter coefficient could be reflected by the change of scattering elements and may be employed as a parameter for characterizing tissues and their physiological states. Therefore the high frequency image system is a useful device for high-resolution image and tissue characterization within the high frequency range.
Footnotes
Supported by the National Natural Science Foundation of China, No. 10204014
S- Editor Guo SY L- Editor Wang XL E- Editor Cao L
References
- 1.Shung KK, Reid JM. Ultrasound Scattering from tissues. Ultrasonic Symposium proceedings (IEEE Cat. 77 CH1264-ISU) 1977. p. 203. [Google Scholar]
- 2.Fei DY, Shung KK. Ultrasonic backscatter from mammalian tissues. J Acoust Soc Am. 1985;78:871–876. doi: 10.1121/1.393115. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell M, Mimbs JW, Miller JG. The relationship between collagen and ultrasonic attenuation in myocardial tissue. J Acoust Soc Am. 1979;65:512–517. doi: 10.1121/1.382352. [DOI] [PubMed] [Google Scholar]
- 4.Madaras EI, Perez J, Sobel BE, Mottley JG, Miller JG. Anisotropy of the ultrasonic backscatter of myocardial tissue: II. Measurements in vivo. J Acoust Soc Am. 1988;83:762–769. doi: 10.1121/1.396119. [DOI] [PubMed] [Google Scholar]
- 5.Barger JE. Brain tissues classification by its ultrasonic backscatter. IEEE Trans on sonics & ultrasonics. 1981;28:311–315. [Google Scholar]
- 6.Nicholas D. Evaluation of backscattering coefficient for excised human tissues: results interpretations and associated measurement. Ultrasound in Med and Biol. 1982;1:17–28. [Google Scholar]
- 7.D'Astous FT, Foster FS. Frequency dependence of ultrasound attenuation and backscatter in breast tissue. Ultrasound Med Biol. 1986;12:795–808. doi: 10.1016/0301-5629(86)90077-3. [DOI] [PubMed] [Google Scholar]
- 8.Foster FS, Strban M, Austin G. The ultrasound macroscope: initial studies of breast tissue. Ultrason Imaging. 1984;6:243–261. doi: 10.1177/016173468400600301. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull DH. Ultrasound backscatter microscopy of mouse embryos. Methods Mol Biol. 2000;135:235–243. doi: 10.1385/1-59259-685-1:235. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull DH, Ramsay JA, Shivji GS, Bloomfield TS, From L, Sauder DN, Foster FS. Ultrasound backscatter microscope analysis of mouse melanoma progression. Ultrasound Med Biol. 1996;22:845–853. doi: 10.1016/0301-5629(96)00107-x. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull DH, Bloomfield TS, Baldwin HS, Foster FS, Joyner AL. Ultrasound backscatter microscope analysis of early mouse embryonic brain development. Proc Natl Acad Sci U S A. 1995;92:2239–2243. doi: 10.1073/pnas.92.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbull DH, Starkoski BG, Harasiewicz KA, Semple JL, From L, Gupta AK, Sauder DN, Foster FS. A 40-100 MHz B-scan ultrasound backscatter microscope for skin imaging. Ultrasound Med Biol. 1995;21:79–88. doi: 10.1016/0301-5629(94)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.Ye SG, Harasiewicz KA, Pavlin CJ, Foster FS. Ultrasonic characterization of normal ocular tissue in the frequency range from 50MHz to 100MHz. IEEE Transactions on ultrasonics, ferroelectrics and frequency control. 1995;42:8–14. [Google Scholar]
- 14.Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98:287–295. doi: 10.1016/s0161-6420(91)32298-x. [DOI] [PubMed] [Google Scholar]
- 15.Pavlin CJ, Sherar MD, Foster FS. Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology. 1990;97:244–250. doi: 10.1016/s0161-6420(90)32598-8. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood GR, Ryan LK, Hunt JW, Foster FS. Measurement of the ultrasonic properties of vascular tissues and blood from 35-65 MHz. Ultrasound Med Biol. 1991;17:653–666. doi: 10.1016/0301-5629(91)90096-f. [DOI] [PubMed] [Google Scholar]
- 17.Meyer CR, Chiang EH, Fechner KP, Fitting DW, Williams DM, Buda AJ. Feasibility of high-resolution, intravascular ultrasonic imaging catheters. Radiology. 1988;168:113–116. doi: 10.1148/radiology.168.1.3289084. [DOI] [PubMed] [Google Scholar]
- 18.Yano T, Fukukita H, Umeo S, Fukumoto A. 40MHz ultrasound diagnostic system for dermatology examination. Proc IEEE Ultrason Symp. 1987:875–878. [Google Scholar]
- 19.Liu XZ, Ye SG, Gong XF, Zhang WY, Jia YQ. High frequency ultrasound image and tissue characterization for human thyroid. Zhonghua Shenwu Yixue Gongcheng. 2001;20:110–115. [Google Scholar]
- 20.Sherar MD, Starkoski BG, Taylor WB, Foster FS. A 100 MHz B-scan ultrasound backscatter microscope. Ultrason Imaging. 1989;11:95–105. doi: 10.1177/016173468901100202. [DOI] [PubMed] [Google Scholar]
- 21.Sherar MD, Noss MB, Foster FS. Ultrasound backscatter microscopy images the internal structure of living tumour spheroids. Nature. 1987;330:493–495. doi: 10.1038/330493a0. [DOI] [PubMed] [Google Scholar]
- 22.Akashi N, Kushibiki J, Chubachi N, Dunn F. Acoustic properties of selected bovine tissues in the frequency range 20-200 MHz. J Acoust Soc Am. 1995;98:3035–3039. doi: 10.1121/1.413827. [DOI] [PubMed] [Google Scholar]
- 23.Frizzell LA, Carstensen EL, Davis JD. Ultrasonic absorption in liver tissue. J Acoust Soc Am. 1979;65:1309–1312. doi: 10.1121/1.382749. [DOI] [PubMed] [Google Scholar]
- 24.Wear KA, Garra BS, Hall TJ. Measurements of ultrasonic backscatter coefficients in human liver and kidney in vivo. J Acoust Soc Am. 1995;98:1852–1857. doi: 10.1121/1.413372. [DOI] [PubMed] [Google Scholar]
- 25.Hosokawa T, Sigel B, Machi J, Kitamura H, Kolecki RV, Justin JR, Feleppa EJ, Tuszynski G, Kakegawa T. Experimental assessment of spectrum analysis of ultrasonic echoes as a method for estimating scatterer properties. Ultrasound Med Biol. 1994;20:463–470. doi: 10.1016/0301-5629(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 26.Lizzi FL, Greenebaum M, Feleppa EJ, Elbaum M, Coleman DJ. Theoretical framework for spectrum analysis in ultrasonic tissue characterization. J Acoust Soc Am. 1983;73:1366–1373. doi: 10.1121/1.389241. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien WD, Erdman JW, Hebner TB. Ultrasonic propagation properties (@ 100 MHz) in excessively fatty rat liver. J Acoust Soc Am. 1988;83:1159–1166. doi: 10.1121/1.396060. [DOI] [PubMed] [Google Scholar]
- 28.Tervola KM, Gummer MA, Erdman JW, O'Brien WD. Ultrasonic attenuation and velocity properties in rat liver as a function of fat concentration: a study at 100 MHz using a scanning laser acoustic microscope. J Acoust Soc Am. 1985;77:307–313. doi: 10.1121/1.392229. [DOI] [PubMed] [Google Scholar]
- 29.Pohlhammer J, O'Brien WD. Dependence of the ultrasonic scatter coefficient on collagen concentration in mammalian tissues. J Acoust Soc Am. 1981;69:283–285. doi: 10.1121/1.385349. [DOI] [PubMed] [Google Scholar]
- 30.Varghese T, Donohue KD. Mean-scatterer spacing estimates with spectral correlation. J Acoust Soc Am. 1994;96:3504–3515. doi: 10.1121/1.410611. [DOI] [PubMed] [Google Scholar]


